Abstract
We report the results of repeated ambulatory continuous 24-h IOP monitoring with a contact lens sensor in a female glaucoma patient who engaged in sexual activity during each of three monitoring sessions. Our findings show that IOP patterns seemed to decrease during the period of sexual activity.
The recent development of a contact lens sensor (CLS) for near-continuous ambulatory 24-h monitoring of intraocular pressure (IOP) patterns (SENSIMED Triggerfish®, Sensimed, Switzerland) facilitates the study of activity-related effects on IOP.(Mansouri & Shaarawy 2011; Mansouri et al. 2012) Every five minutes and for a continuous period of 30 seconds, the CLS measures IOP changes indirectly through circumferential changes at the corneo-scleral junction.(Mansouri & Shaarawy 2011) For the first time, the effect of sexual activity on IOP patterns in a patient with primary open-angle glaucoma (POAG) is described.
Report of a Case
A 66-year old female patient with POAG underwent repeated 26-hour monitoring of IOP patterns with the CLS in the right eye (OD). As part of a prospective trial evaluating day-to-day IOP patterns and the effect of IOP-lowering medications on them, the patient was instructed to follow her routine daily activities. As the CLS provides its output in arbitrary units corresponding to μVolts, IOP was measured with the Goldmann applanation tonometer (GAT) before sensor placement and after removal.
Session 1
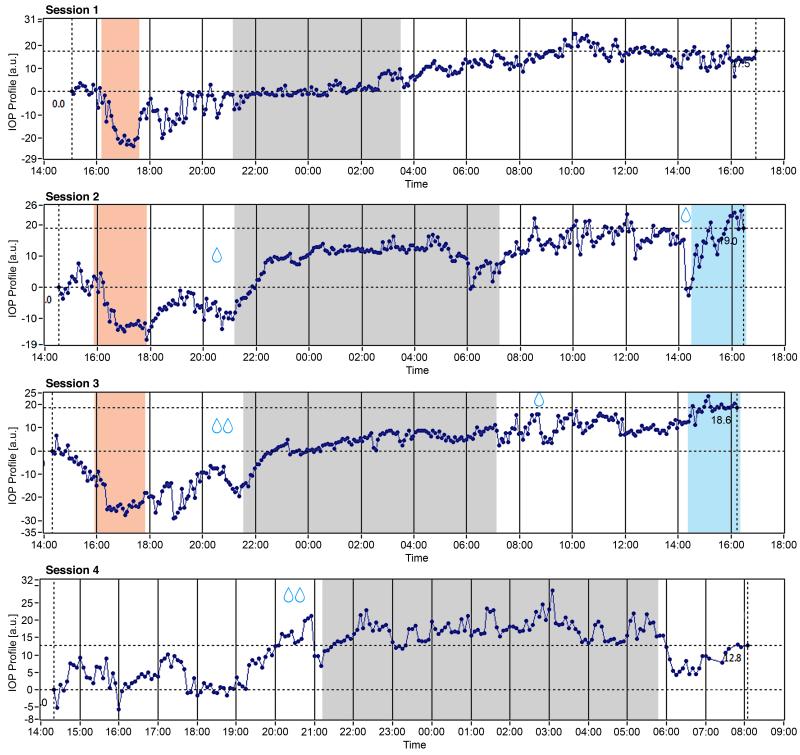
After a 1-month washout period, start and end GAT values were 27 mmHg and 30 mmHg (OD). The interpretation of the 26-h IOP patterns by two independent and masked graders revealed a pronounced signal drop starting at 1620 maintained over the next hour, before returning to initial values (Figure 1, first row). The remainder of the 26-h curve was relatively flat. On questioning, the patient revealed that after returning home from the clinic, she had consumed two glasses of Champagne wine followed by sexual activity with her partner. The timing and duration of this activity was recorded in the patient diary. According to study protocol, the patient was requested to reproduce the same activities in subsequent study sessions. The patient was started on twice-daily brinzolamide 1% (Alcon Inc.).
Figure 1.
Results of 26-h monitoring of IOP patterns at four different sessions, each 1 month apart. A characteristic pattern of signal drop and subsequent increase is observed at sessions 1 to 3 with the time course corresponding to reported times of consumption of Champagne wine followed by sexual activity. At session 4, this pattern was absent. Red-shaded zones correspond to the duration of sexual activity, grey-shaded zones to the sleep period, and blue-shaded zones to the 2-hour period following the water drinking test. Drop signs indicate the time when intraocular pressure-lowering eyedrops were applied.
Session 2 (after 4 weeks)
Start and end GAT values were 22 mmHg and 29 mmHg OD. The patient had failed to instill her prescribed drops in the 24-hours preceding the session. A pattern of signal drop and increase between 1610 and 1800 similar to session 1 was observed and corresponded to the reported time-course of sexual activity. At hour 24, the patient underwent a water drinking test (WDT; ingestion of 1 L of water in 5 minutes). Note a signal increase at 1420 corresponding to the start of the WDT. (Figure 1, second row) The patient was then started on once-daily bimatoprost 0.01% (Lumigan, Allergan) and maintained on twice-daily brinzolamide.
Session 3 (after 4 weeks)
Start and end GAT values were 12 mmHg and 19 mmHg. Reproducing the same routine, the patient consumed 2 glasses of the same Champagne wine at 1530 followed by sexual activity, which lasted until 1730. The timing of the largest drop (1610) corresponded to the recorded moment of orgasm. At 2050, the patient consecutively instilled her brinzolamide and bimatoprost drops and went to bed/sleep shortly before 2200. The patient woke up at 0700 and instilled one drop of brinzolamide at 0830. (Figure 1, third row)
Session 4 (after 4 weeks)
Start GAT was 16 mmHg and 17 mmHg at removal after 20 hours of CLS monitoring. At this visit, the patient was requested to abstain from sex but was permitted to and did consume 2 glasses of Champagne wine (around 1600). Despite a short drop in the signal, the previous pattern was not observed by masked grading. She instilled her drops at 2050. (Figure 1, last row)
Comment
We report the first case of monitoring IOP patterns during sexual activity in a patient with POAG. At all 3 visits, during which sexual activity succeeded the consumption of Champagne wine, a similar pattern of signal drop, stabilization and subsequent increase was observed. We can only speculate whether the observed patterns are related to the sexual activity; however the fact that they were not observed in the absence of sexual activity, reduces (but not eliminates) the possibility of the observed pattern being part of the patient’s normal circadian rhythm or wine consumption.(Vauzour et al. 2010) Acute angle closure after orgasm in a hyperope female has previously been reported.(Ritch et al. 2007) However, the mechanisms for a putative effect of sexual activity on IOP are unknown in POAG. Acute vasodilation leading to a drop in blood pressure may account for a concomitant drop in IOP,(Liu & Dacus 1989) as can the release of hormones with IOP-lowering properties, such as dopamine and prolactin.(Mekki & Turner 1985; Stojek et al. 1991) A rapid and maintained increase of prolactin levels after orgasm has been demonstrated.(Kruger et al. 2005) The neuro-chemical mechanisms underlying sexual arousal and IOP behavior are extremely complex and warrant further investigation. Despite lack of further evidence on potentially beneficial effects of sexual activity on glaucoma, the present data suggest that sexual activity may (at the least) not cause worsening of the IOP-dependent mechanism of glaucoma injury.
Acknowledgments
Supported in part by NIH EY021818 (FAM)
Footnotes
DISCLOSURES:
Sensimed AG, Switzerland provided free material for this study. Kaweh Mansouri and Robert N. Weinreb are consultants for Sensimed AG. Velux Foundation, Switzerland provided an unrestricted research grant to Kaweh Mansouri.
REFERENCES
- Kruger TH, Hartmann U, Schedlowski M. Prolactinergic and dopaminergic mechanisms underlying sexual arousal and orgasm in humans. World J Urol. 2005;23:130–8. doi: 10.1007/s00345-004-0496-7. [DOI] [PubMed] [Google Scholar]
- Liu JH, Dacus AC. Intramuscular injection of chlorpromazine decreases intraocular pressure by lowering systemic blood pressure. Curr Eye Res. 1989;8:1315–21. doi: 10.3109/02713688909013912. [DOI] [PubMed] [Google Scholar]
- Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-Hour Monitoring of Intraocular Pressure Patterns With a Contact Lens Sensor: Safety, Tolerability, and Reproducibility in Patients With Glaucoma. Arch Ophthalmol. 2012:1–6. doi: 10.1001/jamaophthalmol.2013.1350. [DOI] [PubMed] [Google Scholar]
- Mansouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. Br J Ophthalmol. 2011;95:627–9. doi: 10.1136/bjo.2010.192922. [DOI] [PubMed] [Google Scholar]
- Mekki QA, Turner P. Stimulation of dopamine receptors (type 2) lowers human intraocular pressure. Br J Ophthalmol. 1985;69:909–10. doi: 10.1136/bjo.69.12.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritch R, Dorairaj SK, Liebmann JM. Angle-closure triggered by orgasm: a new provocative test? Eye (Lond) 2007;21:872–4. doi: 10.1038/sj.eye.6702744. [DOI] [PubMed] [Google Scholar]
- Stojek A, Kasprzak B, Slabikowski A. Intraocular pressure and prolactin measures in seasonal affective disorder. Psychiatr Pol. 1991;25:8–12. [PubMed] [Google Scholar]
- Vauzour D, Houseman EJ, George TW, Corona G, Garnotel R, Jackson KG, Sellier C, Gillery P, Kennedy OB, Lovegrove JA, Spencer JP. Moderate Champagne consumption promotes an acute improvement in acute endothelial-independent vascular function in healthy human volunteers. Br J Nutr. 2010;103:1168–78. doi: 10.1017/S0007114509992959. [DOI] [PubMed] [Google Scholar]