Abstract
Divergent effects of α– and β–myosin heavy chain (MHC) isoforms on contractile behavior arise mainly because of their impact on thin filament cooperativity. The N terminus of cardiac troponin T (cTnT) also modulates thin filament cooperativity. Our hypothesis is that the impact of the N terminus of cTnT on thin filament activation is modulated by a shift from α- to β-MHC isoform. We engineered two recombinant proteins by deleting residues 1–43 and 44–73 in rat cTnT (RcTnT): RcTnT1–43Δ and RcTnT44–73Δ, respectively. Dynamic and steady-state contractile parameters were measured at sarcomere length of 2.3 µm after reconstituting proteins into detergent-skinned muscle fibers from normal (α-MHC) and propylthiouracil-treated (β-MHC) rat hearts. α-MHC attenuated Ca2+-activated maximal tension (∼46%) in RcTnT1–43Δ fibers. In contrast, β-MHC decreased tension only by 19% in RcTnT1–43Δ fibers. Both α- and β-MHC did not affect tension in RcTnT44–73Δ fibers. The instantaneous muscle fiber stiffness measurements corroborated the divergent impact of α- and β-MHC on tension in RcTnT1–43Δ fibers. pCa50 (-log of [Ca2+]free required for half-maximal activation) decreased significantly by 0.13 pCa units in α-MHC + RcTnT1–43Δ fibers but remained unaltered in β-MHC + RcTnT1–43Δ fibers, demonstrating that β-MHC counteracted the attenuating effect of RcTnT1–43Δ on myofilament Ca2+ sensitivity. β-MHC did not alter the sudden stretch–mediated recruitment of new cross-bridges (ER) in RcTnT1–43Δ fibers, but α-MHC attenuated ER by 36% in RcTnT1–43Δ fibers. The divergent impact of α- and β-MHC on how the N terminus of cTnT modulates contractile dynamics has implications for heart disease; alterations in cTnT and MHC are known to occur via changes in isoform expression or mutations.
INTRODUCTION
We recently demonstrated the existence of two distinct regions in the N terminus of mouse cardiac troponin T (cTnT) that have diverse roles in mediating cardiac contractile activation (Mamidi et al., 2013a). For example, using normal adult mouse cardiac muscle fibers (α–myosin heavy chain [MHC]), we demonstrated that the region 1–44 in mouse cTnT modulated the recruitment of force-bearing cross-bridges (XBs). Our data also showed that the cardiac-specific region 45–74 in mouse cTnT slowed XB recruitment mechanisms by augmenting thin filament cooperative processes. cTnT-induced slowing of XB recruitment mechanisms may be important for linking the dynamics of cardiac contraction to the heart rate (Mamidi et al., 2013a).
Apart from cTnT, XBs also have a major effect on cardiac thin filaments through cooperative mechanisms, a process in which the strongly bound XBs promote the recruitment of other strong XBs by affecting the movement of tropomyosin (Tm) on the actin filament (Gordon et al., 2000; Fitzsimons et al., 2001; Moss et al., 2004; Fitzsimons and Moss, 2007). Because of their major effects on thin filament activation, differences in the expression of XB isoforms are associated with tissue-specific functions. For example, fast skeletal muscle type expresses a fast XB isoform (α-MHC), and slow skeletal muscle type expresses a slow XB isoform (β-MHC). Moreover, the hearts of smaller mammals, with rapid heart rates, primarily express a faster cycling α-MHC isoform; larger mammals, with slower heart rates, primarily express a slower cycling β-MHC isoform. Because of their significant differences in XB dwell times (shorter for α-MHC isoform vs. longer for β-MHC isoform) in the force-producing state, fast and slow XB isoforms are expected to have divergent effects on cooperative processes in the thin filament (Ford et al., 2012).
Whether it is cTnT or a given MHC isoform, cooperative effects modulated by these proteins do not work in isolation. Therefore, the effects of both cTnT and XBs on thin filament cooperativity takes on a new significance when we consider the cTnT–XB interplay effects on cardiac contractile dynamics (Chandra et al., 2007). Collectively, these arguments lead us to hypothesize that the modulating effects of the N terminus of cTnT on contractile dynamics is affected differently by α- and β-MHC isoforms. Our hypothesis has significant implications for understanding how contractile dynamics are tuned by species-specific variation in the expression of fast versus slow MHC isoforms in small versus large animals. In this regard, it is worth noting that hearts of both small and large animals contain the cTnT isoform that has the cardiac-specific 1–44 and 45–74 regions—albeit with minor changes—but express MHC isoforms that possess vastly differing kinetic rates.
Additional support for our hypothesis comes from the following critical observations: (a) the functional impact of disease-related mutations in the N terminus of cTnT is influenced by the type of MHC isoform (Tschirgi et al., 2006; Rice et al., 2010; Ford et al., 2012); (b) the composition of MHC isoform is a key determinant of cardiac contractile activation (Stelzer et al., 2007); (c) small changes in the level of MHC isoform induce large changes in cardiac function both at the myofilament level (Fitzsimons et al., 1998; Rundell et al., 2005; Tschirgi et al., 2006) and at the whole heart level (Tardiff et al., 2000; Korte et al., 2005; Rice et al., 2010); and (d) mutations in sarcomeric proteins that cause human heart failure are often accompanied by a near-complete shift to β-MHC (Miyata et al., 2000; Reiser et al., 2001).
To test our hypothesis, we deleted two specific regions (regions 1–43 and 44–73) in the N terminus of rat cTnT (RcTnT) to generate RcTnT1–43Δ and RcTnT44–73Δ proteins, respectively. Steady-state and dynamic contractile measurements were made in cardiac muscle fibers (containing either α- or β-MHC isoforms) reconstituted with RcTnT1–43Δ and RcTnT44–73Δ proteins. Novel findings from our study demonstrate that α- or β-MHC isoforms have divergent effects on how the N terminus of cTnT modulates contractile function. As demonstrated by the decrease in Ca2+-activated maximal tension and myofilament Ca2+ sensitivity, the thin filament activation was significantly attenuated by RcTnT1–43Δ fibers in the presence of α-MHC. In contrast, the attenuating effect of RcTnT1–43Δ was counteracted by β-MHC. The sudden stretch–mediated recruitment of new force-bearing XBs (ER) was attenuated in α-MHC + RcTnT1–43Δ fibers but not in β-MHC + RcTnT1–43Δ fibers. We discuss these data in terms of the differential synergy between α-/β-MHC isoforms and the N terminus of cTnT.
MATERIALS AND METHODS
Ethical approval and animal treatment protocols
Papillary muscle bundles were collected from the left ventricles of young adult male Sprague-Dawley rats. Rats used in this study were split into two groups: one group consisted of normal rats that predominantly expressed α-MHC, and the other group consisted of propylthiouracil (PTU)-treated rats (see below) that predominantly expressed β-MHC in their left ventricles. Rats were carefully handled to minimize the pain and suffering, according to the established guidelines of the National Academy of Sciences: Guide for the Care and Use of Laboratory animals.
PTU treatment
PTU was administered in drinking water (0.2 g L−1) and in solid feed (0.15% PTU; Harlan Laboratories) for ∼5 wk. PTU treatment has been shown to induce a shift in the ventricular MHC isoform from predominantly α- to β-MHC, without altering other myofilament proteins (Metzger et al., 1999; Herron et al., 2001; Ford et al., 2012).
Preparation of detergent-skinned cardiac muscle fiber bundles
Detergent-skinned cardiac muscle fibers were prepared as described previously (Chandra et al., 2006, 2007). In brief, rats were deeply anesthetized by inhalation of isoflurane. The depth of anesthesia was evaluated by a lack of pedal withdrawal reflex. Hearts were quickly removed and placed into an ice-cold high relaxing (HR) solution containing (mM): 20 2, 3-butanedione monoxime (BDM), 50 N,N-bis (2-hydroxyethyl)-2-amino-ethane-sulfonic acid (BES), 20 EGTA, 6.29 MgCl2, 6.09 Na2ATP, 30.83 potassium propionate, 10 sodium azide, 1.0 DTT, and 4 benzamidine-HCl. The pH was adjusted to 7.0 with KOH. A fresh cocktail of protease inhibitors (µM: 5 bestatin, 2 E-64, 10 leupeptin, 1 pepstatin, and 200 PMSF) was added to the HR solution. Papillary muscle bundles were removed from the left ventricles. Papillary muscle bundles were further dissected into smaller muscle fiber bundles of ∼0.15 mm in width and 2 mm in length. All procedures were performed in ice-cold solution. Muscle fiber bundles were then chemically skinned overnight at 4°C in HR containing 1% Triton X-100.
MHC isoform composition
After removing the hearts from normal (α-MHC) and PTU-treated (β-MHC) rats, left ventricular tissue was rapidly frozen and stored at −80°C until they were used. To prepare protein samples, frozen tissue was submerged in liquid nitrogen, thoroughly crushed, and minced using a pestle and mortar (Ford et al., 2012). The minced tissue was then resuspended in 10 µl of a protein extraction buffer per 1 mg of tissue. The protein extraction buffer contained the following: 2.5% SDS, 10% glycerol, 50 mM Tris base, pH 6.8 at 4°C, 1 mM DTT, 1 mM PMSF, 4 mM benzamidine-HCl, and protease inhibitors (E-64, leupeptin, and bestatin). The resuspended tissue was further homogenized on ice using a Tissue Tearor (model 985370–395; Biospec Products, Inc.), sonicated in a water bath at 4°C, and followed by centrifugation at 10,000 rpm. Protein samples from normal and PTU-treated rats were run on a large 6% SDS gel to assess the left ventricular MHC isoform composition (Chandra et al., 2007; Ford and Chandra, 2013).
Purification of recombinant rat cardiac troponin (Tn) subunits
Recombinant c-myc–tagged RcTnT (c-myc RcTnT), rat cardiac TnI (RcTnI), and rat cardiac TnC (RcTnC)—all cloned into pSBETa vector—were expressed in BL21*DE3 cells (Invitrogen) for protein synthesis (Chandra et al., 2006). Deletions of amino acid residues 1–43 (RcTnT1–43Δ) and 44–73 (RcTnT44–73Δ) in RcTnT were performed using a standard polymerase chain reaction procedure. For protein preparations, cultured cells were spun down and sonicated in a buffer containing 50 mM Tris base, 6 M urea, and 5 mM EDTA, pH 8.0 at 4°C. The sonication buffer also contained a cocktail of protease inhibitors: 0.2 mM PMSF, 5 mM benzamidine-HCl, 10 µM leupeptin, 1 µM pepstatin, 5 µM bestatin, 2 µM E-64, and 1 mM DTT. The sonicated preparation was then centrifuged to remove the insoluble cellular material. The supernatant was used for ammonium sulfate fractionation. c-myc RcTnT and RcTnT44–73Δ were purified as follows: the pellet from 60% ammonium sulfate fractionation was dissolved in 50 mM Tris base, pH 8.0 at 4°C, 6 M urea, 1 mM EDTA, 0.2 mM PMSF, 5 mM benzamidine-HCl, 0.01% sodium azide, and 1 mM DTT, and then purified by ion-exchange chromatography on a DEAE-fast sepharose column (GE Healthcare). c-myc RcTnT and RcTnT44–73Δ were eluted with a 0–0.4-M NaCl gradient. The procedure used for the purification of RcTnT1–43Δ was similar to that of c-myc RcTnT, except that a CM sepharose column (GE Healthcare) was used for ion-exchange chromatography. RcTnI was purified as described previously (Chandra et al., 2007). RcTnC protein was purified as described previously (Mamidi et al., 2013a). Samples from eluted fractions were run on a 12.5% SDS gel to determine their purity. Pure fractions were pooled and dialyzed extensively against deionized water containing 15 mM β-mercaptoethanol, lyophilized, and stored at −80°C.
Reconstitution of recombinant rat cardiac Tn subunits into detergent-skinned rat cardiac muscle fibers
Reconstitution was performed as described previously (Chandra et al., 2007). c-myc–tagged WT RcTnT (RcTnTWT) was used as a control so that the incorporation of the exogenously added WT Tn subunits could be assessed by the differential gel migration pattern of c-myc RcTnT. It has been shown that the use of cTnT with an 11–amino acid c-myc epitope at the N terminus does not affect cardiac function (Tardiff et al., 1998; Montgomery et al., 2001). The replacement of endogenous Tn was performed using an extraction solution containing RcTnT (c-myc RcTnT, RcTnT1–43Δ, or RcTnT44–73Δ) (0.9 mg/ml, wt/vol) and RcTnI (1.0 mg/ml, wt/vol) that were first dissolved in buffer 1, which contained 50 mM Tris-HCl, pH 8.0, 6 M urea, 1.0 M KCl, 10 mM DTT, and a cocktail of protease inhibitors. High salt and urea in the extraction solution were removed by successive dialysis against the following buffers: 50 mM Tris-HCl, pH 8.0, 4 M urea, 0.7 M KCl, 0.2 mM PMSF, 2 mM benzamidine-HCl, 1 mM DTT, and 0.01% NaN3 (buffer 2), followed by 50 mM Tris-HCl, pH 8.0, 2 M urea, 0.5 M KCl, 0.2 mM PMSF, 2 mM benzamidine-HCl, 1 mM DTT, and 0.01% NaN3 (buffer 3), and finally dialyzed against an extraction buffer containing 50 mM BES, pH 7.0 at 20°C, 200 mM KCl, 10 mM BDM, 6.27 mM MgCl2, 5 mM EGTA, 0.2 mM PMSF, 2 mM benzamidine-HCl, 1 mM DTT, and 0.01% NaN3 (buffer 4). After the final dialysis, 6.13 mM MgATP2− and a cocktail of protease inhibitors were added to the supernatant containing RcTnT and RcTnI. Undissolved protein was removed by spinning the protein solution at 3,000 rpm for 15 min. Detergent-skinned fibers were then treated with the extraction solution containing RcTnT and RcTnI for ∼3 h at room temperature (20°C), with constant stirring. RcTnT–RcTnI-treated muscle fibers were then washed three times using buffer 4 for 10 min at room temperature. To complete the reconstitution procedure, RcTnT–RcTnI-treated fibers were incubated with RcTnC (3.0 mg/ml, wt/vol) overnight at 4°C.
Fibers reconstituted with RcTnT1–43Δ + RcTnI + RcTnC are referred to as “RcTnT1–43Δ,” and those reconstituted with RcTnT44–73Δ + RcTnI+RcTnC are referred to as “RcTnT44–73Δ.” Fibers reconstituted with c-myc RcTnT + RcTnI + RcTnC are referred to as “RcTnTWT” and were used as controls. Reconstituted fibers were denatured and solubilized in 2% SDS solution (10 µl/fiber) for SDS-PAGE (Mamidi et al., 2012). SDS-solubilized fibers were mixed with an equal volume of protein loading dye that contained 125 mM Tris-HCl, pH 6.8, 20% glycerol, 2% SDS, 0.01% bromophenol blue, and 50 mM β-mercaptoethanol. Equal quantities of protein were separated on an 8% SDS gel and stained with Bio-Safe Coomassie blue G-250 (Bio-Rad Laboratories).
Proteins separated on the SDS gel were transferred onto a PVDF membrane for Western blot analysis using a Trans-Blot Turbo Transfer System (Bio-Rad Laboratories). The incorporation of c-myc RcTnT, RcTnT1–43Δ, or RcTnT44–73Δ was assessed using a monoclonal anti-TnT primary antibody (clone JLT-12; Sigma-Aldrich), followed by a horseradish peroxidase–labeled anti–mouse secondary antibody (GE Healthcare). Densitometric analysis of Western blots was performed using the ImageJ software (acquired from National Institutes of Health at: http://rsbweb.nih.gov/ij/) (Mamidi et al., 2013b).
Measurement of steady-state isometric tension and ATPase activity
Isometric steady-state tension and ATPase activity were measured as described previously (Chandra et al., 2007, 2009). In brief, T-shaped aluminum clips were used to attach the muscle fiber between a motor arm (322C; Aurora Scientific Inc.) and a force transducer (AE 801; Sensor One Technologies Corp.). The sarcomere length (SL) of the muscle fibers was set to 2.3 µm under relaxing conditions as described previously (Chandra et al., 2007, 2009). After two cycles of maximal activation and relaxation, the SL was readjusted if necessary. The muscle fiber was then immersed in a constantly stirred chamber containing Ca2+ solutions, the concentration of which ranged from pCa 4.3 to 9.0 (pCa = −log of [Ca2+]free). The composition of various pCa solutions was calculated using methods described previously (Fabiato and Fabiato, 1979). The composition of the maximal Ca2+ activation solution (pCa 4.3) was (mM): 31 potassium propionate, 5.95 Na2ATP, 6.61 MgCl2, 10 EGTA, 10.11 CaCl2, 50 BES, 5 sodium azide, and 10 phosphoenol pyruvate (PEP). The composition of the relaxing solution (pCa 9.0) was (mM): 51.14 potassium propionate, 5.83 Na2ATP, 6.87 MgCl2, 10 EGTA, 0.024 CaCl2, 50 BES, 5 NaN3, and 10 PEP. The activation and relaxing solutions also contained 0.5 mg/ml pyruvate kinase (500 U/mg), 0.05 mg/ml lactate dehydrogenase (870 U/mg), 20 µM diadenosine pentaphosphate, 10 µM oligomycin, and a cocktail of protease inhibitors. The pH of the Ca2+ solutions was adjusted to 7.0 using KOH, and the ionic strength was 180 mM. Ca2+-activated tension was digitally recorded on a computer. All measurements were made at 20°C.
Steady-state isometric ATPase activity was measured according to a protocol described previously (de Tombe and Stienen, 1995; Kirk et al., 2009; Rodgers et al., 2009; Gollapudi et al., 2012). In brief, near-UV light (340 nm) was projected through the muscle chamber, and then split (50:50) via a beam splitter and detected at 340 nm (sensitive to changes in NADH) and at 400 nm (insensitive to changes in NADH). ATPase activity was measured as follows: ATP regeneration from ADP was coupled to the breakdown of PEP to pyruvate and ATP catalyzed by pyruvate kinase, which was linked to the synthesis of lactate catalyzed by lactate dehydrogenase. The breakdown of NADH during the synthesis of lactate was proportional to the ATP consumption and was measured by changes in UV absorbance at 340 nm. The signal for NADH was calibrated by multiple injections of 250 pmol of ADP.
Measurement of muscle fiber mechano-dynamics
Once the force developed by the muscle fiber reached steady state (pCa 4.3), step-like length changes were applied (Ford et al., 2010). In brief, the motor arm was commanded to change the muscle length (ML) in a step-like pattern; the changes in ML were on the order of ±0.5, 1.0, 1.5, and 2.0% of the initial ML. The ML was initially increased by 0.5% ML (stretched) and held at the increased length for 5 s, and then the motor arm was commanded to rapidly return (released) to the initial ML. The same protocol was repeated for the remaining step-like length changes of 1.0, 1.5, and 2.0% ML. A nonlinear recruitment distortion (NLRD) model (Ford et al., 2010) was fitted to the elicited force responses to estimate the following model parameters: b, the rate by which new XBs are recruited into the force-bearing state because of a change in ML; c, the rate by which the stiffness of distorted XBs dissipate; ED, the magnitude of an instantaneous increase in muscle fiber stiffness caused by a sudden change in ML; and ER, the magnitude of an increase in steady-state force caused by the sudden stretch–mediated recruitment of additional XBs.
Measurement of rate of tension redevelopment (ktr)
The measurement of ktr (pCa 4.3) was performed according to a modified protocol described originally by Brenner and Eisenberg (1986). Upon attaining a steady-state isometric force, the motor arm was commanded to slacken the muscle fiber by 10% of the ML using a high-speed length-control device (Aurora Scientific Inc.). After a brief shortening period of 25 ms, the motor arm was commanded to rapidly (within 0.5 ms) swing past the original set point by a 10% stretch. The stretch was applied to ensure that any remaining XBs were mechanically detached and the residual force was not more than ∼10% of the initial steady-state isometric force. ktr was determined by fitting the following mono-exponential equation to the force redevelopment:
where F is the force at time t, Fss is the steady-state isometric force, and Fres is the residual force from which the redevelopment of force occurs.
Data analysis
Contractile and mechano-dynamic parameters were analyzed using a two-way ANOVA. One factor in this analysis was the RcTnT variant (c-myc RcTnT, RcTnT1–43Δ, or RcTnT44–73Δ), and the other was the MHC isoform (α- or β-MHC). After analysis by two-way ANOVA, post-hoc tests (planned multiple pairwise comparisons) were made using uncorrected Fisher’s LSD method. Comparisons were made to determine the effects of RcTnT variants and MHC isoforms on various contractile and mechano-dynamic parameters. The Hill equation was fitted to normalized pCa–tension data to estimate pCa50 (−log of [Ca2+]free required for half-maximal activation) and the Hill coefficient (nH). Values are reported as mean ± SEM. The criterion for statistical significance was set at P < 0.05.
Online supplemental material
The online supplemental material describes the development of the NLRD model, which was used to estimate the dynamic contractile parameters (Ford et al., 2010). It also contains characteristic features of the force responses of muscle fibers to step-like length perturbations. The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201310971/DC1.
RESULTS
Rationale for the generation of RcTnT1–43Δ and RcTnT44–73Δ deletion mutants
Recently, we identified two distinct functional regions (1–44 and 45–74) in the N terminus of mouse cTnT; the interplay between these two regions and the overlapping ends of contiguous Tm dimers were shown to affect thin filament activation and myofilament cooperativity (Mamidi et al., 2013b). Based on the sequence comparison shown in Fig. 1, we identified the corresponding regions in RcTnT: (a) a highly acidic peptide region comprising 1–43 amino acids, which shares only 51% sequence similarity with rat fast skeletal TnT (RfsTnT); and (b) a region comprising 44–73 amino acids, which is absent in RfsTnT. Residues 1–43 and 44–73 were deleted in RcTnT to generate RcTnT1–43Δ and RcTnT44–73Δ, respectively. Deletion of these two distinct regions (RcTnT1–43Δ and RcTnT44–73Δ) amounted to a net removal of 22 and 6 acidic residues, respectively.
Figure 1.
Rationale for the generation of RcTnT1–43Δ and RcTnT44–73Δ deletion mutants. Sequence comparison of amino acids at the N-terminal ends of RcTnT and RfsTnT revealed two distinct regions in the N-terminal end region of RcTnT: (1) a highly negatively charged region comprising 1–43 amino acids in RcTnT that corresponds to 1–41 amino acids in RfsTnT; and (2) a region comprising 44–73 amino acids in RcTnT that is missing in RfsTnT. To understand how the functions of these two regions are modulated by α- and β-MHC isoforms, we deleted these regions in RcTnT to generate RcTnT1–43Δ and RcTnT44–73Δ deletion mutants. :, identical amino acids; ., conservative substitution; empty space, nonconservative substitution; –, deletion introduced to maximize sequence similarities.
To understand how α- and β-MHC isoforms affect the interplay between the N terminus of cTnT and the overlapping ends of Tm, we reconstituted these mutants into detergent-skinned rat cardiac muscle fibers from normal rats (α-MHC) and PTU-treated rats (β-MHC).
Shift from α- to β-MHC in PTU-treated rat hearts
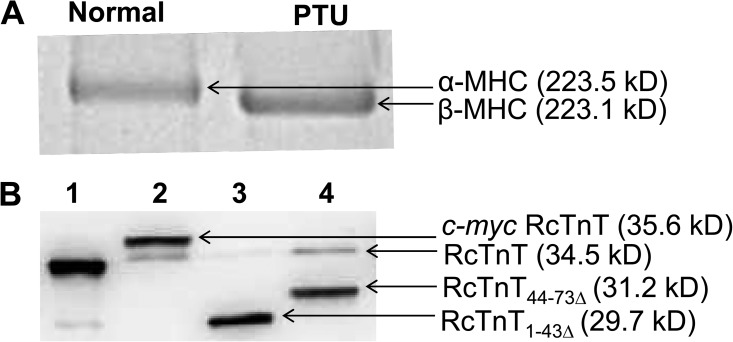
PTU treatment has been shown to induce a shift from α- to β-MHC isoform, without any significant changes in the expression of other myofilament proteins (Metzger et al., 1999; Herron et al., 2001; Ford et al., 2012). PTU-induced hypothyroidism silences the α-MHC promoter and activates the β-MHC promoter, resulting in the myocardial expression of β-MHC (Chizzonite and Zak, 1984). As shown in Fig. 2 A, PTU treatment of rats induced a near-complete shift to β-MHC isoform in the left ventricles.
Figure 2.
SDS-PAGE analysis of MHC isoform expression and Western blot analysis of reconstituted fibers. Solubilized muscle fiber samples from normal (α-MHC) and PTU-treated (β-MHC) rat hearts were run on a 6% SDS gel to estimate the isoform levels of MHC (Ford and Chandra, 2013). (A) SDS gel showing the isoform expression of MHC in the left ventricles of normal and PTU-treated rats. Rats fed on a PTU diet show a near-complete shift to β-MHC isoform. (B) Western blot analysis of samples from reconstituted fibers expressing α-MHC. Reconstituted fibers were solubilized using 2% SDS (Mamidi et al., 2012). Solubilized samples were run on an 8% SDS gel and transferred onto a PVDF membrane for Western blot analysis. Lane 1, purified recombinant RcTnT protein; lanes 2–4, samples from RcTnTWT-, RcTnT1–43Δ-, and RcTnT44–73Δ-reconstituted fibers. Molecular weights of proteins are indicated in parentheses.
Western blot analysis of reconstituted rat cardiac muscle fibers
The c-myc–tagged WT RcTnT (RcTnTWT) was used as the control. In our reconstitution protocol, the exogenously added Tn complex consisted of RcTnT (RcTnTWT, RcTnT1–43Δ, or RcTnT44–73Δ), RcTnI, and RcTnC proteins. We have shown previously that the endogenous Tn complex is replaced when a vast excess of exogenously added cTnT competes with the endogenous cTnT (Chandra et al., 1999). Therefore, the level of RcTnT incorporated may be used as an index of the amount of endogenous Tn replaced. The differential migration pattern of c-myc–tagged RcTnTWT and the endogenous RcTnT allowed us to assess the level of incorporation of RcTnTWT. Because of significant differences in molecular weights, RcTnT1–43Δ and RcTnT44–73Δ migrated differently when compared with the endogenous RcTnT; therefore, RcTnT1–43Δ and RcTnT44–73Δ were not tagged with the c-myc tag.
SDS-solubilized muscle fibers were run on an 8% SDS gel and transferred to a PVDF membrane for the Western blot analysis. The ImageJ software was used for densitometric analysis of protein bands in the Western blot. The total optical band intensity (i.e., the total amount of RcTnT in a single lane) was assumed to be the sum of the optical band intensities of both the endogenous RcTnT and the incorporated RcTnT (RcTnTWT, RcTnT1–43Δ, or RcTnT44–73Δ) bands. The extent of incorporation was determined by dividing the optical band intensity of the exogenously added RcTnT with the total band intensity. This approach permitted us to precisely estimate the level of incorporation of different proteins in reconstituted fibers. In fibers from normal rats (α-MHC), the level of incorporation was as follows: 80% for RcTnTWT (lane 2 of Fig. 2 B), 99% for RcTnT1–43Δ (lane 3 of Fig. 2 B), and 80% for RcTnT44–73Δ (lane 4 of Fig. 2 B). The level of incorporation of exogenously added RcTnT proteins in PTU-treated rat fibers (β-MHC) was similar to that observed in the normal rat fibers.
Divergent effects of α- and β-MHC isoforms on Ca2+-activated maximal tension and ATPase activity in RcTnT1–43Δ- or RcTnT44–73Δ-reconstituted rat cardiac muscle fibers
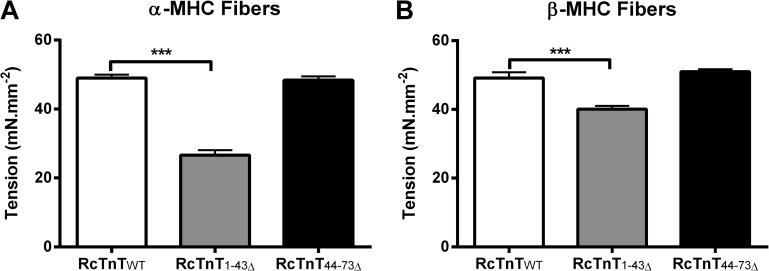
Ca2+-activated maximal tension was measured at pCa 4.3. Two-way ANOVA revealed a significant interaction effect (P < 0.001), demonstrating a divergent impact of α- and β-MHC isoforms on how RcTnT1–43Δ and RcTnT44–73Δ modulated Ca2+-activated maximal tension. To determine the factor that was responsible for the significant interaction effect, we performed subsequent post-hoc tests using multiple pairwise comparisons (uncorrected Fischer’s LSD). Post-hoc tests revealed that α- and β-MHC isoforms had differential impact on maximal tension in RcTnT1–43Δ fibers but not in RcTnT44–73Δ fibers. Ca2+-activated maximal tension decreased by ∼46% in α-MHC + RcTnT1–43Δ fibers (Fig. 3 A) and by only 18% in β-MHC + RcTnT1–43Δ fibers (Fig. 3 B). Thus, β-MHC significantly counteracted the attenuating effect of RcTnT1–43Δ on maximal tension. On the other hand, both α- and β-MHC isoforms did not alter Ca2+-activated maximal tension in RcTnT44–73Δ fibers (Fig. 3, A and B).
Figure 3.
Effect of RcTnT1–43Δ and RcTnT44–73Δ on Ca2+-activated maximal tension in the presence of α- or β-MHC isoform. Ca2+-activated maximal tension was measured at pCa 4.3 and SL of 2.3 µm. (A) Ca2+-activated maximal tension in fibers containing α-MHC. (B) Ca2+-activated maximal tension in fibers containing β-MHC. RcTnT1–43Δ decreased maximal tension by ∼46% in α-MHC fibers, whereas RcTnT1–43Δ decreased maximal tension by only 18% in β-MHC fibers. Number of determinations was at least 13 for each group. Values are reported as mean ± SEM. ***, P < 0.001.
Ca2+-activated maximal ATPase activity was measured in reconstituted fibers at pCa 4.3. Two-way ANOVA revealed a significant interaction effect (P < 0.01), demonstrating a divergent impact of α- and β-MHC isoforms on how RcTnT1–43Δ or RcTnT44–73Δ affected Ca2+-activated maximal ATPase activity. Post-hoc multiple comparisons revealed that a shift from α- to β-MHC had a differential impact on ATPase activity. In the presence of α-MHC, ATPase values (in pmol × mm−3 × s−1) were 174.89 ± 4.87, 118.23 ± 4.31, and 162.43 ± 5.70 for RcTnTWT, RcTnT1–43Δ, and RcTnT44–73Δ fibers, respectively. However, in the presence of β-MHC, ATPase values were 98.84 ± 6.71, 73.38 ± 3.00, and 93.27 ± 4.09 for RcTnTWT, RcTnT1–43Δ, and RcTnT44–73Δ fibers, respectively. When compared with the control fibers, RcTnT1–43Δ decreased ATPase activity by ∼32% in the presence of α-MHC and by ∼26% in the presence of β-MHC. In the presence of α- or β-MHC, RcTnT44–73Δ did not alter maximal ATPase activity when compared with the respective control fibers. Therefore, Ca2+-activated maximal ATPase activity showed trends that were similar to Ca2+-activated maximal tension.
Divergent effects of α- and β-MHC isoforms on the magnitude of an instantaneous increase in muscle fiber stiffness (ED) in RcTnT1–43Δ- or RcTnT44–73Δ-reconstituted rat cardiac muscle fibers
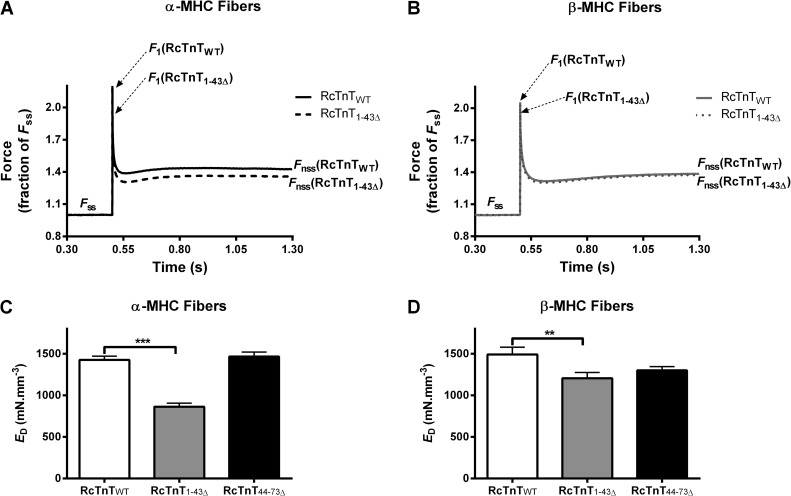
To examine whether the effect of RcTnT1–43Δ on maximal tension was caused by changes in the number of strongly bound XBs, we measured ED by imposing step-like length perturbations in constantly activated muscle fibers (Ford et al., 2010). The amplitude of the ML-induced instantaneous force (see F1 in Fig. 4 and Fig. S1) increases with the amount of stretch and with the level of Ca2+ activation because it is an approximate function of the number of force-bearing XBs. Therefore, a comparison of F1 in Fig. 4 (A and B) suggests that the number of strongly bound XBs is attenuated more in α-MHC + RcTnT1–43Δ fibers. ED was estimated as described in Fig. 4 and Fig. S1. Two-way ANOVA revealed a significant interaction effect (P < 0.001), suggesting a divergent impact of α- and β-MHC isoforms on how RcTnT1–43Δ or RcTnT44–73Δ altered ED. Post-hoc multiple comparisons revealed that α- and β-MHC isoforms differentially affected ED in RcTnT1–43Δ fibers but not in RcTnT44–73Δ fibers. ED decreased by ∼39% in α-MHC + RcTnT1–43Δ fibers (Fig. 4 C). On the other hand, ED decreased by only 19% in β-MHC + RcTnT1–43Δ fibers (Fig. 4 D). Thus, our estimates of ED suggest that the attenuation of maximal tension in RcTnT1–43Δ-reconstituted fibers is caused by a decrease in the number of force-bearing XBs. Furthermore, multiple comparisons also revealed that the ED of β-MHC + RcTnT1–43Δ fibers is significantly higher when compared with that of α-MHC + RcTnT1–43Δ fibers. As observed for maximal tension, both α- and β-MHC isoforms did not alter ED in RcTnT44–73Δ fibers (Fig. 4, C and D).
Figure 4.
Effect of RcTnT1–43Δ and RcTnT44–73Δ on ED in the presence of α- or β-MHC isoform. ED was measured at pCa 4.3. Step-like length perturbation protocol was as described previously (Ford et al., 2010). F1, Fss, and Fnss are described in the supplemental text. (A) Force responses to a 2% stretch in ML of α-MHC fibers reconstituted with RcTnTWT and RcTnT1–43Δ. (B) Force responses to a 2% stretch in ML of β-MHC fibers reconstituted with RcTnTWT and RcTnT1–43Δ. Force traces shown in A and B are the averaged responses measured from at least 11 fibers. F1 decreases to a greater extent in RcTnT1–43Δ + α-MHC fibers. ED (the slope of the relationship between F1–Fss and change in ML) was estimated as described in the supplemental text. (C) ED in fibers containing α-MHC. (D) ED in fibers containing β-MHC. RcTnT1–43Δ decreased ED by ∼39% in α-MHC fibers, whereas RcTnT1–43Δ decreased ED by only 19% in β-MHC fibers. Values are reported as mean ± SEM. ***, P < 0.001; **, P < 0.01.
Divergent effects of α- and β-MHC isoforms on myofilament Ca2+ sensitivity and cooperativity in RcTnT1–43Δ- or RcTnT44–73Δ-reconstituted rat cardiac muscle fibers
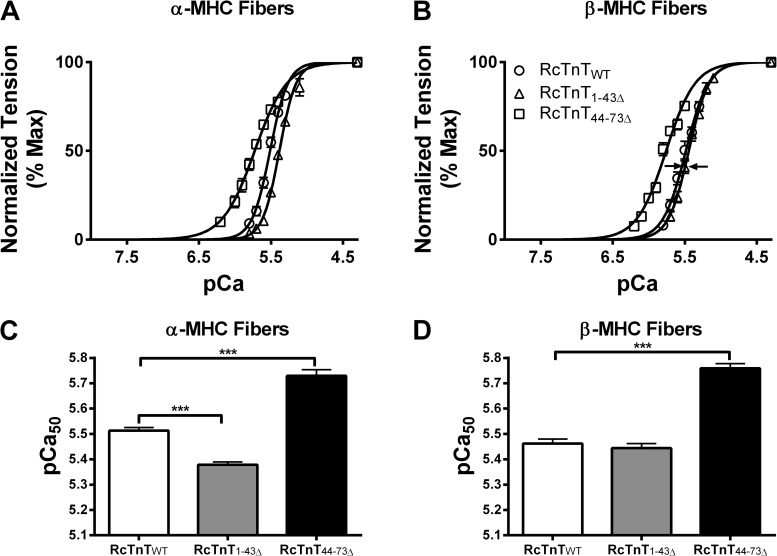
Normalized tension values were plotted against a range of pCa to construct the pCa–tension relations (Fig. 5, A and B). The pCa–tension relation of α-MHC + RcTnT1–43Δ was shifted to the right of α-MHC + RcTnTWT (Fig. 5 A), indicating a desensitization of myofilament Ca2+ sensitivity (pCa50) in α-MHC + RcTnT1–43Δ fibers. This rightward shift of the pCa–tension relation was ablated in β-MHC + RcTnT1–43Δ fibers (indicated by arrows in Fig. 5 B), suggesting that β-MHC counteracted the desensitizing effect of RcTnT1–43Δ on pCa50. Two-way ANOVA revealed a significant interaction effect (P < 0.05) on pCa50, suggesting that α- and β-MHC isoforms divergently affected the way RcTnT1–43Δ and RcTnT44–73Δ altered pCa50 (Fig. 5, C and D). The main contributing factor for the significant interaction effect on pCa50 was caused by the divergent effects of α- and β-MHC isoforms on pCa50 in RcTnT1–43Δ versus RcTnT44–73Δ fibers. Comparison of α-MHC + RcTnT1–43Δ fibers (Fig. 5 C) with β-MHC + RcTnT1–43Δ fibers (Fig. 5 D) shows that β-MHC negated the desensitizing effect of RcTnT1–43Δ on pCa50. In contrast, RcTnT44–73Δ increased pCa50 in the presence of α- or β-MHC (Fig. 5, C and D).
Figure 5.
Effect of RcTnT1–43Δ and RcTnT44–73Δ on pCa50 in the presence of α- or β-MHC isoform. Normalized tension values were plotted against a range of pCa to derive the pCa–tension relationships. (A) pCa–tension relationships in fibers containing α-MHC. (B) pCa–tension relationships in fibers containing β-MHC. Arrows in B indicate that a shift to β-MHC counteracts the desensitizing effect of RcTnT1–43Δ on myofilament Ca2+ sensitivity. Note that the curve for RcTnT1–43Δ is shifted to the right in A, but the rightward shift is attenuated in B (see arrows). The Hill equation was fitted to the pCa–tension relationships to estimate pCa50. (C) Estimates of pCa50 in fibers containing α-MHC. (D) Estimates of pCa50 in fibers containing β-MHC. RcTnT1–43Δ decreased pCa50 significantly in α-MHC fibers, an effect that was counteracted in β-MHC fibers. Number of determinations was at least seven for each group. Values are reported as mean ± SEM. ***, P < 0.001.
The Hill equation was fitted to the pCa–tension relationships to estimate the Hill coefficient (nH). Two-way ANOVA revealed a significant interaction effect (P < 0.05), suggesting that nH was affected differentially in RcTnT1–43Δ and RcTnT44–73Δ fibers, regardless of the type of MHC isoform present. Post-hoc multiple comparisons showed that RcTnT1–43Δ did not affect nH, but RcTnT44–73Δ decreased nH in the presence of α- or β-MHC. In the presence of α-MHC, nH values were 3.62 ± 0.17, 4.10 ± 0.31, and 2.18 ± 0.10 for RcTnTWT, RcTnT1–43Δ, and RcTnT44–73Δ fibers, respectively. In the presence of β-MHC, nH values were 3.32 ± 0.34, 2.98 ± 0.15, and 2.21 ± 0.08 for RcTnTWT, RcTnT1–43Δ, and RcTnT44–73Δ fibers, respectively.
Divergent effects of α- and β-MHC isoforms on the magnitude of sudden stretch–mediated recruitment of new force-bearing XBs (ER) in RcTnT1–43Δ- or RcTnT44–73Δ-reconstituted rat cardiac muscle fibers
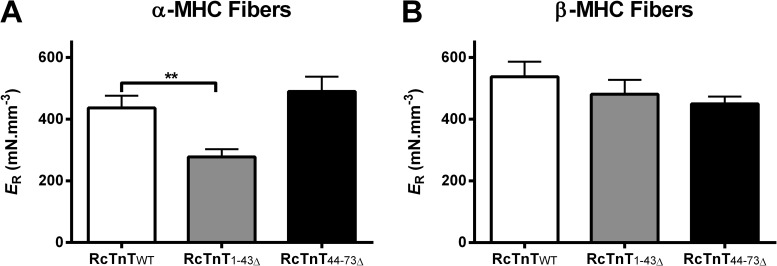
We measured ER to determine if the attenuation of thin filament activation in α-MHC + RcTnT1–43Δ fibers was caused by a decrease in ER. Two-way ANOVA revealed a significant interaction effect (P < 0.05), suggesting a divergent impact of α- and β-MHC isoforms on how RcTnT1–43Δ or RcTnT44–73Δ altered ER. Post-hoc multiple comparisons revealed that α- and β-MHC isoforms had differential impact on ER in RcTnT1–43Δ fibers but not in RcTnT44–73Δ fibers. ER decreased significantly (∼36%) in α-MHC + RcTnT1–43Δ fibers (Fig. 6 A) but not in β-MHC + RcTnT1–43Δ fibers (Fig. 6 B). Furthermore, multiple comparisons also revealed that the ER of β-MHC + RcTnT1–43Δ fibers is significantly higher when compared with that of α-MHC + RcTnT1–43Δ fibers. Thus, β-MHC counteracted the attenuating effect of RcTnT1–43Δ on ER. On the other hand, both α- and β-MHC isoforms did not alter ER in RcTnT44–73Δ fibers (Fig. 6, A and B).
Figure 6.
Effect of RcTnT1–43Δ and RcTnT44–73Δ on ER in α- or β-MHC fibers. ER was measured at pCa 4.3 as described previously (Ford et al., 2010). ER represents the magnitude of the sudden stretch–mediated increase in the recruitment of additional force-bearing XBs (see the supplemental text for a detailed description). (A) ER in fibers containing α-MHC. (B) ER in fibers containing β-MHC. RcTnT1–43Δ decreased ER by ∼36% in α-MHC fibers, an effect that was counteracted in β-MHC fibers. Number of determinations was at least 10 for each group. Values are reported as mean ± SEM. **, P < 0.01.
Effects of α- and β-MHC isoforms on the rates of XB recruitment and detachment dynamics in RcTnT1–43Δ- or RcTnT44–73Δ-reconstituted rat cardiac muscle fibers
The rate constant of XB recruitment (b) was measured by fitting the NLRD model to force responses elicited by step-like ML perturbations (see the supplemental text for details). Two-way ANOVA showed no significant interaction effect but revealed a significant main effect of MHC isoforms on b. Post-hoc multiple comparisons demonstrated that b decreased in all groups of β-MHC–containing fibers (Table 1). This is not surprising because β-MHC has an intrinsic ability to slow b (Chandra et al., 2007; Stelzer et al., 2007). In the presence of β-MHC, b decreased uniformly in RcTnTWT or RcTnT1–43Δ or RcTnT44–73Δ fibers, demonstrating that b was not affected by β-MHC. Another way to look at this is that RcTnT1–43Δ or RcTnT44–73Δ had no effect on b in the presence of α- or β-MHC (Table 1). This observation was corroborated by the rate of tension redevelopment (ktr). Two-way ANOVA revealed no significant interaction effect but revealed a significant main effect of MHC isoforms on ktr. Post-hoc multiple comparisons demonstrated that ktr decreased uniformly in all three different groups of β-MHC fibers when compared with the corresponding groups of α-MHC fibers (Table 1). As observed for b, RcTnT1–43Δ and RcTnT44–73Δ did not alter ktr in α- or β-MHC fibers.
Table 1.
Estimates of dynamic contractile parameters from α- and β-MHC–containing fibers reconstituted with RcTnT1–43Δ and RcTnT44–73Δ
| Dynamic contractile parameters | RcTnTWT | RcTnT1–43Δ | RcTnT44–73Δ |
| α-MHC | |||
| b (s−1) | 18.14 ± 0.62 | 18.38 ± 1.10 | 17.46 ± 0.43 |
| ktr (s−1) | 7.32 ± 0.17 | 7.25 ± 0.31 | 6.79 ± 0.29 |
| c (s−1) | 21.41 ± 0.83 | 20.60 ± 1.53 | 20.14 ± 0.07 |
| β-MHC | |||
| b (s−1) | 7.55 ± 0.34a | 7.84 ± 0.17a | 7.53 ± 0.22a |
| ktr (s−1) | 4.15 ± 0.11a | 4.38 ± 0.08a | 4.40 ± 0.11a |
| c (s−1) | 10.72 ± 0.40a | 10.70 ± 0.35a | 11.68 ± 0.30a |
The SL of the muscle fiber was set to 2.3 µm. b and c were estimated as described previously (Ford et al., 2010). ktr was estimated using a rapid slack/restretch protocol (Brenner and Eisenberg, 1986). Number of determinations was at least 11 for each group. Values are reported as mean ± SEM.
P < 0.001 when compared to the corresponding groups in α-MHC fibers.
A shift from α- to β-MHC isoform affects cardiac contractile dynamics by slowing b (Chandra et al., 2007; Stelzer et al., 2007). Therefore, we sought to determine if the impact of α- and β-MHC on XB detachment kinetics had any effect on how RcTnT1–43Δ and RcTnT44–73Δ modulated contractile dynamics. The rate of XB distortion dynamics (c) was measured by fitting the NLRD model to force responses elicited by step-like ML perturbations (Ford et al., 2010). Two-way ANOVA showed no significant interaction effect but revealed a significant main effect of α- and β-MHC on c. Post-hoc multiple comparisons showed that c decreased in all groups of β-MHC–containing fibers when compared with the corresponding groups of α-MHC–containing fibers. However, RcTnT1–43Δ and RcTnT44–73Δ did not alter c in α- or β-MHC fibers (Table 1).
DISCUSSION
Conclusions drawn from our data have significant implications for the regulation of cardiac thin filament activation. The most important finding from our study is that α- and β-MHC isoforms have a divergent impact on how the cardiac-specific N terminus of cTnT modulates cardiac thin filament activation. The magnitude of cardiac thin filament activation depends on how cooperative mechanisms are modulated by the actions of both cTnT and XBs. Thus, how the 1–44 and 45–74 regions of cTnT modulate thin filament activation and desensitize cardiac thin filaments to Ca2+ (Mamidi et al., 2013a) will not only depend on how these two regions by themselves exert their effects on the thin filament but also on how such actions are further modulated by the MHC isoform–mediated effects on the thin filament.
A shift from α- to β-MHC counteracts the attenuating effect of RcTnT1–43Δ on thin filament activation
The functional significance of the N terminus of cTnT is demonstrated by the findings that deletions in the N terminus of cTnT attenuate thin filament activation by depressing both Ca2+-activated maximal tension (Chandra et al., 1999; Communal et al., 2002; Sumandea et al., 2009; Mamidi et al., 2013a) and myofilament Ca2+ sensitivity (Mamidi et al., 2013a). Deletions in the N terminus of cTnT have been shown to alter Tn–Tm interactions (Chandra et al., 1999), as well as Tn–Tm interaction with actin (Mamidi et al., 2013a). In view of the observation that cardiac contractile dynamics are tuned by a significant interplay between Tn isoforms and MHC (Chandra et al., 2007), and that α- and β-MHC isoforms divergently affect thin filament activation (Ford and Chandra, 2013), we sought to determine how the impact of deletions in the N terminus of cTnT is modified by a shift from α- to β-MHC background. An interesting observation in the present study is that the attenuating effect of RcTnT1–43Δ on Ca2+-activated maximal tension—observed under an α-MHC background (Mamidi et al., 2013a)—is suppressed by a shift to β-MHC background (Fig. 3, A and B). Specifically, RcTnT1–43Δ attenuates maximal tension by ∼46% under an α-MHC background (Fig. 3 A), whereas a shift to β-MHC background attenuates maximal tension by only ∼18% (Fig. 3 B). Thus, our data demonstrate that RcTnT1–43Δ attenuates thin filament activation to a greater extent in the presence of α-MHC.
How does the β-MHC isoform counteract the RcTnT1–43Δ-mediated attenuation of cardiac thin filament activation? The answer may lie in the innate property of β-MHC to slow XB cycling dynamics (Moss et al., 2004; Rundell et al., 2005; Chandra et al., 2007; Stelzer et al., 2007; Locher et al., 2009). XB cycling dynamics—as estimated by the rate of XB recruitment (b) and the rate of XB detachment (c)—are significantly slower in fibers containing β-MHC (Table 1 and Fig. S2). This slowing effect on XB cycling dynamics is independent of the deletion in cTnT because both b and c are unaffected by RcTnT1–43Δ (Table 1). The slower cycling β-MHC may amplify cooperative mechanisms in the thin filament because of its longer XB dwell time in the strongly bound state; such an effect on thin filament cooperativity is expected to cause an increase in the recruitment of additional force-bearing XBs in RcTnT1–43Δ-reconstituted fibers (schematically depicted in Fig. S3). This notion is supported by our ED estimates, which demonstrate that the number of force-bearing XBs is significantly higher in RcTnT1–43Δ + β-MHC fibers when compared with RcTnT1–43Δ + α-MHC fibers (Fig. 4, C and D). Furthermore, the ML-mediated increase in stiffness (ER) is significantly higher in RcTnT1–43Δ + β-MHC fibers when compared with RcTnT1–43Δ + α-MHC fibers (Fig. 6). Mechanisms other than changes in thin filament cooperativity may also be involved. Recent studies (Coffee Castro-Zena and Root, 2013) show that the myosin lever arm tends to attain the pre–power stroke orientation when bound closer to the Tn complex but assumes the post–power stroke orientation when bound away from the Tn complex, suggesting that Tn is responsible for the formation of myosin-binding target zones along the thin filament. Whether or not charge changes in cTnT (caused by deletions in the N terminus of cTnT in our study) may have influenced the XB formation requires further investigation. Collectively, our data demonstrate that α- and β-MHC isoforms divergently modulate the activity of RcTnT1–43Δ because of intrinsic differences in their kinetic properties.
A shift from α- to β-MHC ablates the RcTnT1–43Δ-mediated desensitization of myofilament Ca2+ sensitivity
Another novel finding in this study is that the attenuation of myofilament Ca2+ sensitivity—observed in α-MHC + RcTnT1–43Δ fibers (Fig. 5, A and C)—is ablated in β-MHC + RcTnT1–43Δ fibers (Fig. 5, B and D). These observations clearly demonstrate that α- and β-MHC isoforms have a divergent impact on myofilament Ca2+ sensitivity. Based on our recent studies, the attenuation of myofilament Ca2+ sensitivity by RcTnT1–43Δ may be explained by the RcTnT1–43Δ-mediated increase in the rigidity of Tm (Mamidi et al., 2013a). Such an impact on Tm would shift the OFF/ON equilibrium of the regulatory units (RUs; Tm–Tn) more toward the OFF state, thereby attenuating myofilament Ca2+ sensitivity in α-MHC + RcTnT1–43Δ fibers. pCa50 decreased significantly by 0.13 pCa units in α-MHC + RcTnT1–43Δ fibers (Fig. 5 C) but remained unaffected in β-MHC + RcTnT1–43Δ fibers (Fig. 5 D), demonstrating that the augmenting effect of β-MHC on thin filament cooperativity negated the attenuating effect of RcTnT1–43Δ on myofilament Ca2+ sensitivity. If this observation is true, we expect β-MHC to further increase myofilament Ca2+ sensitivity in RcTnT44–73Δ fibers. Indeed, that is exactly what we observe: for example, RcTnT44–73Δ increases pCa50 by 0.22 pCa units in the presence of α-MHC and by 0.29 pCa units in the presence of β-MHC. Inferences drawn from the above analysis lead us to conclude that β-MHC affects the transition between OFF/ON states of the RU.
A shift from α- to β-MHC ablates the negative effect of RcTnT1–43Δ on the sudden stretch–mediated recruitment of new force-bearing XBs
The effects mediated by the N terminus of cTnT on the rate of RU ON/OFF transitions may be transduced through a direct effect on the overlapping ends of two contiguous Tm dimers (Mamidi et al., 2013b). Moreover, strong XBs themselves affect the balance between RU OFF and ON states through cooperative activation (Fitzsimons and Moss, 1998; Gordon et al., 2000; Razumova et al., 2000; Moss et al., 2004). These observations suggest that some aspect of RU-related cooperativity may modulate the recruitment of XBs and, therefore, the length-dependent activation. One way to determine this is by estimating ER from sudden stretch experiments performed at SL of 2.3 µm. ER is the slope of the relationship between new steady-state force (Fnss) and ΔL (see Fig. 6 and supplemental text). As described previously, ER is an index of the sensitivity of sudden stretch–mediated recruitment of additional XBs into the force-bearing state (Ford et al., 2010). It is interesting to note that ER was different in α- and β-MHC fibers. Specifically, α- and β-MHC isoforms had a divergent impact on ER in RcTnT1–43Δ fibers but not in RcTnT44–73Δ fibers. Note that both pCa50 and ER decrease significantly in α-MHC + RcTnT1–43Δ fibers (Figs. 5 C and 6 A) but not in β-MHC + RcTnT1–43Δ fibers (Figs. 5 D and 6 B). Conclusions drawn from this observation suggest that the β-MHC–mediated effect on the thin filament negates the attenuating effect of RcTnT1–43Δ on ER. Thus, our results demonstrate that the interplay between the N terminus of cTnT and MHC isoform–mediated effects on the thin filament has a divergent impact on mechanisms that modulate both the sudden stretch–mediated XB recruitment and myofilament Ca2+ sensitivity. Collectively, these observations have implications for understanding how cTnT-mediated thin filament activation is modulated in hearts of different species that express either a fast or a slow MHC isoform.
Summary
Novel findings from our study demonstrate that α- and β-MHC isoforms have divergent effects on how the N terminus of cTnT modulates cardiac contractile dynamics. Inferences made from our study also have significant relevance to cardiac health and disease; for example, alterations in MHC and cTnT are known to occur via changes in isoform expression or mutations (Miyata et al., 2000; Wei and Jin, 2011).
Acknowledgments
We thank Sri Lakshmi Mallampalli for excellent technical assistance and Dr. Sampath Gollapudi for help in generating figures using MATLAB.
This work was funded by the National Heart, Lung, and Blood Institute (grant R01-HL-075643 to M. Chandra) and a Poncin Fellowship (to R. Mamidi).
Richard L. Moss served as editor.
Footnotes
Abbreviations used in this paper:
- cTnT
- cardiac troponin T
- MHC
- myosin heavy chain
- ML
- muscle length
- NLRD
- nonlinear recruitment distortion
- PTU
- propylthiouracil
- RcTnT
- rat cTnT
- RU
- regulatory unit
- SL
- sarcomere length
- Tm
- tropomyosin
- Tn
- troponin
- XB
- cross-bridge
References
- Brenner B., Eisenberg E. 1986. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. USA. 83:3542–3546 10.1073/pnas.83.10.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M., Montgomery D.E., Kim J.J., Solaro R.J. 1999. The N-terminal region of troponin T is essential for the maximal activation of rat cardiac myofilaments. J. Mol. Cell. Cardiol. 31:867–880 10.1006/jmcc.1999.0928 [DOI] [PubMed] [Google Scholar]
- Chandra M., Tschirgi M.L., Rajapakse I., Campbell K.B. 2006. Troponin T modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle. Biophys. J. 90:2867–2876 10.1529/biophysj.105.076950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M., Tschirgi M.L., Ford S.J., Slinker B.K., Campbell K.B. 2007. Interaction between myosin heavy chain and troponin isoforms modulate cardiac myofiber contractile dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293:R1595–R1607 10.1152/ajpregu.00157.2007 [DOI] [PubMed] [Google Scholar]
- Chandra M., Mamidi R., Ford S., Hidalgo C., Witt C., Ottenheijm C., Labeit S., Granzier H. 2009. Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. J. Biol. Chem. 284:30889–30896 10.1074/jbc.M109.049718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzonite R.A., Zak R. 1984. Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J. Biol. Chem. 259:12628–12632 [PubMed] [Google Scholar]
- Coffee Castro-Zena P.G., Root D.D. 2013. Asymmetric myosin binding to the thin filament as revealed by a fluorescent nanocircuit. Arch. Biochem. Biophys. 535:14–21 10.1016/j.abb.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communal C., Sumandea M., de Tombe P., Narula J., Solaro R.J., Hajjar R.J. 2002. Functional consequences of caspase activation in cardiac myocytes. Proc. Natl. Acad. Sci. USA. 99:6252–6256 10.1073/pnas.092022999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe P.P., Stienen G.J. 1995. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ. Res. 76:734–741 10.1161/01.RES.76.5.734 [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. 1979. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris). 75:463–505 [PubMed] [Google Scholar]
- Fitzsimons D.P., Moss R.L. 1998. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ. Res. 83:602–607 10.1161/01.RES.83.6.602 [DOI] [PubMed] [Google Scholar]
- Fitzsimons D.P., Moss R.L. 2007. Cooperativity in the regulation of force and the kinetics of force development in heart and skeletal muscles: cross-bridge activation of force. Adv. Exp. Med. Biol. 592:177–189 10.1007/978-4-431-38453-3_16 [DOI] [PubMed] [Google Scholar]
- Fitzsimons D.P., Patel J.R., Moss R.L. 1998. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J. Physiol. 513:171–183 10.1111/j.1469-7793.1998.171by.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons D.P., Patel J.R., Campbell K.S., Moss R.L. 2001. Cooperative mechanisms in the activation dependence of the rate of force development in rabbit skinned skeletal muscle fibers. J. Gen. Physiol. 117:133–148 10.1085/jgp.117.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S.J., Chandra M. 2013. Length-dependent effects on cardiac contractile dynamics are different in cardiac muscle containing α- or β-myosin heavy chain. Arch. Biochem. Biophys. 535:3–13 10.1016/j.abb.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S.J., Chandra M., Mamidi R., Dong W., Campbell K.B. 2010. Model representation of the nonlinear step response in cardiac muscle. J. Gen. Physiol. 136:159–177 10.1085/jgp.201010467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S.J., Mamidi R., Jimenez J., Tardiff J.C., Chandra M. 2012. Effects of R92 mutations in mouse cardiac troponin T are influenced by changes in myosin heavy chain isoform. J. Mol. Cell. Cardiol. 53:542–551 10.1016/j.yjmcc.2012.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., Mamidi R., Mallampalli S.L., Chandra M. 2012. The N-terminal extension of cardiac troponin T stabilizes the blocked state of cardiac thin filament. Biophys. J. 103:940–948 10.1016/j.bpj.2012.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A.M., Homsher E., Regnier M. 2000. Regulation of contraction in striated muscle. Physiol. Rev. 80:853–924 [DOI] [PubMed] [Google Scholar]
- Herron T.J., Korte F.S., McDonald K.S. 2001. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. Am. J. Physiol. Heart Circ. Physiol. 281:H1217–H1222 [DOI] [PubMed] [Google Scholar]
- Kirk J.A., MacGowan G.A., Evans C., Smith S.H., Warren C.M., Mamidi R., Chandra M., Stewart A.F., Solaro R.J., Shroff S.G. 2009. Left ventricular and myocardial function in mice expressing constitutively pseudophosphorylated cardiac troponin I. Circ. Res. 105:1232–1239 10.1161/CIRCRESAHA.109.205427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte F.S., Herron T.J., Rovetto M.J., McDonald K.S. 2005. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am. J. Physiol. Heart Circ. Physiol. 289:H801–H812 10.1152/ajpheart.01227.2004 [DOI] [PubMed] [Google Scholar]
- Locher M.R., Razumova M.V., Stelzer J.E., Norman H.S., Patel J.R., Moss R.L. 2009. Determination of rate constants for turnover of myosin isoforms in rat myocardium: implications for in vivo contractile kinetics. Am. J. Physiol. Heart Circ. Physiol. 297:H247–H256 10.1152/ajpheart.00922.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi R., Gollapudi S.K., Mallampalli S.L., Chandra M. 2012. Alanine or aspartic acid substitutions at serine23/24 of cardiac troponin I decrease thin filament activation, with no effect on crossbridge detachment kinetics. Arch. Biochem. Biophys. 525:1–8 10.1016/j.abb.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi R., Mallampalli S.L., Wieczorek D.F., Chandra M. 2013a. Identification of two new regions in the N-terminus of cardiac troponin T that have divergent effects on cardiac contractile function. J. Physiol. 591:1217–1234 10.1113/jphysiol.2012.243394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi R., Michael J.J., Muthuchamy M., Chandra M. 2013b. Interplay between the overlapping ends of tropomyosin and the N terminus of cardiac troponin T affects tropomyosin states on actin. FASEB J. 27:3848–3859 10.1096/fj.13-232363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J.M., Wahr P.A., Michele D.E., Albayya F., Westfall M.V. 1999. Effects of myosin heavy chain isoform switching on Ca2+-activated tension development in single adult cardiac myocytes. Circ. Res. 84:1310–1317 10.1161/01.RES.84.11.1310 [DOI] [PubMed] [Google Scholar]
- Miyata S., Minobe W., Bristow M.R., Leinwand L.A. 2000. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ. Res. 86:386–390 10.1161/01.RES.86.4.386 [DOI] [PubMed] [Google Scholar]
- Montgomery D.E., Tardiff J.C., Chandra M. 2001. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J. Physiol. 536:583–592 10.1111/j.1469-7793.2001.0583c.xd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R.L., Razumova M., Fitzsimons D.P. 2004. Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ. Res. 94:1290–1300 10.1161/01.RES.0000127125.61647.4F [DOI] [PubMed] [Google Scholar]
- Razumova M.V., Bukatina A.E., Campbell K.B. 2000. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys. J. 78:3120–3137 10.1016/S0006-3495(00)76849-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser P.J., Portman M.A., Ning X.H., Schomisch Moravec C. 2001. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am. J. Physiol. Heart Circ. Physiol. 280:H1814–H1820 [DOI] [PubMed] [Google Scholar]
- Rice R., Guinto P., Dowell-Martino C., He H., Hoyer K., Krenz M., Robbins J., Ingwall J.S., Tardiff J.C. 2010. Cardiac myosin heavy chain isoform exchange alters the phenotype of cTnT-related cardiomyopathies in mouse hearts. J. Mol. Cell. Cardiol. 48:979–988 10.1016/j.yjmcc.2009.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers B.D., Interlichia J.P., Garikipati D.K., Mamidi R., Chandra M., Nelson O.L., Murry C.E., Santana L.F. 2009. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J. Physiol. 587:4873–4886 10.1113/jphysiol.2009.172544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell V.L., Manaves V., Martin A.F., de Tombe P.P. 2005. Impact of beta-myosin heavy chain isoform expression on cross-bridge cycling kinetics. Am. J. Physiol. Heart Circ. Physiol. 288:H896–H903 10.1152/ajpheart.00407.2004 [DOI] [PubMed] [Google Scholar]
- Stelzer J.E., Brickson S.L., Locher M.R., Moss R.L. 2007. Role of myosin heavy chain composition in the stretch activation response of rat myocardium. J. Physiol. 579:161–173 10.1113/jphysiol.2006.119719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumandea M.P., Vahebi S., Sumandea C.A., Garcia-Cazarin M.L., Staidle J., Homsher E. 2009. Impact of cardiac troponin T N-terminal deletion and phosphorylation on myofilament function. Biochemistry. 48:7722–7731 10.1021/bi900516n [DOI] [PubMed] [Google Scholar]
- Tardiff J.C., Factor S.M., Tompkins B.D., Hewett T.E., Palmer B.M., Moore R.L., Schwartz S., Robbins J., Leinwand L.A. 1998. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J. Clin. Invest. 101:2800–2811 10.1172/JCI2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff J.C., Hewett T.E., Factor S.M., Vikstrom K.L., Robbins J., Leinwand L.A. 2000. Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am. J. Physiol. Heart Circ. Physiol. 278:H412–H419 [DOI] [PubMed] [Google Scholar]
- Tschirgi M.L., Rajapakse I., Chandra M. 2006. Functional consequence of mutation in rat cardiac troponin T is affected differently by myosin heavy chain isoforms. J. Physiol. 574:263–273 10.1113/jphysiol.2006.107417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Jin J.P. 2011. Troponin T isoforms and posttranscriptional modifications: Evolution, regulation and function. Arch. Biochem. Biophys. 505:144–154 10.1016/j.abb.2010.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]