Abstract
Increased attention has been drawn to the important role played by regulatory T-cells (Treg) in immune homoeostasis. However, the small numbers of Tregs make them elusive to study. We investigated the cryostability of Tregs and whether they can be expanded from cryopreserved peripheral blood mononuclear cells (PBMCs). Further, to elucidate if there is a difference in ex-vivo frequency or in vitro expansion of Tregs among T1D children (n=9), high-risk (n=7) and healthy (n=10) individuals, Tregs defined as CD4+CD25+CD127lo/− were isolated from cryopreserved PBMCs.
Cryopreserved PBMCs maintained a stable expression of Treg-markers. Tregs were efficiently expanded in vitro from all donors and Tregs from T1D children acquired higher FOXP3 expression compared to healthy subjects. T1D children had a significantly lower percentage of Tregs among CD4+ T-cells and also lower Treg to CD4+CD25− cell ratios compared to healthy individuals.
Keywords: Cryopreservation, Regulatory T-cells, Expansion, Type 1 diabetes
Highlights
► PBMCs maintain stable expression of Treg-markers following cryopreservation. ► Efficient in vitro expansion of Treg from cryopreserved PBMC of T1D children. ► Lower percentage of Tregs among CD4+ T-cells in T1D, compared to healthy individuals. ► Lower Treg to CD4+CD25− cell ratios in T1D, compared to healthy individuals.
1. Introduction
To maintain self-tolerance along with a sufficient protection against the many threats encountered in everyday life, the immune system needs to keep a plastic balance between up- and down-regulating mechanisms. Loss of this plasticity may result in autoimmunity. Type 1 Diabetes (T1D) is connected to an autoimmune process towards the insulin producing pancreatic β-cells and is the most common chronic disease in children in developed countries. T1D is associated with a significant burden of daily insulin injections, regular and controlled meals and close monitoring of blood glucose values. Despite great efforts to keep blood glucose in check, children with T1D are often affected by acute complications (e.g. hypoglycaemia) and chronic micro- and macro-vascular complications.
The autoimmune attack on the beta-cells is considered to be of a T-helper (Th) 1-like effector origin, i.e. connected to cell-mediated immunity and an interferon-γ (IFN-γ) and tumour necrosis factor (TNF) rich milieu [1–3]. It is also associated with the presence of islet cell autoantibodies towards glutamic acid decarboxylase (GAD65), insulin, the islet tyrosine phosphatase IA-2 [4] and zinc transporter 8 (ZnT8) [5]. We have previously reported a Th1-like dominated profile in high-risk first-degree relatives of T1D children, characterized by high production of IFN-γ [6]. We and others have, however, observed that this strong bias towards Th1 immunity vanishes close to T1D onset and remains suppressed in newly diagnosed patients, as documented by reduced IFN-γ mRNA expression and secretion both in unstimulated conditions and after in vitro mitogen stimulation [6–8].
Establishment of T-cell tolerance takes place both centrally in the thymus and through various mechanisms in the periphery [9]. The strongly suppressive regulatory T-cells (Tregs) are bridging central and peripheral tolerance mechanisms through thymic derived and peripherally induced subsets. A common description of the Treg phenotype is the concurrent expression of CD4 and high constitutive expression of the alpha chain of the IL-2 receptor (CD25) [10]. To further identify a regulatory population devoid of activated effector T-cells, a number of markers have been described, most notably absent or low expression of the IL-7 receptor CD127 [11,12]. However, pinpointing a pure Treg population is a complex task in humans due to the lack of a specific and unique Treg marker together with the heterogeneity of this population. The transcription factor forkhead box P3 (FOXP3), found to be expressed in CD4+CD25+ Tregs [13], was also found to be transiently expressed in activated effector T-cells [14,15]. FOXP3+ Tregs also constitutively express the inhibitory molecule CTLA-4 [16], while other T-cell subsets express this protein only transiently upon activation. In mouse models completely deprived of Tregs, a variety of autoimmune diseases develop and, likewise, FOXP3 mutations in man cause immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome [17,18]. Furthermore, a variety of diseases and autoimmune states have been associated with impaired Treg function [10,17–20], while re-introduction of CD4+CD25hi Treg cells into non-obese diabetic (NOD) mice has shown to prevent the development of T1D and to inhibit the production of IFN-γ [21].
In the research field of T1D, where study subjects are often children, blood volumes available for T-cell studies are limited and samples given must be carefully handled and used to its full extent. Cryopreservation of peripheral blood mononuclear cells (PBMC) in liquid nitrogen allows for batch analysis of samples, but the number of Tregs that can be recovered is limited (∼5–7% of CD4+ T-cells) [22]. In vitro expansion of these cells would therefore be most valuable for biological investigations, and, possibly, for adoptive cell therapies.
In the present study, we sought to investigate the cryostability of Treg associated markers and further to sort and expand Tregs from cryopreserved PBMC of T1D, high-risk and healthy individuals. The aim was to efficiently expand Tregs and to detect any difference in T-cell number and composition among the studied subjects.
2. Materials and methods
2.1. Subjects
For the pre-study, sodium-heparinized venous blood samples were obtained from 16 healthy adults (8 females and 8 males, median age 26.5 years, range 23.5–31).
For isolation and expansion, sodium-heparinized venous blood samples were obtained from 9 T1D children (4 females and 5 males, median age 10 years, range 4–14) between 20 days and 10 months after diagnosis (median disease duration 3 months). These patients were compared with 7 high-risk individuals (3 females and 4 males, median age 18 years, range 11–48) who had previously participated in the European Nicotinamide Diabetes Intervention Trial (ENDIT) [23] and who had up to a 40% risk of developing T1D within five years (≥20 ICA IJDF units), and with 10 healthy subjects (5 females and 5 males, median age 23.5 years, range 8–31) without a family history of T1D (Table 1). Blood samples from children with T1D were taken during visits to the Linköping diabetes clinic, and blood samples from healthy individuals were taken at school or at the work place, when possible during the morning hours to avoid time-of-day differences. Blood samples from high-risk individuals were transported to Linköping within 24 h of blood sampling. None of the study subjects showed any signs of cold or other infections, at the time of sampling.
Table 1.
Study subjects.
| Healthy | Age | Gender | T1D | Duration | Age | Gender | High-risk | Age | Gender | Treatment | Developed T1D | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC1 | 9 | Female | T1D1 | 3 months | 4 | Female | HR1 | 17 | Female | Placebo | Yes | 8.5 years post-sampling |
| HC2 | 13 | Male | T1D2 | 6 months | 11 | Female | HR2 | 41 | Female | Nicotinamide | Yes | 10 years post-sampling |
| HC3 | 8 | Male | T1D3 | 9 months | 10 | Male | HR3 | 12 | Male | Nicotinamide | Yes | 4 years post-sampling |
| HC4 | 15 | Male | T1D4 | 5 months | 4 | Male | HR4 | 42 | Female | Placebo | Yes | 1.5 years post-sampling |
| HC5 | 27 | Female | T1D5 | 10 months | 14 | Male | HR5 | 18 | Male | Placebo | No | |
| HC6 | 23 | Female | T1D6 | 1 month | 11 | Male | HR6 | 11 | Male | Placebo | No | |
| HC7 | 25 | Female | T1D7 | 3 months | 13 | Female | HR7 | 48 | Male | Nicotinamide | No | |
| HC8 | 25 | Male | T1D8 | 20 days | 9 | Female | ||||||
| HC9 | 31 | Female | T1D9 | 1 month | 5 | Male | ||||||
| HC10 | 24 | Male | ||||||||||
Composition of study population (healthy (HC), Type 1 diabetes (T1D) and high-risk (HR)), regarding age (years), gender (female/male), disease duration (days/months) and treatment with nicotinamide versus placebo, and development of T1D in high-risk individuals after inclusion in the study cohort (years).
2.2. PBMC isolation, cryopreservation and thawing
PBMCs were isolated by Ficoll Paque density gradient centrifugation (Amersham/Pharmacia), as described previously [6]. For cryopreservation, freezing medium (40% RPMI 1640 (Invitrogen), 10% DMSO (dimethyl sulphoxide, Sigma) and 50% foetal calf serum (FCS) (Gibco/Invitrogen)) were added dropwise to PBMC resulting in a cell suspension of 5×106 cells/ml, divided into aliquots of 1 ml in cryotubes and freezed at −70 °C in a pre-cooled (4 °C) freezing container (Mr Frosty NALGENE Labware), allowing a lowering of the temperature of 1 °C/min. The following day the cryotubes were transferred into liquid nitrogen for storage until further use.
Cryopreserved PBMCs were thawed directly under gentle agitation in a 37 °C water bath and immediately washed in RPMI 1640 supplemented with 10% human serum (HS) before staining and sorting.
2.3. Antibodies
For staining and sorting, fluorescein isothiocyanate (FITC)-conjugated anti-CD127, allophycocyanin (APC)-conjugated anti-CD25 (both from eBioscience) and pacific blue (PB)-conjugated anti-CD4 (clone OKT4, produced in-house) and Alexa 700-conjugated anti-FOXP3 (eBioscience) mAbs were used. For comparison of marker expression in the pre-study, FITC-conjugated anti-FOXP3 (Nordic BioSite), APC-conjugated anti-CD25 and PerCP-conjugated anti-CD4 (Becton Dickinson (BD) Biosciences) mAbs were used. Cells were stained for 30 min at 4 °C and washed in phosphate buffered saline (PBS) supplemented with 2% HS. For intracellular staining, Alexa 700-conjugated anti-FOXP3 was used following fixation and permeabilization with appropriate buffers (Miltenyi).
2.4. Flow cytometry acquisition and sorting
PBMCs were analysed and sorted using a FACSAria (Becton Dickinson) equipped with 488, 633 and 407 nm lasers. Lymphocytes were gated based on forward (FSC) and side scatter (SSC). For examination of Treg-marker expression before and after cryopreservation, in the pre-study, CD25hi cells were gated as CD4+ T lymphocyte subsets expressing higher levels of CD25 than the discrete population of CD4− cells expressing CD25. FOXP3 expression was then analysed in this gate. Also for sorting, CD4 expressing lymphocytes were gated to further obtain a dot plot of CD25 and CD127 fluorescence. Tregs were gated as CD4+CD25+CD127lo/− and compared to CD4+CD25− and CD4+CD25+CD127+ subpopulations (Fig. 1). The concurrent expressions of CD25 and FOXP3, following expansion of CD4+CD25+CD127lo/− and CD4+CD25− cultures, were analysed and compared to each other, as seen in Fig. 5a–c. The cut-offs for the gates were set after the fluorescence of a biologically FOXP3 negative and CD25 negative population. Data was analysed using the FlowJo software (Tree Star) and expressed as mean fluorescence intensity (MFI; geometrical and standard mean) and percentages of cells expressing each marker.
Fig. 1.
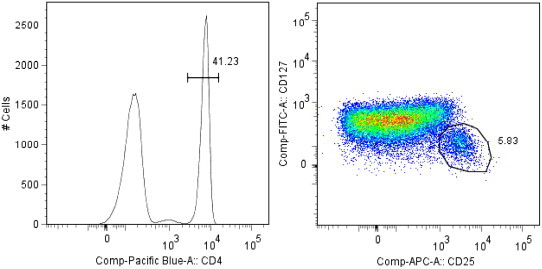
Gating strategy for sorting of CD4+CD25+CD127lo/− Treg. Lymphocyte population was gated based on forward and side scatter and from this gate CD4+ cells were isolated and further gated for expression or no expression of CD25 and CD127.
Fig. 5.
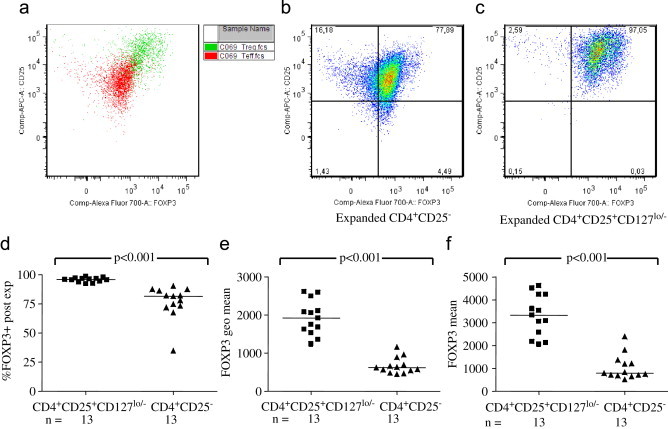
A big part of the sorted CD4+CD25− cells had become FOXP3+CD25+ after 15 days of expansion, but the cells expanded starting with sorted CD4+CD25+CD127lo/− Tregs had a higher FOXP3 expression intensity, as well as a higher percentage of positive cells. (a) Shows a representative overlay of FOXP3 expression in expanded cells starting with sorted CD4+CD25+CD127lo/− and CD4+CD25− of the same individual, and in (b) FOXP3+CD25+ expression after expansion of sorted CD4+CD25− and (c) after expansion of sorted CD4+CD25+CD127lo/−. Cumulative data of the expansion of sorted CD4+CD25+CD127lo/− T-cells (d) confirmed that a higher percentage of sorted and expanded CD4+CD25+CD127lo/− was FOXP3 positive (p<0.001). Expanded CD4+CD25+CD127lo/− had a higher mean fluorescent intensity (MFI) of FOXP3, both expressed as (e) geometrical mean (p<0.001) and (f) mean (p<0.001), compared to expanded cells starting with sorted CD4+CD25−.
2.5. In vitro expansion
Tregs were expanded according to a protocol adapted from Putnam et al. [24]. Briefly, on day 0 sorted cells were resuspended in AIM-V medium (Gibco/Invitrogen) containing 10% HS and amphotericin B and plated according to Table 2. Dynabeads® Human Treg Expander anti-CD3/anti-CD28 coated microbeads (Invitrogen; catalogue number 111.61D) were added at a 1:1 bead to cell ratio. When Treg numbers were lower than 40.000, 96-well flat-bottomed plates were used.
Table 2.
Expansion of CD4+CD25+CD127lo/−.
| Cell number | Vessel | Media volume | Dyanabeads (μl) | rhIL-2 (U/ml) |
|---|---|---|---|---|
| 40,000 | 96 well | 125 μl×2 | 1 | 300 |
| 100,000–150,000 | 48 well | 500 μl×2 | 2.5–3.75 | 300 |
| 200,000–300,000 | 24 well | 1 ml×2 | 5–7.5 | 300 |
| 400,000 | 12 well | 1.5 ml×2 | 10 | 300 |
| 800,000 | 6 well | 2 ml×2 | 20 | 300 |
| 1,200,000 | Vertical T25 | 3 ml×2 | 30 | 300 |
| 2,400,000 | Horizontal T25 | 5 ml×2 | 60 | 300 |
| 7,500,000 | T75 | 15 ml×2 | 187.5 | 300 |
| 17,000,000 | T175 | 35 ml×2 | 425 | 300 |
| Media: AIM-V medium containing 10% HS and amphotericin B | ||||
| Dynabeads® Human Treg Expander | ||||
Description of the experimental set-up for expansion of CD4+CD25+CD127lo/−.
×2=the same volume were added at day 0 and again at day 2.
For culture split or restimulation, the double volume was added.
The cell culture volume was doubled at day 2 and IL-2 (Proleukin, Chiron Therapeutics) added at a final concentration of 300 U/ml. On days 5 and 7, cells were counted, washed in AIM-V and resuspended as above, adding fresh IL-2. Restimulation with anti-CD3/anti-CD28 coated microbeads, was performed on day 9, as described for day 0. Cells were counted again on day 11 and 13, washed, resuspended according to Table 2, and supplemented with fresh IL-2. Cultures were terminated on day 15 and cells stained for FOXP3 analysis.
CD4+CD25− cells were expanded according to a scheme similar to Tregs, with the following alterations. As anti-CD3/anti-CD28 coated microbeads caused overstimulation and activation-induced apoptosis, CD4+CD25− cells were expanded using anti-CD3 (OKT3, 10 μg/ml) coated culturing vessels (Table 3) and soluble anti-CD28 (1 μg/ml). IL-2 addition was added at a concentration of 30 U/ml.
Table 3.
Expansion of CD4+CD25−.
| Cell number | Vessel | Media volume | CD28 (μg/ml) | rhIL-2 (U/ml) |
|---|---|---|---|---|
| <500,000 | 96 well | 125 μl×2 | 1 | 30 |
| 500,000 | 48 well | 500 μl×2 | 1 | 30 |
| 1×106 | 24 well | 1 ml×2 | 1 | 30 |
| When cells exceed 2.5×106 or medium turns yellow, split back to 1×106/24 well | ||||
| 50×106 | T175 | 40 ml×2 | 1 | 30 |
| Media: AIM-V medium containing 10% HS and amphotericin B | ||||
| Vessels precoated with antiCD-3 (OKT3 10 μg/ml) | ||||
Description of the experimental set-up for expansion of CD4+CD25− cells.
×2=the same volume was added at day 0 and again at day 2.
For culture split or restimulation, the double volume was added.
2.6. Statistics
As expression of Treg-markers was not normally distributed, two-group comparisons were performed with the Mann–Whitney U-test, while three or more groups were compared using the Kruskal–Wallis test for unpaired observations. For pair-wise comparisons, Wilcoxon signed rank test was used. A probability level <0.05 was considered statistically significant. Calculations were performed using the statistical package GraphPad Prism version 5.01 for Windows (GraphPad Software, Inc.).
2.7. Ethics
The study was approved by the Research Ethics Committee of the Faculty of Health Sciences, Linköping University. Informed consent was obtained from all volunteers and/or their parents.
3. Results
3.1. Marker expression following cryopreservation
Before sorting of Tregs, a small pre-study of healthy volunteering adults was performed assessing the stability of Treg-markers through cryopreservation and thawing. We did not find cryopreservation and thawing of PBMC to yield any differences in the percentage of FOXP3 expressing cells or the FOXP3 MFI, in the CD4+CD25hi cell population (Fig. 2a,b). Neither the MFI of CD25 in the CD4+CD25hi Treg population displayed any differences after cryopreservation (Fig. 2c). These data encouraged our belief in the feasibility of the use of cryopreserved PBMCs for this type of applications.
Fig. 2.
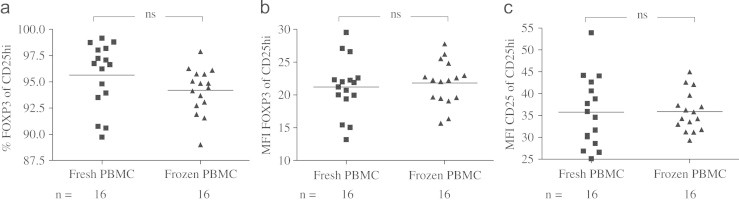
No difference in percentage of FOXP3 expressing cells (a), mean fluorescence intensity (MFI) of FOXP3 expression (b) or MFI of CD25 expression (c), in CD4+CD25hi cells before and after cryopreservation of isolated peripheral blood mononuclear cells.
In the pre-study, we examined the eventual interference of cryopreservation on Tregs based on FOXP3 expression in the strict CD4+CD25hi gate. This same strategy was not optimal for sorting due to the demand for membrane permeabilization and the limited sample sizes from the study cohort and the very limited cell numbers obtainable in this gate. Therefore, based on previous studies showing the IL-7 receptor CD127 to be strongly negatively correlated to FOXP3 expression, we choose to define Tregs as CD4+CD25+CD127lo/− cells. Before settling, we compared the appearance of the chosen sorting gate and the percentages and MFIs of these markers (CD4, CD25 and CD127) and FOXP3 expression, before and after cryopreservation, in two healthy adults. This experiment showed no contradiction between fresh and cryopreserved samples, confirming our strategy (data not shown).
3.2. Expansion of CD4+CD25+CD127lo/− Treg and CD4+CD25−
Following sorting of CD4+CD25+CD127lo/− Tregs and CD4+CD25−, cells were cultured for expansion according to a fifteen day long protocol (Tables 2 and 3). All individuals, independent of study group, were able to achieve a great increase (mean fold change 144, median fold change 104) in CD4+CD25+CD127lo/− numbers (Fig. 3a), even starting with as few as four thousands sorted CD4+CD25+CD127lo/− T-cells. CD4+CD25− however, were harder to expand, never reaching the same magnitude in fold increase as did the expansion of CD4+CD25+CD127lo/− T-cells (Fig. 3b). No differences in fold increase of CD4+CD25+CD127lo/− or CD4+CD25− were seen between the study groups (data not shown) even if CD4+CD25+CD127lo/− T-cells of healthy individuals seem to reach a higher fold increase (3c).
Fig. 3.
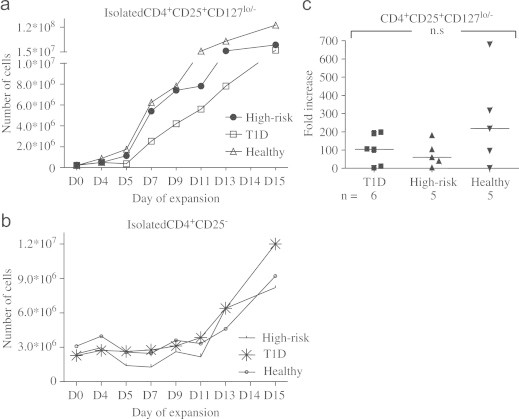
Representative expansion curves of sorted (a) CD4+CD25+CD127lo/− Treg and (b) CD4+CD25− cells. Fold increase (c) of Treg did not differ significantly between T1D, healthy and high-risk individuals, even if observations for healthy individuals visually appeared to be higher.
3.3. Composition of CD25+ and/or CD127+ expressing CD4+ cells
Analysing the composition of CD4+ cells in the three study groups, differences became apparent. T1D children showed a lower percentage of CD4+CD25+CD127lo/− cells in the CD4+ fraction of lymphocytes, compared to healthy individuals (p<0.05, Fig. 4a). Lower CD4+CD25+CD127lo/− cell counts in T1D were also true comparing CD4+CD25+CD127lo/− cells to CD4+CD25− (p<0.01, Fig. 4b). Further, T1D was associated with significantly higher percentages of CD4+CD25−, compared to both healthy (p<0.0001) and high-risk (p<0.01) individuals (Fig. 4c). In line with these results, T1D was associated with a lower percentage of the total CD4+CD25+ cell count, compared to the one seen in healthy individuals (p<0.05, Fig. 4d) and also tended to compared to in individuals at high risk of developing the disease (p<0.1, Fig. 4d). Moreover, T1D was associated with lower fractions of CD25+CD127+ cells in the CD4+ population, when compared to healthy individuals (p<0.05, Fig. 4e).
Fig. 4.
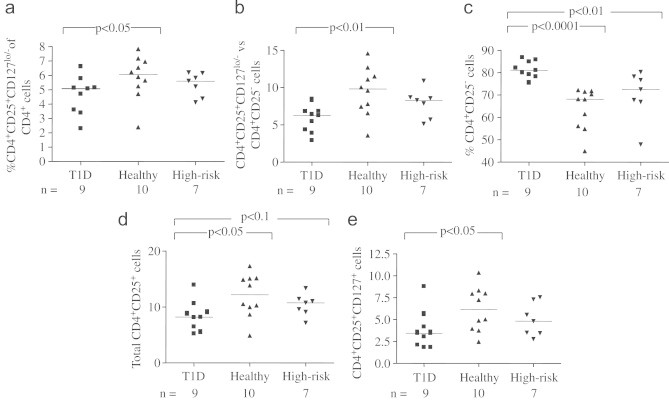
The percentage of CD4+CD25+CD127lo/− Treg in the CD4+ fraction (a), was lower in T1D compared to healthy individuals (p<0.05). Lower CD4+CD25+CD127lo/− quota in T1D were also true comparing CD4+CD25+CD127lo/− to CD4+CD25− (b) (p<0.01). Further, T1D was associated with significantly higher percentages of (c), compared to both healthy (p<0.0001) and high-risk (p<0.01) individuals. T1D was also associated with a lower percentage of the total CD4+CD25+ cell count (d), compared to the one seen in healthy individuals (p<0.05) and also tended to compared to the individuals at high risk of developing the disease (p<0.1) Moreover, T1D was associated with lower fractions of CD25+CD127+ (e) in the CD4 positive population, when compared to healthy individuals (p<0.05).
3.4. Post-expansion expression of FOXP3
After fifteen days, expansion cultures of sorted CD4+CD25+CD127lo/− or CD4+CD25− cells were terminated and FOXP3 expression analysed. Post-expansion, a big part of the sorted CD4+CD25− had become FOXP3+CD25+ but a higher percentage of the sorted Tregs (CD4+CD25+CD127lo/− T-cells) expressed FOXP3 and also had a higher FOXP3 expression, as shown in Fig. 5a, displaying an overlay of the FOXP3+CD25+ expression after expansion of sorted CD4+CD25+CD127lo/− cells and CD4+CD25− cells, from the same individual. Fig. 5b shows the FOXP3+CD25+ expression after expansion of sorted CD4+CD25− cells and Fig. 5c of sorted CD4+CD25+CD127lo/− cells. Cumulative data of the expansion of sorted CD4+CD25+CD127lo/− and CD4+CD25− confirmed that a higher percentage of sorted and expanded CD4+CD25+CD127lo/− was FOXP3 positive (p<0.001, Fig. 5d). Moreover, expanded CD4+CD25+CD127lo/− had a higher mean fluorescent intensity (MFI) of FOXP3, both expressed as geometrical mean (p<0.001, Fig. 5e) and mean (p<0.001, Fig. 5f), compared to expanded CD4+CD25−.
The percentage of FOXP3 expressing cells in the expanded CD4+CD25+CD127lo/− cultures did not differ significantly between T1D, healthy and high-risk individuals, even if T1D visually appeared to be higher (Fig. 6a). Post-expansion, CD4+CD25+CD127lo/− from T1D in contrast tended to display higher FOXP3 MFI, both expressed as geometrical mean (p=0.05, Fig. 6b) and mean (p=0.05, Fig. 6c), compared to healthy individuals.
Fig. 6.
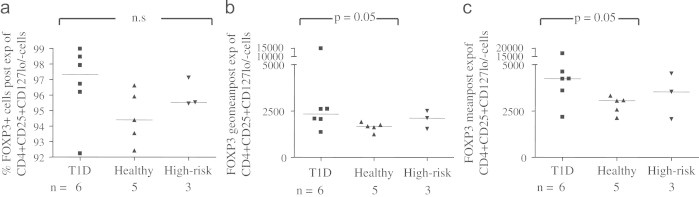
The percentage of FOXP3 expressing cells in the expanded CD4+CD25+CD127lo/− Treg cultures (a) did not differ significantly between T1D, healthy and high-risk individuals. Post-expansion, CD4+CD25+CD127lo/− from T1D tended to display higher FOXP3 MFI, both expressed as (b) geometrical mean (p=0.05) and (c) mean (p=0.05), compared to healthy individuals.
Expansion of CD4+CD25− T-cells did not yield any significant differences in the percentage of FOXP3 expressing cells or in FOXP3 MFI, neither expressed as geometrical mean nor mean (data not shown).
3.5. Influence of age, nicotinamide treatment and development of T1D
No differences, in regard to age distribution, were seen in composition of the studied T-cell phenotypes in healthy or high-risk individuals (data not shown). Neither was fold increase of CD4+CD25+CD127lo/− T-cells correlated to age among the high-risk, healthy or T1D individuals, nor in the total study population as a group (data not shown).
The high-risk group was further split in regard to development of T1D after inclusion in the study as well as split for treatment with nicotinamide or placebo but showed no differences in T-cell composition (data not shown).
4. Discussion
During recent years, the interest for Tregs has increased, and their role and function have been thoroughly scrutinized in a plethora of studies. While their suppressive functions and importance in maintaining immune homoeostasis in experimental models are generally acknowledged, their actual involvement in human autoimmune disease is more disputed and reported findings are non-unanimous. The development of T1D is associated with an imbalance in the immune system connected to an autoimmune attack on the insulin producing β-cells. A vast number of studies have identified pieces of the immunological puzzle of T1D, seeking to unravel the secret of the autoimmune riddle. However the origin, the failing regulatory mechanism rendering subjects non-tolerant to self remains elusive. Therefore, all pieces that can be added to this puzzle will be important for the picture to appear.
Due to costly methods and logistics in sample collection, isolated cells often need to be stored until further analysis can be performed. Cryopreservation offers an opportunity to preserve and store cells. In the research field of Treg, however, one deals with a quite small proportion of the total cell count and every sample is highly valuable. Cryopreservation is a rather harsh process to the handled cells that potentially could induce changes in the marker expression, phenotype and function [25].
To be able to study Tregs, starting with small sample sizes due to restricted sampling from T1D children, one goal of the study was to gain a significant expansion of Treg numbers. At times, the only logical option when working with patient material is to cryopreserve PBMCs. Cryopreservation may restrict the amount of cells available, therefore, efficient methods are of utmost importance.
An important concern regarding flow cytometry analysis of cryopreserved cells is that the expression of surface- and intracellular markers could be affected by the cryopreservation and thereby alter the phenotypes of the studied cells. Hence, we sought to establish the cryostability of Tregs. In our pre-study, the Treg marker FOXP3 was analysed in the CD4+CD25hi population. We were pleased to find that the expression of FOXP3 was not altered with regard to the percentage of expressing cells or MFI. Neither did MFI of CD25 change markedly from sampling to post-cryopreservation. Others have reported a somewhat diminished suppressive function directly upon thawing but this will be restored upon expansion. Further, if Treg was expanded prior to cryopreservation, the suppressive effect was unaffected upon thawing [26]. These results are positive, suggesting that Tregs are stable for applications such as flow cytometry and cell sorting following cryopreservation and thawing. Importantly, others also have shown that isolated Treg can survive cryopreservation [26].
While the so-called classic Treg gating strategy is based on the concurrent expression of CD4 and the highest expression of CD25, as only about 1–2% of the CD4+ cells, Liu et al. [11] demonstrated that Tregs defined by the concurrent expression CD4+CD25+CD127lo/−, as gated in Fig. 1, comprised a larger cell number but were as suppressive. Further it has been shown that the exclusion of CD127hi expressing cells, as done with this type of gating, allowed for isolation of Tregs without contamination of memory effector cells [24]. Beside the above mentioned findings, we found this gating strategy for Tregs (Fig. 1) to be solid and it was therefore chosen over the so-called classic gating strategy with CD4+ cells expressing the highest levels of CD25. We were pleased that cryopreserved PBMCs showed to be a suitable material for sorting and expanding Tregs. Further, we were able to achieve powerful expansion of Tregs from all individuals, independent of study group, even when starting with as few as four thousand sorted Tregs. These results are important, supporting the efficaciousness of the method. A great increase in Treg numbers despite a highly limited starting material is also an important starting-point, should autologous Treg therapy ever be the goal.
Although there was no statistical difference in fold increase of Tregs between the study groups, Tregs of healthy study subjects might be more prone to a higher fold increase than Tregs of T1D subjects based on the display of higher fold expansion in half the group in comparison to expansions seen from T1D and high-risk individuals. A previous study asserted that fold expansion of CD4+CD127lo/−CD25+ T-cells was negatively correlated to age [24]. However, we could not see any such negative correlation, or a positive one, between ages and fold expansion in our study cohort. Certainly it was not the youngest subjects in the healthy group that expanded the most. Further, no difference in fold expansion of CD4+CD25− T-cells between the groups was observed. This indicates that despite the higher proportion of CD4+CD25− observed in our cohort of T1D, there does not seem to be an altered proliferation rate to engagement of CD3 and CD28.
Following expansion, almost all of the sorted CD4+CD25+CD127lo/− Tregs expressed FOXP3 as compared to expanded CD4+CD25− responder cells, where a big but significantly lower percentage expressed FOXP3. Not only did a higher percentage of sorted and expanded Tregs express FOXP3 compared to CD4+CD25− T-cells post-expansion, but they also exhibited significantly higher intensity of FOXP3. This makes a strong case for the use of sorted CD4+CD25+CD127lo/−Tregs for expansion, since they seem to generate cells with strong CD25+FOXP3+ expression. Further, it could perhaps be speculated that the observed skewing of the CD4+ T-cell composition towards a larger proportion of CD4+CD25− cells in T1D children might render these individuals more susceptible to certain threats. This could be speculated since they hold a larger proportion of cells that upon engagement of CD3 and CD28 induce fewer FOXP3 expressing cells with lower FOXP3 intensity, in comparison to CD4+CD25+CD127lo/− cells.
Statistically, we did not see any difference between the study groups, in the percentage of FOXP3 expressing cells in the expanded Treg cultures. However, half of the observations for T1D individuals were higher than all the other observations and T1D also showed a tendency to higher FOXP3 intensity. No such differences were seen for the sorted and expanded CD4+CD25− cells. Taken together, although T1D may be associated with a smaller Treg proportion, they were able to achieve a great Treg expansion and even acquired higher FOXP3 expression than healthy individuals. Considering the variation of the T-cell composition between the groups, one might hypothesize that the Tregs of T1D are predominantly naïve or resting Tregs which could explain their good expansion potential and higher FOXP3 upregulation [27]. With this in mind, one could speculate that T1D subjects possibly could benefit from in vitro expansion and re-introduction of the autologous Tregs to keep the immunological process towards the pancreatic β-cells in check. It would have been elegant and of great importance, to be able to show functional data of the expanded CD4+CD25+CD127lo/− Tregs to establish the efficaciousness of the method and the possibility to use the expanded cells as Tregs, in future applications. Unfortunately, due to limited sample sizes, we were not able to show such results.
Our study displayed a lower percentage of Tregs (CD4+CD25+CD127lo/−) among the CD4+ cells in T1D children. This is in line with studies describing reduced numbers of, or possibly functionally weak, CD4+CD25hi Tregs in diseased individuals bearing autoimmune disorders such as T1D, multiple sclerosis and autoimmune polyglandular syndrome II [28–30]. This suggests that Tregs could be part of the explanation of the failed ability to maintain local self-tolerance during T1D development. However, to make the picture more complex, there are also studies that fail to present such differences for T1D patients [11,31]. One possible explanation for the lack of consensus for Tregs in T1D, and other disorders, could be the various ways of characterizing the cell type and defining the questions we ask on the impact of these cells. In studies where Tregs are defined as CD4+CD25hi, a certain spectrum of cells will be included in the calculations, compared to studies further adding FOXP3 and/or CD127 expression to the definition. Given that different ways of describing the cell type are used, part of the explanation for dissimilar findings could be as simple as diverse definition of Tregs. Moreover, it is conceivable that part of the explanation may be the diverse paths taken in the quest to obtain the truth. While we are describing a lower fraction of Tregs in T1D as a lower percentage of CD4+CD25+CD127lo/− cells in the CD4+ T-cell population, Liu et al. [11] addresses the question as FOXP3 expression in the CD4+CD25+CD127lo/−, CD4+CD25−CD127lo/−, CD4+CD25+CD127+ and CD4+CD25−CD127+ T-cell subsets.
In addition to a lower proportion of CD4+CD25+CD127lo/− cells in the total CD4+ population, we also found that the relationship of CD4+CD25+CD127lo/− cells to CD4+CD25− cells to be lower in T1D, confirming the low Treg (CD4+CD25+CD127lo/−) proportion seen in the total CD4+ population. This skewed ratio, as compared with the healthy individuals, may be explained by an elevated proportion of CD4+CD25− cells accompanying T1D and further strengthens the findings of a lower proportion of CD4+CD25+CD127lo/− Tregs. The CD4+CD25− T-cells were recently described as a composition of responder cells with a lower proliferation rate and slower IL-2 response to in vitro stimulation, compared to already in vivo activated CD4+ responder T-cells expressing low amounts of CD25 [32]. It was recently shown that strong CD28 costimulation suppressed induction of (i)Treg from CD4+CD25− T-cells [33]. At the same time costimulation of CD28 is critical in the thymic development of natural Tregs and their peripheral maintenance [34] and have been suggested to be required in low levels for iTreg generation [35]. Viral infections have been suggested in T1D development and could perhaps explain the observations of skewed proportions of CD4+ subsets, due to the high levels of CD28 costimulation taking place during acute infection, causing expansion of Teffs and hindering iTreg generation. One could also speculate that if the suggested skewing towards higher proportions of CD4+CD25− T-cells takes place before T1D development, this could explain why a viral infection could induce such a powerful response without gaining sufficient downregulation to protect the β-cells from an autoimmune attack.
Following these results, it came as no surprise that T1D subjects exhibited a total CD4+CD25+ cell count lower than the one seen in healthy individuals and furthermore a poorer percentage of the T-cell subset of CD4+CD25+CD127+ cells. CD127 is down-regulated on activated T-cells but is re-expressed in memory T-cells, IL2R(CD25)loIL7R(CD127)+, while Tregs remain CD127lo/− and a dichotomy between them has been suggested [36]. The reduced CD25+CD127+ population seen in our study is likely to contain activated memory cells. Together these results are consistent and suggestive of a shifted composition of the CD4+ T-cell subsets as a part of the disease picture of T1D. One could contemplate whether such alteration in the T-cell composition could be part of rendering an individual incapable to mount a sufficient suppressive and protective response to an autoimmune attack. One explanation for this might be that T1D has less activated (CD25+) memory T-cells and more resting (CD4+CD25−) ones because they do not activate so well. However, as the study was not designed to detect co-expression of activation-, naïve- or memory markers, this remains a speculation. Previous findings suggested that aging is associated with a shift in T-cell phenotype from a predominantly naïve T-cell subset (CD45RA+) to a memory (CD45RO+) T-cell phenotype [37]. However, in our study we could not derive any differences in the T-cell composition to age, neither in the different study groups by themselves or in the whole study population as a group.
We could not derive any correlations or differences in the study material to age, nicotinamide treatment in high-risk individuals or whether high-risk individuals developed disease or not. Thus, the results in our study cohort do not seem to be derived from age related factors, but rather be connected to T1D. In line with our study, Lindley et al. [38] did not find a relationship between age and CD25 expression, or between age and activation markers on CD4+CD25+.
In conclusion, based on the results from our study, cryopreserved PBMC seems to be feasible for flow cytometric analysis, sorting and expansion of Tregs Marker expression was stable through cryopreservation and thawing and an efficient fold expansion with preserved high FOXP3 expression was achieved. Further, our study suggests that T1D is associated with a lower percentage of Tregs, however, the ones present expanded well and even acquired higher FOXP3 upregulation. Whereas we found an altered composition of CD4+ subsets, biased towards a higher CD4+CD25− ratio to CD4+CD25+CD127lo/− Tregs, the importance of the said alteration remains to be shown.
Acknowledgement
This project was generously supported by the Albert Renold Travel Fellowship, the Swedish Child Diabetes Foundation (Barndiabetesfonden) and the Medical Research Fund of the County of Östergötland.
The authors would like to thank Dr. R. Mallone (INSERM U986, DeAR Lab Avenir, Saint Vincent de Paul Hospital, 75014 Paris, France) and the members of his research group for input in study design, technical assistance and lab space during sorting and expansion experiments.
Contributor Information
Anna Rydén, Email: anna.ryden@liu.se.
Maria Faresjö, Email: maria.faresjo@hhj.hj.se.
References
- 1.Eisenbarth G.S. Type I diabetes mellitus: a chronic autoimmune disease. New Engl J Med. 1986;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Castano L., Eisenbarth G.S. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 3.Bradley L.M. Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. J Immunol. 1999;162(5):2511–2520. [PubMed] [Google Scholar]
- 4.Bonifacio E., Bingley P.J. Islet autoantibodies and their use in predicting insulin-dependent diabetes. Acta Diabetol. 1997;34(3):185–193. doi: 10.1007/s005920050072. [DOI] [PubMed] [Google Scholar]
- 5.Wenzlau J.M. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104(43):17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsson M.G., Lawesson S.S., Ludvigsson J. Th1-like dominance in high-risk first-degree relatives of type I diabetic patients. Diabetologia. 2000;43(6):742–749. doi: 10.1007/s001250051372. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson Faresjo M.G., Ludvigsson J. Diminished Th1-like response to autoantigens in children with a high risk of developing type 1 diabetes. Scand J Immunol. 2005;61(2):173–179. doi: 10.1111/j.0300-9475.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- 8.Hedman M., Ludvigsson J., Faresjo M.K. Nicotinamide reduces high secretion of IFN-gamma in high-risk relatives even though it does not prevent type 1 diabetes. J Interferon Cytokine Res. 2006;26(4):207–213. doi: 10.1089/jir.2006.26.207. [DOI] [PubMed] [Google Scholar]
- 9.Walker L.S., Abbas A.K. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2(1):11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 11.Liu W. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seddiki N. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yagi H. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16(11):1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 14.Mallone R. Functional avidity directs T-cell fate in autoreactive CD4+ T cells. Blood. 2005;106(8):2798–2805. doi: 10.1182/blood-2004-12-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allan S.E. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19(4):345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 16.Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett C.L. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 18.Wildin R.S. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 19.Feuerer M. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31(4):654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol. 2009;183(12):7631–7634. doi: 10.4049/jimmunol.0804308. [DOI] [PubMed] [Google Scholar]
- 21.Liu H., Leung B.P. CD4+CD25+ regulatory T cells in health and disease. Clin Exp Pharmacol Physiol. 2006;33(5–6):519–524. doi: 10.1111/j.1440-1681.2006.04401.x. [DOI] [PubMed] [Google Scholar]
- 22.Baecher-Allan C. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 23.Gale E.A. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 24.Putnam A.L. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58(3):652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costantini A. Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods. 2003;278(1-2):145–155. doi: 10.1016/s0022-1759(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 26.Peters J.H. Clinical grade Treg: GMP isolation, improvement of purity by CD127 Depletion, Treg expansion, and Treg cryopreservation. PLoS ONE. 2008;3(9):e3161. doi: 10.1371/journal.pone.0003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyara M. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Kukreja A. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109(1):131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas J. Reduced suppressive effect of CD4+CD25 high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35(11):3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 30.Kriegel M.A. Defective suppressor function of human CD4+CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199(9):1285–1291. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Putnam A.L. CD4+CD25 high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24(1):55–62. doi: 10.1016/j.jaut.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Jana S. The type of responder T-cell has a significant impact in a human in vitro suppression assay. PLoS ONE. 2010;5(12):e15154. doi: 10.1371/journal.pone.0015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semple K, et al. Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood. [DOI] [PMC free article] [PubMed]
- 34.Bour-Jordan H., Bluestone J.A. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229(1):41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo F. CD28 controls differentiation of regulatory T cells from naive CD4 T cells. J Immunol. 2008;181(4):2285–2291. doi: 10.4049/jimmunol.181.4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziegler S.F. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 37.Brusko T. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56(3):604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 38.Lindley S. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54(1):92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]


