Abstract
Metabotropic glutamate (mGlu) receptors were discovered in the mid 1980s and originally described as glutamate receptors coupled to polyphosphoinositide hydrolysis. Almost 6500 articles have been published since then, and subtype-selective mGlu receptor ligands are now under clinical development for the treatment of a variety of disorders such as Fragile-X syndrome, schizophrenia, Parkinson’s disease and L-DOPA-induced dyskinesias, generalized anxiety disorder, chronic pain, and gastroesophageal reflux disorder. Prof. Erminio Costa was linked to the early times of the mGlu receptor history, when a few research groups challenged the general belief that glutamate could only activate ionotropic receptors and all metabolic responses to glutamate were secondary to calcium entry. This review moves from those nostalgic times to the most recent advances in the physiology and pharmacology of mGlu receptors, and highlights the role of individual mGlu receptor subtypes in the pathophysiology of human disorders. This article is part of a Special Issue entitled ‘Trends in Neuropharmacology: In Memory of Erminio Costa’.
Keywords: Metabotropic glutamate receptors, Receptor structure, mGlu receptor ligands, Clinical studies
1. Historical background
The evidence that quisqualate and glutamate stimulated inositol phosphate formation in cultured striatal neurons (Sladeczek et al., 1985) offered the first demonstration that excitatory amino acids could activate receptors other than the classical ligand-gated ion channels (named NMDA, “quisqualate”and kainate receptors at that time). In the meantime, ibotenic acid, a heterocyclic amino acid naturally occurring in the mushrooms Amanita muscaria and Amanita pantherina, was found to stimulate inositol phosphate formation in hippocampal slices (Nicoletti et al., 1986a,b). Glutamate was virtually inactive in adult brain slices, but was able to stimulate polyphosphoinositide (PI) hydrolysis to a great extent (as much as 20 fold in some experiments) in slices from 7- to 9-day old rats (Nicoletti et al., 1986a). The latter findings were the product of the intuition, creativity, and long experience of Prof. Erminio Costa, who, at that time, was the Director of the Laboratory of Preclinical Pharmacology of NIMH, St. Elizabeth’s Hospital, Washington, DC. Independent work by Carl Cotman and his associates demonstrated that L-2-amino-4-phosphonobutanoate (L-AP4) and L-serine-O-phosphate (L-SOP), which at that time were considered as excitatory amino acid receptor antagonists, reduced excitatory synaptic transmission at mossy fibre/CA3 pyramidal cell synapses of the guinea pig hippocampus (Cotman et al., 1986), thus providing the first indirect evidence for the existence or presynaptic group-III mGlu autoreceptors (see below). In 1987, Prof. Sugyiama and his colleagues introduced the terminology of “metabotropic glutamate receptors” to indicate quisqualate-sensitive receptors expressed in Xenopus oocytes injected with rat brain mRNA (Sugiyama et al., 1987). The cloning of the first metabotropic glutamate subtypes (named mGluR1 or mGlu1 receptor) in the lab of Prof. Nakanishi (Masu et al., 1991) was the milestone for any further growth of the field. The eight mGlu receptor subtypes identified so far are divided into three groups, with group I including mGlu1 and mGlu5, group II including mGlu2 and mGlu3, and group III including mGlu4, mGlu6, mGlu7, and mGlu8. mGlu1 and mGlu5 receptors are coupled to Gq/G11, whereas all other subtypes are coupled to Gi/Go in heterologous expression systems (see below). The pharmacology of mGlu receptors has expanded dramatically in the last few years, and subtype-selective ligands are now under clinical development. As described above, quisqualate and ibotenic acid were the first reported molecules active at mGlu receptors coupled to PI hydrolysis. These molecules, however, are not selective and show activity at ionotropic glutamate receptors. The first breakthrough in the pharmacology of mGlu receptors was the discovery that trans-1-amino-cyclopentanedicarboxylic acid (trans-ACPD) could activate PI hydrolysis in brain slices with no effect at ionotropic glutamate receptors (Schoepp et al., 1991). One of the isomers of trans-ACPD, 1S,3R-ACPD, activates both group-I and group-II mGlu receptors (reviewed by Schoepp et al., 1999), and is still widely used as a non-selective mGlu receptor agonist. L-AP4, L-SOP and L-2-amino-3-phosphonopropionic acid (L-AP3) were described as antagonists of mGlu receptors coupled to PI hydrolysis in brain slices (Nicoletti et al., 1986a,b,c; Schoepp and Johnson, 1989). L-AP3 is now obsolete, whereas L-AP4 and L-SOP are considered as prototypical agonists of group-III mGlu receptors. Jeff Watkins and his associates introduced a series of phenylglycine derivatives as pharmacological tools for investigating the role of mGlu receptors in the CNS (Birse et al., 1993). One of these derivatives, R,S-α-methyl-4-carboxyphenylglycine (MCPG), has been widely used as a non-subtype-selective mGlu receptor antagonist (Eaton et al., 1993). The modern pharmacology of mGlu receptors coincides with the advent of orthosteric ligands bearing a cyclopropyl moiety in their structure. (2S,3S,4S)-2-(Carboxycyclopropyl)glycine (L-CCG-I) and (2S,1′R,2′R,3′R)-2-(2,3-dicarboxycyclopropyl)glycine (DCG-IV), synthesized in the lab of Prof. Shinozaki (Shinozaki and Ishida, 1993), have been used for many years, and are still used, as potent mGlu2/3 receptor agonists. More recent compounds synthesized by Jim Monn at Eli Lilly, e.g. (1S,2S,5R,6S)-2-amino-bicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740) and (1R,4R,5S,6R)-4-amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268), show nanomolar potency as mGlu2/3 receptor agonists and are systemically active (reviewed by Schoepp et al., 1999). Other drugs, including positive and negative allosteric modulators (PAMs and NAMs) of individual mGlu receptor subtypes, are described in the appropriate sections below. By definition, these drugs bind to mGlu receptor sites distinct from the primary glutamate recognition site. No endogenous ligands (“endocoids”) of these modulatory sites have been identified, as yet. Their existence is unlikely because the 7-transmembrane (7TM) region, which contains the binding sites of most PAMs and NAMs, is not highly conserved among the eight mGlu receptor subtypes. Now, after 25 years from the discovery of mGlu receptors, a number of potent and subtype-selective ligands of mGlu receptors are under clinical development and are among the most promising drugs in the treatment of neurological and psychiatric disorders.
2. Structure and activation mechanism of mGluRs
Sequence analysis of most class CG-protein coupled receptors (GPCRs), including the 8 mGlu receptor subtypes, the Ca2+-sensing receptor, the taste T1R receptors, and the basic amino acid receptor GPCR6a, revealed that these receptors have a large extracellular domain made of a Venus Fly Trap (VFT) domain similar to the periplasmic bacterial leucine/isoleucine/valine binding protein (LIVBP) linked to the first TM domain via a cysteine-rich domain (CRD) containing 9 highly conserved cysteine residues (Fig. 1c). A number of 3D modeling studies (O’Hara et al., 1993), but most importantly, the resolution of a crystal structure of the mGlu1 extracellular domain (Kunishima et al., 2000; Tsuchiya et al., 2002) confirmed that the glutamate binding domain is structurally similar to LIVBP-like proteins (Fig. 1a). Later on, the structures of mGlu3 and mGlu7 receptor VFTs were reported (Muto et al., 2007). The VFT domain is composed of two lobes, each made of α-helices around a large β-sheet, with the glutamate binding site located in the cleft between the two lobes. Structural analysis of the binding pocket revealed 5 residues that directly contact the amino acid moiety and are conserved in a large variety of amino acid binding LIVBP-like proteins. The structure of the CRD was solved together with the VFT of the mGlu3 receptor (Muto et al., 2007), and revealed a domain made of two subdomains, each composed of 4 β-strands stabilized by 2 disulfide bridges. This domain is further linked to the VFT via an additional highly conserved disulfide bond, likely rigidifying the connection between the VFT and the CRD. The 7-TM domain of mGlu receptors shows very low sequence similarity with the 7-TM domain of other GPCRs. However, the 7-TM domains of mGlu receptors and other GPCRs share a similar general fold, with a relatively central helix 3, and a possible amphipathic helix 8 (Pin et al., 2003). Indeed, when the 7-TM domain alone is expressed in cells (after deletion of both the extra and intracellular parts), it folds correctly, and can be directly activated by ligands known as positive allosteric modulators (PAMs) of the full length receptors (Goudet et al., 2004), indicating that a change in conformation in such an isolated domain is sufficient for G-protein activation. In addition, as observed in many rhodopsin-like GPCRs, an ionic interaction between residues at the intracellular side of helices 3 and 6 is important to maintain the receptor in an inactive conformation (Binet et al., 2007). Lastly, as observed in other GPCRs, both the intracellular loops 2 and 3, as well as the helix 8 are important in G-protein coupling, being also involved in coupling selectivity (Pin et al., 2003).
Fig. 1.
Modular structure of mGlu receptors. a) Ribbon view of the open (left) and closed (right) mGlu1 receptor Venus Fly Trap (VFT) bound with glutamate (back). Images were prepared using the coordinates of the glutamate-bound mGlu1 receptor VFT dimer (pdb 1EWK), in which one VFT is closed while the other is open. b) Side view of the mGlu1 receptor dimer bearing the VFT in its empty “resting” state (left) (pdb 1EWT), or agonist occupied “active” orientation (right) (pdb 1EWK). The front VFT is in light grey, while the one in the back is black. c) General organization of an mGlu receptor deduced from the structure of the dimeric mGlu3 extracellular domain (VFT + CRD) (pdb 1E4U) associated with two rhodopsinlike 7-TM domains.
Not only are mGlu receptors complex proteins because of the modular structural organization, but also because they form dimers stabilized by an intersubunit disulfide bond (Romano et al., 1996a). Such a dimeric organization was shown not only in transfected cells, but also in the brain. Using energy transfer technologies, together with systems to control receptor subunit composition, it was shown that mGlu receptors form dimers but not larger complexes, as opposed, for example, to the related GABAB receptor (Maurel et al., 2008). Early studies suggested that mGlu receptors only form homodimers, then limiting the number of possible mGlu receptors in the brain to the eight major subtypes (not taking into account the splice variants). However, a recent report by Doumazane et al. (2010) demonstrates that, similarly to GABAB receptors and sweet and umami taste receptors, mGlu receptor subtypes can form heterodimers. Subtypes of the same group of mGlu receptors can form intragroup heterodimers (e.g., mGlu1 with mGlu5 and mGlu2 with mGlu3 receptors), and, interestingly, group-II and group-III mGlu receptors can form intergroup heterodimers (e.g., mGlu2 with mGlu4 receptors). No heterodimers can be formed between group-I and group-II/III mGlu receptors (e.g., mGlu1 and mGlu2 or mGlu4), suggesting that only mGlu receptor subtypes coupled to the same G protein can form heterodimers. Of interest, different mGlu receptor subunits have been colocalized in specific subdomains in neurons, suggesting the existence of mGlu receptor heterodimers in the brain. Further work is however necessary before the functional significance of such heterodimeric receptor entities is identified.
The dimeric organization of the mGlu receptors raises the question of how receptor function is infiuenced by ligand binding stoichiometry. Kniazeff et al. (2004a) have shown that a single agonist per dimer (i.e. a single VFT stabilized in the closed state) is sufficient for receptor activation, although the presence of two agonist molecules (i.e. both VFTs in the closed state) leads to a 3-fold higher coupling efficiency. It has even been suggested that the two states (one or two VFTs in the closed state) activate different signalling pathways, e.g. mGlu1 receptor coupling to Gs and Gq, respectively (Tateyama and Kubo, 2006). How can glutamate, by interacting into the cleft of the VFT, induce the necessary change in conformation at the 7-TM level to activate G-proteins? A first important step was discovered through the resolution of the VFT structure occupied either by an agonist or an antagonist (Fig. 1a, and Table 1) (Kunishima et al., 2000; Tsuchiya et al., 2002). Indeed, as observed for most LIVBP-like domains, ligand binding stabilizes the VFT domain in a closed conformation. This is well illustrated by the observation that many structures with bound agonists are in the closed form (Tables 1 and 2). Although open conformations with bound agonist have been identified for mGlu1 receptors, this may simply reflect the ability of this domain to oscillate between a closed and an open state, as already reported for many other LIVBP-like proteins. In contrast, all antagonist bound structures correspond to open VFTs. Of note, removing the steric or ionic hindrance that prevents VFT closure by site directed mutagenesis was found sufficient to convert the group-III mGlu receptor antagonists, (S)-2-amino-2-methyl-4-phosphonobutanoic acid (MAP4) and (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-I) into full agonists, consistent again with the close state corresponding to the active state (Bessis et al., 2002). As a further support to this conclusion, the fully activated state of the related GABAB receptor was obtained by inserting a disulfide bond expected to lock the VFT in its closed state (Kniazeff et al., 2004b).
Table 1.
Conformations of the Venus Fly Traps of mGlu receptor subtypes bound to agonists or antagonists.
| Receptor | Ligand | Ligand activity | Conformation | PDB entry |
|---|---|---|---|---|
| mGlu1 | – | – | Dimer Roo | 1EWT |
| – | – | Dimer Aco | 1EWV | |
| Glu | Agonist | Dimer Aco | 1EWK | |
| Glu + Gd3+ | Agonist | Dimer Acc | 1ISR | |
| MCPG | Antagonist | Dimer Roo | 1ISS | |
| LY341495 | Antagonist | Dimer Aoo | 3KS9 | |
| mGlu5 | Glu | Agonist | Dimer Acc | 3LMK |
| mGlu3 | Glu | Agonist | Dimer Rcc | 2E4U |
| DCG-IV | Agonist | Dimer Rcc | 2E4V | |
| 1S,3S-ACPD | Agonist | Dimer Rcc | 2E4W | |
| 1S,3R-ACPD | Agonist | Dimer Rcc | 2E4X | |
| 2R,4R-APDC | Agonist | Dimer Rcc | 2E4Y | |
| mGlu7 | – | – | Dimer Roo | 2E4Z |
| LY341495 | Antagonist | Monomer open | 3MQ4 |
Glu = Glutamate; R = resting; A = active; o = open; c = closed.
Table 2.
Subtype-selective mGlu receptor ligands.
| Subtype | Orthosteric agonists | Orthosteric antagonists | PAMs | NAMs |
|---|---|---|---|---|
| mGlu1 | DHPG (group-selective) | LY367385, AIDA, MATIDA | Ro-0711401, Ro-674853, Ro-677476, VU71 | CPCCOEt, BAY367620, JNJ6259685 |
| mGlu5 | DHPG (group-selective) | None | DFB, CPPHA, CDPPB, VU29 | MPEP, SIB1757, SIB1893, Fenobam, AFQ056, AZD2516, AZD2066, STX107, ADX10059, ADX48621 |
| CHPG | ADX47273, ADX63365 | |||
| mGlu2 | LY354740, LY379268, LY404039 | LY341495, MGS0039 | LY566332, LY487379, BINA | |
| mGlu3 | MCPG | Antagonist | Dimer Roo | 1ISS |
| LY341495 | Antagonist | Dimer Aoo | 3KS9 | |
| mGlu5 | Glu | Agonist | Dimer Acc | 3LMK |
| mGlu3 | Glu | Agonist | Dimer Rcc | 2E4U |
| DCG-IV | Agonist | Dimer Rcc | 2E4V | |
| 1S,3S-ACPD | Agonist | Dimer Rcc | 2E4W | |
| 1S,3R-ACPD | Agonist | Dimer Rcc | 2E4X | |
| 2R,4R-APDC | Agonist | Dimer Rcc | 2E4Y | |
| mGlu7 | – | – | Dimer Roo | 2E4Z |
| LY341495 | Antagonist | Monomer open | 3MQ4 |
The structures of mGlu1 VFT solved in the presence of agonists and antagonists suggested that the dimeric organization plays a pivotal role in the activation process. Indeed, an important reorientation of the VFTs in the dimer, from resting (R) to active (A) (Fig. 1b) was observed when at least one VFT was in a closed state (Fig. 1b) (Kunishima et al., 2000; Tsuchiya et al., 2002). However, recent new structures of dimeric mGlu VFTs cast some doubts on this proposed activation mechanism. Indeed, the active orientation was observed with the dimeric mGlu1 receptor VFTs bound with the antagonist, (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495) (unpublished observation, pdb accession number 3KS9), and the resting orientation was systematically observed with the mGlu3 receptor VFT dimer bound with 5 different agonists (Muto et al., 2007). Although a change in the relative position between the VFTs is expected, as suggested by a study performed on the related GABAB receptor (Rondard et al., 2008), the movement may not necessarily be as important as that observed in the crystal structures. As shown in Fig. 1c, it is not possible to have both 7-TM domains of a dimeric mGlu receptor to be in close proximity according to the solved structure of the mGlu3 receptor extracellular domain composed of both the VFT and the CRD. This appears surprising and not consistent with the FRET data obtained with GFP fusion mGlu receptor subunits (Marcaggi et al., 2009; Tateyama et al., 2004). A relative movement between the mGlu receptor subunits during activation is indeed supported by FRET measurements between their intracellular sides (Marcaggi et al., 2009; Tateyama et al., 2004). However, whether the change with FRET is solely due to a change in the distance between the fused GFPs, or to a change in their relative orientation due to conformational changes of the intracellular loops remains unclear. Whatever the real mechanism, it leads to the activation of only one 7TM within the dimer (Hlavackova et al., 2005), which strongly suggests a direct contact between the 7TMs in the dimer. Further work is necessary to understand how agonist binding leads to the activation of the 7TM.
3. Detailed description of mGlu receptor subtypes
3.1. mGlu1 receptor
Gene name: GRM1 (human); Grm1 (rat, mouse). Accession numbers: NP_000829 (human); NP_058707 (rat); NP_058672 (mouse). Chromosomal location: 6q24 (human); 1p13 (rat); 10A2 (mouse) (Masu et al., 1991; Kuramoto et al., 1994; Desai et al., 1995; Stephan et al., 1996).
3.1.1. Splice variants (Ferraguti et al., 2008
mGlu1α or mGlu1a (longest isoform): 1199 and 1194 amino acids in rodents and humans, respectively. C-terminus intracellular domain formed by 360 amino acids. mGlu1β1 or mGlu1b: 906 amino acids. C-terminus domain: 68 amino acids. mGlu1β2 or mGlu1f: 906 amino acids. C-terminus domain: 68 amino acids. mGlu1γ or mGlu1d: 908 amino acids. C-terminus domain: 70 amino acids. mGlu1δ or mGlu1E55: 321 amino acids. Possible secreted protein.
3.1.2. Receptor signalling and interacting proteins
mGlu1 receptors are primarily coupled to Gq/G11 proteins (Masu et al., 1991). Receptor activation stimulates phospholipase Cβ (PLCβ), the enzyme that cleaves phosphatidylinositol-4,5-bisphosphate with the ensuing formation of the intracellular second messengers, inositol-1,4,5-trisphosphate (Ins-1,4,5-P3) and diacylglycerol (DAG). Ins-1,4,5-P3 releases Ca2+ from intracellular stores, whereas DAG activates protein kinase C. Stimulation of cAMP formation, arachidonic acid release, the mitogen-activated protein kinase (MAPK) pathway, and L-type voltage-sensitive Ca2+ channels has also been reported in response to mGlu1 receptor activation (reviewed by Ferraguti et al., 2008). The mGlu1 receptor negatively modulates a variety of K+ channels, including M-type voltage-gated K+ channels (Ikeda et al., 1995) and the “tandem-pore” TASK and TREK K+ channels (Talley et al., 2000; Chemin et al., 2003). In cultured neurons, mGlu1 (and mGlu5) receptors negatively modulate voltage-sensitive Ca2+ channels via mechanisms that involve pertussis toxin (PTX)-sensitive and PTX-insensitive G proteins and are regulated by Homer proteins (Choi and Lovinger, 1996; Kammermeier et al., 2000).
Different isoforms of G-protein coupled receptor kinases (GRKs), including GRK2, GRK4, and GRK5, mediate homologous desensitization of mGlu1 receptors (Dale et al., 2000; Sallese et al., 2000; Iacovelli et al., 2003). GRK4 is required for desensitization of native mGlu1 receptors in cerebellar Purkinje cells (Sallese et al., 2000). The Regulator of G-protein Signalling, RGS-4, which increases the GTPase activity of Gαq, inhibits mGlu1 receptor signalling (Saugstad et al., 1998).
A number of proteins have been shown to interact with mGlu1 receptors and to modify their surface expression and intracellular signal transduction. Homer proteins, which contain both PDZ (from PSD-95, DlgA, and Zo-1 proteins) and EVH-1 (from Ena/Vasp Homology-1) domains, bind to a proline-rich motif in the C-terminal tail of mGlu1a and mGlu5 receptors (Brakeman et al., 1997; Kato et al., 1998; Xiao et al., 1998; Tu et al., 1998). All members of the Homer family, with the exception of Homer1a, contain a C-terminal coiled-coil domain, which allows multimerization (Kato et al., 1998; Tu et al., 1998). Thus, Homer proteins form a linking bridge between mGlu1α receptors and other proteins that are involved in receptor signalling, such as PLCβ3 and −4, the InsP3 receptor, and the TrpC1 channel (reviewed by Ferraguti et al., 2008) (Fig. 2). The latter likely behaves as a store-operated channel, providing a potential route to restore intracellular Ca2+ stores after the opening of InsP3-gated ion channels. mGlu1α receptors can also activate the phosphatidylinoisol-3-kinase (PtdIns-3-K) pathway via the interaction with Homer protein, and the PtdIns-3-K enhancer, PIKE-L (Rong et al., 2003) (Fig. 2). All these interactions are disrupted by the short Homer isoform, Homer1a, which is rapidly induced in response to synaptic activation and shares with other Homers the ability to interact with mGlu1α receptors, but cannot form multimeric complexes. mGlu1α receptors can also associate with tamaline (Kitano et al., 2002), the cytoskeletal protein, 4.1G (Lu et al., 2004), and the ubiquitin ligase, Siah-1A (Seven In Ansentia Homologue-1A) (Ishikawa et al., 1999; Kammermeier and Ikeda, 2001). The mGlu1 receptor co-immuno-precipitates and functionally interacts with ephrin-B2 (Calò et al., 2005), a member of the ephrin/Eph receptor family of trans-membrane proteins, which mediate processes of cell-to-cell interaction during development and in the adult life. Activation of ephrin-B2 by its receptor partner, Eph-B1, amplifies mGlu1 receptor signalling in brain tissue and cultured neurons. This interaction might have interesting implications for processes of developmental plasticity (Calò et al., 2005, 2006).
Fig. 2.

Protein–protein interactions involving group-I mGlu receptors in the post-synaptic densities. Long isoforms of Homer proteins allows the formation of multi-molecular complexes including mGlu1 and mGlu5 receptors. Interactions with NMDA receptors, TrpC ion channels, inositol-1,4,5-trisphosphate receptors (InsP3R), or PIKE-L are shown. Short Homer1a lacking the coiled-coil domain disrupts the formation of the multimolecular complex, thereby affecting mGlu1/5 receptor signalling.
3.1.3. Functional anatomy
Expression of mGlu1 receptors is extensive in cerebellar Purkinje cells and in the mitral/tufted cells of the olfactory bulb. Strong expression is also found in the pars compacta of the substantia nigra, lateral septum, globus pallidum, and thalamic relay nuclei (Martin et al., 1992; Shigemoto et al., 1992; Baude et al., 1993). Neuroendocrine regions of the hypothalamus express higher levels of mGlu1β than mGlu1α receptors (Mateos et al., 1998; Van den Pol, 1994). The subcellular localization of the mGlu1 receptor has been consistently associated with postsynaptic specialization of excitatory synapses, where the receptor appears to be concentrated in perisynaptic and extrasynaptic areas. Thus, mGlu receptors are recruited by high concentrations of glutamate that escape the clearance mechanisms and spread to the sides of the synaptic cleft (Ferraguti et al., 2008 and references therein).
The function of the mGlu1 receptor has been most extensively studied in the cerebellar cortex, where receptor activation is required for the induction of long-term depression (LTD) of excitatory neurotransmission at parallel fibre-Purkinje cell synapses. This particular form of synaptic plasticity underlies motor learning, vestibulo-ocular reflex adaptation, and eye-blink conditioning (reviewed by Kano et al., 2008). Activation of mGlu1 receptors in Purkinje cells leads to formation of diacylglycerol, which is then cleaved by diacylglycerol lipase into 2-arachidonylglycerol (2-AG), the major endocannabinoid species of the CNS. 2-AG diffuses back to parallel fibre nerve terminals, where it activates type-1 cannabinoid receptors, thereby inhibiting glutamate release (reviewed by Kano et al., 2008). Gene-targeted deletion of mGlu1 receptors impairs LTD at parallel fibers-Purkinje cell synapses resulting into a severe motor incoordination (Aiba et al., 1994; Conquet et al., 1994). Selective re-introduction of mGlu1α receptors in Purkinje cells restores LTD and corrects motor impairment (Ichise et al., 2000). In addition, mice lacking mGlu1 receptors show a defect in the elimination of supranumerary climbing fibers innervating Purkinje cells that physiologically occurs in the developing cerebellum (Kano et al., 1997). Thus, the presence of mGlu1 receptors is essential for the correct development of the cerebellar cortex. In addition to a role in synaptic plasticity, mGlu1 receptors also mediate a slow EPSP at synapses made between parallel fibers and Purkinje cells (Batchelor et al., 1997). Therefore, mGlu1 receptors are involved in both acute and long-term regulation of synaptic transmission. While this synaptic response is induced by repetitive stimuli, it can be induced by as few as 3 stimuli (Batchelor and Garthwaite, 1997) which demonstrates that synaptically released L-glutamate can readily access these receptors. The mGlu1-receptor mediated EPSC is subject to pronounced synaptic plasticity. Thus, it was shown that depolarization of Purkinje cells by climbing fibre stimulation elicits near complete inhibition of this synaptic current (Jin et al., 2007). These various functions of mGlu1 receptors (role in synaptic transmission, expression of synaptic plasticity, trigger for synaptic plasticity) could all contribute to the role of these receptors in physiological and pathological processes.
3.1.4. Pharmacology
3,5-Dihydroxyphenylglycine (DHPG) binds to the glutamate recognition site of mGlu1 receptors behaving as an orthosteric agonist. However, this drug is not subtype selective, and also activates mGlu5 receptors. To our knowledge, there are no orthosteric agonists selective for mGlu1 receptors. (S)-2-(4-Fluoro-phenyl)-1-(toluene-4-sulfonyl)-pyrrolidine (Ro67-7476), (9H-xanthene-9-carbonyl)-carbamic acid butyl ester (Ro-674853), diphenyl-acetyl-carbamic acid ethyl ester (Ro-01-6128), and 4-nitro-N-(1,4-diphenyl-1H-pyrazol-5-yl) benzamide (VU71) are examples of selective mGlu1 receptor PAMs (or “enhancers”), which require the presence of an orthosteric agonist to be active (reviewed by Niswender and Conn, 2010). These drugs, if given alone, exclusively recruit mGlu1 receptors that are activated by endogenous glutamate, i.e. their action is activity-dependent. 4-(Amino-carboxy-methyl)-3-methyl-benzoic acid (LY367385), 1-ami-noindane-1,5,-dicarboxylic acid (AIDA), and its derivative, α-amino-5-carboxy-3-methyl-2-thiopheneacetic acid (3-MATIDA) are orthosteric antagonists of mGlu1 receptors with little or no activity at mGlu5 receptors (reviewed by Schoepp et al., 1999). 7-(Hydroxyimino) cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) is the prototype of a growing list of drugs acting as negative allosteric modulators (NAMs) of mGlu1 receptors (reviewed by Niswender and Conn, 2010). Some of these drugs, including (3aS,6aS)-hexahydro-5-methylene-6a-(2-naphthalenylmethyl)-1H-cyclopenta[c]furan-1-one (BAY367620) (Carroll et al., 2001) and (3,4-dihydro-2H-pyrano[2,3-b] quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone (JNJ16259685) (Lavreysen et al., 2004), display high receptor affinity and are systemically active.
3.1.5. Relevance to human disorders
3.1.5.1. Inherited ataxia and autoimmune cerebellar disorders
Animal studies suggest that inherited ataxia can be associated with a defective expression or function of mGlu1 receptors in Purkinje cells. Ataxic transgenic mice show mutations of the Grm1 gene or a reduced expression/activity of mGlu1 receptors or downstream effector molecules in Purkinje cells (Conti et al., 2006; Sachs et al., 2007; Kurnellas et al., 2007). Interestingly, in conditional spino-cerebellar ataxia type 1 (SCA1) mutant mice, recovery from the disease after stopping transgene expression correlates with localization of the mGlu1α receptor to the Purkinje cell-parallel fibre synapse (Zu et al., 2004). Although these data suggest that the GRM1 is a candidate gene for inherited cerebellar disorders, it should be highlighted that no causative mutations in the GRM1 gene have been found in children with idiopathic early-onset ataxia (Rossi et al., 2010). In three patients, cerebellar ataxia has been associated with the presence of autoantibodies directed against mGlu1 receptors. Two of these patients had Hodgkin’s lymphoma with paraneoplastic cerebellar ataxia (Sillevis Smitt et al., 2000). The third patient had a primary autoimmune disorder (Marignier et al., 2010). mGlu1 receptor PAMs are potential candidates for the treatment of those forms of ataxia that are associated with defective mGlu1 receptors. Mice developing experimental autoimmune encephalomyelitis (EAE) after immunization with the myelin oligodendrocyte glycoprotein (MOG) show a reduced expression of mGlu1α receptors in Purkinje cells and an impaired motor coordination at the rotarod test. Motor impairment is corrected by a systemic treatment with a selective mGlu1 receptor PAM (Fazio et al., 2008).
3.1.5.2. Malignant melanomas
Suzy Chen and her associates (Pollock et al., 2003) showed that insertional mutant mice predisposed to develop malignant melanomas had a deletion of 70 kb in intron 3 of Grm1, resulting into an ectopic expression of mGlu1 receptors in melanoma cells. Mutant mice in which expression of the Grm1 gene is driven by the promoter of dopachrome tautomerase (an enzyme specifically expressed in melanocytes) also develop multiple melanomas. Expression of mGlu1 receptors has been detected in human melanomas, but not in benign nevi (Pollock et al., 2003). The oncogenic activity of mGlu1 receptors in melanoma cells depends on the activation of multiple transduction pathways that include PKC-ε, the MAPK pathway, and the PtdIns-3-K/Akt pathway (Marín et al., 2006; Shin et al., 2010). Treatment of human melanoma cells with mGlu1 receptor NAMs or with riluzole, which inhibits glutamate release, reduces cell proliferation (Namkoong et al., 2007). No clinical data with mGlu1 receptor antagonists are available, as yet. However, data from a phase 0 clinical trial show that a 14-day treatment with riluzole inhibits mGlu1 receptor signalling and metabolic activity in post-treatment tumor samples (Yip et al., 2009).
3.1.5.3. Neurodegeneration/neuroprotection
mGlu1 receptor antagonists protect hippocampal neurons against “post-ischemic” degeneration in both hippocampal slices exposed to oxygen–glucose deprivation and in vivo models of transient global ischemia (reviewed by Pellegrini-Giampietro, 2003). Pharmacological blockade of mGlu1 receptors enhances GABAergic transmission in the hippocampus by preventing the mGlu1-receptor mediated formation of endocannabinoids and the ensuing activation of inhibitory CB1 receptors localized on GABAergic nerve terminals (Landucci et al., 2009). In contrast, it is the activation of mGlu1 receptors that mediates the mechanism of ischemic tolerance observed with the paradigm of “ischemic preconditioning” in hippocampal slices (Werner et al., 2007), whereas activation of both mGlu1 and mGlu5 receptors mediates mechanisms of “ischemic post-conditioning” (Scartabelli et al., 2008). In cultured neurons challenged with excitotoxins mGlu1 receptor antagonists are consistently neuroprotective, whereas agonists can be neurotoxic or neuroprotective depending on the experimental paradigm (reviewed by Nicoletti et al., 1999). Michel Baudry and his associates have shown that mGlu1α receptors activate two pathways that differentially affect neurodegeneration: (i) a protective pathway mediated by the activation of PtdIns-3-K; and (ii) a toxic pathway mediated by intracellular Ca2+ release. The calpain-mediated truncation of mGlu1α receptors prevents the activation of the PtdIns-3-K pathway, leaving the Ca2+pathway intact (Xu et al., 2007). Recent evidence also indicates that mGlu1α may produce dual neuroprotective and neurotoxic signalling in cerebellar and cortical neurons (Pshenichkin et al., 2008) exhibiting the properties of a dependence receptor by inducing apoptosis in the absence of glutamate, while promoting neuronal survival in its presence. The mechanism of the proapoptotic action has not been elucidated, but, similarly to other dependence receptors, it may involve an agonist-independent interaction of the C-terminal receptor domain with intracellular targets. Instead, the prosurvival action of mGlu1 occurs via a novel, G-protein independent, mechanism mediated by β-arrestin1-dependent, persistent, activation of the MAPK pathway (Emery et al., 2010) with a pharmacology indicating the presence of a ligand bias whereby glutamate, but not quisqualate can activate the signal transduction mechanism. This suggests a role for receptor desensitization and internalization – that critically depend on the extent and duration of agonist exposure – in mechanisms of neurodegeneration/neuroprotection. Moreover, the proapoptotic action of mGlu1α may play a role in developmental apoptosis in the CNS.
3.1.5.4. Schizophrenia
Recent evidence corroborates the hypothesis that inhibition of mGlu1 receptors could be a novel treatment for schizophrenia. Indeed, mGlu1 receptor antagonists were shown to selectively improve prepulse inhibition in DBA/2J mice (Hikichi et al., 2010), an animal model for impaired sensorimotor gating. Sensorimotor gating is a fundamental form of information processing that is deficient in patients with schizophrenia (Braff et al., 1992) and which can be modeled in animals using acoustic pre-pulse inhibition of the startle (PPI). Deficits in PPI were also observed in mice with gene-targeted deletion of the mGlu1 receptor, which was evident as early as 6 weeks postnatal and remained impaired till adulthood (Brody et al., 2003). Moreover, allosteric mGlu1 receptor antagonists reduced methamphetamine-induced hyperlocomotion (Satow et al., 2009) and behave similarly to the atypical antipsychotic, clozapine, in activating neurons in the nucleus accumbens and medial prefrontal cortex (Suzuki et al., 2010). Although these findings are still at a relatively early stage, mGlu1 receptor antagonists hold promise as novel putative antipsychotic drugs.
3.2. mGlu5 receptor
Gene name: GRM5 (human); Grm5 (rat, mouse). Accession numbers: NP_000833 (human); NP_058708 (rat); NP_001074883 (mouse). Chromosomal location: 11q14.2 (human); 1q32 (rat); 7D3 (mouse) (Abe et al., 1992; Kuramoto et al., 1994).
3.2.1. Splice variants
mGlu5a: 1212 amino acids in humans; 1203 amino acids in rats; highly and diffusely expressed in the rat brain during early postnatal development (Romano et al., 1996b).
mGlu5b: a variant that contains a 32 amino acid fragment inserted into the cytoplasmic tail 50 residues after the 7th TM; more abundant in the adult brain (Joly et al., 1995; Minakami et al., 1995; Romano et al., 1996b).
mGlu5d: identified in human cerebellum and hippocampus; C-terminus domain 267 amino acids shorter than human mGlu5a receptors (Malherbe et al., 2002).
3.2.2. Receptor signalling and interacting proteins
The mGlu5 receptor is coupled to Gq/G11 protein and its activation stimulates PI hydrolysis. In recombinant cells, activation of mGlu5 receptors induces oscillatory increases in intracellular Ca2+ release, as a result of a PKC-mediated receptor phosphorylation (Kawabata et al., 1996). Both mGlu5a and mGlu5b receptors are characterized by a long C-terminus domain that allows the interaction with Homer proteins similarly to mGlu1α receptors. In the postsynaptic elements, mGlu5 receptors are physically linked to the NR2 subunit of NMDA receptors via a chain of interacting proteins, which include PSD-95, Shank, and Homer (Tu et al., 1999) (Fig. 2). Calcium ions that enter the cell via the NMDA-gated ion channel activate protein phosphatase 2B, which dephosphorylates mGlu5 receptors thereby limiting mGlu5-receptor desensitization (Alagarsamy et al., 2005). In addition, a large body of evidence indicates that activation of mGlu5 receptors enhances NMDA receptor function (Doherty et al., 1997; Ugolini et al., 1999; Awad et al., 2000; Attucci et al., 2001; Mannaioni et al., 2001; Pisani et al., 2001). The neuron-specific protein, Norbin, physically interacts with mGlu5 receptors in vivo and increases both membrane localization and signalling of mGlu5 receptors (Wang et al., 2009). The mGlu5 receptors are selectively desensitized by members of the GRK2 family (GRK2 and GRK3), through a mechanism that involves phosphorylation of the Threo 840 residue (Sorensen and Conn, 2003).
3.2.3. Functional anatomy
The mGlu5a receptor is highly expressed in the CNS during early postnatal life, and likely mediates the robust PI response to excitatory amino acids found in all brain regions early after birth (Casabona et al., 1997). The mGlu5b receptor is abundant in the adult hippocampus, corpus striatum, and cerebral cortex (Romano et al., 1996b), and is therefore the main target for pharmacological intervention in the adult brain. Partly, because of their functional interaction with NMDA receptors (Collett and Collingridge, 2004), mGlu5 receptors are involved in the regulation of synaptic plasticity (Bortolotto et al., 2005; Manahan-Vaughan and Braunewell, 2005; Bikbaev et al., 2008). Mice with genetic deletion of mGlu5 receptors show a mild deficit in long-term potentiation (LTP) in the hippocampus and an impaired spatial learning (Jia et al., 1998). However, pharmacological inhibition of mGlu5 receptors does not affect LTP in this pathway, suggesting that the deficit in the knockout has a developmental origin (Bortolotto et al., 2005).
Pharmacological activation of mGlu5 receptors induces a robust form of LTD at the Schaffer collateral-CA1 pyramidal cell synapse in the hippocampus (Palmer et al., 1997). This form of synaptic plasticity can also be induced by synaptic activity and has been shown to be dependent on dendritic protein synthesis under certain circumstances (Huber et al., 2000) but independent of protein synthesis under others (Moult et al., 2008). A selective amplification of mGlu5-receptor mediated LTD in the hippocampus has been suggested to underlie cognitive dysfunction in Fragile-X mutant mice and represents a promising target for therapeutic intervention (see below). A variety of different signalling mechanisms have been found to be involved in mGluR-LTD in the hippocampus, including the activation of p38 MAPK (Bolshakov et al., 2000; Rush et al., 2002) and tyrosine phosphatases (Moult et al., 2008), including the Striatal Enriched tyrosine Phosphatase, STEP (Zhang et al., 2008a,b).
In addition to direct roles in LTP and LTD at CA1 synapses, mGlu5 receptors have been demonstrated to play a crucial role in some forms of metaplasticity. Metaplasticity is the plasticity of synaptic plasticity and comes in many varieties (Abraham, 2008). One rather unusual manifestation of metaplasticity has been termed the molecular switch, whereby the induction of LTP makes the induction of subsequent LTP at the same pathway resistant to inhibition by α-methyl-4-carboxyphenylglycine (MCPG) (Bortolotto et al., 1994). This conditioning effect of the first tetanus relies on activation of mGlu5 receptors since it is blocked by 2-methyl-6-(phenyl-ethynyl)-pyridine (MPEP) and is absent in the mGlu5 knockout mouse (Bortolotto et al., 2005). Like the activation of the slow EPSC in cerebellar Purkinje cells described above, the setting of the molecular switch by activation of mGlu5 receptors can be achieved by very few synaptic responses (Bortolotto et al., 2008). This once again demonstrates that mGlu receptors can be activated relatively easily, despite their more peripheral location at synapses. A second form of metaplasticity at these synapses, that may utilise similar mechanism, is where prior stimulation of group-I mGlu receptors leads to a greater magnitude LTP (Cohen et al., 1998). The mechanisms by which activation of mGlu5 results is metaplasticity are not fully understood but might involve both Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Bortolotto and Collingridge, 1998) and PKC (Bortolotto and Collingridge, 2000).
The expression and function of mGlu5 receptors in the basal ganglia motor circuits and in the pain neuraxis is described below. Interestingly, mGlu5 receptors are also expressed in non-neuronal cells, including astrocytes, oligodendrocytes, and microglia, stem-progenitor cells, and a variety of peripheral cells. Expression and function of mGlu5 receptors in astrocytes is highly plastic and changes dramatically in response to cell activation or under pathological conditions (Miller et al., 1996; Balázs et al., 1997; Aronica et al., 2000, 2003; Geurts et al., 2003). Mice expressing a mutated form of human superoxide dismutase associated with amyotrophic lateral sclerosis (ALS) show astrocytic degeneration in the ventral horns of the spinal cord, which likely depends on the activation of mGlu5 receptors by endogenous glutamate. Blocking mGlu5 receptors in vivo delays the onset of motor neuron disease and increases survival of these mice (Rossi et al., 2008). Embryonic stem (ES) cells (pluripotent stem cells derived from the inner mass of the blastocysts) grown under proliferating conditions express mGlu5 receptor and no other mGlu receptor subtypes. Pharmacological blockade of mGlu5 receptors inhibits self-renewal of ES cells and promotes cell differentiation towards mesoderm and endoderm lineages (Cappuccio et al., 2005). mGlu5 receptors are also expressed by neural stem cells, i.e. stem cells resident in the CNS, which can give rise to neurons, astrocytes, and oligodendrocytes. Mice lacking mGlu5 receptors have a lower number of proliferating neuroprogenitors in zones of active neurogenesis of the adult brain (Di Giorgi-Gerevini et al., 2005). A recent review (Julio-Pieper et al., in press) highlights the role of mGlu receptors in peripheral organs. The regulation of the lower esophageal sphincter by mGlu5 receptor is of great relevance from a therapeutical standpoint (see below). mGlu5 receptors are also found in the liver, and hepatocytes lacking mGlu5 receptors are less sensitive to hypoxic damage (Storto et al., 2004). In addition, pharmacological blockade of mGlu5 receptors reduces liver damage caused by lipopolysaccharide (Jesse et al., 2009) or acetaminophen (Storto et al., 2003). mGlu5 receptors are expressed in many other peripheral cells, including cells of the male germinal line, insulinoma cell lines, immune cells, and brain endothelial cells, where their precise function remains to be determined (Julio-Pieper et al., in press).
3.2.4. Pharmacology
There are no selective orthosteric agonists of mGlu5 receptors with the exception of (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), which displays low affinity and is active in the high micromolar range (reviewed by Schoepp et al., 1999). A number of mGlu5-receptor enhancers have been developed, which include [(3-fluorophenyl) methylene]hydrazone-3-fluorobenzaldehyde (DFB), N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydrox-ybenzamide (CPPHA), (S)-(4-fluorophenyl)-(3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]piperidin-1-yl)methanone (ADX47273), 3-cy-ano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB), 4-nitro-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (VU29), and ADX63365 behave as mGlu5-receptor enhancers (reviewed by Niswender and Conn, 2010), whereas MPEP, 3-((2-methyl-1,3-thiazol-4-yl)ethynyl) pyridine hydrochloride (MTEP), 6-methyl-2-(phenylazo)-3-pyridinol (SIB-1757), and (E)-2-methyl-6-(2-phenylethenyl)pyridine (SIB-1893) are the prototypes of a growing list of mGlu5 receptors NAMs, which also includes fenobam, AFQ056, AZD2516, AZD2066, STX107, ADX10059, and ADX48621. Many of these drugs are systemically active and are under clinical development.
3.2.5. Relevance to human disorders and clinical perspectives
3.2.5.1. Fragile-X syndrome (FXS)
FXS is the most frequent inherited cause of mental retardation (incidence = 1:3600 in males and 1:4000–6000 in females) caused by the transcriptional silencing of the FMR1 gene, which encodes the fragile X mental retardation protein (FMRP) (Pieretti et al., 1991). Prominent signs and symptoms include cognitive dysfunction, autism, behavioural problems, seizures, dysmorphic features, and macroorchidism. Current medications include antipsychotic, anticonvulsant, antidepressant, and psychostimulant drugs, which, however, do not correct cognitive impairment associated with FXS (reviewed by Dölen and Bear, 2008). Fmr1 knockout mice show a selective amplification of mGlu5-receptor mediated LTD in the Schaffer collateral-CA1 pyramidal cell synapses in the hippocampus. Remarkably, other forms of synaptic plasticity, including the more classical NMDA-receptor dependent LTD, show no abnormalities in the hippocampus of Fmr1 knockout mice (Huber et al., 2002; reviewed by Waung and Huber, 2009). The mGlu5-receptor mediated LTD is, under certain circumstances, dependent of protein synthesis occurring in the dendrites of CA1 pyramidal neurons as a result of local mRNA translation. FMRP is one of the dendritic proteins synthesized in response to mGlu5-receptor activation and has the specific function of acting as a translational suppressor of other LTD-associated proteins, such as PostSynaptic Density-95 (PSD-95), the amyloid precursor protein, microtubule-associated protein 1b (MAP1b), the elongation factor 1a, and the cytoskeleton-associated protein, Arc. In the absence of FMRP, these proteins are constitutively and highly expressed, and this makes the mGlu5-receptor mediated LTD insensitive to protein synthesis inhibitors (reviewed by Waung and Huber, 2009). A series of recent elegant studies have characterized the signalling pathways leading to dendritic protein synthesis in response to mGlu5-receptor activation. This includes the association of mGlu5 receptors with Homer proteins, and the activation of upstream regulators of dendritic mRNA translation, such as the ERK/MAPK-interacting kinase (Mnk1)/eukaryotic initiation factor 4E (eIF4E) pathway, and the PtdIns-3-K/mammalian target of rapamycin (mTOR)/p70S6K pathway. Many steps along these pathways are abnormal in Fmr1 knockout mice (Giuffrida et al., 2005; Ronesi and Huber, 2008; Sharma et al., 2010). The lack of one of the two Grm5 alleles as well as systemic treatment with MPEP or other mGlu5-receptor antagonists corrects most of the phenotypes associated with the lack of FMRP, including cognitive dysfunction, epileptic seizures, and morphological abnormalities (Yan et al., 2005; Tucker et al., 2006; Dölen et al., 2007; de Vrij et al., 2008). Most of the mGlu5-receptor NAMs listed in the previous paragraph are under clinical development for the treatment of FXS. Interestingly, non-sedative doses of MPEP block repetitive self-grooming behavior in BTBR T + tfJ mice, which show multiple behavioural traits with face validity for the diagnostic symptoms of autism (Silverman et al., 2010). This raises the possibility that forms of autism other than FXS may be amenable of treatment with mGlu5-receptor antagonists.
3.2.5.2. Parkinson’s disease and L-DOPA-induced dyskinesia
mGlu5 receptors are highly expressed in the basal ganglia motor circuit and are involved in the regulation of motor behavior (Smith et al., 2000; Paquet and Smith, 2003; reviewed by Conn et al., 2005). Seminal work by the groups of David Lovinger and Paolo Calabresi, Antonio Pisani and other associates have shed lights into the role of group-I mGlu receptors in processes of striatal synaptic plasticity underlying habit memory and motor learning (reviewed by Gubellini et al., 2004; Bonsi et al., 2008; Lovinger, 2010). The neostriatum (caudate nucleus and putamen), which is the primary input region of the basal ganglia, is connected to output nuclei (internal globus pallidus and substantia nigra pars reticulata) by a “direct” inhibitory GABAergic pathway, and by an “indirect” pathway, which includes a striatal GABAergic projection to the external globus pallidus, an additional GABAergic projection from the external globus pallidus to the subthalamic nucleus, and an excitatory glutamatergic projection from the subthalamic nucleus to the output nuclei. Dopamine released from the nigro-striatal pathway stimulates the direct pathway acting at D1 receptors and inhibits the indirect pathway acting at D2 receptors. The progressive loss of nigro-striatal neurons associated with Parkinson’s disease leads to a decreased activity of the direct pathway and an increased activity of the indirect pathway, which lead to inhibition of thalamocortical neurons and motor dysfunction (bradykinesia, rigidity, and resting tremor). Dopamine replacement therapy with L-3,4-dihydroxyphenylalanine (L-DOPA) is highly effective in relieving parkinsonian symptoms in the first few years of treatment (the “honeymoon”). Afterwards, L-DOPA treatment is complicated by loss of efficacy and the occurrence of stereotyped involuntary movements (monophasic and diphasic L-DOPA-induced dyskinesias), which likely depends on a hyperactivity of the direct pathway (Cenci, 2007). mGlu5 receptors are expressed in the neostriatum by medium spiny projection neurons and interneurons (Smith et al., 2000), and are also found in output nuclei and in nuclei of the indirect pathway (reviewed by Conn et al., 2005). In striatal projection neurons of the indirect pathway, mGlu5 receptors are functionally linked to NMDA receptors and A2A adenosine receptors, forming a receptor complex that counteracts the activity of D2 receptors (Ferré et al., 2002; Nishi et al., 2003). In striatal projection neurons of the direct pathway, mGlu5 receptors affect synaptic responses to dopamine by modulating the activity of dopamine-and cAMP-regulated phosphoprotein, DARPP-32 (Liu et al., 2001). mGlu5 receptors inhibit neurons of the external globus pallidus by a mechanism of cross-desensitization with mGlu1 receptors (Poisik et al., 2003), whereas their activation produces membrane depolarization and an increase in burst firing in neurons of the subthalamic nucleus (Awad et al., 2000). A growing body of evidence indicates that systemic treatment with mGlu5-receptor NAMs is highly effective in relieving motor symptoms and L-DOPA-induced dyskinesias in rodent and primate models of parkinsonism (Breysse et al., 2002, 2003; Mela et al., 2007; Yamamoto and Soghomonian, 2009; Ouattara et al., 2009; Ambrosi et al., 2010; Johnston et al., 2010; Rylander et al., 2010). The mGlu5 NAMs, AFQ056, AZD2516, and ADX48621, are currently in phase I/II of clinical development for the treatment of Parkinson’s disease and L-DOPA-induced dyskinesias. Remarkably, genetic deletion of mGlu5 receptors or systemic treatment with mGlu5-receptor blockers is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and methamphetamine mouse models of toxicological parkinsonism (Battaglia et al., 2002, 2004). Although these are acute models of nigro-striatal degeneration, the possibility that mGlu5-receptor blockade attenuates the progressive loss of nigral neurons associated with Parkinson’s disease is attractive and warrants further investigation.
3.2.5.3. Chronic pain
Chronicization of pain reflects the development of “nociceptive sensitization”, i.e. the amplification of pain transmission that occurs along the entire pain neuraxis and underlies the hallmark features of chronic pain, such as primary and secondary hyperalgesia, mechanical and thermal allodynia, and spontaneous pain (reviewed by Basbaum et al., 2009). All mGlu receptor subtypes (with the exception of mGlu6 receptors) are widely distributed in pain centers of the brain and spinal cord, and are implicated in the induction, expression, and maintenance of nociceptive sensitization (Neugebauer, 2001; Varney and Gereau, 2002; Goudet et al., 2009; Chiechio et al., 2010). In peripheral nociceptors (the peripheral terminals of dorsal root ganglia neurons) mGlu1 and mGlu5 receptors respond to ambient glutamate by triggering a cascade of reactions leading to sensitization of TrpV1 channels, which sense noxious heat and also respond to inflammatory molecules and capsaicin (Bhave et al., 2001; Hu et al., 2002). In nociceptive neurons of the dorsal horns of the spinal cord, the mGlu5 receptor is activated by glutamate released from primary afferent fibers or by local excitatory interneurons expressing PKC-γ. Activation of mGlu5 receptors leads to inhibition of Kv4.2 voltage-sensitive potassium channels via a phosphorylation mechanism mediated by the MAPK pathway (Hu et al., 2007). The resulting increase in neuronal excitability contributes to nociceptive sensitization in spinal cord neurons. mGlu5 receptors are also found in supraspinal brain centers, such as dorsal raphe nuclei and amygdala, where their activation sustains chronic pain (de Novellis et al., 2005; Ansah et al., 2009, 2010). Pharmacological blockade of mGlu5 receptors is analgesic in models of inflammatory and neuropathic pain, and inhibits the development of tolerance to the analgesic activity of morphine (Fisher et al., 2002; Kozela et al., 2003; de Novellis et al., 2004; Smith et al., 2004; Varty et al., 2005; Han and Neugebauer, 2005; Sevostianova and Danysz, 2006; Osikowicz et al., 2008; Montana et al., 2009). Based on these preclinical data, mGlu5-receptor NAMs such as AZD2516 and AZD2066 are under clinical development for the treatment of neuropathic pain (pain originating from structural or functional lesions of the pain pathways). In a phase IIa clinical trial, the mGlu5-receptor NAM, ADX100159, has shown better efficacy than placebo in the treatment of acute pain associated with migraine.
3.2.5.4. Drug addiction
Drug addiction or “substance dependence” (Diagnostic and Statistical Manual of Mental Disorders, fourth edition, 1994) is a chronic, relapsing disorder characterized by compulsive drugs seeking behavior, loss of control in taking the drug, and emergence of a negative emotional state when access to the drug is prevented (reviewed by Koob and Le Moal, 2008). From a therapeutic standpoint, it is helpful to subdivide the addiction process into three phases: (i) an “intoxication phase” (e.g. a cocaine binge), dominated by the reinforcing properties of the drug; (ii) an abstinence phase, in which mechanisms of negative reinforcement sustain drug craving; and (iii) a phase of enduring vulnerability to drug intake, in which drug reinstatement may be induced by stress, drug priming, or environmental cues previously paired with drug intake. Activation of the dopaminergic mesolimbic system, projecting from the ventral tegmental area (VTA) to the shell of nucleus accumbens, mediates the reinforcing properties of drugs of abuse (Di Chiara and Imperato,1988), whereas a dysregulation of synaptic plasticity in the nucleus accumbens contributes to the phase of enduring vulnerability to drug intake (reviewed by Kauer and Malenka, 2007; Kalivas, 2009). Chiamulera et al. (2001) have shown that mice lacking mGlu5 receptors fail to self-administer cocaine without showing a defect in the ability of cocaine to increase extracellular dopamine levels in the nucleus accumbens. Data obtained with MPEP in experimental paradigms measuring acute drug reward (e.g., self-administration of drugs of abuse, measurements of brain reward thresholds, and conditioned-place preference) indicate that activation of mGlu5 receptors is required for incentive motivation for the reinforcer (reviewed by Kenny and Markou, 2004; Kenny et al., 2005). mGlu5-receptor NAMs exert anti-reinforcing effects at multiple levels including VTA neurons, where group-I mGlu receptors are involved in mechanisms of synaptic plasticity underlying reward-based conditioning and the development of drug addiction (Kauer and Malenka, 2007; Ahn et al., 2010). Accordingly, cocaine-mediated synaptic potentiation is absent in VTA neurons from mGlu5-defient mice (Bird et al., 2010). mGlu5-receptor blockade in the nucleus accumbens is effective in preventing relapse to drug seeking as well as neurochemical changes associated with drug reinstatement (Bespalov et al., 2005; Schroeder et al., 2008). mGlu5-receptor NAMs hold promise in the treatment of drug addiction, and AFQ056 is under clinical development for the treatment of tobacco smoking (Kenny, 2009). However, it should be highlighted that mGlu5-receptor blockade increases somatic signs and reward deficits associated with nicotine withdrawal (Liechti and Markou, 2006), suggesting that mGlu5-receptor NAMs should be associated with drugs that lower the reward threshold (e.g., bupropion) in the acute phase of nicotine withdrawal.
3.2.5.5. Anxiety
Drugs that block mGlu5 receptors consistently show anxiolytic-like activity in a variety of animal models such as fear potentiated startle, contextual fear conditioning, elevated plus maze, conflict tests, and stress-induced hyperthermia (Spooren et al., 2000; Tatarczyńska et al., 2001; Busse et al., 2004; Roppe et al., 2004; Pietraszek et al., 2005; Stachowicz et al., 2007; Spanka et al., 2010). Mice lacking mGlu5 receptors also show a reduced stress-induced hyperthermia (Brodkin et al., 2002). Remarkably, fenobam, a clinically validated anxiolytic drug, behaves as a potent and selective mGlu5-receptor NAM (Porter et al., 2005). A critical issue is whether mGlu5-receptor antagonists show a better profile of safety and tolerability than benzodiazepines and selective serotonin reuptake inhibitors (SSRIs), which are widely prescribed for the treatment of generalized anxiety disorders and panic attacks. A potential advantage with respect to benzodiaze-pines is that, at least in preclinical models, mGlu5-receptor NAMs retain their anxiolytic activity after repeated dosing (i.e. there is no development of tolerance) (Nordquist et al., 2007). As opposed to diazepam, MPEP produces a robust anxiolytic effect in rat conflict tests at doses that do not impair working memory and spatial learning (Ballard et al., 2005). Fenobam, however, was shown in one study to produce anxiolytic activity at doses that impaired spatial learning (Jacob et al., 2009). Finally, little is known on the interaction between mGlu5-receptor NAMs and drug metabolizing enzymes (e.g. the various isotypes of cytochrome-P450) and drug efflux pumps. Thus, the development of mGlu5-receptor NAMs as novel anxiolytic agents warrants further investigations.
3.2.5.6. Schizophrenia
The interest for mGlu5 receptors in schizophrenia stems from the evidence that mGlu5-receptor knockout mice show a disruption in PPI, which reflects a defect in sensory-motor gating (Kinney et al., 2003; Brody et al., 2004). mGlu5-receptor blockade enhances the psychotomimetic effect of phencyclidine (Campbell et al., 2004), and mice with genetic deletion of Norbin, which enhances mGlu5-receptor signalling, also show a defect in PPI (Wang et al., 2009). GRM5 has been mapped to 11q15 neighbouring a translocation that segregates with schizophrenia in a large Scottish family (Devon et al., 2001). mGlu5 and NMDA receptors may act synergistically in regulating neuronal activity in the prefrontal cortex, a region that is critically involved in the pathophysiology of cognitive dysfunction in schizophrenic patients. Accordingly, pretreatment with a selective mGlu5-receptor enhancer prevents the abnormality in neuronal firing induced by NMDA receptor blockade in the medial prefrontal cortex (Lecourtier et al., 2007). The prediction that amplification of mGlu5-receptor function could be beneficial in the treatment of schizophrenia led to the development of a series of mGlu5-receptor PAMs (reviewed by Conn et al., 2009). Among these, CDPPB and ADX47273 are active in preclinical models that predict efficacy in the treatment of positive symptoms and cognitive dysfunction associated with schizophrenia (Liu et al., 2008; Schlumberger et al., 2009a,b; Vardigan et al., 2010), and ADX63365 is currently developed for the treatment of schizophrenia. It should be highlighted that the therapeutic activity of conventional antipsychotics on cognitive dysfunction associated with schizophrenia is negligible, and therefore, the use of mGlu5-receptor PAMs may represent a new avenue in the treatment of schizophrenic syndromes.
3.2.5.7. Gastroesophageal reflux disorder (GERD)
GERD is a common disorder affecting 3–7% of the U.S. population, which is caused by an abnormal reflux of stomach acid to the esophagus due to a transient relaxation of the lower esophageal sphincter (LES). The most common symptoms are regurgitation, heartburn, and dysphagia. In some cases, GERD causes injury of the esophageal epithelium leading to esophagitis, esophageal strictures, metaplastic changes in the esophageal epithelium (Barrett’s esophagus), and esophageal adenocarcinoma. Proton pump inhibitors are commonly used in the treatment of GERD. These drugs, however, inhibit acid secretion without affecting LES tone. Interestingly, glutamate is a potent activator of the vagal pathway mediating LES relaxation (Partosoedarso and Blackshaw, 2000), and mGlu receptors, including mGlu5, are expressed by gastric vagal afferents (Page et al., 2005). Preclinical studies have shown that mGlu5-receptor NAMs such as MPEP and MTEP increase basal LES pressure and inhibit LES relaxation (Frisby et al., 2005; Jensen et al., 2005). In a clinical study, treatment with the mGlu5 NAM, ADX100159, reduced the number and duration of symptomatic reflux episodes as well as nocturnal and postprandial esophageal acid exposure (Keywood et al., 2009). A modified release formulation of ADX100159 showed a better profile of tolerability and is therefore particular suitable for long-term treatments in patients with GERD (Zerbib et al., 2010).
3.3. mGlu2 and mGlu3 receptors
3.3.1. mGlu2 receptor
Gene name: GRM2 (human); Grm2 (rat, mouse). Accession numbers: NP_000830 (human); NP_001099181 (rat); NP_001153825 (mouse). Chromosomal location: 3q21.31 (human); 8q32 (rat); 9 (mouse) (Tanabe et al., 1992; Kuramoto et al., 1994; Flor et al., 1995a; Martí et al., 2002).
3.3.2. mGlu3 receptor
Gene name: GRM3 (human); Grm3 (rat, mouse). Accession numbers: NP_000831 (human); NP_001099182 (rat); NP_862898 (mouse). Chromosomal location: 7q21.1–q21.2 (human); 4q32 (rat); 5A1-h (mouse) (Tanabe et al., 1992; Kuramoto et al., 1994; Scherer et al., 1996; Emile et al., 1996; Corti et al., 2000).
3.3.2.1. Splice variants
Three splice variants of GRM3 have been reported in human brain and B lymphoblasts. The most abundant variant lacks exon 4 (GRM3Δ4) and encodes for a 60 kDa protein lacking the 7-TM domain of mGlu3 receptors (Sartorius et al., 2006).
3.3.2.2. Signal transduction and interacting proteins
mGlu2 and mGlu3 receptors are coupled to Gi/Go proteins in heterologous expression systems (reviewed by Tanabe et al., 1992). Their activation inhibits cAMP formation, inhibits voltage-sensitive Ca2+ channels, activates K+ channels, and can also activate the MAPK and PtdIns-3-K pathways (reviewed by Pin and Duvoisin, 1995). mGlu2/3 receptor agonists do not stimulate PI hydrolysis on their own, but amplify the stimulation of PI hydrolysis mediated by mGlu1/5 receptors in brain slices (Genazzani et al., 1994; Schoepp et al., 1996). Recent findings suggest that mGlu2 and mGlu3 receptors show a different sensitivity to processes of homologous desensitization mediated by GRK2/β-arrestin, with mGlu2 receptors being resistant to desensitization. However, this difference is visible only when inhibition of cAMP formation is measured as a read-out of mGlu2/3 receptor activation (Iacovelli et al., 2009). Group-II mGlu receptors were shown to interact with calmodulin, protein phosphatase 2C, and the Ran binding protein in the microtubule-organizing center (RanBPM) (reviewed by Niswender and Conn, 2010).
3.3.2.3. Functional anatomy
mGlu2 and mGlu3 receptors are diffusely expressed in the CNS. mGlu2 receptors are uniquely localized in neurons and particularly in the pre-terminal region of axons, far from the active zone of neurotransmitter release (Luján et al., 1997; Tamaru et al., 2001), whereas mGlu3 receptors are found at both presynaptic and postsynaptic sites as well as in glial cells (Ohishi et al., 1993a,b; 1994; Neki et al., 1996; Petralia et al., 1996; Ferraguti and Shigemoto, 2006). Presynaptic mGlu2/3 receptors can be activated by an excess of synaptic glutamate, or, alternatively, by the glutamate released from astrocytes via the cystine–glutamate membrane antiporter (see Kalivas, 2009) (Fig. 3). Changes in the expression and/or activity of the cystine–glutamate antiporter may affect the function of mGlu2/3 receptors in brain regions that are critically involved in drug addiction (see below). A major function of presynaptic mGlu2/3 receptor is to inhibit neurotransmitter release. Both receptors have an established role in the regulation of synaptic plasticity, particularly in the induction of LTD of excitatory synaptic transmission (Yokoi et al., 1996; Manahan-Vaughan, 1998; Kahn et al., 2001; Kilbride et al., 2001; Renger et al., 2002; Robbe et al., 2002; Grueter and Winder, 2005; Nicholls et al., 2006; Altinbilek and Manahan-Vaughan, 2009).
Fig. 3.
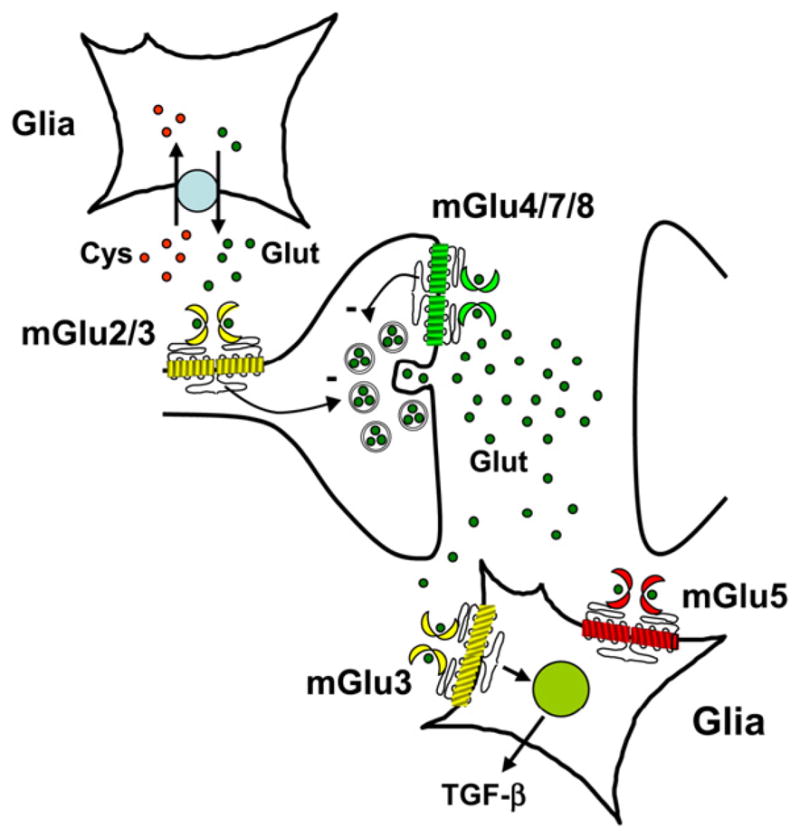
Synaptic distribution of group-II and group-III mGlu receptors. Note that presynaptic mGlu2/3 receptors are located in the pre-terminal regions of the axons, where they can be activated by glutamate released from astrocytes via the cystine/ glutamate antiporter. In contrast, presynaptic mGlu4/7/8 receptors are located near to the active zone of neurotransmitter release. Glial mGlu3 receptors induce the formation and secretion of TGF-β. The presence of mGlu5 receptors in glial cells is also shown.
A particular form of synaptic plasticity has been described in the mouse accessory olfactory bulb, where activation of mGlu2 receptors relieves the GABAergic inhibition of mitral cells, thus permitting the formation of a specific olfactory memory that faithfully reflects the memory of male pheromones formed at mating (Hayashi et al., 1993; Kaba et al., 1994). mGlu3 receptors were also found in embryonic stem cells and glioma-initiating cells, where their activation limits cell differentiation by negatively regulating type-4 bone-morphogenetic protein (BMP-4) receptor signalling (reviewed by Melchiorri et al., 2007).
3.3.2.4. Pharmacology
There are no marketed orthosteric agonists or antagonists that can differentiate between mGlu2 and mGlu3 receptors, with the exception of N-acetylaspartateglutamate (NAAG), which selectively activates mGlu3 receptors (Wroblewska et al.,1997; but see also Chopra et al., 2009). Orthosteric agonists of mGlu2 and mGlu3 receptors include 2R,4R-APDC, and the carboxycyclopropylglycine derivatives, DGC-IV and L-CCG-I. DCG-IV is active in the nanomolar range, but lacks specificity because it also activates NMDA receptors. L-CCG-I also activates group-I mGlu receptors (reviewed by Schoepp et al., 1999). LY354740 and LY379268 are conformationally constrained glutamate analogues in which the glutamate backbone is locked into a fully extended state by incorporation into a bicycle[3.1.0] hexane ring system. Both compounds behave as potent mGlu2/3 receptor agonists and are systemically active (reviewed by Schoepp et al., 1999). (−)-(1R,4S,5S,6S)-4-Amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404-039), which is pharmacologically similar to LY379268, is under clinical development for the treatment of schizophrenia (see below). LY341495 is an orthosteric antagonist with nanomolar affinity for mGlu2 and mGlu3 receptors, but is not subtype selective and may recruit other mGlu receptor subtypes (reviewed by Schoepp et al., 1999). (3-(3,4-Dichlorobenzyloxy)-2-amino-6-fluorobicyclo[3.1.0] hexane-2,6-dicarboxylic acid) MGS0039 (Nakazato et al., 2004), is a potent and selective mGlu2/3 receptor antagonist, which holds promise as a potential antidepressant drug (see below). 2,2,2-Trifluoro-N-[4-(2-methoxyphenoxy)phenyl]-N-(3-pyridinylmethyl) ethanesulfonamide hydrochloride (LY487379) and 3′-([(2-cyclo-pentyl-6-7-dimethyl-1-oxo-2,3-dihydro-1H-inden-5-yl)oxy]methy-l)biphenyl l-4-carboxylate (BINA) are prototypes of selective mGlu2 receptor PAMs (reviewed by Niswender and Conn, 2010), which are also under development in the treatment of schizophrenia (see below). No mGlu3 receptor enhancers are currently available.
3.3.2.5. Relevance to human disorders and clinical perspectives
3.3.2.5.1. Anxiety
mGlu2/3 receptors regulate synaptic transmission and plasticity in the amygdala (Wang and Gean, 1999; Lin et al., 2000, 2005), a brain region that encodes fear memory and is critically involved in the pathophysiology of anxiety disorders. Systemic treatment with the mGlu2/3 receptor agonist, LY354740, enhances Fos protein expression – a non-specific marker of cell activation – in GABAergic neurons of the lateral portion of central amygdala (CeL) (Linden et al., 2005, 2006). Thus, activation of mGlu2/3 receptors inhibits GABA release at basolateral amygdala (BLA)-CeL synapses, leading to an increased activity of GABAergic interneurons in the CeL. This, in turn, reduces the activity of output neurons in the medial central amygdala, which project to brain regions mediating the motor, autonomic, and endocrine features of anxiety. Treatment with LY354740 is highly effective in several animal models of anxiety and panic disorders with no reductions of activity on repeated dosing (reviewed by Swanson et al., 2005). The anxiolytic activity of LY354740 requires the presence of both mGlu2 and mGlu3 receptors (Linden et al., 2005), and, as predicted by the above mechanism, is reversed by the benzodiazepine antagonist, flumazenil (Ferris et al., 2001). LY354740 has progressed into phase II clinical trials with demonstration of good efficacy in the treatment of generalized anxiety disorder before discontinuation based on findings of convulsions in preclinical studies (Dunayevich et al., 2008). If convulsions represent an off-target effect of LY354740 or rather develop as a result of chronic activation (or desensitization) of mGlu2 or mGlu3 receptors remains to be determined.
3.3.2.5.2. Schizophrenia
The development of mGlu2/3 receptor agonists as antipsychotic drugs moved from the observation that LY354740 inhibits glutamate release (Battaglia et al., 1997), allowing for testing the hyperglutamatergic theory in schizophrenia (Aghajanian and Marek, 1999). Moghaddam and Adams (1998) found that LY354740 attenuates the disruptive effect of phencyclidine on working memory, locomotion, stereotypies, and cortical glutamate efflux. Since then, a number of mGlu2/3 receptor agonists have shown robust activity in models that are used to predict efficacy of potential antipsychotic agents (Schoepp and Marek, 2002; Conn et al., 2008, 2009). A pioneer phase II clinical study has shown that the a 21-day treatment with LY2140023, the oral prodrug of the potent and selective mGlu2/3 receptor agonist, LY404039, was nearly as effective as treatment with the antipsychotic olanzapine in reducing both positive and negative symptoms in schizophrenic patients (Patil et al., 2007). As opposed to olanzapine, LY2140023 did not increase body weight and blood triglyceride levels, two effects that seriously limit the use of atypical antipsychotic drugs in the treatment of schizophrenia (Patil et al., 2007). The antipsychotic activity of mGlu2/3 receptor agonists has been proposed to involve a negative interaction between mGlu2/3 receptors and 5-HT2A receptors. 5-HT2A receptors are activated by hallucinogenic drugs, such as lysergic acid diethylamide, psilocin, and (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and are antagonized by clozapine and other atypical antipsychotics. Pharmacological activation of mGlu2/3 receptors attenuates 5-HT2A-receptor-mediated excitatory post-synaptic currents recorded in layer V pyramidal neurons of the prefrontal cortex (Marek et al., 2000), and reduces electrophysiological and behavioural effects of hallucinogens (Gewirtz and Marek, 2000; Winter et al., 2004; Kłodzinska et al., 2002; Zhai et al., 2003; Benneyworth et al., 2007). Variations in the GRM3 gene (encoding human mGlu3 receptors) have been consistently associated with the risk of schizophrenia as well as with the response to antipsychotic medication (Egan et al., 2004; Bishop et al., 2005, 2007; Nicodemus et al., 2007; Fijal et al., 2009; Jönsson et al., 2009). However, preclinical studies suggest that it is the mGlu2 receptor that specifically mediates the antipsychotic activity of mGlu2/3 receptor agonists. González-Maeso et al. (2008) have shown that mGlu2 and 5-HT2A receptors form a heterodimeric complex in the cell membrane, and that activation of mGlu2 receptors inhibits a particular signalling pathway activated by 5- HT2A receptors in response to hallucinogens. In addition, selective mGlu2 receptor PAMs mimic the action of mGlu2/3 receptor agonists in inhibiting 5-HT2A receptor function (Zhai et al., 2003; Benneyworth et al., 2007). In one paper, however, the ability of mGlu2/3 agonists to inhibit DOI-stimulated PI hydrolysis in vivo is preserved in both mGlu2 and mGlu3 receptor knockout mice, although it is lost in double mGlu2/3 knockout mice (Molinaro et al., 2009). In line with these findings, an altered dimerization of mGlu3 receptors was detected in the prefrontal of schizophrenic patients (Corti et al., 2007a). Whether or not dual mGlu2/3 receptor agonists and selective mGlu2 receptor PAMs are comparable in terms of efficacy and tolerability in the treatment of schizophrenia is an interesting issue that warrants further investigation.
3.3.2.5.3. Depression
Multiple classes of drugs are available in the treatment of major depression, yet no drug has a fast onset of antidepressant activity, and a substantial percentage of depressed patients are resistant to medication. Dr. Chaki and his associates have shown that systemic treatment with the selective mGlu2/3 receptor antagonist, MGS0039, exerts antidepressant effects in models that are predictive of clinical efficacy, such as the learned helplessness paradigm, the forced swim test, and the tail suspension test (Chaki et al., 2004; Yoshimizu et al., 2006; reviewed by Pilc et al., 2008). Treatment with MGS0039 increases dopamine release in the nucleus accumbens and neurogenesis in the hippocampal dentate gyrus (Yoshimizu and Chaki, 2004; Karasawa et al., 2006), two effects that are consistent with an antidepressant activity. In combination studies, low doses of the mGlu2/3 receptor agonist, LY379268, shorten the temporal latency of classical antidepressants in reducing the expression of β1-adrenergic receptors in the hippocampus (a classical biochemical marker of antidepressant-induced neuroadaptation) and reducing the immobility time in the forced swim test (Matrisciano et al., 2005, 2007). Whether repeated dosing of LY379268 potentiate the activity of classical antidepressants by desensitizing mGlu2/3 receptors remains to be determined.
3.3.2.5.4. Drug addiction
mGlu2 receptors negatively regulates the activity of the reward pathway (i.e. the mesolimbic dopaminergic system) as shown by the increased reinforcing properties of cocaine shown by mGlu2 receptor knockout mice (Morishima et al., 2005). As predicted from this finding, pharmacological activation of mGlu2/3 receptors reduces self-administration of nicotine and cocaine (Liechti and Markou, 2006; Adewale et al., 2006), but precipitates the deficit in brain reward function associated with nicotine withdrawal (Kenny et al., 2003). Thus, mGlu2/3 receptor agonists may be valuable in the treatment of the “intoxication” phase of nicotine or cocaine addiction, but may increase drug craving during the early phase of drug withdrawal. Interestingly, however, mGlu2/3 receptor agonists reduce the somatic signs of opiate withdrawal and the associated hyperactivity of locus coeruleus neurons (Fundytus and Coderre, 1997; Vandergriff and Rasmussen, 1999), suggesting that activation of mGlu2/3 receptors differentially affects various aspects of drug withdrawal (reviewed by Kenny and Markou, 2004). An elegant series of experiments by Peter Kalivas and his associates has linked a defective activation of mGlu2/3 receptors to abnormalities of synaptic plasticity in the core of nucleus accumbens underlying the enduring vulnerability to drug reinstatement in addiction (reviewed by Kalivas, 2009). Rats withdrawn from chronic cocaine self-administration fail to develop LTP in the nucleus accumbens core after in vivo stimulation of the prefrontal cortex because of a pre-existing potentiation of synaptic transmission, which results form a reduced ability of presynaptic mGlu2/3 receptors to negatively regulate glutamate release. This derives (i) from a reduced expression of the xCT subunit of the glial cystine–glutamate antiporter transport system , which releases the glutamate necessary for the activation of presynaptic mGlu2/3 receptors, and (ii) from a reduced expression of type-3 Activator of G-protein Signalling (AGS3), which, in spite of its name, negatively regulates mGlu2/3 receptor signalling. Treatment with N-acetylcysteine, which provides the cystine substrate for residual cystine–glutamate exchangers, restores synaptic plasticity in the nucleus accumbens core and prevents cocaine relapse via the activation of mGlu2/3 receptors (Moran et al., 2005; Moussawi et al., 2009; reviewed by Kalivas, 2009). N-Acetylcysteine is under clinical development for the treatment of nicotine and cocaine addiction (LaRowe et al., 2006, 2007; Mardikian et al., 2007; Karila et al., 2008; Knackstedt et al., 2009). Curiously, N-acetylcysteine is marketed as an expectorant, and is also helpful in improving bronchial secretions associated with tobacco smoking.
3.3.2.5.5. Chronic pain
mGlu2/3 receptors are found in many stations of the pain neuraxis, from peripheral nociceptors to the amygdala, which encodes the emotional aspects of pain. In nociceptors, activation of mGlu2/3 receptors negatively regulate TrpV1 channels via the inhibition of cAMP formation (Yang and Gereau 4th, 2002; Carlton et al., 2009), whereas activation of presynaptic mGlu2/3 receptors localized on primary afferent fibers inhibits neurotransmitter release in the dorsal horns of the spinal cord (review by Chiechio et al., 2010). Systemic treatment with mGlu2/3 receptor agonists consistently produces analgesia in animal models of acute and chronic pain, but their use is limited by the development of tolerance in response to repeated dosing (Jones et al., 2005). This limitation can be overcome by a novel analgesic strategy based on the transcriptional activation of the Grm2 gene. L-Acetylcarnitine (LAC), a drug marketed for the treatment of neuropathic pain, induces the expression of mGlu2 receptors in dorsal root ganglia and in the dorsal horns of the spinal cord by enhancing acetylation of the p65/Rel subunit of the NFκB family of transcription factors. Remarkably, LAC-induced analgesia requires multiple dosing and is abolished by the mGlu2/3 receptor antagonist, LY341495 (Chiechio et al., 2002, 2006). Identical results are obtained with inhibitors of histone deacetylase, which also cause analgesia by inducing mGlu2 receptors in the spinal cord (Chiechio et al., 2009). Transcriptional regulation of mGlu2 receptors has been highlighted as an epigenetic path to novel treatments for chronic pain (Chiechio et al., 2010).
3.3.2.5.6. Brain tumors
Grade III and IV gliomas (anaplastic astrocytoma and glioblastoma multiforme, respectively) are highly infiltrating malignant brain tumors that represent the 3rd largest cause of all cancer-related deaths. It is generally believed that glioblastoma multiforme arises from a particular population of transformed neural stem cells named “glioma stem cells”, which sustain tumor growth and are intrinsically resistant to radiotherapy and chemotherapy (reviewed by Stiles and Rowitch, 2008). Purified glioma stem cells express mGlu3 receptors, the activation of which restrains the pro-differentiating activity of type-4 bone-morphogenetic protein (BMP-4) via a mechanism of receptor–receptor interaction. Systemic treatment with the preferential mGlu2/3 receptor antagonist, LY341495, reduces the growth of brain tumors originating from U87MG glioma cells (Arcella et al., 2005) or human glioma stem cells (Ciceroni et al., 2008) in mice. More recent findings suggest that mGlu3 receptor antagonists act synergistically with DNA-alkylating agents in killing glioma stem cells (Ciceroni C. et al., unpublished). The possibility that mGlu3 receptor blockade is used as a strategy for the treatment of malignant gliomas is attractive and warrants further investigation.
3.3.2.5.7. Neurodegenerative disorders
The role of mGlu2/3 receptors in mechanisms of neurodegeneration/neuroprotection is highlighted in a previous review (Bruno et al., 2001). It is disappointing that the robust neuroprotective activity of mGlu2/3 receptor agonists found in cell cultures (Bruno et al., 1995; Copani et al., 1995) is only partially seen in in vivo models of acute or chronic neurodegenerative disorders (Bond et al., 1999, 2000; Murray et al., 2002; Battaglia et al., 2003). The use of knockout mice unravelled distinct roles for mGlu2 and mGlu3 receptors in mechanisms of neurodegeneration/neuroprotection, with neuroprotection being entirely mediated by glial mGlu3 receptors in cultures challenged with mGlu2/3 receptor agonists (Corti et al., 2007b). In contrast, the lack of mGlu2 receptors in neurons attenuates excitotoxic death (Corti et al., 2007b), and treatment of neuronal cultures with a selective mGlu2 receptor enhancer amplifies β-amyloid toxicity (Caraci F. et al., unpublished). Activation of glial mGlu3 receptors enhances the production of transforming growth-factor-β (TGF-β) (Fig. 3), which acts as a paracrine protective factor on neighbour neurons (Bruno et al., 1998; D’Onofrio et al., 2001). Interestingly, systemic treatment with the mGlu2/3 receptor agonist, LY379268, prevents neurodegeneration and increases the expression of TGF-β2 in the entorhinal cortex of rats exposed to an alcohol binge (Cippitelli et al., 2010). Glial mGlu3 receptors are promising targets for the treatment of Alzheimer’s disease (AD) because TGF-β protects neurons against β-amyloid toxicity (reviewed by Caraci et al., 2009), and type-2 TGF-β receptors are defective in the AD brain (Tesseur et al., 2006). In addition, abnormalities in the mGlu3/TGF-β axis might contribute to the pathophysiology of Huntington’s disease (HD) because TGF-β1 levels are reduced in the plasma and brain tissue of HD patients, and activation of mGlu3 receptors fails to enhance the production of TGF-β1 in brain tissue of HD mutant mice (Battaglia et al., 2010). Production of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial-derived neurotrophic factor (GDNF) is also positively regulated by mGlu3 receptors (Ciccarelli et al., 1999; Battaglia et al., 2009; Di Liberto et al., 2010). GDNF is a potent prosurvival factor for nigral dopaminergic neurons (Lin et al., 1993), and intraputaminal infusion of GDNF attenuated parkinsonian symptoms in two phase-I clinical trials (Gill et al., 2003; Slevin et al., 2005). Thus, drugs that selectively activate mGlu3 receptors might produce neuroprotective effects in all major neurodegenerative disorders of the CNS.
3.4. mGlu4, mGlu7, and mGlu8 receptors
3.4.1. mGlu4 receptor
Gene name: GRM4 (human); Grm4 (rat, mouse). Accession numbers: NP_000832 (human); NP_073157 (rat); NP_001013403 (mouse). Chromosomal location: 6p21.3 (human); 20p12 (rat); 17A3.3 (mouse) (Tanabe et al., 1992; Kuramoto et al., 1994; Flor et al., 1995b; Barbon et al., 2000a).
3.4.2. mGlu7 receptor
Gene name: GRM7 (human); Grm7 (rat, mouse). Accession numbers: NP_000835 (human); NP_112302 (rat); NP_796302 (mouse). Chromosomal location: 3p26.1–p25-1 (human); 4q42 (rat); 6E3 (mouse) (Okamoto et al., 1994; Saugstad et al., 1994; Barbon et al., 2000b).
3.4.3. mGlu8 receptor
Gene name: GRM8 (human); Grm8 (rat, mouse). Accession numbers: NP_000836 (human); NP_071538 (rat); NP_032200 (mouse). Chromosomal location: 7q31.3–q32 (human); 4q22 (rat); 6A3 (mouse) (Duvoisin et al., 1995; Saugstad et al., 1997; Scherer et al., 1996, 1997).
3.4.3.1. Splice variants
There are no variants of mGlu4 receptors. Five splice variants of mGlu7 receptors have been described so far (mGlu7a–e). In mGlu7b, alternative splice originates from an out-of-frame insertion in the C-terminus domain, which results in the replacement of the last 16 amino acids of mGlu7a with 23 amino acids (Flor et al., 1997; Corti et al., 1998). Expression of mGlu7 receptors in non-neuronal tissue (see below) is restricted to variants mGlu7c and mGlu7d (Schulz et al., 2002). A splice variant of mGlu8 receptors (mGlu8b) has been described in rats and humans, in which an out-of-frame insertion of 55 bp results in the substitution of the last 16 amino acids of mGlu8a with 16 different amino acids (Corti et al., 1998; Malherbe et al., 1999). An additional variant has been described in the human mGlu8 receptor, in which an insertion of 74 bp introduces a frame shift in the predicted translation resulting in termination of the polypeptide before the 7-TM region (Malherbe et al., 1999).
3.4.3.2. Signal transduction and interacting proteins
mGlu4, mGlu7, and mGlu8 receptors are coupled to Gi/Go proteins in heterologous expression systems (reviewed by Pin and Duvoisin, 1995; Niswender and Conn, 2010). Activation of the MAPK and PtdIns-3-K pathways mediated by native mGlu4 receptors has been reported in cultured cerebellar granule cells (Iacovelli et al., 2002). mGlu7 receptors bind to a number of interacting proteins that include filamin A, protein phosphatase 1C, protein interacting with protein kinase C 1 (PICK1), syntenin, the βγ subunits of G proteins, and MacMARCKS (macrophage myristoylated alanine rich C kinase substrate) (Boudin et al., 2000; El Far et al., 2001; Enz and Croci, 2003; Bertaso et al., 2006). Proteins interacting with mGlu8 receptors include Band 4.1, which facilitates cell surface receptor expression and inhibits mGlu8-receptor signalling (Rose et al., 2008), and Pias-1 and other proteins of the sumoylation cascade (Tang et al., 2005).
3.4.3.3. Functional anatomy
mGlu4, mGlu7, and mGlu8 receptors are localized presynaptically at the active zone of neurotransmitter release. Synaptic glutamate can therefore activate all three receptor subtypes, thus negatively regulating its own release (reviewed by Niswender and Conn, 2010). However, glutamate binds with relatively high affinity to mGlu4 and mGlu8 receptors, but displays very low affinity for mGlu7 receptors (reviewed by Schoepp et al., 1999). This suggests that mGlu7 receptors can only be recruited by high concentrations of glutamate released under conditions of high synaptic activity. Not surprisingly, mice lacking mGlu7 receptors show an increased susceptibility to epileptic seizures (Sansig et al., 2001). A presynaptic integration exists among mGlu7 receptors, GABAB receptors and A1 adenosine receptors, which functionally interact in reducing glutamate release mediated by N-type voltage-sensitive Ca2+ channels (Martín et al., 2008). However, repeated agonist exposure discloses a facilitatory mGlu7 signal on glutamate release, which is mediated by stimulation of PI hydrolysis and translocation of munc-13-1, a protein that is essential for priming of synaptic vesicles (Martín et al., 2010). Thus, the regulation of glutamate release by mGlu7 receptors is not univocal and is strictly activity-dependent.
The mGlu4 receptor is highly expressed in the cerebellum, where its activation inhibits glutamate release at the synapse between parallel fibers (the axons of cerebellar granule cells) and Purkinje cells. High-to-moderate levels of expression are also found in the thalamus, basal ganglia, and olfactory bulb. mGlu4 receptors found in nerve terminals of striatal projection neurons innervating the external globus pallidus are particularly relevant from a clinical standpoint (see below). A truncated form of mGlu4 receptors lacking a great portion of the glutamate binding domain has been found in taste buds (Chaudhari et al., 2009). mGlu4 receptors were also present in medulloblastoma cells (Iacovelli et al., 2006), immune cells (see below), and in pancreatic α-cells, where they inhibit glucagons secretion (Uehara et al., 2004). mGlu7 are highly expressed in the CNS, and are also found in peripheral organs. mGlu7 receptors are found in hair cells and in spiral ganglion cells of the inner ear, and common single nucleotide polymorphisms (SNPs) of GRM7 are strongly associated with the risk of developing age-related hearing impairment (presbycusis), the most prevalent sensory impairment in the elderly (Friedman et al., 2009). Recent findings suggest that mGlu7 receptors are expressed in the colon mucosa, where receptor activation increases fecal water content in a stress-induced defecation paradigm (Julio-Pieper et al., in press). It has been suggested that mGlu7 receptors play a role in functional disorders of the gut, such as the irritable bowel syndrome (IBS), which are often associated with chronic stress.
The mGlu8 receptor is widely, but unevenly, distributed in the CNS. For example, mGlu8-receptor expressing nerve terminals target specific subsets of GABAergic neurons in the hippocampus (Ferraguti et al., 2005).
3.4.3.4. Pharmacology
L-AP4 and L-SOP (an endogenous product of phospholipids cleavage which accumulates in the Alzheimer’s brain) are the prototypical, non-subtype-selective orthosteric agonists of group-III mGlu receptors (reviewed by Schoepp et al., 1999). As outlined in the Historical background, these two compounds are also able to block excitatory amino acid-stimulated PI hydrolysis in brain slices (Nicoletti et al., 1986a). Whether this particular effect derives from an interaction between group-I and group-III mGlu receptors is unknown. (1S,3R,4S)-1-Amino-cyclopentane-1,3,4-tricarboxylic acid ACTP-I and (R,S)-4-phosphonophenylglycine (PPG) also behave as non-subtype-selective orthosteric agonists of group-III mGlu receptors (reviewed by Schoepp et al., 1999). In spite of the new insights into the activation mechanisms of group-III mGlu receptors (Selvam et al., 2007; Frauli et al., 2007), there are only a few orthosteric agonists that can discriminate between mGlu4 and mGlu8 receptors. (S)-3,4-dicarboxyphenylglycine (DCPG) has ≥100 fold greater affinity for mGlu8 receptors than for all other group-III mGlu receptor subtypes (Thomas et al., 2001), and is widely used as a tool to selectively activate mGlu8 receptors. The available orthosteric antagonists of group-III mGlu receptors display low receptor affinity or lack specificity. For example, (R,S)-α-cyclopropyl-4-phosphonophenylglycine (CPPG), which is often used as an orthosteric antagonist of group-III mGlu receptors, can also antagonize group-II mGlu receptors with nanomolar affinity (Toms et al., 1996). N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC) is the prototype of a growing list of selective mGlu4 receptor PAMs that hold promise for the treatment of Parkinson’s disease and other disorders (reviewed by Niswender and Conn, 2010). Because of its structure similarity with CPCCOEt, PHCCC can also have off-target effects by blocking mGlu1 receptors at high concentrations. The mGlu5-receptor NAMs, MPEP and SIB1893, also behave as mGlu4 receptor PAMs in the high micromolar range (Mathiesen et al., 2003). Only few drugs behave as selective ligands of mGlu7 receptors. These include the “allosteric agonist” N,N′-Bis(diphenylmethyl)-1,2-ethanediamine dihydrochloride (AMN082), which directly activate mGlu7 receptors by interacting with a site different from the glutamate binding site, and the putative mGlu7 receptor NAM, 6-(4-methoxyphenyl)-5-methyl-3-(4-pyridinyl)-isoxazolo [4,5]pyridine-4(5H)-one (MMPIP) (Mitsukawa et al., 2005; Suzuki et al., 2007). A bias inherent to the use of AMN082 is that the drug induces a rapid internalization of mGlu7 receptors (Pelkey et al., 2007). This may partly explain some unexpected findings in experiments using AMN082 (see below).
3.4.3.5. Relevance to human disorders and clinical perspectives
3.4.3.5.1. Parkinson’s disease
Activation of mGlu4 receptors inhibits GABA release at the synapse between stratial projection neurons and neurons of the external globus pallidus. This is the first synapse of the indirect pathway of the basal ganglia motor circuit, which is overactive in Parkinson’s disease (reviewed by Conn et al., 2005). Drugs that activate mGlu4 receptors including orthosteric agonists and selective PAMs cause a robust depression of inhibitory synaptic transmission in the external globus pallidus and relieve motor symptoms in animal models of parkinsonism (Marino et al., 2003; Valenti et al., 2003; Niswender et al., 2008; Beurrier et al., 2009). Because the inhibitory action of mGlu4 receptors on synaptic transmission in the external globus pallidus is not affected by dopamine loss, one can predict that mGlu4 receptor agonists retain their therapeutic activity in Parkinsonian patients for a long time without causing dyskinesias or other motor side effects that typically develop in response to L-DOPA or other dopaminergic drugs. It is now believed that during the early “compensated” phase of Parkinson’s disease a number of mechanisms reduce the activity of the indirect pathway. These include an increased production of endogenous opioids, which suppress synaptic transmission in the external globus pallidus by activating presynaptic μ opioid receptors (Sandyk, 1988; Schroeder and Schneider, 2002). Whether mGlu4 receptors undergo plastic modification in this specific setting is unknown. An early treatment with mGlu4 receptor agonists might reinforce the defensive mechanisms in the early phases of Parkinson’s disease, thus delaying the clinical onset of the disorder. Activation of mGlu4 receptors might produce a dual benefit in Parkinson’s disease by relieving motor symptoms and attenuating the progressive degeneration of nigro-striatal dopa-minergic neurons at the same time. In mice challenged with the toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), systemic treatment with PHCCC reduces the degeneration of nigro-striatal dopaminergic neurons. PHCCC retains its protective activity when locally injected in the external globus pallidus, suggesting that inhibition of synaptic transmission in the external globus pallidus leads to a reduced activity of excitatory neurons of the subthalamic nucleus, thereby inducing neuroprotection (Battaglia et al., 2004). However, mGlu4 receptors also reduce excitatory synaptic transmission in the pars compacta of the substantia nigra, a mechanism that may contribute to restrain excitotoxic death of nigral dopaminergic neurons (Valenti et al., 2005). Finally, preclinical data raise the interesting possibility that mGlu4 receptor agonists may be helpful for the prophylaxis of drug-induced parkinsonism occurring in schizophrenic patients receiving first-generation antipsychotic drugs. These patients are usually treated with anticholinergic drugs, which, however, cause autonomic adverse effects and cognitive dysfunctions.
3.4.3.5.2. Absence epilepsy
Absence seizures, which are characterized by synchronous spike-and-wave discharges (SWDs) at the EEG, are generated by an abnormal oscillatory activity of a thalamocortical loop that comprises ventrobasal thalamic nuclei, the cerebral cortex, and the reticular thalamic nucleus (RTN) (reviewed by Blumenfeld, 2005). Drugs that block T-type voltage-sensitive Ca2+ channels, such as ethosuximide and valproate, as well as clonazepam and lamotrigine are commonly used in the treatment of absence epilepsy; however, these drugs cause class-related adverse effects as sedation and ataxia, and >20% of patients with absence seizures are resistant to medication. Recent evidence suggests that mGlu receptors in general, and group-III mGlu receptors in particular, are novel potential targets for treatment of absence epilepsy. Genetic deletion of mGlu4 receptors or intra-RTN injection of a group-III mGlu receptor antagonist protects mice against absence seizures induced by low doses of pentylentetrazol (Snead et al., 2000). In addition, WAG/Rij rats, which spontaneously develop SWDs after 2–3 months of age, show an increased expression of mGlu4 receptors in the RTN, and respond to pharmacological activation of mGlu4 receptors with an increased frequency of absence seizures (Ngomba et al., 2008). Interestingly, the GRM4 gene is localized within the EJM1 susceptibility focus for juvenile myoclonic epilepsy (Wong et al., 2001; Izzi et al., 2003), which is characterized by absence seizures combined with myoclonic and tonic–clonic seizures. These data stimulate the search for molecules that selectively antagonize mGlu4 receptors as potential anti-absence drugs.
Recent evidence indicates that absence seizures develop in mice under conditions that disrupt the interaction of mGlu7 receptors with PICK1 (Bertaso et al., 2008). In addition, mutant mice lacking the PDZ domain in mGlu7 receptors (necessary for the interaction with PICK1 and other proteins) are more sensitive to pentylentetrazol-induced absence seizures (Zhang et al., 2008a,b). This suggests that interaction between mGlu7 receptors and PICK1 is protective against paroxysmal activity of the thalamocortical network underlying absence seizures.
3.4.3.5.3. Pain
Recent evidence suggests that group-III mGlu receptors regulate pain transmission and are potential targets for analgesic drugs (reviewed by Goudet et al., 2009). However, the individual role of mGlu4, mGlu7, and mGlu8 receptors in the regulation of pain threshold is not univocal. Intrathecal injection of PHCCC causes analgesia in models of inflammatory or neuropathic pain (Goudet et al., 2008), suggesting that mGlu4 receptors in the spinal cord negatively modulate pain transmission. The role of mGlu7 and mGlu8 receptors has been investigated by experiments with microinfusion of subtype-selective ligands in two supraspinal centers that lie along the pain neuraxis, i.e. the amygdala and the periaqueductal grey. In both regions, activation of mGlu7 receptors with the “allosteric agonist” AMN082 amplifies nocifensive behavior, whereas selective activation of mGlu8 receptors with DCPG produces opposite effects (Marabese et al., 2007a; Palazzo et al., 2008). Systemic treatment with DCPG also causes analgesia (Marabese et al., 2007b). Thus, compounds that activate mGlu4 and mGlu8 receptors but have no activity at mGlu7 receptors can be developed as novel analgesic drugs.
3.4.3.5.4. Anxiety
A role for group-IIII mGlu receptors in anxiety was suggested by the evidence that intrahippocampal injection of the non-subtype-selective agonist, L-SOP, had an anticonflict effect in the rat Vogel test (Tatarczyńska et al., 2001). Both mGlu8 and mGlu4 receptors appear to negatively modulate anxiety in rodents. mGlu8-receptor knockout mice exposed to the elevated plus maze test showed anxiety-related behavior and a higher number of c-Fos-positive neurons in the centromedial thalamic nucleus as compared to wild-type mice (Linden et al., 2002, 2003; Duvoisin et al., 2005). In addition, the mGlu8-receptor agonist, DCPG, and the novel mGlu8-receptor enhancer, AZ12216052, reduced anxiety-like behavior in mice (Duvoisin et al., 2010; see Stachowicz et al., 2005 for contrasting data). Selective activation of mGlu4 receptors in the rat amygdala produced an anticonflict effect in the Vogel test (see Linden and Schoepp, 2006 for a comprehensive review). The role of mGlu7 receptors in anxiety is uncertain because treatment with the mGlu7 receptor agonist, AMN082, relieves anxiety and genetic deletion of mGlu7 receptors also relieves anxiety (reviewed by Lavreysen and Dautzenberg, 2008). Functional antagonism of mGlu7 receptors by AMN082 resulting from receptor internalization (see above) may help reconcile these contrasting findings.
3.4.3.5.5. Neuroinflammation and regulation of the immune system
A report by Fallarino et al. (2010) discloses a new mechanism that highlights the cross-talk between the nervous and immune systems. These authors have shown that activation of mGlu4 receptors expressed on dendritic cells, a cell type crucial for supporting an immune response, drives the differentiation of antigen-specific T cells into T regulatory (Treg) cells at the expenses of autoreactive TH17 cells, thus reinforcing mechanisms of immune tolerance. Mice lacking mGlu4 receptors immunized with a myelin-specific antigen develop a more severe experimental autoimmune encephalomyelitis (EAE), whereas wild-type mice treated with the mGlu4 receptor PAM, PHCCC, are protected against EAE. Remarkably, PHCCC administered at the onset of motor symptoms reduces the frequency and severity of relapses in a relapsing-remitting model of EAE. These data are particularly relevant to the treatment of relapsing-remitting multiple sclerosis, a demyelinating disorder of the CNS mediated inter alia by autoreactive clones of and TH17 cells. The central role of mGlu4 receptors in the immunological synapses between dendritic cells and T lymphocytes (Fallarino et al., 2010; see also Hansen and Caspi, 2010) suggests a therapeutic activity of mGlu4 receptor enhancers in autoimmune disorders other than multiple sclerosis.
3.4.3.5.6. Smith–Lemli–Opitz syndrome and retinitis pigmentosa
GRM8, the human gene encoding the mGlu8 receptor, encompasses approximately 1000 kb of DNA at the boundary of the q31.3–q32.1 bands of chromosome 7, the same region where the Smith–Lemli–Opitz syndrome and an autosomal form of retinite pigmentosa (Scherer et al., 1997). The Smith–Lemli–Opitz syndrome is a relatively rare developmental disorder due to deficiency of the enzyme 7-dehydrocholesterol reductase. Retinite pigmentosa is a group of genetic eye disorders characterized by abnormalities of photoreceptors or retinal pigment epithelium. It should be highlighted that the mGlu8 receptor has been first identified in the retina (Duvoisin et al., 1995), where it shows multiple localizations (Quraishi et al., 2007). Interestingly, mGlu8 receptors are found in photoreceptor nerve terminals, where they modulate neurotransmitter release by interacting with calcium channels (Koulen et al., 1999, 2005). Whether abnormalities of mGlu8 receptors are related to the progressive blindness associated with autosomal retinite pigmentosa remains to be established.
3.5. mGlu6 receptor
Gene name: GRM6 (human); Grm6 (rat, mouse). Accession numbers: NP_000834 (human); NP_075209 (rat); NP_775548 (mouse). Chromosomal location: 5q35 (human); 10 (rat); 11B1.3 (mouse) (Nakajima et al., 1993; Hashimoto et al., 1997; Laurie et al., 1997).
3.5.1. Splice variants
mGlu6 receptor variants have been identified in rats and humans. The rat receptor variant is characterized by an additional exon of 88 nucleotides which contains an in frame stop codon, resulting in a truncated protein of 508 amino acids. The two human variants lack 97 nucleotides of exon 6 and include 5 nucleotides of intron 5, respectively. mRNAs of these two variants encode truncated proteins of 425 and 405 amino acids, respectively. All truncated variants lack the 7-TM and the C-terminus domain of the receptor (Valerio et al., 2001).
3.5.2. Pharmacology
The mGlu6 receptor exhibits the same pharmacological profile as other group-III mGlu receptors, being activated by L-AP4, L-SOP, and PPG, and antagonized by CPPG, (RS)-α-methylserine-O-phosphate (MSOP), and (S)-2-amino-2-methyl-4-phosphonobutanoic acid (MAP4).
3.5.3. Functional anatomy and relevance to human disorders
The mGlu6 receptor is exclusively localized in the dendrites of ON-bipolar cells of the retina and responds to glutamate released from rod and cone photoreceptor cells in the dark (Nakajima et al., 1993; Nomura et al., 1994; Vardi et al., 2000). Activation of mGlu6 receptors reduces excitability of ON-bipolar cells by negatively regulating a membrane non-selective cation channel, which likely corresponds to the TrpM1 channel (Shen et al., 2009; Morgans et al., 2009; Koike et al., 2010). This effect is mediated by a Go protein-dependent activation of the Ca2+-stimulated protein phosphatase, calcineurin (Dhingra et al., 2004; Nawy, 1999, 2000) (Fig. 4). Light reduces glutamate stimulation of mGlu6 receptors, leading to opening of cation channels and depolarization of ON-bipolar cells. The effect of light is amplified by inactivation of the mGlu6 receptor signalling mediated by the transmembrane protein R9AP, which facilitates the GTPase activity of RGS11 complexed with the Gβ5 subunit of Go (Cao et al., 2009; Masuho et al., 2010; Zhang et al., 2010). mGlu6 receptor knockout mice show a loss of ON responses but an unchanged response to light (Masu et al., 1995), and a mouse screened for the lack of the b-wave at the electroretinogram showed a splice error in the Grm6 and the lack of mGlu6 receptors in the retina (Maddox et al., 2008). The b-wave of the electroretinogram reflects the light-induced depolarization of ON-bipolar cells (Stockton and Slaughter, 1989). Interestingly, mutations in the gene encoding the mGlu6 receptor is associated with autosomal recessive congenital stationary night blindness characterized by abnormal electroretinogram ON responses in humans (Dryja et al., 2005; Zeitz et al., 2005; O’Connor et al., 2006). Mutations of the TrpM1 gene are also associated with autosomal recessive congenital stationary night blindness (Van Genderen et al., 2009). In conclusion, these are exciting times for mGlu receptors. After 25 years we can truly believe that subtype-selective mGlu receptor ligands will soon enter the clinic. Perhaps the treatment of FXS will be the launching platform for the use of mGlu drugs in highly prevalent disorders, such as schizophrenia, Parkinson’s disease, chronic pain, and drug addiction. It is sad that Prof. Costa will not be a witness of the clinical era of mGlu receptors.
Fig. 4.

Localization and function of mGlu6 receptors in retinal ON-bipolar cells. mGlu6 receptors are present in the dendrites of the ON-bipolar cells of the retina, where their activation negatively modulates TrpM1 channels through a chain of events that include intracellular calcium release and activation of the protein phosphatase, calcineurin.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group II metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Ahn KC, Bernier BE, Harnett MT, Morikawa H. IP3 receptor sensitization during in vivo amphetamine experience enhances NMDA receptor plasticity in dopamine neurons of the ventral tegmental area. J Neurosci. 2010;30:6689–6699. doi: 10.1523/JNEUROSCI.4453-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Alagarsamy S, Saugstad J, Warren L, Mansuy IM, Gereau RW, 4th, Conn PJ. NMDA-induced potentiation of mGluR5 is mediated by activation of protein phosphatase 2B/calcineurin. Neuropharmacology. 2005;49 (Suppl 1):135–145. doi: 10.1016/j.neuropharm.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altinbilek B, Manahan-Vaughan D. A specific role for group II metabotropic glutamate receptors in hippocampal long-term depression and spatial memory. Neuroscience. 2009;158:149–158. doi: 10.1016/j.neuroscience.2008.07.045. [DOI] [PubMed] [Google Scholar]
- Ambrosi G, Armentero MT, Levandis G, Bramanti P, Nappi G, Blandini F. Effects of early and delayed treatment with an mGluR5 antagonist on motor impairment, nigrostriatal damage and neuroinflammation in a rodent model of Parkinson’s disease. Brain Res Bull. 2010;82:29–38. doi: 10.1016/j.brainresbull.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Ansah OB, Bourbia N, Gonçalves L, Almeida A, Pertovaara A. Influence of amygdaloid glutamatergic receptors on sensory and emotional pain-related behavior in the neuropathic rat. Behav Brain Res. 2010;209:174–178. doi: 10.1016/j.bbr.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Ansah OB, Gonçalves L, Almeida A, Pertovaara A. Enhanced pronoci-ception by amygdaloid group I metabotropic glutamate receptors in nerve-injured animals. Exp Neurol. 2009;216:66–74. doi: 10.1016/j.expneurol.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Arcella A, Carpinelli G, Battaglia G, D’Onofrio M, Santoro F, Ngomba RT, Bruno V, Casolini P, Giangaspero F, Nicoletti F. Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo. Neuro Oncol. 2005;7:236–245. doi: 10.1215/S1152851704000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Jansen GH, van Veelen CW, van Rijen PC, Ramkema M, Troost D. Expression and cell distribution of group I and group II metabotropic glutamate receptor subtypes in taylor-type focal cortical dysplasia. Epilepsia. 2003;44:785–795. doi: 10.1046/j.1528-1157.2003.54802.x. [DOI] [PubMed] [Google Scholar]
- Attucci S, Carlà V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br J Pharmacol. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs R, Miller S, Romano C, de Vries A, Chun Y, Cotman CW. Metabotropic glutamate receptor mGluR5 in astrocytes: pharmacological properties and agonist regulation. J Neurochem. 1997;69:151–163. doi: 10.1046/j.1471-4159.1997.69010151.x. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Woolley ML, Prinssen E, Huwyler J, Porter R, Spooren W. The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison. Psychopharmacology (Berl) 2005;179:218–229. doi: 10.1007/s00213-005-2211-9. [DOI] [PubMed] [Google Scholar]
- Barbon A, Ferraboli S, Barlati S. Assignment of the human metabotropic glutamate receptor gene GRM4 to chromosome 6 band p21.3 by radiation hybrid mapping. Cytogenet Cell Genet. 2000a;88:210. doi: 10.1159/000015551. [DOI] [PubMed] [Google Scholar]
- Barbon A, Ferraboli S, Barlati S. Assignment of the human metabotropic glutamate receptor gene GRM7 to chromosome 3p26.1 → p25.2 by radiation hybrid mapping. Cytogenet Cell Genet. 2000b;88:288. doi: 10.1159/000015541. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. doi: 10.1038/385074a0. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Knöpfel T, Gasparini F, Garthwaite J. Pharmacological characterization of synaptic transmission through mGluRs in rat cerebellar slices. Neuropharmacology. 1997;36:401–403. doi: 10.1016/s0028-3908(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci Lett. 1997;229:161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, De Blasi A, Melchiorri D, Nicoletti F. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci. 2002;22:2135–2141. doi: 10.1523/JNEUROSCI.22-06-02135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Busceti CL, Pontarelli F, Biagioni F, Fornai F, Paparelli A, Bruno V, Ruggieri S, Nicoletti F. Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45:155–166. doi: 10.1016/s0028-3908(03)00146-1. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Busceti CL, Molinaro G, Biagioni F, Storto M, Fornai F, Nicoletti F, Bruno V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24:828–835. doi: 10.1523/JNEUROSCI.3831-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Molinaro G, Riozzi B, Storto M, Busceti CL, Spinsanti P, Bucci D, Di Liberto V, Mudò G, Corti C, Corsi M, Nicoletti F, Belluardo N, Bruno V. Activation of mGlu3 receptors stimulates the production of GDNF in striatal neurons. PLoS One. 2009;4 (8):e6591. doi: 10.1371/journal.pone.0006591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Cannella M, Riozzi B, Orobello S, Maat-Schieman ML, Aronica E, Busceti CL, Ciarmiello A, Alberti S, Amico E, Sassone J, Sipione S, Bruno V, Frati L, Nicoletti F, Squitieri F. Early defect of transforming growth factor beta-1 formation in Huntington’s disease. J Cell Mol Med. 2010 Jan 15; doi: 10.1111/j.1582-4934.2010.01011.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at peri-synaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Bertaso F, Lill Y, Airas JM, Espeut J, Blahos J, Bockaert J, Fagni L, Betz H, El-Far O. MacMARCKS interacts with the metabotropic glutamate receptor type 7 and modulates G protein-mediated constitutive inhibition of calcium channels. J Neurochem. 2006;99:288–298. doi: 10.1111/j.1471-4159.2006.04121.x. [DOI] [PubMed] [Google Scholar]
- Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, Marin P, Huganir RL, Betz H, Bockaert J, Fagni L, Lerner-Natoli M. PICK1 uncoupling from mGluR7a causes absence-like seizures. Nat Neurosci. 2008;11:940–948. doi: 10.1038/nn.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49 (Suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bessis AS, Rondard P, Gaven F, Brabet I, Triballeau N, Prézeau L, Acher F, Pin JP. Closure of the Venus flytrap module of mGlu8 receptor and the activation process: insights from mutations converting antagonists into agonists. Proc Natl Acad Sci U S A. 2002;99:11097–11102. doi: 10.1073/pnas.162138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Lopez S, Révy D, Selvam C, Goudet C, Lhérondel M, Gubellini P, Kerkerian-LeGoff L, Acher F, Pin JP, Amalric M. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23:3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RW., 4th Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;2001 (4):417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Bikbaev A, Neyman S, Ngomba RT, Conn PJ, Nicoletti F, Manahan-Vaughan D. MGluR5 mediates the interaction between late-LTP, network activity, and learning. PLoS One. 2008;3 (5):e2155. doi: 10.1371/journal.pone.0002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet V, Duthey B, Lecaillon J, Vol C, Quoyer J, Labesse G, Pin JP, Prézeau L. Common structural requirements for heptahelical domain function in class A and class C G protein-coupled receptors. J Biol Chem. 2007;282:12154–12163. doi: 10.1074/jbc.M611071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Reid CA, Chen F, Tan HO, Petrou S, Lawrence AJ. Cocaine-mediated synaptic potentiation is absent in VTA neurons from mGlu5-deficient mice. Int J Neuropsychopharmacol. 2010;13:133–141. doi: 10.1017/S1461145709990162. [DOI] [PubMed] [Google Scholar]
- Birse EF, Eaton SA, Jane DE, Jones PL, Porter RH, Pook PC, Sunter DC, Udvarhelyi PM, Wharton B, Roberts PJ, Salt TE, Watkins JC. Phenylglycine derivatives as new pharmacological tools for investigating the role of metabotropic glutamate receptors in the central nervous system. Neuroscience. 1993;52:481–488. doi: 10.1016/0306-4522(93)90400-a. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Ellingrod VL, Moline J, Miller D. Association between the polymorphic GRM3 gene and negative symptom improvement during olanzapine treatment. Schizophr Res. 2005;77:253–260. doi: 10.1016/j.schres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Wang K, Moline J, Ellingrod VL. Association analysis of the metabotropic glutamate receptor type 3 gene (GRM3) with schizophrenia. Psychiatr Genet. 2007;17:358. doi: 10.1097/YPG.0b013e3281ac231e. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;9:21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Carboni L, Cobb MH, Siegelbaum SA, Belardetti F. Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nat Neurosci. 2000;3:1107–1112. doi: 10.1038/80624. [DOI] [PubMed] [Google Scholar]
- Bond A, Jones NM, Hicks CA, Whiffin GM, Ward MA, O’Neill MF, Kingston AE, Monn JA, Ornstein PL, Schoepp DD, Lodge D, O’Neill MJ. Neuroprotective effects of LY379268, a selective mGlu2/3 receptor agonist: investigations into possible mechanism of action in vivo. J Pharmacol Exp Ther. 2000;294:800–809. [PubMed] [Google Scholar]
- Bond A, Ragumoorthy N, Monn JA, Hicks CA, Ward MA, Lodge D, O’Neill MJ. LY379268, a potent and selective group II metabotropic glutamate receptor agonist, is neuroprotective in gerbil global, but not focal, cerebral ischaemia. Neurosci Lett. 1999;273:191–194. doi: 10.1016/s0304-3940(99)00663-1. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A, Bernardi G, Pisani A. Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology. 2008;55:392–395. doi: 10.1016/j.neuropharm.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collingridge GL. Involvement of calcium/calmodulin-dependent protein kinases in the setting of a molecular switch involved in hippocampal LTP. Neuropharmacology. 1998;37:535–544. doi: 10.1016/s0028-3908(98)00058-6. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collingridge GL. A role for protein kinase C in a form of metaplasticity that regulates the induction of long-term potentiation at CA1 synapses of the adult rat hippocampus. Eur J Neurosci. 2000;12:4055–4062. doi: 10.1046/j.1460-9568.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collett VJ, Conquet F, Jia Z, van der Putten H, Collingridge GL. The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology. 2005;49 (Suppl 1):13–25. doi: 10.1016/j.neuropharm.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collett VJ, Conquet F, Jia Z, Collingridge GL. An analysis of the stimulus requirements for setting the molecular switch reveals a lower threshold for metaplasticity than synaptic plasticity. Neuropharmacology. 2008;55:454–458. doi: 10.1016/j.neuropharm.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Boudin H, Doan A, Xia J, Shigemoto R, Huganir RL, Worley P, Craig AM. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28:485–497. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Breysse N, Amalric M, Salin P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J Neurosci. 2003;23:8302–8309. doi: 10.1523/JNEUROSCI.23-23-08302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M. Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci. 2002;22:5669–5678. doi: 10.1523/JNEUROSCI.22-13-05669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin J, Busse C, Sukoff SJ, Varney MA. Anxiolytic-like activity of the mGluR5 antagonist MPEP: a comparison with diazepam and buspirone. Pharmacol Biochem Behav. 2002;73:359–366. doi: 10.1016/s0091-3057(02)00828-6. [DOI] [PubMed] [Google Scholar]
- Brody SA, Conquet F, Geyer MA. Disruption of prepulse inhibition in mice lacking mGluR1. Eur J Neurosci. 2003;18:3361–3366. doi: 10.1111/j.1460-9568.2003.03073.x. [DOI] [PubMed] [Google Scholar]
- Brody SA, Dulawa SC, Conquet F, Geyer MA. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry. 2004;9:35–41. doi: 10.1038/sj.mp.4001404. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, Giffard RG, Raciti G, Raffaele R, Shinozaki H, Nicoletti F. Activation of class II or III metabotropic glutamate receptors protects cultured cortical neurons against excitotoxic degeneration. Eur J Neurosci. 1995;7:1906–1913. doi: 10.1111/j.1460-9568.1995.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Casabona G, Copani A, Caciagli F, Nicoletti F. Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-beta. J Neurosci. 1998;18:9594–9600. doi: 10.1523/JNEUROSCI.18-23-09594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, D’Onofrio M, Di Iorio P, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology. 2004;29:1971–1979. doi: 10.1038/sj.npp.1300540. [DOI] [PubMed] [Google Scholar]
- Calò L, Bruno V, Spinsanti P, Molinari G, Korkhov V, Esposito Z, Patanè M, Melchiorri D, Freissmuth M, Nicoletti F. Interactions between ephrin-B and metabotropic glutamate 1 receptors in brain tissue and cultured neurons. J Neurosci. 2005;25:2245–2254. doi: 10.1523/JNEUROSCI.4956-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò L, Cinque C, Patanè M, Schillaci D, Battaglia G, Melchiorri D, Nicoletti F, Bruno V. Interaction between ephrins/Eph receptors and excitatory amino acid receptors: possible relevance in the regulation of synaptic plasticity and in the pathophysiology of neuronal degeneration. J Neurochem. 2006;98:1–10. doi: 10.1111/j.1471-4159.2006.03844.x. [DOI] [PubMed] [Google Scholar]
- Cao Y, Masuho I, Okawa H, Xie K, Asami J, Kammermeier PJ, Maddox DM, Furukawa T, Inoue T, Sampath AP, Martemyanov KA. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29:9301–9313. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl) 2004;175:310–318. doi: 10.1007/s00213-004-1827-5. [DOI] [PubMed] [Google Scholar]
- Cappuccio I, Spinsanti P, Porcellini A, Desiderati F, De Vita T, Storto M, Capobianco L, Battaglia G, Nicoletti F, Melchiorri D. Endogenous activation of mGlu5 metabotropic glutamate receptors supports self-renewal of cultured mouse embryonic stem cells. Neuropharmacology. 2005;49 (Suppl 1):196–205. doi: 10.1016/j.neuropharm.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Caraci F, Battaglia G, Bruno V, Bosco P, Carbonaro V, Giuffrida ML, Drago F, Sortino MA, Nicoletti F, Copani A. TGF-beta1 pathway as a new target for neuroprotection in Alzheimer’s disease. CNS Neurosci Ther. 2009 doi: 10.1111/j.1755-5949.2009.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Du J, Zhou S. Group II metabotropic glutamate receptor activation on peripheral nociceptors modulates TRPV1 function. Brain Res. 2009;1248:86–95. doi: 10.1016/j.brainres.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FY, Stolle A, Beart PM, Voerste A, Brabet I, Mauler F, Joly C, Antonicek H, Bockaert J, Müller T, Pin JP, Prézeau L. BAY36-7620: a potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol Pharmacol. 2001;59:965–973. [PMC free article] [PubMed] [Google Scholar]
- Casabona G, Knöpfel T, Kuhn R, Gasparini F, Baumann P, Sortino MA, Copani A, Nicoletti F. Expression and coupling to polyphosphoinositide hydrolysis of group I metabotropic glutamate receptors in early postnatal and adult rat brain. Eur J Neurosci. 1997;9:12–17. doi: 10.1111/j.1460-9568.1997.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in l-DOPA-induced dyskinesia. Trends Neurosci. 2007;30:236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, Nakanishi S, Nakazato A. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46:457–467. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr. 2009;90:738S–742S. doi: 10.3945/ajcn.2009.27462H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003;22:5403–5411. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Chiechio S, Caricasole A, Barletta E, Storto M, Catania MV, Copani A, Vertechy M, Nicolai R, Calvani M, Melchiorri D, Nicoletti F. l-Ace-tylcarnitine induces analgesia by selectively up-regulating mGlu2 metabotropic glutamate receptors. Mol Pharmacol. 2002;61:989–996. doi: 10.1124/mol.61.5.989. [DOI] [PubMed] [Google Scholar]
- Chiechio S, Copani A, De Petris L, Morales ME, Nicoletti F, Gereau RW., 4th Transcriptional regulation of metabotropic glutamate receptor 2/3 expression by the NF-kappaB pathway in primary dorsal root ganglia neurons: a possible mechanism for the analgesic effect of l-acetylcarnitine. Mol Pain. 2006;2:20. doi: 10.1186/1744-8069-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RW, 4th, Copani A, Nicoletti F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol. 2009;75:1014–1020. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- Chiechio S, Copani A, Zammataro M, Battaglia G, Gereau RW, 4th, Nicoletti F. Transcriptional regulation of type-2 metabotropic glutamate receptors: an epigenetic path to novel treatments for chronic pain. Trends Pharmacol Sci. 2010;31:153–160. doi: 10.1016/j.tips.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Metabotropic glutamate receptor modulation of voltage-gated Ca2+ channels involves multiple receptor subtypes in cortical neurons. J Neurosci. 1996;16:36–45. doi: 10.1523/JNEUROSCI.16-01-00036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M, Yao Y, Blake TJ, Hampson DR, Johnson EC. The neuroactive peptide N-acetylaspartylglutamate is not an agonist at the metabotropic glutamate receptor subtype 3 of metabotropic glutamate receptor. J Pharmacol Exp Ther. 2009;330:212–219. doi: 10.1124/jpet.109.152553. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, Bruno V, Battaglia G, D’Alimonte I, D’Onofrio M, Nicoletti F, Caciagli F. Activation of A(1) adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia. 1999;27:275–281. [PubMed] [Google Scholar]
- Ciceroni C, Arcella A, Mosillo P, Battaglia G, Mastrantoni E, Oliva MA, Carpinelli G, Santoro F, Sale P, Ricci-Vitiani L, De Maria R, Pallini R, Giangaspero F, Nicoletti F, Melchiorri D. Type-3 metabotropic glutamate receptors negatively modulate bone morphogenetic protein receptor signaling and support the tumourigenic potential of glioma-initiating cells. Neuropharmacology. 2008;55:568–576. doi: 10.1016/j.neuropharm.2008.06.064. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Frankola K, Goldstein A, Thorsell A, Singley E, Eskay RL, Heilig M. Alcohol-induced neurodegeneration, suppression of transforming growth factor-beta, and cognitive impairment in rats: prevention by group II metabotropic glutamate receptor activation. Biol Psychiatry. 2010;67:823–830. doi: 10.1016/j.biopsych.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Raymond CR, Abraham WC. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C. Hippocampus. 1998;8:160–170. doi: 10.1002/(SICI)1098-1063(1998)8:2<160::AID-HIPO8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Collett VJ, Collingridge GL. Interactions between NMDA receptors and mGlu5 receptors expressed in HEK293 cells. Br J Pharmacol. 2004;142:991–1001. doi: 10.1038/sj.bjp.0705861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophrenia: moving beyond monoamine antagonists. Mol Interv. 2008;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Condé F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Conti V, Aghaie A, Cilli M, Martin N, Caridi G, Musante L, Candiano G, Castagna M, Fairen A, Ravazzolo R, Guenet JL, Puliti A. crv4, a mouse model for human ataxia associated with kyphoscoliosis caused by an mRNA splicing mutation of the metabotropic glutamate receptor 1 (Grm1) Int J Mol Med. 2006;18:593–600. [PubMed] [Google Scholar]
- Copani A, Bruno V, Battaglia G, Leanza G, Pellitteri R, Russo A, Stanzani S, Nicoletti F. Activation of metabotropic glutamate receptors protects cultured neurons against apoptosis induced by beta-amyloid peptide. Mol Pharmacol. 1995;47:890–897. [PubMed] [Google Scholar]
- Corti C, Restituito S, Rimland JM, Brabet I, Corsi M, Pin JP, Ferraguti F. Cloning and characterization of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. Eur J Neurosci. 1998;10:3629–3641. doi: 10.1046/j.1460-9568.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- Corti C, Sala CF, Yang F, Corsi M, Xuereb JH, Ferraguti F. Genomic organization of the human metabotropic glutamate receptor subtype 3. J Neurogenet. 2000;14:207–225. doi: 10.3109/01677060009084499. [DOI] [PubMed] [Google Scholar]
- Corti C, Crepaldi L, Mion S, Roth AL, Xuereb JH, Ferraguti F. Altered dimerization of metabotropic glutamate receptor 3 in schizophrenia. Biol Psychiatry. 2007a;62:747–755. doi: 10.1016/j.biopsych.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, Mugnaini M, Nicoletti F, Bruno V. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 2007b;27:8297–8308. doi: 10.1523/JNEUROSCI.1889-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Flatman JA, Ganong AH, Perkins MN. Effects of excitatory amino acid antagonists on evoked and spontaneous excitatory potentials in guinea-pig hippocampus. J Physiol. 1986;378:403–415. doi: 10.1113/jphysiol.1986.sp016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Anborgh PH, Murdoch B, Bhatia M, Nakanishi S, Ferguson SS. G protein-coupled receptor kinase-mediated desensitization of metabotropic glutamate receptor 1A protects against cell death. J Biol Chem. 2000;275:38213–38220. doi: 10.1074/jbc.M006075200. [DOI] [PubMed] [Google Scholar]
- de Novellis V, Siniscalco D, Galderisi U, Fuccio C, Nolano M, Santoro L, Cascino A, Roth KA, Rossi F, Maione S. Blockade of glutamate mGlu5 receptors in a rat model of neuropathic pain prevents early over-expression of pro-apoptotic genes and morphological changes in dorsal horn lamina II. Neuropharmacology. 2004;46:468–479. doi: 10.1016/j.neuropharm.2003.10.014. [DOI] [PubMed] [Google Scholar]
- de Novellis V, Mariani L, Palazzo E, Vita D, Marabese I, Scafuro M, Rossi F, Maione S. Periaqueductal grey CB1 cannabinoid and metabotropic glutamate subtype 5 receptors modulate changes in rostral ventromedial medulla neuronal activities induced by subcutaneous formalin in the rat. Neuroscience. 2005;134:269–281. doi: 10.1016/j.neuroscience.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Desai MA, Burnett JP, Mayne NG, Schoepp DD. Cloning and expression of a human metabotropic glutamate receptor 1 alpha: enhanced coupling on co-transfection with a glutamate transporter. Mol Pharmacol. 1995;48:648–657. [PubMed] [Google Scholar]
- Devon RS, Anderson S, Teague PW, Muir WJ, Murray V, Pelosi AJ, Blackwood DH, Porteous DJ. The genomic organisation of the metabotropic glutamate receptor subtype 5 gene, and its association with schizophrenia. Mol Psychiatry. 2001;6:311–314. doi: 10.1038/sj.mp.4000848. [DOI] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Faurobert E, Dascal N, Sterling P, Vardi N. A retinal-specific regulator of G-protein signaling interacts with Galpha(o) and accelerates an expressed metabotropic glutamate receptor 6 cascade. J Neurosci. 2004;24:5684–5693. doi: 10.1523/JNEUROSCI.0492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgi-Gerevini V, Melchiorri D, Battaglia G, Ricci-Vitiani L, Ciceroni C, Busceti CL, Biagioni F, Iacovelli L, Canudas AM, Parati E, De Maria R, Nicoletti F. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12:1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- Di Liberto V, Bonomo A, Frinchi M, Belluardo N, Mudò G. Group II metabotropic glutamate receptor activation by agonist LY379268 treatment increases the expression of brain derived neurotrophic factor in the mouse brain. Neuroscience. 2010;165:863–873. doi: 10.1016/j.neuroscience.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-Chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but no mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio M, Cuomo L, Battaglia G, Ngomba RT, Storto M, Kingston AE, Orzi F, De Blasi A, Di Iorio P, Nicoletti F, Bruno V. Neuroprotection mediated by glial group-II metabotropic glutamate receptors requires the activation of the MAP kinase and the phosphatidylinositol-3-kinase pathways. J Neurochem. 2001;78:435–445. doi: 10.1046/j.1471-4159.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier J, Trinquet E, Rondard P, Pin JP. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2010;25:1–12. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Berson EL, Fishman GA, Sandberg MA, Alexander KR, Derlacki DJ, Rajagopalan AS. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci U S A. 2005;102:4884–4889. doi: 10.1073/pnas.0501233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2008;33:1603–1610. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Zhang C, Ramonell K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J Neurosci. 1995;15:3075–3083. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Zhang C, Pfankuch TF, O’Connor H, Gayet-Primo J, Quraishi S, Raber J. Increased measures of anxiety and weight gain in mice lacking the group III metabotropic glutamate receptor mGluR8. Eur J Neurosci. 2005;22:425–436. doi: 10.1111/j.1460-9568.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Pfankuch T, Wilson JM, Grabell J, Chhajlani V, Brown DG, Johnson E, Raber J. Acute pharmacological modulation of mGluR8 reduces measures of anxiety. Behav Brain Res. 2010;212:168–173. doi: 10.1016/j.bbr.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SA, Jane DE, Jones PL, Porter RH, Pook PC, Sunter DC, Udvarhelyi PM, Roberts PJ, Salt TE, Watkins JC. Competitive antagonism at metabotropic glutamate receptors by (S)-4-carboxyphenylglycine and (RS)-alpha-methyl-4-carboxyphenylglycine. Eur J Pharmacol. 1993;244:195–197. doi: 10.1016/0922-4106(93)90028-8. [DOI] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Far O, Bofill-Cardona E, Airas JM, O’Connor V, Boehm S, Freissmuth M, Nanoff C, Betz H. Mapping of calmodulin and Gbetagamma binding domains within the C-terminal region of the metabotropic glutamate receptor 7A. J Biol Chem. 2001;276:30662–30669. doi: 10.1074/jbc.M102573200. [DOI] [PubMed] [Google Scholar]
- Emery AC, Pshenichkin S, Takoudjou GR, Grajkowska E, Wolfe BB, Wroblewski JT. The protective signaling of metabotropic glutamate receptor 1 is mediated by sustained, β-arrestin-1-dependent ERK phosphorylation. J Biol Chem. 2010;285:26041–26048. doi: 10.1074/jbc.M110.139899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emile L, Mercken L, Apiou F, Pradier L, Bock MD, Menager J, Clot J, Doble A, Blanchard JC. Molecular cloning, functional expression, pharmacological characterization and chromosomal localization of the human metabotropic glutamate receptor type 3. Neuropharmacology. 1996;35:523–530. doi: 10.1016/0028-3908(96)84622-3. [DOI] [PubMed] [Google Scholar]
- Enz R, Croci C. Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. Biochem J. 2003;372:183–191. doi: 10.1042/BJ20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Volpi C, Fazio F, Notartomaso S, Vacca C, Busceti C, Bicciato S, Battaglia G, Bruno V, Puccetti P, Fioretti MC, Nicoletti F, Grohmann U, Di Marco R. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat Med. 2010;16:897–902. doi: 10.1038/nm.2183. [DOI] [PubMed] [Google Scholar]
- Fazio F, Notartomaso S, Aronica E, Storto M, Battaglia G, Vieira E, Gatti S, Bruno V, Biagioni F, Gradini R, Nicoletti F, Di Marco R. Switch in the expression of mGlu1 and mGlu5 metabotropic glutamate receptors in the cerebellum of mice developing experimental autoimmune encephalomyelitis and in autoptic cerebellar samples from patients with multiple sclerosis. Neuropharmacology. 2008;55:491–499. doi: 10.1016/j.neuropharm.2008.06.066. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Klausberger T, Cobden P, Baude A, Roberts JD, Szucs P, Kinoshita A, Shigemoto R, Somogyi P, Dalezios Y. Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J Neurosci. 2005;25:10520–10536. doi: 10.1523/JNEUROSCI.2547-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Crepaldi L, Nicoletti F. Metabotropic glutamate 1 receptor: current concepts and perspectives. Pharmacol Rev. 2008;60:536–581. doi: 10.1124/pr.108.000166. [DOI] [PubMed] [Google Scholar]
- Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueño J, Gutiérrez MA, Casadó V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris P, Seward E, Dawson GR. Interactions between LY354740, a group II metabotropic agonist and the GABA(A)-benzodiazepine receptor complex in the rat elevated plus-maze. J Psychopharmacol. 2001;15:76–82. doi: 10.1177/026988110101500203. [DOI] [PubMed] [Google Scholar]
- Fijal BA, Kinon BJ, Kapur S, Stauffer VL, Conley RR, Jamal HH, Kane JM, Witte MM, Houston JP. Candidate-gene association analysis of response to risperidone in African-American and white patients with schizophrenia. Pharmacogenomics J. 2009;9:311–318. doi: 10.1038/tpj.2009.24. [DOI] [PubMed] [Google Scholar]
- Fisher K, Lefebvre C, Coderre TJ. Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol Biochem Behav. 2002;73:411–418. doi: 10.1016/s0091-3057(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Flor PJ, Lindauer K, Püttner I, Rüegg D, Lukic S, Knöpfel T, Kuhn R. Molecular cloning, functional expression and pharmacological characterization of the human metabotropic glutamate receptor type 2. Eur J Neurosci. 1995a;7:622–629. doi: 10.1111/j.1460-9568.1995.tb00666.x. [DOI] [PubMed] [Google Scholar]
- Flor PJ, Lukic S, Rüegg D, Leonhardt T, Knöpfel T, Kuhn R. Molecular cloning, functional expression and pharmacological characterization of the human metabotropic glutamate receptor type 4. Neuropharmacology. 1995b;34:149–155. doi: 10.1016/0028-3908(94)00149-m. [DOI] [PubMed] [Google Scholar]
- Flor PJ, Van Der Putten H, Rüegg D, Lukic S, Leonhardt T, Bence M, Sansig G, Knöpfel T, Kuhn RA. A novel splice variant of a metabotropic glutamate receptor, human mGluR7b. Neuropharmacology. 1997;36:153–159. doi: 10.1016/s0028-3908(96)00176-1. [DOI] [PubMed] [Google Scholar]
- Frauli M, Hubert N, Schann S, Triballeau N, Bertrand HO, Acher F, Neuville P, Pin JP, Prézeau L. Amino-pyrrolidine tricarboxylic acids give new insight into group III metabotropic glutamate receptor activation mechanism. Mol Pharmacol. 2007;71:704–712. doi: 10.1124/mol.106.030254. [DOI] [PubMed] [Google Scholar]
- Friedman RA, Van Laer L, Huentelman MJ, Sheth SS, Van Eyken E, Corneveaux JJ, Tembe WD, Halperin RF, Thorburn AQ, Thys S, Bonneux S, Fransen E, Huyghe J, Pyykkö I, Cremers CW, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning PH, Makmura L, Ohmen JD, Linthicum FH, Jr, Fayad JN, Pearson JV, Craig DW, Stephan DA, Van Camp G. GRM7 variants confer susceptibility to age-related hearing impairment. Hum Mol Genet. 2009;18:785–796. doi: 10.1093/hmg/ddn402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby CL, Mattsson JP, Jensen JM, Lehmann A, Dent J, Blackshaw LA. Inhibition of transient lower esophageal sphincter relaxation and gastroesophageal reflux by metabotropic glutamate receptor ligands. Gastroenterology. 2005 Sep;129 (3):995–1004. doi: 10.1053/j.gastro.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Coderre TJ. Attenuation of precipitated morphine withdrawal symptoms by acute i. c.v administration of a group II mGluR agonist. Br J Pharmacol. 1997;121:511–514. doi: 10.1038/sj.bjp.0701174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AA, L’Episcopo MR, Casabona G, Shinozaki H, Nicoletti F. (2S,1′R,2′R,3′R)-2-(2,3-dicarboxycyclopropyl) glycine positively modulates metabotropic glutamate receptors coupled to polyphosphoinositide hydrolysis in rat hippocampal slices. Brain Res. 1994;659:10–16. doi: 10.1016/0006-8993(94)90857-5. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Wolswijk G, Bö L, van der Valk P, Polman CH, Troost D, Aronica E. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain. 2003;126:1755–1766. doi: 10.1093/brain/awg179. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C, Gaven F, Kniazeff J, Vol C, Liu J, Cohen-Gonsaud M, Acher F, Prézeau L, Pin JP. Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors. Proc Natl Acad Sci U S A. 2004;101:378–383. doi: 10.1073/pnas.0304699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C, Chapuy E, Alloui A, Acher F, Pin JP, Eschalier A. Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain. 2008;137:112–124. doi: 10.1016/j.pain.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, 4th, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Winder DG. Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:1302–1311. doi: 10.1038/sj.npp.1300672. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol. 2004;74:271–300. doi: 10.1016/j.pneurobio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain. 2005;113:211–222. doi: 10.1016/j.pain.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Caspi RR. Glutamate joins the ranks of immunomodulators. Nat Med. 2010;16:856–858. doi: 10.1038/nm0810-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Inazawa J, Okamoto N, Tagawa Y, Bessho Y, Honda Y, Nakanishi S. The whole nucleotide sequence and chromosomal localization of the gene for human metabotropic glutamate receptor subtype 6. Eur J Neurosci. 1997;9:1226–1235. doi: 10.1111/j.1460-9568.1997.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Momiyama A, Takahashi T, Ohishi H, Ogawa-Meguro R, Shigemoto R, Mizuno N, Nakanishi S. Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature. 1993;366:687–690. doi: 10.1038/366687a0. [DOI] [PubMed] [Google Scholar]
- Hikichi H, Nishino M, Fukushima M, Satow A, Maehara S, Kawamoto H, Ohta H. Pharmacological effects of metabotropic glutamate receptor ligands on prepulse inhibition in DBA/2j mice. Eur J Pharmacol. 2010;639:99–105. doi: 10.1016/j.ejphar.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Hlavackova V, Goudet C, Kniazeff J, Zikova A, Maurel D, Vol C, Trojanova J, Prézeau L, Pin JP, Blahos J. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 2005;24:499–509. doi: 10.1038/sj.emboj.7600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, Gereau RW., 4th Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW., 4th Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci. 2007;27:13181–13191. doi: 10.1523/JNEUROSCI.0269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Bruno V, Salvatore L, Melchiorri D, Gradini R, Caricasole A, Barletta E, De Blasi A, Nicoletti F. Native group-III metabotropic glutamate receptors are coupled to the mitogen-activated protein kinase/ phosphatidylinositol-3-kinase pathways. J Neurochem. 2002;82:216–223. doi: 10.1046/j.1471-4159.2002.00929.x. [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, Mariggiò S, Sallese M, Porcellini A, Nicoletti F, De Blasi A. Role of G protein-coupled receptor kinase 4 and beta-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem. 2003;278:12433–12442. doi: 10.1074/jbc.M203992200. [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Arcella A, Battaglia G, Pazzaglia S, Aronica E, Spinsanti P, Caruso A, De Smaele E, Saran A, Gulino A, D’Onofrio M, Giangaspero F, Nicoletti F. Pharmacological activation of mGlu4 metabotropic glutamate receptors inhibits the growth of medulloblastomas. J Neurosci. 2006;26:8388–8397. doi: 10.1523/JNEUROSCI.2285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Molinaro G, Battaglia G, Motolese M, Di Menna L, Alfiero M, Blahos J, Matrisciano F, Corsi M, Corti C, Bruno V, De Blasi A, Nicoletti F. Regulation of group II metabotropic glutamate receptors by G protein-coupled receptor kinases: mGlu2 receptors are resistant to homologous desensitization. Mol Pharmacol. 2009;75:991–1003. doi: 10.1124/mol.108.052316. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Lovinger DM, McCool BA, Lewis DL. Heterologous expression of metabotropic glutamate receptors in adult rat sympathetic neurons: subtype-specific coupling to ion channels. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Nash SR, Nishimune A, Neki A, Kaneko S, Nakanishi S. Competitive interaction of seven in absentia homolog-1A and Ca2+/calmodulin with the cytoplasmic tail of group 1 metabotropic glutamate receptors. Genes Cells. 1999;4:381–390. doi: 10.1046/j.1365-2443.1999.00269.x. [DOI] [PubMed] [Google Scholar]
- Izzi C, Barbon A, Toliat MR, Heils A, Becker C, Nürnberg P, Sander T, Barlati S. Candidate gene analysis of the human metabotropic glutamate receptor type 4 (GRM4) in patients with juvenile myoclonic epilepsy. Am J Med Genet B Neuropsychiatr Genet. 2003;123:59–63. doi: 10.1002/ajmg.b.20024. [DOI] [PubMed] [Google Scholar]
- Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E, Malyshkin A, Greco S, Barberi C, Danysz W. The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology. 2009;57:97–108. doi: 10.1016/j.neuropharm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Jensen J, Lehmann A, Uvebrant A, Carlsson A, Jerndal G, Nilsson K, Frisby C, Blackshaw LA, Mattsson JP. Transient lower esophageal sphincter relaxations in dogs are inhibited by a metabotropic glutamate receptor 5 antagonist. Eur J Pharmacol. 2005;519:154–157. doi: 10.1016/j.ejphar.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Jesse CR, Wilhelm EA, Bortolatto CF, Savegnago L, Nogueira CW. Selective blockade of mGlu5 metabotropic glutamate receptors is hepatoprotective against fulminant hepatic failure induced by lipopolysaccharide and d-galactosamine in mice. J Appl Toxicol. 2009;29:323–329. doi: 10.1002/jat.1413. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Kim SJ, Kim J, Worley PF, Linden DJ. Long-term depression of mGluR1 signaling. Neuron. 2007;55:277–287. doi: 10.1016/j.neuron.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin JP. Molecular, functional, and pharmacological characterization of the metabotropic glutamate receptor type 5 splice variants: comparison with mGluR1. J Neurosci. 1995;15:3970–3981. doi: 10.1523/JNEUROSCI.15-05-03970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Eberle EL, Peters SC, Monn JA, Shannon HE. Analgesic effects of the selective group II (mGlu2/3) metabotropic glutamate receptor agonists LY379268 and LY389795 in persistent and inflammatory pain models after acute and repeated dosing. Neuropharmacology. 2005;49 (Suppl 1):206–218. doi: 10.1016/j.neuropharm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Saetre P, Vares M, Andreou D, Larsson K, Timm S, Rasmussen HB, Djurovic S, Melle I, Andreassen OA, Agartz I, Werge T, Hall H, Terenius L. DTNBP1, NRG1, DAOA, DAO and GRM3 polymorphisms and schizophrenia: an association study. Neuropsychobiology. 2009;59:142–150. doi: 10.1159/000218076. [DOI] [PubMed] [Google Scholar]
- Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM. Reduction of l-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther. 2010;333:865–873. doi: 10.1124/jpet.110.166629. [DOI] [PubMed] [Google Scholar]
- Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. doi: 10.1124/pr.110.004036. in press. [DOI] [PubMed] [Google Scholar]
- Kaba H, Hayashi Y, Higuchi T, Nakanishi S. Induction of an olfactory memory by the activation of a metabotropic glutamate receptor. Science. 1994;265:262–264. doi: 10.1126/science.8023145. [DOI] [PubMed] [Google Scholar]
- Kahn L, Alonso G, Robbe D, Bockaert J, Manzoni OJ. Group 2 metabotropic glutamate receptors induced long term depression in mouse striatal slices. Neurosci Lett. 2001;316:178–182. doi: 10.1016/s0304-3940(01)02397-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Ikeda SR. A role for Seven in Absentia Homolog (Siah1a) in metabotropic glutamate receptor signaling. BMC Neurosci. 2001;2:15. doi: 10.1186/1471-2202-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Tabata T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos Trans R Soc Lond B Biol Sci. 2008;363:2173–2186. doi: 10.1098/rstb.2008.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Yoshimizu T, Chaki SA. The metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neurosci Lett. 2006;393:127–130. doi: 10.1016/j.neulet.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lépine JP. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature. 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Emergic therapeutic targets for the treatments of nicotine addiction. Expert Rev Clin Pharmacol. 2009;2:221–225. doi: 10.1586/ecp.09.6. [DOI] [PubMed] [Google Scholar]
- Keywood C, Wakefield M, Tack J. A proof-of-concept study evaluating the effect of ADX10059, a metabotropic glutamate receptor-5 negative allosteric modulator, on acid exposure and symptoms in gastrooesophageal reflux disease. Gut. 2009;58:1192–1199. doi: 10.1136/gut.2008.162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbride J, Rush AM, Rowan MJ, Anwyl R. Presynaptic group II mGluR inhibition of short-term depression in the medial perforant path of the dentate gyrus in vitro. J Neurophysiol. 2001;85:2509–2515. doi: 10.1152/jn.2001.85.6.2509. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, Nakanishi S. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J Neurosci. 2002;22:1280–1289. doi: 10.1523/JNEUROSCI.22-04-01280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kłodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol. 2004a;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Saintot PP, Goudet C, Liu J, Charnet A, Guillon G, Pin JP. Locking the dimeric GABAB G-protein coupled receptor in its active state. J Neurosci. 2004b;24:370–377. doi: 10.1523/JNEUROSCI.3141-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koulen P, Kuhn R, Wässle H, Brandstätter JH. Modulation of the intracellular calcium concentration in photoreceptor terminals by a presynaptic metabotropic glutamate receptor. Proc Natl Acad Sci U S A. 1999;96:9909–9914. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Liu J, Nixon E, Madry C. Interaction between mGluR8 and calcium channels in photoreceptors is sensitive to pertussis toxin and occurs via G protein betagamma subunit signaling. Invest Ophthalmol Vis Sci. 2005;46:287–291. doi: 10.1167/iovs.04-0963. [DOI] [PubMed] [Google Scholar]
- Kozela E, Pilc A, Popik P. Inhibitory effects of MPEP, an mGluR5 antagonist, and memantine, an N-methyl-d-aspartate receptor antagonist, on morphine antinociceptive tolerance in mice. Psychopharmacology (Berl) 2003;165:245–251. doi: 10.1007/s00213-002-1287-8. [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Kuramoto T, Maihara T, Masu M, Nakanishi S, Serikawa T. Gene mapping of NMDA receptors and metabotropic glutamate receptors in the rat (Rattus norvegicus) Genomics. 1994;19:358–361. doi: 10.1006/geno.1994.1069. [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Lee AK, Li H, Deng L, Ehrlich DJ, Elkabes S. Molecular alterations in the cerebellum of the plasma membrane calcium ATPase 2 (PMCA2)-null mouse indicate abnormalities in Purkinje neurons. Mol Cell Neurosci. 2007;34:178–188. doi: 10.1016/j.mcn.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landucci E, Boscia F, Gerace E, Scartabelli T, Cozzi A, Moroni F, Mannaioni G, Pellegrini-Giampietro DE. Involvement of endocanna-binoid signaling in the neuroprotective effects of subtype 1 metabotropic glutamate receptor antagonists in models of cerebral ischemia. Int Rev Neurobiol. 2009;85:337–350. doi: 10.1016/S0074-7742(09)85023-X. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW. Malcolm R.Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Schoeffter P, Wiederhold KH, Sommer B. Cloning, distribution and functional expression of the human mGlu6 metabotropic glutamate receptor. Neuropharmacology. 1997;36:145–152. doi: 10.1016/s0028-3908(96)00172-4. [DOI] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nóbrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lavreysen H, Dautzenberg FM. Therapeutic potential of group III metabotropic glutamate receptors. Curr Med Chem. 2008;15:671–684. doi: 10.2174/092986708783885246. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-d-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry. 2007;62:739–746. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2006;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lin HC, Wang SJ, Luo MZ, Gean PW. Activation of group II metabotropic glutamate receptors induces long-term depression of synaptic transmission in the rat amygdala. J Neurosci. 2000;20:9017–9024. doi: 10.1523/JNEUROSCI.20-24-09017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Huang YC, Wang SJ, Gean PW. Activation of group II metabotropic glutamate receptors induces depotentiation in amygdala slices and reduces fear-potentiated startle in rats. Learn Mem. 2005;12:130–137. doi: 10.1101/lm.85304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Johnson BG, Peters SC, Shannon HE, Tian M, Wang Y, Yu JL, Köster A, Baez M, Schoepp DD. Increased anxiety-related behavior in mice deficient for metabotropic glutamate 8 (mGlu8) receptor. Neuropharmacology. 2002;43:251–259. doi: 10.1016/s0028-3908(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Linden AM, Baez M, Bergeron M, Schoepp DD. Increased anxiety-related behavior in the elevated plus maze associated with the increased C-FOS expression in the centromedial nucleus of the thalamus in mGlu8 receptor knockout mice. Neuroscience. 2003;121:167–178. doi: 10.1016/s0306-4522(03)00393-2. [DOI] [PubMed] [Google Scholar]
- Linden AM, Bergeron M, Schoepp DD. Comparison of c-Fos induction in the brain by the mGlu2/3 receptor antagonist LY341495 and agonist LY354740: evidence for widespread endogenous tone at brain mGlu2/3 receptors in vivo. Neuropharmacology. 2005;49 (Suppl 1):120–134. doi: 10.1016/j.neuropharm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Linden AM, Baez M, Bergeron M, Schoepp DD. Effects of mGlu2 or mGlu3 receptor deletions on mGlu2/3 receptor agonist (LY354740)-induced brain c-Fos expression: specific roles for mGlu2 in the amygdala and subcortical nuclei, and mGlu3 in the hippocampus. Neuropharmacology. 2006;51:213–228. doi: 10.1016/j.neuropharm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Linden AM, Schoepp DD. Metabotropic glutamate receptors targets for neuropsychiatric disorders. Drug Discov Today Ther Strateg. 2006;3:507–517. [Google Scholar]
- Liu F, Ma XH, Ule J, Bibb JA, Nishi A, DeMaggio AJ, Yan Z, Nairn AC, Greengard P. Regulation of cyclin-dependent kinase 5 and casein kinase 1 by metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2001;98:11062–11068. doi: 10.1073/pnas.191353898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and precognitive activities. J Pharmacol Exp Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Yan H, Othman T, Rivkees SA. Cytoskeletal protein 4.1G is a binding partner of the metabotropic glutamate receptor subtype 1 alpha. J Neurosci Res. 2004;78:49–55. doi: 10.1002/jnr.20230. [DOI] [PubMed] [Google Scholar]
- Luján R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- Maddox DM, Vessey KA, Yarbrough GL, Invergo BM, Cantrell DR, Inayat S, Balannik V, Hicks WL, Hawes NL, Byers S, Smith RS, Hurd R, Howell D, Gregg RG, Chang B, Naggert JK, Troy JB, Pinto LH, Nishina PM, McCall MA. Allelic variance between GRM6 mutants, Grm6nob3 and Grm6nob4 results in differences in retinal ganglion cell visual responses. J Physiol. 2008;586:4409–4424. doi: 10.1113/jphysiol.2008.157289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe P, Kratzeisen C, Lundstrom K, Richards JG, Faull RL, Mutel V. Cloning and functional expression of alternative spliced variants of the human metabotropic glutamate receptor 8. Brain Res Mol Brain Res. 1999;67:201–210. doi: 10.1016/s0169-328x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Kew JN, Richards JG, Knoflach F, Kratzeisen C, Zenner MT, Faull RL, Kemp JA, Mutel V. Identification and characterization of a novel splice variant of the metabotropic glutamate receptor 5 gene in human hippocampus and cerebellum. Brain Res Mol Brain Res. 2002;109:168–178. doi: 10.1016/s0169-328x(02)00557-0. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Priming of group 2 metabotropic glutamate receptors facilitates induction of long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology. 1998;37:1459–1464. doi: 10.1016/s0028-3908(98)00150-6. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabese I, Rossi F, Palazzo E, de Novellis V, Starowicz K, Cristino L, Vita D, Gatta L, Guida F, Di Marzo V, Rossi F, Maione S. Periaqueductal gray metabotropic glutamate receptor subtype 7 and 8 mediate opposite effects on amino acid release, rostral ventromedial medulla cell activities, and thermal nociception. J Neurophysiol. 2007a;98:43–53. doi: 10.1152/jn.00356.2007. [DOI] [PubMed] [Google Scholar]
- Marabese I, de Novellis V, Palazzo E, Scafuro MA, Vita D, Rossi F, Maione S. Effects of (S)-3,4-DCPG, an mGlu8 receptor agonist, on inflammatory and neuropathic pain in mice. Neuropharmacology. 2007b;52:253–262. doi: 10.1016/j.neuropharm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Mutoh H, Dimitrov D, Beato M, Knopfel T. Optical measurement of mGluR1 conformational changes reveals fast activation, slow deactivation, and sensitization. Proc Natl Acad Sci U S A. 2009;106:11388–11393. doi: 10.1073/pnas.0901290106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Marignier R, Chenevier F, Rogemond V, Sillevis Smitt P, Renoux C, Cavillon G, Androdias G, Vukusic S, Graus F, Honnorat J, Confavreux C. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67:627–630. doi: 10.1001/archneurol.2010.51. [DOI] [PubMed] [Google Scholar]
- Marín YE, Namkoong J, Cohen-Solal K, Shin SS, Martino JJ, Oka M, Chen S. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal. 2006;18:1279–1286. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Williams DL, Jr, O’Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson’s disease treatment. Proc Natl Acad Sci U S A. 2003;100:13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí SB, Cichon S, Propping P, Nöthen M. Human metabotropic glutamate receptor 2 gene (GRM2): chromosomal sublocalization (3p21.1-p21.2) and genomic organization. Am J Med Genet. 2002;114:12–14. doi: 10.1002/ajmg.1622. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- Martín R, Ladera C, Bartolomé-Martín D, Torres M, Sánchez-Prieto J. The inhibition of release by mGlu7 receptors is independent of the Ca2+ channel type but associated to GABAB and adenosine A1 receptors. Neuropharmacology. 2008;55:464–473. doi: 10.1016/j.neuropharm.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Martín R, Durroux T, Ciruela F, Torres M, Pin JP, Sánchez-Prieto J. The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc-13-1 protein, and potentiates glutamate release at cerebrocortical nerve terminals. J Biol Chem. 2010;285:17907–17917. doi: 10.1074/jbc.M109.080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- Masuho I, Celver J, Kovoor A, Martemyanov KA. Membrane anchor R9AP potentiates GTPase-accelerating protein activity of RGS11 × Gbeta5 complex and accelerates inactivation of the mGluR6-G(o) signaling. J Biol Chem. 2010;285:4781–4787. doi: 10.1074/jbc.M109.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos JM, Azkue J, Benítez R, Sarría R, Losada J, Conquet F, Ferraguti F, Kuhn R, Knöpfel T, Grandes P. Immunocytochemical localization of the mGluR1b metabotropic glutamate receptor in the rat hypothalamus. J Comp Neurol. 1998;390:225–233. doi: 10.1002/(sici)1096-9861(19980112)390:2<225::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mathiesen JM, Svendsen N, Bräuner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol. 2003;138:1026–1030. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Scaccianoce S, Del Bianco P, Panaccione I, Canudas AM, Battaglia G, Riozzi B, Ngomba RT, Molinaro G, Tatarelli R, Melchiorri D, Nicoletti F. Metabotropic glutamate receptors and neuroadaptation to antidepressants: imipramine-induced down-regulation of beta-adrenergic receptors in mice treated with metabotropic glutamate 2/3 receptor ligands. J Neurochem. 2005;93:1345–1352. doi: 10.1111/j.1471-4159.2005.03141.x. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Panaccione I, Zusso M, Giusti P, Tatarelli R, Iacovelli L, Mathé AA, Gruber SH, Nicoletti F, Girardi P. Group-II metabotropic glutamate receptor ligands as adjunctive drugs in the treatment of depression: a new strategy to shorten the latency of antidepressant medication? Mol Psychiatry. 2007;12:704–706. doi: 10.1038/sj.mp.4002005. [DOI] [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prézeau L, Trinquet E, Pin JP. Cell surface protein-protein interaction analysis with combined time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem. 2007;101:483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- Melchiorri D, Cappuccio I, Ciceroni C, Spinsanti P, Mosillo P, Sarichelou I, Sale P, Nicoletti F. Metabotropic glutamate receptors in stem/progenitor cells. Neuropharmacology. 2007;53:473–480. doi: 10.1016/j.neuropharm.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Miller S, Sehati N, Romano C, Cotman CW. Exposure of astrocytes to thrombin reduces levels of the metabotropic glutamate receptor mGluR5. J Neurochem. 1996;67:1435–1447. doi: 10.1046/j.1471-4159.1996.67041435.x. [DOI] [PubMed] [Google Scholar]
- Minakami R, Iida K, Hirakawa N, Sugiyama H. The expression of two splice variants of metabotropic glutamate receptor subtype 5 in the rat brain and neuronal cells during development. J Neurochem. 1995;65:1536–1542. doi: 10.1046/j.1471-4159.1995.65041536.x. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Yamamoto R, Ofner S, Nozulak J, Pescott O, Lukic S, Stoehr N, Mombereau C, Kuhn R, McAllister KH, van der Putten H, Cryan JF, Flor PJ. A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc Natl Acad Sci U S A. 2005;102:18712–18717. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G. Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice. Mol Pharmacol. 2009;76:379–387. doi: 10.1124/mol.109.056580. [DOI] [PubMed] [Google Scholar]
- Montana MC, Cavallone LF, Stubbert KK, Stefanescu AD, Kharasch ED, Gereau RW., 4th The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity compared with the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J Pharmacol Exp Ther. 2009;330:834–843. doi: 10.1124/jpet.109.154138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A. 2005;102:4170–4175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Corrêa SA, Collingridge GL, Fitzjohn SM, Bashir ZI. Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabotropic glutamate receptor-dependent long-term depression. J Physiol. 2008;586:2499–2510. doi: 10.1113/jphysiol.2008.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TK, Messenger MJ, Ward MA, Woodhouse S, Osborne DJ, Duty S, O’Neill MJ. Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson’s disease. Pharmacol Biochem Behav. 2002;73:455–466. doi: 10.1016/s0091-3057(02)00842-0. [DOI] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for l-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nakazato A, Sakagami K, Yasuhara A, Ohta H, Yoshikawa R, Itoh M, Nakamura M, Chaki S. Synthesis, in vitro pharmacology, structure-activity relationships, and pharmacokinetics of 3-alkoxy-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives as potent and selective group II metabotropic glutamate receptor antagonists. J Med Chem. 2004;47:4570–4587. doi: 10.1021/jm0400294. [DOI] [PubMed] [Google Scholar]
- Namkoong J, Shin SS, Lee HJ, Marín YE, Wall BA, Goydos JS, Chen S. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- Nawy S. Regulation of the ON bipolar cell mGluR6 pathway by Ca2+ J Neurosci. 2000;20:4471–4479. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999;19:2938–2944. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Pre-and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: an immunohistochemical study with a monoclonal antibody. Neurosci Lett. 1996;202:197–200. doi: 10.1016/0304-3940(95)12248-6. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Peripheral metabotropic glutamate receptors: fight the pain where it hurts. Trends Neurosci. 2001;24:550–552. doi: 10.1016/s0166-2236(00)02007-5. [DOI] [PubMed] [Google Scholar]
- Ngomba RT, Ferraguti F, Badura A, Citraro R, Santolini I, Battaglia G, Bruno V, De Sarro G, Simonyi A, van Luijtelaar G, Nicoletti F. Positive allosteric modulation of metabotropic glutamate 4 (mGlu4) receptors enhances spontaneous and evoked absence seizures. Neuropharmacology. 2008;54:344–354. doi: 10.1016/j.neuropharm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Nicholls RE, Zhang XL, Bailey CP, Conklin BR, Kandel ER, Stanton PK. mGluR2 acts through inhibitory Galpha subunits to regulate transmission and long-term plasticity at hippocampal mossy fiber-CA3 synapses. Proc Natl Acad Sci U S A. 2006;103:6380–6385. doi: 10.1073/pnas.0601267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Iadarola MJ, Wroblewski JT, Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986a;83:1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Meek JL, Iadarola MJ, Chuang DM, Roth BL, Costa E. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J Neurochem. 1986b;46:40–46. doi: 10.1111/j.1471-4159.1986.tb12922.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Wroblewski JT, Iadarola MJ, Costa E. Serine-O-phosphate, an endogenous metabolite, inhibits the stimulation of inositol phospholipid hydrolysis elicited by ibotenic acid in rat hippocampal slices. Neuropharmacology. 1986c;25:335–338. doi: 10.1016/0028-3908(86)90262-5. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bruno V, Catania MV, Battaglia G, Copani A, Barbagallo G, Ceña V, Sanchez-Prieto J, Spano PF, Pizzi M. Group-I metabotropic glutamate receptors: hypotheses to explain their dual role in neurotoxicity and neuroprotection. Neuropharmacology. 1999;38:1477–1484. doi: 10.1016/s0028-3908(99)00102-1. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Kolachana BS, Vakkalanka R, Straub RE, Giegling I, Egan MF, Rujescu D, Weinberger DR. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet. 2007;120:889–906. doi: 10.1007/s00439-006-0257-3. [DOI] [PubMed] [Google Scholar]
- Nishi A, Liu F, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci U S A. 2003;100:1322–1327. doi: 10.1073/pnas.0237126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, Days EL, Nalywajko T, Austin CA, Williams MB, Ayala JE, Williams R, Lindsley CW, Conn PJ. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74:1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Durkin S, Jaeschke G, Spooren W. Stress-induced hyperthermia: effects of acute and repeated dosing of MPEP. Eur J Pharmacol. 2007;568:199–202. doi: 10.1016/j.ejphar.2007.04.034. [DOI] [PubMed] [Google Scholar]
- O’Connor E, Allen LE, Bradshaw K, Boylan J, Moore AT, Trump D. Congenital stationary night blindness associated with mutations in GRM6 encoding glutamate receptor MGluR6. Br J Ophthalmol. 2006;90:653–654. doi: 10.1136/bjo.2005.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara PJ, Sheppard PO, Thøgersen H, Venezia D, Haldeman BA, McGrane V, Houamed KM, Thomsen C, Gilbert TL, Mulvihill ER. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron. 1993;11:41–52. doi: 10.1016/0896-6273(93)90269-w. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Ogawa-Meguro R, Shigemoto R, Kaneko T, Nakanishi S, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron. 1994;13:55–66. doi: 10.1016/0896-6273(94)90459-6. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993a;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993b;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol Chem. 1994;269:1231–1236. [PubMed] [Google Scholar]
- Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008;139:117–126. doi: 10.1016/j.pain.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Ouattara B, Grégoire L, Morissette M, Gasparini F, Vranesic I, Bilbe G, Johns DR. Metabotropic glutamate receptor type 5 in levodopa-induced motor complications. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.07.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Page AJ, Young RL, Martin CM, Umaerus M, O’Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005;128:402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology. 2008;55:537–545. doi: 10.1016/j.neuropharm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Partosoedarso ER, Blackshaw LA. Roles of central glutamate, acetylcholine and CGRP receptors in gastrointestinal afferent inputs to vagal preganglionic neurones. Auton Neurosci. 2000;83:37–48. doi: 10.1016/S0165-1838(00)00109-0. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Yuan X, Lavezzari G, Roche KW, McBain CJ. mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology. 2007;52:108–117. doi: 10.1016/j.neuropharm.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE. The distinct role of mGlu1 receptors in post-ischemic neuronal death. Trends Pharmacol Sci. 2003;24:461–470. doi: 10.1016/S0165-6147(03)00231-1. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey T, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Pietraszek M, Sukhanov I, Maciejak P, Szyndler J, Gravius A, Wisłowska A, PłaŸnik A, Bespalov AY, Danysz W. Anxiolytic-like effects of mGlu1 and mGlu5 receptor antagonists in rats. Eur J Pharmacol. 2005;514:25–34. doi: 10.1016/j.ejphar.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Pin JP, Galvez T, Prézeau L. Evolution, structure and activation mechanism of family 3/C G-protein coupled receptors. Pharmacol Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-d-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Poisik V, Mannaioni G, Traynelis S, Smith Y, Conn PJ. Distinct functional roles of the metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. J Neurosci. 2003;23:122–130. doi: 10.1523/JNEUROSCI.23-01-00122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, Zhu H, Robbins C, Makalowska I, Shin SS, Marin Y, Roberts KG, Yudt LM, Chen A, Cheng J, Incao A, Pinkett HW, Graham CL, Dunn K, Crespo-Carbone SM, Mackason KR, Ryan KB, Sinsimer D, Goydos J, Reuhl KR, Eckhaus M, Meltzer PS, Pavan WJ, Trent JM, Chen S. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Büttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Mühlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated non-benzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Pshenichkin S, Dolińska M, Klauzińska M, Luchenko V, Grajkowska E, Wroblewski JT. Dual neurotoxic and neuroprotective role of metabotropic glutamate receptor 1 in conditions of trophic deprivation – possible role as a dependence receptor. Neuropharmacology. 2008;55:500–508. doi: 10.1016/j.neuropharm.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishi S, Gayet J, Morgans CW, Duvoisin RM. Distribution of group-III metabotropic glutamate receptors in the retina. J Comp Neurol. 2007;501:931–943. doi: 10.1002/cne.21274. [DOI] [PubMed] [Google Scholar]
- Renger JJ, Hartman KN, Tsuchimoto Y, Yokoi M, Nakanishi S, Hensch TK. Experience-dependent plasticity without long-term depression by type 2 metabotropic glutamate receptors in developing visual cortex. Proc Natl Acad Sci U S A. 2002;99:1041–1046. doi: 10.1073/pnas.022618799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ. Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci. 2002;22:4346–4356. doi: 10.1523/JNEUROSCI.22-11-04346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Yang WL, O’Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem. 1996a;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Romano C, van den Pol AN, O’Malley KL. Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: protein, mRNA splice variants, and regional distribution. J Comp Neurol. 1996b;367:403–412. doi: 10.1002/(SICI)1096-9861(19960408)367:3<403::AID-CNE6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Rondard P, Huang S, Monnier C, Tu H, Blanchard B, Oueslati N, Malhaire F, Li Y, Maurel D, Trinquet E, Labesse G, Pin JP, Liu J. Functioning of the dimeric GABAB receptor extracellular domain revealed by glycan wedge scanning. EMBO J. 2008;27:1321–1332. doi: 10.1038/emboj.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Roppe JR, Wang B, Huang D, Tehrani L, Kamenecka T, Schweiger EJ, Anderson JJ, Brodkin J, Jiang X, Cramer M, Chung J, Reyes-Manalo G, Munoz B, Cosford ND. 5-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-2,3′-bipyridine: a highly potent, orally active metabotropic glutamate subtype 5 (mGlu5) receptor antagonist with anxiolytic activity. Bioorg Med Chem Lett. 2004;14:3993–3996. doi: 10.1016/j.bmcl.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Rose M, Dütting E, Enz R. Band 4.1 proteins are expressed in the retina and interact with both isoforms of the metabotropic glutamate receptor type 8. J Neurochem. 2008;105:2375–2387. doi: 10.1111/j.1471-4159.2008.05331.x. [DOI] [PubMed] [Google Scholar]
- Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, Bredesen DE, Volterra A. Focal degeneration of astrocytes in amyo-trophic lateral sclerosis. Cell Death Differ. 2008;15:1691–1700. doi: 10.1038/cdd.2008.99. [DOI] [PubMed] [Google Scholar]
- Rossi PI, Vaccari CM, Terracciano A, Doria-Lamba L, Facchinetti S, Priolo M, Ayuso C, De Jorge L, Gimelli S, Santorelli FM, Ravazzolo R, Puliti A. The metabotropic glutamate receptor 1, GRM1: evaluation as a candidate gene for inherited forms of cerebellar ataxia. J Neurol. 2010;257:598–602. doi: 10.1007/s00415-009-5380-3. [DOI] [PubMed] [Google Scholar]
- Rush AM, Wu J, Rowan MJ, Anwyl R. Group I metabotropic glutamate receptor(mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. J Neurosci. 2002;22:6121–6128. doi: 10.1523/JNEUROSCI.22-14-06121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, Baishen R, Danysz W, Bezard E, Cenci MA. A mGluR5 antagonist under clinical development improves l-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010;39:352–361. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Sachs AJ, Schwendinger JK, Yang AW, Haider NB, Nystuen AM. The mouse mutants recoil wobbler and nmf373 represent a series of Grm1 mutations. Mamm Genome. 2007;18:749–756. doi: 10.1007/s00335-007-9064-y. [DOI] [PubMed] [Google Scholar]
- Sallese M, Salvatore L, D’Urbano E, Sala G, Storto M, Launey T, Nicoletti F, Knöpfel T, De Blasi A. The G-protein-coupled receptor kinase GRK4 mediates homologous desensitization of metabotropic glutamate receptor 1. FASEB J. 2000;14:2569–2580. doi: 10.1096/fj.00-0072com. [DOI] [PubMed] [Google Scholar]
- Sandyk R. Enkephalinergic mechanisms in the “compensated” phase of Parkinson’s disease. Int J Neurosci. 1988;42:301–303. doi: 10.3109/00207458808991604. [DOI] [PubMed] [Google Scholar]
- Sansig G, Bushell TJ, Clarke VR, Rozov A, Burnashev N, Portet C, Gasparini F, Schmutz M, Klebs K, Shigemoto R, Flor PJ, Kuhn R, Knoepfel T, Schroeder M, Hampson DR, Collett VJ, Zhang C, Duvoisin RM, Collingridge GL, van Der Putten H. Increased seizure susceptibility in mice lacking metabotropic glutamate receptor 7. J Neurosci. 2001;21:8734–8745. doi: 10.1523/JNEUROSCI.21-22-08734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius LJ, Nagappan G, Lipska BK, Lu B, Sei Y, Ren-Patterson R, Li Z, Weinberger DR, Harrison PJ. Alternative splicing of human metabotropic glutamate receptor 3. J Neurochem. 2006;96:1139–1148. doi: 10.1111/j.1471-4159.2005.03609.x. [DOI] [PubMed] [Google Scholar]
- Satow A, Suzuki G, Maehara S, Hikichi H, Murai T, Murai T, Kawagoe-Takaki H, Hata M, Ito S, Ozaki S, Kawamoto H, Ohta H. Unique antipsychotic activities of the selective metabotropic glutamate receptor 1 allosteric antagonist 2-cyclopropyl-5-[1-(2-fluoro-3-pyridinyl)-5-methyl-1H-1,2,3-triazol-4-yl]-2,3-dihydro-1H-isoindol-1-one. J Pharmacol Exp Ther. 2009;330:179–190. doi: 10.1124/jpet.109.151118. [DOI] [PubMed] [Google Scholar]
- Saugstad JA, Kinzie JM, Mulvihill ER, Segerson TP, Westbrook GL. Cloning and expression of a new member of the l-2-amino-4-phosphonobutyric acid-sensitive class of metabotropic glutamate receptors. Mol Pharmacol. 1994;45:367–372. [PubMed] [Google Scholar]
- Saugstad JA, Kinzie JM, Shinohara MM, Segerson TP, Westbrook GL. Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Mol Pharmacol. 1997;51:119–125. doi: 10.1124/mol.51.1.119. [DOI] [PubMed] [Google Scholar]
- Saugstad JA, Marino MJ, Folk JA, Hepler JR, Conn PJ. RGS4 inhibits signalling by group I metabotropic glutamate receptors. J Neurosci. 1998;18:905–913. doi: 10.1523/JNEUROSCI.18-03-00905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scartabelli T, Gerace E, Landucci E, Moroni F, Pellegrini-Giampietro DE. Neuroprotection by group I mGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3K-Akt signaling pathway: a novel postconditioning strategy? Neuropharmacology. 2008;55:509–516. doi: 10.1016/j.neuropharm.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Scherer SW, Duvoisin RM, Kuhn R, Heng HH, Belloni E, Tsui LC. Localization of two metabotropic glutamate receptor genes, GRM3 and GRM8, to human chromosome 7q. Genomics. 1996;31:230–233. doi: 10.1006/geno.1996.0036. [DOI] [PubMed] [Google Scholar]
- Scherer SW, Soder S, Duvoisin RM, Huizenga JJ, Tsui LC. The human metabotropic glutamate receptor 8 (GRM8) gene: a disproportionately large gene located at 7q31.3-q32.1. Genomics. 1997;44:232–236. doi: 10.1006/geno.1997.4842. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Pietraszek M, Gravius A, Danysz W. Effects of a positive allosteric modulator of mGluR5 ADX47273 on conditioned avoidance response and PCP-induced hyperlocomotion in the rat as models for schizophrenia. Pharmacol Biochem Behav. 2009a;95:23–30. doi: 10.1016/j.pbb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Pietraszek M, Gravius A, Klein KU, Greco S, Morè L, Danysz W. Comparison of the mGlu(5) receptor positive allosteric modulator ADX47273 and the mGlu(2/3) receptor agonist LY354740 in tests for antipsychotic-like activity. Eur J Pharmacol. 2009b;623:73–83. doi: 10.1016/j.ejphar.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Johnson BG. Comparison of excitatory amino acid-stimulated phosphoinositide hydrolysis and N-[3H]acetylaspartylglutamate binding in rat brain: selective inhibition of phosphoinositide hydrolysis by 2-amino-3-phos-phonopropionate. J Neurochem. 1989;53:273–278. doi: 10.1111/j.1471-4159.1989.tb07324.x. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Johnson BG, Salhoff CR, McDonald JW, Johnston MV. In vitro and in vivo pharmacology of trans- and cis-(±)-1-amino-1,3-cyclo-pentanedicarboxylic acid: dissociation of metabotropic and ionotropic excitatory amino acid receptor effects. J Neurochem. 1991;56:1789–1796. doi: 10.1111/j.1471-4159.1991.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Salhoff CR, Wright RA, Johnson BG, Burnett JP, Mayne NG, Belagaje R, Wu S, Monn JA. The novel metabotropic glutamate receptor agonist 2R,4R-APDC potentiates stimulation of phosphoinositide hydrolysis in the rat hippocampus by 3,5-dihydroxyphenylglycine: evidence for a synergistic interaction between group 1 and group 2 receptors. Neuropharmacology. 1996;35:1661–1672. doi: 10.1016/s0028-3908(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Marek GJ. Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Curr Drug Targets CNS Neurol Disord. 2002;1:215–225. doi: 10.2174/1568007024606177. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Schneider JS. GABA-opioid interactions in the globus pallidus: [d-Ala2]-met-enkephalinamide attenuates potassium-evoked GABA release after nigrostriatal lesion. J Neurochem. 2002;82:666–673. doi: 10.1046/j.1471-4159.2002.01010.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz HL, Stohr H, Weber BH. Characterization of three novel isoforms of the metabotrobic glutamate receptor 7 (GRM7) Neurosci Lett. 2002;326:37–40. doi: 10.1016/s0304-3940(02)00306-3. [DOI] [PubMed] [Google Scholar]
- Selvam C, Goudet C, Oueslati N, Pin JP, Acher FC. l-(+)-2-Amino-4-thi-ophosphonobutyric acid (l-thioAP4), a new potent agonist of group III metabotropic glutamate receptors: increased distal acidity affords enhanced potency. J Med Chem. 2007;50:4656–4664. doi: 10.1021/jm070400y. [DOI] [PubMed] [Google Scholar]
- Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006;51:623–630. doi: 10.1016/j.neuropharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy SA. Transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Shin SS, Wall BA, Goydos JS, Chen S. AKT2 is a downstream target of metabotropic glutamate receptor 1 (Grm1) Pigment Cell Melanoma Res. 2010;23:103–111. doi: 10.1111/j.1755-148X.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki H, Ishida M. Excitatory amino acids: physiological and pharmacological probes for neuroscience research. Acta Neurobiol Exp (Wars) 1993;53:43–51. [PubMed] [Google Scholar]
- Sillevis Smitt P, Kinoshita A, De Leeuw B, Moll W, Coesmans M, Jaarsma D, Henzen-Logmans S, Vecht C, De Zeeuw C, Sekiyama N, Nakanishi S, Shigemoto R. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342:21–27. doi: 10.1056/NEJM200001063420104. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladeczek F, Pin JP, Récasens M, Bockaert J, Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985;317:717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- Smith Y, Charara A, Hanson JE, Paquet M, Levey AI. GABA(B) and group I metabotropic glutamate receptors in the striatopallidal complex in primates. J Anat. 2000;196:555–576. doi: 10.1046/j.1469-7580.2000.19640555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Smith PA, Dewey WL, Javed RR. Effects of mGlu1 and mGlu5 metabotropic glutamate antagonists to reverse morphine tolerance in mice. Eur J Pharmacol. 2004;492:137–142. doi: 10.1016/j.ejphar.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Banerjee PK, Burnham M, Hampson D. Modulation of absence seizures by the GABA(A) receptor: a critical role for metabotropic glutamate receptor 4 (mGluR4) J Neurosci. 2000;20:6218–6224. doi: 10.1523/JNEUROSCI.20-16-06218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SD, Conn PJ. G protein-coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology. 2003;44:699–706. doi: 10.1016/s0028-3908(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Spanka C, Glatthar R, Desrayaud S, Fendt M, Orain D, Troxler T, Vranesic I. Piperidyl amides as novel, potent and orally active mGlu5 receptor antagonists with anxiolytic-like activity. Bioorg Med Chem Lett. 2010;20:184–188. doi: 10.1016/j.bmcl.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- Stachowicz K, Gołembiowska K, Sowa M, Nowak G, Chojnacka-Wójcik E, Pilc A. Anxiolytic-like action of MTEP expressed in the conflict drinking Vogel test in rats is serotonin dependent. Neuropharmacology. 2007;53:741–748. doi: 10.1016/j.neuropharm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Stachowicz K, Kłak K, Pilc A, Chojnacka-Wójcik E. Lack of the antianxiety-like effect of (S)-3,4-DCPG, an mGlu8 receptor agonist, after central administration in rats. Pharmacol Rep. 2005;57:856–860. [PubMed] [Google Scholar]
- Stephan D, Bon C, Holzwarth JA, Galvan M, Pruss RM. Human metabotropic glutamate receptor 1: mRNA distribution, chromosome localization and functional expression of two splice variants. Neuropharmacology. 1996;35:1649–1660. doi: 10.1016/s0028-3908(96)00108-6. [DOI] [PubMed] [Google Scholar]
- Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Stockton RA, Slaughter MM. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J Gen Physiol. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storto M, Ngomba RT, Battaglia G, Freitas I, Griffini P, Richelmi P, Nicoletti F, Vairetti M. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against acetaminophen hepatotoxicity in mice. J Hepatol. 2003;38:179–187. doi: 10.1016/s0168-8278(02)00384-7. [DOI] [PubMed] [Google Scholar]
- Storto M, Battaglia G, Gradini R, Bruno V, Nicoletti F, Vairetti M. Mouse hepatocytes lacking mGlu5 metabotropic glutamate receptors are less sensitive to hypoxic damage. Eur J Pharmacol. 2004;497:25–27. doi: 10.1016/j.ejphar.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Ito I, Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987;325:531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Tsukamoto N, Fushiki H, Kawagishi A, Nakamura M, Kurihara H, Mitsuya M, Ohkubo M, Ohta H. In vitro pharmacological characterization of novel isoxazolopyridone derivatives as allosteric metabotropic glutamate receptor 7 antagonists. J Pharmacol Exp Ther. 2007;323:147–156. doi: 10.1124/jpet.107.124701. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Satow A, Ohta H. Effect of CFMTI, an allosteric metabotropic glutamate receptor 1 antagonist with antipsychotic activity, on Fos expression in regions of the brain related to schizophrenia. Neuroscience. 2010;168:787–796. doi: 10.1016/j.neuroscience.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992;8:169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- Tang Z, El Far O, Betz H, Scheschonka A. Pias1 interaction and sumoylation of metabotropic glutamate receptor 8. J Biol Chem. 2005;280:38153–38159. doi: 10.1074/jbc.M508168200. [DOI] [PubMed] [Google Scholar]
- Tatarczyńska E, Klodzińska A, Chojnacka-Wójcik E, Palucha A, Gasparini F, Kuhn R, Pilc A. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol. 2001;132:1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateyama M, Abe H, Nakata H, Saito O, Kubo Y. Ligand-induced rearrangement of the dimeric metabotropic glutamate receptor 1alpha. Nat Struct Mol Biol. 2004;11:637–642. doi: 10.1038/nsmb770. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Kubo Y. Dual signaling is differentially activated by different active states of the metabotropic glutamate receptor 1. Proc Natl Acad Sci U S A. 2006;103:1124–1128. doi: 10.1073/pnas.0505925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NK, Wright RA, Howson PA, Kingston AE, Schoepp DD, Jane DE. (S)-3,4-DCPG, a potent and selective mGlu8a receptor agonist, activates metabotropic glutamate receptors on primary afferent terminals in the neonatal rat spinal cord. Neuropharmacology. 2001;40:311–318. doi: 10.1016/s0028-3908(00)00169-6. [DOI] [PubMed] [Google Scholar]
- Toms NJ, Jane DE, Kemp MC, Bedingfield JS, Roberts PJ. The effects of (RS)-alpha-cyclopropyl-4-phosphonophenylglycine ((RS)-CPPG), a potent and selective metabotropic glutamate receptor antagonist. Br J Pharmacol. 1996;119:851–854. doi: 10.1111/j.1476-5381.1996.tb15750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ Proc Natl Acad Sci U S A. 2002;99:2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/ Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Tucker B, Richards RI, Lardelli M. Contribution of mGluR and Fmr1 functional pathways to neurite morphogenesis, craniofacial development and fragile X syndrome. Hum Mol Genet. 2006;15:3446–3458. doi: 10.1093/hmg/ddl422. [DOI] [PubMed] [Google Scholar]
- Uehara S, Muroyama A, Echigo N, Morimoto R, Otsuka M, Yatsushiro S, Moriyama Y. Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by alpha-cells of islet of Langerhans. Diabetes. 2004;53:998–1006. doi: 10.2337/diabetes.53.4.998. [DOI] [PubMed] [Google Scholar]
- Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by the specific mGluR5 agonist CHPG in spinal cord motoneurons. Neuropharmacology. 1999;38:1569–1576. doi: 10.1016/s0028-3908(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN. Metabotropic glutamate receptor mGluR1 distribution and ultrastructural localization in hypothalamus. J Comp Neurol. 1994;349:615–632. doi: 10.1002/cne.903490409. [DOI] [PubMed] [Google Scholar]
- Vandergriff J, Rasmussen K. The selective mGlu2/3 receptor agonist LY354740 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropharmacology. 1999;38:217–222. doi: 10.1016/s0028-3908(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Valenti O, Mannaioni G, Seabrook GR, Conn PJ, Marino MJ. Group III metabotropic glutamate-receptor-mediated modulation of excitatory transmission in rodent substantia nigra pars compacta dopamine neurons. J Pharmacol Exp Ther. 2005;313:1296–1304. doi: 10.1124/jpet.104.080481. [DOI] [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Ferraboli S, Paterlini M, Spano P, Barlati S. Identification of novel alternatively-spliced mRNA isoforms of metabotropic glutamate receptor 6 gene in rat and human retina. Gene. 2001;262:99–106. doi: 10.1016/s0378-1119(00)00547-3. [DOI] [PubMed] [Google Scholar]
- Van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, Kamermans M. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, d-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacol Biochem Behav. 2010;95:223–229. doi: 10.1016/j.pbb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Varney MA, Gereau RW., 4th Metabotropic glutamate receptor involvement in models of acute and persistent pain: prospects for the development of novel analgesics. Curr Drug Targets CNS Neurol Disord. 2002;1:283–296. doi: 10.2174/1568007023339300. [DOI] [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, Hodgson RA, Lu SX, Nicolussi E, Pond AJ, Parker EM, Hunter JC, Higgins GA, Reggiani A, Bertorelli R. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl) 2005;179:207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Gean PW. Long-term depression of excitatory synaptic transmission in the rat amygdala. J Neurosci. 1999;19:10656–10663. doi: 10.1523/JNEUROSCI.19-24-10656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Westin L, Nong Y, Birnbaum S, Bendor J, Brismar H, Nestler E, Aperia A, Flajolet M, Greengard P. Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science. 2009;326:1554–1557. doi: 10.1126/science.1178496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner CG, Scartabelli T, Pancani T, Landucci E, Moroni F, Pellegrini-Giampietro DE. Differential role of mGlu1 and mGlu5 receptors in rat hippocampal slice models of ischemic tolerance. Eur J Neurosci. 2007;25:3597–3604. doi: 10.1111/j.1460-9568.2007.05614.x. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl) 2004;172:233–240. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Wong CG, Scherer SW, Snead OC, 3rd, Hampson DR. Localization of the human mGluR4 gene within an epilepsy susceptibility locus(1) Brain Res Mol Brain Res. 2001;87:109–116. doi: 10.1016/s0169-328x(00)00283-7. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-Acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xu W, Wong TP, Chery N, Gaertner T, Wang YT, Baudry M. Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 2007;53:399–412. doi: 10.1016/j.neuron.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Soghomonian JJ. Metabotropic glutamate mGluR5 receptor blockade opposes abnormal involuntary movements and the increases in glutamic acid decarboxylase mRNA levels induced by l-DOPA in striatal neurons of 6-hydroxydopamine-lesioned rats. Neuroscience. 2009;163:1171–1180. doi: 10.1016/j.neuroscience.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yang D, Gereau RW., 4th Peripheral group II metabotropic glutamate receptors (mGluR2/3) regulate prostaglandin E2-mediated sensitization of capsaicin responses and thermal nociception. J Neurosci. 2002;22:6388–6393. doi: 10.1523/JNEUROSCI.22-15-06388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip D, Le MN, Chan JL, Lee JH, Mehnert JA, Yudd A, Kempf J, Shih WJ, Chen S, Goydos JS. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res. 2009;15:3896–3902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Chaki S. Increased cell proliferation in the adult mouse hippocampus following chronic administration of group II metabotropic glutamate receptor antagonist, MGS0039. Biochem Biophys Res Commun. 2004;315:493–496. doi: 10.1016/j.bbrc.2004.01.073. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Shimazaki T, Ito A, Chaki S. An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berl) 2006;186:587–593. doi: 10.1007/s00213-006-0390-7. [DOI] [PubMed] [Google Scholar]
- Zeitz C, van Genderen M, Neidhardt J, Luhmann UF, Hoeben F, Forster U, Wycisk K, Mátyás G, Hoyng CB, Riemslag F, Meire F, Cremers FP, Berger W. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electro-retinogram. Invest Ophthalmol Vis Sci. 2005;46:4328–4335. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]
- Zerbib F, Keywood C, Strabach G. Efficacy, tolerability and pharma-cokinetics of a modified release formulation of ADX10059, a negative allosteric modulator of metabotropic glutamate receptor 5: an esophageal pH-impedance study in healthy subjects. Neurogastroenterol Motil. 2010;22:859–865. doi: 10.1111/j.1365-2982.2010.01484.x. [DOI] [PubMed] [Google Scholar]
- Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology. 2003;28:45–52. doi: 10.1038/sj.npp.1300013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008a;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CS, Bertaso F, Eulenburg V, Lerner-Natoli M, Herin GA, Bauer L, Bockaert J, Fagni L, Betz H, Scheschonka A. Knock-in mice lacking the PDZ-ligand motif of mGluR7a show impaired PKC-dependent auto-inhibition of glutamate release, spatial working memory deficits, and increased susceptibility to pentylenetetrazol. J Neurosci. 2008b;28:8604–8614. doi: 10.1523/JNEUROSCI.0628-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jeffrey BG, Morgans CW, Burke NS, Haley TL, Duvoisin RM, Brown RL. RGS7 and -11 complexes accelerate the ON-bipolar cell light response. Invest Ophthalmol Vis Sci. 2010;51:1121–1129. doi: 10.1167/iovs.09-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, Orr HT. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J Neurosci. 2004;24:8853–8861. doi: 10.1523/JNEUROSCI.2978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]



