Abstract
Purpose
To present and evaluate a new method of estimating rates of retinal ganglion cell (RGC) loss in glaucoma by combining structural and functional measurements.
Design
Observational cohort study
Methods
The study included 213 eyes of 213 glaucoma patients followed for an average of 4.5±0.8 years with standard automated perimetry (SAP) visual fields and optical coherence tomography (OCT). A control group of 33 eyes of 33 glaucoma patients had repeated tests over a short period of time to test the specificity of the method. An additional group of 52 eyes from 52 healthy subjects followed for an average of 4.0±0.7 years was used to estimate age-related losses of RGCs. Estimates of RGC counts were obtained from SAP and OCT and a weighted average was used to obtain a final estimate of the number of RGCs for each eye. The rate of RGC loss was calculated for each eye using linear regression. Progression was defined by a statistically significant slope faster than the age-expected loss of RGCs.
Results
From the 213 eyes, 47 (22.1%) showed rates of RGC loss that were faster than the age-expected decline. A larger proportion of glaucomatous eyes showed progression based on rates of RGC loss than based on isolated parameters from SAP (8.5%) or OCT (14.6%; P<0.01), while maintaining similar specificities in the stable group.
Conclusion
The rate of RGC loss estimated from combining structure and function performed better than either isolated structural or functional measures for detecting progressive glaucomatous damage.
INTRODUCTION
Glaucoma is an optic neuropathy characterized by progressive neuroretinal rim thinning, excavation of the optic nerve head, and loss of the retinal nerve fibers.1 These structural changes are usually accompanied by functional losses, which may ultimately result in significant decrease in vision-related quality of life. Although both the characteristic structural and functional changes seen in the disease are ultimately related to the pathological loss of retinal ganglion cell (RGC) somas and axons, the measurements of structural and functional change are somewhat variable and have an imperfect relationship to one another, both for recognizing damage and for detecting disease progression over time. Standard automated perimetry (SAP) remains the usual method for monitoring functional changes in the disease. However, patients may present structural changes in the optic nerve or retinal nerve fiber layer (RNFL) before changes are detected with SAP.2–10 On the other hand, several patients show evidence of functional deterioration without measurable changes in currently available structural tests.5,6,11
The imperfect relationship between structural and functional measurements of the disease seem to be largely derived from the different algorithms and measurement scales, as well as the different variability characteristics of the tests commonly used to assess structural and functional losses. In fact, Harwerth and colleagues12 demonstrated that structural and functional tests are in agreement as long as one uses appropriate measurement scales for neural and sensitivity losses and considers factors such as the effect of aging and eccentricity on estimates of neural losses. In a series of investigations, they demonstrated that estimates of RGC losses obtained from clinical perimetry agreed closely with estimates of RGC losses obtained from RNFL assessment by optical coherence tomography (OCT).12 The results of their model provided a common domain for expressing results of structural and functional tests, i.e., the estimates of RGC losses, opening the possibility of combining these different tests to improve the reliability and accuracy of estimates of the amount of neural losses in glaucoma.
In the current study, we combine measurements of structural and functional tests to provide an estimate of the rate of RGC loss in glaucoma patients followed over time. We show that the calculated estimates of the rate of RGC loss performed significantly better than isolated measures of structure or of function to detect disease progression over time.
METHODS
This was an observational study. Participants from this study were included in two prospective longitudinal studies designed to evaluate optic nerve structure and visual function in glaucoma (the African Descent and Glaucoma Evaluation Study [ADAGES] and the Diagnostic Innovations in Glaucoma Study [DIGS]). The 3-site ADAGES collaboration includes the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California-San Diego (UCSD) (data coordinating center), the New York Eye and Ear Infirmary and the Department of Ophthalmology, University of Alabama, Birmingham (UAB). Although the DIGS includes only patients recruited at UCSD, the protocols of the two studies are identical. Methodological details have been described previously.13
At each visit during follow-up, subjects underwent a comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, gonioscopy, dilated fundoscopic examination, stereoscopic optic disc photography, and automated perimetry using Swedish Interactive Threshold Algorithm (SITA Standard 24-2). Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented with a best-corrected visual acuity less than 20/40, spherical refraction outside ± 5.0 diopters and/or cylinder correction outside 3.0 diopters, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
Participants
The study included 3 groups of participants. The main study group was composed of 213 eyes of 213 glaucoma patients from the DIGS/ADAGES cohort followed for an average of 4.5 ± 0.8 years. Eyes were classified as glaucomatous if they had evidence of glaucomatous optic neuropathy based on masked grading of optic disc stereophotographs and/or repeatable abnormal visual field test results on the baseline visit. Glaucomatous optic neuropathy was diagnosed based on the presence of neuroretinal rim thinning, excavation or retinal nerve fiber layer defects. Abnormal visual field was defined as a pattern standard deviation (PSD) outside of the 95% normal confidence limits, or a Glaucoma Hemifield Test result outside normal limits. All eyes were followed at approximately annual intervals with SAP and OCT testing and were required to have a minimum of 5 SAP and 5 OCTs during follow-up.
A control group of 33 eyes from 33 stable glaucoma patients was used to evaluate the specificity of our method. This set consisted of eyes with 5 serial visual fields and OCT exams collected under an IRB approved protocol within a maximum period of eight weeks from individuals seen at the Department of Ophthalmology, University of Miami Miller School of Medicine. All participating subjects were fully informed, and each signed a consent form. All participants had previous experience with visual field testing. Each eye also had to have evidence of glaucoma at baseline based on ocular examination and the presence of repeated visual field loss as defined above. Mean MD and PSD values at the first visit were −7.4dB and 8.4dB. There was a wide range of disease severity in these eyes, with MD values ranging from −30.43dB to 0.91dB. The assumption was made that the disease was not progressing in these eyes over such a short time, and that any change noted would be due to the variability in the visual fields or OCT measurements in stable glaucoma. Therefore, the order of testing would be exchangeable and a permutation technique was used to provide a larger dataset to evaluate specificity. We generated all possible permutations of the order of the tests so that 3960 different sequences were obtained. For evaluation of rates of change in these eyes, the visits were annualized.
An additional group of 52 eyes from 52 healthy subjects followed for an average of 4.0 ± 0.7 years was used to evaluate the effect of aging on the rate of RGC loss. All eyes were followed at approximately annual intervals with SAP and OCT testing and had an average of 4.4 ± 0.6 tests acquired during follow-up. These subjects were recruited from the general population and were required to have a normal ophthalmologic examination, IOP below 22mmHg in both eyes and normal visual field tests. Normal visual fields were defined as MD and PSD with P greater than 0.05 and GHT within normal limits.
Visual Field Testing
All patients underwent SAP testing using SITA-standard 24-2 strategy less than 6 months apart from imaging. All visual fields were evaluated by the UCSD Visual Field Assessment Center (VisFACT).14 Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors were excluded. The only exception was the inclusion of visual fields with false-negative errors of more than 33% when the field showed advanced disease (MD lower than −12dB).15 Visual fields exhibiting a learning effect (i.e., initial tests showing consistent improvement on visual field indexes) were also excluded. Visual fields were further reviewed for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia), and inattention. The VisFACT requested repeats of unreliable visual field test results, and these were obtained whenever possible.
Optical Coherence Tomography
Subjects underwent ocular imaging with dilated pupils using the optical coherence tomograph StratusOCT™ (Carl Zeiss Meditec, Dublin, CA).16 Quality assessment of Stratus OCT scans was evaluated by an experienced examiner masked to the subject’s results of the other tests. Good quality scans had to have focused images from the ocular fundus, signal strength greater than 7 and presence of a centered circular ring around the optic disc. The fast RNFL algorithm was used to obtain RNFL thickness measurements with Stratus OCT. Three images were acquired from each subject, with each image consisting of 256 A-scans along a 3.4mm-diameter circular ring around the optic disc. The average parapapillary RNFL thickness (360° measure) was automatically calculated by the software and used in the study. RNFL scans were also evaluated as to the adequacy of the algorithm for detection of the RNFL. Only scans without overt algorithm failure in detecting the retinal borders were included in the study.
Combined Structure and Function Estimate of RGC Counts
The development of the combined structure and function estimate of RGC counts was based on previous work by Harwerth and colleagues4,12 on the development and validation of a model linking structure and function in glaucoma. Based on experimental studies in monkeys, the authors first derived an empirical model relating sensitivity measurements in SAP to histological RGC counts as a function of retinal eccentricities. The experimental results were then translated to clinical perimetry in humans. The following formulas were proposed to estimate the number of RGC somas in an area of the retina corresponding to a specific SAP test field location at eccentricity ec with sensitivity s in dB:
In the above formulas, m and b represent the slope and intercept, respectively, of the linear function relating ganglion cell quantity (gc) in dB to the visual field sensitivity (s) in dB at a given eccentricity. To account for the total number of ganglion cells in an area of the retina, the cell density derived from each perimetry measurement was considered to be uniform over an area of retina corresponding to an area of 6×6 degrees of visual space that separates test locations in SAP. By applying the above formulas, a SAP-derived estimate of the total number of RGCs (SAPrgc) was obtained by adding the estimates from all locations in the visual field. The structural part of the model consisted in estimating the number of RGC axons from RNFL thickness measurements obtained by optical coherence tomography. The model took into account the effect of aging in the axonal density and the effect of disease severity on the relationship between the neuronal and non-neuronal components of the RNFL thickness estimates obtained by OCT. To derive the total number of RGC axons from the global RNFL thickness measurement obtained by OCT (OCTrgc), we applied the following formulas:
In the above formulas, d corresponds to the axonal density (axons/μm2) and c is a correction factor for the severity of disease to take into account remodeling of the RNFL axonal and non-axonal composition. The average RNFL thickness corresponds to the 360° measure automatically calculated by the OCT software. These calculations provide an estimate of the number of RGCs from two sources, one functional and one structural, and a strong relationship was demonstrated between the two estimates in external validation cohorts.12 However, although Harwerth et al12 proposed a model linking structure and function, no attempt was made to obtain a combined estimate derived from structural and functional tests that could be clinically used to stage glaucoma severity and detect change over time. We developed such a combined measure by averaging the estimates of RGC numbers obtained from SAP and from OCT, but weighting according to severity of disease. Because clinical perimetry and imaging tests accuracies are inversely related to disease severity, we used a weighted scale that combined the estimates of RGC numbers from both tests:
The weights were chosen to reflect the inverse relationship with disease severity of SAP and OCT estimates, along the scale of MD values ranging from 0 to −30dB. Therefore, in early disease, the OCT-derived RGC estimates will have greater weight than those obtained by SAP. In contrast, in advanced disease, SAP estimates will carry greater weight than those obtained from OCT.
After the combined estimates of RGC number were obtained, a linear mixed effects model17 was run to evaluate the effect of aging on RGC loss in the 52 healthy eyes followed longitudinally. The purpose was to calculate the effect of normal aging on the rate of RGC loss so that glaucomatous progression would be considered to occur if the rate of RGC loss was greater than the expected age-related loss. The linear mixed effects model showed a significant effect of age on the number of RGCs over time with a loss of 7877 RGCs per 1 year older (P<0.001). For each eye, we obtained the slope of change using ordinary least squares (OLS) linear regression of the combined RGC counts over time. An eye was considered to have progressed if the slope of RGC loss was significantly faster than the age-expected decline of RGC counts with P<0.05.
Slopes were also calculated for the raw values of OCT average thickness and for the SAP visual field index (VFI) provided by the Humphrey perimeter (Carl-Zeiss Meditec, Inc., Dublin, CA). 18 The VFI represents the percent of normal age-corrected visual function and is the method currently used for calculating rates of progression in the Humphrey visual field printout. Details of the calculation of the VFI have been described elsewhere. 18 The VFI can range from 100% (normal visual field) to 0% (perimetrically blind field). Progression by OCT average thickness or by VFI was defined based on the presence of a statistically significant negative slope with P<0.05.
All statistical analyses were performed with commercially available software (Stata version 12; StataCorp, College Station, TX). Cluster-correlated robust estimates of variance were used to adjust for correlated data when necessary.19 The alpha level (type I error) was set at 0.05.
RESULTS
The main study group was composed of 213 eyes with mean age of 60 ± 11 years at baseline. Average MD and PSD values of the baseline visual field test were −2.51dB and 3.34dB. Average baseline RNFL thickness was 88μm (±15μm). These eyes had a wide range of disease severities at baseline with MD values ranging from −20.1dB to 2.14dB. A median number of 5 pairs of SAP and OCT tests were available during follow-up for these eyes, ranging from 5 to 8.
There was a strong correlation between RGC estimates obtained from SAP and OCT data for all exams from the 213 eyes included in the study group (r = 0.80; P<0.001) (Figure 1). Figure 2 shows a histogram of calculated RGC numbers combining structural and functional tests at the baseline visit for these eyes. The mean number of RGCs was 765745 (± 270029) at baseline which was significantly lower than the mean number of RGCs in the 52 healthy eyes (1123504 ± 172667; P<0.001).
Figure 1.
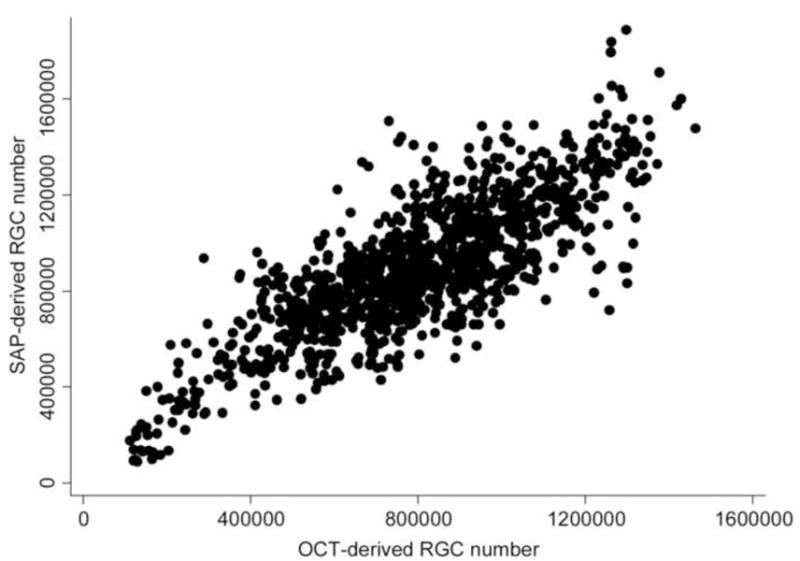
Scatterplot illustrating the relationship between estimates of the number of retinal ganglion cells (RGC) obtained by standard automated perimetry (SAP) and optical coherence tomography (OCT).
Figure 2.
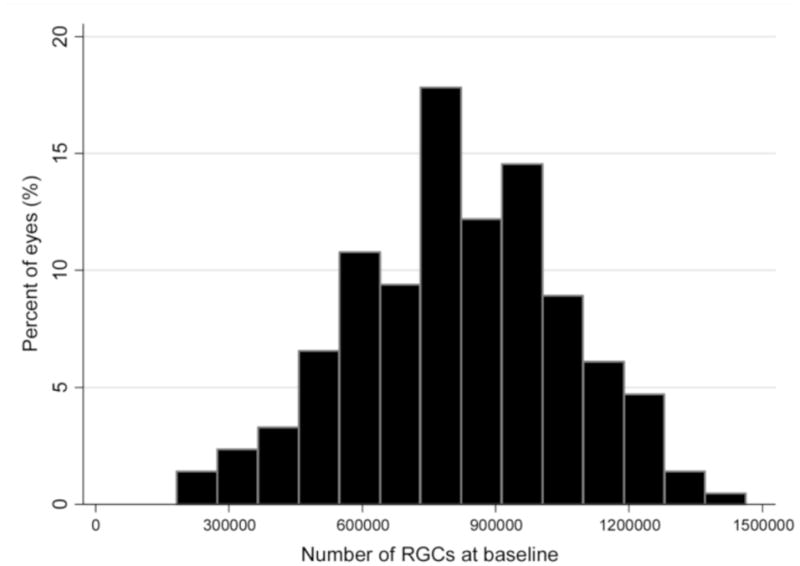
Histogram of the estimates of baseline retinal ganglion cell (RGC) number combining structure and function measurements in the 213 eyes of the study group.
From the 213 eyes, 47 (22.1%) showed statistically significant rates of RGC loss that were faster than the age-expected decline. The mean rate of RGC loss in these eyes was −33369 cells/year (range: −8332 cells/year to −80636 cells/year). There was no statistically significant difference between mean baseline RGC counts for progressing versus non-progressing eyes (797229 vs. 758527; P=0.377). We estimated a percent rate of RGC loss by dividing the calculated rate of RGC loss by the baseline RGC count. The mean percent rate of RGC loss was −4.4%/year for the 47 progressing eyes, ranging from −1.4%/year to −8.9%/year.
The VFI was able to detect progression in only 18 (8.5%) of the 213 eyes whereas the OCT parameter average RNFL thickness detected progression in 31 eyes (14.6%). Figure 3 shows a proportional Venn diagram with the number of eyes detected as progressing by each method. Figure 4 shows an example of an eye with significant rate of RGC loss which also progressed by VFI and OCT average thickness.
Figure 3.
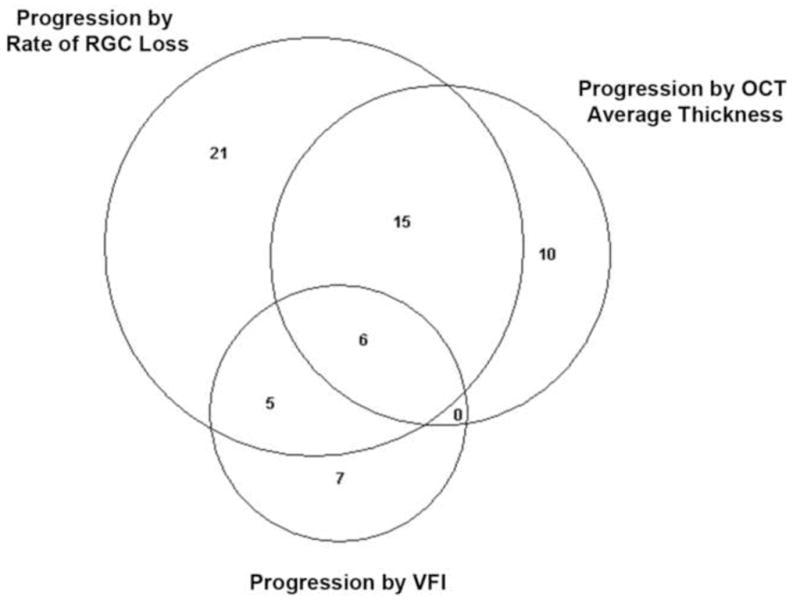
Proportional Venn diagram illustrating the number of eyes detected as progressing according to the rates of retinal ganglion cell (RGC) loss, optical coherence tomography (OCT) average thickness parameter and standard automated perimetry visual field index (VFI).
Figure 4.
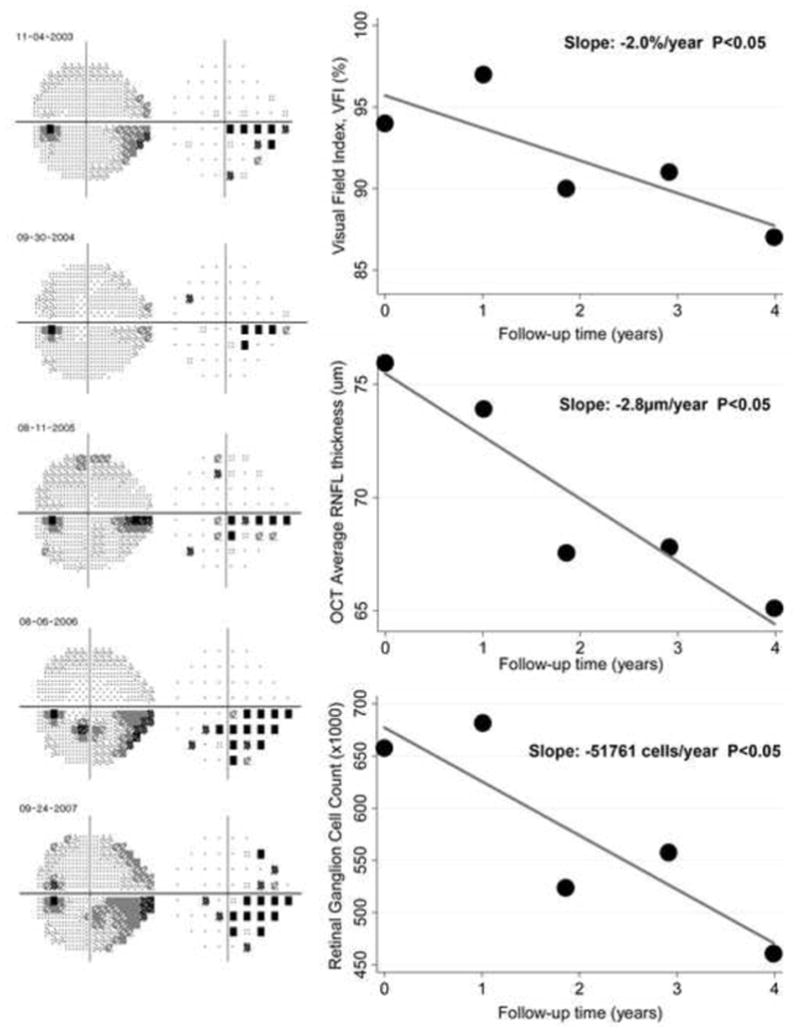
Eye detected as having progression during follow-up according to the rate of retinal ganglion cell (RGC) loss with a slope of −51761 cells/year (P<0.05). The eye also had progression according to the Visual Field Index (VFI) with slope of −2.0%/year and the optical coherence tomography parameter average thickness (slope of −2.8μm/year).
Thirty-six eyes had progression detected by the rate of RGC loss but not by the VFI. These eyes had a mean rate of RGC loss of −32310 cells/year. Seven eyes had progression detected by the VFI but not by the rate of RGC loss. These eyes had a rate of RGC loss of only −3393 cells/year. A comparison between these two groups also revealed that eyes progressing only by the rate of RGC loss had significantly faster rates of structural change than those progressing only by VFI as measured by OCT average RNFL thickness (−1.89μm/year versus 0.37μm/year, respectively; P=0.002). Figure 5 shows an example of an eye detected as progressing according to the estimated rate of RGC loss but not by the VFI.
Figure 5.
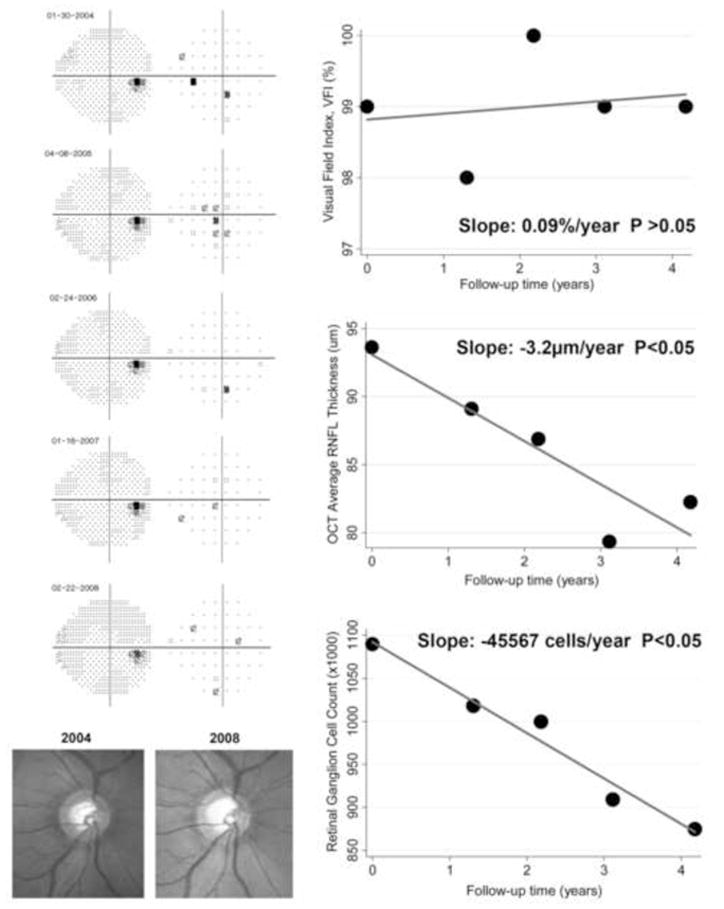
Eye detected as progressing by the rate of retinal ganglion cell loss with a slope of −45567 cells/year (P<0.05), but not by the Visual Field Index. The eye had early glaucomatous damage and showed progressive neuroretinal rim thinning as seen on the optic disc stereophotographs. The optical coherence tomography parameter average thickness showed a statistically significant slope of −3.2μm/year.
Twenty-six eyes had progression detected by the rate of RGC loss but not by OCT average thickness, whereas 10 eyes had progression detected by OCT average thickness but not by the rate of RGC loss. The former group had a mean rate of RGC loss of −32486 cells/year versus −7539 cells/year in the latter. A comparison between these two groups also revealed that eyes progressing only by the rate of RGC loss had significantly faster rates of functional change than those progressing only by OCT as measured by the VFI (−0.65%/year versus 0.55%/year; P = 0.003). Figure 6 shows an example of an eye detected as progressing according to the estimated rate of RGC loss but not by the OCT parameter average thickness.
Figure 6.
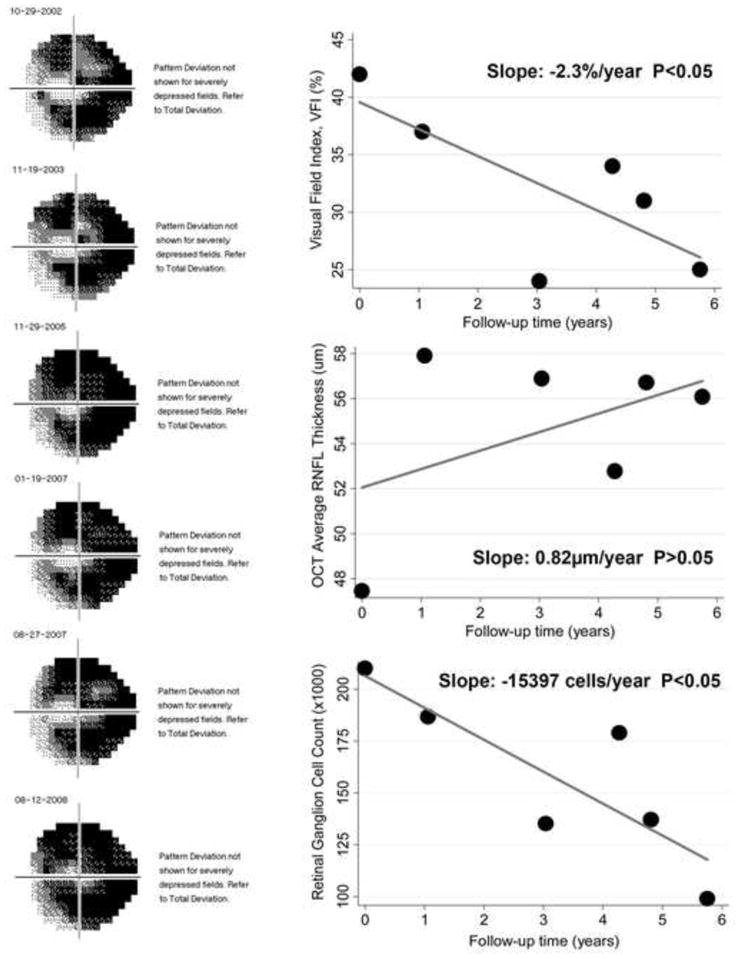
Eye detected as progressing by the rate of retinal ganglion cell (RGC) loss with a slope of −15397 cells/year (P<0.05), but not by the optical coherence tomography average thickness parameter. The eye had advanced visual field loss and a statistically significant slope of change with the Visual Field Index (−2.3%/year).
Evaluation of Specificity
Specificity for detection of change was evaluated in the 3960 sequences of tests generated from the 33 eyes of the stable data. The rate of RGC loss was statistically significant in 203 (5%) of the 3960 sequences resulting in specificity of 95%. The OCT parameter average thickness detected change in 199 sequences (specificity of 95%) and the VFI detected change in 174 sequences (specificity of 96%).
We compared the proportions of eyes from the main study group that were detected as progressing by each method at the matched specificities. The proportion progressing by rates of RGC loss was larger than that progressing only by OCT average thickness (22.1% vs. 14.6%; P=0.01) and by VFI (22.1% vs. 8.5%; P<0.001).
DISCUSSION
In the current study, we demonstrated that the evaluation of rates of neuronal loss based on estimates of RGC counts combining structure and function was able to detect a larger number of glaucomatous eyes as progressing compared to the use of isolated measures of SAP or OCT, while maintaining comparable specificity in a group of stable eyes. To our knowledge this is the first study to develop and evaluate the ability of a single measure of RGC count combining structure and function for detection of glaucoma progression.
Several studies have shown that considerable disagreement is present when different structural and functional tests are used to detect disease progression.7–9,11,20,21 More specifically, SAP seems to be relatively insensitive to detect change in early stages of the disease, whereas structural assessment by imaging instruments seem to perform relatively worse at advanced stages of damage. The disagreement between structure and function, however, seems to be largely derived from the different algorithms and measurement scales of the tests commonly used to assess losses. In fact, Harwerth and colleagues12 demonstrated a strong correlation between structural and functional tests when appropriate measurement scales for neural and sensitivity losses were used. Our present results agree with those previously published by Harwerth et al, as shown by the strong linear relationship between RGC estimates obtained from SAP and OCT data. The linear relationship suggests that the lack of sensitivity of SAP for detection of progression in early disease is most likely not the result of true structural changes occurring in the absence of functional losses, but is rather related to the logarithmic scale used for SAP sensitivity measurements. Such result has also been suggested by other authors.3,22 The logarithmic scale compresses the range of losses in early stages of the disease while expanding the range in later stages. In principle, this could suggest that a simple linearization of SAP data could improve detection of early losses. However, this is usually not the case.23 As SAP data is originally acquired using staircase procedures based on a logarithmic scale (dB), SAP is not good at estimating small amounts of ganglion cell losses at early stages of the disease. In contrast, by expanding the range of the scale at later stages, SAP might be more sensitive to small changes in the number of RGCs which do not seem to produce detectable changes in RNFL thickness. This highlights the need for a combined approach using structure and function to detect disease progression.24–26 The ability to express results of functional and structural tests in the same domain opens the possibility of combining the information from the two tests to increase the precision of RGC estimates, as performed in our study. By combining the estimates, one increases the precision of the final estimate of neuronal losses to better detect change over time. However, instead of simply averaging the two estimates, we used a weighting scheme based on MD values. This was done in order to take into consideration differences in performance of SAP and imaging tests at different stages of the disease for the reasons described above.
Our estimates of RGC losses detected a significantly larger number of glaucomatous eyes as progressing compared to isolated measures of structure and of function, despite having the same specificity in the stable data. It detected the majority of eyes progressing by VFI or OCT. However, some disagreement was seen among the different methods as seen on Figure 3. Interestingly, 36 eyes had progression detected by rates of RGC loss but not by the VFI, whereas 7 eyes had progression detected by the VFI but not by the rate of RGC loss. A comparison between these two groups revealed that eyes progressing only by rates of RGC loss had concomitant evidence of structural change, whereas in eyes progressing only by VFI no such evidence was present. It should be noted that at 95% specificity, approximately 10 of the 213 eyes would be expected to show significant slopes just by chance. In the absence of supportive concomitant structural changes, it is likely that the 7 eyes showing progression by the VFI but not by rates of RGC loss could represent just false positives. Similarly, eyes progressing only based on the rates of RGC loss had significantly faster rates of functional change than those progressing only by OCT as measured by the VFI. The presence of concomitant structural and functional change in eyes progressing by rates of RGC loss provides stronger support suggesting that these eyes represented true progressors compared to those progressing only by VFI or by OCT. Twenty-one eyes had progression by rates of RGC loss but neither by VFI nor by OCT average thickness. These eyes had a mean rate of RGC loss of −31009 cells/year. The mean rates of VFI and OCT average thickness change were −0.51%/year and −0.98μm/year, respectively. It is likely that the amount of change in these eyes was not enough to declare progression based only on the results of the structural or the functional test. However, the combination of measurements from both tests allowed detection of significant change in these eyes. It is also important to note that no eye was detected as progressing by VFI and OCT average thickness, but not by the calculated rate of RGC loss, as shown on Figure 3.
Clinicians are frequently faced with the task of integrating results from structural and functional testing to detect glaucoma progression. This is done routinely as they attempt to correlate changes in their examinations of the optic nerve to those occurring in the visual field, so that if changes over time are seen in both methods, they are more reassuring to indicate true deterioration. However, clinicians are frequently uncertain about how to interpret apparently conflicting results coming from different tests. Also, the use of many different tests can increase the chance of a type I error, i.e., declaring as significant a change that actually has occurred by chance. In fact, if we had declared progression based on the presence of significant change on either SAP VFI or OCT average thickness, the specificity in the stable dataset would have decreased to 90.7%. That is, from the 213 eyes, approximately 20 eyes would be expected to be false positives. By providing a single index of RGC loss combining structural and functional information, we are able to better control type I error. In fact, by setting the alpha to 0.05 to declare the slope of RGC loss as statistically significant, we were able to maintain a specificity of 95%, as demonstrated in the stable group. In addition, we also required that the slopes of RGC loss had to be faster than the age-expected RGC losses for an eye to be considered progressing. This may also represent an additional advantage of our method compared to detection of change based on raw indexes such as OCT average thickness, for example, especially when a large series of tests is being evaluated over a long period of time.
An ideal method for detection of glaucomatous progression should not only give an indication of whether the eye or the patient is likely showing progression, but also needs to give an estimate of the rate of deterioration. Although most glaucoma patients will show some evidence of progression if followed long enough, the rate of deterioration can be highly variable among them.10,27–30 While most patients progress relatively slowly, others have aggressive disease with fast deterioration which can eventually result in blindness or substantial impairment unless appropriate interventions take place. The proposed index allows estimation of the rate of RGC loss over time from structural and functional measurements and has an intuitive meaning which should facilitate the interpretation of rates of change by clinicians. From the 47 eyes detected as progressing by rates of RGC loss, 14 (30%) had rates faster than −5%/year. In principle, these eyes could be considered fast progressors, as their rate of progression would result in 50% loss of their RGCs from the baseline value in a 10-year period. It is important to emphasize, however, that when assessing the clinical relevance of an estimated rate of RGC loss, clinicians also need to consider other factors, such as life expectancy and the patient’s expectations with regard to treatment.
We used the VFI to evaluate rates of visual field loss using standard automated perimetry. This index has been incorporated into the Guided Progression Analysis (GPA) software and is the current method used to analyze rates of visual field loss with the Humphrey perimeters. A recent study, however, has suggested that the reliance of the VFI on pattern deviation probability maps may cause a ceiling effect that may reduce its sensitivity to change in eyes with early damage.31 Therefore, we have also analyzed rates of visual field loss using the parameter MD. For a specificity of 95% in the stable group, only 16 (7.5%) of the 213 eyes had progression based on rates of MD change, a number significantly lower than that found using combined estimates of RGC loss (P<0.001).
We have previously combined structural and functional measurements for detection of glaucoma progression using Bayesian methodology.24 The Bayesian approach provided an effective method of combining results of different tests to improve estimates of rate of progression and also incorporate risk factors for detection of change. Compared to the Bayesian method, the current approach has the potential advantage of using a single estimate of RGC counts obtained from structural and functional tests which potentially facilitates clinical interpretation. However, the Bayesian approach provides the flexibility of combining multiple different tests including structural measurements derived by other imaging technologies such as confocal scanning laser ophthalmoscopy or scanning laser polarimetry and function-specific perimetric tests. Although the principles outlined in our study could in theory be applied to these other tests, the specific methods for translating measurements to RGC counts have not yet been established. It should be noted, however, that a combination of the two methodologies should be possible, such as incorporating risk factors to improve estimation of rates of RGC loss, but the benefits of such approach would have to be evaluated on an independent sample of patients.
Our study has limitations. We used empirically-derived formulas to estimate the number of RGCs from SAP and OCT data. Although these estimates have been validated in histologic studies in monkeys and also applied to multiple external cohorts in humans,12 such validation was not based on direct histologic RGC counts in humans. However, this limitation applies to most measurements obtained in clinical practice from imaging devices and other instruments. In our study, we clearly showed a benefit of our method in detecting glaucoma progression and even though a full histologic validation is not available at this time, this should not preclude its utility in clinical practice. It is interesting to note that despite absence of histologic validation, the age-related loss of RGCs (7877 RGCs per year) found in our study was very similar to that found in previous histologic studies in humans.32 It is possible that other weighting schemes for combination of SAP and OCT estimates of RGC counts could perform better than the one proposed in our study. When we performed an analysis using a simple average of RGC counts from SAP and OCT without weighting, the method detected progression in only 28 eyes compared to 47 eyes for our proposed weighting scheme, at similar specificities. When the weighting system was based on antilog MD values, the performance was also inferior, detecting only 28 eyes as progressing for similar specificity. Further studies should evaluate other methods of combining SAP and OCT estimates of RGC loss and test them on independent populations. In addition, further developments in perimetry and imaging techniques may potentially improve estimates of RGC counts obtained by these instruments leading to improved detection of change.33
We used OCT measurements based on the time-domain version of this technology. The use of spectral-domain OCT (SDOCT) has resulted in faster and more reproducible scans compared to time-domain OCT.34 In a previous cross-sectional study, we developed a combined index of RGC count which used SDOCT measurements along with SAP results. The index performed better than isolated measures of structure and function to stage disease severity.23 However, due to the relatively recent introduction of SDOCT, longitudinal data was not available to perform the current study using this technology. Another potential limitation of our study is that we used only global measures of visual function and structural damage. A sectorial analysis may provide a better representation of localized damage and improved detection of progression. However, sectorial information will be more variable and not necessarily better for monitoring changes over time. Further studies should evaluate whether a combination of sectorial structure and function data could improve detection of glaucomatous change.
In conclusion, an index estimating the rate of RGC loss combining structure and function performed better than isolated structural and functional measures for detecting progressive glaucomatous damage. The use of such index may improve detection of change in clinical practice and in trials evaluating disease progression.
Acknowledgments
A. Funding support: Supported in part by NIH/NEI grants EY021818 (FAM), EY11008 (LMZ), EY14267 (LMZ), EY13959 (CAG), unrestricted grant from Research to Prevent Blindness to the University of California San Diego, the Eyesight Foundation of Alabama, Pfizer, David and Marilyn Dunn Fund, Grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck and Santen.
Biography

Felipe A. Medeiros, M.D., Ph.D. is Professor of Ophthalmology at the University of California San Diego (UCSD). He is also Medical Director and Director of Visual Function Research of the Hamilton Glaucoma Center at UCSD. His research interests encompass many different areas in glaucoma, including identification of risk factors for development and progression of disease, methods and strategies for diagnosis, follow-up and management of glaucoma and evaluation of functional impairment from the disease.
Footnotes
C. Author contributions: design and conduct of the study (FAM, LMZ, CAG, JML, RNW); collection, management, analysis, and interpretation of the data (FAM, DRA, LMZ, CAG, JML, RSH, MJF, RNW) and preparation, review, or approval of the manuscript (FAM, DRA, LMZ, CAG, JML, RSH, MJF, RNW)
D. This prospectively designed study received IRB approval at all involved sites. The methodology adhered to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act. All patients signed an informed consent before participation in the research.
B. Financial Disclosures: Research support from Carl-Zeiss Meditec (FAM, LMZ, CAG, JML, RNW). Research support from Heidelberg Engineering (LMZ, RNW, JML). Consultant to Carl-Zeiss Meditec, Inc. (RNW, JML).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999;43(4):293–320. doi: 10.1016/s0039-6257(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros FA, Alencar LM, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009;127(10):1250–1256. doi: 10.1001/archophthalmol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harwerth RS, Carter-Dawson L, Smith EL, 3rd, Barnes G, Holt WF, Crawford ML. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45(9):3152–3160. doi: 10.1167/iovs.04-0227. [DOI] [PubMed] [Google Scholar]
- 5.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 6.Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112(3):366–375. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Wollstein G, Schuman JS, Price LL, Aydin A, Stark PC, Hertzmark E, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123(4):464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Invest Ophthalmol Vis Sci. 2006;47(7):2904–2910. doi: 10.1167/iovs.05-1584. [DOI] [PubMed] [Google Scholar]
- 9.Leung CK, Cheung CY, Weinreb RN, Qiu K, Liu S, Li H, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a study on optical coherence tomography guided progression analysis. Invest Ophthalmol Vis Sci. 2010;51(1):217–222. doi: 10.1167/iovs.09-3468. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros FA, Alencar LM, Zangwill LM, Sample PA, Weinreb RN. The Relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116(6):1125–1133. e1121–1123. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005;24(3):333–354. doi: 10.1016/j.preteyeres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Harwerth RS, Wheat JL, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010;29(4):249–271. doi: 10.1016/j.preteyeres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sample PA, Girkin CA, Zangwill LM, Jain S, Racette L, Becerra LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127(9):1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racette L, Liebmann JM, Girkin CA, Zangwill LM, Jain S, Becerra LM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128(5):551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengtsson B, Heijl A. False-negative responses in glaucoma perimetry: indicators of patient performance or test reliability? Invest Ophthalmol Vis Sci. 2000;41(8):2201–2204. [PubMed] [Google Scholar]
- 16.Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna R, Jr, Weinreb RN. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139(1):44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros FA, Zangwill LM, Alencar LM, Bowd C, Sample PA, Susanna R, Jr, et al. Detection of glaucoma progression with stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci. 2009;50(12):5741–5748. doi: 10.1167/iovs.09-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145(2):343–353. doi: 10.1016/j.ajo.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 20.Alencar LM, Zangwill LM, Weinreb RN, Bowd C, Vizzeri G, Sample PA, et al. Agreement for detecting glaucoma progression with the GDx guided progression analysis, automated perimetry, and optic disc photography. Ophthalmology. 2010;117(3):462–470. doi: 10.1016/j.ophtha.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fayers T, Strouthidis NG, Garway-Heath DF. Monitoring glaucomatous progression using a novel Heidelberg Retina Tomograph event analysis. Ophthalmology. 2007;114(11):1973–1980. doi: 10.1016/j.ophtha.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41(7):1774–1782. [PubMed] [Google Scholar]
- 23.Medeiros FA, Lisboa R, Weinreb RN, Girkin CA, Liebmann JM, Zangwilll LM. A Combined Index of Structure and Function for Staging Glaucomatous Damage. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining Structural and Functional Measurements to Improve Detection of Glaucoma Progression using Bayesian Hierarchical Models. Invest Ophthalmol Vis Sci. 2011;52(8):5794–5803. doi: 10.1167/iovs.10-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boland MV, Quigley HA. Evaluation of a combined index of optic nerve structure and function for glaucoma diagnosis. BMC Ophthalmol. 2011;11:6. doi: 10.1186/1471-2415-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu H, Crabb DP, Fredette MJ, Anderson DR, Garway-Heath DF. Quantifying discordance between structure and function measurements in the clinical assessment of glaucoma. Arch Ophthalmol. 2011;129(9):1167–1174. doi: 10.1001/archophthalmol.2011.112. [DOI] [PubMed] [Google Scholar]
- 27.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 28.Anderson DR, Drance SM, Schulzer M. Natural history of normal-tension glaucoma. Ophthalmology. 2001;108(2):247–253. doi: 10.1016/s0161-6420(00)00518-2. [DOI] [PubMed] [Google Scholar]
- 29.Heijl A, Bengtsson B, Hyman L, Leske MC. Natural history of open-angle glaucoma. Ophthalmology. 2009;116(12):2271–2276. doi: 10.1016/j.ophtha.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 30.Medeiros FA, Zangwill LM, Alencar LM, Sample PA, Weinreb RN. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149(6):908–915. doi: 10.1016/j.ajo.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Artes PH, O’Leary N, Hutchison DM, Heckler L, Sharpe GP, Nicolela MT, et al. Properties of the statpac visual field index. Invest Ophthalmol Vis Sci. 2011;52(7):4030–4038. doi: 10.1167/iovs.10-6905. [DOI] [PubMed] [Google Scholar]
- 32.Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41(3):741–748. [PubMed] [Google Scholar]
- 33.Malik R, Swanson WH, Garway-Heath DF. Development and evaluation of a linear staircase strategy for the measurement of perimetric sensitivity. Vision Res. 2006;46(18):2956–2967. doi: 10.1016/j.visres.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JS, Ishikawa H, Sung KR, Xu J, Wollstein G, Bilonick RA, et al. Retinal nerve fibre layer thickness measurement reproducibility improved with spectral domain optical coherence tomography. Br J Ophthalmol. 2009;93(8):1057–1063. doi: 10.1136/bjo.2009.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]


