Abstract
TLR3 is known to respond to dsRNA from viruses, apoptotic cells, and/or necrotic cells. Dying cells are a rich source of ligands that can activate TLRs, such as TLR3. TLR3 expressed in the liver is likely to be a mediator of innate activation and inflammation in the liver. The importance of this function of TLR3 during acute hepatitis has not previously been fully explored. We used the mouse model of Con A-induced hepatitis and observed a novel role for TLR3 in hepatocyte damage in the absence of an exogenous viral stimulus. Interestingly, TLR3 expression in liver mononuclear cells and sinus endothelial cells was up-regulated after Con A injection and TLR3−/− mice were protected from Con A-induced hepatitis. Moreover, splenocytes from TLR3−/− mice proliferated less to Con A stimulation in the presence of RNA derived from damaged liver tissue compared with wild-type (WT) mice. To determine the relative contribution of TLR3 expression by hematopoietic cells or nonhematopoietic to liver damage during Con A-induced hepatitis, we generated bone marrow chimeric mice. TLR3−/− mice engrafted with WT hematopoietic cells were protected in a similar manner to WT mice reconstituted with TLR3−/− bone marrow, indicating that TLR3 signaling in both nonhematopoietic and hematopoietic cells plays an important role in mediating liver damage. In summary, our data suggest that TLR3 signaling is necessary for Con A-induced liver damage in vivo and that TLR3 regulates inflammation and the adaptive T cell immune response in the absence of viral infection.
Introduction
T cell-mediated fulminant hepatitis is a life-threatening liver disease (1). It has been estimated that ~1% of people infected with hepatitis viruses develop acute fulminant hepatitis without effective treatment (2). In mice, fulminant T cell-mediated hepatitis can be induced by injection of the T cell mitogenic plant lectin Con A, which rapidly induces elevation of liver transaminase activities, massive T cell infiltration, and secondary necrosis (3). Con A-induced hepatitis in mice has been considered to be an animal model for human acute autoimmune hepatitis (4).
TLRs are germline-encoded pattern recognition receptors that are highly conserved in species as diverse as Drosophila and humans (5). They recognize pathogen-associated molecular patterns (PAMPs)3 and lead to key inflammatory responses. They also shape adaptive immunity (6, 7). TLR3 is expressed in cells of the immune system, such as macrophages, dendritic cells, neutrophils, B cells, and NK cells, and it is also expressed in tissue cells, such as biliary epithelial cells, sinusoidal endothelial cells, and hepatocytes (8, 9). TLR3 signaling pathways are active in hepatic immune disorders (10) and the immunoprivileged status of the liver is likely to be controlled by TLR3 signaling (11). Therefore, activation of TLR3 could provoke liver damage by production of type I IFN and/or by highly activated effector T cells (12). Increasingly, experimental evidence indicates endogenous ligands released from damaged/stressed tissues, designated as damaged tissue-associated molecular patterns (DAMPs), can also signal through TLRs (13, 14). Engagement of the TLRs by endogenous ligands, such as DAMPs, could be a major trigger of inflammation. Recent studies have suggested that RNA released from damaged tissue or contained within endocytosed cells might serve as a ligand for TLR3 (15).
Nonobese diabetic (NOD) mice develop spontaneous autoimmune diabetes, and the genetic makeup of NOD mice renders them susceptible to developing other autoimmune syndromes, such as autoimmune sialitis, thyroiditis, peripheral polyneuropathy, a systemic lupus erythematosus-like disease, and possibly other autoimmune disorders (16, 17). In this study, we used wild-type (WT) and TLR3−/− mice on both NOD and C57BL/6 genetic backgrounds, and tested the hypothesis that endogenous ligands released in the course of Con A-induced hepatocyte damage can activate innate immunity via TLR3 signaling. Our results showed that in the absence of an exogenous viral pathogen, TLR3 activation was important for the initiation and the amplification of hepatic inflammation possibly via recognition of cellular by-products from apoptotic and necrotic cells. Our results also support a novel notion that TLR3 is an endogenous inflammatory regulator in acute inflammation, such as Con A-induced autoimmune hepatitis.
Materials and Methods
Animals
NOD/Caj mice, originally obtained from The Jackson Laboratory, have been maintained at Yale University for >20 years. C57BL/6 (B6) mice were purchased from The Jackson Laboratory and maintained at Yale University for over 4 years. TLR3−/− B6 mice (18) were backcrossed >10 generations onto the NOD genetic background and intercrossed to obtain TLR3−/− NOD homozygotes. The mice used in the study were 6–10 wk old and housed in specific pathogen-free conditions with autoclaved food and bedding. The use of the animals and the procedures applied in this study were approved by the Institutional Animal Care and Use Committee of Yale University.
Induction of hepatitis
Con A (Sigma-Aldrich) was dissolved in sterile PBS and injected i.v. to mice at a dose of 15 mg/kg. Mice were bled 0, 8, and 24 h following Con A administration. Control mice were injected with PBS. Mice were sacrificed 24 h after Con A injection. Liver was perfused and divided into three portions: 1) embedded and frozen in OCT compound (Sakura Finetek), 2) fixed in 10% neutral-buffered formalin for paraffin embedding, and 3) used for isolation of liver mononuclear cells (MNCs).
Assessment of liver function
Serum alanine aminotransferase (ALT) was measured using a standard kit (Biotron Diagnostics).
Isolation of liver MNCs
Perfused livers were passed through a 200-gauge stainless steel mesh. The cells were resuspended in 30% Percoll (GE Healthcare) and 70% Percoll was gently placed under the suspension. After gradient centrifugation, liver MNCs were collected from the interface and washed with PBS.
Flow cytometric analysis
All fluorescence-conjugated Abs used in this study were purchased from eBioscience unless otherwise specified. After blocking with anti-FcR (clone 2.4G2), surface markers were identified by specific mAbs conjugated with different fluorochromes. Cells were analyzed by flow cytometry using a FACSCalibur (BD Biosciences). To analyze intracellular proteins, cells were first fixed and permeabilized and then stained with appropriate mAbs using a Cytofix/Cytoperm plus kit (eBioscience). Flow cytometric analysis was performed using FlowJo software (Tree Star).
Histological examination and immunostaining
Livers embedded in paraffin were sectioned at 3 μm and stained with H&E by standard methods. For immunostaining, perfused liver was fixed overnight in periodate-lysine-paraformaldehyde fixative (2% paraformaldehyde, 0.075 M lysine, 0.037M sodium phosphate, and 0.01 M periodate). After embedding in OCT compound (Sakura Finetek), the liver tissue was snap frozen. Cryosections (10 μm) were rehydrated with PBS followed by blocking with 2% goat serum (Sigma-Aldrich). The following primary Abs were used to stain the liver sections: rat anti-mouse ICAM (Invitrogen), rat anti-mouse ICAM-FITC, rat anti-mouse CD11b-allophycocyanin (eBioscience), rat anti-mouse TLR3 (Imgenex) followed by goat anti-rat IgG conjugated with Alexa Fluor 546 or goat anti-FITC IgG conjugated with Alexa Fluor 488. Liver sections were examined and photographed using a META510 confocal microscope (Zeiss). Analysis of the cellular infiltrate was performed in a blinded manner by assessing 20 consecutive high-power fields (under ×63 water immersion objective) on each section. The number of cells staining positively for each Ab were counted and expressed as cells per 10 high-power fields.
Analysis of apoptosis of hepatocytes
Hepatic cell nuclei positive for DNA strand breaks was determined by TUNEL assay using a fluorescence detection kit (Roche Applied Science). Sections were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. To the TUNEL reaction mixture containing TdT, fluorescein-dUTP was added to the sections and incubated for 60 min at 37°C in a humidified chamber in the dark. The sections were rinsed three times in PBS. Following embedding, sections were visualized with a META510 confocal microscope. DNase I and labeling solution were used as positive and negative controls, respectively. To determine the percentage of apoptotic cells, the TUNEL-positive nuclei and TUNEL-negative cells were counted using ImagePro image analysis software (Media Cybernetics).
Measurement of serum cytokine levels
Serum cytokine levels for IL-6, IFN-γ, TNF-α, and IL-17 were detected using Luminex technology. In brief, 30 μl of mouse serum (diluted 1/5) or 30 μl each of a 12-point standard cytokine dilution series was incubated in the required number of wells in a MultiScreen HTS plate (Millipore) in the presence of an aliquot of capture Ab-conjugated beads (anti-IL-6, IFN-γ, TNF-α, and IL-17; Millipore). After incubation, the beads were washed with PBS. Cytokines bound to the beads were further incubated with a mix of four biotinylated anti-cytokines (Millipore). Streptavidin-PE (1/100; Caltag Laboratories) was used for detection of bound cytokines. Bead fluorescence was detected and analyzed with a Bio-Plex reader with provided software (Bio-Rad). The results are presented as median fluorescence intensities. IFN-α was measured using an ELISA kit (R&D Systems) according to the manufacturer’s instructions.
Generation of bone marrow (BM) chimeric mice
BM cells were harvested from WT or TLR3−/− mice by flushing femurs and tibiae with PBS. Chimeric mice were generated by transferring donor BM cells into irradiated recipients using the following recipient/BM-donor combinations of WT and TLR3−/− NOD mice: WT/WT BM, WT/TLR3−/− BM, TLR3−/−/WT BM, and TLR3−/−/TLR3−/− BM. The recipients were lethally irradiated (1200 cGy) using an X-320 irradiator (Agfa NDT). Twenty-four hours after irradiation, ~107 BM cells were injected i.v. into the recipients. The animals were allowed to recover for 8–10 wk to ensure full reconstitution of hematopoietic cells. Reconstitution was confirmed by sampling peripheral blood of chimeric mice and examined for full reconstitution of T and B cell compartments.
Effect of RNA from damaged liver tissue on splenocyte response to Con A stimulation
To test whether RNA from damaged liver tissue would affect the response of lymphocytes to Con A stimulation, we performed proliferation assays. Splenocytes (1 × 105/well, 96-well culture plate) from WT or TLR3−/− mice were stimulated with Con A (1 μg/ml) in the presence or absence of total cellular RNA (30 μg/ml) isolated from liver tissue damaged by repeated freezing and thawing. The cells were cultured for 48 h and pulsed with [3H]thymidine (0.5μCi/well) for the last 12 h of culture. Proliferation was determined by [3H]thymidine incorporation using a Wallac 1205 liquid scintillation counter.
Statistical analysis
Data were mostly presented as mean ± SEM. Differences between groups were assessed using ANOVA followed by Bonferroni post hoc test or Student’s t test wherever appropriate. A p < 0.05 was considered statistically significant.
Results
Up-regulation of TLR3 upon induction of Con A hepatitis
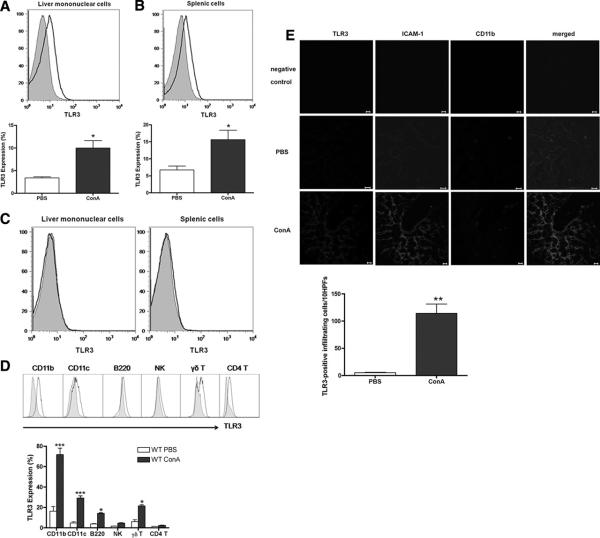
Growing evidence has suggested that host-derived RNA (15, 19, 20) from apoptotic and/or necrotic cells serves as a major stimulus for TLR3 activation. To assess the expression pattern of TLR3 during the development of Con A-induced hepatitis, we examined the level of TLR3 expression in the infiltrating liver MNCs and splenocytes. At 24 h, Con A-injected WT mice showed increased intracellular expression of TLR3 in liver MNCs and splenocytes (Fig. 1⇓, A and B, respectively) when compared with PBS-injected mice. As expected, no expression of TLR3 was detected in TLR3−/− mice (Fig. 1⇓C). As shown in Fig. 1⇓D, TLR3 was widely expressed in various subsets of liver MNCs, including macrophages (CD11b+), dendritic cells (CD11c+), B cells (B220+), NK cells, γδ, and αβ T cells. Moreover, the expression of TLR3 in these cells was up-regulated after Con A injection in WT mice. In addition to the immune cells of liver MNCs, we also found that TLR3 was expressed in sinusoidal endothelial cells of liver as demonstrated by double-positive staining of TLR3 and ICAM-1 (a marker of endothelial cells) and morphology of the cells (Fig. 1⇓E). ICAM-1 and TLR3 were present at low levels in the leukocytes and sinus endothelial cells of PBS control mice (Fig. 1⇓E) and the expression level was greatly increased after Con A injection (Fig. 1⇓E).
FIGURE 1.
TLR3 is required for Con A-induced hepatitis. A, Intracellular TLR3 expression in liver MNCs of WT mice 24 h after PBS or Con A injection. *, p < 0.05 for Con A- vs PBS-injected mice. B, TLR3 expression in splenocytes of WT mice at 24 h after PBS or Con A injection. *, p < 0.05 for Con A- vs PBS-injected mice. C, TLR3 expression in liver MNCs and splenocytes of TLR3−/− mice at 24 h after PBS and Con A injection. Shaded, PBS-injected mice; black line, Con A-injected mice. D, TLR3 expression in various subsets of liver MNCs (macrophages, dendritic cells, B cells, NK cells, γδT cells, and CD4 T cells) which were gated using CD11b+, CD11c+, B220+, pan-NK+, γδTCR+, and CD4+ populations, respectively. ***, p < 0.001 for Con A- vs PBS-injected mice; *, p < 0.05 for Con A- vs PBS-injected mice. Shaded, PBS-injected mice; black line, Con A-injected mice. E, Immunostaining of liver sections in mice injected with PBS or Con A. A higher level of TLR3 expression was found in sinus endothelial cells (ICAM+) and macrophages (CD11b+) after Con A injection and the number of macrophages was also increased in the liver after Con A injection. The numbers of cells expressing TLR3 and CD11b were analyzed. **, p < 0.01 for Con A- vs PBS-injected mice. Data are means ± SEM, n = 4–5 in each group. Similar results were also observed in mice on B6 background.
We also examined the expression of other TLRs (TLR2, TLR4, and TLR9) in liver MNCs and splenocytes in this nonviral-induced hepatitis model. It was interesting that the expression of TLR9 was increased significantly after Con A injection in both WT and TLR3−/− mice, although this was not significantly different between WT and TLR3−/− mice (supplemental Fig. 1).4
TLR3−/− mice are protected from Con A-induced hepatitis
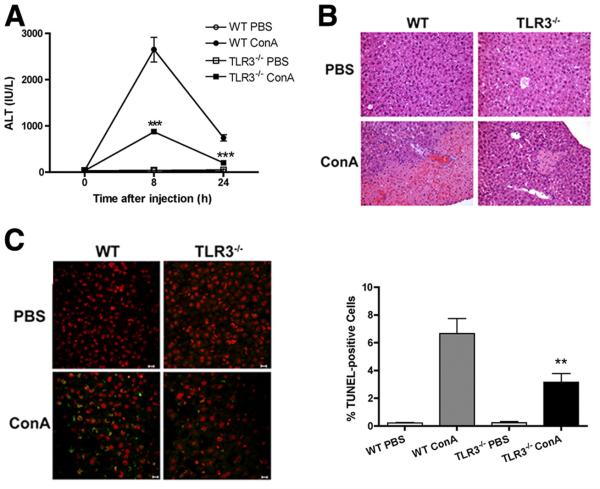
To determine the role of TLR3, as a receptor for DAMPs, in the sterile inflammation of Con A-induced hepatitis, we tested two strains of mice, NOD and C57BL/6. The induction of hepatitis and liver injury was evaluated by elevated levels of ALT, a clinical readout of abnormal liver function. Following Con A administration, the ALT level rapidly increased in WT mice of both strains, reaching a peak at 8 h. In contrast, the increase in the ALT level was significantly less in TLR3−/− mice (Fig. 2⇓A, NOD genetic background). Accompanying the elevated ALT level, large areas of necrosis were observed in the livers of WT mice as seen in H&E-stained sections while only mild lesions were found in TLR3−/− mice after Con A injection (Fig. 2⇓B). TUNEL staining revealed many apoptotic hepatocytes in WT mice. In contrast, there were significantly fewer TUNEL-positive cells in the livers of TLR3−/− mice (Fig. 2⇓C). These results suggest that in the absence of TLR3 expression, the mice were protected from Con A-induced hepatitis and liver damage.
FIGURE 2.
Liver injury was observed after the administration of Con A in mice on the NOD genetic background. A, Serum ALT level was measured at the indicated time points (n = 6–8); ***, p < 0.001 for TLR3−/− Con A- vs WT Con A-injected mice. B, Histological changes in the liver 24 h after Con A injection in WT and TLR3−/− mice. Light micrographs of livers stained with H&E (original magnification, ×200) are shown from one of five experiments. C, TUNEL staining and percentage of TUNEL-positive cells (stained green) in the non-necrotic area (**, p < 0.01, Con A-treated TLR3−/− vs Con A-treated WT mice). Three mice were used in each group. Scale bar, 10 μm.
Activation of hepatic T cells in Con A-induced hepatitis
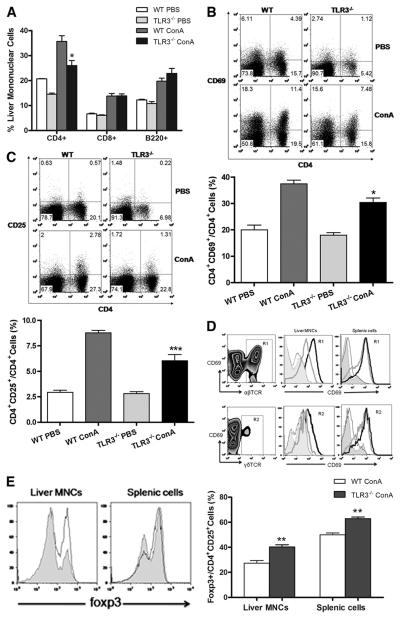
ConA is a T cell mitogen that induces polyclonal T cell activation and liver injury. Concordant with the lower ALT levels, TLR3−/− mice had less hepatic T cell infiltration after Con A injection compared with WT mice, in particular with CD4+ T cells (Fig. 3⇓A). Interestingly, there was no difference in the number of CD8+ T cells and B cells infiltrating the livers between WT and TLR3−/− mice after Con A injection. Moreover, the expression of the T cell activation markers CD69 and CD25 on infiltrating CD4+ T cells in TLR3−/− mice was also significantly lower compared with WT mice, as shown in Fig. 3⇓, B and C.
FIGURE 3.
Activation of hepatic lymphocytes after Con A injection. A, Analysis of different lymphocyte populations at 24 h after Con A injection. *, p < 0.05 Con A-treated TLR3−/− vs Con A-treated WT mice. B, Induction of CD69 expression on CD4+ cells after Con A injection. C, Induction of CD25 expression on CD4+ cells after Con A injection. (*, p < 0.05, Con A-treated TLR3−/− vs Con A-treated WT). D, A representative staining of Con A-treated liver MNCs and splenic cells with anti-CD69 Ab (ordinate) and anti-αβTCR or anti-γδTCR Ab (abscissa). The gates for all αβTCR-positive cells (R1) and γδTCR-positive cells (R2) are indicated. CD69 expression was analyzed on gated R1 or R2 cells. Histograms show PBS-treated TLR3−/− (shaded), PBS-treated WT (thin black line), Con A-treated TLR3−/− (gray line), and Con A-treated WT mice (thick black line), respectively. E, Foxp3 expression was analyzed on gated CD4+CD25+ populations of Con A-treated liver MNCs and splenic cells. **, p < 0.01 for TLR3−/− Con A- vs WT Con A-injected mice. Shaded, WT Con A-injected mice; black line, TLR3−/− Con A-injected mice.
We further investigated T cell subsets in the infiltration and found that both activated αβ and γδ T cells were increased after Con A injection. However, WT mice showed a greater increase in the percentage of activated (CD69-positive) αβ and γδ T cells in liver MNCs compared with TLR3−/− mice (Fig. 3⇑D). As expected, activated αβ and γδ T cells in splenocytes of WT and TLR3−/− mice also increased after Con A injection, although the difference between WT and TLR3−/− was not statistically significant (Fig. 3⇑D). These results suggest that T cell activation was attenuated in the liver in the absence of TLR3 in the Con A-induced hepatitis model system. We also investigated regulatory T cells (Tregs; Foxp3+CD4+CD25+) and found TLR3−/− mice had significantly more Tregs in liver MNCs after Con A injection compared with WT mice (Fig. 3⇑E). This result supports a recent report that Tregs mediate tolerance in Con A-induced hepatitis (21). There was also an increase in splenic Tregs in TLR3−/− mice compared with WT mice after Con A injection (Fig. 3⇑E).
Activation of costimulatory and adhesion molecules in Con A-induced hepatitis
To investigate whether alterations in costimulatory molecule expression contributed to the attenuated T cell activation seen in TLR3−/− mice in Con A-induced hepatitis, we examined the expression of a panel of costimulatory molecules in liver MNCs including B7.2 (CD86), CTLA-4, ICOS, programmed cell death 1, programmed cell death ligand 1 (PDL-1), and the MHC class II molecule I-Ag7. All of the costimulatory molecules were modestly up-regulated except PDL-1 which was strikingly up-regulated after Con A stimulation in vivo in both WT and TLR3−/− mice, although WT mice appeared to express higher levels compared with TLR3−/− mice. In contrast, programmed death 1 was down-regulated. The overall changes in costimulatory molecules were not statistically different between WT and TLR3−/− mice (supplemental Fig. 2A). However, expression of the adhesion molecule ICAM-1 was markedly up-regulated on endothelial cells, hepatocytes, and infiltrating lymphocytes after Con A injection in WT mice, whereas ICAM expression was attenuated in TLR3−/− mice (supplemental Fig. 2B). ICAM-1 has been reported to play a critical role in the infiltration of leukocytes into the liver in Con A-induced hepatitis (22, 23) and the attenuated up-regulation of ICAM-1 after Con A injection in TLR3−/− mice is likely to contribute to the reduced inflammation in the liver of these mice.
Attenuated proinflammatory cytokine production in TLR3-deficient mice in Con A-induced hepatitis
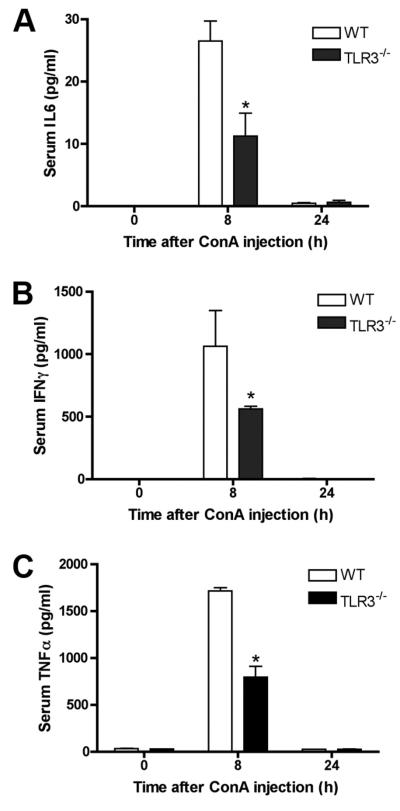
Proinflammatory cytokines play an important role in Con A-induced T cell-mediated hepatitis (24). To investigate the role of TLR3 in proinflammatory cytokine production, we examined serum levels of a panel of proinflammatory cytokines in WT and TLR3−/− mice. The levels of serum IL-6, IFN-γ, and TNF-α were dramatically increased 8 h after Con A injection, which paralleled the peak increase of liver enzymes. However, a significant difference was observed between WT mice and TLR3−/− mice, where the TLR3−/− mice again showed attenuated production of proinflammatory cytokines during the induction of hepatitis (Fig. 4⇓). We also tested serum IFN-α and IL-17 and their levels were negligible (data not shown).
FIGURE 4.
Inflammatory cytokine production in WT and TLR3−/− NOD mice after Con A administration. Serum levels of IL-6 (A), IFN-γ (B), and TNF-α (C) were measured at the indicated time points. *, p < 0.05 TLR3−/− vs WT, four mice were used in each group.
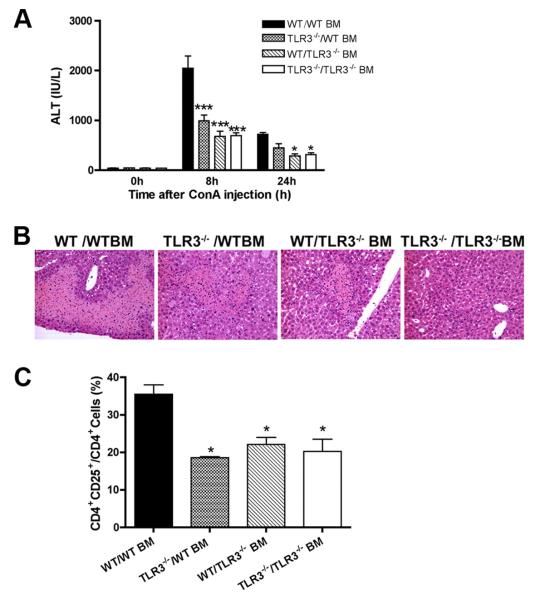
TLR3-mediated liver injury requires functional TLR3 signaling in both hematopoietic and nonhematopoietic cells
Since TLR3 is expressed in both hematopoietic cells and nonhematopoietic cells, we next investigated the importance of signaling through TLR3 in nonhematopoietic cells and BM-derived hematopoietic cells in the pathogenesis of Con A hepatitis by generating BM chimeric mice. We generated mice with TLR3 present in hematopoietic cells but absent in nonhematopoietic cells (lethally irradiated TLR3−/− recipients reconstituted with WT BM: TLR3−/−/WT BM), and mice with TLR3 present in nonhematopoietic cells but not in hematopoietic cells (lethally irradiated WT recipients reconstituted with TLR3−/− BM: WT/TLR3−/− BM). Two additional control groups of mice were also generated where TLR3 was present or absent in all cells (WT/WT BM or TLR3−/−/TLR3−/− BM). Upon confirming full BM engraftment, Con A-induced hepatitis was studied in the chimeric mice 8 wk after BM transplantation. WT/WT BM mice showed significantly abnormal liver function and tissue injury after Con A injection, whereas TLR3−/−/TLR3−/− BM chimeric mice were protected from liver injury as shown by lower serum ALT (Fig. 5⇓, A and B), fewer CD25+ activated CD4+ T cells (Fig. 5⇓C). Consistent with the results obtained from unmanipulated mice, there was no difference in the expression of costimulatory molecules (data not shown). Thus, the phenotype of the control group BM chimeras recapitulated those observed in unmanipulated WT and TLR3−/− mice. However, hepatitis induced by Con A in both TLR3−/−/WT BM and WT/TLR3−/− BM chimeras was attenuated, and the phenotype was similar to that of TLR3−/−/TLR3−/− BM mice (Fig. 5⇓). These results suggest that functional TLR3 in both hematopoietic and nonhematopoietic cells is important in liver damage in this model system. Moreover, these results indicate a novel role of TLR3 in the acute inflammation, which is not associated with viral infection or mediated by type 1 IFN. Our results suggest that TLR3 is not only a receptor for PAMPs, such as viral dsRNA, but likely to be a receptor for DAMPs, which induce inflammation in the Con A-induced hepatitis experimental system.
FIGURE 5.
Functional TLR3 in nonhematopoietic cells or hematopoietic cells contributed to liver damage in NOD mice. WT/WT BM mice showed significant liver dysfunction after Con A injection, while TLR3−/−/TLR3−/− BM chimeric mice were protected from liver injury as measured by serum ALT level (A) and liver damage shown by histology (B). For ALT: ***, P < 0.001, vs WT/WT BM at 8 h after Con A injection; *, p < 0.05 vs WT/WT BM at the 24-h time point. C, Induction of T cell activation marker CD25 expression on CD4+ liver MNCs after Con A injection. *, p < 0.05. Data are shown as mean ± SEM. n = 6–12/group.
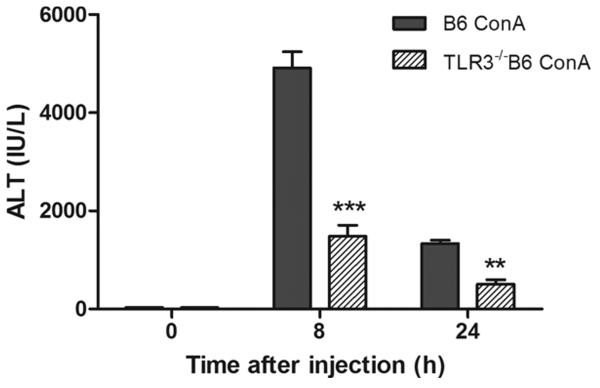
TLR3−/−B6 mice also developed attenuated hepatitis
Con A-induced hepatitis had been considered as a model of autoimmune hepatitis (4). Given the fact that the NOD genetic background harbors many autoimmune susceptibility genes, we devised many of the experiments described above in mice on the NOD genetic background. However, to confirm that the role of TLR3 in Con A-induced hepatitis was not due to the NOD genetic background, we tested the model in B6 WT and TLR3−/− mice. Similar to NOD mice, removal of TLR3 in B6 mice significantly attenuated liver inflammation and tissue damage induced by Con A, as evidenced by lower ALT levels (Fig. 6⇓) and less liver pathology (data not shown). Like TLR3−/− NOD mice, TLR3−/− B6 mice also showed fewer activated T cells and NKT cells and more Tregs compared with the WT B6 mice during Con A-induced hepatitis (data not shown). Our data in B6 mice further support the notion that TLR3 signaling plays a dominant role in regulating inflammation and liver damage regardless of the genetic background of the mouse strains.
FIGURE 6.
TLR3−/− also attenuates Con A-induced hepatitis in B6 mice. The serum ALT level in WT and TLR3−/− B6 mice was measured at the indicated time points (n = 4). ***, p < 0.001 vs B6 Con A-injected mice and **, p < 0.01 vs TLR3−/−B6 Con A-injected mice.
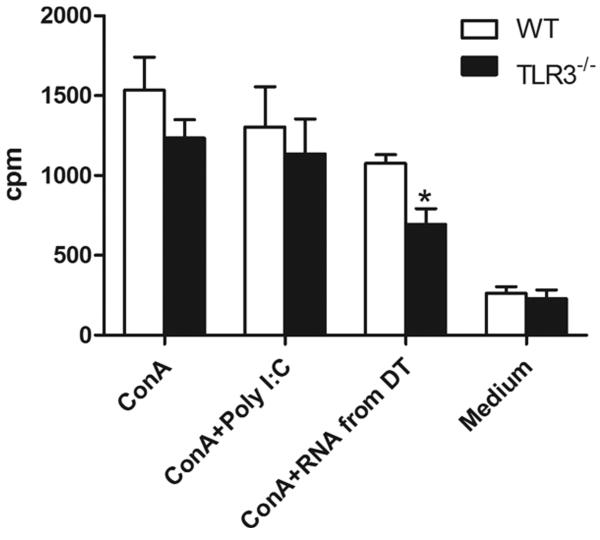
Attenuated in vitro response of splenocytes to Con A stimulation in the presence of RNA from necrotic liver tissue
To test whether we could mimic the attenuated in vivo immune response in an in vitro setting, we examined the proliferative responses of splenocytes from WT and TLR3−/− mice to Con A stimulation in the presence or absence of exogenous RNA isolated from damaged liver tissue. It is interesting that the proliferation of splenocytes from TLR3−/− mice to Con A stimulation was attenuated in the presence of RNA from damaged liver tissue compared with cells from the WT mice (Fig. 7⇓).
FIGURE 7.
Proliferation assay of splenocytes to Con A stimulation. Splenocytes (1 × 105/well) from WT and TLR3−/− mice were stimulated with Con A (1 μg/ml) in the presence or absence of poly(I:C) (30 μg/ml) or RNA (30 μg/ml) isolated from liver tissue damaged by repeated freezing and thawing (damaged tissue (DT)). The cells were cultured for 48 h and pulsed with [3H]thymidine (0.5 μCi/well) for the last 12 h. Proliferation was determined by [3H]thymidine incorporation using a Wallac 1205 liquid scintillation counter. Results are presented as mean ± SEM; n = 3–4 samples/group. *, p < 0.05 TLR3−/− vs WT.
Discussion
In the present study, we have examined the role of TLR3 in Con A-induced hepatitis, a model of autoimmune liver disease, where a large number of hepatocytes undergo apoptosis and necrosis. TLR3 is a receptor for dsRNA (18) and recent studies have suggested that TLR3 also recognizes endogenous nucleic acids from cells (15). Thus, we were interested in whether TLR3, as a receptor for nucleic acids, may be important in this disease. We have shown that 1) TLR3 mediates acute liver inflammation and that the tissue damage was markedly attenuated in the absence of TLR3; 2) the expression of TLR3 in both hematopoietic and nonhematopoietic cells was equally important for the acute inflammation, as the inflammation was attenuated in the absence of TLR3 when the effect was tested separately in each type of cell; 3) activation of liver-infiltrating T cells was impaired in the absence of TLR3 as demonstrated by reduced up-regulation of T cell activation markers CD69 and CD25, reduced proinflammatory cytokine production, altered costimulatory molecules, and an increase in the number of Tregs; 4) expression of the adhesion molecule ICAM-1, necessary for the infiltration of inflammatory cells into the liver, is considerably reduced in TLR3−/− mice on T cells as well as endothelial cells and hepatocytes; 5) the attenuation of Con A-induced hepatitis in TLR3−/− mice is similar on the B6 or NOD genetic backgrounds, indicating that this reduction in severity of inflammation is not related to genetic background; and 6) the response of T cells from TLR3−/− mice to Con A stimulation along with RNA from damaged liver tissue is reduced in vitro compared with responses in T cells from WT mice.
In view of the fact that TLR3 as an innate immune receptor plays an important role in determining the severity of the inflammation in Con A-induced hepatitis, we investigated other TLRs including, TLR2, TLR4, and TLR9. It is interesting that only TLR9 was up-regulated after Con A injection in liver MNCs. This was consistent with a recent report by Jiang et al. (25) that Con A induced up-regulation of TLR9 expression in liver MNCs of normal B6 mice. TLR9 recognizes bacterial or viral DNA and unmethylated CpG-containing DNA sequences (26). High-mobility group box (HMGB1), a nuclear DNA-binding protein released from necrotic cells, is an essential component of DNA containing immune complexes that stimulate cytokine production through TLR9 (27). In contrast to TLR3, TLR9 appears to negatively regulate nonpathogen-associated inflammation, including autoimmunity, since accelerated inflammation and/or autoimmunity was found in the absence of TLR9 (13). Thus, TLR9 is also a receptor for DAMPs, which may have an opposite effect to TLR3 in sterile inflammation and/or autoimmunity. In this study, the up-regulation of TLR9 in TLR3−/− mice was comparable to that in WT mice and this was true for both genetic backgrounds. These results suggest that TLR9 does not contribute to the protected phenotype observed in TLR3−/− mice. In addition to TLRs, the nucleotide-binding and oligomerization domain (NOD)-like receptors and the retinoic acid-inducible gene (RIG) I-like receptors have been recently identified to be additional innate immune receptor families that recognize PAMPs in intracellular compartments (28). NOD-like receptors also detect endogenous danger signals (29). Retinoid acid -inducible gene I-like receptors can discriminate endogenous RNA from foreign viral RNA and initiate signaling cascades, leading to the induction of type I IFNs (30). Together with TLRs, these receptors play a vital role in cellular defense both at the plasma membrane and within the cell and are key players in the innate immune response. It would be important in the future to examine whether these may also play a role in this type of inflammation.
The processes in which TLR3 is involved in the generation of Con A-induced hepatitis are multiple and include effects in both hematopoietic and nonhematopoietic cells. In terms of the hematopoietic cells, it has been shown that both T and NKT cells play essential roles in Con A-induced hepatitis (23, 31, 32). In addition to the suppressed production of inflammatory cytokines in the absence of TLR3, our results showed that T cell activation was compromised in inflamed tissue in TLR3−/− mice. TLR3 is clearly expressed in various types of immune cells that include macrophages (CD11b+), dendritic cells (CD11c+), and B cells (B220+). It is conceivable that TLR3 deficiency may lead to hyporesponsiveness of these cells to environmental insults and fail to provide the appropriate adhesion signals for maximal T cell activation, as there was reduced expression of adhesion molecules. Moreover, TLR3 is also expressed in T cells and in the absence of TLR3, T cells in the liver cannot be fully activated by Con A, supported by the reduced expression of T cell activation markers CD25 and CD69. The number of infiltrating T cells, in particular CD4+ T cells, was also much lower in TLR3−/− mice than in WT controls. The liver harbors a large number of γδ T cells (33). We found that the number of activated infiltrating γδ T cells was also considerably lower in TLR3−/− mice. Emerging evidence suggests that γδ T cells are important for innate immune responses against viral or bacterial infections and also against tumor formation (34, 35). These cells function as an important link between the innate and adaptive immune response. It is possible that TLR3 also regulates inflammation through these cells at the interface between innate and adaptive immune responses. In respect of the nonhematopoietic cells, it is striking that one of the largest differences observed between TLR3−/− mice and WT mice was the lower expression of ICAM-1 on both endothelial cells and hepatocytes in addition to the reduced expression on infiltrating lymphocytes. ICAM-1 has been reported to play a critical role in the infiltration of leukocytes into the liver in Con A-induced hepatitis (22, 23) and the attenuated up-regulation of ICAM-1 after Con A injection in TLR3−/− mice is likely to contribute to the reduced inflammation in the liver of these mice.
In summary, our results document an important role of TLR3 as a possible receptor for DAMP-mediated signaling in the pathogenesis of Con A-induced hepatitis and suggest that this pathway is central in the innate immune response that leads to liver injury. Our study supports a very recent report using a gut ischemia model system, which also implies a novel role for TLR3 as a possible receptor for DAMPs (20). More importantly, using this model system, we have revealed a novel regulatory function of TLR3 in nonviral-induced inflammation and our study indicates that targeting TLR3 could potentially provide a new anti-inflammatory tool.
Supplementary Material
Acknowledgments
We thank Bin Lu for dedicated care of the animals used in this study and Dr. Hongmei Li (Internal Medicine, Yale University, New Haven, CT) for assistance with the graphs.
Footnotes
Disclosures The authors have no financial conflict of interest.
This work was supported by a pilot grant from the Yale Liver Center (P38, DK034989) and Animal Genetic Core of Yale Diabetes Endocrine Research Center (DK-02-003). X.X. is a scholarship recipient from the China Scholarship Council (2007-102113).
Abbreviations used in this paper: PAMP, pathogen-associated molecular pattern; ALT, alanine aminotransferase; MNC, mononuclear cell; WT, wild type; DAMP, damaged tissue-associated molecular pattern; BM, bone marrow; Treg, regulatory T cell; PDL-1, programmed cell death ligand 1.
The online version of this article contains supplemental material.
References
- 1.Schiodt FV, Lee WM. Fulminant liver disease. Clin. Liver Dis. 2003;7:331–349. vi. doi: 10.1016/s1089-3261(03)00026-6. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 2.Diao J, Garces R, Richardson CD. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001;12:189–205. doi: 10.1016/s1359-6101(00)00034-4. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 3.Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 2003;9:347–351. doi: 10.1038/nm828. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 4.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Invest. 1992;90:196–203. doi: 10.1172/JCI115836. Yale Links Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 7.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 8.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 9.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takii Y, Nakamura M, Ito M, Yokoyama T, Komori A, Shimizu-Yoshida Y, Nakao R, Kusumoto K, Nagaoka S, et al. Enhanced expression of type I interferon and Toll-like receptor-3 in primary biliary cirrhosis. Lab. Invest. 2005;85:908–920. doi: 10.1038/labinvest.3700285. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 11.Lang KS, Georgiev P, Recher M, Navarini AA, Bergthaler A, Heikenwalder M, Harris NL, Junt T, Odermatt B, Clavien PA, et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Invest. 2006;116:2456–2463. doi: 10.1172/JCI28349. Yale Links CrossRef Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertolino P, Holz LE. Toll-like receptor-3 and the regulation of intrahepatic immunity: implications for interferon-α therapy. Hepatology. 2007;45:250–251. doi: 10.1002/hep.21504. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 13.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 2006;6:823–835. doi: 10.1038/nri1957. Yale Links CrossRef Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 15.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 16.Silveira PA, Baxter AG. The NOD mouse as a model of SLE. Autoimmunity. 2001;34:53–64. doi: 10.3109/08916930108994126. Yale Links Medline. [DOI] [PubMed] [Google Scholar]
- 17.Rosignoli F, Roca V, Meiss R, Leceta J, Gomariz RP, Leiros C. Perez. Defective signalling in salivary glands precedes the autoimmune response in the non-obese diabetic mouse model of sialadenitis. Clin. Exp. Immunol. 2005;142:411–418. doi: 10.1111/j.1365-2249.2005.02930.x. Yale Links CrossRef Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 19.Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52:2656–2665. doi: 10.1002/art.21273. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 20.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 22.Jaruga B, Hong F, Kim WH, Gao B. IFN-γ/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am. J. Physiol. 2004;287:G1044–G1052. doi: 10.1152/ajpgi.00184.2004. Yale Links. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto G, Tsunematsu S, Tsukinoki K, Ohmi Y, Iwamiya M, Oliveirados-Santos A, Tone D, Shindo J, Penninger JM. Essential role of the adhesion receptor LFA-1 for T cell-dependent fulminant hepatitis. J. Immunol. 2002;169:7087–7096. doi: 10.4049/jimmunol.169.12.7087. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 24.Torisu T, Nakaya M, Watanabe S, Hashimoto M, Yoshida H, Chinen T, Yoshida R, Okamoto F, Hanada T, Torisu K, et al. Suppressor of cytokine signaling 1 protects mice against concanavalin A-induced hepatitis by inhibiting apoptosis. Hepatology. 2008;47:1644–1654. doi: 10.1002/hep.22214. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W, Sun R, Zhou R, Wei H, Tian Z. TLR-9 activation aggravates concanavalin A-induced hepatitis via promoting accumulation and activation of liver CD4+ NKT cells. J. Immunol. 2009;182:3768–3774. doi: 10.4049/jimmunol.0800973. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 26.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 2006;7:49–56. doi: 10.1038/ni1280. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 27.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–496. doi: 10.1038/ni1457. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 28.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 29.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 30.Myong S, Cui S, Cornish PV, Kirchhofer A, Gack MU, Jung JU, Hopfner KP, Ha T. Cytosolic viral sensor RIG-I is a 5′-triphosphate-dependent translocase on double-stranded RNA. Science. 2009;323:1070–1074. doi: 10.1126/science.1168352. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc. Natl. Acad. Sci. USA. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margalit M, Ghazala SA, Alper R, Elinav E, Klein A, Doviner V, Sherman Y, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Glucocerebroside treatment ameliorates ConA hepatitis by inhibition of NKT lymphocytes. Am. J.Physiol. 2005;289:G917–G925. doi: 10.1152/ajpgi.00105.2005. Yale Links. [DOI] [PubMed] [Google Scholar]
- 33.Wen L, Ma Y, Bogdanos DP, Wong FS, Demaine A, Mieli-Vergani G, Vergani D. Pediatric autoimmune liver diseases: the molecular basis of humoral and cellular immunity. Curr. Mol. Med. 2001;1:379–389. doi: 10.2174/1566524013363672. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 34.Born WK, Reardon CL, O’Brien RL. The function of γδ T cells in innate immunity. Curr. Opin. Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
- 35.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. Yale Links CrossRef Medline. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.