Abstract
Deregulation of calcium has been implicated in neurodegenerative diseases, including Alzheimer’s disease (AD). Previously, we showed that saturated free-fatty acid, palmitate, causes AD-like changes in primary cortical neurons mediated by astrocytes. However, the molecular mechanisms by which conditioned media from astrocytes cultured in palmitate induces AD-like changes in neurons are unknown. This study demonstrates that this condition media from astrocytes elevates calcium level in the neurons, which subsequently increases calpain activity, a calcium-dependent protease, leading to enhance p25/Cdk5 activity and phosphorylation and activation of the STAT3 (signal transducer and activator of transcription) transcription factor. Inhibiting calpain or Cdk5 significantly reduces the upregulation in nuclear level of pSTAT3, which we found to transcriptionally regulate both BACE1 and presenilin-1, the latter is a catalytic subunit of γ-secretase. Decreasing pSTAT3 levels reduced the mRNA levels of both BACE1 and presenilin-1 to near control levels. These data demonstrate a signal pathway leading to the activation of STAT3, and the generation of the amyloid peptide. Thus, our results suggest that STAT3 is an important potential therapeutic target of AD pathogenesis.
Keywords: Alzheimer’s disease, fatty acids, calcium, STAT3, BACE1, presenilin-1
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, characterized by the deposition of amyloid β (Aβ) peptide generated through cleavage by BACE1 (β-site amyloid precursor protein cleaving enzyme) and γ-secretase. AD begins with mild memory deficits and ultimately results in total loss of cognition and executive functions. One in 85 people worldwide are predicted to be affected with AD by year 2050 (Alzheimer’s, 2012). The etiology of AD is unknown and no effective cures currently exist (Berridge, 2010). Calcium signaling is involved in numerous neuronal functions, and the dysregulation of neuronal calcium homeostasis is a hallmark of AD pathology and found in both AD patients and in AD animal models (Berridge, 2010), impairs neuronal activity, accelerates Aβ formation (Demuro et al., 2010) and is linked to the neurodegenerative process in AD (Fedrizzi and Carafoli, 2011). Nevertheless, the mechanism by which abnormal level of calcium leads to amyloidogenesis is unclear.
Calpain, a calcium-dependent protease that can be triggered by abnormally high calcium levels, is over-activated during early stages of the AD process (Medeiros et al., 2012; Querfurth and LaFerla; Trinchese et al., 2008). Pharmacological inhibitors of calpain improve cognition and reduce amyloid plaque load in AD transgenic mice model, implicating their potential therapeutic application for AD (Liang et al., 2010). P25, the proteolytic cleavage product from p35 generated by calpain, activates cyclin-dependent kinase 5 (Cdk5) and was reported to be upregulated in human AD brain (Cruz et al., 2006). Cdk5, a proline-directed serine/threonine kinase, has diverse functions in the central nervous system, including neuronal migration, synaptic plasticity and cognition (Crews and Masliah, 2010; Giusti-Rodriguez et al., 2011). In support of its role in the pathogenesis of AD, the activity of Cdk5 is upregulated in AD patient’s brain (Wen et al., 2008). Activation of Cdk5 increases while inhibiting Cdk5 activity attenuates Aβ generation (Cheung and Ip, 2012).
Aβ peptide is generated from sequential proteolytic cleavages of the Aβ precursor protein by BACE1 and γ-secretase (Cole and Vassar, 2007; Selkoe, 2001). The expression of BACE1 is cell-type and stimuli-specific (Christensen et al., 2004; Ge et al., 2004; Sambamurti et al., 2004), and is tightly regulated at the transcriptional level (Bourne et al., 2007; Sinha et al., 1999; Sun et al., 2012). The promoter region of BACE1 contains many putative transcription factor binding sites that are conserved among different species, including rat, mouse and human (Bourne et al., 2007; Chami et al., 2012; Sun et al., 2012). Several putative transcription factors of BACE1, such as SP1 and Yin Yang 1, have been confirmed in neuroblastoma cells (Christensen et al., 2004; Nowak et al., 2006). Similarly, p25/Cdk5 has been reported to phosphorylate and activate the transcription factor, signal transducer and activator of transcription 3 (STAT3), to positively regulate the transcription of the BACE1 gene, also, in neuroblastoma cells (Wen et al., 2008).
γ-secretase consists of presenilin, nicastrin, APH-1 (anterior pharynx-defective 1) and PEN-2 (presenilin enhancer 2). The catalytic core of γ-secretase consists of presenilin-1 which is essential for the activity of γ-secretase. The expression of the presenilin-1 gene increases during aging and during brain injury (Das, 2008). The expression of presenilin-1 can be regulated by the Ets transcription factor (Pastorcic and Das, 2000), and further p53 has been shown to be involved in the regulation of presenilin-1 expression (Pastorcic and Das, 2000), while inhibiting c-Jun N-terminal kinase (JNK) represses presenilin-1 expression (Lee and Das, 2010).
The AD brain is characterized by high fatty acid content, and significant increases in fatty acids from brain trauma have been suggested as a risk factor for AD (Jellinger, 2004; Morris and Tangney, 2010). In animal models, the consumption of saturated fatty acids increases the pathophysiological changes associated with AD (Takechi et al., 2009). Previously our group confirmed that saturated fatty acids, e.g. palmitate, induced AD-like changes in primary rat neurons mediated by conditioned media from astrocytes, although it was not determined whether palmitate directly increases BACE1 and hyperphosphorylation of tau in the primary neurons (Liu et al., 2013; Patil et al., 2007). Further, the conditioned media from astrocytes treated with palmitate increased sphingomyelinase activities and enhanced the stability of BACE1, to propagate the deleterious effects of palmitate in primary neurons (Liu et al., 2013). The process, however, by which Aβ is generated in primary neurons cultured in conditioned media from palmitate-treated astrocytes, is unclear. Here we show that the conditioned media from these astrocytes rapidly increases the calcium levels, enhances the activity of calpain and of Cdk5 through p25 (the cleavage product of calpain) and in turn activates STAT3 in the neurons. Further we found that the STAT3 transcription factor could regulate the expression of both BACE1 and presenilin-1, a core subunit of γ-secretase, thereby supporting pSTAT3 as a potential therapeutic target.
MATERIALS AND METHODS
Isolation and culture of primary rat astrocytes and neurons
All procedures in the cell isolation were approved by the Institutional Animal Care and Use Committee at Michigan State University. Primary cortical astrocytes were isolated from postnatal day 0-2 newborn Sprague-Dawley rats as previously described (Liu et al., 2013). Briefly, the cortices were digested with papain (10units/ml; Worthington, NJ, USA) and DNaseI (100units/ml; Roche, IN, USA) for 30min at 37°C, and washed with DMEM/F12 (Invitrogen, Carlsbad, CA, USA). The cells were seeded on poly-L-lysine (PLL, 50g/ml; Cultrex, Gaithersburg, MD, USA) coated plate for 1hr and the media was changed to fresh complete DMEM/F12 media (DMEM/F12 supplemented with 10% fetal bovine serum, 100μg/mL streptomycin and 100U/mL penicillin (Invitrogen)) to remove dead cells. The cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C, the cultured medium changed every 3 days. The purity of the astrocytes were >90% (Liu et al., 2013), and ready for use in experiments when the cells reached around 80% confluence.
Cortices from postnatal day 0 Sprague Dawley rats were used for the neuronal culture. Briefly, the cortical tissues were digested with papain and DNaseI for 30min at 37°C, and washed with Neurobasal A medium (Invitrogen) three times. The cells were seeded on PLL coated plates for 1hr and the media was changed to fresh complete cell culture media (neurobasalA medium with B27, 0.5mM glutamine, and 100μg/mL streptomycin and 100U/mL penicillin). The purity of the neurons was >90% (Liu et al., 2013) and used for experiments after 3 or 4 days of culture.
Astrocyte conditioned media (CM) and Materials
Neuronal cell culture medium (DMEM 10313 supplemented with 10% horse serum, 10mM HEPES, 2mM glutamine, 100μg/mL streptomycin and 100U/mL penicillin) was used to culture primary neurons and astrocytes for 24hr prior to treatment. BSA (fatty acid-free bovine serum albumin) (Millipore, Billerica, MA, USA), or 0.2mM PA (Sigma, St. Louis, MO, USA) plus BSA as a carrier protein (molar ration is 3:1) was used to incubate the astrocytes for 12hr. This astrocyte conditioned media (CM-B or CM-P) were used to culture the neurons.
Roscovitine (Cat# R7772, Sigma) and butyrolactone-1 (Cat# BML-CC210, Enzo life sci, Farmingdale, NY, USA) are specific inhibitors of Cdk5 and were used at concentrations of 10μM. Calpeptin (Cat# 03-34-0051, Calbiochem, Billerica, MA, USA) and PD150606 (Cat#CAS 659-22-3, Santa Cruz, Dallas, Texas, USA) are specific inhibitors of calpain and were used at 20μM and 100μM, respectively. Cell permeable STAT3 inhibitor (Calbiochem 573095) was used to inhibit the dimerization of STAT3 at 235μM. STAT3 inhibitor VI (Calbiochem 573102) was used to inhibit STAT3 activity at 100μM. ActinomycinD (ActD) (Cat#9415, Sigma) was used to inhibit transcription at a concentration of 1μg/ml.
Total mRNA Extraction and Quantitative real time PCR
Primary neurons were incubated for 12hr with conditioned media from astrocytes. Then the cells were lysed and total mRNA from the cells was extracted using the RNeasy Plus kit (QiaGen, Valencia, CA, USA) according to the manufacturer’s instructions and the total mRNA was reverse transcribed into cDNA using the cDNA synthesis kit (BioRad, Hercules, CA, USA) as described (Wu et al., 2011). The following primer sets (Operon, Huntsville, AL, USA) were used for PCR: actin (5′-ctcttccagccttccttcct-3′and 5′-aatgcctgggtacatggtg-3′), BACE1 (5′-aatcagtccttccgcatcac-3′ and 5′-ggctcgatcaaagaccacat-3′), presenilin-1 (5′-ggtacccaaaaaccccaagt-3′and 5′-agtgaggatgctcccagaaa-3′), Quantitative real-time PCR was performed using iQSYBR Green Supermix and Real-Time PCR Detection System (BioRad). The cycle threshold values were determined by the MyIQ software. Three independent experiments were performed for statistical analysis.
Western blot
Primary neurons were cultured in conditioned media at the indicated time in the figures. Then the cells were lysed and protein concentrations from whole cell extracts were determined by Bradford assay (BioRad). 15-30μg of protein samples were used for Western blot analysis as previously described (Bilgin et al., 2013; Liu et al., 2013; Wu et al., 2013) using specific antibodies for BACE1 (Cat#ab2077, Abcam, Cambridge, MA, USA), pSTAT3 (Cat#9131, Cell signaling, Danvers, MA, USA), spectrin (Cat# MAB1622, Millipore, Billerica, MA, USA), Histone H1 (Cat#ab11079, Abcam, Cambridge, MA, USA), C99 (Cat#2136, QED Bioscience, San Diego, CA, USA), and beta-actin (Cat# NB600-501H, Novus Biologicals, Littleton, CO, USA). Anti-mouse and anti-rabbit HRP-conjugated secondary antibodies were purchased from Thermo Scientific (Cat#31430, 31460, Asheville, NC, USA). The blots were visualized by Super Signal West Femto maximum sensitivity substrate (Thermo Scientific).
Nuclear extracts
Primary rat neurons were cultured in conditioned media at the indicated time in the figures. Then the cells were lysed and nuclear extraction was performed according to the protocol described in (Zhang et al., 2011). Briefly, cells were suspended in buffer A (10mM HEPES (pH=8.0), 1.5mM MgCl2, 10mM KCl, protease and phosphatase inhibitor cocktail) and kept on ice for 15 minutes. The cells were lysed with a 25-gauge 5/8 inch needle and the nuclear pellets were collected by centrifugation. Nuclear pellets were re-suspended in buffer C (20mM HEPES (pH=8.0), 1.5mM MgCl2, 25% (v/v) glycerol, 420mM NaCl, 0.2mM EDTA (pH=8.0), protease and phosphatase inhibitor cocktails) and incubated on ice for 30min, then spun down to obtain the nuclear extracts.
Calpain activity assay
Calpain activity in primary neurons cultured for 30min in conditioned media from astrocytes was detected by a calpain activity assay kit according to the manufacturer’s instructions (Cat#K240, BioVision, Milpitas, CA, USA). Briefly, cells were lysed in lysis buffer for 20 min on ice, and the lysates were incubated with the calpain substrate, fluorogenic peptide (Ac-LLY-AFC), and reaction buffer for 1hr at 37°C in the dark. Upon the cleavage of the substrate, the fluorogenic portion produces fluorescence at a wavelength of 505nm and excitation at 400nm. The fluorescence emission was measured by Spectra MAX GEMINI EM plate reader. All readings were normalized to the protein levels obtained by Bradford assay.
Enzyme-linked immunosorbent assay (ELISA) and Aβ42 assay
Primary rat neurons were cultured for 12hr in conditioned media from astrocytes. Then the cultured media was used for the ELISA assay. The level of Aβ42 was detected by Aβ42 ELISA kit (Invitrogen) according to the manufacture’s instruction. Optical densities were measured at 450nm wavelength by Spectra MAX Plus384 plate reader. Each sample concentration was calculated based on a standard curve of Aβ42 standards. All readings were normalized to the total protein levels determined by Bradford’s assay, and the data then were normalized to the control. Three independent experiments were performed for statistical analysis.
Calcium imaging
Calcium imaging was performed according to the protocol described in (Zhang et al., 2011). Briefly, neurons were cultured in 4-well chambered cover-glass (Thermo Fisher Scientific). 4μM non-ratiometric dye Fluo-4 (Invitrogen) was added to the cultured media for 30min at 37°C. Excess dye was removed by washing with PBS, and the cells were incubated with complete neuronal media and placed in a chamber on a 37°C stage of an Olympus FluoView 1000. Images were captured and fluorescence intensity is represented by a spectral table (warmer colors represent higher intensity whereas cooler colors represent lower intensity). The completed neuronal media were replaced with CM-P or CM-B, and incubate for 20min prior to imaging.
Quantitative analysis of calcium was measured with Fluo-4. Briefly, neurons were cultured in 12-well plates. 4μM non-ratiometric dye Fluo-4 was added to the cultured media for 30min at 37°C, excess dye was removed by washing with PBS, and neurons were incubated with complete neuronal media. The fluorescence signal (F0) was detected by Spectra MAX GEMINI EM plate reader at 520nm and excitation at 480nm. Completed neuronal media were replaced with CM-P or CM-B, and incubate for 20min. The fluorescence signal (F) was measured again. Changes in the fluorescence intensity of the Ca2+ signal are represented as F/F0. Three or more independent experiments were performed.
Statistical analysis
All experiments were performed at least three times, and representative results are shown. Statistical analysis was performed using an unpaired, two tail student t-test * indicates p<0.05, ** indicates p<0.01 and *** indicates p<0.001.
Results
3.1 Abnormal calcium and calpain levels in primary neurons treated with CM-P
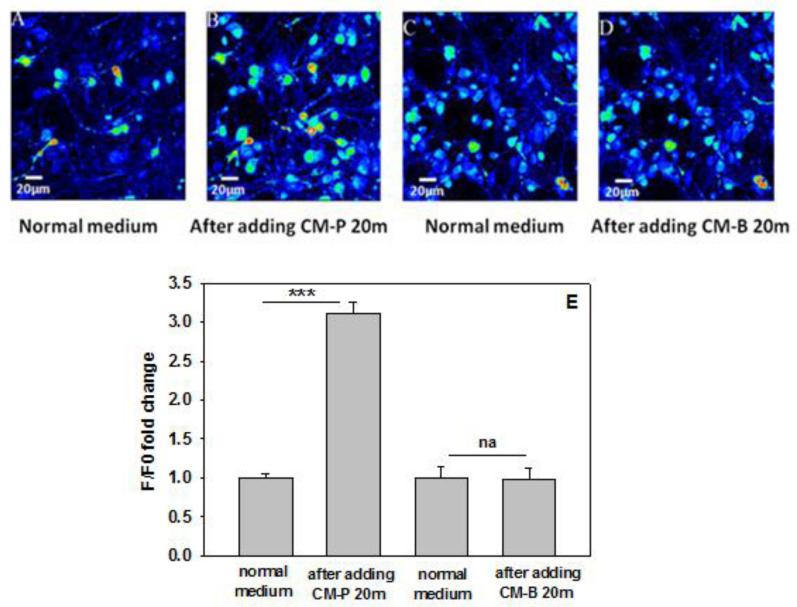
Condition media from astrocytes cultured in palmitate or control media (BSA) is herein denoted as CM-P or CM-B, respectively. We reported previously that primary neurons cultured in CM-P developed AD-like changes (Patil et al., 2007). Upregulated levels of calcium have been implicated in neurodegenerative diseases including AD. We observe the calcium level is upregulated in neuronal cells incubated in CM-P (Figure 1). Elevated levels of calcium could enhance calcium-dependent protein activities that trigger signaling cascades, such as calpain, a calcium-dependent protease. Mounting evidences indicate that abnormal activation of calpain is involved in AD pathogenesis (Querfurth and LaFerla; Trinchese et al., 2008). To validate whether the activity of calpain is upregulated in neurons cultured in conditioned media from astrocytes, the activity of calpain was measured and the results show a significant increase in calpain activity in the primary neurons (Figure 2A). This increased calpain activity is likely due to elevated calcium levels. Calpain activity comes from two main isoforms of calpain in the cells, μ-calpain and m-calpain. The primary difference between the two isoforms is the amount of calcium required for activation. μ-calpain can be activated by micromolar concentration of Ca2+, while m-calpain is activated by millimolar concentration of Ca2+. We detected m-calpain protein level in primary neurons cultured for 30mins with condition media from astrocytes. The western blot results show that m-calpain protein levels are increased significantly in primary neurons cultured in CM-P (Figure 2B). In further support, to monitor the activity of calpain in neurons treated with CM-P, spectrin, an intracellular substrate of calpain that can be cleaved by calpain to indicate elevated calpain activity, was detected by western blot analysis. A cleavage fragment of around 150kDa from the 220kDa spectrin protein was detected in primary neurons cultured for 30 min in CM-P (Figure 2C, D), suggesting increased calpain activity. To determine if spectrin is cleaved by calpain, two specific calpain inhibitors, calpeptin (20μM) and PD150606 (100μM) were used individually to pre- and co-treat the primary neurons cultured in CM-P, and found to decrease significantly the 150kDa spectrin fragment (Figure 2C, D), indicating that CM-P increased the activity of calpain in primary cortical neurons.
Figure 1. Calcium imaging of neurons in response to conditioned media from astrocytes.
(A) Representative fluorescence image of primary neurons cultured with normal cell culture media, after capturing the image, the normal cell cultured media was replaced with conditioned media from astrocytes cultured in palmitate (CM-P) for 20min, and the image of the same field of view as (A) was captured and presented in (B). (C) Representative image of primary neurons cultured with normal cell culture media, after capturing the image, the normal cell cultured media was replaced with control conditioned media from astrocytes cultured in DMEM/BSA (CM-B) for 20min, then the image of the same field of view as (C) was captured and presented in (D). Fluorescence images represented by a spectral table; the warmer colors indicate the higher fluorescence intensities and cooler colors indicate the lower fluorescence intensities. (E) Relative signal intensity (F/F0) with Fluo-4 loaded neurons cultured with conditioned media from astrocytes. The fluorescence intensity was measured with a plate reader. ***p<0.001. A line indicates a comparison between the 2 bars connected by the line.
Figure 2. Calpain activity increases in primary neurons.
(A) Primary neurons were incubated for 30min in conditioned media from astrocytes cultured in DMEM/BSA (CM-B) or in palmitate (CM-P) and the activity of calpain was measured. (B) m-calpain level in primary neurons. Primary neurons were incubated with CM-B (control) or CM-P for 30min and the cells were lysed. m-calpain levels were detected by western blot. Actin was used as a loading control. (C, D) Spectrin levels in neurons. Primary neurons were incubated with CM-B (control) or CM-P for 30min. Calpeptin (20μM) or PD150606 (100μM), specific inhibitors of calpain, was used to pre- and co-treated the neurons with CM-P for 30min individually. Then the cells were lysed and representative western blot results of spectrin are shown in (C). The results were normalized to control (CM-B). *p<0.05, **p<0.01, ***p<0.001.
3.2 CM-P elevates C99 and BACE1 levels, and calpain is involved in APP processing in neurons
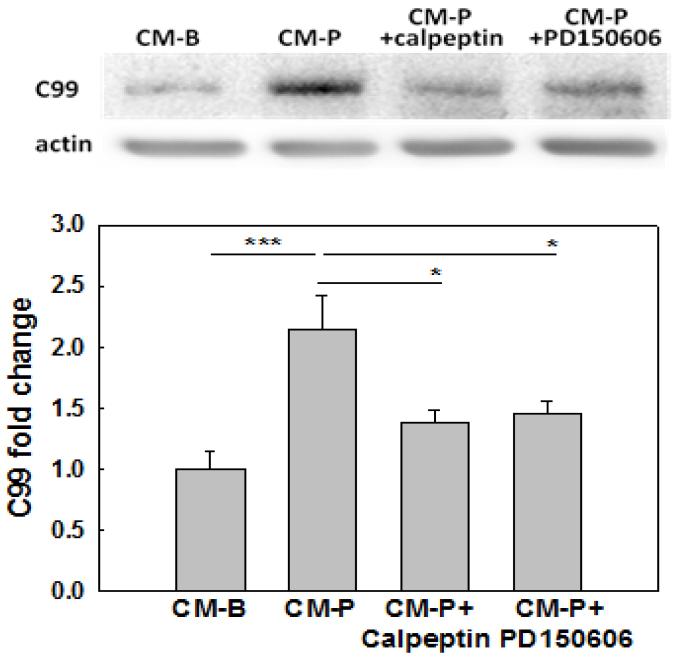
We previously reported that BACE1 protein levels increased in a time-dependent manner in primary neurons cultured in CM-P (Liu et al., 2013). BACE1 initiates the production of Aβ by cleaving the Aβ precursor protein (APP) to generate a membrane bound carboxy-terminal fragment (C99). A higher level of C99 indicates higher BACE1 activity. Incubating the primary neurons in CM-P significantly elevated, while inhibiting calpain with specific inhibitors, calpeptin at 20μM, or PD150606 at 100μM significantly reduced, the accumulation of C99 (Figure 3). This suggests that calpain could be involved in regulating C99 level by modulating BACE1 activity.
Figure 3. C99 level in neurons.
Neurons were treated with conditioned media (CM-B or CM-P) for 12hr. Calpeptin (20μM) or PD150606 (100μM), specific inhibitors of calpain, was used to pre- and co-treated the neurons with CM-P (CM-P+Calpeptin) or (CM-P+PD150606) for 12hr. Then the cells were lysed and representative western blot result of C99 is shown (n=3). *p<0.05, **p<0.01, ***p<0.001. A line indicates comparison between the 2 bars connected by the line.
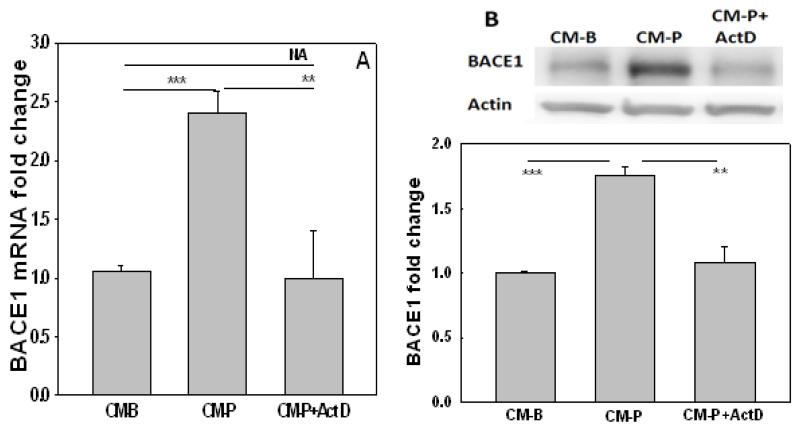
To determine if the increase in BACE1 mRNA level is transcriptionally regulated, a transcriptional inhibitor, actinomycinD (ActD, 1μg/ml) was incubated with the neurons cultured in CM-P. Transcriptional inhibition with ActD significantly decreased the upregulation in BACE1 mRNA (Figure 4A) and protein levels (Figure 4B) suggesting BACE1 is regulated at the transcriptional level in primary neurons cultured in CM-P.
Figure 4. The expression of BACE1 is regulated at the transcriptional level in neurons cultured in conditioned media from astrocytes.
(A) The mRNA expression of BACE1 in neurons treated with CM-B (control) or CM-P, or CM-P plus actinomycinD (ActD) for 12hr. Then the mRNA was extracted and mRNA levels were detected by real-time PCR. (B) Representative western blot results of BACE1 level in control cells (CM-B), in cells treated with CM-P, in cells treated with CM-P in the presence of ActD (1μg/ml) for 12hr (n=3). *p<0.05, **p<0.01, ***p<0.001. A line indicates comparison between the 2 bars connected by the line.
3.3 pSTAT3 is involved in the transcriptional regulation of BACE1
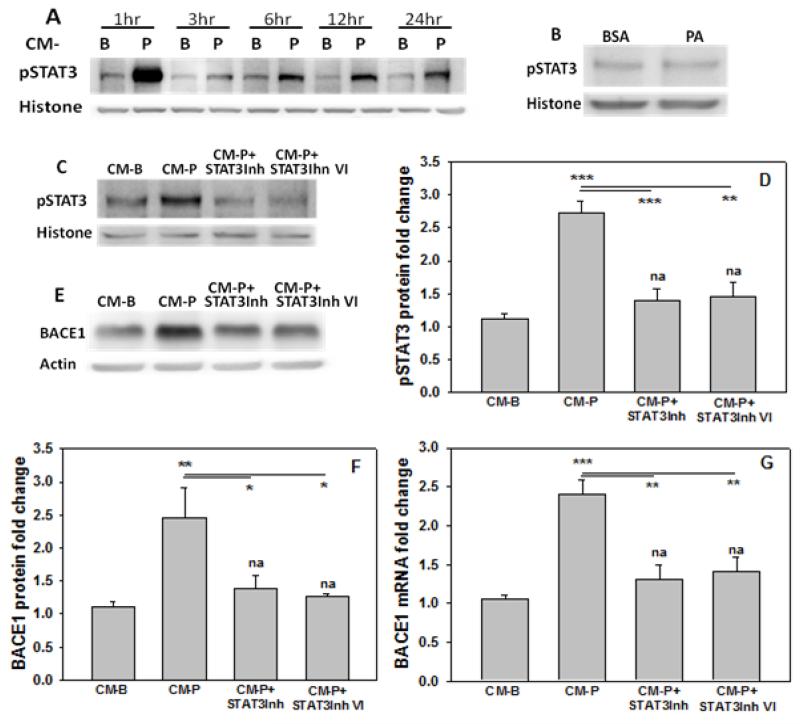
The transcription factor STAT3 is known to regulate numerous genes (Fu et al., 2004), including BACE1 expression in neuroblastoma cells (Wen et al., 2008). Nevertheless, the regulation of BACE1 expression is cell-type and stimuli-specific (Lahiri et al., 2006; Wen et al., 2008). To determine if STAT3 could regulate BACE1 in primary cortical neurons treated with CM-P, nuclear level of pSTAT3 was measured after 1hr of culture. Cytoplasmic STAT3 is phosphorylated at the serine 727 site by kinases (Lim and Cao, 1999; Lim and Cao, 2001), and the phosphorylation of this site induces its dimer formation, which causes auto-phosphorylation at multiple tyrosine sites, including tyrosine 705, leading to translocation of STAT3 to the nucleus (Brierley and Fish, 2005; Fu et al., 2004). Therefore, pSTAT(Y705) was measured in the nucleus and found to increase (Figure 5A). In contrast, treating primary neurons directly with palmitate did not change the pSTAT3 level (Figure 5B). To determine if the upregulated pSTAT3 level could regulate BACE1, STAT3 inhibitor at 235μM, and STAT3 inhibitor VI at 100μM were used individually to pre- and co-treated the primary neurons for 12hr in CM-P. Western blot results showed that phosphorylated STAT3 levels were significantly reduced (Figure 5C, D). Upon inhibition, the mRNA and protein levels of BACE1 reduced significantly as compared to the control-treated neurons (CM-B) (Figure 5E, F and G), suggesting that pSTAT3 could transcriptionally regulate BACE1 expression.
Figure 5. STAT3 is involved in regulating BACE1.
(A) pSTAT3 levels in the nucleus of primary neurons incubated in control (CM-B) or CM-P for the indicated time. Histone was used as loading control for the nuclear extracts. (B) pSTAT3 levels in the nucleus of primary neurons cultured in BSA (control) or 0.2mM palmitate (PA) for 12hr. Then the cells were lysed and western blot for pSTAT3 was performed. Histone was used as loading control for the nuclear extracts. (C, D) pSTAT3 levels in the nucleus. Primary neurons treated with control (CM-B), CM-P or CM-P plus specific inhibitors of STAT3 (STAT3Inh at 235μM or STAT3Inh VI at 100μM) for 12hr. Then the cells were lysed and pSTAT3 levels were measured by western blot and histone was used as loading control for the nuclear extracts. (E, F,G) BACE1 mRNA or protein levels in primary neurons treated with control (CM-B), CM-P or CM-P plus specific inhibitors of STAT3 (STAT3Inh at 235μM or STAT3Inh VI at 100μM) for 12hr. Then the cells were lysed and BACE1 protein levels were measured by western blotting of whole cell lysates with actin used as a loading control. The mRNA of BACE1 was detected by real-time PCR. The results were normalized to control (CM-B) and * or na (not significant) above the bar indicates the significance. A line indicates a comparison between the 2 bars connected by the line. (n=3). *p<0.05, **p<0.01, ***p<0.001.
3.4 Elevated calpain and p25/Cdk5 leads to phosphorylation of STAT3
pSTAT3 is elevated in neurons cultured in CM-P, and this elevated pSTAT3 level correlates with increased BACE1 accumulation (Figure 5). p25/Cdk5 is known to activate STAT3 (Wen et al., 2008) and p25, an activator of Cdk5, increases significantly in primary neurons cultured in CM-P (Patil and Chan, 2005). To confirm whether the elevated pSTAT3 level is due to p25/Cdk5, specific inhibitors of Cdk5, roscovitine (10μM) or butyrolactone-1(10μM), was used to pre- and co-treated the primary neurons cultured in CM-P for 12hr. The protein level of pSTAT3 and the mRNA level of BACE1 reduced significantly upon inhibition of Cdk5, (Figure 6). This suggests that p25/Cdk5 is involved in upregulating pSTAT3 level. The activator of Cdk5, p25, is cleaved from p35 by calpain (Cruz et al., 2003). Since the activity of calpain is upregulated in neurons treated with CM-P (Figure 2), we propose a potential signaling pathway in which the upregulated calpain activity in neurons treated with CM-P enhances p25 production, which elevates Cdk5 activity and in turn pSTAT3 level. To evaluate whether the increased pSTAT3 level is due to upregulated calpain activity, primary neurons were pre- and co-treated with calpeptin (20μM) or PD150606 (100μM), specific calpain inhibitors, along with CM-P for 12hr. The calpain inhibitors significantly decreased pSTAT3 as well as BACE1 mRNA levels (Figure 6). These findings support that both the increased calpain and p25/Cdk5 activities are involved in the upregulation of pSTAT3 activity and BACE1 mRNA level in primary neurons treated with CM-P. The upregulated calcium level in primary neurons (Figure 1) could enhance calpain activity, and induce the signaling cascade of calcium-calpain-p25/Cdk5-pSTAT3 to transcriptionally regulate BACE1 in primary neurons treated with CM-P.
Figure 6. Elevated calpain and p25/Cdk5 activity correlated with STAT3 levels and BACE1 mRNA level.
Neurons were incubated with conditioned media, CM-B (control), CM-P, CM-P plus specific inhibitors of Cdk5, Roscovitine at 10μM or butyrolactone-1 at 10μM (CM-P+Ros or CM-P+BL-1, respectively), or CM-P plus specific inhibitors of calpain, calpeptin at 20μM or PD150606 at 100μM (CM-P+calpeptin or CM-P+PD150606, respectively) for 12hr. Then the cells were lysed. (A-D) pSTAT3 levels in the nucleus of primary neurons. Histone is a loading control for the nuclear extracts. (E) BACE1 mRNA fold change. The results were normalized to control (CM-B), and * or na (not significant) above the bar indicates the significance. A line indicates comparison between the 2 bars connected by the line. (n=3). *p<0.05, **p<0.01, ***p<0.001.
3.5 pSTAT3 could regulate the transcription of γ-secretase
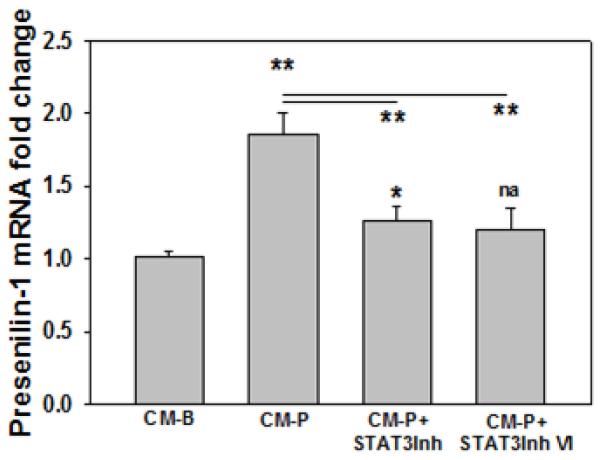
We found the increased C99 level corresponded with the elevated BACE1 mRNA and protein levels in neurons treated with CM-P. In addition, we also observe increased Aβ42 level in primary cortical neurons cultured in CM-P (Figure 7), which is consistent with the increased mRNA presenilin-1 levels observed (Figure 8), the latter is a catalytic subunit of γ-secretase that cleaves C99 to generate amyloid peptide. Thus we proposed that STAT3 could regulate presenilin-1. We analyzed the promoter region of presenilin-1 with JASPAR and TRANSFAC databases, and found that STAT3 could be a potential putative transcription factor of presenilin-1. Inhibiting pSTAT3 significantly reduces the mRNA level of presenilin-1 in neurons cultured in CM-P, suggesting that pSTAT3 could regulate presenilin-1 (Figure 8).
Figure 7. Aβ42 fold change.
Primary neurons were treated with conditioned media CM-B (control) or CM-P for 12hr. Then the cells were lysed and Aβ42 levels were detected by ELISA (n=3). **p<0.01.
Figure 8. Presenilin-1 expression in neurons.
Primary neurons were cultured in conditioned media CM-B (control), CM-P or CM-P plus STAT3 inhibitor or STAT3 inhibitor VI (CM-P+STAT3Inh at 235μM, CM-P+STAT3Inh VI at 100μM, respectively) for 12hr. Then the cells were lysed and the mRNA level of presenilin-1 was monitored by real-time PCR. The results were normalized to control (CM-B), and * or na (not significant) above the bar indicates the significance. A line indicates comparison between the 2 bars connected by the line. (n=3). *p<0.05, **p<0.01, ***p<0.001.
Discussion
Astrocytes are the most abundant cells in the central nervous system. Mounting evidence suggest that local inflammatory responses are mediated by astrocytes and that these astrocytes are not simply passive supportive cells, but contribute to the pathological processes of neurodegenerative diseases (Zaheer et al., 2012) and precede neuronal alterations and behavioral impairment in the progression of AD (Brambilla et al., 2012). Epidemiological studies suggest that saturated free fatty acids may increase the risk of AD (Takechi et al., 2009). To date, the level of palmitate in AD brain has not been reported. However, traumatic brain injury, considered an independent risk factor for AD, is characterized by elevated levels of palmitate in the brain (Homayoun et al., 1997), with palmitate increasing from ~60 to 180 μM (Lipton, 1999). Further supporting reports demonstrated that in traumatic brain injury, free fatty acid (FFA) levels increase significantly in the cerebrospinal fluid (CSF), and are recognized as markers of brain injury. In particular, the concentration of palmitate almost doubled as compared to control, above 1100 μg/L in the CSF of traumatic brain injury versus around 600 μg/L in control (Pilitsis et al., 2003; Pilitsis et al., 2001; Zamir et al., 1991). FFAs in plasma can cross the blood-brain barrier (Dhopeshwarkar and Mead, 1973; Smith and Nagura, 2001), and high fat diets increase the uptake of fatty acids by the brain from the plasma (Karmi et al., 2010; Wang et al., 1994). The concentration of FFAs in normal human plasma in vivo generally ranges between 0.3-1.0mM (Dole, 1956; Shultz, 1991; Tikanoja et al., 1989). In the brain, astrocytes readily take up and metabolize fatty acids. In fact peripheral administration of radio-labeled saturated fatty acid was found to accumulate primarily in astrocytes (Bernoud et al., 1998; Morand et al., 1979). Previously our group showed that palmitate does not directly induce AD-like changes in primary cortical neurons, whereas, it does upregulate Aβ levels in primary neurons mediated by astrocytes (Liu et al., 2013; Patil et al., 2007). Furthermore, the condition media from palmitate-treated astrocytes increased ceramide levels in neurons through sphingomyelinase, which could stabilize BACE1 and promote Aβ biogenesis (Ding et al., 2012; Liu et al., 2013; Puglielli et al., 2003). Aβ peptide accumulation derived from BACE1 and γ-secretase is a well-established pathological hallmark of AD patient brains. Here we demonstrate a role of STAT3 in transcriptionally regulating not only the expression of BACE1, but also the expression of presenilin-1, a core protein of the γ-secretase complex in primary cortical neurons cultured in CM-P. Inhibiting pSTAT3 reduced the upregulation in the mRNA levels of both BACE1 and presenilin-1 in neurons cultured in CM-P. Thus, suggesting that reducing the activity of STAT3 might decrease the amyloidogenic processing, and be a possible therapeutic target for AD pathogenesis. Although we found pSTAT3 could regulate the expression of BACE1 and presenilin-1, it does not preclude the possibility that pSTAT3 could be mediating the expression of BACE1 and presenilin-1 through other proteins. STAT3 can be phosphorylated by Cdk5 kinase, and our results suggest that calpain, a calcium-dependent protease, could increase Cdk5 activity mediated by p25. We observed that inhibiting calpain and Cdk5 reduced the upregulated pSTAT3 levels in the nucleus, suggesting that p25/Cdk5 and calpain could be upstream components in the signaling cascade to activate STAT3.
Deregulation of calcium-mediated signaling has been implicated in many neurodegenerative diseases including AD (Hermes et al., 2010). Calcium generates diverse intracellular signals involved in a variety of cellular functions. The concentration of cytosolic calcium is tightly regulated by the balance between calcium influx and efflux, and by the exchange of calcium with the endoplasmic reticulum (ER) and mitochondria. Several reports have indicated that elevated calcium promotes amyloidogenesis and Aβ aggregation. In turn, Aβ can increase cytosolic levels of calcium by forming voltage-independent cation channels in the cell membrane, causing calcium influx and degeneration of the neuritis (LaFerla, 2002; Lin et al., 2001; Querfurth and LaFerla, 2010). Aβ can also stimulate the release of ER calcium into the cytoplasm (LaFerla, 2002). In addition, factors, such as aging, ceramide and cytokines have been shown to disrupt brain calcium homoeostasis (Beskina et al., 2007; Liu et al., 1999; Wong et al., 1995). Our previous study showed that the ceramide levels increased significantly in primary cortical neurons cultured in CM-P (Liu et al., 2013). Furthermore, palmitate triggered cytokine secretion from the astrocytes into the CM-P, in particular IL-1β and TNFα (Liu et al., 2013). The upregulation in the levels of cytokines and intracellular ceramides could trigger deregulation of calcium homeostasis in the neurons cultured in CM-P. Indeed here we observed the calcium level is upregulated in primary cortical neurons cultured in CM-P.
Abnormally high calcium levels can over-activate calpain, a calcium-dependent protease (Medeiros et al., 2012). In the central nervous system, upregulated calpain activity has been linked to a number of diseases, including AD (Medeiros et al., 2012). Furthermore, selective calpain inhibitors have been shown to mitigate the AD-like changes and cognitive decline in animal model (Medeiros et al., 2012). Aβ can activate calpain to enhance the accumulation of p25 (Lee et al., 2000) by cleaving p35 to p25, to form the p25/Cdk5 complex (Lee et al., 2000). P25/Cdk5 has many substrates which have been shown to be involved in AD pathogenesis. For example, p25/Cdk5 can phosphorylate APP, and BACE1 preferentially cleaves phosphorylated APP to increase Aβ production (Lee et al., 2003), and could also phosphorylate BACE1 and tau, leading to altered BACE1 trafficking or sorting with the former (Pastorino et al., 2002; Walter et al., 2001), and tau hyperphosphorylation with the latter (Wen et al., 2008). Elevated p25/Cdk5 has also been reported in animal model of ischemia, and ischemia has been shown to increase amyloidogenesis and is considered a risk factor for AD (Wang et al., 2003; Wen et al., 2004). Both calpain and p25/Cdk5 play multiple roles in the pathogenesis of AD and have been suggested to be potential therapeutic targets of AD. In this study, calpain activity is increased significantly, and our group previously showed p25 level is upregulated in primary cortical neurons incubated in CM-P (Patil et al., 2007). Inhibiting Cdk5 and calpain significantly reduces pSTAT3 levels in primary cortical neurons cultured in CM-P. Thus, it further supports the role of Cdk5 and calpain in AD pathogenesis.
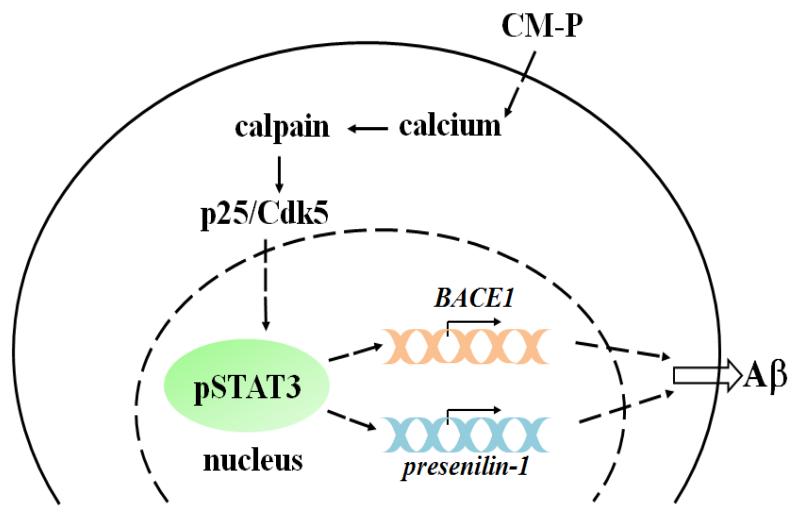
In conclusion, our previous studies showed that the conditioned media from astrocytes cultured in palmitate increased the AD-like changes in primary neurons (Patil et al., 2007). Therefore, to further elucidate the mechanism by which CM-P induces AD-like changes, and based on our findings and previously published results, we propose the following mechanism (Figure 9). In summary, conditioned media from palmitate-treated astrocytes elevates the calcium level in primary cortical neurons, to increase calpain activity, a calcium-dependent protease, which subsequently enhances p25/Cdk5 activity. STAT3 is a substrate of p25/Cdk5 and upon phosphorylation becomes activated and translocates into the nucleus. Elevated pSTAT3 level in the nucleus could transcriptionally upregulate both BACE1 and presenilin-1, directly or indirectly, enhancing the production of Aβ. Elevated Aβ level could further trigger neurons to disrupt calcium homeostasis (LaFerla, 2002; Lin et al., 2001; Querfurth and LaFerla) and reinforce a calcium-Aβ-calcium cascade, and exacerbate the AD pathology. In this signaling cascade STAT3 regulates both BACE1 and presenilin-1, thereby suggesting that STAT3 could be a potential therapeutic target for AD.
Figure 9. Scheme of neuronal responses upon CM-P treatment.
CM-P elevates calcium level in primary neurons. Elevated calcium increases calpain activity, a calcium dependent protease, which in turn enhances p25/Cdk5 activity. STAT3 is phosphorylated by p25/Cdk5 and translocates into the nucleus. Elevated pSTAT3 in the nucleus transcriptionally upregulates both BACE1 and presenilin-1 mRNA, which could further enhance the production of Aβ.
Highlights.
Conditioned media from palmitate-treated astrocytes (CM-P) cause AD-like changes
CM-P triggers an abnormal calcium level which elevates calpain activity in neurons
pSTAT3 transcriptionally regulates both BACE1 and presenilin-1 expression
Signaling of calcium-calpain-p25/Cdk5-STAT3→BACE1 & γ-secretase and amyloidogenesis
Acknowledgement
This study was supported in part by the National Institute of Health (R01GM079688 and R01GM089866), the National Science Foundation (CBET 0941055).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest and author contributions
The authors declare no actual or potential conflicts of interest.
Reference
- Alzheimer’s A. Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Bernoud N, Fenart L, Benistant C, Pageaux JF, Dehouck MP, Moliere P, Lagarde M, Cecchelli R, Lecerf J. Astrocytes are mainly responsible for the polyunsaturated fatty acid enrichment in blood-brain barrier endothelial cells in vitro. J Lipid Res. 1998;39:1816–1824. [PubMed] [Google Scholar]
- Berridge MJ. Calcium hypothesis of Alzheimer’s disease. Pflugers Arch. 2010;459:441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- Beskina O, Miller A, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Mechanisms of interleukin-1beta-induced Ca2+ signals in mouse cortical astrocytes: roles of store- and receptor-operated Ca2+ entry. Am J Physiol Cell Physiol. 2007;293:C1103–1111. doi: 10.1152/ajpcell.00249.2007. [DOI] [PubMed] [Google Scholar]
- Bilgin B, Liu L, Chan C, Walton SP. Quantitative, solution-phase profiling of multiple transcription factors in parallel. Anal Bioanal Chem. 2013;405:2461–2468. doi: 10.1007/s00216-013-6712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne KZ, Ferrari DC, Lange-Dohna C, Rossner S, Wood TG, Perez-Polo JR. Differential regulation of BACE1 promoter activity by nuclear factor-kappaB in neurons and glia upon exposure to beta-amyloid peptides. J Neurosci Res. 2007;85:1194–1204. doi: 10.1002/jnr.21252. [DOI] [PubMed] [Google Scholar]
- Brambilla L, Martorana F, Rossi D. Astrocyte signaling and neurodegeneration: New insights into CNS disorders. Prion. 2012:7. doi: 10.4161/pri.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley MM, Fish EN. Stats: multifaceted regulators of transcription. J Interferon Cytokine Res. 2005;25:733–744. doi: 10.1089/jir.2005.25.733. [DOI] [PubMed] [Google Scholar]
- Chami L, Buggia-Prevot V, Duplan E, Delprete D, Chami M, Peyron JF, Checler F. Nuclear factor-kappaB regulates betaAPP and beta- and gamma-secretases differently at physiological and supraphysiological Abeta concentrations. J Biol Chem. 2012;287:24573–24584. doi: 10.1074/jbc.M111.333054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY. Cdk5: a multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 2012;22:169–175. doi: 10.1016/j.tcb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Christensen MA, Zhou W, Qing H, Lehman A, Philipsen S, Song W. Transcriptional regulation of BACE1, the beta-amyloid precursor protein beta-secretase, by Sp1. Mol Cell Biol. 2004;24:865–874. doi: 10.1128/MCB.24.2.865-874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet. 2010;19:R12–20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Kim D, Moy LY, Dobbin MM, Sun X, Bronson RT, Tsai LH. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J Neurosci. 2006;26:10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Das HK. Transcriptional regulation of the presenilin-1 gene: implication in Alzheimer’s disease. Front Biosci. 2008;13:822–832. doi: 10.2741/2723. [DOI] [PubMed] [Google Scholar]
- Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. J Biol Chem. 2010;285:12463–12468. doi: 10.1074/jbc.R109.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhopeshwarkar GA, Mead JF. Uptake and transport of fatty acids into the brain and the role of the blood-brain barrier system. Adv Lipid Res. 1973;11:109–142. doi: 10.1016/b978-0-12-024911-4.50010-6. [DOI] [PubMed] [Google Scholar]
- Ding Y, Ko MH, Pehar M, Kotch F, Peters NR, Luo Y, Salamat SM, Puglielli L. Biochemical inhibition of the acetyltransferases ATase1 and ATase2 reduces beta-secretase (BACE1) levels and Abeta generation. J Biol Chem. 2012;287:8424–8433. doi: 10.1074/jbc.M111.310136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956;35:150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedrizzi L, Carafoli E. Ca2+ dysfunction in neurodegenerative disorders: Alzheimer’s disease. Biofactors. 2011;37:189–196. doi: 10.1002/biof.157. [DOI] [PubMed] [Google Scholar]
- Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, Ip NY. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Natl Acad Sci U S A. 2004;101:6728–6733. doi: 10.1073/pnas.0307606100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge YW, Maloney B, Sambamurti K, Lahiri DK. Functional characterization of the 5′ flanking region of the BACE gene: identification of a 91 bp fragment involved in basal level of BACE promoter expression. FASEB J. 2004;18:1037–1039. doi: 10.1096/fj.03-1379fje. [DOI] [PubMed] [Google Scholar]
- Giusti-Rodriguez P, Gao J, Graff J, Rei D, Soda T, Tsai LH. Synaptic deficits are rescued in the p25/Cdk5 model of neurodegeneration by the reduction of beta-secretase (BACE1) J Neurosci. 2011;31:15751–15756. doi: 10.1523/JNEUROSCI.3588-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes M, Eichhoff G, Garaschuk O. Intracellular calcium signalling in Alzheimer’s disease. J Cell Mol Med. 2010;14:30–41. doi: 10.1111/j.1582-4934.2009.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun P, Rodriguez de Turco EB, Parkins NE, Lane DC, Soblosky J, Carey ME, Bazan NG. Delayed phospholipid degradation in rat brain after traumatic brain injury. J Neurochem. 1997;69:199–205. doi: 10.1046/j.1471-4159.1997.69010199.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Head injury and dementia. Curr Opin Neurol. 2004;17:719–723. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Nagren K, Solin O, Nuutila P. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–2177. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Ge YW. Functional domains of the BACE1 and BACE2 promoters and mechanisms of transcriptional suppression of the BACE2 promoter in normal neuronal cells. J Mol Neurosci. 2006;29:65–80. doi: 10.1385/JMN:29:1:65. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Lee S, Das HK. Transcriptional regulation of the presenilin-1 gene controls gamma-secretase activity. Front Biosci (Elite Ed) 2010;2:22–35. doi: 10.2741/e61. [DOI] [PubMed] [Google Scholar]
- Liang B, Duan BY, Zhou XP, Gong JX, Luo ZG. Calpain activation promotes BACE1 expression, amyloid precursor protein processing, and amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2010;285:27737–27744. doi: 10.1074/jbc.M110.117960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CP, Cao X. Serine phosphorylation and negative regulation of Stat3 by JNK. J Biol Chem. 1999;274:31055–31061. doi: 10.1074/jbc.274.43.31055. [DOI] [PubMed] [Google Scholar]
- Lim CP, Cao X. Regulation of Stat3 activation by MEK kinase 1. J Biol Chem. 2001;276:21004–21011. doi: 10.1074/jbc.M007592200. [DOI] [PubMed] [Google Scholar]
- Lin H, Bhatia R, Lal R. Amyloid beta protein forms ion channels: implications for Alzheimer’s disease pathophysiology. FASEB J. 2001;15:2433–2444. doi: 10.1096/fj.01-0377com. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu G, Kleine L, Hebert RL. Advances in the signal transduction of ceramide and related sphingolipids. Crit Rev Clin Lab Sci. 1999;36:511–573. doi: 10.1080/10408369991239240. [DOI] [PubMed] [Google Scholar]
- Liu L, Martin R, Chan C. Palmitate-activated astrocytes via serine palmitoyltransferase increase BACE1 in primary neurons by sphingomyelinases. Neurobiol Aging. 2013;34:540–550. doi: 10.1016/j.neurobiolaging.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros R, Kitazawa M, Chabrier MA, Cheng D, Baglietto-Vargas D, Kling A, Moeller A, Green KN, LaFerla FM. Calpain inhibitor A-705253 mitigates Alzheimer’s disease-like pathology and cognitive decline in aged 3xTgAD mice. Am J Pathol. 2012;181:616–625. doi: 10.1016/j.ajpath.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Morand O, Baumann N, Bourre JM. In vivo incorporation of exogenous [1-14C]stearic acid into neurons and astrocytes. Neurosci Lett. 1979;13:177–181. doi: 10.1016/0304-3940(79)90038-7. [DOI] [PubMed] [Google Scholar]
- Morris MC, Tangney CC. Diet and prevention of Alzheimer disease. JAMA. 2010;303:2519–2520. doi: 10.1001/jama.2010.844. [DOI] [PubMed] [Google Scholar]
- Nowak K, Lange-Dohna C, Zeitschel U, Gunther A, Luscher B, Robitzki A, Perez-Polo R, Rossner S. The transcription factor Yin Yang 1 is an activator of BACE1 expression. J Neurochem. 2006;96:1696–1707. doi: 10.1111/j.1471-4159.2006.03692.x. [DOI] [PubMed] [Google Scholar]
- Pastorcic M, Das HK. Regulation of transcription of the human presenilin-1 gene by ets transcription factors and the p53 protooncogene. J Biol Chem. 2000;275:34938–34945. doi: 10.1074/jbc.M005411200. [DOI] [PubMed] [Google Scholar]
- Pastorino L, Ikin AF, Nairn AC, Pursnani A, Buxbaum JD. The carboxyl-terminus of BACE contains a sorting signal that regulates BACE trafficking but not the formation of total A(beta) Mol Cell Neurosci. 2002;19:175–185. doi: 10.1006/mcne.2001.1065. [DOI] [PubMed] [Google Scholar]
- Patil S, Chan C. Palmitic and stearic fatty acids induce Alzheimer-like hyperphosphorylation of tau in primary rat cortical neurons. Neurosci Lett. 2005;384:288–293. doi: 10.1016/j.neulet.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Patil S, Melrose J, Chan C. Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur J Neurosci. 2007;26:2131–2141. doi: 10.1111/j.1460-9568.2007.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilitsis JG, Coplin WM, O’Regan MH, Wellwood JM, Diaz FG, Fairfax MR, Michael DB, Phillis JW. Free fatty acids in cerebrospinal fluids from patients with traumatic brain injury. Neurosci Lett. 2003;349:136–138. doi: 10.1016/s0304-3940(03)00803-6. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Diaz FG, Wellwood JM, Oregan MH, Fairfax MR, Phillis JW, Coplin WM. Quantification of free fatty acids in human cerebrospinal fluid. Neurochem Res. 2001;26:1265–1270. doi: 10.1023/a:1014227231130. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Kinsey R, Maloney B, Ge YW, Lahiri DK. Gene structure and organization of the human beta-secretase (BACE) promoter. FASEB J. 2004;18:1034–1036. doi: 10.1096/fj.03-1378fje. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shultz TD. Physiological free fatty acid concentrations do not increase free estradiol in plasma. J Clin Endocrinol Metab. 1991;72:65–68. doi: 10.1210/jcem-72-1-65. [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Smith QR, Nagura H. Fatty acid uptake and incorporation in brain: studies with the perfusion model. J Mol Neurosci. 2001;16:167–172. doi: 10.1385/JMN:16:2-3:167. discussion 215-121. [DOI] [PubMed] [Google Scholar]
- Sun X, Bromley-Brits K, Song W. Regulation of beta-site APP-cleaving enzyme 1 gene expression and its role in Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):62–70. doi: 10.1111/j.1471-4159.2011.07515.x. [DOI] [PubMed] [Google Scholar]
- Takechi R, Galloway S, Pallebage-Gamarallage MM, Lam V, Mamo JC. Dietary fats, cerebrovasculature integrity and Alzheimer’s disease risk. Prog Lipid Res. 2009;49:159–170. doi: 10.1016/j.plipres.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Tikanoja SH, Joutti A, Liewendahl BK. Association between increased concentrations of free thyroxine and unsaturated free fatty acids in non-thyroidal illnesses: role of albumin. Clin Chim Acta. 1989;179:33–43. doi: 10.1016/0009-8981(89)90020-x. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Fa M, Liu S, Zhang H, Hidalgo A, Schmidt SD, Yamaguchi H, Yoshii N, Mathews PM, Nixon RA, Arancio O. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, Lammich S, Multhaup G, Haass C. Phosphorylation regulates intracellular trafficking of beta-secretase. J Biol Chem. 2001;276:14634–14641. doi: 10.1074/jbc.M011116200. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci. 2003;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- Wang SW, Wang M, Grossman BM, Martin RJ. Effects of dietary fat on food intake and brain uptake and oxidation of fatty acids. Physiol Behav. 1994;56:517–522. doi: 10.1016/0031-9384(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yu WH, Maloney B, Bailey J, Ma J, Marie I, Maurin T, Wang L, Figueroa H, Herman M, Krishnamurthy P, Liu L, Planel E, Lau LF, Lahiri DK, Duff K. Transcriptional regulation of beta-secretase by p25/cdk5 leads to enhanced amyloidogenic processing. Neuron. 2008;57:680–690. doi: 10.1016/j.neuron.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Li XB, Hunchuk N. N-acetylsphingosine (C2-ceramide) inhibited neutrophil superoxide formation and calcium influx. J Biol Chem. 1995;270:3056–3062. doi: 10.1074/jbc.270.7.3056. [DOI] [PubMed] [Google Scholar]
- Wu M, Liu L, Chan C. Identification of novel targets for breast cancer by exploring gene switches on a genome scale. BMC Genomics. 2011;12:547. doi: 10.1186/1471-2164-12-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Liu L, Hijazi H, Chan C. A multi-layer inference approach to reconstruct condition-specific genes and their regulation. Bioinformatics. 2013 doi: 10.1093/bioinformatics/btt186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer S, Thangavel R, Wu Y, Khan MM, Kempuraj D, Zaheer A. Enhanced Expression of Glia Maturation Factor Correlates with Glial Activation in the Brain of Triple Transgenic Alzheimer’s Disease Mice. Neurochem Res. 2012 doi: 10.1007/s11064-012-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir I, Grushka E, Cividalli G. High-performance liquid chromatographic analysis of free palmitic and stearic acids in cerebrospinal fluid. J Chromatogr. 1991;565:424–429. doi: 10.1016/0378-4347(91)80404-z. [DOI] [PubMed] [Google Scholar]
- Zhang L, Seitz LC, Abramczyk AM, Liu L, Chan C. cAMP initiates early phase neuron-like morphology changes and late phase neural differentiation in mesenchymal stem cells. Cell Mol Life Sci. 2011;68:863–876. doi: 10.1007/s00018-010-0497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]