Abstract
The underlying mechanisms of schizophrenia pathogenesis are not well understood. Increasing evidence supports the glutamatergic hypothesis that posits a hypofunction of the N-methyl D-aspartate (NMDA) receptor on specific gamma amino-butyric acid (GABA)-ergic neurons may be responsible for the disorder. Alterations in the GABAergic system have been observed in schizophrenia, most notably a change in the expression of parvalbumin (PV) in the cortex and hippocampus. Several reports also suggest abnormal neuronal migration may play a role in the etiology of schizophrenia. The current study examined the positioning and distribution of PV-positive cells in the hippocampus following chronic treatment with the NMDA receptor antagonist ketamine. A robust increase was found in the number of PV-positive interneurons located outside the stratum oriens (SO), the layer where most of these cells are normally localized, as well as an overall numerical increase in CA3 PV cells. These results suggest ketamine leads to an abnormal distribution of PV-positive cells, which may be indicative of aberrant migratory activity and possibly related to the Morris water maze deficits observed. These findings may also be relevant to alterations observed in schizophrenia populations.
Keywords: ketamine, schizophrenia, parvalbumin, hippocampus
Introduction
Schizophrenia is a severe neuropsychiatric disorder for which the underlying mechanisms are not well understood [35]. Post-mortem investigations of patients with schizophrenia have identified disruptions in glutamatergic signaling, most notably a hypofunction of the N-methyl D-aspartate (NMDA) glutamate receptor [1,4,6,11,13]. Abnormal activity of NMDA receptors on gamma amino-butyric acid (GABA)-ergic interneurons in particular has been observed in the brains of schizophrenic patients [49]. A reduction in the expression of the calcium-binding protein parvalbumin (PV) in fast-spiking GABAergic interneurons is another consistent pathological feature of the disorder [2,15,29,50]. This change is commonly reported as a decrease in mRNA expression or the number of PV-positive cells [2,15,29], while more recent reports suggest the reduction is in the mean PV intensity per cell [25,40,48]. These findings collectively suggest that aberrant GABA functioning may play an influential role in aspects of schizophrenia.
Neurodevelopmental hypotheses of schizophrenia indicate abnormal migration of newborn neurons may play a role in the disorder [18]. The expression of reelin, a protein implicated in neuronal migration during development [9,16], shows profound reductions in post-mortem brains of schizophrenia patients [17], particularly in the prefrontal cortex [14] and hippocampus [12]. Genetic linkage studies with schizophrenia patients have reported additional risk factors tied to migratory regulation such as MDGA1 [20,30] and Disrupted-in-Schizophrenia-1 (DISC1) [7,45]. DISC1 in particular has been implicated in normal neuronal migration in the hippocampus [10,24,27], suggesting DISC1 mutations may impair migration and alter network function in schizophrenia.
Sub-anesthetic doses of noncompetitive NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine, produce neurological and behavioral alterations consistent with schizophrenia in humans [19,28,47]. Investigations of disease processes in rodents have also benefitted from pharmacological approaches with these drugs [3,8,37,44]. Modeling aspects of schizophrenia in various animal systems has demonstrated heterotopic expression of newborn neurons during development [21,33] and adulthood [31,36]; however, the types of abnormally migrated neurons have not been well elucidated. Based on the altered expression of PV in schizophrenic brains, we examined the distribution and localization of PV-positive neurons within the CA3 region of the hippocampus and cortex following chronic administration of ketamine. In this exploratory study, we observed differences in the number and positioning of the PV-positive cells which may be indicative of migratory deficits, and could be linked to altered connectivity.
Methods
Subjects
Forty male Sprague-Dawley rats (n=10) from Charles River Laboratories (Hollister, CA, USA) weighing between 250 and 350 g were pair-housed in a standard animal facility with a 12–12h light-dark cycle, and with food and water available ad libitum. All procedures were performed in accordance with the institutional Animal Care and Use Committee and NIH guidelines for ethical treatment of research subjects.
Drugs
Ketamine HCl (Henry–Schein, Indianapolis, IN) was diluted in physiological saline (VWR, Bridgeport, NJ) to achieve a final concentration of 8 mg/ml as has been previously demonstrated to impair sensorimotor gating and spatial learning [42]. Ketamine or saline was administered subcutaneously once daily at a dose of 1 ml/kg of body weight beginning on the first day of behavioral testing through the completion of testing, a total of 18 days consistent with previous investigations [42].
Morris Water Maze
The Morris water maze (MWM) was conducted as described previously [42]. In experiment 1, subjects underwent 5 days of acquisition followed by a probe trial an average of 5 hours later. Ketamine-administered rats were then retrained for 7 additional days in an effort to determine if equivalent performance to controls could be achieved prior to 3 days of reversal training and 2 days of visible training. Based on the data from the first experiment a second experiment was performed to replicate and verify tissue results. In experiment 2, small variations were made in reversal training in order to parse out ketamine-induced deficits, but overall number of days of drug administration and training days was consistent.
Immunohistochemistry
Half of the animals run in each of the Morris water maze (n=5) experiments were randomly selected for transcardial perfusion with 4% paraformaldehyde following CO2 asphyxiation. Brains were then removed and placed in 4% paraformaldehyde at 4° C for 48 hours before being moved to a 30% sucrose solution until sectioning. Whole brains were sectioned at a thickness of 15 μm on a cryostat and sections were stored at 4° C until the immunohistochemistry (IHC) experiments. Sections were placed in plastic wells and remained free floating until the completion of the IHC procedure. Sections were initially blocked for 45 minutes in a 5% normal goat serum (NGS) solution and then incubated overnight at room temperature with a primary monoclonal antibody raised in mouse directed against PV (1:1000; Sigma-Aldrich, St. Louis, MO). Following five washes, fluorescent labeling was performed with Alexafluor 488 anti-mouse secondary antibody (1:2000; Invitrogen, Grand Island, NY) for 45 minutes at room temperature. Following five washes, sections were mounted onto slides for fluorescent imaging.
Serial sections beginning at the anterior hippocampus (2.5 mm posterior of Bregma) were evaluated by two independent observers blind to treatment. PV-positive neuron number and positioning were examined in the CA3 region of the hippocampus due to their abundance and typical localization in this area. PV-positive neuron number was also examined in the retrosplenial cortex, directly superior to the hippocampus. For each individual section, the area of interest, encompassing the CA3 region medial to and including the stratum oriens (SO), was determined by alignment of the outer shell of the CA3 region of the hippocampus along the border of the field of view using a 10X objective. All images were captured at an objective of 10X using a Zeiss Axioskop II Plus microscope (Carl Zeiss MicroImaging, Inc, Thornwood, NY). A cell was determined to be outside the SO if no part of the cell fell within the clearly defined SO layer. A minimum of six sections per subject was utilized to determine the number and location of PV-positive cells.
Statistical Analyses
MWM acquisition and visible training data were analyzed using the SPSS statistical software package by a repeated measures analysis of variance (ANOVA), while probe trial data were analyzed with one-way ANOVA. One-way ANOVA was also performed to detect differences in the amount or distribution of PV-positive cells in the hippocampus between groups. Tukey post-hoc comparisons were performed to analyzed probe within group probe trial performance.
Results
Morris Water Maze
Experiment 1
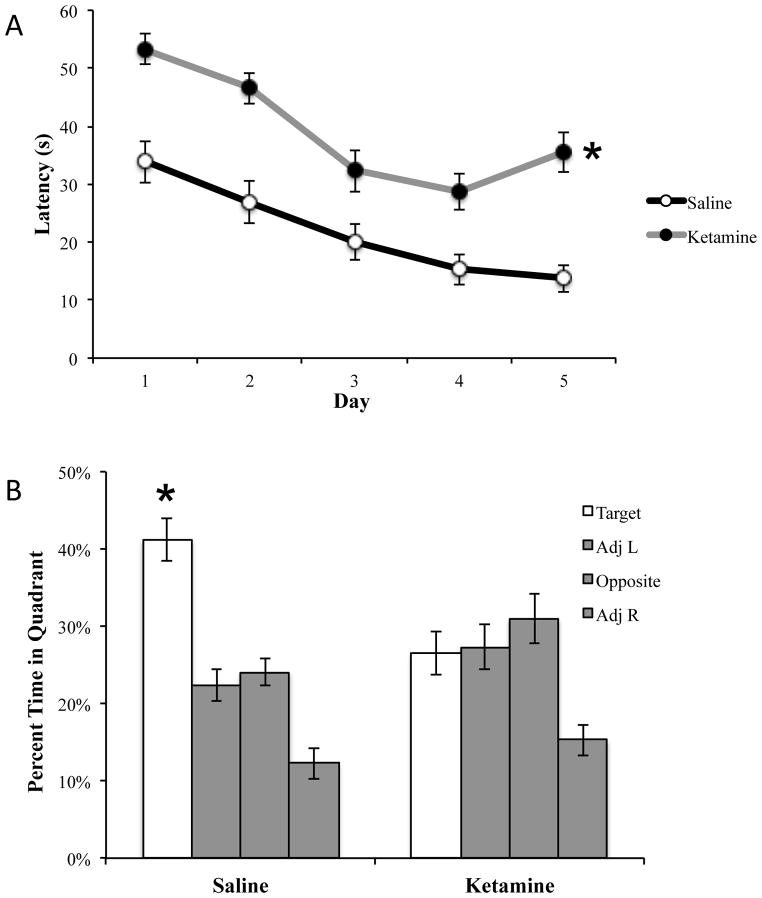
Ketamine produced a deficit in MWM consistent with previous investigations [5,42]. Significant deficits were observed in acquisition training versus saline (F1,78 = 40.04, p<0.01; see figure 1A). During the probe trial (see figure 1B), ketamine-administered rats did not perform a selective search (F3,36 = 6.118, p<0.01; Tukey post-hoc analyses of quadrant revealed that animals did not spent significantly more time in the target quadrant than the adjacent left (p=0.997) or opposite quadrant (p=0.661)), whereas rats administered saline did show a preference for the target quadrant (F3,36 = 29.635, p<0.01; Tukey post-hocs indicated target versus each quadrant at p<0.01).
Figure 1.
Ketamine produced deficits in the Morris water maze. (A) Ketamine-treated rats displayed significantly longer latency to find the platform during acquisition. (B) Saline-treated rats exhibited a selective search on the probe trial, indicating a significant preference for the target quadrant, while ketamine produced a deficit. Saline: n=10; ketamine: n=10; *p<0.01
Experiment 2
Ketamine produced similar impairments in experiment 2 with a significant deficit during acquisition (F1,78 = 64.741, p<0.01; data not shown) and the probe trial (F3,36 = 4.892, p<0.01; Tukey post-hocs revealed that rats spent significantly more time in the opposite quadrant than the target quadrant (p<0.01).
Immunohistochemistry
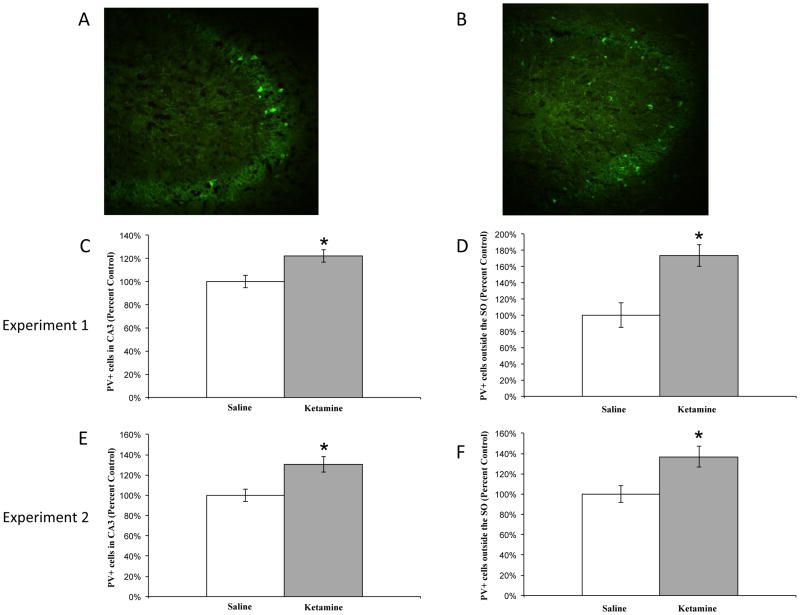
Cell counts for immunofluorescent PV-positive cells from both experiments were performed within the left and right CA3 region of the hippocampus and left and right cortices to assess changes in number. For the hippocampus, representative images for saline and ketamine are displayed in figure 2A and 2B, respectively. Ketamine produced an increase in the total number of PV-positive neurons in CA3 compared to controls in experiment 1 (F1,45 = 8.169, p<0.01; see figure 2C) and experiment 2 (F1,45 = 9.609, p<0.01; see figure 2E). Ketamine also produced a change in the localization of these neurons. The number of PV-positive cells outside the SO was significantly greater following chronic ketamine administration as compared to saline controls in experiment 1 (F1,45 = 7.659, p<0.01; see figure 2D) and experiment 2 (F1,45 = 7.921, p<0.01; see figure 2F).
Figure 2.
Number and positioning of PV-positive neurons in the CA3 region of hippocampus. (A) Representative image of saline-treated subject. (B) Representative image of ketamine-treated subject. Ketamine produced a significant increase in PV-positive neurons in CA3 in experiment 1 (C) and experiment 2 (E) compared to saline. Ketamine also led to a percent increase in these cells outside the stratum oriens as compared to saline in experiment 1 (D) and experiment 2 (F). Saline: n=10; ketamine: n=10; *p<0.01
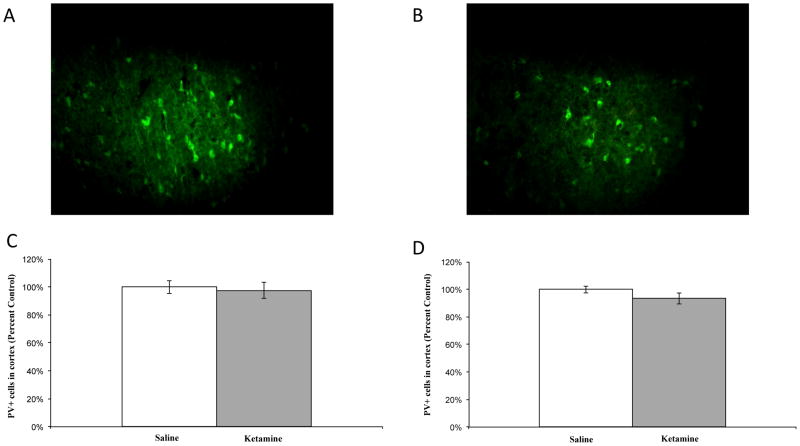
Cell counts were also performed in the retrosplenial cortex; representative images are depicted in figure 3A and 3B, respectively. No significant differences were observed between groups in the number of PV-positive neurons in the cortex in experiment 1 (F1,45 = 0.107, p=.745; see figure 3C) or experiment 2 (F1,45 = 1.276, p=.264; see figure 3D), suggesting the ketamine-induced changes are region-specific. Table 1 depicts the raw cell count data from both experiments.
Figure 3.
PV-positive neurons in the cortex. (A) Representative image of saline-treated subject. (B) Representative image of ketamine-treated subject. No significant differences were observed in PV-positive neurons in the cortex in experiment 1 (C) or experiment 2 (D), p>.05. Saline: n=5; ketamine: n=5.
Table 1.
PV + cell count data from experiments 1 and 2 (expressed as mean ± SEM).
| Experiment | Group | CA3 | CA3 outside SO | Cortex |
|---|---|---|---|---|
| 1 | Saline | 26.71 ± 1.46 | 3.50 ± 0.52 | 64.13 ± 2.94 |
| Ketamine | 32.61 ± 1.46 | 6.09 ± 0.78 | 62.59 ± 3.70 | |
| 2 | Saline | 24.65 ± 0.65 | 3.65 ± 0.30 | 66.88 ± 1.70 |
| Ketamine | 36.33 ± 2.20 | 5.00 ± 0.37 | 62.50 ± 2.70 |
Discussion
The current study investigated the expression and positioning of PV-positive cells in the hippocampus following chronic ketamine administration and a spatial learning task. Our findings reveal an increase in number and a shift in the precise localization of these GABAergic neurons, which may be indicative of abnormal migratory activity. The significant increase in the number of PV-positive neurons in CA3 in two distinct experiments is in contrast to findings of decreased hippocampal PV density in animal models [22,38] and schizophrenia populations [26]. The increased number of PV cells reported here suggests ketamine may alter neurogenesis in these studies, as previously reported [23], resulting in the increased number and aberrant positioning in CA3. This interpretation is supported by the lack of a ketamine-induced increase in number in the cortex. An overall increase the in total number of PV-positive neurons specifically in the hippocampus is particularly interesting as it may related to the spatial learning and memory deficits. The consequence of increased PV-positive neurons is difficult to estimate, however, alterations in overall network function would be likely if these neurons are functional. The increased number of PV-positive cells outside the SO in the ketamine-treated group suggests a heterotopic distribution of newborn neurons in the CA3 region of the hippocampus. These neuronal displacements could lead to altered connectivity within the hippocampus and disrupted network activity, a phenomenon that may be occurring in schizophrenia [46].
The location of PV neurons within the hippocampus is generally restricted to the SO and stratum pyramidale with sparse distribution in other layers such as the stratum radiatum [32,34,43]. The heterotopic nature of PV-positive neurons observed in the current study suggests there may be disrupted migration following chronic ketamine administration. The protein reelin, which regulates the migration and positioning of immature neurons, is only expressed in GABAergic cells in the adult rat hippocampus [39]. These findings, along with post-mortem studies from schizophrenia brains showing reductions in PV [2,15,29] and reelin expression [12,14,17], may present a link between PV-mediated network dysfunction and abnormal migration.
Previous reports suggest the NMDA receptor plays an integral role in regulating the migration of newborn neurons in the adult hippocampus [36] and developing cortex [41]. Our findings suggest that chronic NMDA receptor blockade with ketamine may impair neuronal migration. The current study, as well as previous studies from our laboratory, has demonstrated that ketamine produces robust impairments in sensorimotor gating, spatial learning, and emotional learning and memory [5,42]. It is possible that aberrant network function due to migratory deficits may be important in these cognitive impairments. Future studies may be able to elucidate if abnormal migration plays any role in these deficits.
Although the data reported here suggest the altered distribution of PV-positive neurons may be a result of aberrant migration, more studies are necessary to confirm this hypothesis. The co-administration of 5-bromo-2-deoxyuridine (BrDU) with ketamine may allow a clearer picture of whether these heterotopic PV-positive cells are newborn neurons; previous studies suggest ketamine enhances neurogenesis, though without altering newborn cell location or showing BrDU-PV co-localization in the dentate gyrus [23]. Furthermore, more extensive stereological analyses in future studies may be able to detect quantitative differences produced by ketamine. Additional investigations are needed to determine if the above changes persist once ketamine administration and behavioral testing have been completed similar to previous work [23]. Despite these limitations, the current study strengthens the link between ketamine-induced disruptions in PV and schizophrenia.
Research Highlights.
Ketamine SC at 8mg/kg impaired spatial learning consistent with previous findings.
Ketamine induced a significant increase in the number of PV+ neurons in CA3 field of hippocampus.
Ketamine induced a significant increase in PV+ neurons outside the SO of the hippocampus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jr, Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. Journal of Neuroscience. 1996;16(1):19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24(3):349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 3.Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27(4):687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 4.Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32(9):1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 5.Bolton MM, Heaney CF, Sabbagh JJ, Murtishaw AS, Magcalas CM, Kinney JW. Deficits in emotional learning and memory in an animal model of schizophrenia. Behav Brain Res. 2012;233(1):35–44. doi: 10.1016/j.bbr.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry. 2008;165(12):1594–1603. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- 7.Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, Goldberg TE, Weinberger DR. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102(24):8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cilia J, Hatcher P, Reavill C, Jones DNC. (±) Ketamine-induced prepulse inhibition deficits of an acoustic startle response in rats are not reversed by antipsychotics. Journal of Psychopharmacology. 2007;21(3):302–311. doi: 10.1177/0269881107077718. [DOI] [PubMed] [Google Scholar]
- 9.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374(6524):719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 10.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: Further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res. 2005;73(2–3):159–172. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5(6):654–63. 571. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: Effects of schizophrenia. Am J Psychiatry. 2000;157(7):1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 14.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24(2):481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 17.Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95(26):15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakob H, Beckmann H. Circumscribed malformation and nerve cell alterations in the entorhinal cortex of schizophrenics. Pathogenetic and clinical aspects. J Neural Transm Gen Sect. 1994;98(2):83–106. doi: 10.1007/BF01277013. [DOI] [PubMed] [Google Scholar]
- 19.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 20.Kahler AK, Djurovic S, Kulle B, Jonsson EG, Agartz I, Hall H, Opjordsmoen S, Jakobsen KD, Hansen T, Melle I, Werge T, Steen VM, Andreassen OA. Association analysis of schizophrenia on 18 genes involved in neuronal migration: MDGA1 as a new susceptibility gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1089–1100. doi: 10.1002/ajmg.b.30726. [DOI] [PubMed] [Google Scholar]
- 21.Kang WY, Kim SS, Cho SK, Kim S, Suh-Kim H, Lee YD. Migratory defect of mesencephalic dopaminergic neurons in developing reeler mice. Anat Cell Biol. 2010;43(3):241–251. doi: 10.5115/acb.2010.43.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126(3):591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Keilhoff G, Bernstein HG, Becker A, Grecksch G, Wolf G. Increased neurogenesis in a rat ketamine model of schizophrenia. Biol Psychiatry. 2004;56(5):317–322. doi: 10.1016/j.biopsych.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63(6):761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26(5):1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131(1–3):165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105(19):7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahti AC, Weiler MA, Michaelidis T, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 29.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Liu J, Feng G, Li T, Zhao Q, Li Y, Hu Z, Zheng L, Zeng Z, He L, Wang T, Shi Y. The MDGA1 gene confers risk to schizophrenia and bipolar disorder. Schizophr Res. 2011;125(2–3):194–200. doi: 10.1016/j.schres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Manning EE, Ransome MI, Burrows EL, Hannan AJ. Increased adult hippocampal neurogenesis and abnormal migration of adult-born granule neurons is associated with hippocampal-specific cognitive deficits in phospholipase C-beta1 knockout mice. Hippocampus. 2012;22(2):309–319. doi: 10.1002/hipo.20900. [DOI] [PubMed] [Google Scholar]
- 32.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2(1):11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 33.Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci U S A. 2009;106(38):16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercer A, Eastlake K, Trigg HL, Thomson AM. Local circuitry involving parvalbumin-positive basket cells in the CA2 region of the hippocampus. Hippocampus. 2012;22(1):43–56. doi: 10.1002/hipo.20841. [DOI] [PubMed] [Google Scholar]
- 35.Moller H. The psychopathology of schizophrenia: An integrated view on positive symptoms and negative symptoms. Int Clin Psychopharmacol. 1995;10(SUPPL 3):57–64. [PubMed] [Google Scholar]
- 36.Namba T, Ming GL, Song H, Waga C, Enomoto A, Kaibuchi K, Kohsaka S, Uchino S. NMDA receptor regulates migration of newly generated neurons in the adult hippocampus via Disrupted-In-Schizophrenia 1 (DISC1) J Neurochem. 2011;118(1):34–44. doi: 10.1111/j.1471-4159.2011.07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128(3):419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Nullmeier S, Panther P, Dobrowolny H, Frotscher M, Zhao S, Schwegler H, Wolf R. Region-specific alteration of GABAergic markers in the brain of heterozygous reeler mice. Eur J Neurosci. 2011;33(4):689–698. doi: 10.1111/j.1460-9568.2010.07563.x. [DOI] [PubMed] [Google Scholar]
- 39.Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci U S A. 1998;95(6):3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62(3):1322–1331. doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiprich P, Kilb W, Luhmann HJ. Neonatal NMDA receptor blockade disturbs neuronal migration in rat somatosensory cortex in vivo. Cereb Cortex. 2005;15(3):349–358. doi: 10.1093/cercor/bhh137. [DOI] [PubMed] [Google Scholar]
- 42.Sabbagh JJ, Heaney CF, Bolton MM, Murtishaw AS, Kinney JW. Examination of ketamine-induced deficits in sensorimotor gating and spatial learning. Physiol Behav. 2012;107(3):355–363. doi: 10.1016/j.physbeh.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280(2):183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- 44.Swerdlow NR, Bakshi V, Waikar M, Taaid N, Geyer MA. Seroquel, clozapine and chlorpromazine restore sensorimotor gating in ketamine-treated rats. Psychopharmacology (Berl) 1998;140(1):75–80. doi: 10.1007/s002130050741. [DOI] [PubMed] [Google Scholar]
- 45.Tomita K, Kubo K, Ishii K, Nakajima K. Disrupted-in-Schizophrenia-1 (Disc1) is necessary for migration of the pyramidal neurons during mouse hippocampal development. Hum Mol Genet. 2011;20(14):2834–2845. doi: 10.1093/hmg/ddr194. [DOI] [PubMed] [Google Scholar]
- 46.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34(5):927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Berckel BN, Oranje B, van Ree JM, Verbaten MN, Kahn RS. The effects of low dose ketamine on sensory gating, neuroendocrine secretion and behavior in healthy human subjects. Psychopharmacology (Berl) 1998;137(3):271–281. doi: 10.1007/s002130050620. [DOI] [PubMed] [Google Scholar]
- 48.Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci. 2011;31(49):18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo T-W, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218(C):267–277. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55(1–2):1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]