Abstract
Despite early detection programs, many patients with prostate cancer present with intermediate- or high-risk disease. We prospectively investigated whether 11C-acetate PET/CT predicts lymph-node (LN) metastasis and treatment failure in men planned for radical prostatectomy.
Methods
107 men with intermediate-or-high-risk localized prostate cancer with negative conventional imaging underwent PET/CT with 11C-acetate. Five underwent LN staging only and 102 LN staging and prostatectomy. PET/CT findings were correlated with pathologic nodal status. Treatment-failure-free survival (TFFS) was estimated by Kaplan-Meier method. The ability of PET/CT to predict outcomes was evaluated by multivariate Cox proportional hazards analysis.
Results
PET/CT was positive for pelvic LN or distant metastasis in 36 of 107 patients (33.6%). LN metastasis was present histopathologically in 25 (23.4%). The sensitivity, specificity, positive- and negative-predictive values of PET/CT for detecting LN metastasis were 68.0%, 78.1%, 48.6% and 88.9% respectively. 64 patients failed: 25 with metastasis, 17 with persistent post-prostatectomy prostate specific antigen (PSA) >0.20 ng/mL, and 22 with biochemical recurrence (PSA >0.20 ng/mL after nadir) during follow-up for a median of 44.0 months. TFFS was worse in PET-positive than in PET-negative patients (p<0.0001) and in those with false-positive versus true-negative scans (p<0.01), suggesting that PET may have demonstrated nodal disease not removed surgically or identified pathologically. PET positivity independently predicted failure in preoperative (hazard ratio=3.26, p<0.0001) and postoperative (HR=3.07, p=0.0001) multivariate models.
Conclusion
In patients planned for or completing prostatectomy, 11C-acetate-PET/CT detects LN metastasis not identified by conventional imaging and independently predicts TTFS.
Keywords: prostatic cancer, PET, acetate, cancer staging, lymphatic metastasis
INTRODUCTION
While many patients in the United States with newly diagnosed prostate cancer have low-risk disease, 40-50% have intermediate- or high-risk localized disease (1, 2). Up to 20% of these patients have metastatic disease, usually in lymph nodes. Identification of lymph node (LN) involvement is important for treatment planning (3, 4). These patients typically undergo computed tomography (CT) and/or magnetic resonance imaging (MRI). However, neither is sensitive for detecting nodal metastasis unless the nodes are enlarged (4). In a recent meta-analysis, the sensitivity of CT and MRI was 39-42% for detecting pelvic lymph nodes (5). MRI with ultra-small superparamagnetic iron oxide contrast (which is not available in the United States) and diffusion-weighted MRI appear to have improved sensitivity (6), but experience is still limited.
Because of the unreliability of imaging, nomograms based on clinical parameters, such as prostate specific antigen (PSA), T stage and Gleason score, are used to estimate the risk of nodal metastasis (3, 7, 8) and may justify omission of lymphadenectomy in patients with estimated risk <5% since there is an 8-20% complication rate of lymphadenectomy (9, 10). This approach is not ideal, however, as some men will be understaged.
Positron emission tomography (PET) allows for detection of characteristic biochemical attributes of malignant cells and is not dependent on size criteria alone. PET with 18F-fluorodeoxyglucose (FDG) effectively stages many cancers, but has limited utility for initial staging of prostate cancer because urinary excretion may obscure nodal uptake; additionally, most prostate cancers have low rates of glucose metabolism, and FDG uptake is similar in prostate cancer, benign prostatic enlargement and inflammation (11, 12).
Because of these limitations, other radiopharmaceuticals have been investigated for prostate cancer imaging, including 11C-acetate (11, 13). Acetate enters the biochemical pathways of fatty acid metabolism, which are consistently upregulated in prostate cancer cell lines (14), and 11C-acetate has minimal urinary excretion (15). Although multiple studies have demonstrated promising results with 11C-acetate-PET for diagnosing local and distant disease after initial treatment failure(16-20), less is known about its value for initial prostate cancer staging (21).
The purpose of this prospective study was to investigate PET/CT with 11C-acetate for nodal staging and as a biomarker for prediction of treatment failure in patients with newly diagnosed intermediate- or high-risk disease, who were planned for radical prostatectomy and in whom conventional staging was negative for metastasis.
MATERIALS AND METHODS
This study was conducted within a larger prospective study (http://www.clinicaltrials.gov, NCT00121212) investigating 11C-acetate-PET/CT for staging of patients with newly diagnosed prostate cancer planned for radical prostatectomy or radiation therapy. The study was approved by the institutional review board at Washington University. All subjects gave written informed consent.
Patients and Eligibility Criteria
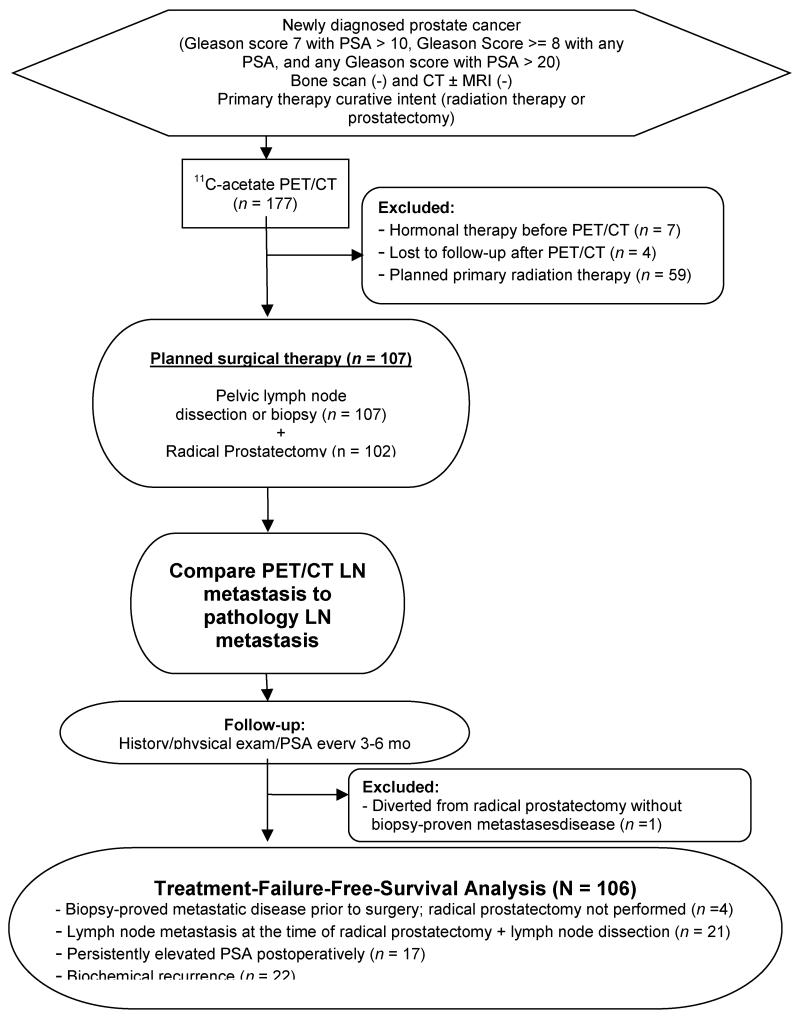
The patient flow diagram of the study is shown in Figure 1. From October 2003 to November 2009, 177 men with newly diagnosed biopsy-proved prostate cancer judged to be at intermediate or high risk for nodal metastasis with negative abdominopelvic CT (and/or pelvic MRI) and bone scintigraphy underwent 11C-acetate-PET/CT. Intermediate- or high-risk patients were defined as those with: Gleason score 7 and PSA ≥10 ng/mL, Gleason score ≥8 with any PSA, or any Gleason score with PSA >20 ng/mL. Patients were excluded from this analysis if they elected to have primary radiation therapy (n=59), had hormonal therapy prior to PET/CT (n=7), or were lost to follow-up after PET/CT (n=4). The remaining 107 patients planned for radical prostatectomy comprise the cohort of this report. All of these patients underwent CT (rather than MRI) for pre-enrollment staging.
Figure 1.
11C-Acetate-PET/CT
PET/CT was performed on either a Siemens Biograph Duo (n=110) or a Biograph 40 (n=67) scanner. 11C-acetate, 1.48 GBq for studies on the Biograph Duo and 0.74 GBq for studies on the Biograph 40, was administered intravenously. Approximately 10-15 minutes post injection, a 10-20 s CT topogram was obtained from the skull base through the pelvis. Based on the topogram, the tube current for the CT scan was adjusted so that the effective mAs was between 95 and 111. The tube voltage was 130 kVp (Biograph Duo) or 120 kVp (Biograph 40). Reconstructed images had 5-mm slice thickness. Immediately after the CT scan, emission images beginning at the pelvis and proceeding cranially were obtained. Emission images ranged from 2 to 5 minutes in duration for each of the 6-8 bed positions.
Imaging Interpretation
Each PET/CT study was reviewed preoperatively by an experienced nuclear radiologist and a genitourinary radiologist, first independently and then jointly. Their consensus interpretation was used for this analysis. The radiologists were aware of the diagnosis of prostate cancer, Gleason score, and PSA level, as defined by the study eligibility criteria. Considering the normal biodistribution of 11C-acetate, the radiologists graded the presence or absence of metastasis on PET/CT imaging (pelvic or distant LN or distant organ metastasis) on a five-point ordinal scale from 0 to 4, where 0=definitely normal and 4=definitely abnormal. Scans graded 2 to 4 were considered PET positive. In addition, all PET images were evaluated semiquantitatively by determination of the maximum standardized uptake value (SUVmax) within the prostate gland.
Treatment and Pathologic Evaluation
To allow for assessment of the impact of PET on patient management, neither patients nor their treating physicians were blinded to the results of 11C-acetate-PET/CT. The information was available for clinical decision making, such that patients could be diverted from prostatectomy if PET demonstrated metastasis that was confirmed before surgery by biopsy. The management of each patient was documented by medical record review.
The surgical approach, laparoscopic or open, and lymphadenectomy boundaries were left to the discretion of the surgeon. However, lymphadenectomy involved at minimum removal of nodes within the obturator fossae (external iliac artery anteriorly, pelvic sidewall laterally, obturator nerve inferiorly and Cooper’s ligament distally). Nodal metastatic status was based on final biopsy or lymphadenectomy pathology. Pathologists were blinded to the results of PET/CT.
Follow-up and Assessment of Treatment Failure
Patients were followed every three to six months postoperatively with physical examination and serum PSA measurement.
Treatment failure was defined as one of the following events: pathologic diagnosis of metastasis by biopsy or surgery; persistently detectable PSA> 0.20 ng/mL ≥4 weeks after surgery; or subsequent biochemical recurrence with PSA > 0.20 ng/mL and rising (with the failure event dated to the first occurrence). The time to treatment failure was defined from the time of PET/CT until the failure event. Patients given adjuvant therapy without biochemical recurrence, because of high pathologic T stage or positive margins in the prostatectomy specimen, were not considered failures. For assessment of treatment-failure-free survival (TFFS), patients who did not have treatment failure were censored at the last follow-up date. Seven patients died after either their date of disease progression or their last follow-up visit; thus, none of these deaths was considered to be within the study interval.
Statistical Analysis
The 11C-acetate-PET/CT findings of LN metastasis were correlated with the histological findings on a per patient basis to calculate the sensitivity, specificity, positive and negative predictive values for PET detection of nodal metastasis. Continuous and categorical variables between PET-positive and PET-negative patients (with respect to nodal or distant metastasis) were compared by a Kruskal-Wallis test and the Fisher’s Exact test, respectively. Analysis of treatment-failure-free survival (TFFS) for PET-positive and PET-negative patients was performed using Kaplan-Meier analysis and a log-rank test. Univariate predictors of treatment failure included biopsy Gleason score, percent biopsy core positive, PSA level, clinical stage, PET positivity, prostate SUVmax, pathological Gleason score, pathological stage, and surgical margin status. Preoperative and postoperative multivariate Cox proportional hazards multivariate analyses evaluated the independent predictive ability of PET status while controlling for these covariates. The assumption of proportionality was tested and met for Cox analysis. P<0.05 was considered statistically significant for all study outcomes, and all statistical tests were two-sided. SAS Version 9. (Cary, NC) was used to perform statistical analyses.
RESULTS
Demographic, clinical, and pathological data of the patients are summarized in Table 1. Median age was 61 years and median PSA was 11.8 ng/mL. Clinical T stage was T1 in 66.7% of patients and biopsy Gleason scores were 8-10 in 58.9%.
Table 1.
Clinical and Pathological Demographic Information
| PET Positive for Metastasis |
PET Negative for Metastasis |
P value* |
||
|---|---|---|---|---|
| Patients (N) | 107 | 36 | 71 | |
|
| ||||
| Age (years) | ||||
|
| ||||
| Median (range) |
61 (46-80) |
61.5 (47-78) |
61 (46-80) |
0.9544 |
|
| ||||
| PSA (ng/mL) | ||||
|
| ||||
| Median (range) |
11.8 (1.4-225.4) |
11.25 (1.4-82.4) |
11.9 (2.9-225.4) |
0.3556 |
|
| ||||
|
Clinical
Stage (N) |
105* | 35 | 70 | |
|
| ||||
| T1 | 70 (66.7%) | 21 (60.0%) | 49 (70.0%) | 0.2909 |
|
| ||||
| T2 | 34 (32.4%) | 13 (37.1%) | 21 (30.0%) | |
|
| ||||
| T3 | 1 (0.9%) | 1 (2.9%) | 0 (0.0%) | |
|
| ||||
|
Biopsy
Gleason Score (N) |
107 | 36 | 71 | |
|
| ||||
| 6 | 6 (5.6%) | 1 (2.78%) | 5 (7.04%) | 0.8364 |
|
| ||||
| 7 | 38 (35.5%) | 13 (36.11%) | 25 (35.21%) | |
|
| ||||
| 8-10 | 63 (58.9%) | 22 (61.11%) | 41 (57.75%) | |
|
| ||||
|
Prostatecto my Pathology Gleason Score (N) |
102** | 31 | 71 | |
|
| ||||
| 6 | 6 (5.9%) | 1 (3.23%) | 5 (7.0%) | 0.0346 |
|
| ||||
| 7 | 53 (52.0%) | 11 (35.48%) | 42 (59.2%) | |
|
| ||||
| 8-10 | 43 (42.1%) | 19 (61.3%) | 24 (33.8%) | |
|
| ||||
|
Pathological
Stage (N) |
102** | 31 | 71 | |
|
| ||||
| T2 | 41 (40.2%) | 7 (22.58%) | 34 (47.89%) | 0.0273 |
|
| ||||
| T3a | 28 (27.5%) | 9 (29.03%) | 19 (26.76%) | |
|
| ||||
| T3b | 32 (31.4%) | 14 (45.16%) | 18 (25.35%) | |
|
| ||||
| T4 | 1 (1.0%) | 1 (3.23%) | 0 (0.00%) | |
|
Time from
PET/CT to surgery (days) |
106 | 35 | 71 | |
|
| ||||
| Median (range) |
13 (1-290) |
13 (1-290) |
14 (1-79) |
0.3550 |
Data unavailable in 2 patients
**102 patients underwent prostatectomy while 5 underwent LN sampling or biopsy only.
11C-Acetate-PET/CT Findings
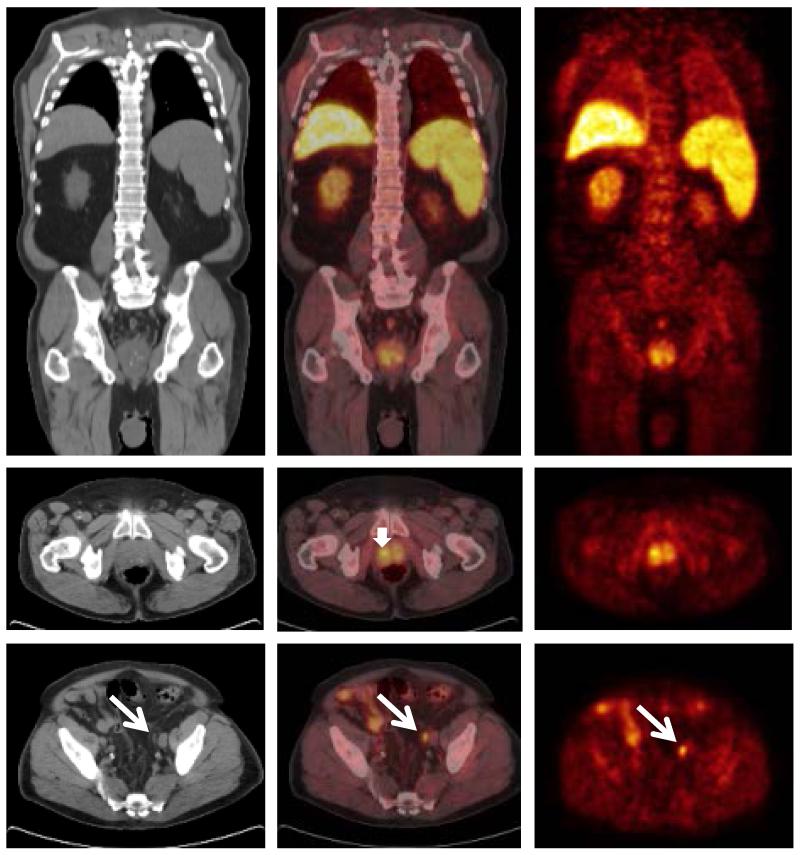
A representative 11C-acetate-PET/CT positive in both the prostate gland and in a pelvic LN is shown in Figure 2.
Figure 2.
On the 11C-acetate-PET/CT images, focally or diffusely increased activity, presumably representing abnormal tracer uptake in the primary tumor, was recorded in the prostate gland in 103 of the 107 patients (96.3%). The mean SUVmax ± standard deviation in these 103 prostate glands was 5.4 ± 1.8. There were no instances where 11C-acetate-PET/CT was positive in LNs but negative in the prostate gland.
11C-acetate-PET/CT demonstrated metastasis in 36 (33.6%) of 107 patients. Of these, 35 had LN metastasis [32 in pelvic LNs only, 2 in pelvic LNs and a distant node (1 supraclavicular and 1 paratracheal), and 1 in pelvic LNs and bone], and one patient had bone metastasis only. The two patients with PET findings of bone metastasis had negative MRI of the skeletal foci, and underwent prostatectomy as planned; both were considered to have failed treatment on the basis of persistently increased PSA after prostatectomy. As is shown in Table 1, the 36 PET-positive patients were more likely than the 71 PET-negative patients to have higher prostatectomy Gleason scores and higher pathological stage, but otherwise had similar clinical features.
Pathology Findings
One-hundred-two patients underwent prostatectomy and lymphadenectomy and 5 underwent LN sampling only (1 fine-needle aspiration of distant nodes only, and 4 with pelvic lymphadenectomy). Four of the 5 patients with nodal sampling only had metastases and were defined as treatment failures. The remaining patient had negative pathology but opted for radiation therapy (and was excluded from the treatment failure analysis). . Of the 102 patients undergoing prostatectomy and lymphadenectomy, 21 (20.6%) had nodal metastasis.
11C-Acetate-PET/CT Lymph Node Staging
On a per patient basis, the overall sensitivity, specificity, positive-predictive value (PPV) and negative-predictive value (NPV) of PET/CT for detection of lymph node metastasis were 68.0%, 78.1%, 48.6% and 88.9%, respectively (Table 2).
Table 2.
Correlation of PET/CT and Pathological Results for Nodal Metastasis (per patient basis)
| LN Positive Pathology |
LN Negative Pathology |
Total | |
|---|---|---|---|
|
LN Positive
PET/CT |
17 | 18 | 35 |
|
LN Negative
PET/CT |
8 | 64 | 72 |
| Total | 25 | 82 | 107 |
Sensitivity = 68.0 % (95% CI, 46.5% - 85.1%)
Specificity = 78.1% (67.5% - 86.4%)
PPV = 48.6% (31.4% - 66.0%)
NPV = 88.9 % (79.3% - 95.1%)
Treatment-Failure-Free Survival Analysis
Failure Events
The cohort for treatment failure analysis consisted of 106 patients; as noted above, one patient with a positive PET scan had a negative LN biopsy, opted for radiotherapy, and was excluded from the failure analysis. Treatment failure occurred in 64 of the 106 patients: 4 diverted from prostatectomy with biopsy-confirmed metastasis, 21 with confirmed nodal metastasis at lymphadenectomy, and 17 with persistently detectable PSA after prostatectomy. Of the remaining 64 patients, 22 had biochemical recurrence during a median follow-up of 44.0 months (range 1.2-93.5 months). Three patients received adjuvant therapy for positive margins without biochemical recurrence and were not considered to have failed at that time.
Univariate Analysis
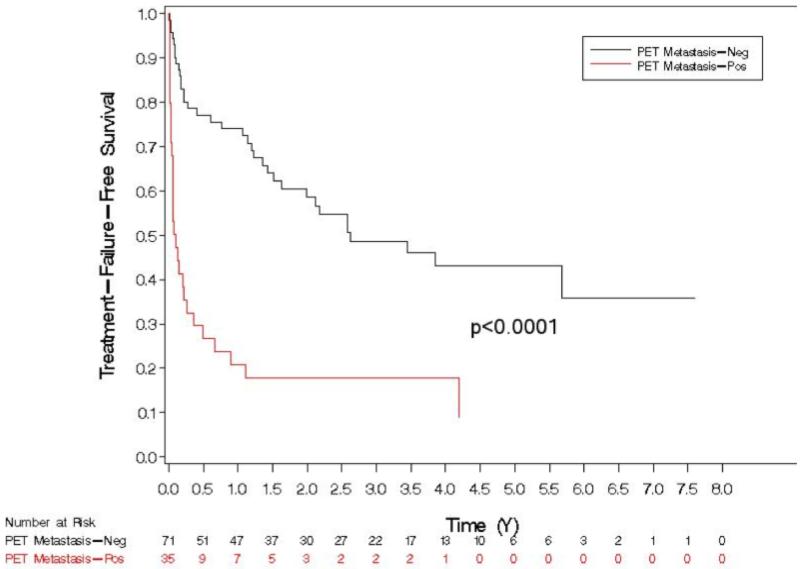
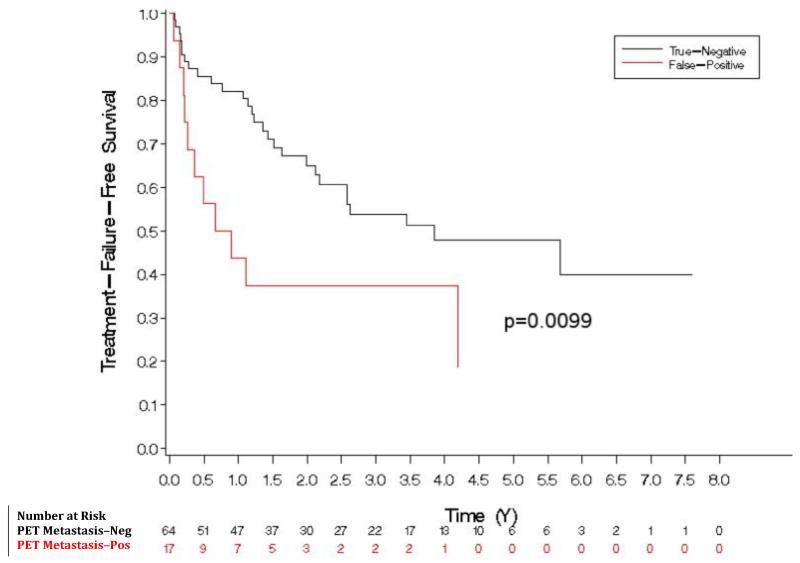
On univariate analysis, factors predicting treatment failure are shown in Table 3. PET/CT positivity for any site of metastasis was significantly associated with poorer TFFS on univariate analysis (HR 3.830, p < 0.001) (Figure 3). The three-year failure probabilities of PET-negative and PET-positive patients are 51% and 82%, respectively. The median TFFS for PET-positive patients was 1.3 months, while that for PET-negative patients was 31.5 months. Patients with false-positive PET/CT (relative to the pathological reference standard) had worse TFFS than patients with true-negative PET/CT (p<0.01), suggesting that PET/CT may have demonstrated nodal disease not removed at surgery or not identified in the pathologic specimen (Figure 4).
Table 3.
Univariate Model for Predicting Treatment Failure
| Variable | *N | HR (95% CI) | P value |
|---|---|---|---|
|
| |||
| PET metastasis (any site) | 106 | 3.83 (2.30, 6.38) | <.0001 |
|
| |||
| Clinical Gleason Score | 106 | 0.1092 | |
| 6 vs. 7 | 0.46 (0.11, 1.97) | ||
| (8, 9, 10) vs. 7 | 1.51 (0.89, 2.55) | ||
|
| |||
| Percent cores positive | 101 | 1.01 (1.00, 1.02) | 0.0140 |
|
| |||
| PSA (ng/mL) | 106 | 1.01 (1.00, 1.01) | 0.0563 |
|
| |||
| Clinical Stage (cT2 vs cT1c) | 103 | 1.39 (0.814, 2.36) | 0.2294 |
|
| |||
| SUV (primary prostate cancer) | 106 | 1.11 (0.97, 1.26) | 0.1285 |
|
| |||
| Pathology Gleason Score | 102 | <.0001 | |
| 6 vs. 7 | 0.26 (0.04, 1.96) | ||
| (8, 9, 10) vs. 7 | 2.82(1.67, 4.76) | ||
|
| |||
| Pathology Stage | 101 | <.0001 | |
| 3a vs. 2 | 2.93 (1.39, 6.20) | ||
| 3b vs. 2 | 9.15 (4.47, 18.70) | ||
|
| |||
| Surgical Margins | 102 | 2.56 (1.50, 4.37) | 0.0006 |
Number of patients for which variable was available.
Figure 3.
Figure 4.
Multivariate Analysis
On the preoperative multivariate Cox proportional hazards model (Table 4), PET metastasis was significantly associated with a 3.26-fold (95% CI 1.86-5.72, p < .0001) increased risk of treatment failure while controlling for other known preoperative factors (PSA, clinical stage, clinical Gleason score, and percent cores positive).
Table 4.
Preoperative Multivariate Cox Proportional Hazards Model for Predicting Treatment Failure (N = 98)
| Variable | HR (95% CI) | P value |
|---|---|---|
|
| ||
| PET metastasis (any site) | 3.26 (1.86, 5.72) | <.0001 |
|
| ||
| PSA (ng/mL) | 1.01 (1.00, 1.02) | 0.0043 |
|
| ||
| Clinical Stage (cT2 vs cT1c) | 1.16 (0.66, 2.06) | 0.6065 |
|
| ||
| Clinical Gleason Score | ||
| 6 vs. 7 | 0.76 (0.17, 3.48) | 0.0199 |
| (8, 9, 10) vs. 7 | 2.14 (1.20, 3.81) | |
|
| ||
| Percent cores positive | 1.01 (1.00, 1.02) | 0.1089 |
On the postoperative multivariate Cox proportional hazards model (Table 5), PET metastasis was significantly associated with a 3.07-fold (1.73-5.46, p = 0.0001) increased risk of treatment failure while controlling for other postoperative factors (PSA, pathology stage, pathology Gleason score, surgical margins).
Table 5.
Postoperative Multivariate Cox Proportional Hazards Model for Predicting Treatment Failure (N = 101).
| Variable | HR (95% CI) | P value |
|---|---|---|
|
| ||
| PET metastasis (any site) | 3.07 (1.73, 5.46) | 0.0001 |
|
| ||
| PSA (ng/mL) | 1.00 (1.00,1.01) | 0.3722 |
|
| ||
| Pathology Stage | ||
| 3a vs. 2 | 2.45 (1.07, 5.62) | <.0001 |
| 3b vs. 2 | 8.15 (3.67, 18.11) | |
|
| ||
| Pathology Gleason Score | ||
| 6 vs. 7 | 0.31 (0.04, 2.48) | 0.0021 |
| (8, 9, 10) vs. 7 | t:v="b"2.45 (1.39, 4.31) | |
|
| ||
| Surgical Margins (Positive vs. Negative) | 1.18 (0.64, 2.18) | 0.5912 |
DISCUSSION
Accurate LN staging is important for management of patients with newly diagnosed prostate cancer. CT, the current standard imaging modality, relies primarily on nodal size to identify nodal metastasis and is neither sufficiently sensitive nor specific (22). Conventional MRI and diffusion-weighted MRI have similar limitations (23).
Our study indicates that 11C-acetate-PET/CT can detect LN metastasis in patients with negative CT with sensitivity and specificity of 68% and 78%, respectively. While the PPV of PET/CT based on pathologic correlation was only 49%, the Cox-proportional hazards model for predicting treatment failure showed that patients with an apparent false-positive PET had a significantly worse prognosis than did those with true-negative PET scans, suggesting that the relatively low PPV might be due to inadequate lymphadenectomy and/or lack of pathological identification of positive nodes.
Several other studies employing either 11C-acetate-PET or 11C-choline-PET have confirmed the potential of PET for diagnosing metastatic prostate cancer (13). Neither tracer has significant urinary excretion and both enter into the biochemical pathways of fatty acid metabolism, which are consistently upregulated in prostate cancer cell lines (13). 11C-choline is incorporated into cellular membranes as phosphatidylcholine through overexpression of fatty acid synthetase (FAS) while 11C-acetate participates in cytoplasmic lipid synthesis, which is believed to be increased in tumors (24).
Considering studies using 11C-acetate-PET, ours uniquely evaluates the staging of patients with newly diagnosed prostate cancer prospectively by comparing PET with pathological nodal status as the reference standard. Multiple other studies have evaluated the effectiveness of 11C-acetate-PET in prostate cancer (14-21, 25). However, most of these studies have focused on patients with recurrent disease; have included heterogeneous patient populations (local recurrence, nodal disease, bone or visceral metastasis) or reference standards; and have been limited by small sample sizes. These studies do not have extensive confirmatory pathological evaluation of PET-positive disease, and rely on the tracer uptake value or follow-up findings to infer that PET positivity represents a true-positive finding (21, 25).
11C-choline-PET has been studied more extensively for preoperative LN staging. De Jong et al. examined 67 patients with newly diagnosed prostate cancer who underwent 11C-choline-PET/CT, and found a sensitivity of 80% and specificity of 96% for detecting nodal metastasis. However, only 24 patients in the study underwent lymphadenectomy, and scan results were assumed to be true-negative in 14 without pathology based on the clinical scenario or to be true positive in 10 patients who declined surgery and had gross disease on preoperative CT scan. Approximately half the patients with nodal metastasis had lymphadenopathy already detectable by standard imaging (CT or MRI) and therefore would have been excluded from our study. Also, the patients appeared to be at higher risk for nodal metastasis than ours, with average PSA 34 (range 3-500) ng/mL, PSA >20 ng/mL in 60% of patients, and cT2/3 disease in 75% of the population (26). Schiavina et al., examined 11C-choline-PET in 57 patients with newly diagnosed prostate cancer who had previously negative bone scintigraphy and underwent extended pelvic lymphadenectomy, and found a sensitivity of 60% and specificity of 97.6% (27). This sensitivity was similar to that found in our study with 11C-acetate-PET (60% vs. 68%), while the specificity and PPV were higher than in our study (97.6% vs. 78% and 90% vs. 48%, respectively). However, their patient population overall was at lower risk for nodal metastasis than our population and included more patients with biopsy Gleason scores of 5 or 6 (22 of 57) and fewer with biopsy Gleason scores of 8-10 (8 of 57), likely explaining their high specificity rates. Schiavina’s higher PPV may also reflect the use of a standardized approach to extended pelvic lymphadenectomy rather than allowing for surgical discretion, as in our study. Whether the differences in sensitivity, specificity, and PPV between these studies are also attributable to differences in performance of these two tracers is unclear. Besides differential uptake of 11C-acetate intracellularly and 11C-choline in the cellular membrane, clinically relevant distinctions between these two tracers have not been elucidated at this time. However, one small study indicates that they may be equivalent (28). Similar results for LN metastasis detection have been reported for PET/CT performed with 18F-fluorocholine derivatives (29).
In addition to providing anatomic localization of metastatic disease, the results of 11C-acetate-PET/CT were also found in our study to be independently predictive of failure of planned or completed radical prostatectomy in both preoperative and postoperative multivariate models. To our knowledge, this is the first study in which the results of initial staging PET were correlated to treatment failure in prostate cancer patients. Most of the patients with PET/CT-positive disease failed early, either immediately perioperatively or within a few months of surgery. By one year, 79% of PET/CT-positive patients had treatment failure while 26% of PET/CT-negative patients had treatment failure. 11C-acetate-PET/CT may allow for better treatment counseling in patients with high-risk prostate cancer. Knowing that their PET/CT studies were positive, five of our patients elected to undergo biopsy before having definitive surgery. Four of these patients were confirmed to have metastasis and received radiotherapy or hormonal therapy, sparing them the potential complications of surgery. 11C-acetate-PET/CT may also help to identify patients likely to benefit from multimodality therapy. Currently, studies on neoadjuvant therapy before radical prostatectomy focus on clinical parameters (PSA, biopsy Gleason score, and clinical stage) and are investigational (30, 31). While clinical parameters may identify patients with high risk of metastasis, they do not provide confirmation of metastasis as PET/CT may.
While our study avoids some of the pitfalls of past 11C-acetate and 11C-choline-PET studies, it is not without limitations. First, patients and surgeons were deliberately not blinded to the results of the 11C-acetate-PET/CT studies in order to gain an appreciation of the real-world utility of this technology, and this may have introduced verification bias. Second, treating urologists were allowed to individualize the limits of lymphadenectomy based, at least in part on the PET findings. This may have contributed to the low PPV of our PET results
PET/CT for initial staging of prostate cancer is not without its drawbacks. Because of the short half-life of 11C (T1/2 20 minutes), the widespread use of 11C-acetate (and 11C-choline) is largely limited to centers with on-site cyclotrons. Despite this limitation, it is of interest that 11C-choline recently was approved by the U.S. Food and Drug administration at a single site for use as an adjunct for evaluation of patients with suspected recurrent prostate cancer (32). Newer radiopharmaceuticals labeled with fluorine-18 (T1/2 110 min) would overcome this limitation, but none has yet been commercialized (33, 34). Additionally, the routine use of PET/CT staging would add a substantial cost to the management of prostate cancer. Only carefully designed studies can determine whether the benefits of PET/CT with 11C-acetate or another radiopharmaceutical for detecting LN metastasis and predicting treatment failure outweigh the expense of the procedure. Until then, pelvic lymph node dissection, although invasive, remains the gold standard for diagnosing LN disease.
CONCLUSION
While pelvic lymphadenectomy is the gold standard for diagnosing nodal metastases, 11C-acetate-PET/CT is more sensitive than current imaging methods for staging of men with medium-to high-risk prostate cancer. It may guide initial treatment planning by prompting surgeons to perform a more extended pelvic lymphadenectomy. In patients planned for or completing prostatectomy after negative conventional imaging, 11C-acetate-PET/CT independently predicts TTFS.
Acknowledgments
This work was supported by National Institutes of Health grant CA101734 and the Alvin J. Siteman Cancer Center Biostatistics Core and Imaging and Response Assessment Core.
REFERENCES
- 1.Gallina A, Chun FK, Suardi N, et al. Comparison of stage migration patterns between Europe and the USA: an analysis of 11 350 men treated with radical prostatectomy for prostate cancer. BJU Int. 2008;101:1513–1518. doi: 10.1111/j.1464-410X.2008.07519.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makarov DV, Trock BJ, Humphreys EB, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker CC, Husband J, Dearnaley DP. Lymph node staging in clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 1999;2:191–199. doi: 10.1038/sj.pcan.4500311. [DOI] [PubMed] [Google Scholar]
- 5.Hovels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Thoeny HC, Triantafyllou M, Birkhaeuser FD, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol. 2009;55:761–769. doi: 10.1016/j.eururo.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Briganti A, Chun FK, Salonia A, et al. Validation of a nomogram predicting the probability of lymph node invasion among patients undergoing radical prostatectomy and an extended pelvic lymphadenectomy. Eur Urol. 2006;49:1019–1026. doi: 10.1016/j.eururo.2006.01.043. discussion 1026-1027. [DOI] [PubMed] [Google Scholar]
- 8.Cagiannos I, Karakiewicz P, Eastham JA, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–1803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 9.Bishoff JT, Reyes A, Thompson IM, et al. Pelvic lymphadenectomy can be omitted in selected patients with carcinoma of the prostate: development of a system of patient selection. Urology. 1995;45:270–274. doi: 10.1016/0090-4295(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 10.Briganti A, Chun FK, Salonia A, et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol. 2006;50:1006–1013. doi: 10.1016/j.eururo.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51:1511–1520. doi: 10.1016/j.eururo.2007.01.061. discussion 1520-1521. [DOI] [PubMed] [Google Scholar]
- 12.Effert PJ, Bares R, Handt S, Wolff JM, Bull U, Jakse G. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorine-labeled deoxyglucose. J Urol. 1996;155:994–998. [PubMed] [Google Scholar]
- 13.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–89. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vavere AL, Kridel SJ, Wheeler FB, Lewis JS. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med. 2008;49:327–334. doi: 10.2967/jnumed.107.046672. [DOI] [PubMed] [Google Scholar]
- 15.Seltzer MA, Jahan SA, Sparks R, et al. Radiation dose estimates in humans for (11)C-acetate whole-body PET. J Nucl Med. 2004;45:1233–1236. [PubMed] [Google Scholar]
- 16.Fricke E, Machtens S, Hofmann M, et al. Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2003;30:607–611. doi: 10.1007/s00259-002-1104-y. [DOI] [PubMed] [Google Scholar]
- 17.Kotzerke J, Volkmer BG, Neumaier B, Gschwend JE, Hautmann RE, Reske SN. Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2002;29:1380–1384. doi: 10.1007/s00259-002-0882-6. [DOI] [PubMed] [Google Scholar]
- 18.Oyama N, Akino H, Kanamaru H, et al. 11C-acetate PET imaging of prostate cancer. J Nucl Med. 2002;43:181–186. [PubMed] [Google Scholar]
- 19.Oyama N, Miller TR, Dehdashti F, et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med. 2003;44:549–555. [PubMed] [Google Scholar]
- 20.Sandblom G, Sorensen J, Lundin N, Haggman M, Malmstrom PU. Positron emission tomography with C11-acetate for tumor detection and localization in patients with prostate-specific antigen relapse after radical prostatectomy. Urology. 2006;67:996–1000. doi: 10.1016/j.urology.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Morris MJ, Scher HI. (11)C-acetate PET imaging in prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34:181–184. doi: 10.1007/s00259-006-0281-5. [DOI] [PubMed] [Google Scholar]
- 22.Wolf JS, Jr., Cher M, Dall’era M, Presti JC, Jr., Hricak H, Carroll PR. The use and accuracy of cross-sectional imaging and fine needle aspiration cytology for detection of pelvic lymph node metastases before radical prostatectomy. J Urol. 1995;153(3 Pt 2):993–999. [PubMed] [Google Scholar]
- 23.Budiharto T, Joniau S, Lerut E, et al. Prospective evaluation of 11C-choline positron emission tomography/computed tomography and diffusion-weighted magnetic resonance imaging for the nodal staging of prostate cancer with a high risk of lymph node metastases. Eur Urol. 2011;60:125–130. doi: 10.1016/j.eururo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht S, Buchegger F, Soloviev D, et al. (11)C-acetate PET in the early evaluation of prostate cancer recurrence. Eur J Nucl Med Mol Imaging. 2007;34:185–196. doi: 10.1007/s00259-006-0163-x. [DOI] [PubMed] [Google Scholar]
- 26.de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J Nucl Med. 2003;44:331–335. [PubMed] [Google Scholar]
- 27.Schiavina R, Scattoni V, Castellucci P, et al. 11C-choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol. 2008;54:392–401. doi: 10.1016/j.eururo.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Kotzerke J, Volkmer BG, Glatting G, et al. Intraindividual comparison of [11C]acetate and [11C]choline PET for detection of metastases of prostate cancer. Nuklearmedizin. 2003;42:25–30. [PubMed] [Google Scholar]
- 29.Beheshti M, Imamovic L, Broinger G, et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology. 2010;254:925–933. doi: 10.1148/radiol.09090413. [DOI] [PubMed] [Google Scholar]
- 30.Womble PR, VanVeldhuizen PJ, Nisbet AA, Reed GA, Thrasher JB, Holzbeierlein JM. A phase II clinical trial of neoadjuvant ketoconazole and docetaxel chemotherapy before radical prostatectomy in high risk patients. J Urol. 2011;186:882–887. doi: 10.1016/j.juro.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 31.Eastham JA, Kelly WK, Grossfeld GD, Small EJ. Cancer and Leukemia Group B (CALGB) 90203: a randomized phase 3 study of radical prostatectomy alone versus estramustine and docetaxel before radical prostatectomy for patients with high-risk localized disease. Urology. 2003;62(Suppl 1):55–62. doi: 10.1016/j.urology.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 32. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm3201.htm.
- 33.Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–7653. doi: 10.1158/1078-0432.CCR-11-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuster DM, Votaw JR, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63. [PubMed] [Google Scholar]