Abstract
Alkaptonuria (AKU) is an ultra-rare disease developed from the lack of homogentisic acid oxidase activity, causing homogentisic acid (HGA) accumulation that produces a HGA-melanin ochronotic pigment, of unknown composition. There is no therapy for AKU. Our aim was to verify if AKU implied a secondary amyloidosis. Congo Red, Thioflavin-T staining and TEM were performed to assess amyloid presence in AKU specimens (cartilage, synovia, periumbelical fat, salivary gland) and in HGA-treated human chondrocytes and cartilage. SAA and SAP deposition was examined using immunofluorescence and their levels were evaluated in the patients' plasma by ELISA. 2D electrophoresis was undertaken in AKU cells to evaluate the levels of proteins involved in amyloidogenesis. AKU osteoarticular tissues contained SAA-amyloid in 7/7 patients. Ochronotic pigment and amyloid co-localized in AKU osteoarticular tissues. SAA and SAP composition of the deposits assessed secondary type of amyloidosis. High levels of SAA and SAP were found in AKU patients' plasma. Systemic amyloidosis was assessed by Congo Red staining of patients' abdominal fat and salivary gland. AKU is the second pathology after Parkinson's disease where amyloid is associated with a form of melanin. Aberrant expression of proteins involved in amyloidogenesis has been found in AKU cells. Our findings on alkaptonuria as a novel type II AA amyloidosis open new important perspectives for its therapy, since methotrexate treatment proved to significantly reduce in vitro HGA-induced A-amyloid aggregates.
Keywords: Ochronosis, Amyloidosis, Homogentisic acid
Highlights
► Alkaptonuria (no therapy) is a novel type II secondary amyloidosis. ► Ochronotic melanin-based pigment and SAA/SAP amyloid co-localize in Alkaptonuria. ► High levels of SAA and SAP were found in AKU patients' plasma. ► Systemic amyloidosis was assessed in patients' abdominal fat and salivary gland. ► Methotrexate reduces (- 97%) HGA-induced A-amyloid aggregates.
1. Introduction
Alkaptonuria (AKU; MIM no. 203500) is a rare disease (1:250,000–1,000,000 incidence) resulting from a deficiency of the enzyme homogentisate1,2-dioxygenase (HGO) that splits the aromatic ring of homogentisic acid (HGA, 2,5-dihydroxyphenylacetic acid), an intermediary product of tyrosine and phenylalanine catabolism in the liver [1]. This leads to the accumulation of HGA that cannot be further metabolized. A portion of HGA is excreted daily in the urine where it imparts a characteristic black discoloration upon oxidation. In urine, as in tissues, HGA oxidizes to benzoquinone acetic acid (BQA), which in turn forms HGA-melanin-based polymers [2], deposited in the connective tissue, most commonly the joints, cardiovascular system, kidney and skin [3], causing a pigmentation known as “ochronosis”. Polymer deposition in cartilage leads to degeneration, chronic inflammation and osteoarthritis. Musculoskeletal involvement is the most serious complication, leading to a severe and sometimes crippling form of arthropathy, which is the most common clinical presentation of AKU and often mimics ankylosing spondylitis [4]. AKU patients sometimes suffer from cardiovascular disease (frequent cause of death [5]) and kidney disease [6].
Although AKU pathological features are clinically described, its molecular basis has not been explored to any significant degree, because of the lack of suitable models to study the disease. We introduced novel human ochronotic cell, tissue and serum models and undertook pre-clinical testing of potential antioxidant therapies for AKU [1,7–11]. These models contributed to understanding HGA effects on cell viability [9,12], cell protein expression [9,10,12] and joint destruction in AKU [2]. Both intra- and extra-cellular pigmented deposition indicates that HGA cannot be the sole factor causing it and suggests the potential role/presence of other unidentified proteins [13].
There is no effective cure for AKU at the moment. Treatment is symptomatic, although this is recommended for early-stage of the disease while for the end-stage, total joint replacement is required.
Secondary amyloid-A (AA) amyloidosis is a serious complication of chronic inflammatory conditions such as rheumatoid arthritis (RA) and its amyloid deposition process involves a cleaved product of the acute-phase protein serum amyloid A (SAA) [14]. AA amyloidosis occurs in patients with poorly controlled chronic inflammatory disease, mainly RA, ankylosing spondylitis, and familial Mediterranean fever.
In the present paper, we provided experimental evidence that AKU osteoarticular tissue contains AA-amyloid deposits. This is the first report, to the best of our knowledge, of secondary amyloidosis associated with AKU. This opens new perspectives for AKU therapy and we also showed that methotrexate was able to significantly prevent in vitro HGA-induced A-amyloid aggregates.
2. Materials and methods
The whole study was conducted following the approval of the local University Hospital Ethics Committee. All patients gave a written informed consent prior to inclusion in the study.
All reagents were from Sigma-Aldrich (St. Louis, MO), if not differently specified.
2.1. AKU samples
Alkaptonuric specimens were obtained from seven AKU patients (Table 1). Healthy human articular cartilage was obtained from patients without any history of rheumatic diseases, who underwent surgical knee joint sampling. Tissue was removed only from healthy, glossy and completely intact articular cartilage surface.
Table 1.
Alkaptonuria patients enrolled for the study and their characteristics. F: female, M: male, Syn: synovia, Car: cartilage, 1: SAA, 2: SAP, 3: beta2-microglobulin, 4: a-synuclein, 5: Ig-l light chain, 6: prealbumin, 7: Pmel17, N: negative, P: positive, Asc: ascorbic acid (1 g/day), Nim: nimesulide (100 mg × 2/day), n.d.: not determined.
| Features | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age | 62 | 45 | 60 | 52 | 69 | 58 | 61 |
| Sex | F | M | M | M | F | F | M |
| Backbone impairment | 4/4 | 2/4 | 4/4 | 2/4 | 4/4 | 4/4 | 3/4 |
| Articular joints impairment | 4/4 | 2/4 | 4/4 | 3/4 | 4/4 | n.d. | 3/4 |
| Orthopedic surgical interventions | 2 | 1 | 5 | 2 | 2 | 1 | 3 |
| Cardiovascular involvement | 2/4 | 0/4 | 0/4 | 3/4 | 2/4 | n.d. | 1/4 |
| Serology | |||||||
| SAA (mg/L) | 65.64 | 3.43 | 134.69 | 99.36 | 117.70 | 87.14 | 97.44 |
| SAP (mg/L) | 37.008 | 36.362 | 68.042 | 46.615 | 47.996 | 25.434 | 39.996 |
| Histology | |||||||
| Congo Red staining | SynP, CarP | SynP, CarP | SynP, CarP | SynP, CarP | SynP, CarP | SynP, CarP | SynP, CarP |
| Th-T staining | SynP, CarP | SynP, CarP | SynP, CarP | SynP, CarP | SynP, CarP | SynP, CarP | SynP, CarP |
| 1P,2P, | 1P,2P, | 1P,2P, | 1P,2P, | 1P,2P, | 1P,2P, | 1P,2P, | |
| Immunohistology | 3N,4N, | 3N,4N, | 3N,4N, | 3N,4N, | 3N,4N, | 3N,4N, | 3N,4N, |
| 5N,6N,7N | 5N,6N,7N | 5N,6N,7N | 5N,6N,7N | 5N,6N,7N | 5N,6N,7N | 5N,6N,7N | |
| Location of amyloid | Hip | Knee | Hip | Knee | Knee | Hip | Knee |
| Treatment and medication | Asc | Asc, Nim | No | Asc | No | Asc | No |
2.2. AKU cell and tissue models
We previously developed original cell and organotypic ex vivo AKU models based on human chondrocytes or articular cartilage treated with 0.33 mM HGA up to the development of ochronosis, as described [10–12].
2.3. Congo Red (CR) staining
Amyloid fibrils appear as twisted rods composed of cross-beta sheet structures that selectively bound the dye Congo Red and Thioflavin-T. A version of Romhányi's original CR staining method [15] modified according to Bély and Apáthy [16] was adopted. Sections of 3–5 μm thickness of fresh cartilage, synovia, abdominal fat and salivary gland specimens were fixed in cooled 96% ethanol 10 min, rinsed in distilled water, incubated in 1% CR for 40 min, washed in water, incubated 10 s in 1 mL 1% sodium hydroxide in 100 mL of 50% ethanol, incubated 30 s in Mayer's hematoxylin, sequentially washed in 50%, 75%, 95% ethanol, mounted and observed under a polarized light microscope (Zeiss Axio Lab.A1, Arese, Milano).
2.4. Thioflavin T (Th-T) staining
Samples incubated in 1% Th-T [17,18] were mounted and observed under a fluorescence microscope (excitation 450 nm, emission 482 nm).
2.5. Fluorescence microscopy
Synovia and cartilage samples in paraffin were cut in 3–5 μm slices and used for double immunofluorescence staining with anti-SAA and anti-serum amyloid P (SAP) antibodies (Santa Cruz Biotechnology, CA). Additional immunofluorescence assays were performed using anti-immunoglobulin light chain, anti-pre-albumin, anti-α-synuclein, anti-beta-2 microglobulin and anti-Pmel17 antibodies (all by Santa Cruz Biotechnology, CA). Intrinsic HGA-melanin fluorescence (excitation 633 nm and emission between 650 and 742 nm) was observed under a Rhodamine 123 filter.
2.6. Biochemical assays
Plasma SAA and SAP in AKU patients were measured by ELISA (Invitrogen-Life Technologies, Carlsbad, CA).
2.7. Statistical analysis
Student's t-test was used when appropriate. Two-tailed analysis with P value lower than 0.05 was considered significant. Correlation analysis was performed using Pearson's correlation.
2.8. Transmission electron microscopy (TEM)
AKU cartilage was fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (CB) pH 7.2 for 3 h at 4 °C. After rinsing in CB, samples were post-fixed in 1% osmium tetroxide in CB for 2 h at 4 °C, dehydrated in a graded series of ethanol and embedded in a mixture of Epon–Araldite resins. Thin sections, obtained with a Reichert ultramicrotome, were stained with uranyl acetate and lead citrate and observed with a TEM FeiTecnai G2 spirit at 80 Kv.
2.9. Chondrocyte proteomic analysis
Cell cultures of AKU or healthy (control) chondrocytes were washed twice with sterile PBS and resuspended in a buffer containing 65 mM DTE, 65 mM CHAPS, 9 M urea, and 35 mM Tris-base. Cell disruption was achieved by sonicating in an ice bath and protein content was assessed. A total of 50 μg of protein samples were submitted to 2D electrophoresis (2DE), as described [9]. Digitalized images were obtained by ImageScanner III (GE-Healthcare, Milano) and then qualitatively and quantitatively analyzed by the ImageMaster software (GE-Healthcare). The increasing/decreasing index (fold change) was calculated as the ratio of spot relative volume between the different gel maps. Protein spot identification was obtained as described [9,19].
3. Results
3.1. Congo Red stained AKU cartilage, synovia and chondrocytes
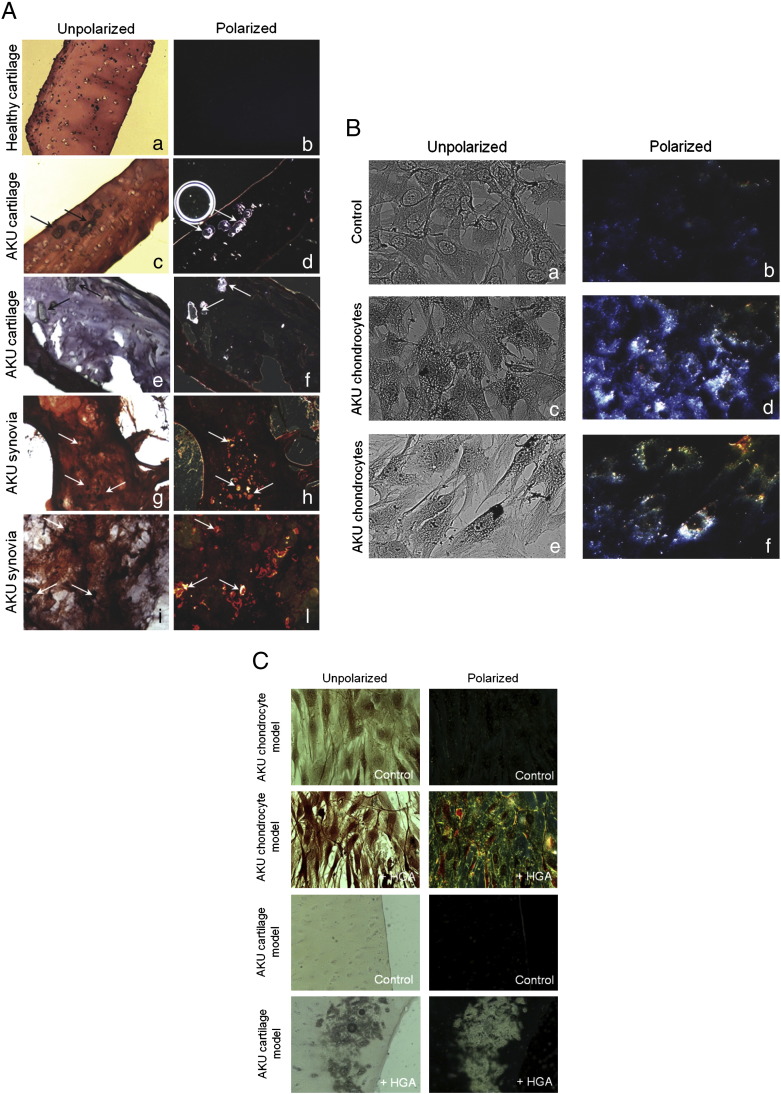
CR staining under polarized light of AKU cartilage of elderly patients (58 to 69 years) showed green birefringence as well as ochronotic cartilage fragments (shards) while control healthy cartilage did not. The size and the prevalence of cartilagineous amyloid in AKU patients seemed to be related to disease progress. We observed interconnected amyloid deposits in AKU synovial tissues and ochronotic cartilage shards embedded in severely degraded synovium [Fig. 1A(H,L)]. Amyloid deposits appeared along the surface and more deeply [Fig. 1A(H,L)]. CR birefringence was superimposing the ochronotic shards (Fig. 1A, compare G with H and I with L). CR-positive amyloid deposits were revealed in AKU chondrocytes isolated from patients (Fig. 1B).
Fig. 1.
A) Congo Red stained AKU cartilage and synovia. A, B: Healthy cartilage; C, D: cartilage from Patient 4; E, F: cartilage from Patient 5. G, H: Synovia from Patient 4; I, L: synovia from Patient 5. Analogous results were obtained from specimens of other patients. M–P: Congo Red staining of HGA-treated human healthy cartilage sections. M: Control, untreated cartilage model; O, P: HGA-treated cartilage model. In O an ochronotic shard is well visible showing a remarkable birefringence in P. Arrows indicate ochronotic shards. Magnification 20 ×. Representative images from a triplicate set are shown. B) Congo Red stained AKU chondrocytes. Congo Red birefringence was observable in ex vivo AKU chondrocytes. A, B: Control, healthy human chondrocytes; C, D: chondrocytes from AKU Patient 1; E, F: chondrocytes from AKU Patient 7; Analogous results were obtained from specimens of other patients. Magnification 20 ×. Representative images from a triplicate set are shown. C) Congo Red stained cell and cartilage AKU models. Upper panels) Congo Red birefringence was observable in human primary cultured chondrocytes treated with 0.33 mM HGA. Control: untreated chondrocytes. Magnification 10 ×; Lower panels) Congo Red staining of 0.33 mM HGA-treated human healthy cartilage sections: an ochronotic shard is well visible showing a remarkable birefringence. Control: untreated cartilage. Magnification 20 ×. Representative images from a triplicate set are shown.
3.2. Congo Red stained cell and cartilage AKU models
Using our AKU models [10–12] we confirmed CR staining of chondrocytic and cartilage pigmented areas (Fig. 1C) and at the same time we proved that the amyloid presence was due to HGA, suggesting its potential role in the formation of amyloid structures in vivo. Similar staining of deposits was visible in AKU patients' cartilage [Fig. 1A(C–F)], perfectly reproducing the ex vivo situation, since CR birefringence of AKU cartilage model exactly overlapped the pigmented areas (Fig. 1C).
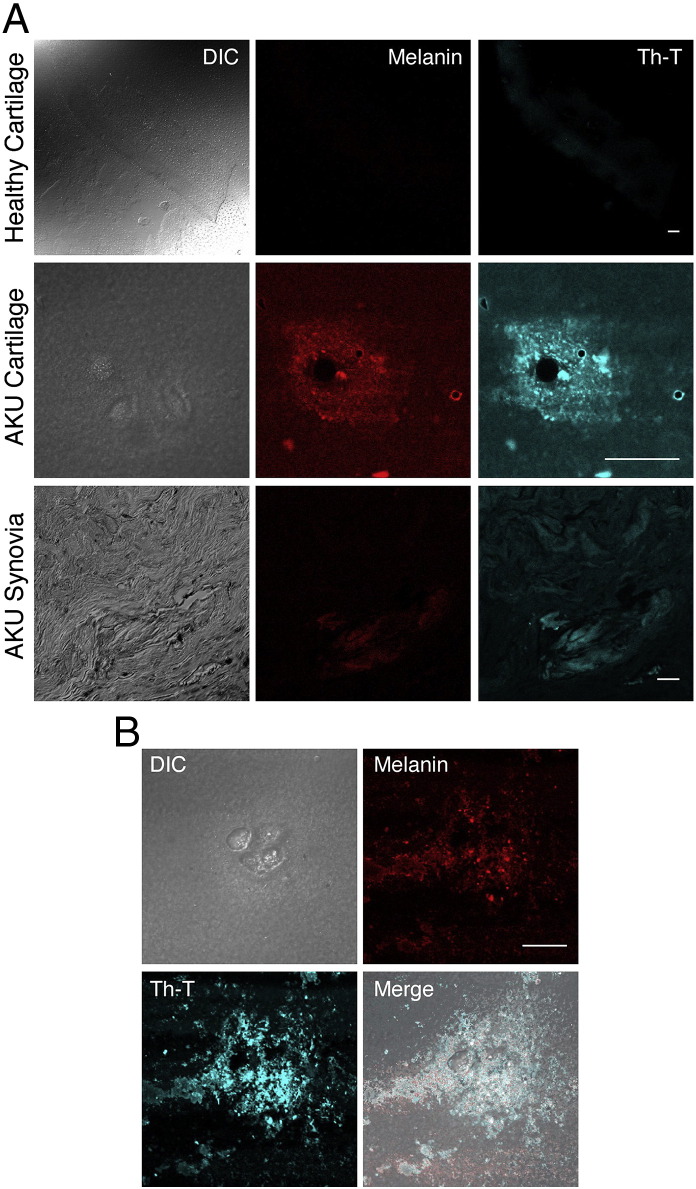
3.3. Thioflavin T stained AKU cartilage and AKU synovia and amyloid co-localized with melanin-like deposits
To confirm the presence of amyloid aggregates in cartilage and synovial tissue from AKU patients we performed the Th-T assay. Th-T fluorescence was evident and perfectly superimposing the ochronotic shards in AKU tissues (Fig. 2A). Ochronotic deposits are defined as melanin‐like pigments and we wanted to ascertain if such structures could potentially co-localize with amyloid deposits in AKU cartilage and synovia. Th-T fluorescence overlapped HGA-melanin fluorescence and double exposure of phase contrast and fluorescence allowed the simultaneous localization of amyloid and ochronotic shards (Fig. 2B).
Fig. 2.
Thioflavin T stained AKU cartilage and AKU synovia and amyloid co-localized with melanin-like deposits. A) Th-T fluorescence of AKU synovial and cartilage specimens shown by confocal microscopy. Melanin fluorescence was revealed under a Rhodamine 123 filter. Cartilage was from AKU Patient 7, synovia was from AKU Patient 3. Analogous results were obtained from specimens of other patients. Bar: 30 μm; B) co-localization of melanin and amyloid was revealed by merge of Th-T and melanin fluorescence. DIC: differential interference contrast. Bar: 22 μm. Representative images from a triplicate set are shown.
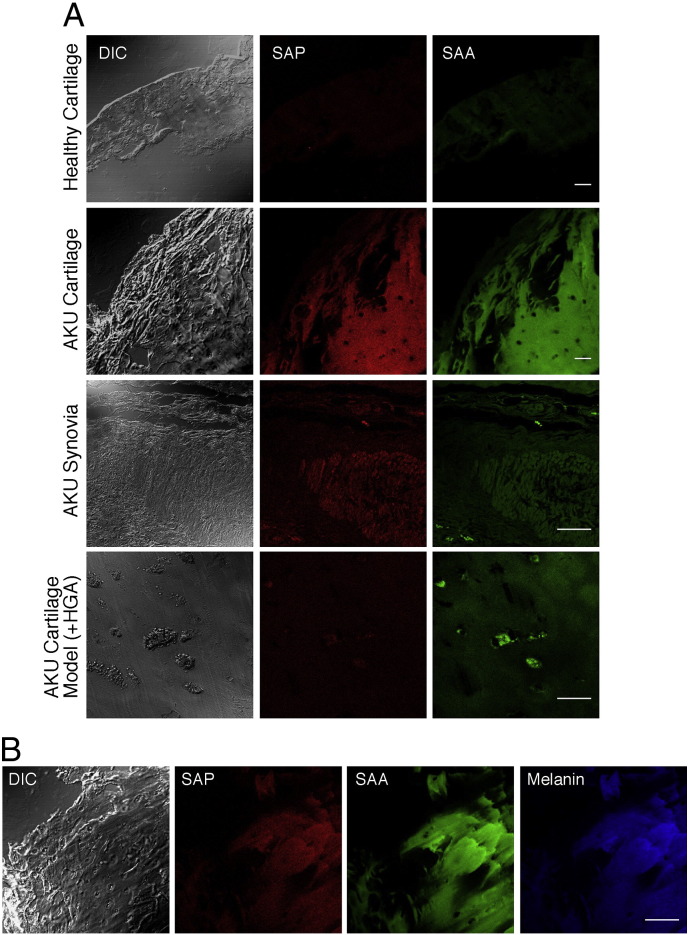
3.4. AKU is a SAA- and SAP-mediated secondary amyloidosis
SAA and SAP deposition in AKU cartilage and synovial specimens was examined using immunofluorescence techniques. Co-localization of SAA with SAP staining was detected in all of the examined tissues (Fig. 3A). No positivity for the presence of immunoglobulin light chains, pre-albumin, α-synuclein, beta-2 microglobulin and Pmel17 was observed (Table 1). The patterns of immunofluorescent staining did not appear to differ between SAP and SAA, although this latter was highly present in the cartilage from any AKU patient, suggesting a strong production and release of SAA by AKU chondrocytes and consequently high SAA and SAP circulating levels. Interestingly, SAA and SAP distribution in amyloid of AKU cartilage perfectly superimposed with HGA-melanin localization (Fig. 3B). Indeed, high plasma levels of both SAA and SAP were found in all AKU patients (Fig. 4A,B). AKU synovial sections showed particularly intensive SAP positivity in correspondence of ochronotic shards (Fig. 3A), especially in patients with high SAA and SAP plasma levels (Table 1, Fig. 4A,B).
Fig. 3.
SAA and SAP were present in amyloid deposits of AKU cartilage and synovia and both co-localized with melanin. A) SAA and SAP deposition in AKU cartilage and synovial specimens was detected by dual immunofluorescence technique. AKU cartilage and synovia showed high levels of SAA deposit superimposing SAP deposits. Positive staining for SAA and SAP was particularly intense in correspondence of ochronotic shards. Bar: 75 μm; B) AKU cartilage sections were dual-stained using antibodies specific for SAA SAP and compared to melanin fluorescence, resulting in a perfect co-localization of amyloid deposits and pigmented areas. Cartilage specimen was from Patient 6 and synovia specimen was from Patient 1. DIC: differential interference contrast. Bar: 150 μm. Representative images from a triplicate set are shown.
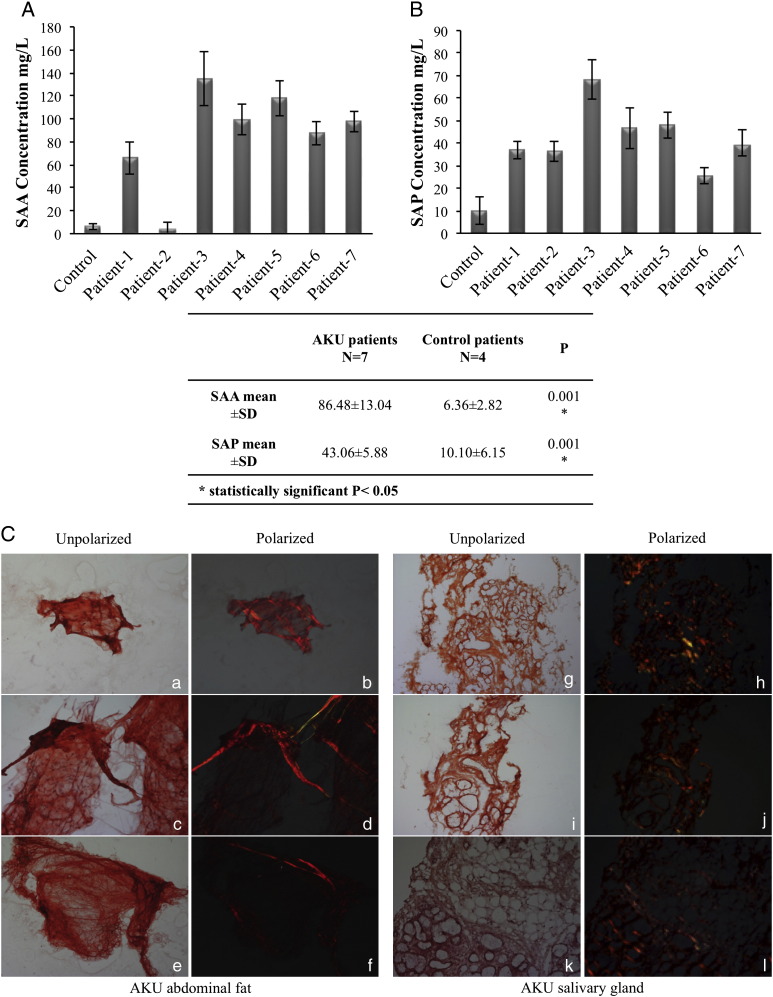
Fig. 4.
A, B) High SAA and SAP plasma levels in AKU patients. SAA (A) and SAP (B) plasma levels related with age and disease severity, thus reflecting also the progressive nature of AA amyloidosis. Experiments were performed in triplicate; data are presented as average values ± standard deviation. C) Congo Red staining of AKU subcutaneous periumbelical fat and AKU salivary gland tissues. Examples of CR fat smears and labial salivary gland biopsy of AKU Patients 3 and 5, under normal and in polarized light. CR staining confirmed the presence of amyloid deposition. Magnification 20 ×, a,b 10 ×. Representative images from a triplicate set are shown.
High plasma levels of both SAA and SAP were found in all AKU patients (Fig. 4A,B). Four patients were receiving oral antioxidant therapy, for at least 6 months before the time of study. Patient 2 had received an anti-inflammatory treatment. Three cases were untreated. The SAA level of AKU patients (Fig. 4, middle panel) ranged 3.43–134.69 mg/L with a mean of 86.48 ± 13.04 mg/L, while those of the control group ranged 4.23–8.92 mg/L with a mean of 6.36 ± 2.82 mg/L; the difference was statistically significant (P = 0.001). SAA levels in patients who had not received any treatment (mean = 116.61 ± 20.44 mg/L) resulted significantly higher than controls (P = 0.043). Correlation between SAA level and some of the disease parameters revealed statistically significant positive correlation for the age (r = 0.362, P = 0.02) and disease duration (r = 0.698, P = 0.0001). This significant correlation indicated that age and disease severity in AKU may be associated with raised SAA levels, thus reflecting also the progressive nature of type AA amyloidosis. Mean serum SAP in AKU patients was 43.06 ± 4.40 mg/L, which was statistically different (P = 0.001) from the values in 4 healthy controls (10 ± 5.6 μg/L). All AKU patients showed high SAP plasma levels, apparently not influencing disease severity (Fig. 4B). In the control population serum SAP was not related to age.
3.5. Congo Red stained periumbelical fat and salivary gland AKU specimens
Confirmation of systemic amyloidosis in AKU patients was obtained by CR staining of AKU abdominal fat aspiration and labial salivary gland biopsies (Fig. 4C), that has been proven as highly sensitive and reliable method for diagnosis of secondary amyloidosis [20,21]. Minor (labial) salivary gland and subcutaneous abdominal fat tissues from AKU patients showed amyloid presence in all samples examined (Fig. 4C).
3.6. Methotrexate (MTX) was able to prevent amyloid and to decrease pro-inflammatory cytokine release in an in vitro AKU chondrocytic model
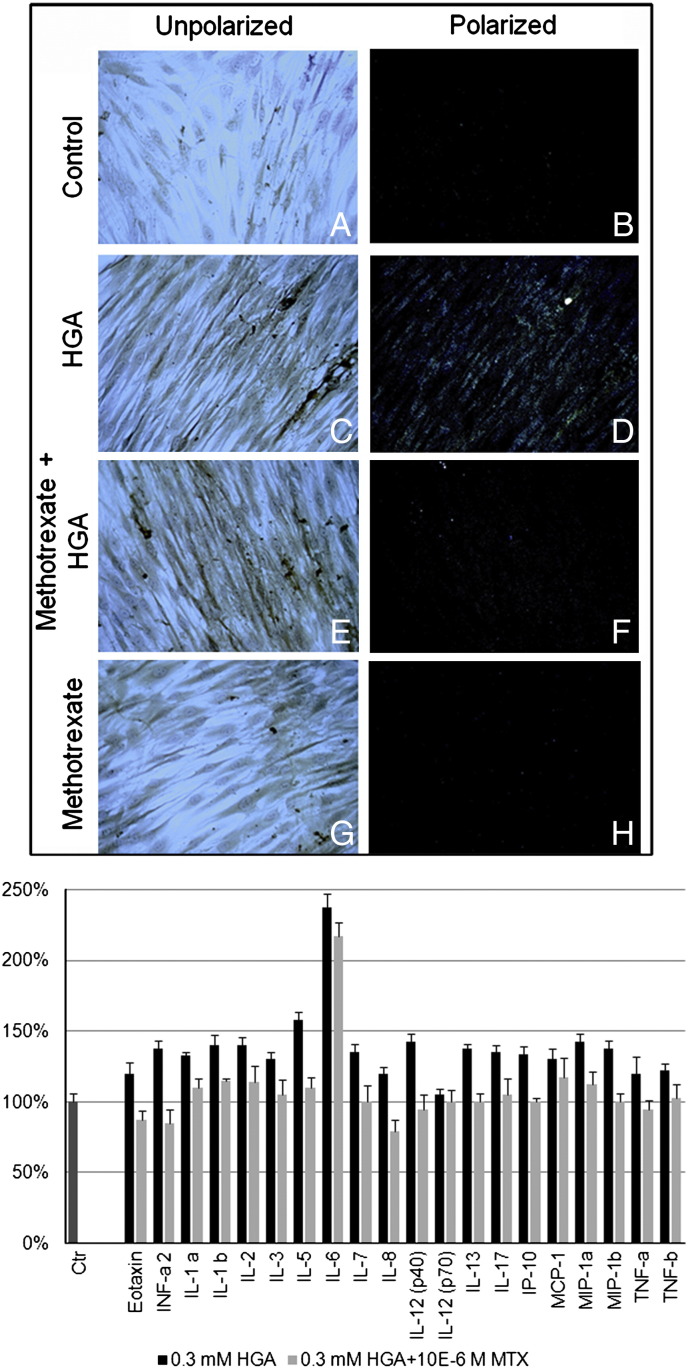
Our in vitro AKU model allowed a semi-quantitative analysis of the production of amyloid due to HGA addition and its reduction (− 97.2%) due to a treatment with 10− 9 M MTX (Fig. 5A), a concentration in the range of that administered to RA patients to keep low SAA plasma levels and thus control/reverse secondary amyloidosis [22]. HGA-treated chondrocytes released high levels of pro-inflammatory cytokines and MTX treatment proved to be able to decrease them or even restore control levels (Fig. 5B).
Fig. 5.
A) MTX treatment was able to prevent HGA-induced amyloid formation. CR birefringence was observable in human primary cultured chondrocytes treated with 0.33 mM HGA. Pre-treatment with 10− 9 M methotrexate was able to significantly inhibit the HGA-induced production of amyloid. Magnification 20 ×. Representative images from a triplicate set are shown; B) MTX reduced HGA-induced pro-inflammatory cytokines. Evaluation of the profile of pro-inflammatory cytokines induced by HGA treatment of human chondrocytes and the positive effect of MTX in significantly reducing or restoring control levels of pro-inflammatory cytokines. Cytokine concentrations were calculated using a standard curve established from serial dilutions of each cytokine standard and expressed as pg/mL. Experiments were performed in triplicate; data are presented as average values ± standard deviation.
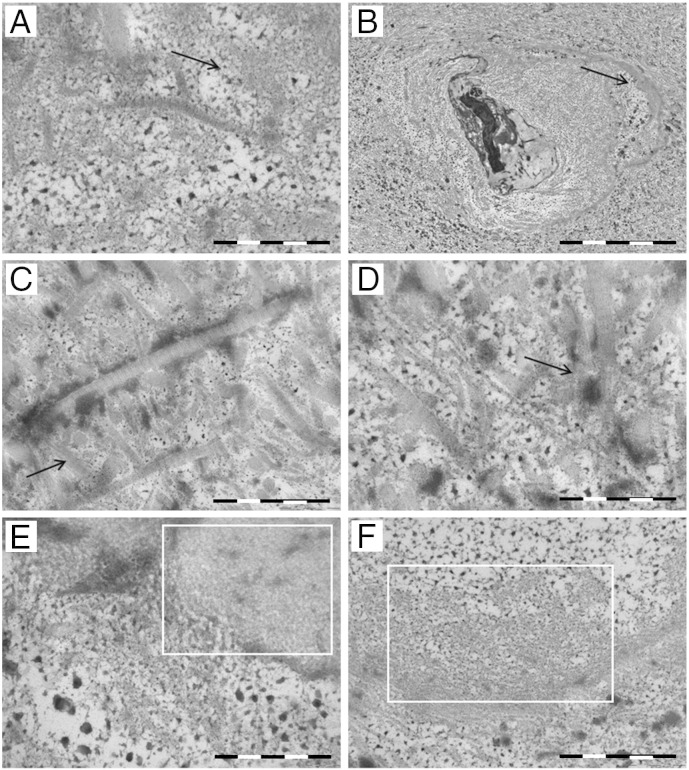
3.7. TEM observation of amyloid deposits in AKU cartilage
The darkness of AKU cartilage is the feature that differentiates ochronotic articular cartilage from other forms of arthritis. To better investigate the ultrastructure of amyloid deposits in AKU tissue, we performed an electron microscopical study of AKU cartilage samples (Fig. 6). Amyloid fibrils were seen in little bundles mainly nearby chondrocytes. In individual chondrocytes, an intense nuclear pigment deposition was visible: nuclei showed remarkable differences from normal chondrocytes, being pyknotic and frequently condensed and irregular (Fig. 6B). Amyloid fibrils in small aggregations without definite polarity spread out in the tissue [Fig. 6B(A,E,F)] as well as in around collagen fibrils [Fig. 6(A,E)] were evident. In several tissue areas, disruption of collagen fibers had occurred as a precursor of osteoarthritic changes [Fig. 6(C–D)] and the ultrastructure of AKU cartilage showed a remarkable sparse dotted pigmentation distributed within the tissue [Fig. 6(A,B,E,F)]; an alteration of collagen fibrils that appeared wavy and sometimes fragmented with loss of periodicity is also visible when amyloid fibrils merge with the collagen fibrils that were always mixed with the dispersed pigment [Fig. 6(A,D)].
Fig. 6.
TEM observation of amyloid deposits in AKU cartilage. Dispersed amyloid fibrils and bundles of parallel fibrils were present in articular cartilage from Patient 3. The collagen meshwork was in disarray and disruption of individual collagen fibrils fragmented where loss of periodicity is evident (A, C, D). The fibrils appeared interspersed with cross-striated collagen fibrils (C, D) and nearby ochronotic deposits in the chondrocytes were observable (B). Amyloid fibrils are blended and superimposed at times with sparsely dotted pigment (E, F). Arrows indicate amyloid and areas surrounded with white squares show the fibrillar nature of the deposits. Scale bars: A: 200 nm; B: 5 μm; C: 500 nm; D: 200 nm; E: 200 nm; F: 500 nm. Representative images from a triplicate set are shown.
3.8. Proteomic analysis of AKU chondrocytes
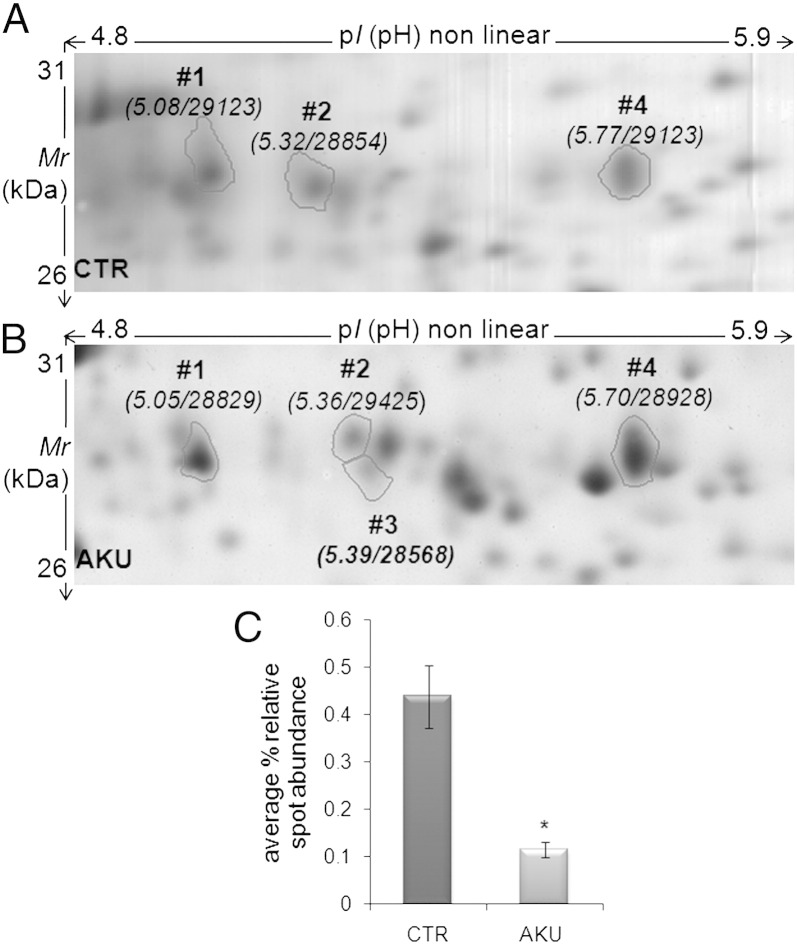
Proteomic analysis of chondrocytes from AKU patients revealed the abnormal expression of proteins involved in amyloidogenic processes (Table 2). One of the most remarkable cases was cathepsin D, that was markedly under-expressed (− 3.8 fold change) in AKU chondrocytes in respect to control (Fig. 7). The global analysis of the protein repertoires of chondrocytes from AKU patients [7] provided further support to our data on alkaptonuric amyloidosis, since several amyloidogenic proteins were found abnormally expressed in diseased cells. Cathepsin D, under-expressed in AKU cells, is necessary for a correct cleavage of SAA and has protective activity against development of type AA amyloid fibrils [23]. AlphaB-crystallin (HSP20), GRP75, HSP74 and ENPL (HSP90), all found under-expressed in AKU cells, are chaperones known to block amyloid aggregation in vitro and in cell and animal models [24,25], like also Protein DJ-I, known to prevent α-synuclein aggregation [26]. AlphaB-crystallin is also a novel mediator of chondrocyte matrix gene expression that may contribute to altered chondrocyte metabolism during OA development [27], but possibly also in AKU. AlphaB-crystallin had been previously found underexpressed in HGA-treated chondrocytes [9]. More generally, in our previous paper we found that HGA induced alteration of protein folding in human chondrocytes and caused production of high molecular weight protein aggregates [9]. Transgelin expression is induced following processing of the amyloid precursor protein in Alzheimer's disease and its overexpression significantly alters actin dynamics and mitochondrial function in neurons [28]. Gelsolin plays an important role in amyloidogenesis and inhibits amyloid-β fibrillization. A relationship between proteolytic cleavage of gelsolin and increased Aβ in the brain has been recently reported [29] and its decrease correlates with rate of decline in Alzheimer's disease [30]. Gelsolin amyloid disease (familial amyloidosis Finnish-type) derives from variants of gelsolin aberrantly processed by furin [31]. The proprotein convertase furin is responsible as well for the correct intra-melanosome processing of Pmel17, a key protein to properly assemble physiological amyloid in DOPA-melanin synthesis [32]. Within melanosomes, Pmel17 forms an amyloid matrix sequestering toxic intermediates produced during DOPA-melanin synthesis and templating melanin production [33,34].
Table 2.
Comparative proteomics of human AKU chondrocytes. Proteins whose synthesis was altered in AKU chondrocytes versus controls.
| Spot | ANa | Gene | Protein | Biological processesb | AKU chondrocytes/ctrc |
|---|---|---|---|---|---|
| GRP75 | P38646 | HSPA9 | Stress-70 protein, heat shock 70 kDa protein 9 or 75 kDa glucose-regulated protein | Implicated in the control of cell proliferation and cellular aging. May also act as a chaperone. Anti-apoptotic functions | − 2.1 |
| PARK7 | Q99497 | PARK7 | Protein DJ-1 | May function as a redox-sensitive chaperone and as a sensor for oxidative stress. Prevents aggregation of SNCA. | − 5.5 |
| PDIA1 | P07237 | P4HB | Protein disulfide-isomerase | Catalyzes the formation, breakage and rearrangement of disulfide bonds. At high concentrations, functions as a chaperone that inhibits aggregation of misfolded proteins. At low concentrations, facilitates aggregation (anti-chaperone activity). | − 2.3 |
| GELS | P06396 | GSN | Gelsolin | Binds to actin and to fibronectin. Calcium-regulated, actin-modulating protein that binds to the plus (or barbed) ends of actin monomers or filaments, preventing monomer exchange (end-blocking or capping). It can promote the assembly of monomers into filaments (nucleation) as well as sever filaments already formed. Defects in GSN are the cause of amyloidosis type 5 (AMYL5) [MIM: 105120], also known as familial amyloidosis Finnish type. | − 2.0 |
| TAGL | Q01995 | TAGLN | Transgelin | Actin cross-linking/gelling protein. Involved in calcium interactions and contractile properties of the cell that may contribute to replicative senescence. | + 17.0 |
| ENPL | P14625 | HSP90B1 | Endoplasmin, 94 kDa glucose-regulated protein, GRP-94, Heat shock protein 90 kDa beta member 1 | Molecular chaperone that functions in the processing and transport of secreted proteins. Functions in endoplasmic reticulum associated degradation (ERAD). Has ATPase activity. Plays a role in protein folding and transport, has anti-apoptotic functions. | − 4.8 |
| HSP74 | P34932 | HSPA4 | Heat shock 70 kDa protein 4 | Stress response, plays a role in the unfolded protein response. | − 5.4 |
| CATD | P07339 | CTSD | Cathepsin D | Acid protease active in intracellular protein breakdown. Involved in the pathogenesis of several diseases, AA amyloidosis included. | − 3.8 |
AN: accession number.
Protein biological processes retrieved by UniProt knowledgebase (http://www.uniprot.org/).
Fold-change in protein % relative abundance (as average values in case of multiple spots); (+) over-expressed proteins, (−) under-expressed protein according to the ratio calculated between AKU and control (ctr) cells.
Fig. 7.
Expression of cathepsin D in AKU chondrocytes. Whole cell protein extracts harvested from healthy human chondrocytes (control, A) and AKU chondrocytes (derived from ochronotic cartilage, B) were resolved through 2D-PAGE. Protein spots corresponding to cathepsin D are indicated with circles and numbers; pI and Mr values are reported in brackets. % relative abundance of each spot, calculated by ImageMaster during image analysis of a triplicate set of gels, is indicated with vertical bars ± standard deviation (C). CTR: control. P value < 0.05. Experiments were performed in triplicate; data are presented as average values ± standard deviation. Representative images from a triplicate set are shown.
4. Discussion
We present here original results showing that alkaptonuria is a novel secondary amyloidosis. All the conventionally adopted and universally accepted methods (CR and Th-T staining, TEM) succeeded in unequivocally assessing the presence of amyloid in our tissue and cellular samples, as well as the SAA and SAP composition of the deposits has been ascertained to assess secondary type of amyloidosis. CR positivity of periumbelical fat and salivary gland AKU specimens unequivocally confirmed systemic amyloidosis. Alkaptonuria is not a local disease, it is actually a complicating inflammatory multisystemic disease, involving many different organs [3,35]. Any body district expressing HGO may be affected in this disease: joints [36], heart [5], kidney [37], liver [38], eyes [4], marrow [39], bladder [40], and lung [41].
It is necessary to correctly define three forms of melanin (two of them are natural): i) DOPA-melanin or eumelanin synthesized in melanosomes of the melanocytes of the skin and in melanosomes of retinal-pigment epithelium, and ii) neuromelanin (NM) or DOPAmine-melanin synthesized in DOPAminergic neurons during all life long (NM accumulates linearly in nervous system during normal aging). A third type is the abnormal melanin, HGA-melanin found only in AKU.
Remarkably, we found that alkaptonuric amyloid co-localized with HGA-melanin ochronotic pigment. Our unprecedented findings are the first case, to our knowledge, in which ochronotic pigment is directly associated with amyloid. This evidence suggests that HGA polymer may be involved in amyloid deposition. The association of AKU and amyloidosis is in keeping with evidence that synoviocytes and chondrocytes may be important producers of amyloid in RA [14].
Reactive systemic AA amyloidosis is one of the most severe complications of several chronic rheumatic disorders [42]. Problems associated with these pathologies may present joint symptoms similar to AKU (stiffness, swelling, and movement limitation) due to the deposition of amyloid in the synovial membranes of the joint or of the tendon sheaths. In AKU AA-amyloidosis, the clinical features could be secondary to the deposition of ochronotic pigment in connective tissues. Amyloidoses are progressive diseases, with a lag period before the appearance of AA amyloidogenesis [42], congruently with the progressive nature of AKU whose symptoms are analogous to other joint diseases with ascertained secondary amyloidosis (RA, ankylosing spondylitis, familial Mediterranean fever). A case of acute anterior uveitis as the initial presentation of AKU mimicking ankylosing spondylitis has been recently reported [4]. Both uveitis and ankylosing spondylitis are SAA secondary amyloidoses.
Alkaptonuric arthritis resembles osteoarthritis (OA), but clinically is more like RA and in most patients with AKU there are frequent periods of acute inflammation as in RA. RA chondrocytes serve as a source of intra-articular SAA, suggesting an active role in RA pathogenesis [14]. Compared to RA, secondary amyloidosis is a new complication of AKU. We detected AA-amyloid in longstanding AKU patients, confirming amyloidosis to be a progressive disease.
Since SAA plasma levels do not correlate with age while SAA serum concentration provides prognostic information [43], the different SAA levels found in our AKU patients could help to grade AKU severity whose scoring system [44,45] is so far not established at the molecular level. The striking co-localization of HGA-melanin and amyloid suggests the participation of fluorescent oxidized HGA pigment in the formation of amyloid aggregates and a link between HGA oxidation and amyloid deposition. Melanin acts by trapping free radicals and its synthesis appears to represent a protective/compensatory process that removes excess reactive oxygen species (ROS).
RA and OA may present yellowish/brownish gray cartilage in some stages of these diseases, suggesting the presence of HGA-melanin. It has been proven that HGA produces melanin [2] and that HGA-melanin enhances inflammation. Consequently, in AKU the chronic accumulation of HGA and its auto-oxidized derivatives may initiate a variety of reactions that promote inflammatory responses and mediate tissue damage. Alkaptonuric arthritis develops after decades of life but its onset may be the consequence of repeated oxidative insults to selected target tissues initiated by HGA auto-oxidation. This may in turn induce the production of HGA-melanin as a reaction of cells to counteract oxidative stress. The chronic presence of HGA-melanin may cause a further inflammatory stimulus resulting in overproduction of SAA and SAP finally causing the formation of amyloid.
HGA, once injected into joints produces disabling damage, ochronosis, necrosis and inflammatory reactions [2]. Indeed, an inflammatory condition, suggesting a chronic inflammatory status, can be observed in AKU synovia [1,3]. In ochronotic arthropathy, macrophages surround the pigmented areas [13] and HGA-treated chondrocytes and synoviocytes show phagocytic features [27]. Interestingly, both gelsolin and cathepsin D are present in macrophages and gelsolin is down-regulated by cathepsin D [46], but we did not evaluate the presence of gelsolin in AKU deposits. This may be relevant not only for the initiation of fibril formation in AKU, but also for the dynamic balance proteinaceous amyloid deposits undergo. AA amyloid fibril may form starting from pre-existing fibrils seeds, and similarly pigment formation and distribution have been recently found to follow a nucleation process in AKU [13].
Proteomic analysis of human cells from AKU patients revealed the aberrant expression of several proteins involved in the control of folding/unfolding and amyloidogenic processes [7]. The under-expression of cathepsin D in cells from AKU patients is very interesting since it has been demonstrated that this protein plays a major key physiological role in completing SAA catabolism, preventing SAA from accumulating and serving as a precursor of AA amyloid fibrils [23,47].
It is intriguing that amyloid plays also a fundamental role in the regulation of melanin synthesis [48,49]. Our findings on AKU are, to the best of our knowledge, the first case of amyloid associated with melanin outside the melanosome compartment under pathological conditions. AKU is the second pathology after Parkinson's disease (PD) where amyloid is associated with a melanin-based pigmentation and also a parallel has been drawn between A-beta and DOPA-melanin with respect to the relation of these molecules and Alzheimer's disease (AD) and PD [48,50]. AD and PD are neurodegenerative diseases traditionally associated with amyloid fibrils, produced by β-amyloid and α-synuclein aggregation-prone proteins, respectively. The destruction of connective tissue by HGA is reminiscent of the neurotoxicity of 6-hydroxyDOPAmine [2]. Indeed, an association of PD and AKU has been reported [50]. Amyloid and melanin have different structures but share several common features with respect to synthesis, accumulation in aging, affinity for metals and roles in cell protection or toxicity, this latter mediated by inflammation by both types of molecules, and they can enter into a physiological or pathological process depending on the cell context [32,35]. In PD the colocalization of α-synuclein, the protein whose aggregation induces the formation of amyloid, and DOPA-melanin may facilitate the precipitation of α-synuclein and the consequent neuronal damage [49,50]. Analogously to PD, it is not clear if HGA-melanin is part of the toxic events that underlie AKU or a protective response that may slow the disease.
We suggest that, analogously to RA, AA is a secondary complication of AKU, due in this case, to a chronic inflammatory status derived from HGA-benzoquinone acetic acid (BQA)-melanin-induced oxidative stress.
5. Conclusions
Our findings on AKU as a novel AA amyloidosis open new perspectives for its treatment. In fact, AA tissue amyloid resolves following the cessation of inflammatory stimuli, the impetus that maintains high SAA plasma levels. This principle is supported by the excellent outcome of liver transplantation in patients affected by some forms of amyloidosis. For AKU amyloidosis, our present findings are supported by the completely successful reversal of ochronotic arthropathy following liver transplantation [41]. The control of the underlying inflammatory disorder can result in regression of the disease, as proven for some secondary amyloidogenic musculoskeletal disorders sharing clinical features with AKU. Suppression of SAA below 10 mg/L halts the progression of the disease and is associated with prolonged survival, with reversal of amyloid deposition and with recovery of organ function [26]. Low doses of MTX are safe and effective for the routine treatment of inflammatory arthritis and it has been successfully adopted to keep low SAA levels in RA in order to prevent and regress secondary amyloidosis [18]. MTX proved to have an excellent efficacy to inhibit the production of amyloid in our AKU model chondrocytes, suggesting the introduction of its use in AKU therapy. This treatment would be useful especially for those symptomatic AKU patients for whom the therapy with nitisinone (the only orphan drug so far recognized for alkaptonuria) failed in a clinical trial [51].
Acknowledgements
This work has been supported by Telethon Italy grant GGP10058. The authors also thank Toscana Life Sciences Orphan_1 project, Fondazione Monte dei Paschi di Siena 2008-2010, FP7 Research & Innovation Grant 304985-2 – DevelopAKUre, and aimAKU – Associazione Italiana Malati di Alcaptonuria (ORPHA263402).
References
- 1.Selvi E., Manganelli S., Mannoni A., Benucci M., Minacci C., Marcolongo R. Chronic ochronotic arthritis: clinical, arthroscopic, and pathologic findings. J. Rheumatol. 2000;27:2272–2274. [PubMed] [Google Scholar]
- 2.Martin J.P., Jr., Batkoff B. Homogentisic acid autoxidation and oxygen radical generation: implications for the etiology of alkaptonuric arthritis. Free Radic. Biol. Med. 1987;3:241–250. doi: 10.1016/s0891-5849(87)80031-x. [DOI] [PubMed] [Google Scholar]
- 3.Helliwell T.R., Gallagher J.A., Ranganath L. Alkaptonuria—a review of surgical and autopsy pathology. Histopathology. 2008;53:503–512. doi: 10.1111/j.1365-2559.2008.03000.x. [DOI] [PubMed] [Google Scholar]
- 4.Mathews J., Padhan P., John S., David S. Acute anterior uveitis as the initial presentation of alkaptonuria. J. Postgrad. Med. 2009;55(1):35–37. doi: 10.4103/0022-3859.48438. [DOI] [PubMed] [Google Scholar]
- 5.Pettit S.J., Fisher M., Gallagher J.A., Ranganath L.R. Cardiovascular manifestations of alkaptonuria. J. Inherit. Metab. Dis. 2011;34:1177–1181. doi: 10.1007/s10545-011-9339-z. [DOI] [PubMed] [Google Scholar]
- 6.Butany J.W., Naseemuddin A., Moshkowitz Y., Nair V. Ochronosis and aortic valve stenosis. J. Card. Surg. 2006;21:182–184. doi: 10.1111/j.1540-8191.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 7.D. Braconi, G. Bernardini, C. Bianchini, M. Laschi, L. Millucci, L. Amato, L. Tinti, T. Serchi, F. Chellini, A. Spreafico, A. Santucci, Biochemical and proteomic characterization of alkaptonuric chondrocytes, J. Cell. Physiol. (2011) 227(9) (2012) 3333–3343. [DOI] [PMC free article] [PubMed]
- 8.Braconi D., Laschi M., Amato L., Bernardini G., Millucci L., Marcolongo R., Cavallo G., Spreafico A., Santucci A. Evaluation of anti-oxidant treatments in an in vitro model of alkaptonuric ochronosis. Rheumatology (Oxford) 2010;49:1975–1983. doi: 10.1093/rheumatology/keq175. [DOI] [PubMed] [Google Scholar]
- 9.Braconi D., Laschi M., Taylor A.M., Bernardini G., Spreafico A., Tinti L., Gallagher J.A., Santucci A. Proteomic and redox-proteomic evaluation of homogentisic acid and ascorbic acid effects on human articular chondrocytes. J. Cell. Biochem. 2010;111:922–932. doi: 10.1002/jcb.22780. [DOI] [PubMed] [Google Scholar]
- 10.Tinti L., Spreafico A., Braconi D., Millucci L., Bernardini G., Chellini F., Cavallo G., Selvi E., Galeazzi M., Marcolongo R., Gallagher J.A., Santucci A. Evaluation of antioxidant drugs for the treatment of ochronotic alkaptonuria in an in vitro human cell model. J. Cell. Physiol. 2010;225:84–91. doi: 10.1002/jcp.22199. [DOI] [PubMed] [Google Scholar]
- 11.Tinti L., Taylor A.M., Santucci A., Wlodarski B., Wilson P.J., Jarvis J.C., Fraser W.D., Davidson J.S., Ranganath L.R., Gallagher J.A. Development of an in vitro model to investigate joint ochronosis in alkaptonuria. Rheumatology (Oxford) 2011;50:271–277. doi: 10.1093/rheumatology/keq246. [DOI] [PubMed] [Google Scholar]
- 12.Tinti L., Spreafico A., Chellini F., Galeazzi M., Santucci A. A novel ex vivo organotypic culture model of alkaptonuria-ochronosis. Clin. Exp. Rheumatol. 2011;29:693–696. [PubMed] [Google Scholar]
- 13.Taylor A.M., Wlodarski B., Prior I.A., Wilson P.J., Jarvis J.C., Ranganath L.R., Gallagher J.A. Ultrastructural examination of tissue in a patient with alkaptonuric arthropathy reveals a distinct pattern of binding of ochronotic pigment. Rheumatology (Oxford) 2010;49:1412–1414. doi: 10.1093/rheumatology/keq027. [DOI] [PubMed] [Google Scholar]
- 14.Momohara S., Okamoto H., Yamanaka H. Chondrocyte of rheumatoid arthritis serve as a source of intra-articular acute-phase serum amyloid A protein. Clin. Chim. Acta. 2008;398:155–156. doi: 10.1016/j.cca.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Romhányi G. Selective differentiation between amyloid and connective tissue structures based on the collagen specific topo-optical staining reaction with Congo Red. Virchows Arch. 1971;354:209–222. doi: 10.1007/BF00544254. [DOI] [PubMed] [Google Scholar]
- 16.Bély M., Apáthy A. Histochemical and immunohistochemical differential diagnosis of amyloidosis—a brief illustrated essay and personal experience with Romhányi's method. Amyloid: Int. J. Exp. Clin. Invest. 2000;7:212–217. doi: 10.3109/13506120009146836. [DOI] [PubMed] [Google Scholar]
- 17.Saeed S.M., Fine G. Thioflavin T for amyloid detection. Am. J. Clin. Pathol. 1967;47:588–593. doi: 10.1093/ajcp/47.5.588. [DOI] [PubMed] [Google Scholar]
- 18.M.M. Picken, G.A. Herrera, Th-T stain: an easier and more sensitive method for amyloid detection, in: M.M. Picken, A. Dogan, G.A. Herrera, Amyloid and Related Disorders: Surgical Pathology and Clinical Correlations, Current Clinical Pathology, Series Editor: A. Giordano, Springer – Humana Press, 2012, pp. 187–190.
- 19.Bernardini G., Braconi D., Spreafico A., Santucci A. Post-genomics of bone metabolic dysfunctions and neoplasias. Proteomics. 2012;12:708–721. doi: 10.1002/pmic.201100358. [DOI] [PubMed] [Google Scholar]
- 20.Hachulla E., Janin A., Flipo R.M., Saile R., Facon T., Bataille D., Vanhille P., Hatron P.Y., Devulder B., Duquesnoy B. Labial salivary gland biopsy is a reliable test for the diagnosis of primary and secondary amyloidosis. A prospective clinical and immunohistologic study in 59 patients. Arthritis Rheum. 1993;36:691–697. doi: 10.1002/art.1780360518. [DOI] [PubMed] [Google Scholar]
- 21.van Gameren I.I., Hazenberg B.P., Bijzet J., Haagsma E.B., Vellenga E., Posthumus M.D., Jager P.L., van Rijswijk M.H. Amyloid load in fat tissue reflects disease severity and predicts survival in amyloidosis. Arthritis Care Res. (Hoboken) 2010;62:296–301. doi: 10.1002/acr.20101. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T. Clinical strategies for amyloid A amyloidosis secondary to rheumatoid arthritis. Mod. Rheumatol. 2008;18:109–118. doi: 10.1007/s10165-008-0035-2. [DOI] [PubMed] [Google Scholar]
- 23.van der Hilst J.C., Kluve-Beckerman B., van der Meer J.W., Simon A. Cathepsin D activity protects against development of type AA amyloid fibrils. Eur. J. Clin. Invest. 2009;39:412–416. doi: 10.1111/j.1365-2362.2009.02104.x. [DOI] [PubMed] [Google Scholar]
- 24.Evans C.G., Wisen S., Gestwicki J.E. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J. Biol. Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 25.Meehan S., Knowles T.P., Baldwin A.J., Smith J.F., Squires A.M., Clements P., Treweek T.M., Ecroyd H., Tartaglia G.G., Vendruscolo M., Macphee C.E., Dobson C.M., Carver J.A. Characterisation of amyloid fibril formation by small heat-shock chaperone proteins human alphaA-, alphaB- and R120G alphaB-crystallins. J. Mol. Biol. 2007;372:470–484. doi: 10.1016/j.jmb.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 26.Lachmann H.J., Goodman H.J., Gilbertson J.A., Gallimore J.R., Sabin C.A., Gillmore J.D., Hawkins P.N. Natural history and outcome in systemic AA amyloidosis. N. Engl. J. Med. 2007;356:2361–2371. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 27.Castillo E.C.G., Kourí J.B. A new role for chondrocytes as non-professional phagocytes. An in vitro study. Microsc. Res. Tech. 2004;64:269–278. doi: 10.1002/jemt.20080. [DOI] [PubMed] [Google Scholar]
- 28.Ward M.W., Concannon C.G., Whyte J., Walsh C.M., Corley B., Prehn J.H. The amyloid precursor protein intracellular domain(AICD) disrupts actin dynamics and mitochondrial bioenergetics. J. Neurochem. 2010;113:275–284. doi: 10.1111/j.1471-4159.2010.06615.x. [DOI] [PubMed] [Google Scholar]
- 29.Ji L., Chauhan V., Mehta P., Wegiel J., Mehta S., Chauhan A. Relationship between proteolytically cleaved gelsolin and levels of amyloid-β protein in the brains of Down syndrome subjects. J. Alzheimers Dis. 2010;22:609–617. doi: 10.3233/JAD-2010-101029. [DOI] [PubMed] [Google Scholar]
- 30.Güntert A., Campbell J., Saleem M., O'Brien D.P., Thompson A.J., Byers H.L., Ward M.A., Lovestone S. Plasma gelsolin is decreased and correlates with rate of decline in Alzheimer's disease. J. Alzheimers Dis. 2010;21:585–596. doi: 10.3233/JAD-2010-100279. [DOI] [PubMed] [Google Scholar]
- 31.Nag S., Ma Q., Wang H., Chumnarnsilpa S., Lee W.L., Larsson M., Kannan B., Hernandez-Valladares M., Burtnick L.D., Robinson R.C. Ca2 + binding by domain 2 plays a critical role in the activation and stabilization of gelsolin. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13713–13718. doi: 10.1073/pnas.0812374106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S.H., Creemers J.W., Chu S., Thinakaran G., Sisodia S.S. Proteolytic processing of familial British dementia-associated BRI variants: evidence for enhanced intracellular accumulation of amyloidogenic peptides. J. Biol. Chem. 2002;277:1872–1877. doi: 10.1074/jbc.M108739200. [DOI] [PubMed] [Google Scholar]
- 33.Watt B., van Niel G., Fowler D.M., Hurbain I., Kelvin K.L., Stayrook S.E., Lemmon M.A., Raposo G., Shorter J., Kelly J.W., Marks M.S. N-terminal domains elicit formation of functional Pmel17 amyloid fibrils. J. Biol. Chem. 2009;284:35543–35555. doi: 10.1074/jbc.M109.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly J.W., Balch W.E. Amyloid as a natural product. J. Cell Biol. 2003;161:461–462. doi: 10.1083/jcb.200304074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaines J.J., Jr. The pathology of alkaptonuric ochronosis. Hum. Pathol. 1989;20:40–46. doi: 10.1016/0046-8177(89)90200-1. [DOI] [PubMed] [Google Scholar]
- 36.Laschi M., Tinti L., Braconi D., Millucci L., Ghezzi L., Amato L., Selvi E., Spreafico A., Bernardini G., Santucci A. Homogentisate 1,2 dioxygenase is expressed in human osteoarticular cells: implications in alkaptonuria. J. Cell. Physiol. 2012;227:3254–3257. doi: 10.1002/jcp.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heng A.E., Courbebaisse M., Kemeny J.L., Matesan R., Bonniol C., Deteix P., Souweine B. Hemolysis in a patient with alkaptonuria and chronic kidney failure. Am. J. Kidney Dis. 2010;56:e1–e4. doi: 10.1053/j.ajkd.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Bulow C., Rosenberg J. Intrahepatic gallstones in patient with alkaptonuria. Ugeskr. Laeger. 2009;171:2198–2199. [PubMed] [Google Scholar]
- 39.Raina S., Mahesh D.M., Kaushal S.S., Gupta D., Dhiman D.S., Negi A., Sharma S. Alkaptonuria and intramedullary calcification. J. Assoc. Physicians India. 2008;56:552–555. [PubMed] [Google Scholar]
- 40.Kolar J., Krizek V. Radiographic symptoms of alkaptomuric ochronosis. Fortschr. Geb. Rontgenstr. Nuklearmed. 1968;109:203–208. [PubMed] [Google Scholar]
- 41.Parambil J.G., Daniels C.E., Zehr K.J., Utz J.P. Alkaptonuria diagnosed by flexible bronchoscopy. Chest. 2005;128:3678–3680. doi: 10.1378/chest.128.5.3678. [DOI] [PubMed] [Google Scholar]
- 42.Obici L., Raimondi S., Lavatelli F., Bellotti V., Merlini G. Susceptibility to AA amyloidosis in rheumatic diseases: a critical overview. Arthritis Rheum. 2009;61:1435–1440. doi: 10.1002/art.24735. [DOI] [PubMed] [Google Scholar]
- 43.Gillmore J.D., Lovat L.B., Persey M.R., Pepys M.B., Hawkins P.N. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet. 2001;358:24–29. doi: 10.1016/S0140-6736(00)05252-1. [DOI] [PubMed] [Google Scholar]
- 44.Cox T.F., Ranganath L. A quantitative assessment of alkaptonuria: testing the reliability of two disease severity scoring systems. J. Inherit. Metab. Dis. 2011;34:1153–1162. doi: 10.1007/s10545-011-9367-8. [DOI] [PubMed] [Google Scholar]
- 45.Ranganath L.R., Cox T.F. Natural history of alkaptonuria revisited: analyses based on scoring systems. J. Inherit. Metab. Dis. 2011;34:1141–1151. doi: 10.1007/s10545-011-9374-9. [DOI] [PubMed] [Google Scholar]
- 46.Bewely M.A., Pham T.K., Marriott H.M., Noirel J., Chu H.-P., Ow S.Y., Ryazanov A.G., Read R.C., Whyte M.K.B., Chain B., Wright P.C., Dockrell D.H. Proteomic evaluation and validation of cathepsin D regulated proteins in macrophages exposed to Streptococcus pneumoniae. Mol. Cell. Proteomics. 2011;10.1074:1–14. doi: 10.1074/mcp.M111.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Hilst J.C. Recent insights into the pathogenesis of type AA amyloidosis. ScientificWorldJournal. 2011;11:641–650. doi: 10.1100/tsw.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fowler D.M., Koulov A.V., Alory-Jost C., Marks M.S., Balch W.E., Kelly J.W. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao K.S., Hegde M.L., Anitha S., Musicco M., Zucca F.A., Turro N.J., Zecca L. Amyloid beta and neuromelanin—toxic or protective molecules? The cellular context makes the difference. Prog. Neurobiol. 2006;78:364–373. doi: 10.1016/j.pneurobio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Aquaron R., Fayet G., Barthet C., Desire S., Viallet F. Parkinson disease and alkaptonuria: fortuitous association or striatonigral ochronosis? Rev. Neurol. (Paris) 1995;151:63–66. [PubMed] [Google Scholar]
- 51.Introne W.J., Perry M.B., Troendle J., Tsilou E., Kayser M.A., Suwannarat P., O'Brien K.E., Bryant J., Sachdev V., Reynolds J.C., Moylan E., Bernardini I., Gahl W.A. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol. Genet. Metab. 2011;103:307–314. doi: 10.1016/j.ymgme.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]