Abstract
Cirrhotic patients (CPs) are susceptible to spontaneous bacterial peritonitis (SBP). Aim of this study was to examine if this susceptibility was related to peritoneal macrophages' (PMs) altered host defence. Absorbance of phagocytosed particles by PMs from CPs was lower than that of control (31.88% vs. 77.2%). Particle opsonisation increased the absorbance to 41% in CPs' PMs, and this value remains lower than the control; 77.2%. Respiratory burst (RB) was expressed as fluorescence index values, and these were higher in PMs from CPs than in controls (82 vs. 41, 73 vs. 26 and 71 vs. 26). IFN-γ made no further increase of RB values in PMs from CPs. CD14 expression was also higher in CPs' PMs. IFN-γ significantly downregulated CD14 expression in both CPs' PMs and control. Reduced phagocytosis by predominantly CD14-positive PMs from CPs could be related to intense RB. Findings suggest altered host defence that could contribute to susceptibility to SBP.
Keywords: Peritoneal macrophages, Yeast phagocytosis, Respiratory burst, CD14 expression, Spontaneous bacterial peritonitis
1. Introduction
Spontaneous bacterial peritonitis (SBP) is a commonly occurring infection among the patients with cirrhosis, and it is associated with high morbidity and mortality. The pathogenesis of SBP is not fully understood, but it is probably multi-factorial. Bacterial translocation from the bowel lumen into the peritoneal cavity has been demonstrated in portal hypertension models [1]. Defects in circulating polymorphonuclear and mononuclear phagocytes have been reported [2,3].
Humeral factors such as the opsonising properties of serum and ascitic fluid, were also found to be deficient [4,5], and the phagocytic function of the hepatic reticuloendothelial system was found to be decreased in approximately 50% of cirrhotic patients [6,7]. Peritoneal macrophages (PMs) are predominantly resident or tissue-type phagocytes and are presumed to constitute the host's first line of defence in the peritoneal cavity.
Unlike circulating neutrophils, macrophages can phagocytose avidly without prior opsonisation of ingestible particles [8]. Furthermore, macrophages undergo respiratory bursts (RBs), which are necessary for microbial killing and are more or less similar to those observed in neutrophils [9,10]. These two functions, phagocytosis and RB, represent the fundamental characteristics of host defence in PMs. In the murine system, these functions have been shown to be influenced by certain cytokines, including granulocyte macrophage colony-stimulating factor (GM-CSF) and interferon-gamma (IFN-γ) [11,12].
Since an alteration in host defence mechanism could contribute to the susceptibility to SBP, the aim of this study was (a) to examine phagocytosis and RB in PM and (b) to examine if applying potentially useful interventions might help in correcting a detectable defect.
2. Theory/calculation
Host defence is studied in a number of ways, and the choice made to examine those two basic characteristics above (Section 1) is justified. First both functions are quantitatively measured using simple and reproducible techniques with a relatively small number of cells. The cells used in this study were recovered from patients with cirrhotic ascites, which is not particularly cell-rich. The results were compared with those of peritoneal cells obtained from a group of otherwise healthy women who were undergoing gynaecological laparoscopic sterilisation. Second, the information that is obtained from this research can be extended into further examining detail components of the respective host defence function. For instance, an impaired phagocytosis may indicate further endocytic receptor studies, which mediate phagocytosis. An altered RB may suggest looking more closely at the pathway that generates reactive oxygen intermediates.
If particle opsonisation corrected a detectable phagocytic defect, the enhancement of the opsonic activity of ascitic fluid could be of practical benefit. The same could apply to the potential use of GM-CSF if it had been shown that this agent would correct a phagocytic defect. Intense RB that is produced by CD14-positive PMs might be harmful, and interventions that optimise RB production may be developed.
3. Materials
Nycoprep (Nycomed UK Ltd., Birmingham, England), Nunclon (InterMed, Roskilde, Denmark). RPMI 1640, DMEM, Hank's Balanced Salt Solution and Foetal calf serum (FCS) were all from Life Technologies Ltd., Paisley, UK. HEPES Buffered Saline (HBS), EDTA, NaOH solution, Trypan blue, Candida albicans, Fluorescein isothiocyanate (FITC), DMF, Zymosan and Lipopolysaccharide (LPS) (Escherichia coli serotype 026 B6) were all from Sigma Chemicals, Poole, UK. Anti-human CD14 monoclonal antibody Leu-M3 FITC (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA), Dihydrorhodamine solution (DHR) (Cambridge Biosciences, Cambridge, UK), Phorbol 12-myristate 13-acetate (PMA) (Fisons Scientific Equipment, Loughborough, UK), Granulocyte macrophage colony-stimulating factor (GM-CSF), a gift from Schering-Plough (UK) Ltd., Welwyn Garden City, Herts, UK, Interferon-gamma (IFN-γ), a gift from Boehringer Ingelheim (UK) Ltd., Bracknell, UK.
4. Patients and methods
4.1. Study population
The patient group comprised thirty patients with ascites, with a mean age (±SD) of 51.4±7.7 years. Seventeen of these patients were male. All patients had biopsy-proven cirrhosis and were clinically graded as Child-Pugh class C. There was no clinical evidence of SBP in any of these patients at the time of paracentesis. A neutrophil cell count of >200 cell/mL in the ascitic fluid or in the control fluid excluded the specimen from analysis. Ascitic fluid was aspirated during therapeutic paracentesis using a strict aseptic technique and collected in 2-litre sterile containers. These fluid samples were placed on ice and transferred immediately to the lab. Phagocytosis was measured in PMs from fourteen patients, and RB was studied in PMs from seven patients. In these seven patients, CD14 phenotype expression was also measured.
The control group comprised twelve female patients, with a mean age of 31±4.5 years, who attended the gynaecology unit as scheduled day cases for laparoscopic fallopian tube ligation. None of these controls had evidence of liver disease or a major system disorder. Peritoneal fluid specimens in these controls were collected by the operating surgeon under direct vision from the pouch of Douglas, as described elsewhere [13]. Phagocytosis was measured in all the controls, and RB and CD14 expression were measured in seven of the control samples. The control samples were collected in sterile universal containers, placed on ice and sent immediately to the laboratory. Neither the patients nor the controls had been on any immune-modulating therapy within three months of the procedure.
Informed written consent was obtained from all patients and controls. Approval for this study was obtained from the King's College Hospital Research Ethics Committee, according to the Declaration of Helsinki [14].
4.2. Peritoneal macrophage (PMs) preparation
The samples were centrifuged twice at 600×g for ten minutes. The cell pellets were re-suspended and layered over a density gradient (Nycoprep, Nycomed UK Ltd., Birmingham, England) and centrifuged at 600g for thirty minutes to obtain a monolayer [15]. The monolayer, which normally consists of monocytes, was carefully aspirated and was washed twice in RPMI 1640. The final cell pellet was re-suspended in RPMI 1640 containing penicillin 100 u/mL, streptomycin 100 μg/mL and 10% foetal calf serum (FCS) (Life Technologies Ltd., Paisley, UK). A cell count was then performed using a haemocytometer, and the cell morphology was examined on cytocentrifuge preparations stained with May Grunwald Giemsa. The purity of PMs was further determined using non-specific esterase staining [16], and the cell viability was determined by trypan blue dye exclusion. At this stage the cell suspension was then kept at 0 °C until assayed.
4.3. Yeast preparation
C. albicans (Sigma Chemicals, Poole, UK) was heat killed by placing it in a hot bath at 60 °C for one hour. The yeast particles were then re-suspended and counted before labelling them with fluorescein isothiocyanate (FITC) (Sigma Chemicals, Poole, UK) according to the method of Ragsdale and Grasso, with some modification [17]. Labelled yeast cells were re-suspended at 5×107 cells/mL in 20% DMEM-FCS (Life Technologies, Paisley, UK) stored in a light-protected environment at 4 °C. This stock was used within ten weeks of preparation. Yeast particles opsonisation was performed with pooled human serum (PHS) collected from healthy laboratory staff personnel and stored at −70 °C [18]. The opsonisation step was done one hour before the phagocytosis assay.
4.4. Phagocytosis assay
PMs, which were prepared (1.3.3), were seeded at 0.5–1×106 cells/well in 24-well tissue culture plates (Nunclon, InterMed, Roskilde, Denmark) in RPMI/FCS at 37 °C and 5% CO2 air for two hours to allow for cell adherence. Non-adherent cells were thereafter gently washed out twice with warm HBSS (Life Technologies, Paisley, UK). The labelled yeast particles were added to wells at a macrophage-to-particles ratio of 1:40 and phagocytosis was allowed to proceed for thirty minutes. In experiments in which GM-CSF was used, the cells were incubated with this cytokine at concentrations of 50, 100 or 500 IU/mL for thirty-six hours, and then phagocytosis was measured [19]. All samples were assayed in duplicates.
At the end of this period, the plates were placed on ice and washed twice with ice-cold HBSS, as described previously [17]. Each wash involved gentle pipetting up and down ten times after which the supernatant was discarded. The plates were examined using an inverted microscope between washings to determine the number of internalised particles and to ensure that non-internalised particles, and not cells, were actually washed away. To ensure that there were no free or non-internalised particles left behind, the wells were rinsed once more with the same buffer in some experiments, and the fluorescence of the supernatant was measured using a spectrofluorometer (RF-540 Shimadzu Corp, Kyoto, Japan) at an excitation wavelength of 482 nm and an emission wavelength of 520 nm [17].
Finally cells were lysed using 0.1 N NaOH, and the fluorescence of the liberated intracellular particles in the supernatant was measured using spectrofluorometer (1.3.4). Values expressed as arbitrary absorbance units’ percentages were calculated using the following formula:
where %A is percent absorbance unit, F1 is the fluorescence in the wells containing both cells and particles, F2 is the fluorescence in the wells containing cells only (auto-fluorescence) and F3 is the fluorescence in the wells containing labelled yeast particles only (total fluorescence).
4.5. CD14 phenotype
PMs (2×104) in HEPES-buffered saline (containing 1.25 mM CaCl2 and 1.25 mM MgCl2) and 2 mM EDTA (Sigma Chemicals, Poole, UK) were incubated with 20 μL of the anti-human CD14 monoclonal antibody Leu-M3 FITC (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) for twenty minutes on ice [20]. The cells were then washed twice and re-suspended in 200 μL of the same solution and vortexed to disrupt any clumps of cells. In experiments in which IFN-γ was used, non-adherent cells were first incubated with this cytokine for forty-eight hours at a concentration of 500 IU/mL. All samples were analysed in a flow cytometer (Becton Dickinson, Immunocytometry Systems, San Jose, CA, USA) and the PMs were identified using a monocyte gate. This gate was based on forward angle light scatter and log 90° light scatter parameters. Cell surface markers used other than CD14 were CD5 and CD15. A significant decrease in forward light scatter was noted, with a smaller change in 90° light scatter, which required a change in the gates. Monocytes were identified utilising the FITC-conjugated anti-CD14.
4.6. Respiratory burst measurement
Respiratory burst measurement was performed according to the method of Banati et al. [21], with some modification. Briefly, aliquots of 2×102 PMs in 200 μL HBS/EDTA were placed in sterile capped flow cytometer tubes and stained for five minutes at 37 °C with 1.0 μL of 40 μM dihydrorhodamine solution (DHR) (Cambridge Biosciences, Cambridge, UK). The DHR loaded-cells were further incubated for thirty minutes at 37 °C with either 5 μL of 15 μM of phorbol 12-myristate 13-acetate (PMA) (Fisons Scientific Equipment, Loughborough, England) in DMF, 10 μL of 12.5 mg/mL zymosan (Sigma Chemicals, Poole, UK) solution in HBS or with 10 μL/mL lipopolysaccharide (LPS) (E. coli serotype 026 B6) (Sigma Chemicals, Poole, UK). In experiments in which two stimuli were used the PMs were first incubated with LPS for one hour and then the second stimulant (PMA or zymosan) was added for a further thirty minutes of incubation.
In experiments in which interferon-gamma (IFN-γ) was used, non-adherent cells were incubated for forty-eight hours with this cytokine at varying concentrations (10–1000 IU/mL) before the RB assay. RB was measured using a fluorescence cell sorter (FACS) with an argon ion laser at an excitation wavelength of 488 nm. A total of 2000 CD14-positive monocytes were analysed at a time. DHR is oxidised intracellularly to rhodamine 123 in the presence of hydrogen peroxide (H2O2) and peroxidase. A 525-nm band-pass filter was used for the green fluorescence from rhodamine 123, which represented the magnitude of RB activity. The data were reported as a mean channel number (MCN) (256 channels, a three-decade log scale in arbitrary log units). The fluorescence index was calculated by subtracting the MCN of the unstimulated cells from the MCN of the stimulated cells (PMA, zymosan and LPS).
5. Statistical analysis
For data description, the mean and a 95% confidence limit were used. The Student's t-test for paired samples and the Wilcoxon's test were used for analysing the results within each group, and the Mann–Whitney U test was used for comparing the results between the groups.
6. Results
6.1. Peritoneal macrophages (PMs) characteristics
Table 1 shows the details of the patients and control groups together with the PMs characteristics. 0.5–1.5 L of the ascitic fluid samples was used, which yielded an average total cell number of 2.24±0.87×104 cells/mL (Table 1). The fluid samples from the control group were smaller, between 7 and 20 mL, and yielded a higher average cell number of 16.8±13.2×104 cells/mL (Table 1). The percentages of PMs in the cell mixtures were 85.2% and 91% (p>0.05) and the percentages of viable cells were 92% and 94% (p=NS) for patients and controls, respectively.
Table 1.
Data related to patients and control groups and PM isolation.
| Patients | Controls | p-Value | |
|---|---|---|---|
| Number | 22 | 12 | |
| Male:female | 11:11 | 0:12 | |
| Age (mean±95% CL) | 51.4±7.7 years | 31±2.7 years | |
| Aetiology of cirrhosis | Alcoholic=13 | Laparoscopic sterilisation=10 | |
| Primary sclerosing cholangitis (PSC)=4 | Diagnostic laparoscopy=2 | ||
| Viral hepatitis=4 | |||
| Cryptogenic=1 | |||
| Total number of cells isolated per mL | 2.24±0.87×104 cells/mL | 16.8±13.2×104 cells/mL | p=0.001 |
| % of macrophages in cell mixture | 85.2±2.4 | 90.1±1.86 | p=0.05 |
| Viability | 92±1.3% | 94.1±1.86% | p>0.05 |
When the plates were examined using an inverted microscope following overnight incubation, we observed morphological changes that became more prominent in the plates to which GM-CSF had been added.
6.2. Yeast phagocytosis
The absorbance readings from internalised particles were expressed as percentages of arbitrary units (AU) (Section 4.4), and a standard mean value ±95% CL was obtained for each group. Fig. 1 shows the number of cases in which the absorbance readings fall within the corresponding absorbance range on the horizontal axis, where blue curve line represents patients' PMs readings and green curve line represents control PMs'. The mean absorbance reading of the patient PMs (n=14) was significantly lower than that of the control PMs (n=12) (31.88±8.0% vs. 77.2±5.64%, p<0.01). The absorbance in the patients' PMs increased following particle opsonisation (brown-red curve line, Fig. 1) (from 28.08±8.0% before to 41.24±13.35% after opsonisation, p<0.05), although the absorbance remained significantly lower than that for unopsonised control PMs (41.24% vs. 77.2%, p<0.05).
Fig. 1.
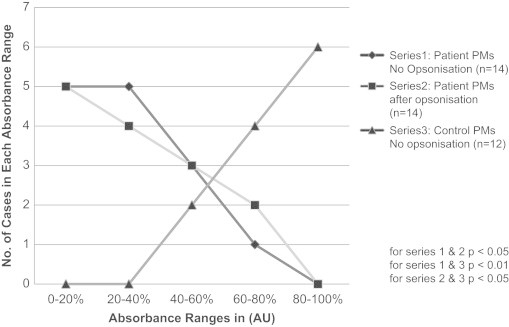
Phagocytosis measurement in patient and control PMs.
Absorbance readings of supernatant fluid aspirated from the final plate washings, and representing non-internalised particles, were negligible (0.2–0.5%). A parallel qualitative observation was made when phagocytosis was assessed using direct microscopy examination and manually counting the ingested particles (data not shown). Pre-incubation of the patient PMs (n=10) with GM-CSF had insignificant impact on phagocytosis (30±9.4% before compared to 36±2.8.9% after, p=0.998).
6.3. CD14 phenotype
CD14 expression was significantly higher in the patient PMs than in the control PMs (180 vs. 118 MCN, p<0.05) (Fig. 2). After IFN-γ treatment, the expression of CD14 was significantly downregulated in the two groups (from 180 to 80 MCN in the patient PMs (p<0.05), and from 118 to 20 MCN in the control PMs (p<0.05) (Fig. 2). The downregulation was greater in the control PMs than in the patients PMs (83% and 50%, respectively; p<0.05).
Fig. 2.
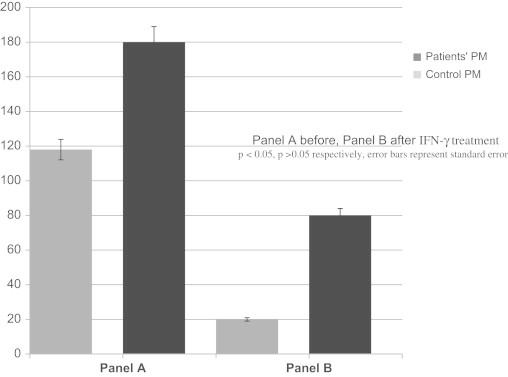
CD14 phenotype expression in patient's PM (dark bars) and controls (light bars) before and after INF-γ treatment.
6.4. Respiratory burst
The RB fluorescence index values for patient PMs were significantly higher than those for the control PMs following stimulation with a single agent (PMA, 82 vs. 41; LPS, 73 vs., 44; zymosan, 71 vs. 26; p<0.005) (Fig. 3).When two agents, PMA and LPS, were used in combination for stimulation, the RB index values further increased by 40% (from 82 to 115) in the patient PMs and by 130% in the control PMs (from 41 to 95) (p<0.005) (Fig. 3). When the cells were treated with IFN-γ and then stimulated with PMA alone, the RB index values showed an increase of 6% (from 82 to 87) in the patient PMs and 22% (from 41 to 50, p<0.05) (Fig. 4) in the control PMs.
Fig. 3.
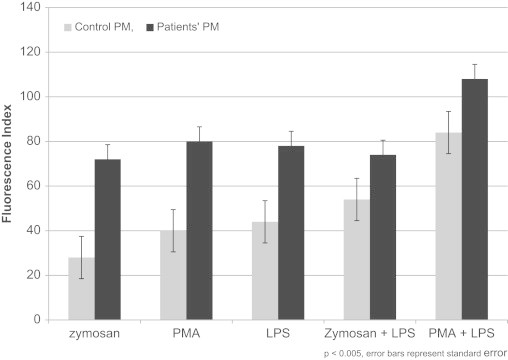
Measurement of Respiratory Burst (RB) in patients' PM (dark bars) and controls (light bars) PM.
Fig. 4.
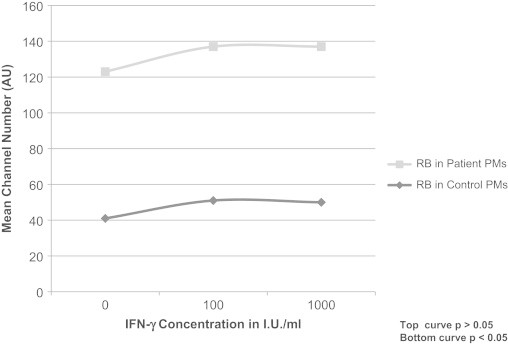
Effect of Interferon-γ (IFN-γ) on Respiratory Burst (RB) in patient and control PMs.
7. Discussion
In this study, we extended the work of other investigators on peritoneal macrophages [22–24]. First, unlike the studies on macrophage function that used cells isolated from healthy donors, this study is one of a few in which the cells were isolated from ill patients. Second, although studies in healthy PMs, and in particular, those from the murine system have provided most of the available knowledge in this area, studying these cells in relation to cirrhotic ascites and SBP is clearly very relevant.
The control PMs used by other investigators were isolated from dialysate fluid, which was removed from renal patients undergoing continuous ambulatory peritoneal dialysis (CAPD). Because exogenous solutions used in CAPD perturb the peritoneal environment, the behaviour of the PMs isolated from dialysate fluid could be influenced [25]. The control PMs used in this study were similar to the patient PMs because both cell populations matured in an in vivo human environment and without the effect of exogenous factors. Not surprisingly, the total number of cells isolated from cirrhotic ascites exceeded that isolated from the controls, because the volume of ascites irrigates a greater surface area of the peritoneum.
Previous studies have reported reduced phagocytosis in peripheral blood monocytes and in the Kupffer cells of cirrhotic patients (CP) [3,6,7]. This reduced phagocytosis was explained by intrinsic cell defects and by the presence of phagocytosis inhibitory factors. These findings may not necessarily be extrapolated to the PMs because the peritoneum environment in which the PMs reside is different from the environments, the circulation and the reticuloendothelial system, in which the peripheral blood monocytes and the Kupffer cells exist. To our knowledge, the finding in this study of reduced phagocytosis in PMs from cirrhotic patients has not been previously reported. Unlike the situation with neutrophils, particle opsonisation is not a pre-requisite for phagocytosis by macrophages. Opsonisation is known to further augment this function [26].
However, in the present study although phagocytosis increased following particle opsonisation it was still much lower than that recorded in the control PMs before opsonisation. This result implies that the defect is probably on the PM cell surface. Phagocytosis is a process mediated via surface receptors that recognise the chemical structure of the particle to be internalised. For example yeast and bacteria are covered in glycoconjugates that are recognised by an endoreceptor macrophage mannose receptor (MR), which mediates phagocytosis [27–29]. Although yeast is not a common SBP-causing pathogen, its structure in relation to the endocytic process may resemble that of the bacteria that cause SBP.
GM-CSF is reported to enhance phagocytosis in normal macrophages [12]. This was not demonstrable in the present study; this result is not surprising as GM-CSF primarily induces monocytes to develop into a more mature and efficient form of phagocytes [8]. In our study, the PMs were already mature. More recently, interleukin-4 (IL-4) has been shown to have an upregulatory effect on the expression of the endocytic receptor MR [30], and currently, this issue is being investigated in our laboratory.
Reactive oxygen intermediates (ROI) are generated during RB, and they would provide a clear indication of the ability of cells to destroy invading organisms and would also reflect the cytotoxic capability. There are, of course, other agents involved in macrophage effector function, including NO and myeloperoxidase. However, RB in particular is a more robust microbicidal mediator, and its measurement could be more reflective of this effector function. The vigorous RB response recorded in patient PMs is of interest. Previous studies have shown that the ability of human PMs to undergo a RB is limited and may even decline if the cell culture period is extended [24]. This finding prompted us to prime cells with LPS first and then add another stimulus, such as PMA to increase the chances of producing the desired RB effect. Furthermore, the period from the beginning of the incubation until the time of the RB assay was kept relatively short to minimise any loss in RB produced. The result was that the RB was even greater in patient PMs. This intense RB was perhaps partly due to the experimental factors above and partly due to a possibility of pre-existing in vivo cell activation. We observed that the cell morphology changed during the short incubation period, even in the wells to which no cytokine had been added. These observed changes may be similar to the morphological changes in activated macrophages that have been reported by other researchers [31,32]. In addition, we observed a small increase in the RB following the treatment of cells with IFN-γ, which is also consistent with an already activated state. Although intense RB activity is a desirable effector function that is essential for microbial killing, it is by no means without a caveat. The expression of relevant endocytic receptors involved in phagocytosis has been shown to be downregulated by intense RB, resulting in reduced phagocytosis [33]. CD14-positive macrophages, as opposed to their CD14-negative counterparts, are more involved in RB. Furthermore, the magnitude of CD14 expression in the mature macrophages is greater than that in the less mature macrophages [34]. It is of interest that CD14 expression was found to be more abundant in the PMs from cirrhotic patients compared to those of controls, and this difference in the magnitude of CD14 expression could contribute to the RB result.
Consistent with other studies, our study has further confirmed the downregulatory effect of IFN-γ on CD14 expression [22,23]. CD14 binds LPS, and as a result, the release of pro-inflammatory cytokines, including TNF-α, is mediated. Because TNF-α is involved in the pathogenesis of shock syndrome [23], the downregulatory effect of IFN-γ on CD14 expression may potentially have a protective effect on the host in complex situations such as shock syndrome.
8. Conclusion
We have shown for the first time that the host defence of PMs from cirrhotic patients with ascites is altered. The predominance of activated CD14 expressing cells has generated a vigorous RB, which in turn had a downregulatory effect on endocytic receptors. This mechanism could account for the reduced phagocytosis reported. Such an alteration in host defence may in part play a role in the increased susceptibility of these patients to SBP.
Acknowledgement
We are grateful to Anne Etches from Haematology Department at King's College Hospital, London, UK, for her help with macrophage characterisation studies. We are also grateful to Schering-Plough (UK) Ltd., Welwyn Garden City, Herts, UK, for providing the GM-CSF and to Boehringer Ingelheim (UK) Ltd., Bracknell, UK, for providing the interferon-gamma.
References
- 1.Garcia-Tsao G., Albillos A., Barden G.E., West A.B. Bacterial translocation in acute and chronic portal hypertension. Hepatology. 1993;17(6):1081–1085. [PubMed] [Google Scholar]
- 2.Rajkovic I.A., Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity of alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252–262. doi: 10.1002/hep.1840060217. [DOI] [PubMed] [Google Scholar]
- 3.Hassner A., Kletter Y., Schlag D., Yedvab M., Aronson M., Shibolet S. Impaired monocyte function in liver cirrhosis. British Medical Journal. 1981;282:1262–1263. doi: 10.1136/bmj.282.6272.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyke R.J., Rajkovic I.A., Williams R. Impaired opsonization by serum from patients with chronic liver disease. Clinical and Experimental Immunology. 1983;51:91–98. [PMC free article] [PubMed] [Google Scholar]
- 5.Simberkoff M.S., Moldover N.H., Weiss G. Bactericidal and opsonic activity of cirrhotic ascites and non-ascitic fluid. Journal of Laboratory and Clinical Medicine. 1978;91:831–839. [PubMed] [Google Scholar]
- 6.Rimola A., Soto R., Bory F., Arroyo B., Piera C., Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4(1):53–58. doi: 10.1002/hep.1840040109. [DOI] [PubMed] [Google Scholar]
- 7.Bolognessi M., Markel C., Bianco S., Angeli P., Sacerdotti D., Amadio P. Clinical significance of the evaluation of hepatic reticuloendothelial removal capacity in patients with cirrhosis. Hepatology. 1994;19(3):628–634. doi: 10.1002/hep.1840190313. [DOI] [PubMed] [Google Scholar]
- 8.Marodi L., Korchak H.M., Johnson R.B., Jr Mechanisms of host defence against Candida species. Phagocytosis by monocyte and monocyte-derived macrophage. Journal of Immunology. 1991;146:2783–2789. [PubMed] [Google Scholar]
- 9.Babior B.M., Kipnes R.S., Curnutte J.T. Biological defence mechanisms. The production of leukocytes of superoxide, a potential bactericidal agent. Journal of Clinical Investigation. 1973;52:741. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fantone J.C., Ward P.A. Role of oxygen-derived free radicles and metabolites in leukocyte-depedent inflammatory reactions. American Journal of Pathology. 1982;107:397. [PMC free article] [PubMed] [Google Scholar]
- 11.Murray H.W. Interferon-gamma, the activated macrophage and host defence against microbial challenge. Annals of Internal Medicine. 1988;108:595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- 12.Weisbart R.H., Gasson J.C., Golde D.W. Colony stimulating factors and host defence. Annals of Internal Medicine. 1989;110:297–303. doi: 10.7326/0003-4819-110-4-297. [DOI] [PubMed] [Google Scholar]
- 13.van Furth R., Raeburn J.A., Van Zwet T.L. Characteristics of human mononuclear phagocytes. Blood. 1979;54:485–498. [PubMed] [Google Scholar]
- 14.Human Experimentation Code of ethics of the World Medical Association, Declaration of Helsinki. British Medical Journal. 1964;ii:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyum A. Isolation of human blood monocytes with Nycodenz. A new non-ionic iodinated gradient medium. Scandinavian Journal of Immunology. 1983;17:429–436. doi: 10.1111/j.1365-3083.1983.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 16.Yam L.T., Li C.Y., Crosby H.W. Cytochemical identification of monocytes and granulocytes. American Journal of Clinical Pathology. 1971;55:283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- 17.Ragsdale R.L., Grasso R.J. An improved spectrofluorometry assay for quantitating yeast phagocytosis in cultures of murine peritoneal macrophages. Journal of Immunological Methods. 1989;123:259–267. doi: 10.1016/0022-1759(89)90230-5. [DOI] [PubMed] [Google Scholar]
- 18.Peterson P.K., Gaziano E., Suh J.A., Devalon M., Peterson L., Keane W.F. Antimicrobial activities of dialysate-elicited and resident human peritoneal macrophages. Infection and Immunity. 1985;49:212–218. doi: 10.1128/iai.49.1.212-218.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M., Friedman H., Djeu J.Y. Enhancement of human monocyte function against Candida albicans by the colony stimulating factors (CSF): IL3, GM-CSF and M-CSF. Journal of Immunology. 1989;143:671–677. [PubMed] [Google Scholar]
- 20.Banati R.B., Rothe G., Valet G., Kreutzberg W. Respiratory burst activity in brain macrophages: a flow cytometric study on cultured rat microglia. Neuropathology and Applied Neurobiology. 1991;17:223–230. doi: 10.1111/j.1365-2990.1991.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 21.Zeller J.M., Rothberg L., Landay A.L. Evaluation of human monocytes oxidative metabolism utilizing a flow cytometric assay. Clinical and Experimental Immunology. 1989;78:91–96. [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan C.F., Murray H.W., Weibe M.E., Rubin B.Y. Identification of IFN-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. Journal of Experimental Medicine. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne J.B., Nichols F.C., Peluso J.F. The effect of interferon-γ and lipopolysaccharide on CD14 expression in human monocytes. Journal of Interferon Research. 1992;12:307–310. doi: 10.1089/jir.1992.12.307. [DOI] [PubMed] [Google Scholar]
- 24.Pruimboom W.M., van Dijk A.P.M., Tak C.J.A.M., Zijlstra F.J., Bonta I.l., Wilson J.H.P. Inflammatory mediators and activity of human peritoneal macrophages. Agents Actions. 1993;38:C86–C88. doi: 10.1007/BF01991146. [DOI] [PubMed] [Google Scholar]
- 25.Peterson P.K., Gaziano E., Suh J.A., Devalon M., Peterson L., Keane W.F. Antimicrobial activities of dialysate-elicited and resident human peritoneal macrophages. Infection and Immunity. 1985;49:212–218. doi: 10.1128/iai.49.1.212-218.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacGowan A.P., Peterson P.K., Keane W., Quie P.G. Human peritoneal macrophage phagocytic, killing and chemiluminescent responses to opsonized Listeria monocytogenes. Infection and Immunity. 1983;40:440–443. doi: 10.1128/iai.40.1.440-443.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stahl P.D., Rodman J.S., Miller M.J., Schreiber P.H. Evidence of receptor-mediated binding of glycoproteins, glycoconjugates and lysosomal glycosidases by alveolar macrophages. Proceedings of the National Academy of Sciences. 1978:1399–1402. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson M.E., Pearson R.D. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. Journal of Immunology. 1986;136:4681–4687. [PubMed] [Google Scholar]
- 29.Speert D.P., Wright S.D., Silverstein S.C., Mah B. Functional characterization of macrophage receptors for in vitro phagocytosis of unopsonized Pseudomonas aeruginosa. Journal of Clinical Investigation. 1988;82:872–879. doi: 10.1172/JCI113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances alternative murine macrophage mannose receptor activity: a marker of immunologic macrophage activation. Journal of Experimental Medicine. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart P.H., Jones J.A., Finlay-Jones J.J. Peritoneal macrophages during peritonitis. Phenotypic studies. Journal of Immunology. 1992;88:484–491. doi: 10.1111/j.1365-2249.1992.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berton G., Gordon S. Superoxide release by peritoneal and bone marrow-derived mouse macrophages. Modulation by adherence and cell activation. Immunology. 1983;49:693–704. [PMC free article] [PubMed] [Google Scholar]
- 33.Ezekowitz R.A.B., Austyn J., Stahl P.D., Gordon S. Surface properties of Bacillus-Calmette–Guerin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors and antigen F4/80 accompanies induction of Ia. Journal of Experimental Medicine. 1981;154:60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eischen A., Duclos B., Schmitt-Goguel M., Rouyer N., Bergerat J.P., Hummel M. Human resident peritoneal macrophages: phenotype and biology. British Journal of Haematology. 1994;88:712–722. doi: 10.1111/j.1365-2141.1994.tb05109.x. [DOI] [PubMed] [Google Scholar]


