Abstract
Background/aims
Pre-ESRD care associates with improved outcomes among patients receiving dialysis. It is unknown what proportion of US micropolitan and rural dialysis patients receive pre-ESRD care and benefit from such care when compared to urban.
Methods
A retrospective cohort study was performed using data from the US Renal Data System. Patients ≥18 years old who initiated dialysis in 2006 and 2007 were classified as rural, micropolitan, or urban and prevalence of pre-ESRD care (early nephrology care >6 months, permanent vascular access, dietary education) was determined using the medical evidence report. The association of pre-ESRD care with dialysis mortality and transplantation was assessed using Cox regression with stratification for geographic residence.
Results
Of 204,463 dialysis patients, 80% were urban, 10.2% were micropolitan, and 9.8% were rural. Overall attainment of pre-ESRD care was poor. After adjustment, there were no significant geographic differences in attainment of early nephrology care or permanent dialysis access. Receiving care reduced all-cause mortality and increased the likelihood of transplantation to a similar degree regardless of geographic residence. Both micropolitan and rural patients received less dietary education (RR 0.80 95% CI 0.76–0.84 and RR 0.85 95% CI 0.80–0.89, respectively).
Conclusion
Among patients who receive dialysis, the prevalence of early nephrology care and permanent dialysis access is poor and does not vary by geographic residence. Micropolitan and rural patients receive less dietary education despite an observed mortality benefit, suggesting that barriers may exist to quality dietary care in more remote locations.
Keywords: rural, disparity, chronic kidney disease
Health outcomes among patients living in remote locations is of increasing focus in the United States (US), as approximately 20% of the population live in micropolitan (small towns) or rural areas[1]. Among end-stage renal disease (ESRD) patients receiving dialysis, micropolitan and rural residence are independently associated with worse mortality in more remote patients, particularly those on peritoneal dialysis[2]. It is unknown if lack of quality of pre-ESRD care could partially explain this increased risk.
Optimal pre-ESRD care includes timely referral to a nephrologist, dialysis and dietary education, placement of a permanent vascular access in patients who prefer hemodialysis, and referral for pre-emptive kidney transplantation[3, 4]. Pre-ESRD nephrology care has been independently associated with decreased dialysis mortality, higher likelihood of pre-emptive kidney transplantation, higher serum albumin concentrations at initiation of dialysis, and higher incidence of AV fistula or graft use for hemodialysis initiation[5–9]. Timely referral and quality care prior to the start of dialysis has been identified as an area requiring improvement in the United States[10].
Micropolitan and rural communities face barriers in receiving specialized care, which may be related to lack of local subspecialists and hospitals with advanced resources[11]. These barriers may limit access to nephrology support required for optimum pre-ESRD care. This study examines the prevalence of pre-ESRD care among a population of urban, micropolitan, and rural dialysis patients and its impact on mortality and kidney transplantation. We hypothesized that micropolitan and rural residence would associate with lower prevalence of pre-ESRD care and reduce the protective effect of such care.
Materials and methods
A retrospective cohort study was performed using patient-level data obtained from the US Renal Data System (USRDS). The design of the study cohort including data sources, patient selection, and determination of rural location has been previously described in more detail[2]. This study was approved by the Institutional Review Board at Vanderbilt University Medical Center and by the US Renal Data System.
Patient selection
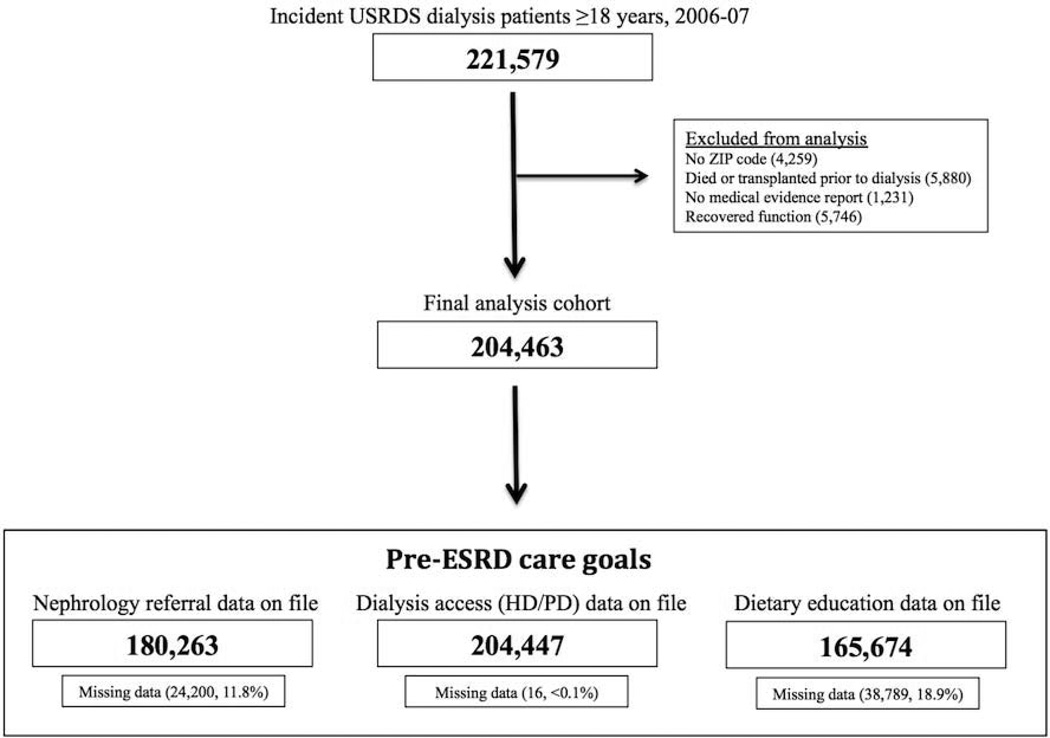
Patients were included if they initiated dialysis for the first time between January 1, 2006 and December 31, 2007 and were 18 years of age or older. Patients were excluded if their residence location could not be determined, the medical evidence report was missing, or they discontinued dialysis during follow-up due to recovery of kidney function. Figure 1 depicts the study flow.
Figure 1.
Study flow
Identification of residence location
Rural and micropolitan residence was determined by the use of rural-urban commuting area (RUCA) codes[12], a robust and flexible method for measuring the degree of rurality in epidemiologic research[13]. ZIP-code specific RUCA codes were used as they represent the smallest feasible geographic area to study[14].
Pre-ESRD care goals
The prevalence of selected pre-ESRD care goals was determined from the medical evidence report, including: (1) early nephrology care >6 months prior to dialysis initiation (yes/no), (2) mature permanent hemodialysis access (AV fistula or graft) at initiation (yes/no), and (3) dietary education prior to dialysis initiation (yes/no).
Mortality and kidney transplantation
Longitudinal outcomes of interest included time-to-death and time-to-kidney transplantation as reported from the first dialysis service date. For survival analysis, patients who received a transplant were censored at the time of their kidney transplant. For transplantation analysis, patients were excluded from the “at risk” population if they were >75 years old at initiation or were deemed unfit for transplant on the medical evidence report. Censoring occurred at the date of the outcome, last follow-up, or October 1, 2009, whichever occurred earlier.
Adjustment covariates
Demographic information included age at initiation, sex, and race. Measures of socio-economic status (SES) that are available in USRDS, such as insurance coverage and employment status, were included. Ecologic surrogates from the US Census were utilized for measures not collected by USRDS, including ZIP-code-median household income and ZIP-code-proportion over age 25 with a high school diploma[15]. Characteristics of kidney disease included reported cause of CKD, estimated GFR (eGFR) at initiation, and dialysis modality at 90 days after initiation. Covariates describing chronic medical comorbidities were included such as heart failure, coronary artery disease, diabetes mellitus, hypertension, chronic-obstructive pulmonary disease, cancer, history of stroke, and use of tobacco products, alcohol, or illicit drugs. Documented history of institutionalization and impairment of activities of daily living were also included as they could impede patients from seeking medical care.
Statistical analysis
Baseline characteristics were summarized as percentages, medians with interquartile ranges, or means with standard deviations, as appropriate based on the variable type and frequency distribution. Comparisons across geographic (urban, micropolitan, and rural) groups were made using Pearson’s chi-square for categorical and Kruskal-Wallis for continuous data due to non-normal distributions. Statistical significance was defined at a p-value <0.05 and all tests were two-tailed.
The unadjusted prevalence of pre-ESRD care by geographic residence was calculated. Adjusted estimates were determined through Poisson regression with robust variances, which provides relative risk estimates and correct confidence intervals [16]. The unadjusted association of pre-ESRD care with longitudinal outcomes was assessed using the Kaplan Meier method for time-to-death and time-to-transplantation with stratification by geographic residence. Multivariable Cox regression models were created with stratification for geographic location (urban, micropolitan, rural). Stratified analyses were performed due to the a-priori hypothesis that differential effects may be present in different geographic strata.
Poisson and Cox regression models were adjusted for demographics (age, sex, race), body mass index, US region (by ESRD network: northeast 1–5, midwest 9–12, south 6–8 and 13–14, and west 15–18), socioeconomic status (insurance, employment, ZIP-code median household income), kidney-disease related factors (presumed primary cause of ESRD, eGFR at initiation) and co-morbidities (as listed in “covariates of interest”). All Cox models were tested for the proportional hazard assumption through use of log-log survival plots and were found to satisfy the assumption.
As depicted in Figure 1, substantial missing data was observed in documentation of nephrology care (11.3% missing) and dietary education (20.1% missing). The characteristics of missing data were assessed for non-randomness using pairwise correlation matrices. There was no independent correlation between missing data in pre-ESRD care variables (nephrology care, permanent vascular access, and dietary education) and other variables in the analysis (correlation coefficients <0.1), suggesting that missing data was mostly random. Missing data was handled through use of list-wise deletion for the multivariable models, with the analysis only including patients with complete data on file. Other covariates were rarely missing (<1%). Documentation of survival and transplantation were based entirely on data from USRDS.
Data management and statistical analysis was performed using Stata version 11.2 (College Station, Texas).
Results
Cohort characteristics
Baseline characteristics of this cohort are summarized in Table 1. Micropolitan and rural patients were modestly older than urban patients, lived in less wealthy communities, and had fewer high school graduates compared to urban communities. Blacks accounted for only 21.6% of micropolitan and 21.1% of rural patients compared to 31.4% of urban patients. Micropolitan and rural patients had higher prevalence of heart failure, heart disease, stroke, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease.
Table 1.
Baseline characteristics of study group
| Urban | Micropolitan | Rural | P-value | |
|---|---|---|---|---|
| Demographics | N=163,592 | N=20,811 | N=20,060 | |
| Age (mean, SD) | 63.0 (15.4) | 63.7 (14.9) | 63.9 (14.6) | <0.001 |
| Sex (% male) | 55.9 | 55 | 55.4 | 0.031 |
| BMI (median, IQR) | 27.0 (23.1–32.3) | 27.8 (23.7–33.3) | 27.9 (23.9–33.4) | <0.001 |
| Race (%) | <0.001 | |||
| White | 62.9 | 74.7 | 73.9 | |
| Black | 31.6 | 21.9 | 20.8 | |
| Other | 5.6 | 3.5 | 5.4 | |
| Has any insurance coverage (%) | 92.1 | 93.3 | 93.0 | <0.001 |
| Employment (%) | <0.001 | |||
| Unemployed | 44.5 | 44.9 | 45.8 | |
| Employed | 15.4 | 13.1 | 12.5 | |
| Retired | 40.1 | 42.0 | 41.7 | |
| Characteristics of kidney disease | ||||
| Primary disease causing ESRD (%) | <0.001 | |||
| Diabetes mellitus | 45.4 | 47.6 | 48.0 | |
| Hypertension | 28.8 | 27.7 | 27.2 | |
| Glomerulonephritis | 6.8 | 6.3 | 5.9 | |
| Cystic disease | 2.1 | 2.2 | 2.1 | |
| Other | 16.9 | 16.2 | 16.8 | |
| Modality as peritoneal dialysis At initiation (%) At 90 days (%) |
5.7 5.9 |
6.3 6.8 |
7.9 8.4 |
<0.001 <0.001 |
| eGFR (ml/min/1.73m2, median, IQR) | 9.9 (7.2–13.2) | 10.2 (7.5–13.5) | 10.1 (7.4–13.3) | <0.001 |
| Medical co-morbidities | ||||
| Congestive heart failure (%) | 33.2 | 36.1 | 35.2 | <0.001 |
| Heart disease (includes valvular) (%) | 32.2 | 37.9 | 37.9 | <0.001 |
| Stroke (%) | 9.5 | 10.6 | 11.1 | <0.001 |
| Hypertension (%) | 84.2 | 85.4 | 85.2 | <0.001 |
| Diabetes mellitus (%) | 52.5 | 56.5 | 56.6 | <0.001 |
| COPD (%) | 8.4 | 11.9 | 12.0 | <0.001 |
| Cancer (%) | 7.2 | 7.8 | 8.3 | <0.001 |
| Needs assistance with ADLs (%) | 13.0 | 15.2 | 15.0 | <0.001 |
| Institutionalized (nursing home) (%) | 6.8 | 7.8 | 7.7 | <0.001 |
| Tobacco use (%) | 5.9 | 7.9 | 8.5 | <0.001 |
| Alcohol use (%) | 1.5 | 1.6 | 1.5 | 0.452 |
| Illicit drug use (%) | 1.5 | 1.0 | 0.8 | <0.001 |
| ZIP-code based measures of community socioeconomic status | ||||
| Household income, thousands (median, IQR) | $40.2 (31.8–51.4) | $33.0 (29.3–37.9) | $30.6 (26.8–35.3) | <0.001 |
| High school diploma (mean %, SD) | 77.3 (13.3) | 75.4 (9.9) | 72.5 (10.5) | <0.001 |
Statistical testing performed by Pearson’s chi-squared for categorical variables, Kruskal–Wallis test for continuous variables, standard deviation (SD) and 25–75th interquartile ranges (ICR) are reported for means and medians, respectively.
Estimates of ZIP-code income and education are based on calculations from the US Census, 2000.
Prevalence of pre-ESRD care
The overall prevalence of pre-ESRD care was poor in the study cohort (nephrology care 53.5%, permanent dialysis access 17.7%, dietary education 11.9%). Table 2 summarizes the prevalence of pre-ESRD care by geographic residential location. The unadjusted prevalence and adjusted relative risk (RR) of early nephrology care and permanent dialysis access (among hemodialysis patients) was similar across geographic groups. Dietary education prior to initiation of dialysis was significantly less likely to occur among micropolitan (RR 0.80 95% CI 0.69–0.93) and rural patients (RR 0.85 95% CI 0.73–0.98). Patients who received dietary education had significantly higher serum albumin at the time of initiation (3.30 g/dL with SD 0.70) compared to those without dietary education (3.11 g/dL with SD 0.72), p<0.001. The serum albumin among patients who received dietary education did not vary by geographic region (urban 3.30 g/dL with SD 0.69, micropolitan 3.29 g/dL with SD 0.70, rural 3.26 g/dL with SD 0.71), p=0.12.
Table 2.
Prevalence of pre-ESRD care goals by geographic location
| Urban | Micropolitan | Rural | |
|---|---|---|---|
| Nephrologist care >6 months prior to initiation of dialysis | |||
| Prevalence (%) | 46.8 | 45.3 | 46.1 |
| Unadjusted | 1.00 (reference) | 1.03 (1.01–1.04) | 1.01 (0.99–1.03) |
| Adjusted | 1.00 (reference) | 1.02 (0.98–1.06) | 1.02 (0.97–1.07) |
| Mature permanent dialysis access (AVF or AVG) used upon initiation of hemodialysis | |||
| Prevalence (%) | 17.7 | 17.8 | 17.9 |
| Unadjusted | 1.00 (reference) | 1.01 (0.98–1.04) | 1.01 (0.98–1.05) |
| Adjusted | 1.00 (reference) | 1.03 (0.95–1.10) | 1.04 (0.96–1.12) |
| Dietary education received prior to initiation of dialysis | |||
| Prevalence (%) | 12.4 | 9.5 | 10.1 |
| Unadjusted | 1.00 (reference) | 0.76 (0.73–0.80) | 0.81 (0.77–0.85) |
| Adjusted | 1.00 (reference) | 0.80 (0.69–0.93) | 0.85 (0.73–0.98) |
Relative risks by Poisson regression (95% confidence intervals).
Multivariable models adjusted for age, sex, race, BMI, insurance coverage, employment status, ZIP-code median household income, presumed primary cause of CKD, eGFR at initiation, modality choice at 90 days after initiation of dialysis, and medical co-morbidities.
Dialysis mortality and likelihood of kidney transplantation
The association of pre-ESRD care on mortality and kidney transplantation was examined. Table 3 summarizes the multivariable Cox models for the association of pre-ESRD care with dialysis mortality and kidney transplantation, stratified by geographic residence. Early nephrology care (HR death 0.79 95% CI 0.78–0.80), permanent dialysis access (HR death 0.63 95% CI 0.62–0.65), and dietary education (HR death 0.90 95% CI 0.88–0.92) are all associated with decreased mortality with no evidence of effect modification by geographic strata. Early nephrology care (HR transplant 1.45 95% CI 1.39–1.52) and dietary education (HR transplant 1.26 95% CI 1.20–1.33) both increase the likelihood of kidney transplantation, an effect that is also demonstrated at similar magnitude across geographic strata. All tests for interaction (comparison between strata) were not statistically significant (p>0.10).
Table 3.
Association of pre-ESRD goal and long-term outcomes with stratification by geographic location
| All locations | Urban | Micropolitan | Rural | ||
|---|---|---|---|---|---|
| Death | |||||
| Early nephrology care | All | 0.79 (0.78–0.80) | 0.79 (0.77–0.80) | 0.81 (0.77–0.85) | 0.82 (0.78–0.86) |
| Permanent dialysis access | HD only | 0.63 (0.62–0.65) | 0.64 (0.62–0.65) | 0.60 (0.56–0.65) | 0.66 (0.62–0.71) |
| Dietary education | All | 0.90 (0.88–0.92) | 0.90 (0.88–0.93) | 0.91 (0.83–0.98) | 0.87 (0.80–0.94) |
| Kidney transplantation | |||||
| Early nephrology care | All | 1.45 (1.39–1.52) | 1.45 (1.38–1.52) | 1.51 (1.32–1.73) | 1.38 (1.21–1.58) |
| Permanent dialysis access | HD only | 1.19 (1.13–1.25) | 1.20 (1.13–1.27) | 1.20 (1.03–1.40) | 1.07 (0.91–1.26) |
| Dietary education | All | 1.26 (1.20–1.33) | 1.25 (1.18–1.33) | 1.30 (1.09–1.54) | 1.33 (1.12–1.57) |
Hazard ratios by Cox regression (95% confidence intervals).
Multivariable models adjusted for age, sex, race, BMI, insurance coverage, employment status, ZIP-code median household income, dialysis modality at 90 days, presumed primary cause of CKD, eGFR at initiation, and medical co-morbidities.
Discussion
Micropolitan and rural dialysis patients obtained early nephrology care and permanent hemodialysis access at comparable rates as urban patients and shared a similar reduction in mortality from such care, suggesting that geographic residence does not substantially impact basic pre-ESRD care in the US dialysis population. The notable exception is dietary education, where micropolitan and rural dialysis patients receive less pre-ESRD dietary care.
Previous studies focusing on rural health have described barriers that may preclude quality care in the micropolitan or rural setting. This includes dependence on community health centers, challenges in subspecialty access[17], clustering of providers in urban areas[18], and lack of access to transportation[19]. A retrospective study in Canada by Tonelli and colleagues found remote-dwelling CKD patients, defined as an eGFR <45 ml/min, were less likely to be referred to a nephrologist and have quality CKD care. In addition, remote patients were more likely to die during follow-up, presumably from higher cardiovascular risk and limitations in healthcare access in rural Canada locales[18]. In contrast, our study of US dialysis patients suggests that micropolitan and rural patients obtain pre-ESRD care from nephrologists and dialysis access surgeons at a similar prevalence as those in urban communities. A number of factors may influence these disparate findings. First, our study focused on dialysis patients who survived advanced CKD to initiate dialysis and not the general CKD population. Second, advanced chronic kidney disease, often with an eGFR<20 ml/min, is associated with significant morbidity and mortality requiring subspecialty and multidisciplinary care. In such circumstances, the proximity to needing dialysis treatments may trump physical remoteness as a barrier to access of pre-ESRD care. Third, dialysis unit penetration into rural areas has advanced over time, allowing increasing access to nephrology services while maintaining similar facility outcomes when compared to urban units[20]. Lastly, it is important to recognize that the overall prevalence of basic pre-ESRD care in the United States is exceedingly low regardless of geographic location, which makes detecting differences between regions less likely and highlights the need for systematic improvements throughout the nation.
Despite the lack of differences in nephrology care and permanent access placement, micropolitan and rural dialysis patients had less pre-ESRD dietary care compared to urban. Pre-ESRD dietary education is recommended for all patients with advanced CKD[21]. It is a complex task that requires attention to nutrition, fluid and protein intake, sodium, potassium, and phosphorus. Counseling can improve the management of hyperkalemia, hyperphosphatemia, and avoidance of protein-energy wasting[21, 22]. Dietary education is associated with lower dialysis mortality, a finding that was first noted in another cohort of USRDS patients by Slinin and colleagues[23] and corroborated in this study population. Patients who receive pre-ESRD dietary education initiate dialysis with higher serum albumin that does not vary with geographic residence. This suggests that receipt of dietary education confers similar benefit regardless of where patients live.
The prevalence of pre-ESRD dietary education was low, suggesting that barriers to dietary education exist in all geographic strata but are more pronounced in micropolitan and rural areas. The mechanisms behind this are less clear, but likely relate to a lack of qualified kidney dieticians in remote locations. Further research is required to determine the barriers to dietary care and to develop strategies to improve receipt of pre-ESRD care in all geographic locations.
While the dialysis mortality benefit from pre-ESRD care is important, kidney transplantation is the only cure for dialysis-dependent kidney disease. Our study demonstrated that better pre-ESRD care independently increases the likelihood of kidney transplantation. The factors that influence this finding are unknown, but could be related to improved participation in care, better educational resources, or avoidance of blood transfusions through use of erythropoiesis stimulating agents. In addition, pre-ESRD care has been associated with pre-emptive kidney transplantation[24], suggesting quality CKD care could drive referrals to kidney transplant centers. Pre-ESRD care among micropolitan and rural patients associates with kidney transplantation similarly to urban patients, suggesting that receiving care confers a similar benefit regardless of where a patient lives.
There are important limitations to this study. First, we focused on patients with kidney disease who survived to dialysis initiation, a subpopulation of the patients with advanced CKD, and is source for survival bias. Second, the source of data regarding pre-ESRD care was the medical evidence report (CMS 2728), which can have inconsistencies and errors, especially when completed by non-physicians[25, 26]. The medical evidence report, however, is the only available source of data within USRDS to study pre-ESRD care in all adults. However, the documentation of longitudinal outcomes, including death and kidney transplantation, are more robust due to internal redundancies within USRDS[27]. Third, we assessed geographic residence at dialysis initiation that did not account for patient movement over time, which could lead to misclassification. While every effort was made to reduce the impact of confounding, unmeasured confounders and the use of community-level surrogates for SES may limit the interpretation of the results. Lastly, the results reported are associations and cannot imply causality.
Conclusions
There is no observed difference in the prevalence of early nephrology care or permanent dialysis access in the pre-ESRD period between micropolitan, rural, and urban dialysis patients. The protective effect of pre-ESRD care is similar across all geographic strata. Micropolitan and rural patients receive less dietary education than urban patients, suggesting that barriers exist to quality dietary care in remote locations.
Acknowledgements
Dr. Maripuri is supported by an institutional training grant from the National Institutes of Health (T32 DK007569). Drs. Cavanaugh and Ikizler are supported by grants from the National Institutes of Health (K23 DK080952 and K24 DK062849, respectively). Dr. Cavanaugh is also supported by the American Society of Nephrology Carl W. Gottschalk Research Scholar Grant. Funding for patient-level data, provided by the US Renal Data System, was supported by the Vanderbilt CTSA grant UL1 RR024975-01 from the National Center for Research Resources, National Institutes of Health. This paper does not represent the opinion of the US Renal Data System or the National Institutes of Health. Drs. Maripuri and Cavanaugh assume full responsibility for data management and statistical analysis.
Footnotes
No authors have any conflicts of interest to disclose.
The findings of this study were presented at the Southern Society for Clinical Investigation 2012 Annual Meeting, New Orleans, LA and National Kidney Foundation 2012 Spring Clinical Meeting, Washington, DC.
REFERENCES
- 1.United States Census Bureau. Census 2000 Summary File (SF-1) 100-Percent Data. 2000
- 2.Maripuri S, Arbogast P, Ikizler TA, Cavanaugh KL. Rural and Micropolitan Residence and Mortality in Patients on Dialysis. Clin J Am Soc Nephol. 2012 doi: 10.2215/CJN.10831011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 4.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 5.Goransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med. 2001;250(2):154–159. doi: 10.1046/j.1365-2796.2001.00869.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen SC, Hwang SJ, Tsai JC, Liu WC, Hwang SC, Chou MC, Lin MY, Chang JM, Chen HC. Early nephrology referral is associated with prolonged survival in hemodialysis patients even after exclusion of lead-time bias. Am J Med Sci. 2010;339(2):123–126. doi: 10.1097/MAJ.0b013e3181c0678a. [DOI] [PubMed] [Google Scholar]
- 7.Frimat L, Loos-Ayav C, Panescu V, Cordebar N, Briancon S, Kessler M. Early referral to a nephrologist is associated with better outcomes in type 2 diabetes patients with end-stage renal disease. Diabetes Metab. 2004;30(1):67–74. doi: 10.1016/s1262-3636(07)70091-5. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzo V, Martn M, Rufino M, Hernandez D, Torres A, Ayus JC. Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: an observational cohort study. Am J Kidney Dis. 2004;43(6):999–1007. doi: 10.1053/j.ajkd.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Arora P, Obrador GT, Ruthazer R, Kausz AT, Meyer KB, Jenuleson CS, Pereira BJ. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol. 1999;10(6):1281–1286. doi: 10.1681/ASN.V1061281. [DOI] [PubMed] [Google Scholar]
- 10.Obrador GT, Ruthazer R, Arora P, Kausz AT, Pereira BJ. Prevalence of and factors associated with suboptimal care before initiation of dialysis in the United States. J Am Soc Nephrol. 1999;10(8):1793–1800. doi: 10.1681/ASN.V1081793. [DOI] [PubMed] [Google Scholar]
- 11.Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45–52. doi: 10.1001/jama.2011.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Measuring rurality: rural-urban commuting area codes. [Accessed: March 8];Economic Research Service, United States Department of Agriculture. 2011 http://www.ers.usda.gov/briefing/Rurality/RuralUrbanCommutingAreas.
- 13.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149–1155. doi: 10.2105/AJPH.2004.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ZIP code RUCA approximation methodology. [Accessed: March 8];Washington Wyoming Alaska Montana Idaho Rural Health Research Center. 2011 http://depts.washington.edu/uwruca/ruca-data.php.
- 15.United States Census Bureau. Census 2000 Summary File (SF-3) Sample Data: Median Household Income (P053) and Education Attainment for Population 25 Years or Older (P037) 2000
- 16.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook NL, Hicks LS, O'Malley AJ, Keegan T, Guadagnoli E, Landon BE. Access to specialty care and medical services in community health centers. Health Aff. 2007;26(5):1459–1468. doi: 10.1377/hlthaff.26.5.1459. [DOI] [PubMed] [Google Scholar]
- 18.Rucker D, Hemmelgarn BR, Lin M, Manns BJ, Klarenbach SW, Ayyalasomayajula B, James MT, Bello A, Gordon D, Jindal KK, Tonelli M. Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int. 2011;79(2):210–217. doi: 10.1038/ki.2010.376. [DOI] [PubMed] [Google Scholar]
- 19.Jennette CE, Vupputuri S, Hogan SL, Shoham DA, Falk RJ, Harward DH. Community perspectives on kidney disease and health promotion from at-risk populations in rural North Carolina, USA. Rural Remote Health. 2010;10(2):1388. [PubMed] [Google Scholar]
- 20.O'Hare AM, Johansen KL, Rodriguez RA. Dialysis and kidney transplantation among patients living in rural areas of the United States. Kidney Int. 2006;69(2):343–349. doi: 10.1038/sj.ki.5000044. [DOI] [PubMed] [Google Scholar]
- 21.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35(6 Suppl 2):S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan C, Sayre SS, Leon JB, Machekano R, Love TE, Porter D, Marbury M, Sehgal AR. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA. 2009;301(6):629–635. doi: 10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 23.Slinin Y, Guo H, Gilbertson DT, Mau LW, Ensrud K, Collins AJ, Ishani A. Prehemodialysis care by dietitians and first-year mortality after initiation of hemodialysis. Am J Kidney Dis. 2011;58(4):583–590. doi: 10.1053/j.ajkd.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosla N, Gordon E, Nishi L, Ghossein C. Impact of a chronic kidney disease clinic on preemptive kidney transplantation and transplant wait times. Progress in Transplantation. 2010;20(3):216–220. doi: 10.1177/152692481002000304. [DOI] [PubMed] [Google Scholar]
- 25.Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, McClellan WM. Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol. 2010;5(11):2046–2052. doi: 10.2215/CJN.03550410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JP, Desai M, Chertow GM, Winkelmayer WC. Validation of Reported Predialysis Nephrology Care of Older Patients Initiating Dialysis. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011080871. [DOI] [PubMed] [Google Scholar]
- 27.Eggers PW. CMS 2728: what good is it? Clin J Am Soc Nephrol. 2010;5(11):1908–1909. doi: 10.2215/CJN.08170910. [DOI] [PubMed] [Google Scholar]