Abstract
Hypoxia-inducible factor (HIF) prolyl hydroxylases (PHDs) are α-ketoglutarate (αKG)-dependent dioxygenases that function as cellular oxygen sensors. However, PHD activity also depends on factors other than oxygen, especially αKG, a key metabolic compound closely linked to amino-acid metabolism. We examined the connection between amino-acid availability and PHD activity. We found that amino-acid starvation leads to αKG depletion and to PHD inactivation but not to HIF stabilization. Furthermore, pharmacologic or genetic inhibition of PHDs induced autophagy and prevented mammalian target of rapamycin complex 1 (mTORC1) activation by amino acids in a HIF-independent manner. Therefore, PHDs sense not only oxygen but also respond to amino acids, constituting a broad intracellular nutrient-sensing network.
Keywords: prolyl hydroxylases, PHD, alpha-ketoglutarate, amino acids, HIF, mTOR
Introduction
Prolyl hydroxylases (PHDs) are considered the oxygen sensors of cells. The cellular response to hypoxia is mediated by the transcription factor hypoxia-inducible factor (HIF)1, constituted by HIF1α and HIF1β. In the presence of molecular oxygen, PHD proteins hydroxylate HIF1α in its oxygen-dependent degradation (ODD) domain allowing for the proteasomal degradation of HIF1α.1, 2, 3 During hypoxia, the lack of molecular oxygen leads to the inhibition of PHDs, the consequent stabilization of HIF1 and the expression of its target genes.4 In humans, the PHD family comprises three different α-ketoglutarate (αKG)-dependent dioxygenases (PHD1, PHD2 and PHD3), requiring iron and ascorbate as cofactors. For the hydroxylation process, oxygen atoms are obtained from molecular oxygen while αKG provides electrons while being decarboxylated to succinate. First discovered in Caenorhabditis elegans, PHDs were later found in many other organisms including mammals, Drosophila, Dictyostelium, fission yeast and even photosynthetic organisms such as Chlamydomonas reinhardtii.5, 6, 7, 8, 9 The presence of PHD proteins in organisms lacking HIF raises the possibility PHD functions beyond HIF regulation.
It was previously discovered that PHDs are inhibited by their product, succinate, and that mutations in the tumor suppressor succinate dehydrogenase lead to the accumulation of succinate and consequently to HIF1 induction due to the inactivation of PHDs.10, 11 This effect is reversed by artificially increasing intracellular αKG levels.12 This finding pointed to the importance of αKG availability for PHD activity.
αKG is a central metabolic hub, involved in several crucial pathways. It participates in the tricarboxylic acid (TCA) cycle and in amino-acid transamination. Furthermore, αKG is the product of glutamine deamination (glutaminolysis), an anaplerotic process, which replenishes the TCA cycle. During glutaminolysis, glutamine is deaminated to αKG in a two steps process catalyzed by glutaminase and glutamate dehydrogenase, the latter is positively regulated by leucine.13 The degradation of other amino acids, such as arginine, histidine or proline, also leads to the production of αKG. Thus, αKG is closely connected to amino-acid metabolism. Indeed, glutamine deprivation causes a marked drop in the levels of different TCA cycle intermediates.14 As PHDs are strictly dependent on αKG as an electron donor for prolyl hydroxylation, we hypothesized that PHDs may be dependent on amino-acid availability. Acting as αKG sensors, PHDs would thus provide not only oxygen-monitoring activity, but a broader metabolic-sensing system that also monitors the TCA cycle status. To date, it is not well understood how cells sense amino-acid availability, although it is known that, the serine/threonine kinase mammalian target of rapamycin complex 1 (mTORC1) is activated by amino acids.15
In this work, we demonstrated that both intracellular αKG levels and PHD activity are strictly dependent on amino-acid availability. We also demonstrated that PHD activity is required for mTORC1 activation by amino acids. We therefore linked amino-acid availability and mTORC1 activity via αKG production and PHD activation.
Results
Amino-acid starvation causes depletion of αKG that leads to PHD inactivation
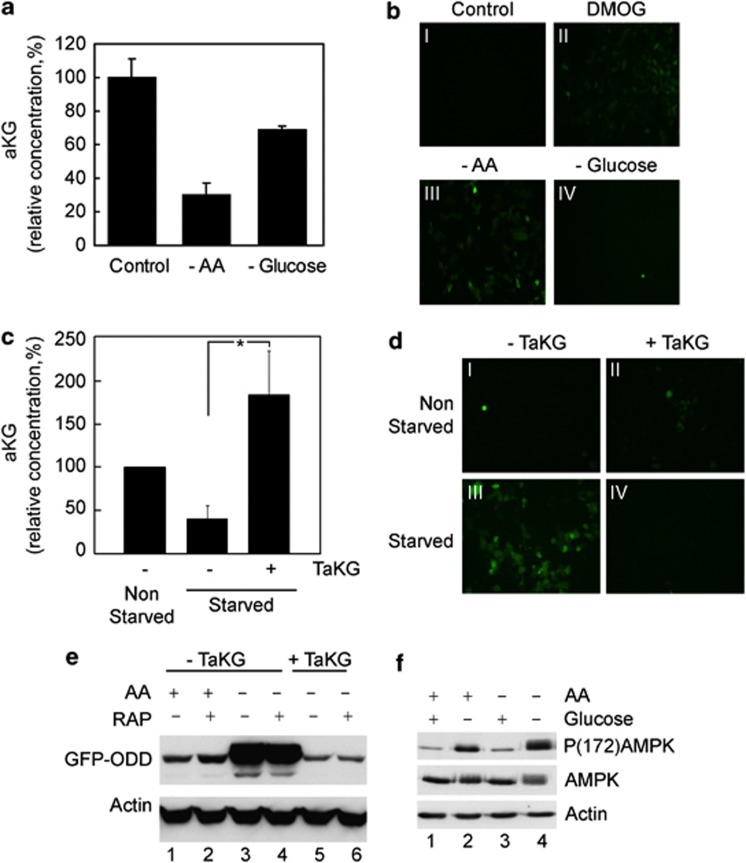
Using the U2OS cell line, we first determined the intracellular αKG levels and PHD activity in response to amino-acid availability. Amino-acid starvation caused a marked decrease in the intracellular levels of αKG (Figure 1a). Interestingly, glucose deprivation did not cause a comparable decrease in αKG (Figure 1a), supporting the notion that amino acids and not glucose are essential for maintaining TCA cycle components by sustaining the levels of αKG.
Figure 1.
Amino-acid starvation decreases αKG levels and inhibit PHD activity. (a) Intracellular αKG levels of U2OS cells after incubation for 3 h under the indicated condition. (b) GFP-ODD accumulation in HCT116 under the indicated condition. (c) Intracellular αKG content of starved cells treated with TαKG as indicated. Non-starved cells were used as control. (d, e) GFP-ODD accumulation examined microscopically (d) or by western blot (e) in fed and starved cells treated with TαKG and rapamycin (RAP) as indicated. (f) Phosphorylation levels of AMPK in U2OS cells incubated in either the presence or absence of amino acids and glucose. Results represent the average and s.d. of three independent experiments (*P<0.05).
As αKG is a co-substrate of PHDs, we next investigated whether amino-acid availability is required for PHD activity. Intracellular PHD activity was monitored using the HCT116 cell line that stably expresses a green fluorescent protein (GFP)–ODD fusion protein,12 the levels of which inversely correlate with PHD activity. In the presence of amino acids, GFP-ODD levels were low, confirming high PHD activity under normal nutritional conditions (Figure 1b, i). After inhibition of PHDs using dimethyloxallyl glycine (DMOG) GFP-ODD levels accumulated (Figure 1b, ii). In accordance with the observed changes in αKG levels (Figure 1a), GFP accumulated only after amino acid but not glucose starvation (Figure 1b, iii–iv) in a dose-dependent manner (Supplementary Figures S1A, B). We therefore concluded that PHD inactivation correlates with αKG reduction during amino-acid starvation.
As amino acids control intracellular αKG levels, we investigated whether PHD inactivation observed after amino-acid withdrawal is a direct consequence of the drop in αKG. For this purpose, the 1-trifluoromethylbenzyl-αKG ester (TαKG), a cell permeable ester of αKG,12 was utilized. The addition of TαKG to U2OS cells replenished αKG levels during amino-acid deprivation (Figure 1c) and restored low GFP-ODD levels (Figures 1d, e). This effect of TαKG was independent of mTORC1 activity, as rapamycin did not alter the GFP-ODD degradation upon TαKG addition (Figure 1e). Similar results were obtained in U2OS cells transiently expressing GFP-ODD (Supplementary Figure S1C). These results confirmed that reduction of PHD activity after amino-acid withdrawal is indeed caused by the loss of αKG.
Amino-acid starvation may have an impact on mitochondrial functions causing changes in the energetic status of cells. Such a bioenergetic stress could signal to PHDs. Indeed, amino-acid-starved cells demonstrated reduced mitochondrial-dependent respiration and ATP levels, however, the addition of αKG to the amino-acid-starved cells did not rescue cells from this bioenergetic deficit (Supplementary Figure S2). Furthermore, unlike glucose, amino-acid starvation did not increase the phosphorylation status of AMP-activated protein kinase (AMPK) (Figure 1f), an energy-sensor enzyme, which is phosphorylated during bioenergetic failure.16 These results further confirmed that the inactivation of PHDs following amino-acid starvation was not mediated by the bioenergetic status of the cell.
Loss of PHD activity during amino-acid starvation does not activate HIF
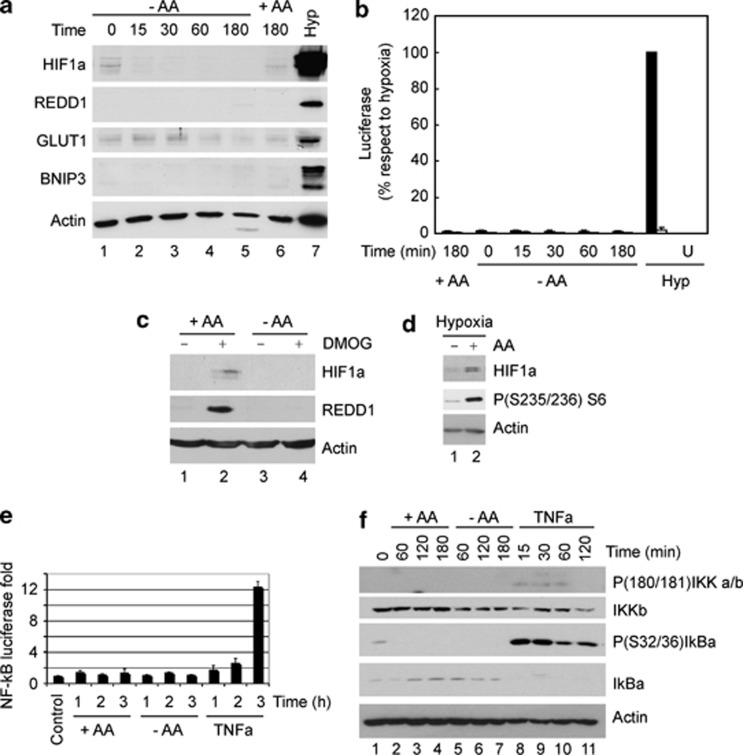
The best characterized PHD targets are the HIFα proteins, which accumulates under hypoxia. Therefore, we studied whether inactivation of PHDs during amino-acid deprivation leads to the accumulation of HIFα and to an increase in HIF transcriptional activity. Despite the inactivation of PHDs (Figures 1a–e), no accumulation of HIF1α was observed under amino-acid starvation (Figure 2a). Analysis of various HIF target genes (REDD1, GLUT1 and BNIP3) as well as of HIF transcriptional activity (using a luciferase reporter) did not reveal changes in HIF activity (Figures 2 a). This suggests that despite the fact that PHDs are inhibited after amino-acid starvation, HIF does not accumulate, likely due to a decrease in protein translation.17 Indeed, the inhibition of the proteasome using MG132 did not induce HIF1α accumulation during amino-acid starvation (Supplementary Figure S3A). In support of this notion, HIF1α and REDD1 levels were not increased by DMOG or hypoxia when cells were starved for amino acids (Figures 2c, d). The transcription factor nuclear factor (NF)-κB is an additional target of PHDs.18 However, amino-acid withdrawal did not cause any change in NF-κB signaling (Figures 2e, f). Therefore, we concluded that neither HIF nor NF-κB is activated upon PHD inhibition in response to amino-acid starvation.
Figure 2.
Inactivation of PHDs during amino-acid starvation does not activate HIF. (a) Protein levels of HIF1α, REDD1, Glut1 and BNIP3 in U2OS cells incubated in the presence or absence of amino acids for the indicated time. A positive control of cells incubated under hypoxic conditions for 12 h (Hyp) was included. (b) U2OS cells expressing a construct containing either a wild-type HRE-luciferase reporter (black bars) or a mutant HRE-luciferase reporter unable to respond to HIF1α (white bars) were incubated in the presence or absence of amino acids for the indicated time and the luciferase intensity was analyzed using a luminometer (values represent average and s.e. of three independent experiments). A positive control of cells incubated in hypoxic conditions for 12 h was included (U=untransfected). (c) Protein levels of HIF1α and REDD1 in U2OS cells incubated in fed or starving conditions for 2 h followed by 30 min of incubation in the presence or absence of DMOG. (d) U2OS cells where incubated in hypoxia (1% O2) in starving conditions for 2 h followed by 1 extra hour of incubation in the presence or absence of amino acids. HIF1α accumulation and S6 phosphorylation were then analyzed by western blot. (e, f) U2OS cells stably expressing NF-κB-reporter construct pGL4.32[luc2P/NF-kB-RE/Hygro] were incubated for the indicated time in KRBB either in the presence or absence of all amino acids or tumor necrosis factor-α (10 ng/ml). Activation of NF-κB was analyzed by luminescence analysis of the NF-κB-luciferase reporter (e) and by phosphorylation of IκB kinase (IKK) and IKβ-α by western blot (f). Graph depicts mean and s.d. of three independent experiments and represents fold activation compared with untreated samples.
PHD inhibition induces autophagy independently of HIF
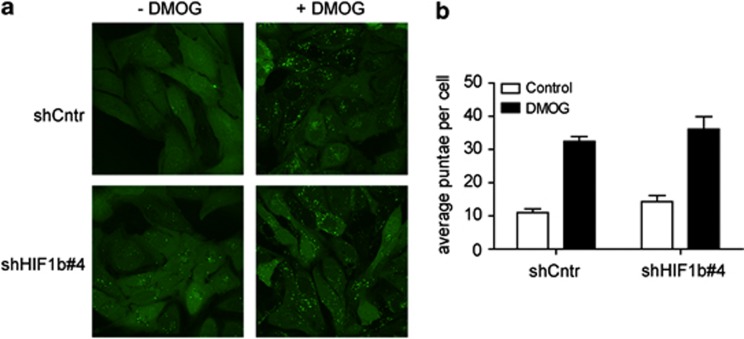
The role of PHDs in the biological response to nutrients deprivation was next analyzed. As amino-acid starvation is known to induce autophagy,19 and the activity of PHDs is inhibited in the absence of amino acid, a relation between PHD inhibition and autophagy was investigated, using the aggregation of the LC3–GFP fusion protein in autophagosomes as a reporter. Indeed, inhibition of PHDs by DMOG for 5 h in U2OS cells was sufficient to markedly induce autophagy to a similar extent as amino-acid starvation (Figures 3a, b and Supplementary Figures S3B, C). As we did not observe HIF accumulation after PHD inhibition during amino-acid starvation, it is unlikely that the corresponding autophagy mediated by PHD inhibition is HIF dependent. Indeed, efficient silencing of HIF activity (Supplementary Figure S4) did not reduce the ability of the PHD inhibitor DMOG to induce autophagy (Figures 3a, b).
Figure 3.
Inhibition of PHD by DMOG induces autophagy independently of HIF. U2OS cells stably expressing GFP-LC3 were transfected with a plasmid encoding control, nontargeting shRNA (shCntr) or transfected with a plasmid encoding shRNA for HIF1b (clone no. 4; shHIF1b no. 4). After 48 h, cells were incubated for 5 h in the presence of amino acids with or without DMOG as indicated. Aggregation of GFP-LC3 was analyzed by fluorescence microscopy (a) and quantified (b). Results represent the average and s.d. of three independent experiments.
Reduction of intracellular αKG levels during amino-acid starvation correlates with mTORC1 inhibition
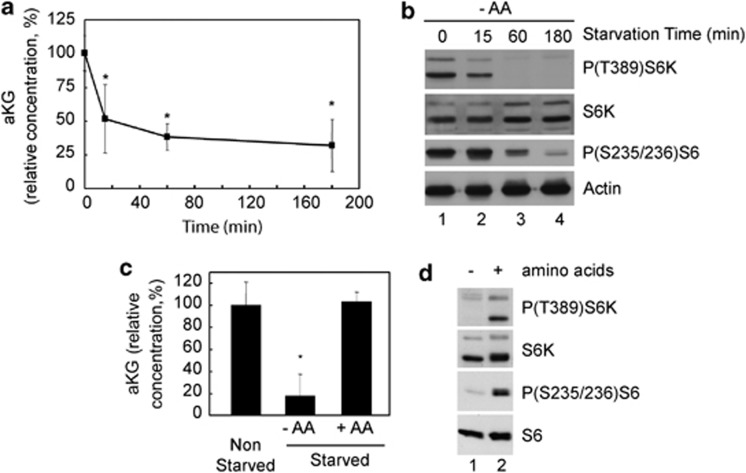
The data presented so far position the αKG-dependent activity of PHDs as a sensor of amino acids. Therefore, we next investigated whether the reduction in intracellular levels of αKG correlates with the well-characterized inhibition of mTORC1 during amino-acid starvation. As shown in Figures 4a, b, the kinetics of the reduction of intracellular αKG levels upon amino-acid withdrawal correlates with the inhibition of mTORC1 (measured by the phosphorylation status of its target proteins S6K and S6). Furthermore, re-addition of amino acids to amino-acid-starved cells restored both αKG levels and mTORC1 activity (Figures 4c, d). As leucine is a key amino acid in the regulation of mTORC1,20 we also investigated the reduction of αKG levels after leucine starvation only. Similar to our observations with complete amino-acid starvation, leucine deprivation resulted in a reduction in αKG that correlated with mTORC1 inhibition (Supplementary Figures S5A, B). This result positioned leucine as a critical amino acid for sustaining αKG production. In support of this, the inhibition of αKG production by 6-diazo-5-oxo-l-norleucine, a glutaminase inhibitor, which blocks αKG production from glutamine, also impaired the activation of mTORC1 by leucine and glutamine (Supplementary Figure S5C). Furthermore, starving U2OS cells of glutamine reduced the intracellular levels glutamate and glutamine, but it had no effect on the levels of intracellular leucine or any other amino acid (Supplementary Figure S6). Therefore, at least in U2OS cells, glutamine is required to sustain the intracellular levels of αKG, but not of leucine, contrary to a previous report.21
Figure 4.
Reduction of intracellular αKG during amino-acid starvation correlates with mTORC1 inhibition. (a, b) Intracellular αKG levels (a) and phosphorylation status of S6K and S6 proteins (b) in U2OS cells at different time points after incubation without amino acids. (c, d) Intracellular αKG levels (c) and phosphorylation status of S6K and S6 proteins (d) in U2OS cells under normal (non-starved) conditions, under starvation for amino acids for 3 h (−AA) or under starvation for amino acid for 2 h followed by re-addition of amino acids for 1 h (+AA).
Inhibition of PHDs blocks the response of mTORC1 to amino acids
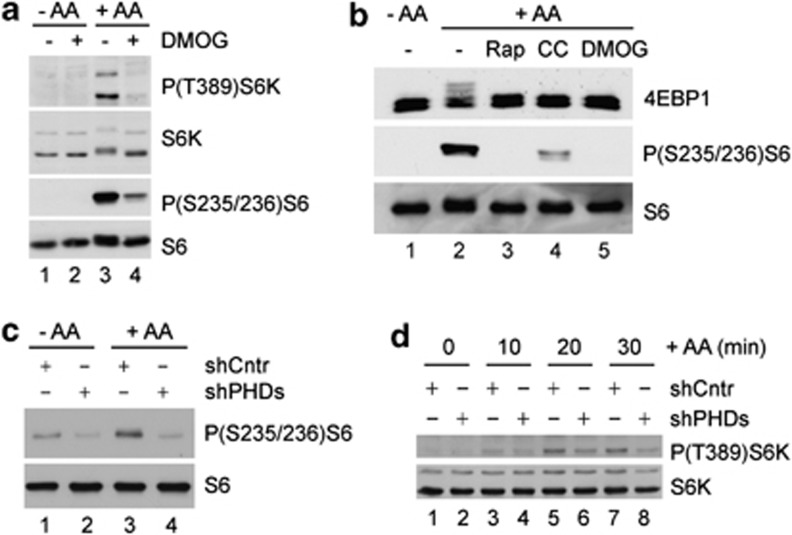
We next studied whether PHD activity is required for mTORC1 activation by amino acids. Inhibition of PHDs by DMOG blocked the ability of amino acids to re-activate mTORC1, as demonstrated by the lack of phosphorylation of the mTORC1 target proteins S6K, S6 and 4EBP1 (Figures 5a, b). Similar results were obtained using cobalt chloride (Figure 5b), a different inhibitor for PHDs. To further substantiate these results, PHDs were silenced with three different shRNAs, efficiently targeting each of the PHDs (Supplementary Figure S7). Although individual silencing of each of the PHDs did not block mTORC1 activation by amino acids (Supplementary Figures S8A–C), the silencing of all three PHDs simultaneously caused a marked inhibition in ribosomal S6 and S6K phosphorylation in response to amino acids (Figures 5c, d). Therefore, the results obtained with both chemical and genetic inhibition of PHDs strongly suggest that PHD activity is required for mTORC1 activation by amino acids. These results also propose a partial redundancy between all three PHDs in their response to amino acids and in mediating mTORC1 activation.
Figure 5.
Inhibition of PHDs blocks the response of mTORC1 to amino acids. (a, b) Phosphorylation state of S6K, S6 and 4EBP1 in cells starved for 2 h and then re-fed with amino acids for one more hour in the presence or absence of DMOG, rapamycin (Rap) or cobalt chloride (CC). (c) U2OS cells were transfected with a plasmid encoding control, nontargeting shRNA (shCntr) or cotransfected with three different plasmids encoding shRNAs for each member of the PHD family (shPHDs). After 48 h, cells were starved for amino acids for 2 h and then incubated for 20 min with (+ AA) or without (− AA) amino acids. The phosphorylation state of S6 was analyzed by western blot. (d) Cells were transfected as in (c) and starved for amino acids for 2 h and then incubated for the indicated time with amino acids (+ AA). The phosphorylation state of S6K was analyzed by western blot.
PHDs signal to mTORC1 via the Rag GTPases
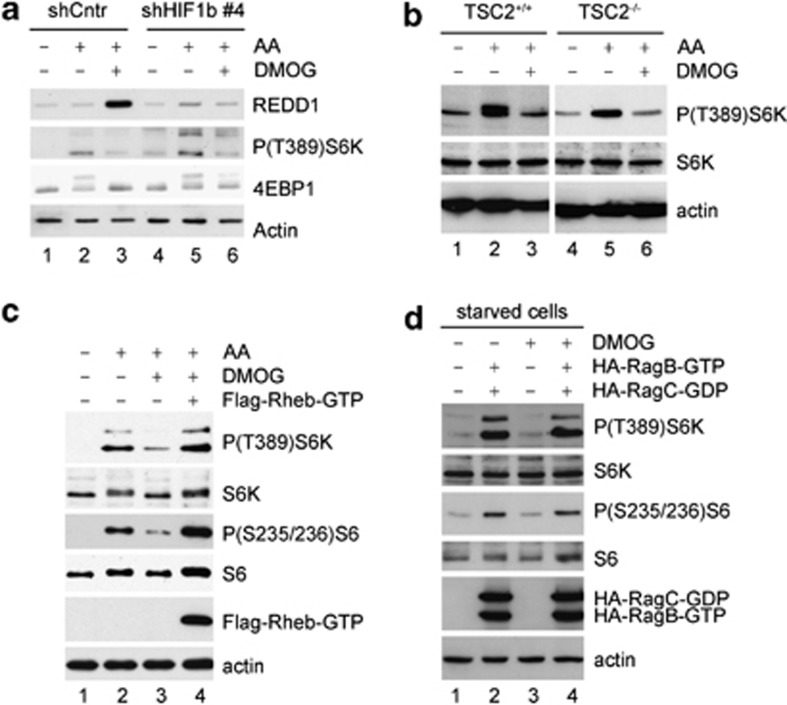
As we demonstrated that HIF was not affected by PHD inhibition during amino-acid starvation, we hypothesized that mTORC1 downregulation during PHD inhibition is independent of HIF, which can inhibit mTORC1 via the activation of the TSC1/2 complex.22 To test this possibility HIF activity was efficiently silenced, as judged by the lack of REDD1 induction by DMOG in the presence of amino acids (Figure 6a) and as expected, HIF silencing did not prevent the inhibition of mTORC1 by DMOG (Figure 6a). Therefore, we concluded that PHDs are required to maintain mTORC1 in a HIF-independent manner. Furthermore, DMOG treatment prevented mTORC1 activation by amino acids in both TSC2+/+ and TSC2−/− mouse embryonic fibroblasts (Figure 6b). In contrast, the expression of a constitutively active mutant of Rheb, which activates mTORC1 even in the absence of amino acids,23 also induced S6K and S6 phosphorylation in the presence of DMOG (Figure 6c). These results suggest that PHDs signal toward mTORC1 through the amino-acid-signaling pathway, which involves the Rag small GTPases,24 and not through the TSC1/2 pathway. Indeed, the expression of a constitutively active Rag also rescued mTORC1 activation in starved cells both in the presence or absence of DMOG (Figure 6d). Altogether, this work suggests that αKG-dependent activity of PHDs is necessary for mTORC1 activation by amino acids through the Rag GTPases and in a HIF/TSC-independent manner.
Figure 6.
PHDs signal to mTORC1 in a HIF/TSC-independent manner. (a) U2OS cells were transfected with a plasmid encoding control, nontargeting shRNA (shCntr) or transfected with a plasmid encoding shRNA for HIF1b (clone no. 4; shHIF1b no. 4). After 48 h, cells were starved for amino acids for 2 h and then incubated for 20 min with (+ AA) or without (− AA) amino acids either in the presence or absence of DMOG. Accumulation of REDD1 and phosphorylation state of S6K and 4EBP1 were analyzed by western blot. (b) Phosphorylation status of S6K in TSC2+/+ and TSC2−/− mouse embryonic fibroblasts starved for 2 h and then re-fed with amino acids for one more hour in the presence or absence of DMOG. (c) U2OS cells were transfected with either empty vector or Flag-Rheb-GTP, as indicated. After 48 h, cells were amino-acid starved for 2 h and re-stimulated for 1 h with amino acids (AA) either in the presence or absence of DMOG, as indicated. Phosphorylation of S6K and S6 was then measured by immunoblotting. (d) U2OS cells were transfected with either empty vector or with HA-RagB-GTP plus HA-RagC-GDP, as indicated. After 48 h, cells were amino-acid starved for 1 h where indicated, DMOG was added 30 min before the collection of the cells. Phosphorylation of S6K and S6 was then measured by immunoblotting.
Discussion
We demonstrate here that the ability of amino acids to induce mTORC1 signaling strictly requires PHD enzymatic activity. We show that αKG is an intracellular indicator of amino-acid availability, and it transduces the information to PHDs, which in turn, are required for mTORC1 pathway activation. Importantly, in a recent report we also demonstrated that αKG production through glutaminolysis is a necessary step for the activation of mTORC1 by leucine and glutamine.25 The results in this study confirm and extend this previous report. Of note, the estimated in vitro KM of PHDs for αKG is ∼50 μℳ,26 while we previously calculated an intracellular αKG concentration in steady state in the range of 100 μℳ,27 indicating that PHDs are good candidates to sense αKG oscillations. In this model, PHD enzymes do not function solely as intracellular oxygen sensors, but also as nutrient sensors (Figure 7). As the effect of amino acids on mTORC1 was severely hindered by DMOG or PHDs silencing, it appears that PHD activity is necessary for a complete mTORC1 response to amino acids. Interestingly, PHD1 regulates energy metabolism in muscle28 in a similar manner to mTORC1.29 Furthermore, like dTOR, the Drosophila homolog of mTOR,30 the Drosophila PHD is also required for cell growth.31 These observations genetically position PHDs and mTORC1 in a similar metabolic axis and together with our new data they propose a biological role of PHDs in turning nutrient availability or scarcity to signals that control cell growth or autophagy.
Figure 7.
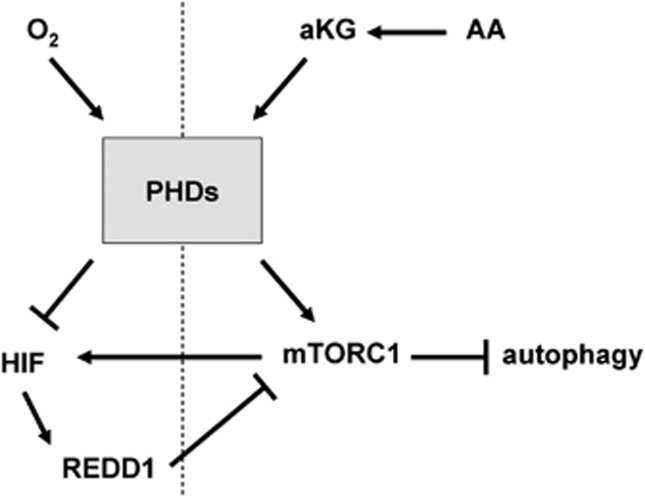
Model for oxygen- and nutrient-sensing mechanisms by PHDs. Left side: PHDs mediate the inhibition of HIF in the presence of oxygen. Under hypoxia, lack of oxygen inhibits PHD activity, resulting in the stabilization of HIF, which inhibits mTORC1 via REDD1 induction. Right side: PHDs are required for the activation of mTORC1 in the presence of amino acids. Under amino-acid starvation, lack of αKG inhibits PHD activity, resulting in the inhibition of mTORC1, hence a reduction in HIF expression. Loss of PHD activity can also mediate the induction of autophagy under amino-acid starvation conditions in a HIF-independent manner.
HIFα is the most-studied substrate of PHD enzymes, yet we have shown that HIF is not involved in the amino-acid-sensing process. The direct substrates of PHDs in the amino-acid-sensing pathway are yet to be identified. Nevertheless, the observation that PHDs clearly respond to amino acids indicates that these enzymes have important roles in metabolic control. The involvement of PHDs in the oxygen- and amino-acid-sensing mechanisms designates them as major cellular metabolic sensors. This dual role for PHDs raises the question how cells distinguish between oxygen- and amino-acid-limiting conditions. In fact, inhibition of PHDs under hypoxia leads to the accumulation of HIF1, which inhibits mTORC1 through REDD1 induction.32 On the other hand, PHD inhibition by amino-acid starvation leads to reduced mTORC1 activity, which prevents HIF1α translation.33 This feedback loop prevents HIF activation under amino-acid starvation, despite the fact that PHDs are inactivated.
In conclusion, we propose that PHD proteins function as general metabolic sensors in the cell, signaling scarcity not only of oxygen but also of amino acids and forming a link between amino-acid availability and the mTORC1 pathway.
Materials and methods
Reagents
Reagents were obtained from the following sources: Antibodies against ribosomal S6, phospho-S235/236 S6, S6K1, phospho-T389 S6K1, 4EBP1, AMP-activated protein kinase, phospho-T172 AMP-activated protein kinase, IκB kinase β, phospho-S180/S181 IκB kinase α/β, IκBα and phospho-S32/36 IκBα were obtained from Cell Signalling Technology, Beverly, MA, USA; anti-GFP and anti-HIF1α were obtained from BD Biosciences, Franklin Lakes, NJ, USA; anti-actin, anti-tubulin and anti-BNIP3 were obtained from Sigma (Gillingham, UK); antibody against REDD1 was obtained from the ProteinTech Group, Chicago, IL, USA; anti-Glut1 was obtained from Abcam, Cambridge, UK; antibody against PHD1 was obtained from Novus, Littleton, CO, USA; PHD2 and PHD3 antibodies were kindly provided by Kevin Gatter; all horseradish peroxidase-conjugated secondary antibodies were from Cell Signalling Technology; every antibody was used at the manufacturer's recommended dilution. TαKG was obtained as described previously.12 6-Diazo-5-oxo-l-norleucine, CoCl2, DMOG and rapamycin were from Sigma.
Plasmids, shRNAs and transfections
The plasmid pGL2/hypoxia responsive element (HRE)-luciferase was described elsewhere.10 pFLAG-PHD2 was obtained from Origene, Rockville, MD, USA. For knockdown experiments, pLKO.1-derived plasmids containing shRNA for PHD1 (RHS3979-9589735), PHD2 (RHS3979-9569473), PHD3 (RHS3979-9569477) and HIF1β/ARNT (RHS4533-NM_178427) were purchased from Open Biosystems, Lafayette, CO, USA. pLKO.1 plasmid containing control shRNA (Addgene plasmid 1864, Cambridge, MA, USA), plasmid expressing HA-RagB-Q99L (RagB-GTP; Addgene plasmid 19303) and plasmid expressing HA-RagC-S75L (RagC-GDP; Addgene plasmid 19305) were kindly provided by David M Sabatini.34 Plasmid expressing FLAG-Rheb-N153T (RhebGTP; Addgene plasmid 19997) was kindly provided by Fuyuhiko Tamanoi. Cells were transfected with lipofectamine (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions.
Cell culture
U2OS, HeLa and mouse embryonic fibroblasts (both TSC2+/+ and TSC2−/−) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 2 mℳℒ-glutamine. HCT116 cells stably expressing GFP-ODD (CL18 cells) were described previously.27 CL18 cells were cultured in RPMI medium supplemented with 10% fetal calf serum and 2 mℳℒ-glutamine. For amino-acid starvation, cells were incubated in Krebs-Ringer Bicarbonate Buffer (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid 25 mℳ, NaCl 111 mℳ, NaHCO3 25 mℳ, KCl 5 mℳ, CaCl2 2.5 mℳ and MgCl2 1 mℳ) supplemented with glucose (4.5 g/l). Standard starvation time was 2 h, unless otherwise indicated. When required, cells were re-fed with a solution with all essential and nonessential amino acids (Invitrogen) during the indicated time. For TαKG re-stimulation purposes, these compounds were added to a final concentration of 1 mℳ for the indicated time. Inhibitors such as DMOG (1 mℳ final concentration), CoCl2 (0.5 mℳ final concentration), 6-diazo-5-oxo-l-norleucine (40 μℳ final concentration) or rapamycin (50 nM final concentration) were added 1 h before the re-stimulation with amino acids. Hypoxia was achieved using a humidified, Invivo2 500 hypoxic chamber (Biotrace, Bridgend, UK) and Ruskin hypoxic gas mixer at 37 1C with 1% O2, 5% CO2 and 94% N2. For U2OS cells stably expressing the NF-κB-luciferase reporter, U2OS cells were transfected with the 2 μg of NF-κB-reporter construct pGL4.32[luc2P/NF-kB-RE/Hygro] (Promega, Madison, WI, USA) using genejuice (Merck Biosciences, Darmstadt, Germany). After 48 h, cells were split and cultivated under 300 μg/ml Hygromycin B (Roche, Indianapolis, IN, USA). Individual clones were picked and selected based on their response to tumor necrosis factor-α.
Protein analysis
For western blot analysis, cells were extracted in Laemmli sample buffer. Following sodium dodecyl sulfate–polyacrylamide gel electrophoresis, proteins were blotted onto nitrocellulose and analyzed with the appropriate antibody, using the ECL kit (GE Healthcare, Piscataway, NJ, USA) for detection.
Measurement of α-ketoglutarate
Intracellular levels of alpha-ketoglutarate were determined by following the method described previously.12 U2OS cells were washed with phosphate-buffered saline and lysed with RIPA buffer (50 mℳ Tris–HCl (pH 7.4), 150 mℳ NaCl, 1% Triton X 100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate supplemented with protease inhibitor cocktail (Invitrogen)). Cell lysates were collected from the plate, vortexed vigorously and centrifuged for 5 min at 15 000 × g at 4 °C. Aliquots of the extracts were analyzed immediately for alpha-ketoglutarate. The assay solution consisted of 100 mℳ KH2PO4 (pH 7.2), 10 mℳ NH4Cl, 5 mℳ MgCl2 and 0.15 mℳ nicotinamide adenine dinucleotide (Roche). Following equilibration at 37 °C with extract, the reaction was started by the addition of 5 units of glutamate dehydrogenase (Sigma). The absorbance decrease was monitored at 340 nm. The intracellular level of α-ketoglutarate was determined from the absorbance decrease in nicotinamide adenine dinucleotide (extinction coefficient, 6.22/mℳ/cm).
Measurement of oxygen consumption rate
A total of 3 × 104 cells were plated onto XF24 plates (Seahorse Bioscience, North Billerica, MA, USA) and incubated at 37 °C, 5% CO2 for 24 h. The medium was then replaced with 675 μl of unbuffered Dulbecco's modified Eagle's medium (Seahorse Bioscience) supplemented with 2 mmol glutamine, 25 mmol glucose and 2% fetal bovine serum (pH was adjusted to 7.4 using sodium hydroxide 0.5 ℳ and cells were incubated at 37 °C in a CO2-free incubator. Basal oxygen consumption rate was then recorded using the XF24 plate reader (Seahorse Bioscience). Every cycle of measurement consisted of 3 min mix, 3 min wait and 4 min measure. Oxygen consumption rate was normalized to cell number calculated at the end of the experiments. Homogeneous plating of the cells and cell count was obtained by fixing the cells with trichloroacetic acid 10% for 1 h at 4 °C and then staining the fixes cells with 0.47% solution of Sulforhodamine B (Sigma).
Measurement of intracellular ATP levels
A total of 2 × 105 cells were grown on six well plates and treated as indicated. ATP was measured from the cell lysates of the same samples using a luciferase-based assay as per manufacturer's instructions (Sigma). Data were normalized to total protein contents of the cell lysate determined by Bicinchoninic Acid Assay (Thermoscientific, Rockford, IL, USA) using bovine serum albumin as standard.
Luciferase assay
For HRE-luciferase assays, U2OS cells were transfected with either wild type or mutant pGL2/HRE-luciferase 48 h before start of the experiment. Luciferase intensity was determined using the Luciferase Assay System (Promega) by following manufacturer's instruction and using a VeritasMicroplateLuminometer (Turner Biosystems, Madison, WI, USA). For NF-κB-luciferase assay, cells were processed as described previously35 using the Luciferase Assay System (Promega).
Measurement of intracellular content of amino acids
U2OS cells were plated in six wells plates (8 × 105 cells per well). After 24 h, cells were rapidly washed with phosphate-buffered saline and incubated for the indicated times in Krebs-Ringer Bicarbonate Buffer supplemented with glucose, essential and nonessential amino acids (Invitrogen), in the presence or absence of 2 mℳ glutamine. At the end of the treatments, the intracellular content of amino acids was determined as described previously.36 Briefly, cells were washed twice with ice cold phosphate-buffered saline, extracted for 5 min on ice with 1 ml of a 6.6-mℳ acetic acid solution in ethanol,and analyzed with a Biochrom 20 Amino Acid Analyzer (Biochrom, Cambridge, UK) using a High-Resolution Column Bio 20 Peek Lithium and the Physiological Fluid Chemical Kit (Biochrom) for elution. The column effluent was mixed with ninhydrin reagent, passed through the high temperature reaction coil and read by the photometer unit. Data are normalized on a standard curve and expressed as nmol/mg of protein.
Autophagy measurements
U2OS cells stably expressing LC3-GFP where cultured in Iwaki glass-bottomed dishes. After 5 h, treatment in starving conditions either in the presence or absence of DMOG, GFP localization was detected using a confocal microscopy using a SP2 DMIRBE laser scanning confocal microscope with SP2 software (Leica, Wetzlar, Germany). GFP aggregation was then quantified.
GFP-ODD quantification
Fluorescence in HCT116 cell line stably expressing a GFP–ODD fusion protein was analyzed directly in culture using an Olympus CX41 Fluorescent Tissue Culture Microscope (Olympus, Tokyo, Japan).
Statistical analyses
Data were expressed as average±s.d. of at least three independent experiments. Student's t-test was used to determine differences among two groups. Significance was judged when P<0.05.
Acknowledgments
PHD2 and PHD3 antibodies were provided by Kevin Gatter. We thank David Sabatini and Fuyuhiko Tamanoi for kindly providing Rag and Rheb plasmids. We thank David Kwiatkowski for TSC2+/+ and TSC2−/− mouse embryonic fibroblasts. This work was supported by the Cancer Research UK and by the Swiss National Science Foundation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- Berra E, Ginouves A, Pouyssegur J. The hypoxia-inducible-factor hydroxylases bring fresh air into hypoxia signalling. EMBO Rep. 2006;7:41–45. doi: 10.1038/sj.embor.7400598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Taylor MS. Characterization and comparative analysis of the EGLN gene family. Gene. 2001;275:125–132. doi: 10.1016/s0378-1119(01)00633-3. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- West CM, van der Wel H, Wang ZA. Prolyl 4-hydroxylase-1 mediates O2 signaling during development of Dictyostelium. Development. 2007;134:3349–3358. doi: 10.1242/dev.000893. [DOI] [PubMed] [Google Scholar]
- Hughes BT, Espenshade PJ. Oxygen-regulated degradation of fission yeast SREBP by Ofd1, a prolyl hydroxylase family member. EMBO J. 2008;27:1491–1501. doi: 10.1038/emboj.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskiaho K, Hieta R, Sormunen R, Myllyharju J. Chlamydomonas reinhardtii has multiple prolyl 4-hydroxylases, one of which is essential for proper cell wall assembly. Plant Cell. 2007;19:256–269. doi: 10.1105/tpc.106.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol. 2007;27:912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A, Malaisse WJ. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288:187–189. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Guan KL. Amino acid signaling in TOR activation. Annu Rev Biochem. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296:E592–E602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Urano J, Sato T, Matsuo T, Otsubo Y, Yamamoto M, Tamanoi F. Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc Natl Acad Sci USA. 2007;104:3514–3519. doi: 10.1073/pnas.0608510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- Tennant DA, Frezza C, MacKenzie ED, Nguyen QD, Zheng L, Selak MA, et al. Reactivating HIF prolyl hydroxylases under hypoxia results in metabolic catastrophe and cell death. Oncogene. 2009;28:4009–4021. doi: 10.1038/onc.2009.250. [DOI] [PubMed] [Google Scholar]
- Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei C, Edgar BA. Drosophila cyclin D/Cdk4 requires Hif-1 prolyl hydroxylase to drive cell growth. Dev Cell. 2004;6:241–251. doi: 10.1016/s1534-5807(03)00409-x. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Rocha S, Martin AM, Meek DW, Perkins ND. p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-kappaB subunit with histone deacetylase 1. Mol Cell Biol. 2003;23:4713–4727. doi: 10.1128/MCB.23.13.4713-4727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Asta V, Bussolati O, Sala R, Parolari A, Alamanni F, Biglioli P, et al. Amino acids are compatible osmolytes for volume recovery after hypertonic shrinkage in vascular endothelial cells. Am J Physiol. 1999;276:C865–C872. doi: 10.1152/ajpcell.1999.276.4.C865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.