Overview
Multiple line of clinical and experimental evidence demonstrates that both acute, moderate and chronic, excessive alcohol use result in various abnormalities in the functions of the immune system. Altered inflammatory cell and adaptive immune responses in turn result in increased incidence and poor outcome of infections and other organ effects after alcohol use. This review article summarizes recent findings relevant to immunomodulation by alcohol and its consequences on host defense against microbial pathogens and tissue injury.
Keywords: innate and adaptive immunity, Inflammation, Toll-like receptors, Macrophages, T cells
Introduction
Alcohol is historically the most commonly abused substance that maintained popularity from ancient Mesopotamian times to the 21st century. An association between heavy alcohol use and poor infection outcomes was noted as early as the 19th century by Robert Koch, who observed significant mortality of alcoholics during the cholera epidemics of 1884 (Howard-Jones, 1984). In the early 1900s, Sir William Osler published in his “Principles of Practice of Medicine that “the most potent predisposing factor is perhaps the lowered resistance in alcoholics”- in pneumonia (Osler, 1902). Indeed, contemporary medical literature widely documented that excessive alcohol use is associated with reduced host defense including antimicrobial defense, antiviral immunity and altered host repair (Capps and Coleman, 1923; reviewed in Cook 1998; Pavia et al., 2004; Szabo 1999).
The immune system serves to protect the host from invading pathogens by mounting a pathogen-specific immune response aimed at elimination of the infectious agent (Medzhitov and Janeway, 1997; Medzhitov and Janeway, 2000). In this complex process pathogens are recognized through pattern recognition receptors expressed on the different cells of the innate immune system including leukocytes, monocytes/macrophages, natural killer cells (Takeuchi and Akira, 2007a; Szabo et al, 2006b). These cell types serve as the first line of host defense and are uniquely equipped to survey their environment and rapidly respond to pathogen-derived danger signals by redistribution in the body to the site of infection, phagocytosis of the pathogen, production of cytokines, chemokines and reactive oxygen radicals. Innate immune cells with antigen presentation capacity (monocytes and dendritic cells) also play a key role in activation of T lymphocytes in the initiation of adaptive immune responses (Medzhitov and Janeway, 2000). Adaptive immune responses involve antigen-specific T-cell proliferation, immunological memory, B cell activation and production of immune antibodies that are all components of an efficient anti-microbial host defense for pathogen elimination and/or protection of the host from future infections. However, this complex process of host immunity can be severely disturbed by the modulating effects of alcohol on the different cellular components of the innate and/or adaptive immune system (Figure 1) (Brown et al, 2006; Cook 1998; Szabo 1999a). The modulating effects of alcohol on immunity occurs not only in adult individuals or in animal models, but there is also evidence for modulation of both innate and adaptive immune cell functions by alcohol use even in the fetal alcohol setting (Chiapelli et al, 1997; Jerrells and Weinberg, 1998).
Figure 1. Induction of immune responses by pathogens.
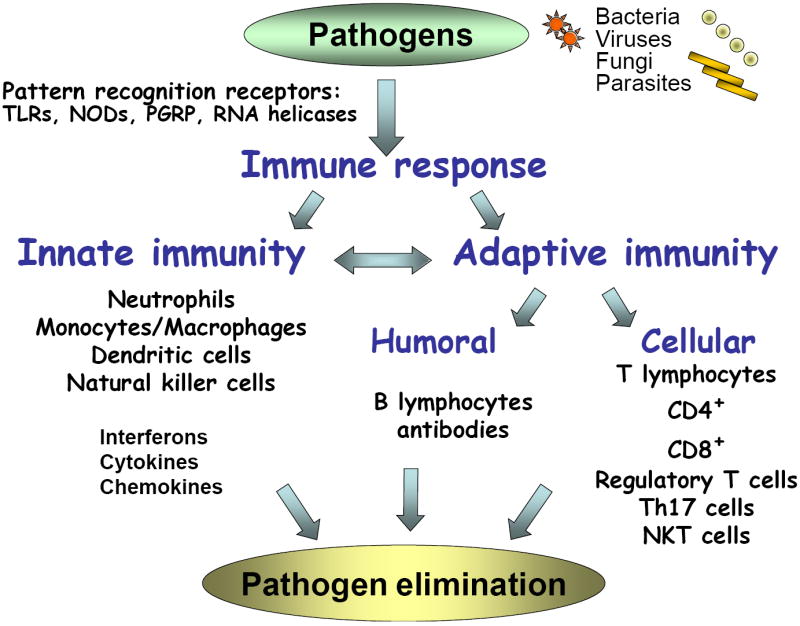
Various pathogens, such as bacteria, viruses, fungi and parasites, trigger host defense through activation of an immune response. Pathogens are recognized by pattern recognition receptors including the family of Toll-like receptors (TLRs) (10 different TLRs in humans and 13 in rodents), NOD-LRR proteins, peptidoglycan recognition receptors (PGRP), and RNA helicases expressed on host cells. PRRs trigger various signaling pathways to initiate activation of innate and/or adaptive immune responses. Innate immunity involves recruitment and activation of neutrophil leukocytes, macrophages, monocytes, dendritic cells and natural killer cells to initiate the immune response. These cells also produce immune mediators aimed at inhibition of the pathogen (interferons), recruitment (chemokines) and modulation (cytokines) of other immune cells. The innate immunity is also pivotal in initiation of adaptive immune responses via the antigen presenting function of dendritic cells and other antigen presenting cell types. Pathogen/antigen-specific T cell activation extends to both the CD4+ and CD8+ T lymphocyte populations and the extent of T cell activation and proliferation can be regulated by regulatory T cell subsets, Th17+ T cells and NKT cells. Adaptive immunity also involves activation of B lymphocytes and induction of immunoglobulin switching leading to production of pathogen-specific antibodies. All of these processes of the innate and adaptive immunity work in concert to ultimately achieve pathogen elimination.
Experimental models of alcohol administration and their clinical relevance
While human alcohol consumption is typically categorized to acute, moderate drinking that is limited to occasional consumption of 1-2 drinks, binge drinking is characterized by consumption of larger amounts of alcohol on consecutive days followed by several sober days (USDA Dietary Guidelines, 2005 6th edition). According to the USDA 2005 dietary guidelines, moderate drinking is considered to be no more than one drink per day for women and no more than two drinks per day for men (USDA Dietary Guidelines, 2005 6th edition). A drink is defined as 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of 80-proof distilled spirits that contain about 15 g of alcohol. Chronic alcohol consumption in humans usually represents daily use of > 3-5 drink equivalents for men and > 2 for women for a prolonged period of time. Studies on the biological effects of alcohol attempt to mimic these basic alcohol use patterns both in vitro and in vivo studies. The different alcohol administration experimental approaches used in in vitro and in vivo studies have been recently reviewed (Nagy 2008). In in vitro studies, acute alcohol administration is considered for up to about 24 hours (Szabo and Mandrekar, 2008) while the definition of chronic alcohol exposure in cell culture systems is less well defined. For mouse macrophages in cell cultures, 48 hours or longer alcohol treatment has been used as a model of chronic alcohol exposure and resulted in functional changes reminiscent of tissue macrophages exposed to in vivo chronic alcohol in mice (Kishore et al, 2004). In human monocytes/macrophages, in vitro alcohol treatment for 5-7 days results in functional changes consistent with chronic in vivo alcohol use (Szabo and Mandrekar, 2008). However, there are no set guidelines or published consensus regarding the relevance of various in vitro and in vivo alcohol administration models to human alcohol use.
In vivo studies mostly concentrated on alcohol administration in mice and rats. Acute alcohol administration through gastric gavage or intraperitoneal injection have been used and resulted in alcohol-related alterations in immune functions (Plackett and Kovacs, 2008). Binge drinking models were achieved by repeated alcohol administration for 3-4 consecutive days using gastric gavage leading to altered innate immune responses (Pruett et al, 2004). Chronic alcohol administration models have been developed by several groups of investigators all resulting in abnormalities in immune functions. For example, the Lieber-DeCarli diet has been widely used in the past both in mice and rats in studies on both liver disease and immune system. The Lieber-deCarli diet is based on a high-fat liquid diet and uses a pair-fed control diet by substituting alcohol-derived calories with maltose-dextrin (DeCarli and Lieber, 1967). This model results in both liver pathology (steatosis and some inflammation) as well as immunologic changes that are reminiscent of human chronic alcohol consumption. In the intragastric continous alcohol feeding model developed by Tsukamoto-French, alcohol administration to rats or mice leads to most features of human alcoholic liver disease including steatohepatitis and necroinflammation, although the extent of liver fibrosis is minimal with any of these models in rodents (Tsukamoto et al, 2008). Another method used in mice is administration of alcohol in the drinking water with regular chow diet (Coleman et al, 2008). This model has recently been proposed as mimicking human chronic alcohol use without liver disease (Cook et al, 2007). Consistent with this, there is lack of features of alcoholic liver disease but there are manifestations of immune alterations including innate and adaptive immune cell defects developing after various length of time of alcohol treatment (Cook et al, 2007; Edsen-Moore et al, 2008).
An additional consideration of the in vivo effects of alcohol is induction of stress responses that may indirectly affect immune functions. Based on animal studies, a role for the stress response has been proposed in cell loss and changes in immune cell functions (Jerrells et al, 1990; Padgett et al, 2000). In human alcohol abusers without significant alcoholic liver disease, an association between the immunosuppressive cytokine, IL-10, and cortisol was found after surgical trauma (Sander et al). In contrast, the animal model of chronic alcohol administration without alcoholic liver disease found no involvement of the stress response in the immune effects caused by very long-term alcohol administration (Cook et al, 2007).
Increased susceptibility to infections
Alcohol use and lung infections
Chronic alcohol consumption is associated with increased incidence of bacterial pneumonias caused by Steptococcus pneumoniae, Klebsiella pneumoniae, Hemophilus influensae, Legionella pneumoniae and other Gram-negative organisms in humans. Alcohol-induced immunosuppression in the lung has been the topic of a recent review (Happel and Nelson, 2005). One of the earliest observations of alcohol and infections was made by Benjamin Rush in 1785 who noted an increased susceptibility to tuberculosis and pneumonias in alcoholics (Rush, 1943). Subsequent epidemiologic studies confirmed that acute and chronic alcohol use predisposes individuals to M. tuberculosis-mediated disease as well as other bacterial pulmonary infections most likely through alterations in specific and nonspecific innate immune responses (Jacobson, 1992; MacGregor, 1986; Moran et al., 2007; Saitz et al., 1997; Schmidt and De Lint, 1972).
In an experimental animal study with Lieber-deCarli diet, Mason et al. showed that alcohol exacerbates pulmonary tuberculosis resulting in higher lung organisms and blunted CD4+ and CD8+ T lymphocyte responses. Lymphocyte proliferation and IFNγ levels were decreased in CD4+ T cells in alcohol-consuming mice (Mason et al., 2004).
The clinical observation of higher prevalence and poorer outcome of lung infections in alcohol-exposed individuals is not unexpected because both innate and adaptive immune responses are affected by alcohol in the lung. Chronic alcohol use impairs phagocytosis and superoxide production in response to a bacterial challenge in alveolar macrophages (Brown et al., 2004; Greenberg et al., 1999a Greenberg et al. 1999b;). TNFα, a product of alveolar macrophages, is a key cytokine in pulmonary host defense against bacterial pathogens. In animal models acute alcohol intoxication resulted in impaired pulmonary TNFα production in response to LPS challenge but no inhibition of TNFα gene expression (Nelson et al., 1989). Considering that both TNFα and optimal PMNL functions are necessary to control bacterial infections in the lung, by dampening these functions, alcohol results in more pathogenic effects. These observations lead to the discovery that acute alcohol prevented cleavage of TNFα from the precursor by the TNFα converting enzyme, TACE (Zhao et al., 2003). Acute alcohol also suppressed the lung’s expression of the chemokines MIP-2 (KC?) and CINC, a rodent equivalent of human IL-8 and Gro-α that attract neutrophils to tissue sites. Additional neutrophil defects were identified as decreased expression of adhesion molecules CD11b/c and CD18 in response to LPS in alcohol-exposed mice (Zhang et al., 1998). It is conceivable that neutrophil defects in chronic alcohol user individuals would also increase susceptibility to secondary bacterial lung infection after viral pneumonia (Grabowska et al., 2006; Navarini et al, 2006). It has been shown that both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. (McNamee and Harmsen, 2006).
Chronic alcohol use results in hypo-responsiveness of neutrophils to chemotactic signals and neutrophils are less able to kill bacteria because of impaired phagocytosis and superoxide generation in humans and in animal models (MacGregor et al., 1978; Sachs et al.,1990; Tamura et al., 1998; Zhang et al., 1999). A recent study also identified defects after acute alcohol intoxication in lung expression of CXC chemokines that are involved in T cell recruitment, (Happel et al., 2007). Alcohol abusing patients are frequently neutropenic and leukopenic that is likely mediated by alcohol-induced suppression of the bone marrow. Indeed, acute alcohol inhibits the expression of granulocyte colony-stimulating factor (G-CSF) and animal studies showed that exogenous administration of this recombinant cytokine can partially restore neutrophil recruitment to the lung in response to a bacterial stimulus (Bagby et al., 1998; Nelson et al., 1991). Chronic alcohol feeding in mice resulted in impaired alveolar macrophage phagocytosis of inactivated Staphylococcus aureus. These macrophages of chronic alcohol-fed mice showed decreased cellular glutathione, impaired lipid peroxidation and increased apoptosis (Brown et al, 2007). Feeding with N-acetylcysteine, a precursor of glutathione, with the alcohol diet reversed the paralysis of macrophage phagocytosis and the loss of viability offering a potential mechanism for amelioration of alcohol-induced macrophage defects (Velasquez et al., 2002). More recently, IL-23, a cytokine produced by activated antigen presenting cells and involved in activation of memory T cell proliferation and T cell-derived IL-17 production, was studied in pneumonia. Acute alcohol intoxication suppressed lung as well as alveolar macrophage IL-23 response to a bacterial challenge with Klebsiella (Happel et al., 2006).
Alcohol exposure of the host can also predispose to pnemonia caused by viral infections. In a mouse model of respiratory syncytial virus (RSV) infection, alcohol provided in the drinking water for 13-16 weeks resulted in increased levels of TNFα and MCP-1 and increased the numbers of neutrophils in the bronchoalveolar fluid after RSV infection compared to the non-alcohol group (Jerrells et al., 2007b). Notably, alcohol-fed mice also showed a failure to clear the RSV infection despite increased IFNα and IFNß induction. Using the same alcohol-in-the water-feeding model, a recent report demonstrated increased susceptibility to influenza infection in mice (Meyerholz et al, 2008). There was increased lethality, increased injury and neutrophil leukocyte inflammation in the lung associated with a progressive loss of CD8+ T cell function in mice with prolonged alcohol administration. These studies demonstrate that experimental alcohol exposure results in impaired immune cell activation in the lung at multiple levels.
Alcohol use and systemic bacterial infections
The increased susceptibility to infections after alcohol consumption is not limited to lung infections. Antimicrobial defense is attenuated to several other pathogens including Salmonella (Sibley et al., 2001). Listeria monocytogenes infection that can be lethal is more severe in human alcoholics resulting in higher mortality from Listeric meningitis or sepsis (Iwarson and Larson, 1979). Animal models showed that alcohol-feeding not only increases the susceptibility to Listeria monocytogenes infection but also attenuates immune mechanisms of the host in elimination of the infection (Saad et al., 1993; Salerno et al, 2001. In experimental models of L. monocytogenes infection model, chronic alcohol feeding with the Lieber-DeCarli diet resulted in increased liver damage and increased bacterial colony counts both in the liver and spleen in alcohol-fed mice (Saad et al., 1993). The investigators found that increased activation of CD8+ T cells contribute to liver damage in alcoholic and other types of hepatitis (reviewed in Jerrells, 2002a,b).
Alcohol and retroviral infection
Studies demonstrated an association between alcohol consumption and the risk of HIV infection and it was noted that the incidence of alcohol abuse among HIV-infected individuals is greater than in the general population (Lefevre et al., 1995; Stinson and DeBakey, 1993). While alcohol abuse has been shown to impair immune defenses, the adverse consequences of heavy alcohol use on HIV-infected patients are poorly understood. One study described a rapid progression of HIV to AIDS in a period of 3 months in a patient with heavy alcohol abuse (Fong et al, 1994). In another study, alcohol withdrawal in HIV infected patients was associated with an increase in blood CD4+ T cell counts (Pol et al., 1996). A recent large observational study also found decreased survival in HIV infected alcohol abusing patients. Non-hazardous drinkers (consume < 5 drinks on drinking days) survived by less than 1 year if the frequency of alcohol use was once per week or greater compared to non-alcohol consuming HIV infected individuals while hazardous drinkers (consume > 5 standard drinks per day) had a 3.3 year reduction in survival (Braithwaite et al., 2007). Alcohol use not only alters adherence to antiretroviral therapy, but studies demonstrated that it also diminishes the benefits of the highly active retroviral therapy (HAART). Heavy alcohol use was associated with a two-fold likelihood of decreased CD4+ T cell count and HAART-treated heavy alcohol users were four times less likely to achieve virological response (Miguez et al., 2003). These observations suggest that alcohol abuse impacts both immunological and virological responses to HAART treatment in HIV infected patients.
Experimental data from Simian Immunodeficiency Virus (SIV) infection demonstrated that alcohol feeding resulted in increased plasma SIV RNA levels in the early phase but not at later timepoints during the SIV infection (Bagby et al., 2006). However, a more rapid progression to end-stage disease was found in alcohol-fed animals compared to the controls. Interestingly, the SIV-related decrease in CD4 T cell counts was similar in both groups (Bagby et al., 2006). In a related study, alcohol-fed SIV-infected animals had significantly higher muscle TNFα mRNA expression compared to the control SIV-infected animals and this correlated with muscle wasting, a major characteristics of advanced HIV disease (Molina et al., 2006).
Alcohol and viral hepatitis
It is generally accepted that alcohol use worsens the clinical outcome of any types of viral hepatitis, particularly chronic infections with hepatitis B (HBV) or hepatitis C virus (HCV) in humans (McCullough et al, 1998; Szabo and Marshal, 2008). Chronic hepatitis C virus (HCV) infection and alcohol use are the two most common causes of chronic liver diseases in the United States and both are recognized as major causes of liver disease worldwide. The prevalence of HCV infection in patients with a history of alcohol abuse is significantly higher than that seen in the general population. Heavy alcohol use accelerates fibrosis progression, increases the risk of cirrhosis, predicts increased mortality, and increases the risk of hepatocellular carcinoma in HCV infection (Szabo et al., 2008). Several studies in humans (Dolganiuc et al., 2003a; Plumlee et al., 2005; Szabo, 2003; Szabo et al., 2006a;) or mice (Aloman et al., 2007;) have evaluated the interaction of HCV and alcohol on host cellular immunity. These studies found that alcohol influences host cellular signal transduction pathways possibly acting with HCV to induce alterations in responses to HCV. Specifically, host dendritic cells are independently modulated by both HCV (Bain et al., 2001) infection and alcohol in humans (Dolganiuc et al., 2003b; Mandrekar et al., 2004). However, experimental data suggests that alcohol and HCV infection can be additive in inhibition of the T cell activating capacity of human myeloid dendritic cells (Dolganiuc et al., 2003a). The reduced T cell activating capacity of dendritic cells of patients with chronic HCV infection was further diminished by exposure of these cells to a physiologically relevant dose (25 mM) of alcohol in vitro (Dolganiuc et al., 2003a). The presence of HCV proteins (core and NS3) reduced dendritic cell differentiation in vitro while alcohol further reduced dendritic cell function by decreasing T cell stimulation and interferon production both in humans and in a mouse model (Aloman et al., 2007; Dolganiuc et al., 2003a). Animal models of long-term alcohol consumption have also demonstrated an impaired cellular immune response to HCV infection attributable to altered dendritic cell function (Aloman et al., 2007). However, in another study, alcohol consumption did not influence T cell immune responses (interferon gamma and IL-10 production) in HCV or HCV/HIV coinfected patients (Graham et al., 2007). Further studies are needed to elucidate the interactions of HCV and alcohol use on immune responses.
In animal models of experimental hepatitis, cytomegalovirus, an otherwise self-limiting hepatitis in immunocompetent mice, resulted in severe hepatitis in ethanol-fed mice (Sibley and Jerrells, 2000; Jerrells, et al. 2002a,b). The CMV-induced hepatitis was more prolonged and it was associated with greater mononuclear cell inflammatory response in alcohol-fed mice. Early induction of IFNγ and IL-12 were reduced in alcohol-fed mice after CMV infection compared to control diet-fed mice (Jerrells, et al. 2002a,b). These data suggest that both innate immunity and antiviral responses are affected in the liver by alcohol administration.
Alcohol-related modulation of trauma and burn injury
Alcohol abuse is a well-established risk factor for traumatic injuries of all types. Not only is the risk of sustaining an injury increased by alcohol use but the severity of trauma-related immune compromise and recovery from trauma-related hospitalization are all negatively affected by alcohol intoxication (Greiffenstein and Molina, 2008; Reviewed in Messingham et al., 2002) The alcohol-induced alterations in host defense after traumatic injury were summarized in a recent review (Greiffenstein and Molina, 2008). Host response to injury involves initial activation of the inflammatory cascade and a subsequent induction of anti-inflammatory mechanisms to allow recovery. Chronic alcohol use was found as an independent risk factor for development of sepsis, septic shock and sepsis-related mortality in critically ill patients (O’Brien et al., 2007). In experimental models binge drinking before trauma/heamorrhage resulted in attenuated levels of TNFα, IL-1 and IL-6 and no change in IL-10 in response to an LPS challenge (Greiffenstein et al., 2007). Acute alcohol intoxication before excisional injury in mice resulted in no change in the numbers of infiltrating neutrophil leukocytes but resulted in decreased myeloperoxidase activity, impaired production of MIP-2, KC, and IL-1ß in mice (Fitzgerald et al., 2007). In humans, monocyte production of TNFα, a major mediator of sepsis and inflammation, was attenuated in alcohol intoxicated trauma victims in the early post-trauma period, however, a delayed increase in monocyte TNFα production was found late during the period of post-trauma immununosupression (Szabo et al., 1994).
Immune responses following burn injury in rats were also altered by alcohol intoxication. On day one after injury, lung tissue IL-18, neutrophil chemokines (CINC-1/CINC-3), adhesion molecules (ICAM-1), neutrophil infiltration, MPO activity and lung edema were significantly increased compared to the alcohol or burn alone groups (Li et al., 2007). Administration of anti-IL-18 neutralizing antibody or an inhibitor of caspase-1, an enzyme involved in cleavage of the bioactive IL-18 from its precursor, prevented the increase in lung IL-18, CINC-2, CINC-3, ICAM-1 and MPO following alcohol plus burn injury. Furthermore, administration of anti-neutrophil serum also attenuated the lung injury in this alcohol plus burn injury model suggesting that IL-18 production and subsequent neutrophil infiltration in the lung play a critical role in increased lung edema following a combined insult of alcohol and burn injury (Li et al., 2007). Increased susceptibility to pulmonary infection after alcohol use and surgery was also found in a different model. Here, mice that received alcohol for 8 days followed by the trauma of laparotomy were challenged with K. pneumoniae infection. Alcohol-treated mice demonstrated worse clinical outcome, deteriorated pulmonary structure, and increased levels of IL-6 and IL-10 compared to the non-alcohol treated group (Spies et al., 2008). In human alcoholics, the immunosuppressive cytokine, IL-10 was also more significantly increased after major surgical intervention compared to non-alcoholic individuals (Sander et al., 2005). Another component of the dysfunctional immune regulation in the setting of alcohol and tissue injury is the reduced T cell proliferation capacity. Alcohol intake both acutely and chronically inhibits antigen presenting cell functions (Heinz and Waltenbaugh, 2007; Mandrekar et al.,2004), and trauma injury alone is associated with poor APC capacity and T cell activation (Bandyopadhyay, 2007; Szabo et al., 1994; Szabo et al.,1995). In mice, acute alcohol intoxication followed by thermal injury resulted in a significant decrease in ConA-induced splenic T cell proliferation and IL-2 production capacity compared to either alcohol or thermal injury alone (Choudhry et al., 2000). These clinical and experimental observations demonstrate that alcohol use (either acute or chronic) negatively affects immune responses in post-trauma recovery.
Immune mechanisms in alcoholic liver disease
Alcoholic liver disease (ALD) is a major heath consequence of chronic alcohol use and approximately 44% of the 26,000 deaths from cirrhosis are due to alcoholic liver disease in the United States (McCullough et al, 1998). Activation of the inflammatory cascade is a key element in alcoholic liver disease (Adachi et al., 1994; Nagy, 2003; Nanji et al., 2002; Thurman 1998). Alcoholic liver disease, particularly, alcoholic hepatitis is associated with massive inflammatory cell (neutrophil leukocytes, monocytes, macrophages) recruitment in the liver. It has been proposed that alcohol-related increased gut permeability results in elevated LPS in portal circulation leading to Kupffer cell activation and initiation of a cycle of inflammatory cytokine and chemokine production, and activation of innate immune cells in the liver. Endotoxin levels are increased in the portal blood of patients with alcoholic liver disease (Bode and Bode, 2005). Consistent with this, chronic alcohol intake in humans results in increased serum levels of pro-inflammatory cytokines TNFα, IL-6, and IL-8 that mediate liver injury in alcoholic liver disease (McClain et al., 1999).
An important role for LPS, a TLR4 ligand, has been established in alcoholic hepatitis by studies of Thurman’s group where both mice deficient in the LPS co-receptor, CD14, and mice with mutation in TLR4 were protected from alcohol-induced early liver inflammation and damage (Uesugi et al., 2001). Activation of Kupffer cells by LPS via TLR4 is a central component in the pro-inflammatory cytokine cascade activation in alcoholic hepatitis and the ongoing hepatic inflammation result in high levels of circulating TNFα, IL-1 IL-6 and IL-8 in these patients (Adachi et al., 1994; Hill et al., 1993; McClain et al., 1999). Sterilization of the gut with antibiotics could prevent alcohol-induced liver inflammation in animal models (Adachi et al., 1995). LPS recognition by TLR4 expressed on sinusoidal endothelial cells and stellate cells may also contribute to progression of alcoholic liver disease (Szabo et al., 2006b). Liver injury and TNFα mRNA induction are also amplified in alcohol-induced fatty liver after stimulation with TLR2/1, TLR2/6, TLR4, TLR7 and TLR9 ligands in mice (Gustot et al., 2006). It has been proposed that suppression of the antifibrotic effects of NK cells and NK-derived IFNg production may amplify the profibrotic effects of Kupffer cells (Reviewed in Jeong et al, 2008). Alcohol metabolism induces malondialdehyde-acetaldehyde adducts that in turn activate liver sinusoidal endothelial cells and Kupffer cells in the presence of LPS to induce cytokines and chemokines (Duryee et al., 2004; Tuma, 2002). These mechanisms suggest a pivotal role for activation of innate immune cells and proinflammatory pathways in alcoholic liver disease.
In addition to innate and inflammatory responses, adaptive immune responses appear to be affected in alcoholic liver disease. In an experimental model, hepatic inflammation and fat deposition with a specific recall immune response was found in mice immune to Listeria monocytogenes and fed with an alcohol containing Lieber-DeCarli diet (Slukvin et al, 2001).
Alcohol and pancreatitis
The pathobiology of alcoholic pancreatitis appears to be complex (reviewed in: Pandol and Raraty, 2007). While acinar cell necrosis and induction of sterile inflammation are key components, there is evidence for ethanol consumption potentiating viral pancreatitis. Experimental models with coxackievirus, which is also a human pathogen, demonstrated that short-term (5-14 days) and subchronic (> 28 days) alcohol administration increased the severity of Coxackie B3 virus-induced pancreatic damage (Clemens and Jerrells, 2004; Jerrells et al 2007a). Studies on the effects of alcohol on tissue necrosis-related activation of the innate immune system and inflammation are yet to be performed.
Moderate alcohol use on cardiovascular disease: a potential link to inflammation
The potential benefit of moderate alcohol use on coronary heat disease and overall mortality has been suggested by several studies while challenged by others (Rimm and Moats, 2007; Mukamal 2007; Klatsky and Udaltsova 2007; Gronbeck 2007). Moderate alcohol use (up to 2 drinks/day in men and one drink/day in women) is associated with health benefits including decreased risk of cardiovascular disease and reduced overall mortality compared to people who do not consume alcohol (Mukamal et al., 2001; Poikolainen, 1995). In contrast, excessive alcohol use (>5 drinks/day) increases mortality and cardiovascular death. The molecular mechanisms underlying this phenomenon are yet to be understood. One of the differences between acute and chronic alcohol use that may contribute to their different biological effects is their opposite effects on inflammatory cell activation. Inflammatory cytokine overproduction and monocyte/macrophage activation (NF-κB and TNFα production) are associated with coronary heart disease where moderate alcohol use has protective effects (Libby, 2002; Ross, 1999). Recent studies found an association between Toll-like receptor (TLR) polymorphism and atherosclerosis and suggested a central role for TLR signaling in coronary heart disease (Bjorbacka. 2006; Vainas et al., 2006). We and others have reported that acute alcohol in vitro as well as in vivo in humans and in experimental animals inhibits induction of pro-inflammatory mediators such as tumor necrosis factor α, interleukin-1 (IL-1), IL-6 and the chemokines, IL-8 and MCP-1 (Verma et al., 1993; Szabo et al., 1996; Szabo et al., 1999b). It has also been shown that the molecular pathways in inhibition of pro-inflammatory cytokines by acute moderate alcohol intake involve inhibition of the nuclear regulatory factor κB, a common element in the promoter region of inflammatory cytokine genes (Mandrekar et al., 1999; Mandrekar et al., 2007). Thus, it is tempting to speculate that such attenuation of inflammatory pathway activation by acute alcohol may have a beneficial role in atherosclerosis where inflammatory pathway activation has been shown to accelerate disease progression and results in destabilization of plaques leading to acute coronary syndrome. Further studies are needed to evaluate the relevance of the finding from the one-occasion acute alcohol administration models to the human consumption of moderate alcohol amounts on a more regular basis.
Innate immune response alterations caused by alcohol intake
Considering that most of the detrimental effects of alcohol on immunity are related to infections, recent studies turned attention to the role of pattern recognition receptors that play a key role in recognition of invading pathogens (Takeuchi and Akira, 2007a,b) and are expressed on innate immune cells as well as on some parencymal cells. The family of Toll-like receptors (TLRs) that includes 11 mammalian receptors has been partially studied in relation to alcohol. Most studies investigated the LPS-induced inflammatory responses in monocytes, macrophages or dendritic cells LPS, a component of Gram-negative bacteria, is recognized by TLR4 with participation of the co-receptors, CD14 and MD2 (Takeuchi and Akira 2007a,b).
Another pivotal component of the innate immune response is the initiation of adaptive immune responses through antigen presenting cell functions (Kaisho and Akira 2003). Dendritic cells are substantially more potent in antigen presentation and antigen-specific T cell activation than monocytes or macrophages (Kabelitz et al., 2006). Impaired delayed-type hypersensitivity response has been shown in alcohol consuming individuals (Tennesen et al, 1992) and in a mouse model of chronic alcohol administration (Jayasinghe et al, 1992). More recently, decreased monocyte antigen presenting cell function was reported in humans after one occasion of alcohol intake (Mandrekar et al., 2004). T cell proliferation induced by mitogen stimulation or superantigens was decreased after alcohol consumption and these effects were mediated by alcohol exposure of the antigen presenting cells, monocytes (Szabo et al., 1995). Classical antigen presentation by monocytes was also impaired after acute alcohol intake or in vitro alcohol treatment in humans (Szabo et al., 2004). Recent studies with different dendritic cell types also found an inhibitory effect of alcohol on DC functions. Human studies showed that acute alcohol intake or prolonged in vitro alcohol treatment both inhibited monocyte-derived myeloid dendritic cell capacity to induce T cell activation (Mandrekar et al., 2004; Szabo et al., 2004). This was associated with increased IL-10 and decreased IL-12 production by alcohol-exposed DCs (Mandrekar et al., 2004). Furthermore, initial exposure of T cells to alcohol-treated dendritic cells induced T cell anergy that resulted in impaired T cell proliferation even to subsequent stimulation with normal DCs; this T cell anergy could be ameliorated by addition of exogenous IL-12 (Mandrekar et al., 2004). Chronic alcohol consumption affected DC functions in the skin in mice. There were decreased numbers and migration of Langerhans cells (skin DCs) and dermal DCs in mice after 35 weeks of alcohol feeding (Ness et al., 2008). Alcohol feeding in mice also resulted in decreased bone marrow-derived DC generation, decreased expression of the co-stimulatory molecules CD80, CD86 and DCs and impaired induction of T cell proliferation and IL-12 production (Lau et al., 2006). These were associated with increased DC production of IL-10, a cytokine with inhibitory actions on DC maturation, antigen presentation and T cell proliferation. Another study found that chronic alcohol consumption altered CD11c+, CD8+ DC function and antigen presentation that was associated with decreased IL-6, IL-12 and increased IL-13 cytokine production (Heinz and Waltenbaugh, 2007). These results suggest that whether acute or chronic, alcohol consumption appears to inhibit differentiation and functions of various types of dendritic cells.
While alcohol use in general results in impaired immune responses, it is important to distinguish between the effects of acute and chronic alcohol use on inflammatory cell responses. Innate immune cells including monocytes and macrophages are a major source of pro-inflammatory cytokine production in response to various pathogen-derived signals (Medzhitov and Janeway, 2000; Takeuchi and Akira, 2007a). While inhibition of phagocytic functions was reported by both acute and chronic alcohol exposure, the effects of alcohol are opposite on inflammatory cytokine production in monocytes and macrophages. A single dose of alcohol or binge drinking results in attenuation of pro-inflammatory pathway activation after a pathogen-derived challenge that leads to the production of TNFα, IL-1 or IL-6 (Pruett et al., 2004a). Chemokine production (MCP-1, MIP-1) in response to an LPS challenge was also inhibited by acute alcohol intoxication in human monocytes (Szabo et al., 1999b). Different types of alcoholic beverages all seem to have the same inhibitory potential on inflammatory response after acute intake as both studies with vodka or beer ingestion had similar findings with respect to inhibition of inflammatory cell activation by acute alcohol intake (Mandrekar et. al. 2006; Romeo et al., 2007a, Romeo et al., 2007b; Winkler et al., 2006).
While studies have general consensus on the inhibitory effects of acute alcohol on LPS/TLR4-mediated inflammatory cascade activation, the effects of acute alcohol on other TLR-induced monocyte/macrophage responses are less clear. In human monocytes TLR2 ligand-induced TNFα production and NF-κB activation was not inhibited by acute alcohol (Oak et al., 2006). In contrast, murine macrophages had attenuated responses to TLR2 or TLR9 ligand stimulation (Goral et al., 2004). In murine macrophages acute alcohol inhibited LPS-induced IL-6 production and this was associated with transient down-regulation of the extracellular regulated kinases 1 and 2 (ERK1/2) and p38 MAPK (Goral et al., 2004). Acute ethanol treatment in RAW 264.7 murine macrophages increased IL-10 production but inhibited IL-6 and IL-12 levels (Pruett et al., 2005). Besides TLR4, acute alcohol altered TLR3-induced gene expression to affect the interferon-related amplification loop which could be responsible for suppression of several effector molecules of inflammation (Pruett et al., 2004b; Pruett et al, 2004c). It has been shown that alcohol inhibits the recruitment of the LPS receptors, TLR4 and CD14 into the lipid rafts on the cell surface (Dai et al., 2006; Dolganiuc et al., 2006; Fernandez-Lizarbe et al., 2008). Interestingly, TLR2 recruitment to the lipid rafts was not affected by acute alcohol; this correlates with the lack of inhibition of TLR2-induced TNFα production (Dolganiuc et al., 2006; Oak et al., 2006)
In contrast to the inhibitory effects of acute alcohol, prolonged alcohol treatment results in an augmentation of macrophage TNFα production and inflammatory cascade activation. In rat and mouse liver macrophages, Kupffer cells, chronic alcohol exposure in vivo resulted in an increased production of TNFα after ex vivo LPS stimulation (Kishore et al., 2004). This was associated with increased Erk and Egr1 activation and Egr1 mice were protected from alcohol-induced liver disease (Prichard and Nagy 2005). Consistent with this, peripheral blood monocytes from patients with alcoholic liver disease show increased production of TNFα (McClain et al., 1999). Chronic ethanol consumption increased expression of co-stimulatory molecules CD80 and CD86 on TLR9 activated CD11b+ splenocytes contributing to systemic immunodysregulation including T cell activation (Cook et al., 2004; Zhu et al., 2004). As discussed above, lung alveolar macrophages are not only suppressed after acute alcohol exposure but even after chronic alcohol exposure, the functions of alveolar macrophages remained impaired (Guidot et al.,2000).
Abnormalities in adaptive immune responses after alcohol use
Adaptive immunity includes T cell and B cell activation, and production of immunoglobulins. There is manifestation of autoimmune processes in chronic alcoholic individuals including the presence of circulating autoantibodies to lymphocytes, brain, DNA, serum lipoproteins and various liver proteins (Vidali et al., 2008). Polyclonal hyperglobulinemia is frequent in alcoholics and deposition of immunoglobulin-A are found in skin, liver and kidney of many patients with alcoholic liver disease (Laskin et al., 1990). Increased immunoglobulins are found in alcoholics with below-normal B cell counts suggesting that the increased immunoglobulin production is not the result of an increase in B cell populations (Cook et al., 1996). It remains to be evaluated in humans whether without alcohol-mediated stress response, B cell functions would be altered. In a mouse model of alcohol-in-the-water-feeding, B cell functions are relatively unaffected by alcohol without a stress response (Cook et al., 2007).
Previous reports indicate that alcoholics without liver disease have an increased percentage of the activated CD8+ T cells measured and expression of HLA-DR (Cook et al., 1996). These patients also have a shift from “naïve” (CD45RA+) to the “memory” phenotype of T cells (CD45RO+) (Cook et al., 1996). This change was present in CD8+ and CD4+ T cells and it was associated with a significant reduction in L-selectin and an increase in CD11b expression on CD8+ T cells.
Studies in mice with chronic alcohol consumption found activated splenic T cells (Song et al., 2002). Administration of ethanol to mice in the drinking water for 3-13 weeks resulted in decreased expression of CD62L and increased percentage of CD44hi T cells. In the CD44hi T cell population the study found more IFNγ, IL-4 but not IL-2 secretion in alcohol-fed compared to control mice (Song et al., 2002). Overall these studies support the contention that in humans and mice, chronic alcohol exposure results in splenic T cell activation or sensitization in vivo and results in an increased percentage of effector/memory cell subset.
In a recent study, chronic alcohol administration in the drinking water evaluated T and B cell functions in various strains of mice. Alcohol feeding from 8-32 weeks resulted in no changes in serum immunoglobulin levels, pre-B cells or in the percentage of double positive thymocytes (Cook et al, 2007). Exposure of mice to similarly low levels of alcohol for 4-8 weeks (5% alcohol in the drinking water) resulted in significant changes in the splenic, thymic and bone marrow T cell subpopulations and increased T cell expression of STAT5 while it decreased STAT5 activation in NK cells (Guo et al., 2002). Chronic alcohol consumption led to increased NK T cell numbers and activity in the liver. These cells express both T-cell and natural killer (NK) cell receptors; they are abundant in the liver and mediate various regulatory and effector functions. NKT cells alone or in conjunction with NK cells modulate responses of the adaptive immune system by secreting cytokines and by mediating cytotoxic effector functions by perforin, tumor necrosis factor (TNF)- and Fas (Minagawa et al., 2004). In an experimental mouse model chronic alcohol feeding lead to an increase of NKT cells in the liver, concomitant with an induction of liver injury (Jaruga et al., 2004). Activation of NKT cells prompted a dramatic increase in liver injury, causing death in most animals. The cells inducing this effect are NKT cells that mediate injury by a Fas and TNF receptor- 1 (TNFR1)–dependent mechanism. Reports on the effects of alcohol on NK cell functions are controversial. Many investigators found that alcohol inhibits NK cell cytolytic function (Ristow et al., 1082; Zhou and Meadows, 2003) but in vitro treatment of human NK cells for 1-7 days resulted in an increased cytolytic activity (Li et al., 1997). A recent study showed that chronic alcohol consumption inhibits hepatic NK cell function in CMV-induced hepatitis (Pan et al., 2006). Further evaluation of the effects of alcohol on NK and NKT cell functions needs investigation in humans and in animal models.
Summary
Experimental and clinical data support the conclusion that alcohol is a potent immunomodulatory agent. Even a single occasion of moderate-to-heavy drinking results in alteration of innate immune response such as attenuation of inflammatory cell function to pathogen-derived signals (Figure 2). Acute and chronic alcohol consumption, however, have opposite effects on inflammatory cell activation wherein acute alcohol is inhibitory whereas chronic alcohol leads to an increase in inflammatory cell responses, particularly to lipopolysaccharide, a TLR4 ligand. The antigen presenting function of innate immune cells including monocytes and macrophages is inhibited by alcohol exposure and such impaired antigen presentation capacity contributes to decreased antigen-specific T cell proliferation. In addition to dendritic cell-mediated induction of anergy, alcohol may directly affect functions of T cells to change the distribution and functions of memory T cells. Natural killer cell functions are also altered by alcohol use further contributing to the alcohol-induced immune defects. While B cell functions appear to be relatively unaffected by alcohol, secondary modification through stress response may still occur. Together, the alcohol-induced specific changes in immune cell functions result in a impaired host defense and inappropriate immune response to invading pathogens leading to higher incidence of pneumonias and other infection as well as prolonged recovery from infections, burn or trauma injury. Further investigation of the specific effects of alcohol on immune functions should help to define potential therapeutic options for amelioration of alcohol-induced immune defects.
Figure 2. Alcohol impairs innate and adaptive immunity.
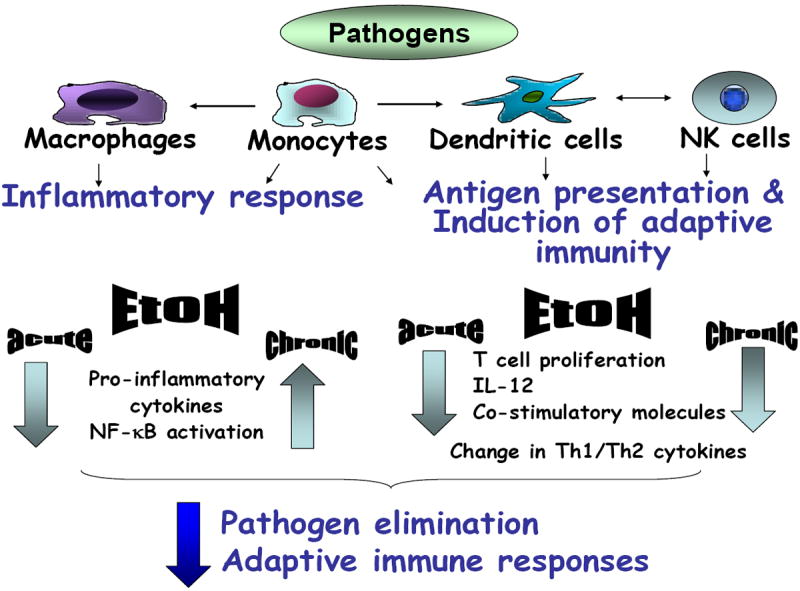
Acute and chronic alcohol has broad immunoregulatory effects. Cells of the innate immune system (macrophages, monocytes, dendritic cells, NK cells) are modulated by alcohol in their capacity to respond to pathogens. Inflammatory cell responses including production of pro-inflammatory cytokines (IL-1, TNFa) and NF-kB activation are inhibited by acute alcohol exposure while chronic alcohol augments these pro-inflammatory responses. The antigen presenting function of both monocytes and dendritic cells is impaired by both acute and chronic alcohol and this contributes to impaired induction of adaptive immune responses. Both acute and chronic alcohol inhibits T cell functions and IL-12 production and results in alterations in Th1 (IFNg) and Th2 (IL-10) cytokine production. These alcohol-induced abnormalities then collectively contribute to impaired pathogen elimination and reduced adaptive immune responses in the alcohol-exposed host.
References
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Aloman C, Gehring S, Wintermeyer P, Kuzushita N, Wands JR. Chronic ethanol consumption impairs cellular immune responses against HCV NS5 protein due to dendritic cell dysfunction. Gastroenterology. 2007;132:698. doi: 10.1053/j.gastro.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;27:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, De A, Laudanski K, Li F, Lentz C, Bankey P, Miller-Graziano C. Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med. 2007;35:794–801. doi: 10.1097/01.CCM.0000256847.61085.A5. [DOI] [PubMed] [Google Scholar]
- Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcoholism Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- Bjorbacka H. Multiple roles of TLR signaling in atherosclerosis. Curr Opin Lipidol. 2006;17:527–533. doi: 10.1097/01.mol.0000245258.25387.ec. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, Rodriguez MC, Rabeneck L, Bryant K, Justice AC. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19:459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Brown LA, Cook RT, Jerrells TR, Kolls JK, Nagy LE, Szabo G, Wands JR, Kovacs EJ. Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res. 2006;30:1624–1631. doi: 10.1111/j.1530-0277.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J physiol Lung Cell Mol Physiol. 2007;292:L824–L832. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- Capps JA, Coleman GH. Influence of alcohol on prognosis of pneumonia in Cook County Hospital. JAMA. 1923;80:750–752. [Google Scholar]
- Chiappelli F, Kung MA, Tio DL, Tritt SH, Yirmiya R, Taylor AN. Fetal alcohol exposure augments the blunting of tumor necrosis factor production in vitro resulting from in vivo priming with lipopolysaccharide in young adult male but not female rats. Alcohol Clin Exp Res. 1997;21:1542–1546. [PubMed] [Google Scholar]
- Choudhry MA, Messingham KAN, Namak S, Colantonti A, Fontanilla CV, Duffner LA, Sayeed MM, Kovacs EJ. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol. 2000;21:239–243. doi: 10.1016/s0741-8329(00)00093-8. [DOI] [PubMed] [Google Scholar]
- Clemens DL, Jerrells TR. Ethanol consumption potentiaties viral pancreatitis and may inhibit pancreas regeneration: preliminary findings. Alcohol. 2004;33:183–189. doi: 10.1016/j.alcohol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Young BM, Turner LE, Cook RT. In: A practical method of chronic ethanol administration in mice in Alcohol, Methods and Protocols. Nagy LE, editor. Humana Press; New Jersey: 2008. pp. 49–59. [DOI] [PubMed] [Google Scholar]
- Cook RT, Waldschmidt TJ, Cook BL, Labrecque DR, McLatchie K. Loss of the CD5+ and CD45RAhi B cell subsets in alcoholics. Clin Exp Immunol. 1996;103:304–310. doi: 10.1046/j.1365-2249.1996.d01-621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Cook RT, Zhu X, Coleman RA, Ballas ZK, Waldschmidt TJ, Ray NB, LaBrecque DR, Cook BL. T-cell activation after chronic ethanol ingestion in mice. Alcohol. 2004;33:175–181. doi: 10.1016/j.alcohol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Cook TR, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion—absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Pruett SB. Ethanol suppresses LPS-induced Toll-like receptor 4 clustering, reorganization of the actin cytoskeleton, and associated TNF-alpha production. Alcohol Clin Exp Res. 2006;30:1436–1444. doi: 10.1111/j.1530-0277.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- DeCarli LM, Lieber CS. Fatty liver in the rat after prolonged intake of ethanol with a nutrionally adequate new diet. J Nutr. 1967;91:331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Kodys K, Kopasz A, Marshall C, Do T, Romics L, Jr, Mandrekar P, Zapp M, Szabo G. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003a;170:5615–5624. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Kodys K, Kopasz A, Marshall C, Mandrekar P, Szabo G. Additive inhibition of dendritic cell allostimulatory capacity by alcohol and hepatitis C is not restored by DC maturation and involves abnormal IL-10 and IL-2 induction. Alcohol Clin Exp Res. 2003b;27:10231031. doi: 10.1097/01.ALC.0000071745.63433.32. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Bakis G, Kodys K, Szabo G. Acute alcohol treatment modulates Toll-like receptor 4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004;28:1931–1938. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- Edsen-Moore MR, Fan J, Ness KJ, Marietta JR, Cook RT, Schlueter AJ. Effects of chronic ethanol feeding on murine dendritic cell numbers, turnover rats and dendropoiesis. 2008;32:1309–1320. doi: 10.1111/j.1530-0277.2008.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Mol Immunol. 2008;45:2007–16. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DJ, Radek KA, Chaar M, Faunce DE, DiPietro LA, Kovacs EJ. Effects of acute ethanol exposure on the early inflammatory response after excisional injury. Alcohol Clin Exp Res. 2007;31:317–323. doi: 10.1111/j.1530-0277.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- Fong IW, Read S, Wainberg MA, Chia WK, Major C. Alcoholism and rapid progression to AIDS after seroconversion. Clin Infect Dis. 1994;19:337–338. doi: 10.1093/clinids/19.2.337. [DOI] [PubMed] [Google Scholar]
- Goral J, Choudhry MA, Kovacs EJ. Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK 1/2 MAPK. J Leukoc Biol. 2004;75:553–559. doi: 10.1189/jlb.0703350. [DOI] [PubMed] [Google Scholar]
- Grabowska K, Hogberg L, Penttinen P, Swensson A, Ekdahl K. Occurrence of invasive pneumococcal disease and number of excess cases due to influenza. BMC Infect Dis. 2006;6:58. doi: 10.1186/1471-2334-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CS, Wells A, Edwards EM, Herren T, Tumilty S, Stuver SO, Samet JH, Nunes D, Horsburgh CR, Jr, Koziel MJ. Effect of exposure to injection drugs or alcohol on antigen-specific immune responses in HIV and hepatitis C virus coinfection. J Infect Dis. 2007;195:847–856. doi: 10.1086/511990. [DOI] [PubMed] [Google Scholar]
- Greenberg SS, Ouyang J, Zhao X, Parrish C, Nelson S, Giles TD. Effects of ethanol on neutrophil recruitment and lung host defense in nitric oxide synthase I and nitric oxide synthase II knockout mice. Alcohol Clin Exp Res. 1999;23:1435–1445. [PubMed] [Google Scholar]
- Greenberg SS, Zhao X, Hua L, Wang JF, Nelson S, Ouyang J. Ethanol inhibits lung clearance of Pseudomonas aeruginosa by a neutrophil and nitric oxide-dependent mechanism, in vivo. Alcohol Clin Exp Res. 1999;23:735–744. [PubMed] [Google Scholar]
- Greiffenstein P, Mathis KW, Vande Slouwe C, Molina Alcohol binge before trauma/hemorrhage impairs integrity of host defense mechanisms during recovery. Alcohol Clin Exp Res. 2007;31:704–715. doi: 10.1111/j.1530-0277.2007.00355.x. [DOI] [PubMed] [Google Scholar]
- Greiffenstein P, Molina PE. Alcohol-induced alterations on host defense after traumatic injury. J Trauma. 2008;64:230–240. doi: 10.1097/TA.0b013e318158a4ad. [DOI] [PubMed] [Google Scholar]
- Gronbaek M. Confounders of the relation between type of alcohol and cardiovascular disease. 2007:S13–S15. [Google Scholar]
- Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol. 2000;279:L127–L135. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- Guo TL, Zhang LX, Chen JP, Nguyen VA, White KL, Jr, Gao B. Differential STAT5 activation and phenotypic marker expression by immune cells following low levels of ethanol consumption in mice. Immunopharmacol Immunotoxicol. 2002;24:121–38. doi: 10.1081/iph-120003408. [DOI] [PubMed] [Google Scholar]
- Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmount E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to Toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–32. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Happel KI, Odden AR, Zhang P, Shellito JE, Bagby GJ, Nelson S. Acute alcohol intoxication suppresses the Interleukin 23 response to Klebsiella pneumonia infection. Alcohol Clin Exp Res. 2006;30:1200–1207. doi: 10.1111/j.1530-0277.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41:325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz R, Waltenbaugh C. Ethanol consumption modifies dendritic cell antigen presentation in mice. Alcohol Clin Exp Res. 2007;31:1739–1771. doi: 10.1111/j.1530-0277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Hill D, Marsano L, McClain C. Increased plasma IL-8 concentrations in alcoholic hepatitis. J Hepatol. 1993;24:377–384. [PubMed] [Google Scholar]
- Howard-Jones RN. Koch and the cholera vibrio: a centenary. Br Med J. 1984;288:379–381. doi: 10.1136/bmj.288.6414.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwarson S, Larsson S. Outcome of Listeria monocytogenes infection in compromised and non-compromised adults; a comparative study of seventy-two cases. Infection. 1979;7:54–56. doi: 10.1007/BF01641612. [DOI] [PubMed] [Google Scholar]
- Jacobson JM. Alcoholism and tuberculosis. Alcohol Health Res World. 1992;16:39–45. [Google Scholar]
- Jaruga B, Hong F, Kim WH, Sun R, Fan S, Gao B. Chronic alcohol consumption accelerates liver injury in T cell-mediated hepatitis: alcohol disregulation of NF-kappaB and STAT3 signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2004;287:G471–G479. doi: 10.1152/ajpgi.00018.2004. [DOI] [PubMed] [Google Scholar]
- Jayasinghe R, Gianutsos G, Hubbard AK. Ethanol-induced suppression of cell-mediated immunity in the mouse. Alcohol Clin Exp Res. 1992;16:331–335. doi: 10.1111/j.1530-0277.1992.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells TR, Smith W, Eckardt MJ. Murine model of ethanol-induced immunosuppression. Alcohol Clin Exp Res. 1990;14:546–550. doi: 10.1111/j.1530-0277.1990.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Weinberg J. Influence of ethanol consumption on immune competence of adult animals exposesd to ethanol in utero. Alcohol Clin Exp Res. 1998;22:391–400. [PubMed] [Google Scholar]
- Jerrells TR. Role of activated CD8+ T cells in the initiation and continuation of hepatic damage. Alcohol. 2002a;27:47–52. doi: 10.1016/s0741-8329(02)00210-0. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Mitchell K, Pavlik J, Jerrells J, Hoerman Influence of ethanol consumption on experimental viral hepatitis. Alcohol Clin Exp Res. 2002b;6:1734–1746. doi: 10.1097/01.ALC.0000037138.62811.9E. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Vidlak D, Strachota JM. Alcoholic pancreatitis: mechanisms of viral infections as cofactors in the development of acute and chronic pancreatitis and fibrosis. J Leukoc Biol. 2007a;81:430–439. doi: 10.1189/jlb.1004622. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Pavlik JA, DeVasure J, Vidlak D, Costello A, Strachota JM, Wyatt TA. Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory synctial virus in mice. Alcohol. 2007b;41:357–369. doi: 10.1016/j.alcohol.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, Oberg H. Regulation of regulatory T cells: role of dendritic cells and toll-like receptors. Crit Rev Immunol. 2006;26:291–306. doi: 10.1615/critrevimmunol.v26.i4.10. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira A. Regulation of dendritic cell function through TLRs. Curr Mol Med. 2003;3:373–85. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Udaltsova N. Alcohol drinking and total mortality risk. Ann Epidemiol. 2007:S63–S67. [Google Scholar]
- Kishore R, McMullen MR, Cocuzzi E, Nagy LE. Lipopolysaccharide-mediated signal transduction: Stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp Hepatol. 2004;1:S31. doi: 10.1186/1476-5926-2-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin CA, Vidins E, Blendis LM, Soloninka CA. Autoantibodies in alcoholic liver disease. Am J Med. 1990;89:129–33. doi: 10.1016/0002-9343(90)90288-o. [DOI] [PubMed] [Google Scholar]
- Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79:911–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- Lefevre F, O’Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J. Alcohol consumption among HIV-infected patients. J Gen Intern Med. 1995;10:458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- Li F, Cook RT, Alber C, Rasmussen W, Stapleton JT, Ballas ZK. Ethanol and natural killer cells. II. Stimulation of human killer activity by ethanol in vitro. Alcohol Clin Exp Res. 1997;21:981–987. [PubMed] [Google Scholar]
- Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication increases interleukin-18-mediated neutrophil infilitration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1193–L1201. doi: 10.1152/ajplung.00408.2006. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Gluckman SJ, Senior JR. Granulocyte function and levels of immunoglobulins and complement in patients admitted for withdrawal from alcohol. J Infect Dis. 1978;138:747–755. doi: 10.1093/infdis/138.6.747. [DOI] [PubMed] [Google Scholar]
- MacGregor RR. Alcohol and immune defense. JAMA. 1986;256:1474–1479. [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Inhibition of LPS-mediated NF-kB activation by ethanol in human monocytes. Int Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function by alcohol correlates with reduced CD80/CD86 expression and decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Jeliazkova V, Catalano D, Szabo G. Acute alcohol exposure exerts anti-inflammatory effects by inhibiting IκB kinase activity and p65 phosphorylation in human monocytes. J Immunol. 2007;178:7686–7693. doi: 10.4049/jimmunol.178.12.7686. [DOI] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Zhang P, Nelson S. Alcohol exacerbates murine pulmonary tuberculosis. Infect Immun. 2004;72:2556–25563. doi: 10.1128/IAI.72.5.2556-2563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain C, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Disease. 1999;19:205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- McCullough AJ, O’Connor JF, Barry Alcoholic liver disease: proposed recommendations for the American College of Gastroentrology. Am J Gastroenterol. 1998;93:2022–2036. doi: 10.1111/j.1572-0241.1998.00587.x. [DOI] [PubMed] [Google Scholar]
- McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun. 2006;74:6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Messingham K, Faunce D, Kovacs E. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic Alcohol Consumption Increases the Severity of Murine Influenza Virus Infections. J Immunol. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- Minagawa M, Deng Q, Liu Z-H, Tsukamoto H, Dennert G. Activated natural killer T cells induce liver injury by Fas and tumor necrosis factor-α during alcohol consumption. Gastroenterology. 2004;126:1367–1399. doi: 10.1053/j.gastro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Molina PE, McNurtan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, Bohm RP, Zhang P, Bagby GJ, Nelson S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res. 2006;30:2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Moran A, Harbour DV, Teeter LD, Musser JM, Graviss EA. Is alcohol use associated with cavitary disease in tuberculosis? Alcohol Clin Exp Res. 2007;31:33–38. doi: 10.1111/j.1530-0277.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- Mukamal K, Maclure M, Muller J, Scherwood J, Mittleman M. Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285:1965–1970. doi: 10.1001/jama.285.15.1965. [DOI] [PubMed] [Google Scholar]
- Mukamal K. Alcohol intake noncoronary cardiovascular disease. Ann Epidemiol. 2007:S8–S15. doi: 10.1016/j.annepidem.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med. 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Alcohol: Methods and Protocols. Humana Press; New Jersey: 2008. [Google Scholar]
- Nanji AA, Su G, Laposta M, French S. Pathogenesis of alcoholic liver disease: recent advances. Alcohol Clin Exp Res. 2002;26:731–736. [PubMed] [Google Scholar]
- Navarini AA, Recher M, Lang KS, Georgiev P, Meury S, Bergthaler A, Flatz L, Bille J, Landmann R, Odermatt B, Hengartner H, Zinkernagel RM. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc Nat Acad Sci USA. 2006;103:155535–15539. doi: 10.1073/pnas.0607325103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Bagby G, Summer WR. Alcohol suppresses lipopolysaccharide-induced tumor necrosis factor activity in serum and lung. Life Sci. 1989;44:673–676. doi: 10.1016/0024-3205(89)90472-4. [DOI] [PubMed] [Google Scholar]
- Nelson S, Summer W, Bagby G, Nakamura C, Stewart L, Lipscomb G, Andresen J. Granulocyte colony stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. 1991;164:901–906. doi: 10.1093/infdis/164.5.901. [DOI] [PubMed] [Google Scholar]
- Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, Schlueter AJ. Chronic ethanol consumption decreases murine langerhans cell numbers and delays migration of langerhans cells as well as dermal dendritic cells. Alcohol Clin Exp Res. 2008;32:1–12. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR-2 and TLR-4 mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–35. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- O’Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- Osler W. The principles and practice of medicine. D. Appleton and Co; New York, NY: 1902. p. 109. [Google Scholar]
- Padgett EL, Sibley DA, Jerrells TR. Effect of adrenalectomy on ethanol-associated changes in lymphocyte cell numbers and subpopulaitons in thymus, spleen, and gut-associated lymphoid tissues. Int J Immunopharmacol. 2000;22:285–298. doi: 10.1016/s0192-0561(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Pan HN, Sun R, Jaruga B, Hong F, Kim WH, Gao B. Chronic ethanol consumption inhibits hepatic natural killer cell activity and accelerates murine cytomegalovirus-induced hepatitis. Alcohol Clin Exp Res. 2006;30:1615–1623. doi: 10.1111/j.1530-0277.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Pandol SJ, Raraty M. Pathobiology of alcoholic pancreatitis. 2007;7:105–114. doi: 10.1159/000104235. [DOI] [PubMed] [Google Scholar]
- Pavia CS, LaMothe M, Kavanagh Influence of alcohol on antimicrobial immunity. Biomed Pharmacother. 2004;58:84–89. doi: 10.1016/j.biopha.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Plackett TP, Kovacs EJ. In: Acute models of ethanol exposure to mice, in Alcohol, Methods and Protocols. Nagy LE, editor. Humana Press; New Jersey: 2008. pp. 3–9. [DOI] [PubMed] [Google Scholar]
- Plumlee CR, Lazaro CA, Fausto N, Polyak SJ. Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells. Virol J. 2005;2:89. doi: 10.1186/1743-422X-2-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poikolainen K. Alcohol and mortality: a review. J Clin Epidemiol. 1995;48:455–465. doi: 10.1016/0895-4356(94)00174-o. [DOI] [PubMed] [Google Scholar]
- Pol S, Artru P, Thépot V, Berthelot P, Nalpas B. Improvement of the CD4 cell count after alcohol withdrawal in HIV-positive alcoholic patients. AIDS. 1996;10:1293–4. doi: 10.1097/00002030-199609000-00019. [DOI] [PubMed] [Google Scholar]
- Pritchard MT, Nagy LE. Ethanol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res. 2005;29:146S–150S. doi: 10.1097/01.alc.0000189286.81943.51. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004a;33:147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Acute exposure to ethanol affects Toll-like receptor signaling and subsequent responses: an overview of recent studies. Alcohol. 2004b;33:235–9. doi: 10.1016/j.alcohol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004c;173:2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan P, Zheng Q, Carlton Schwab Differences in IL-10 and IL-12 production patterns and differences in the effects of acute ethanol treatment on macrophages in vivo and in vitro. Alcohol. 2005;37:1–8. doi: 10.1016/j.alcohol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Moats C. Alcohol and coronary heart disease: drinking patterns and mediators of effect. Ann Epidemiol. 2007;17:S3–S7. [Google Scholar]
- Ristow SS, Starkey JR, Hass GM. Inhibition of natural killer cell activity in vitro by alcohol. Biochem Biophys Res Commun. 1982;105:1315–1321. doi: 10.1016/0006-291x(82)90930-5. [DOI] [PubMed] [Google Scholar]
- Romeo J, Warnberg J, Nova E, Diaz LE, Gonzalez-Gross M, Marcos A. Changes in the immune system after moderate beer consumption. Ann Nutr Metab. 2007a;51:359–366. doi: 10.1159/000107679. [DOI] [PubMed] [Google Scholar]
- Romeo J, Warnberg J, Nova E, Diaz LE, Gomez S, Marcos A. Moderate alcohol consumption and the immune system. A review Brit J Nutr. 2007b;98:S111–S115. doi: 10.1017/S0007114507838049. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis: an inflammatory disease. N Eng J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rush B. An inquiry into the effects of ardent spirits upon the human body and mind. Q J Stud Alcohol. 1943;4:321–341. [Google Scholar]
- Saad AJ, Domiati-Saad R, Jerrells TR. Ethanol ingestion increases susceptibility of mice to Listeria monocytogenes. Alcohol Clin Exp Res. 1993;17:75–85. doi: 10.1111/j.1530-0277.1993.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Sachs CW, Christensen RH, Pratt PC, Lynn WS. Neutrophil elastase activity and superoxide production are diminished in neutrophils of alcoholics. Am Rev Respir Dis. 1990;141:1249–1255. doi: 10.1164/ajrccm/141.5_Pt_1.1249. [DOI] [PubMed] [Google Scholar]
- Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997;157:1446–1457. [PubMed] [Google Scholar]
- Salerno JA, Waltenbaugh C, Cianciotto NP. Ethanol consumption and the susceptibility of mice to Listeria monocytogenes infection. Alcohol Clin Exp Res. 2001;25:464–72. [PubMed] [Google Scholar]
- Sander M, von Heymann C, Neumann T, Braun JP, Kastrup M, Beholz S, Konertz W, Spies CD. Increased interleukin-10 and cortisol in long-term alcoholics after cardiopulmonary bypass: a hint to the increased postoperative infection rate? Alcohol Clin Exp Res. 2005;29:1677–1684. doi: 10.1097/01.alc.0000179365.58403.b2. [DOI] [PubMed] [Google Scholar]
- Schmidt W, De Lint J. Causes of death of alcoholics. Q J Stud Alcohol. 1972;23:171–185. [PubMed] [Google Scholar]
- Sibley DA, Osna N, Kusynski C, Wilkie L, Jerrells TR. Alcohol consumption is associated with alterations in macrophage responses to interferon-gamma and infection by Salmonella typhimurium. FEMS Immunol Med Microbiol. 2001;32:73–83. doi: 10.1111/j.1574-695X.2001.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Sibley D, Jerrells TR. Alcohol consumtion by CS7BL/6 mice is associated with depletion of lymphoid tissues and altered resistance to oral infection with Salmonella typhinurium. J Infect Dis. 2000;182:482–489. doi: 10.1086/315728. [DOI] [PubMed] [Google Scholar]
- Slukvin II, Boor PJ, Jerrells TR. Initiation of alcoholic fatty liver and hepatic inflammation with a specific recall immune response in alcohol-consuming C57BL/6 mice. Clin Exp Immunol. 2001;125:123–133. doi: 10.1046/j.1365-2249.2001.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Spies CD, Lanzke N, Schlichting U, Muenhlbauer S, Pipolo C, vonMettenhein M, Lehmann A, Morawietz L, Nattermann H, Sander M. Effects of ethanol on cytokine production after surgery in a murine model of gram-negative pneumonia. Alcohol Clin Exp Res. 2008;32:331–338. doi: 10.1111/j.1530-0277.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Stinson FS, DeBakey SF. Prevalence of alcohol problems amongselected AIDS risk groups: United States,1988. Addiction. 1993;88:1139–1147. doi: 10.1111/j.1360-0443.1993.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Verma B, Isaac A, Catalano D. Acute Ethanol Consumption Synergizes with Trauma to Increase Monocyte TNFα Production Late Post-injury. J Clin Immunol. 1994;14:340–352. doi: 10.1007/BF01546318. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T cell proliferation and Monocyte IL-1ß, TNF, and IL-6 production by acute ethanol treatment. J Leukoc Biol. 1995;58:342–350. doi: 10.1002/jlb.58.3.342. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Newman L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: Decreased TNFα, IL-1ß and elevated IL-10, and TGFß production. Alcohol Clin Exp Res. 1996;14:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defense. Alcohol Alcoholism. 1999;634:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szabo G, Chavan S, Mandrekar P, Catalano D. Acute alcohol consumption attenuates chemokine induction in response to ex vivo stimulation. J Clin Immunol. 1999;19:67–76. doi: 10.1023/a:1020518703050. [DOI] [PubMed] [Google Scholar]
- Szabo G. Pathogenic interactions between alcohol and hepatitis C. Curr Gastroenterol Rep. 2003;5:86. doi: 10.1007/s11894-003-0014-x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Catalano D, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res. 2004;28:824–28. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- Szabo G, Aloman C, Polyak SJ, Weinman SA, Wands J, Zakhari S. Hepatitis C Infection and Alcohol Use: A Dangerous Mix for the Liver and Antiviral Immunity. Alcohol Clin Exp Res. 2006a;30:709. doi: 10.1111/j.1530-0277.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Mandrekar P. Pattern Recognition Receptors: A Contemporary View on Liver Diseases. Hepatology. 2006b;44:287–98. doi: 10.1002/hep.21308. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. In: Human monocytes, macrophages, and dendritic cells: Alcohol treatment methods, in Alcohol, Methods and Protocols. Nagy LE, editor. Humana Press; New Jersey: 2008. pp. 113–124. [DOI] [PubMed] [Google Scholar]
- Szabo G, Marshal C. Hepatitis C and Alcohol. Up-TO-Date. 2008 in press. [Google Scholar]
- Takeuchi O, Akira S. Signaling pathways activated by microorganisms. Curr Opin Cell Biol. 2007a;19:185–191. doi: 10.1016/j.ceb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007b;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Tamura DY, Moore EE, Patrick DA, Johnson JL, Offner PJ, Harbeck RJ, Silliman CC. Clinically relevant concentrations of ethanol attenuate primed neutrophil bacterial activity. J Trauma. 1998;44:320–324. doi: 10.1097/00005373-199802000-00015. [DOI] [PubMed] [Google Scholar]
- Tennesen H, Kaisser H, Nielsen BB, Pedersen AE. Reversibility of alcohol-induced immune depression. Br J Addict. 1992;87:1025–1028. doi: 10.1111/j.1360-0443.1992.tb03119.x. [DOI] [PubMed] [Google Scholar]
- Thurman RG. Mechanisms of hepatic toxicity. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G613. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Mkrtchyan H, Dynnyk A. In: Intragastric ethanol infusion model in rodents, in Alcohol, Methods and Protocols. Nagy LE, editor. Humana Press; New Jersey: 2008. pp. 33–48. [DOI] [PubMed] [Google Scholar]
- Tuma DJ. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic Biol Med. 2002;32:303–308. doi: 10.1016/s0891-5849(01)00742-0. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans. 6. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- Vainas T, Stassen F, Bruggerman C, Welten R, Pena A, Morre S. Synergistic effect of TLR4 and CD14 polymorphysms on the total atherosclerosis burden in patients with peripheral arterial disease. J Vasc Surg. 2006;44:326–32. doi: 10.1016/j.jvs.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Velasquez A, Bechara RI, Lewis JF, Malloy J, McCaig L, Brown LA, Guidot DM. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- Verma BK, Fogarasi M, Szabo G. Down-regulation of TNFα activity by acute ethanol treatment in human peripheral blood monocytes. J Clin Immunol. 1993;13:8–22. doi: 10.1007/BF00920631. [DOI] [PubMed] [Google Scholar]
- Vidali M, Stewart SF, Albano E. Interplay between oxidative stress and immunity in the progression of alcohol-mediated liver injury. Trends Mol Med. 2008;14:63–71. doi: 10.1016/j.molmed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Winkler C, Wirleitner B, Schroecksnadel K, Schennach H, Fuchs D. Int Immunopharmacol. 2006;6:390–395. doi: 10.1016/j.intimp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Xie M, Stoltz DA, Summer WR, Nelson S. Acute ethanol intoxication inhibits neutrophil beta2-integrin expression in rats during endotoxemia. Alcohol Clin Exp Res. 1998;22:135–141. [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Stolz DA, Summer WR, Nelson S. Granulocyte colony-stimulating factor modulates the pulmonary host response to endotoxin in the absence and presence of acute ethanol intoxication. J Infect Dis. 1999;179:1441–1448. doi: 10.1086/314763. [DOI] [PubMed] [Google Scholar]
- Zhao XJ, Marrero L, Song K, Oliver P, Chin SY, Simon H, Schurr JR, Zhang Z, Thoppil D, Lee S, Nelson S, Kolls JK. Acute alcohol inhibits TNF-alpha processing in human monocytes by inhibiting TNF/TNF-alpha-converting enzyme interactions in the cell membrane. 2003;170:2923–2931. doi: 10.4049/jimmunol.170.6.2923. [DOI] [PubMed] [Google Scholar]
- Zhou J, Meadows GG. Alcohol consumption decreases IL-2-induced NF-kappaB activity in enriched NK cells from C57BL/6 mice. Toxicol Sci. 2003;73:72–9. doi: 10.1093/toxsci/kfg047. [DOI] [PubMed] [Google Scholar]
- Zhu X, Coleman RA, Alber C, Ballas ZK, Waldschmidt TJ, Ray NB, Krieg AM, Cook RT. Chronic ethanol ingestion by mice increases expression of CD80 and CD86 by activated macrophages. Alcohol. 2004;32:91–100. doi: 10.1016/j.alcohol.2004.01.004. [DOI] [PubMed] [Google Scholar]


