Abstract
Background
It is difficult in clinical practice to differentiate patients with newly diagnosed diabetes and ketosis. The aim of this study was to investigate the effect of intensive insulin therapy on islet function in patients with new-onset diabetes and concomitant ketosis, and to determine the value of alternation in islet function in the typing of diabetes.
Material/Methods
A total of 206 inpatients with new-onset diabetes and ketosis were recruited after intensive insulin therapy and followed for 36 months. Patients were divided into type 1 diabetes group (Group A) and type 2 diabetes group (Group B). Islet function was compared between the 2 groups before and after intensive insulin therapy, and the influence of islet function on the typing of diabetes and the selection of therapeutic strategies is discussed.
Results
In group A, the AUCI, AUCC, HOMA-β cell and HOMA-IR were significantly lower than those in Group B before and after intensive insulin therapy. The sensitivity and accuracy of antibody test were at a low level in Group A. An insulin release test was done after intensive insulin therapy. Results showed that the peaks of insulin and C peptide appeared at 0.5–1 h after glucose administration in Group A, which was earlier than that before therapy, but the maximal levels were no more than 2 times those of baseline levels. In Group B, the peaks appeared at 2 h, and the maximal levels were about 10 times those of baseline levels.
Conclusions
Poor islet function, incomplete recovery of islet function after intensive insulin therapy, and a short “honeymoon” period are characteristics of type 1 diabetes. Detection of diabetes-related antibodies is not reliable.
Keywords: diabetic ketosis, typing, islet function, honeymoon period
Background
The age of patients with type 2 diabetes has a decreasing tendency owing to changes in life style. Recently, the incidence of diabetes has been increasing rapidly in China. In adults over age 20 years, the incidence of diabetes is 9.7%, but it is 15.5% in pre-diabetes [1]. Differentiation between type 1 and type 2 diabetes is most important in the diagnosis and treatment of diabetes. The type of diabetes is closely related to the selection of therapeutic regimen. According to different etiologic and pathophysiologic mechanisms, it seems easy to differentiate the 2 types of diabetes.
The American Diabetes Association (ADA) defines patients with type 1 diabetes as those who have an immunologic disorder, such as GAD-Ab positive. Regardless of islet beta cell function and insulin-dependence, ADA also defines ketosis-prone diabetes patients without immunologic evidence as a subset of type 1 diabetes called “idiopathic type 1” or “type 1B” diabetes [2]. Latent autoimmune diabetes in adults (LADA) is a form of autoimmune diabetes that resembles T1DM, but the manifestations of LADA are different from those of type 1 diabetes. LADA shows a later onset and slower progression towards insulin dependence [3]. It appears that age is no longer as important as before in the diagnosis of diabetes. In China, detection of diabetes antibodies has limitations in the sensitivity and accuracy, which makes physicians confused. The positive rate of GAD-Ab and/or IA-2A is only 44.5% in clinical definitive diagnosed type 1 diabetes [4], while it is 13.5% in clinic preliminary diagnosed type 2 diabetes [5].
Clinically, patients with acute onset diabetes and tendency to ketosis usually present with distinct long-term prognosis. The treatment of diabetes in some of them is insulin-independent, but most of them do not require insulin after short-term insulin therapy. The biochemical parameters and islet function in these patients are similar to those in type 2 diabetes patients. Thus, some experts do not agree with the “idiopathic type 1” definition; thus, this classification has not been widely accepted [6,7].
Some American experts have divided ketosis-prone diabetes patients into 4 subtypes by islet beta-cell function (preserved/absent) and immunologic index (islet auto-antibiosis: positive/negative). They defined the islet beta cell function as “preserved” if the fasting serum C-peptide level was 1 ng/ml or the maximum glucagon-stimulated serum C-peptide level was 1.5 ng/ml, or “absent” if the fasting serum C-peptide level was 1 ng/ml or the maximum glucagon-stimulated serum C-peptide level was 1.5 ng/ml, believing that this classification have more clinical value for evaluating prognosis [6].
Therefore, it is difficult in clinical practice to differentiate patients with newly diagnosed diabetes and ketosis to determine the long-term therapeutic regimen. In the present study, subjects with acute diabetes onset and ketosis were followed for 36 months and insulin-dependence status was determined at the end of follow-up. Then, the diagnosis and type of diabetes, as well as the islet function, were retrospectively evaluated, aiming to finding effective indicators for differentiation of type 1 and type 2 diabetes. The aim of this study was to investigate the effect of intensive insulin therapy on islet function in patients with new-onset diabetes and concomitant ketosis, and to determine the value of changes in islet function to the typing of diabetes.
Material and Methods
Subjects
From August 2003 to May 2007, a total of 467 in-patients with newly-onset diabetes and ketosis were recruited. On the basis of diet control and exercise, intensive insulin therapy (IIT) was repeatedly administered shortly before follow-up. Of the 467 patients, 214 were in a honeymoon period (when the immunological attack is stopped, but the deterioration of β cell function continues as long as the immunological reaction is not stopped) after IIT, and then were followed up for 36 months. Among 214 patients, 5 withdrew from the study because of unauthorized medication (1 case) and consent withdrawn (2 cases), and 3 were lost to follow-up. The remaining 206 patients (107 males and 99 females) were included in the final analysis. The mean age was 43.56±5.7 years (range: 17–58 years), the body mass index (BMI) was 19.56–31.22 kg/m2, the course of disease ranged from 0 to 12 months. On the 1st visit, the fasting plasma glucose (FPG) was 9.89–18.78 mmol/L, the 2-h postprandial plasma glucose (PPG) was 16.16–34.11 mmol/L, and the HbA1c was 9.71–15.20%. Diabetes was diagnosed according to the Criteria for Diabetes developed by the WHO in 1999. Patients had no stress, no severe injured liver or kidney functions (alanine aminotransferase [ALT] and aspartate aminotransferase [AST] <2.5 times of normal upper limit; normal kidney function, serum creatinine [Cr] <120 μmol/dL), and diseases affecting the glucose metabolism were excluded.
Examination and treatment
On admission, all patients received physical and biochemical examinations such as height, weight, blood pressure, HbA1c, fasting blood sugar (FBS), 2-h postprandial blood sugar (PBS), triglyceride (TG), high-density lipoprotein cholesterol (HDL-ch), low-density lipoprotein cholesterol (LDL-ch), total cholesterol (Tch), Cr, uric acid (UA), ALT, and AST. The diabetes-related antibodies were detected with enzyme-linked immunosorbent assay (ELISA; Biomerica Corporation) and included GAD-Ab, IAA, and ICA. Ultrasonography was done in the liver, gallbladder, and spleen. When patients developed acidosis, examinations were repeated after the acidosis was cured. Besides fluid supplement, diet control, and physical exercise, patients were subcutaneously treated with Novolin R (Biosynthetic Human Insulin Injection) at 15 minutes before each meal and with Novolin N (Isophane Protamine Biosynthetic Human Insulin Injection) before breakfast and/or before sleep. The blood glucose was measured before and after each meal and before sleep. The dose of insulin was adjusted to control the FBS at 3.3–6.5 mmol/L and 2-h PBS at 3.3–8.0 mmol/L and maintain the glucose level in a stable state.
At 2–5 weeks after IIT, all patients were in a honeymoon period in which the blood glucose (HBA1C <7%) was controlled at a normal level by diet and exercise. Indication for discontinuation of insulin treatment was the daily dose of insulin <0.3 U/kg/patient. Of these patients, 19 required IIT again to control the blood glucose after a 4–9 month honeymoon and were classified as Group A (type 1 diabetes). The remaining 187 patients had a honeymoon period of 7–36 months and did not require IIT to control the blood glucose after the honeymoon. These patients were included in Group B (type 2 diabetes). Before and after IIT, oral glucose tolerance test (OGTT), detection of insulin, and C peptide test were performed. At 1 day before testing, insulin treatment was stopped, and 75 g of oral glucose was administered on the morning of the next day. The serum blood glucose, insulin, and C peptide were measured at 0, 30, 60, 120, and 180 min after glucose administration.
Follow up
Patients were followed for 3 years. The BMI, FBS, and 2-h PBS after 3 meals were measured at least 4 times monthly. The doses of insulin and oral antidiabetics (OAD) were adjusted at each hospital visit. The HbA1c was measured once every 3 months to maintain the blood glucose at a normal level (FBG <7.0 mmol/L, 2-h PBG <10.0 mmol/L, HbA1c <7%). Islet function was evaluated before and after IIT and the influence of islet function on the typing of diabetes and the selection of therapeutic regimen was recorded. The area under the curve (AUC) of insulin (AUCI), AUC of C peptide (AUCC), and AUC following OGTT (AUCG) were used to evaluate islet function. Homeostasis model assessment (HOMA) was used to evaluate the insulin resistance (HOMA-IR) and the β cell function (HOMA-β cell). The relation of islet function after IIT with the type of diabetes and therapeutic efficacy was further evaluated.
Statistical analysis
Quantitative data are expressed as mean ± standard deviation (χ̄±s), and the paired t test was used for the comparisons of data before and after IIT. Data were compared between the 2 groups using the 2 independent samples t test. The INS, CP, and PG had abnormal distribution and then underwent logarithmic transformation before calculation. The insulin sensitivity was calculated as the ratio of AUCG to AUCI HOMA-IR and HOMA-β cell. A value of P<0.05 was considered statistically significant.
The HOMA-IR and HOMA-β cell were calculated as follows: HOMA-IR=FBG×fasting insulin/22.5; HOMA-β cell = 20×fasting insulin/(FBG-3.5). The AUCI, AUCG and AUCC were calculated as follow: AUC=1/4×(S0+2×S30+3×S60+4×S120+2×S180), where S0, S30, S60, S120, and S180 were insulin level at 0, 30, 60, 120 and 180 min, respectively.
Results
All patients presented with polydipsia, polyuria, and/or loss of weight and fatigue of different extents as well as ketosis (urine ketone body: +-++++). Five patients had acidosis (blood pH <7.35), and only 2 developed digestive system symptoms (vomiting and nausea). Fluid supplement and infusion of low-dose insulin were done until the ketosis resolved, gastrointestinal symptoms disappeared, and patients were able to e a normal diet. Then, IIT was done. After IIT, polydipsia, polyuria, and/or loss of weight and fatigue resolved or were significantly improved. Serious hypoglycemia (blood glucose <2.8 mmol/l) was not observed during the IIT due to the guidance of diet control. In Group A, 19 patients (10 males and 9 females) were subcutaneously treated with Novolin or Humulin R before each meal and with Novolin or Humulin N before sleep. In Group B, 187 patients (97 males and 90 females) did not require insulin treatment, of which 82 had favorable blood glucose control with only diet control and exercise, 49 were treated with OAD within 12 months, 32 with OAD at 12 months, and 24 with OAD at 24 months.
Baseline characteristics and findings in biochemical examinations in the 2 groups
The patients in Group A were younger, which did not mean that there were no older patients. For example, 2 patients aged 47 years and 56 years, respectively, were included in Group A. Thus, age was not necessary in the typing of diabetes. Patients in Group A had lower BMI than those in Group B. The proportion of patients with a family history of diabetes, concomitant hypertension, or non-alcoholic fatty liver in Group A was lower than in Group B. The TCH-C, LDL-C, UA, and TG in Group A were significantly lower than in Group B, but the HDL-C was slightly higher. Patients in Group B had a higher ALT level, which might be attributed to the high incidence of non-alcoholic fatty liver in them. The duration of honeymoon period in Group A was shorter than in Group B, but there were no significant differences between the 2 groups in the duration of IIT before honeymoon and maximal daily insulin dose. In addition, only 21.2% and 18.1% of patients in Group A were positive for GAD-Ab and ICA, respectively, and were significantly higher than in Group B. The proportion of patients positive for IAA was comparable between the groups. Thus, detection of diabetes-related antibodies had low sensitivity and accuracy (Tables 1 and 2).
Table 1.
Characteristics of patients in two groups at baseline.
| Group | BMI (kg/m2) | Age (yr) | Family history of diabetes (n, %) | Duration of IIT (day) | Duration of honeymoon period (month) | Maximal daily insulin dose (U/kg/d) | Concomitant NAFLD n (%) | Concomitant hypertension n (%) |
|---|---|---|---|---|---|---|---|---|
| A (n=19) | 22.31±8.24 | 23.2±6.7 | 3 (15.8%) | 21.72±4.58 | 5.9±3.2 | 0.79±5.60 | 1 (5.3) | 2 (10.5) |
| B (n=187) | 26.89±7.12 | 45.6±9.5 | 105 (56.1%) | 20.54±5.44 | 20.9±9.9 | 0.86±8.28 | 69 (36.9) | 39 (20.8) |
| t | −10.237 | −10.831 | 16.425 | −1.677 | −12.481 | −0.198 | 13.247 | 30.915 |
| P | 0.0012 | 0.000 | 0.000 | 0.775 | 0.000 | 0.376 | 0.000 | 0.000 |
Group A – T1DM; Group B – T2DM; NAFLD – non-alcoholic fatty liver disease.
Table 2.
Biochemical and immunological parameters in two groups at baseline.
| GAD-Ab (%) | ICA (%) | IAA (%) | TCH-C | LDL-C | TG | HDL-C | UA | AST | ALT | |
|---|---|---|---|---|---|---|---|---|---|---|
| A (n=19) | 21.1 | 26.3 | 15.8 | 4.42±1.58 | 2.31±0.49 | 1.39±0.30 | 1.30±0.11 | 169.22±39.30 | 26.71±5.23 | 26.27±9.39 |
| B (n=187) | 4.8 | 3.2 | 10.6 | 4.74±2.24 | 2.79±0.93 | 2.86±2.28 | 0.89±0.12 | 385.16±83.57 | 29.21±9.36 | 39.82±15.34 |
| t | 6.089 | 9.877 | 3.787 | −0.351 | −0.369 | −5.536 | 1.102 | −5.162 | −6.777 | −13.907 |
| P | 0.014 | 0.027 | 0.798 | 0.623 | 0.413 | 0.0012 | 0.253 | 0.000 | 0.169 | 0.023 |
Group A – T1DM; Group B – T2DM; GADAb – glutamate decarboxylase antibody; ICA – Islet cell antibodies; IAA – Insulin IgG antibody.
Blood glucose and islet function in the 2 groups before and after treatment
The 206 patients with new-onset diabetes were followed for 36 months after IIT and then retrospectively evaluated. The AUCG, AUCI, AUCC HOMA-β cell, and HOMA-IR were compared between the 2 groups. The AUCI, AUCC, and HOMA-β cell in 2 groups increased significantly and the AUCG and HOMA-IR reduced markedly in the post-treatment honeymoon period. The AUCI, AUCC, and HOMA-β cell in Group A were significantly lower than in Group B before treatment, and the differences in these parameters between the 2 groups were more obvious after treatment. There were no marked differences between the 2 groups in HOMA-IR and AUCG before treatment. However, the HOMA- IR in Group A was markedly reduced when compared with Group B after treatment, and the AUCG in Group B was only slightly lower than in Group A (P>0.05). These findings suggest that the islet function in Group A before IIT and in the honeymoon period after IIT were significantly compromised when compared with Group B. Although the islet function was partially improved after IIT, the islet function was still significantly compromised when compared with Group B, but the sensitivity to insulin in Group A increased dramatically when compared with Group B (Table 3).
Table 3.
Blood glucose and islet function in two groups before and after IIT.
| AUCC (ng·h·mL−1) | AUC I (uIu·h·L−1) | AUCG (mmol·h·L−1) | HOMA- IR | Homa-β cell | |
|---|---|---|---|---|---|
| A before IIT (n=19) | 1.56±0.53 | 10.18±2.36 | 66.89±10.25 | 1.22±0.49 | 3.68±1.08* |
| B before IIT (n=187) | 3.75±0.67 | 20.28±6.89 | 66.35±12.58 | 3.06±1.54 | 18.20±6.59 |
| t | −15.521 | −10.038 | 14.823 | −12.587 | −16.526 |
| P | 0.002 | 0.000 | 0.980 | 0.003 | 0.000 |
| A in honey moon period (n=19) | 3.99±0.79 | 29.86±8.65 | 29.94±3.40 | 0.63±0.56 | 8.50±2.46 |
| B in honey moon period (n=187) | 12.54±3.83 | 93.35±19.42 | 27.14±3.37 | 1.32±0.70 | 56.17±19.42 |
| t | 5.793 | −11.600 | 2.452 | −9.683 | −25.437 |
| P | 0.000 | 0.000 | 0.072 | 0.026 | 0.000 |
Group A – T1DM; Group B – T2DM; AUCC – area under ROC of C peptide; AUCI – area under ROC of insulin; AUCG – area under ROC of OGTT; HOMA-IR – homeostatic model assessment of insulin resistance; HOMA-β – homeostatic model assessment of β cell function.
OGTT, insulin measurement and C peptide test were performed again after IIT
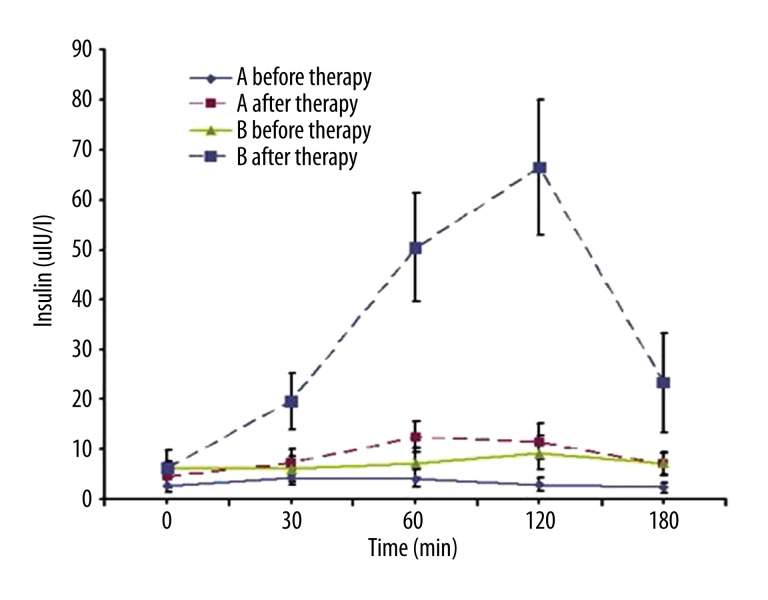
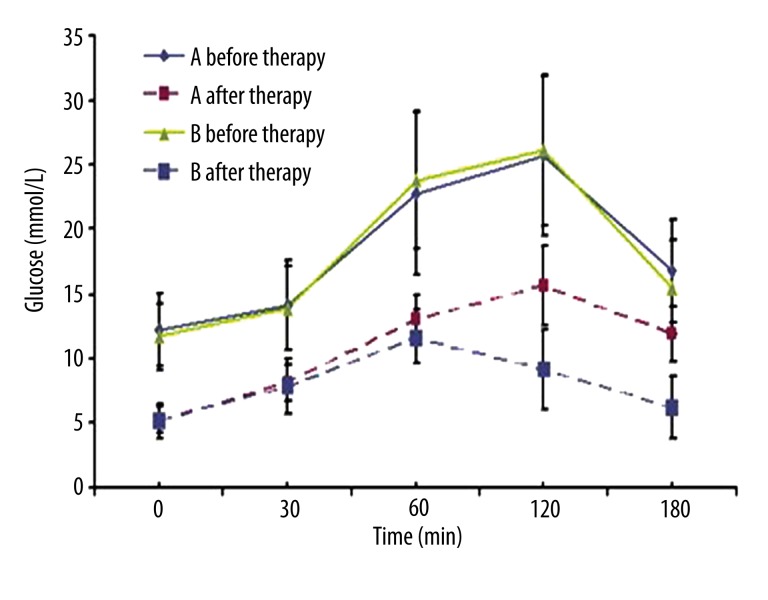
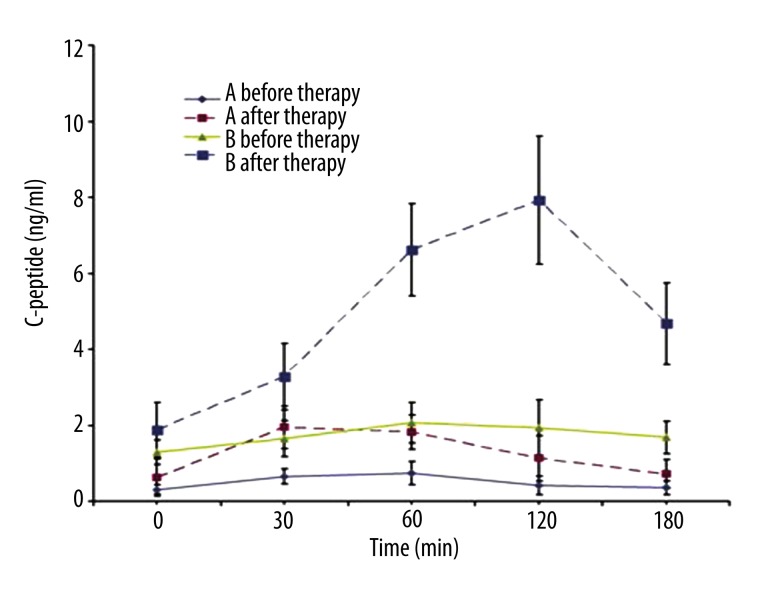
In Group A, the peaks of insulin and C peptide occurred at 0.5–1 h after glucose administration, but the maximum levels of insulin and C peptide were no more than 2 times baseline levels. In Group B, the peaks of insulin and C peptide appeared at 1–2 h after glucose administration, and the maximum levels were 4–10 times baseline levels. There was no significant difference in the FPG, but the 2–3 h PPG in Group A was significantly higher than in Group B (Figures 1–3).
Figure 1.
Insulin release curve of both groups before and after IIT. Before IIT, the ROC of insulin was a straight line in both groups, and no insulin peak was noted after oral glucose administration. In addition, the insulin level in the Group A was markedly lower than that in the Group B at 0, 60, 120 and 180 min after glucose administration (P<0.05). After IIT, the insulin peak was found at 1 h after oral glucose administration in the Group A, but the maximal insulin level was no more than 2 times that at baseline. In the Group B, the insulin peak was noted at 2 h after oral glucose administration and maximal insulin level was about 10 times than at baseline. Group A: T1DM; Group B: T2DM.
Figure 3.
OGTT curve of both groups before and after IIT. OGTT was similar in both groups before IIT. After IIT, the FPG was comparable between two groups, but significant difference was noted in the 2–3 h PG between two groups (P<0.05). Group A: T1DM; Group B: T2DM.
Discussion
In recent years, the incidence of diabetes has risen rapidly, and the age of diabetes patients has been decreasing [1]. Thus, it is imperative to investigate the causes of diabetes and to develop preventive strategies to delay its progression. It is necessary to type the diabetes because the therapeutic regimen, therapeutic efficacy, risk, and prognosis are different between type 1 and type 2 diabetes.
It has been confirmed that autoimmune-mediated damage to the β cells is a basic mechanism underlying the pathogenesis of type 1 diabetes [8], and type 2 diabetes is a metabolic disorder closely related to life-style and characterized by insulin resistance or relatively insufficient insulin secretion. Theoretically, it is easy to differentiate type 1 diabetes from type 2 diabetes. However, the clinical manifestations and pathological process vary among individuals. The differentiation between type 1 and type 2 diabetes has been a challenge in the diagnosis of diabetes, especially in non-Caucasians, including Asians and Africans [6]. In China, the typing of diabetes is also difficult in clinical practice. In the present study, patients with acute onset diabetes and ketosis were recruited and followed for 36 months. At the onset of diabetes, they received IIT to determine whether the diabetes was insulin-dependent, which was then used to type the diabetes. Moreover, the BMI, biochemical parameters, diabetes-related antibodies, and islet function were retrospectively evaluated to provide evidence for the differentiation between type 1 and type 2 diabetes.
Our findings also revealed that the type of diabetes based on the diabetes-related antibodies was largely inconsistent with that based on clinical indicators, which might be attributed to the time and methods of detection, or the low positive rate in Asians. Regardless of the reliability of immunological indicators, classification on the basis of these indicators may not be used to guide the clinical treatment of diabetes. Although the clinical outcome is a reliable indicator for the typing of diabetes, identifying some reliable risks in the early stage of diabetes is still a challenge. It has been demonstrated that IIT may cure ketosis, improve glucose-induced cytotoxicity, and improve islet function enough to discontinue the insulin therapy in the early phase of diabetes, and patients then are in the honeymoon period. However, this condition is temporary for type 1 diabetes patients but long-lasting for type 2 diabetes patients [9]. The mechanism underlying the entry into the honeymoon period is still unclear for patients with type 1 diabetes. The proliferation or regeneration of residual β cells in the islet is due to the remission of inflammation and the improvement of glucose metabolism. However, the immunogenic environment is long-lasting, which provides more antigens for the proliferative β cells. The subsequent intensified immunological attack finally results in the complete loss of β cell function [10]. In type 2 diabetes, there is no or mild autoimmunity against β cells in the islets, and thus the function of residual β cells, or the β cell function after the improvement of glucose metabolism, is better than that in type 1 diabetes. There are differences in the pathogenesis and islet function between type 1 and type 2 diabetes, especially in patients with new-onset diabetes. After IIT, the patients enter the honeymoon period, and the degree of islet function recovery is closely related to the pathological basis. A variety of studies have investigated islet function in the honeymoon period in type 2 diabetes patients. Results reveal that the islet function significantly improves in patients with new onset diabetes after IIT, and the islet function even returns to normal [11]. However, few studies have explored the islet function in the honeymoon period in type 1 diabetes patients. Theoretically, the islet function in the honeymoon period in type 1 diabetes patients is poorer than that in type 2 diabetes patients. In the present study, the islet function was compared after IIT in 2 groups, aiming to validate the above hypothesis. Our results showed the islet function in Group B (type 2 diabetes) was better than that in Group A (type 1 diabetes) before IIT. After IIT, the improvement in islet function of type 2 diabetes patients was more obvious when compared with type 1 diabetes patients. Especially at 1–2 h after glucose administration, the amounts of released insulin and C peptide were significantly increased (4–10 times baseline level) in Group B, but those in Group A were only about 2 times baseline level. HOMA-IR in Group B was higher than in Group A before and after IIT, suggesting the insulin resistance in Group B. Patients in Group A had favorable insulin sensitivity, especially in the honeymoon period. This suggests that islet function improves in the honeymoon period after IIT in type 1 diabetes patients, but is still poorer than that in the type 2 diabetes patients. However, the insulin sensitivity of type 1 diabetes patients is superior to that of type 2 diabetes patients. In addition, the OGTT showed there was no marked difference in the FPG, but the 2-h PPG of Group A was markedly higher than that of Group B. Thus, blood glucose can be favorably controlled on the basis of diet control, which may be identified in the routine measurement of blood glucose. Patients in Group A are susceptible to postprandial hyperglycemia after overeating, which suggests that the relatively insufficient insulin secretion is more evident after meals in type 1 diabetes, even in the honeymoon period. In addition, the honeymoon period in type 1 diabetes patients was shorter than that in type 2 diabetes patients. The honeymoon period is a period when the immunological attack is stopped, but the deterioration of β cell function will continue as long as the immunological reaction is not stopped. However, the honeymoon period in type 2 diabetes patients might reflect the recovery of islet function after the attenuation of glucose- and lipid-induced cytotoxicity. The progression of type 2 diabetes might be delayed or even reversed as long as the environmental factors (such as change in life-style) remain [12]. Thus, islet function in the early phase of diabetes and in the honeymoon period may be a reliable measure for predicting therapeutic efficacy and prognosis.
The findings about the influence of BMI and age on the typing of diabetes were consistent with previously reported. Although the type 2 diabetes patients were older than the type 1 diabetes patients, there were also young patients in the Group B (the youngest patient in Group B was 14 years old). BMI may serve as an indicator for the typing of diabetes. Our findings revealed that there was marked difference in the BMI between the 2 groups, and BMI was closely related to islet function. Waist circumference and waist-to-hip ratio may also serve as good indicators for typing of diabetes [13]. Hypertension, fatty liver, hyperuricemia, and lipid metabolism disorder are the important components of metabolic syndrome and all suggest the presence of insulin resistance. In type 2 diabetes, insulin resistance is a major pathogenesis [14]. Thus, to identify the evidence reflecting insulin resistance or metabolic syndrome is crucial in differentiating type 1 from type 2 diabetes. Although our findings indicated that the patients in Group A had lower BMI than in Group B, the BMI cannot be used to differentiate the 2 types of diabetes when BMI is close to the normal level.
Conclusions
This study concluded that waist circumference, waist-to-hip ratio, concomitant hypertension, fatty liver, hyperuricemia, and lipid metabolism disorder, as well as the opinion of diabetes experts, can be used to effectively differentiate type 1 from type 2 diabetes, which may provide evidence for predicting the duration of the honeymoon period and selection of long-term therapeutic regimen after the honeymoon period. Thus, the age of onset, acute onset, tendency to ketosis, and detection of diabetes-related antibodies may serve as secondary indicators, and ca not be used as major evidence to guide the typing of diabetes and selection of long-term therapeutic regimen. Islet function, especially the islet function after IIT, is crucial for the typing of diabetes and the prediction of therapeutic efficacy and prognosis.
Figure 2.
C-peptide release curve of both groups before and after IIT. Before IIT, the ROC of C peptide was a straight line in both groups, and no C peptide peak was noted after oral glucose administration. In addition, the C peptide level in the Group A was markedly lower than that in the Group B at different time points after glucose administration (P<0.05). After IIT, the C peptide peak was found at 0.5 h after oral glucose administration in the Group A, but the maximal C peptide level was no more than 2 times that at baseline. In the Group B, the C peptide peak was noted at 2 h after oral glucose administration and maximal C peptide level was about 10 times than at baseline. Group A: T1DM; Group B: T2DM.
Footnotes
Conflict of interest
None declared.
Source of support: Departmental sources
References
- 1.Yang W, Lu J, Weng J. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naik RG, Brooks-Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab. 2009;94:4635–44. doi: 10.1210/jc.2009-1120. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Zhou ZG, Lin J, et al. Use of ABC typing to redefine subtypes of acute-onset type 1 diabetes mellitus: study of 308 patients. Zhonghua Yi Xue Za Zhi. 2008;88(12):797–801. [PubMed] [Google Scholar]
- 5.Wu YJ, Hu YF, Zhao L, et al. Relationship between cell function and islet function in diabetes. Zhonghua Nei Fen Mi Dai Xie Za Zhi. 2003;19(1):17–20. [Google Scholar]
- 6.Balasubramanyam A, Garza G, Rodriguez L, et al. Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care. 2006;29:2575–79. doi: 10.2337/dc06-0749. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Román MA, Piñero-Piloña A, Adams-Huet B, Raskin P. Comparison of type 1, type 2, and atypical ketosis-prone diabetes at 4 years of diabetes duration. J Diabetes Complications. 2006;20:137–44. doi: 10.1016/j.jdiacomp.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 9.Wajchenberg BL. Clinical approaches to preserve beta-cell function in diabetes. Adv Exp Med Biol. 2010;654:515–35. doi: 10.1007/978-90-481-3271-3_23. [DOI] [PubMed] [Google Scholar]
- 10.Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57:2883–88. doi: 10.2337/db07-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–60. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 12.Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 13.Mihic M, Modi P. Metabolic syndrome – risk factors for atherosclerosis and diabetes. Curr Diabetes Rev. 2008;4:122–28. doi: 10.2174/157339908784220750. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher EJ, Leroith D, Karnieli E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt Sinai J Med. 2010;77:511–23. doi: 10.1002/msj.20212. [DOI] [PubMed] [Google Scholar]