Abstract
Studies of primate vocal communication systems have generally focused on vocalizations and the information they convey to conspecifics. But the vocalizations are not the only sources of information. Aspects of each species vocal behaviors are also likely to be communicatively rich as well. During vocal interactions, for example, the latency delay between the calls could communicate an important message to the signal receiver, such as an interest and willingness to socialize. Here we employed novel, interactive playback software to address this issue in the antiphonal calling behavior of common marmosets. In these experiments, we parametrically varied the latency delay of antiphonal call stimuli and measured its effects on subjects’ resultant vocal behavior. Results showed that marmosets produced successively fewer antiphonal call responses during test conditions with increasing latency delays. Moreover, although subjects produced significantly more antiphonal than spontaneous calls in conditions with antiphonal call timing delays up to 9s, a longer delay resulted in a significant decline in calling. These data suggest that antiphonal call timing is a salient cue for maintaining antiphonal calling interactions and may be used by marmosets to determine whether a subsequent call is produced in response to or independently of their own.
Keywords: antiphonal calling, call timing, common marmosets, vocal behavior, vocal interactions
Introduction
Vocal communication systems are ubiquitous amongst nonhuman primates. Due to its significance, an extensive effort has been made to characterize various elements of these systems for a large diversity of individual species. This research agenda ranges from acoustic descriptions of a species’ vocalizations (Cleveland and Snowdon 1982; Owren and Bernacki 1988; Hauser 1991; Macedonia, 1993; Norcross and Newman 1993; Pistorio et al. 2006; De la Torre and Snowdon 2009) to ethological studies that characterize the social and behavioral significance of individual call types ( Struhsaker 1967; Pola and Snowdon 1975; Mitani, 1985; Mitani and Gros-Louis 1998; Arnold and Zuberbuhler 2006; Bezera and Souto 2008;) to playback experiments testing combinations of these two aspects of vocalizations (Waser 1975; Seyfarth et al. 1980; Cheney and Seyfarth 1982; Rendall et al. 1996; Fischer, 1998; Zuberbuhler et al. 1999; Fischer et al., 2001; Ghazanfar et al., 2001; Wilson et al. 2001; Miller and Hauser 2004; Miller et al. 2004; Miller et al. 2005). Collectively, though not entirely (Egnor et al. 2007), studies on this topic have largely focused on the vocalization itself, ignoring to some extent the suite of vocal behaviors involved in communication. While the call type and information content of vocal signals certainly carry a wealth of communicative information to conspecific signal receivers (Miller and Cohen 2009), behavioral cues, such as the timing or sequence of the calls, are likely communicatively significant as well.
Vocal interactions are particularly well suited for addressing the significance of behavioral cues in communication. During these social behaviors, two or more individuals produce a sequence of vocalizations from which signal receivers may extract additional information not present in the vocalizations alone. In baboons (Papio cynocephalus), for example, dominant females produce grunts when approaching a subordinate, which may then elicit a fear bark from the subordinate female. Cheney, Seyfarth and colleagues (Cheney et al. 1995; Bergman et al. 2003) designed a series of playback experiments aimed at testing whether conspecifics attend not only to the sequence of the calls in the interaction, but the social relationships of the two animals as well. In these experiments, subjects were broadcast a sequence of socially consistent (dominant grunt followed by subordinate fear bark) or inconsistent (subordinate grunt followed by dominant fear bark) vocalizations from a hidden speaker and were more responsive in the latter than the former context. Subjects were more responsive to the socially inconsistent stimuli suggesting that baboons not only attend to what is being communicated by each call individually, but also to other behavioral cues involved in the sequence of vocalizations, such as the relative dominance relationship of the two callers. Importantly, these data show that conspecific signal receivers are attending to communicative information not explicitly provided by the structure of the vocalizations themselves. Rather they are utilizing to cues that emerge from the interaction of the two callers.
Antiphonal calling is a cooperative, species-typical vocal behavior characterized by the reciprocal exchange of long-distance contact calls between conspecifics. In common marmosets (Callithrix jacchus), antiphonal calling involves a single vocalization – the phee call. Similarly to many contact calls (Miller and Ghazanfar 2002), this vocalization is thought to maintain group contact when visually occluded (Miller and Wang 2006; Bezera and Souto 2008). Upon hearing a phee call, animals will respond by producing the same call type (Figure 1a). That vocal response is the antiphonal call. Previous studies of this behavior in marmosets provide at least two sources of evidence that the latency timing of the antiphonal call response may serve as an important behavioral cue. First, acoustic recordings of two marmosets engaged in antiphonal calling showed that marmosets produce ~90% of antiphonal calls within 6s of the preceding call suggesting a consistent temporal latency between the initiating call and the behavioral response (Miller & Wang 2006). Second, during playback experiments, data showed that subjects were significantly more likely to produce an antiphonal response if phee calls were broadcast in an interactive manner (i.e. the stimuli were played within 2-5s of subjects’ phee production), than if they were played every 15s independent of subjects’ vocal behavior (Miller and Wang 2006). Together these data suggest that antiphonal call timing in marmosets, a behavioral cue that emerges from the vocal interaction, may be behaviorally significant, but more detailed experiments are needed to explicitly test this hypothesis.
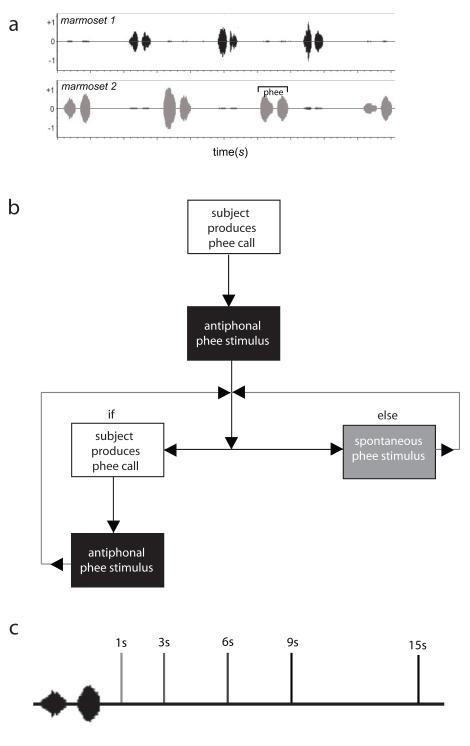
Figure 1.
(a) Acoustic recordings of two marmosets engaged in antiphonal calling. The image demonstrates the reciprocal nature of this vocal behavior. Following the initial phee call produced by ‘marmoset-2’, each subsequent phee would be considered an antiphonal call. (b) Shows a flow diagram of the logic steps for the Interactive Playback Software. (c) A schematic drawing of the latency delays used in each of the 5 test conditions. For each condition, we presented subjects with phee stimuli at a single latency measured from the offset of subjects’ phee call. This schematic shows the amplitude waveform of phee call produced by a subject and the onset of the phee stimulus that would be broadcast in each of the 5 conditions: 1s, 3s, 6s, 9s, and 15s. The onset of the phee call stimulus is shown with a vertical dark line.
In this experiment, we employed custom, interactive playback software to present phee stimuli at specific latency intervals (Figure 1b). The aim was to more precisely test the behavioral significance of antiphonal call timing by presenting stimuli at controlled time intervals. During test sessions, we broadcast a phee call stimulus at a specific latency following the offset of subjects’ phee production. Specifically, the study consisted of five test conditions, each involving a single specific playback latency: 1s, 3s, 6s, 9s, or 15s (Figure 1c). These latencies were selected because they reflect a representative sample of the natural distribution of antiphonal call responses in common marmosets (Miller & Wang 2006). The prediction is that if the latency to call is a behavioral cue used by marmosets during antiphonal calling, subjects should produce significantly more antiphonal calls under particular latency conditions. If, however, this is not the case, no difference in vocal behavior should be observed across the test conditions.
Methods
Subjects
10 adult common marmosets (Callithrix jacchus) participated as subjects in this experiment (5 male/ 5 female). Two subjects (1male/1female) did not participate in the 15s condition as they were our initial pilot subjects, during which time we did not run this test condition. The common marmoset is a small-bodied (~400g), New World primate endemic to the rainforests of northeastern Brazil (Rylands 1993). This highly vocal primate has been the subject of several previous behavioral and neural studies of vocal communication under laboratory conditions (Norcross and Newman 1993, 1997; Wang and Kadia 2001; Eliades and Wang 2003; Miller and Wang 2006; Pistorio et al. 2006; DiMattina and Wang 2006). In our colony, subjects are all housed in social groups consisting of their pair-bonded mates and up to two generations of offspring.
Procedure
We transported subjects from the colony to the testing room in transport cages. The testing room was 7m × 4m in size and had the walls covered completely in acoustic attenuating foam and a carpet floor. Once inside the room, we placed subjects in a wire mesh test cage. We positioned a free-field speaker (Cambridge Soundworks M80, Frequency Range: 40 – 22000 Hz) 2m in front of test cage with a dark curtain equidistant between the subject and the speaker (i.e. both the cage and speaker were 1m each from the curtain). We broadcast stimuli from a computer through a Crown amplifier (Model D-75A) and the free-field speaker at ~90 dB SPL measured at 1m from the speaker. For more details, see Miller & Wang (2006).
Interactive Playback Design
In a previous study (Miller and Wang 2006), we showed that interactive playbacks were significantly more effective at eliciting antiphonal calls than traditional playback experiments. Interactive playbacks differ from traditional playback experiments in that the timing of stimulus presentation is determined entirely by subjects’ behavior and occurs in response to subjects’ vocalizations. In other words, during interactive playback experiments, stimulus presentation timing is based on when subjects produce calls, rather than being presented at a specific timing interval.
Antiphonal calling is an ideal behavior to examine with interactive playbacks because it involves the reciprocal exchange of vocalizations between individuals. For the present study, we developed automated, interactive playback software (Matlab, Mathworks) that effectively mimics antiphonal calling behavior. With this software, we are able to parametrically manipulate a number of different quantifiable parameters in the behavior. Although experiments employing interactive playback software have been used in studies of other taxonomic groups (Moore et al.1989; Todt and Naguib 2000; Schwartz 2001), this study is the first to develop this type of system for a nonhuman primate species.
Prior to the beginning of a test session, two stimulus sets are loaded into the software. These stimulus sets consist of previously recorded phee calls from known animals (10-15 exemplars in each set), in this case from their respective cagemate. One set of stimuli is phee calls produced antiphonally, while the second set is phee calls produced spontaneously. The difference between these calls is that ‘antiphonal’ phee call stimuli are phees produced in response to another phee call, while the ‘spontaneous’ phee calls are phees produced without a preceding phee (Miller and Wang 2006). The difference between these stimulus sets is based on behavioral context, though acoustic analysis of phee calls shows some acoustic differences between calls produced in these contexts (Miller et al. 2009).
The interactive playback system aims to effectively mimic the natural antiphonal calling behavior of common marmosets (Miller and Wang 2006). The logic of the system is as follows (Figure 1b). Subjects initiated the software by producing a phee call. Once that initial phee was produced, the system broadcasted an ‘antiphonal’ phee stimulus at a preset latency interval: ‘antiphonal latency’. Subsequently, each time subjects produced a phee, an ‘antiphonal’ phee stimulus was broadcast at the ‘antiphonal latency’. If subjects did not respond to the ‘antiphonal’ phee stimulus within a predetermined period of time, labeled as ‘spontaneous period 1’, a ‘spontaneous’ phee stimulus was broadcast. If subjects did not respond to two consecutive ‘spontaneous’ phee stimuli, the interval between the ‘spontaneous’ phee stimuli was increased to a preset interval, labeled as ‘spontaneous period 2’, and the level of the stimulus was decreased to a preset gain. If at any point subjects produced a phee, an ‘antiphonal’ phee stimulus was broadcast and the same process for broadcasting an ‘antiphonal’ phee and ‘spontaneous’ phee occurs.
In this experiment we manipulated the latency for the interactive system to playback an ‘antiphonal’ phee stimulus following the detection of a phee produced by a subject. As such, the antiphonal latency period varied between the test conditions. For all conditions, we set ‘spontaneous period 1’ to 15s-20s and ‘spontaneous period 2’ to 30s-35s. The sound level of was reduced by 25% during phee stimuli broadcast following a ‘stimulus period 2’ as this follows our observations of marmoset antiphonal calling. These parameters reflect the natural distribution observed during our earlier observational study of marmoset antiphonal calling (Miller & Wang 2006).
Test Conditions
The experiment consisted of 5 test conditions. For each test condition, we presented subjects with pre-recorded phees from their respective cagemate at a specific latency: 1s, 3s, 6s, 9s, 15s (Figure 1c). We selected these latencies because they reflect a representative sample of the natural distribution of antiphonal call responses in common marmosets (Miller & Wang 2006). The calls of the same respective cagemate were used in all test conditions for each subject. Only a single latency was used in each condition. The order each subject was run on the test conditions was randomized and counterbalanced across subjects. Subjects were run independently and participated in only a single test session on a given day. During each condition, we broadcast a total of 20 phee stimuli to subjects.
Results
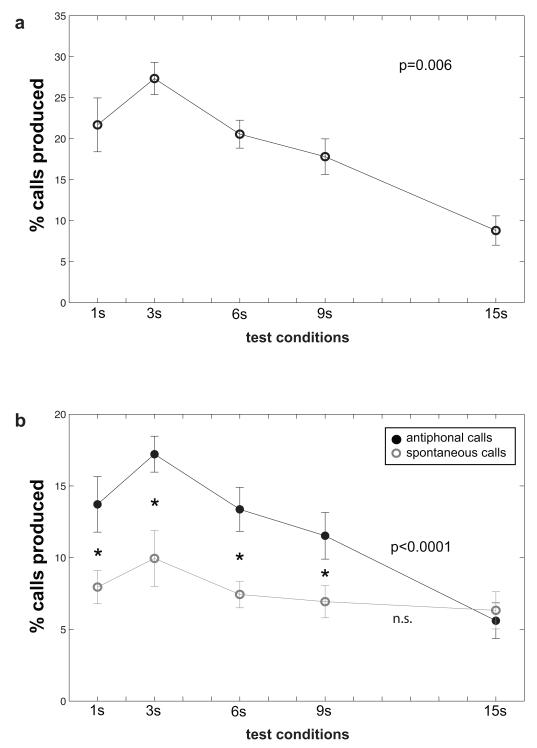
Overall, data showed a general trend that subjects produced successively fewer phee calls in test conditions with longer latency delays (Figure 2a). A Repeated-Measures ANOVA showed a significant difference in the total number of calls produced across the five test conditions (F(4,36)=5.36, p=0.002) suggesting a difference in overall calling based on the latency of stimulus presentation.
Figure 2.
(a) Plots the mean (se) total %of phee calls produced in each of the 5 test conditions. (b) Shows the mean (se) of the % of calls produced for both antiphonal (black closed circle) and spontaneous (grey open circle) calls. Data were normalized to percentages for each individual by dividing the number of calls produced for a particular test condition by the total number of all calls produced by that individual across all conditions: (a) All calls were combined (b) Antiphonal and Spontaneous calls were parsed. Statistical tests were performed on the raw data. * denotes statistically significant differences between the antiphonal and spontaneous calls produced within a particular test condition. P-values denote the results of Repeated-measures ANOVA tests of calls produced across the test conditions: (a) All calls combined (b) Antiphonal and Spontaneous calls were analyzed separately.
Our previous work showed that marmosets produced ~90% of antiphonal calls within 6s following the preceding call (Miller and Wang 2006). As such, we next analyzed the data based on whether subjects produced a vocalization within 6s of a phee stimulus, labeled as antiphonal calls, or after 6s, labeled as spontaneous calls. The general trend for antiphonal calls was consistent with the total phee calls produced. A Repeated-Measure ANOVA showed a significant difference in the number of antiphonal calls produced across the test conditions for the population (F(4,36)=6.7, p<0.0001; Figure 2b). In marked contrast to the antiphonal calls, subjects showed no difference in the number of spontaneous calls produced across the test conditions. A Repeated-Measure ANOVA showed no difference in this aspect of vocal behavior across the conditions (F(4,36)=0.82, p = 0.52, Figure 2b). This suggests that although the number of antiphonal calls produced in each test condition was modulated by the latency delay in the test condition, spontaneous calling remained stable.
As the number of spontaneous calls produced in each test condition did not differ statistically, we used these data as a baseline reference to determine whether the number of antiphonal calls increased above baseline. For this analysis, we calculated the mean number of antiphonal and spontaneous calls produced by each individual in each condition. We then used paired t-tests for each condition to test for statistical significance. This analysis showed that subjects produced significantly more antiphonal calls than spontaneous calls in all test conditions except for the 15s condition [1s: t(9)=2.47, p=0.03; 3s: t(9)=2.92, p=0.01; 6s: t(9)=2.26, p=0.05; 9s: t(9)=2.26, p=0.05] (Figure 2). This suggests that latency delays up to 9s are effective at eliciting antiphonal calls from marmosets.
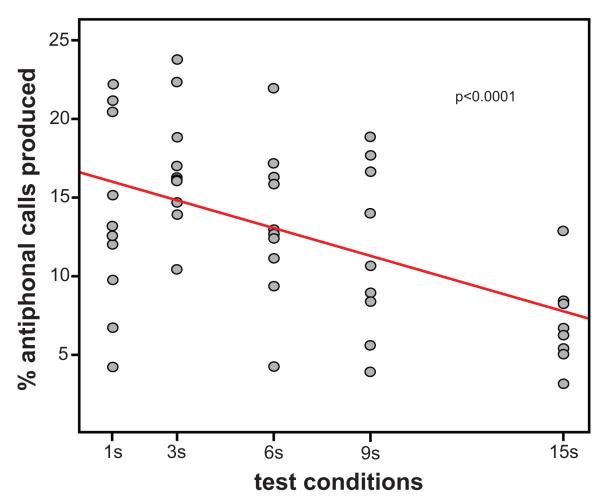
The general pattern we observed was that subjects produced successively fewer antiphonal calls in test conditions employing increasingly longer latency delays (Figure 2). Using trend analysis, we tested whether the negative linear trend was statistically significant (Figure 3). As subjects all varied in the total number of calls produced, we normalized the number of antiphonal calls each subject produced in each test condition as a percent of their total calls across all conditions. A one-way ANOVA with a linear contrast of these data was statistically significant (F(1,47)=16.35, p<0.0001; Figure 3) suggesting that the observation that marmosets produce fewer antiphonal calls as the latency delay increased is statistically reliable.
Figure 3.
Plots the % of antiphonal calls produced for each individual (grey circles) for test test condition. Data were normalized to percentages for each individual by dividing the number of antiphonal calls produced for a particular test condition and call type by the total number of all calls produced by that individual across all conditions. The red line marks the linear contour fit to the antiphonal calling population data.
Discussion
In this study, we tested a relatively simple, but potentially significant behavioral cue in marmoset antiphonal calling – the timing of the vocal response. Employing novel, interactive playback software, we manipulated the latency delay of the antiphonal playback response to test whether marmosets attend to this behavioral cue during their natural antiphonal calling interactions. If the timing of the antiphonal call response was behaviorally significant, we predicted that subjects’ responsiveness would vary across the test conditions, whereas if this cue carries no behavioral meaning for marmosets, no differences in vocal behavior would be observed. Data showed a general trend that subjects produced successively fewer antiphonal calls in test conditions with longer latency delays (Figure 2&3) suggesting that antiphonal call timing is behaviorally meaningful to marmosets. Furthermore, the range over which marmosets consider calls to be antiphonal responses appears to be approximately 9s, as longer latency delays did not elicit significant levels of antiphonal calling. These data show that marmosets attend to antiphonal call timing as a cue and respond only if that latency delay is within a particular time window.
The data shown here are consistent with our earlier report, but provide several additional insights into this vocal behavior. Observational data showed that ~90% of all antiphonal calls produced during natural antiphonal calling interactions occur within the first 6s of the preceding call (Miller and Wang 2006). In the present study, however, we found that even a latency delay of up to 9s elicited a significant number of antiphonal call responses (Figure 2b). This discrepancy may occur for at least the two following reasons. First, the difference between the behavioral observation and playback data may show that marmosets have a broader range over which they perceive a call to be an antiphonal response then the average timing of the call response. Given the inherent variability in antiphonal calling behavior (Miller and Wang 2006), such behavioral flexibility may be important for maintaining the behavior. Second, the original data were recorded from two marmosets engaged in natural antiphonal calling interactions, whereas here the data represent experimentally induced behavioral responses. Similarly to playback experiments testing the perceptual significance of acoustic features for call recognition (Ghazanfar et al. 2002; Miller and Hauser 2004), these experiments are effectively pushing the boundaries of typical antiphonal calling behavior in order to test its limits from the perspective of the animals themselves. As such, subjects’ responses may be somewhat outside their more typical range under natural conditions (Miller et al. 2004). By manipulating the behavior along this dimension, however, we now have a more precise understanding of the species-specific range for antiphonal call timing.
Although the results of this study provide evidence that antiphonal call timing is significant for maintaining this natural vocal behavior, its specific function is not clear from these data. One possibility is that the timing of the response communicates to the initiating signal producer that the antiphonal call is produced specifically as a response. In other words, it provides a cue that a subsequent call is produced in response to -rather than independently of - the preceding vocalization. Determining which, if any, call is produced in response to one’s own call would be imperative for communicative efficacy, particularly given the acoustic environment of marmosets in their natural habitat. This species is highly vocal and typically multiple animals within the group may be producing calls within a small time window. A behavioral cue that signals the subsequent call is a response could be quite useful under these conditions. One possible alternative mechanism for communicating this information would be to match some aspect of the acoustic structure of the initiating call match (Suguira 1998;Janik 2000); . Given the degradation of vocalizations in forest environments (Waser and Brown 1986; Brown and Waser 1988) , however, call matching may not be an effective causal cue for long distance vocal interactions. A more robust cue under these conditions would be the relative timing of the vocal response. Specifically, individuals may perceive a relationship between their own vocalization and a subsequent call if that vocalization is produced within a specific time window. The data presented here would support this latter possibility, though call matching may also contribute to this process as an additional cue. Future studies will explore this possibility.
If marmosets utilize call timing as a cue for determining which vocalizations are produced as an antiphonal response, questions about the mechanisms underlying their perception of this cue remain. One possibility is that antiphonal call timing is innately specified; the animals are genetically programmed to produce and respond to phee calls within a particular time window. This, however, seems unlikely for at least two reasons. First, results from our earlier observational study showed that the latency to antiphonal call actually varied depending on the social relationship of the two animals (e.g. cagemate, non-cagemate of the same sex, etc) (Miller and Wang 2006). If subtleties in the social context have an effect on antiphonal call timing, then it is unlikely that all aspects of the behavior are innate, though it is possible that some parameters may be restricted by innate constraints. Second, during these and previous playback experiments, marmosets typically only respond to 50%-60% of all playback stimuli. The same level of responsiveness was also evident during our previous acoustic recordings of antiphonal calling. At the very least, this shows that antiphonal calling is more complex than a simple stimulus-response behavior, and that more sophisticated mechanisms are mediating the timing and frequency of the behavioral responses.
The perception of antiphonal call timing could be modulated by more complex cognitive mechanisms related to their understanding of marmoset social dynamics. The simplest case would be that marmosets learning the statistical relationships between calls inherent in the behavior and use this to determine when subsequent calls are vocal responses. Alternatively, marmosets may possess a more sophisticated understanding of social dynamics and infer, based on the current social context, what cues determine a vocal response. They would essentially be trying to determine which cues reflected a causal relationship between their own behavior and those of conspecifics. The world is full of causal relationships, but the extent to which our nonhuman primate cousins are also sensitive to these occurrences and use them to guide their behavior is not well understood. Nonhuman primates are known to possess sophisticated cognitive mechanisms for navigating their social worlds (Hare et al. 2001, 2006; Cheney and Seyfarth 2007), but little experimental work addresses their understanding of causality in the social domain (Cheney et al.1995; Bergman et al. 2003). The interactive playback software employed here provides a unique and powerful technique for parametrically testing various aspects of the antiphonal calling behavior, such as their understanding of the causal relationships that exist in this behavior. By actively engaging the species in their own natural behavior, continued work on antiphonal calling will experimentally test the various cognitive and perceptual mechanisms underlying this natural primate social behavior. More broadly, the ability to experimentally engage nonhuman primates in their social behaviors may be critical for elucidating many of the more sophisticated neural mechanisms in cortex, as it is these behaviors that likely had a significant impact on the evolution of the primate brain (Ghazanfar and Santos 2004).
Acknowledgments
We thank Laurie Santos, Michael Osmanski and Yi Zhou for helpful comments on an earlier version of this manuscript and Eric Kim for writing the interactive playback software. This work was supported by grants from the NIH to CTM (R03 DC008404, K99 DC009007) and XW (R01 DC005808). All experimental protocols were approved by the Johns Hopkins University Animal Care and Use Committee.
References
- Arnold K, Zuberbuhler K. Semantic combinations in primate calls. Nature. 2006;441:303. doi: 10.1038/441303a. [DOI] [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. Hierarchical classification by rank and kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- Bezera BM, Souto A. Structure and usage of the vocal repertoire of Callithrix jacchus. Int J Primatol. 2008;29:671–701. [Google Scholar]
- Brown C, Waser P. Environmental influences on the structure of primate vocalizations. In: Todt D, Goedeking P, Symmes D, editors. Primate vocal communication. Springer-Verlag; Berlin, Germany: 1988. [Google Scholar]
- Cheney DL, Seyfarth RM. How vervet monkeys perceive their grunts: Field playback experiments. Anim Behav. 1982;30:739–751. [Google Scholar]
- Cheney DL, Seyfarth RM. Baboon metaphysics: the evolution of a social mind. University of Chicago Press; Chicago: 2007. [Google Scholar]
- Cheney DL, Seyfarth RM, Silk J. The responses of female baboons (Papio cynocephalus ursinus) to anomalous social interactions: Evidence for causal reasoning? J Comp Psych. 1995;109:134–141. doi: 10.1037/0735-7036.109.2.134. [DOI] [PubMed] [Google Scholar]
- Cleveland J, Snowdon CT. The complex vocal repertoire of the adult cotton-top tamarin, Saguinus oedipus oedipus. Zeit Tierpsychol. 1982;58:231–270. [Google Scholar]
- De la Torre S, Snowdon CT. Dialiects in pygmy marmosets? population variation in call structure. Am J Primatol. 2009;71:1–10. doi: 10.1002/ajp.20657. [DOI] [PubMed] [Google Scholar]
- DiMattina C, Wang X. Virtual vocalization stimuli for investigating neural representations of species-specific vocalizations. J Neurophys. 2006;95:1244–1262. doi: 10.1152/jn.00818.2005. [DOI] [PubMed] [Google Scholar]
- Egnor SER, Wickelgren JG, Hauser MD. Tracking silence: adjusting vocal production to avoid acoustic interference. J of Comp Phys A. 2007;193:477–483. doi: 10.1007/s00359-006-0205-7. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophys. 2003;89:2185–2207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- Fischer J. Barbary macaques categorize shrill barks into two call types. Anim Behav. 1998;55:799–807. doi: 10.1006/anbe.1997.0663. [DOI] [PubMed] [Google Scholar]
- Fischer J, Metz M, Cheney DL, Seyfarth RM. Baboon responses to graded bark variants. Anim Behav. 2001;61:925–931. [Google Scholar]
- Ghazanfar AA, Santos LR. Primate brains in the wild: the sensory basis for social interactions. Nat Rev Neuro. 2004;5:603–616. doi: 10.1038/nrn1473. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Smith-Rohrberg D, Hauser MD. The role of temporal cues in rhesus monkey vocal recognition: Orienting asymmetries to reversed calls. Brain Behav Evol. 2001;58:163–172. doi: 10.1159/000047270. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Smith-Rohrberg D, Pollen A, Hauser MD. Temporal cues in the antiphonal calling behaviour of cotton-top tamarins. Anim Behav. 2002;64:427–438. [Google Scholar]
- Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know. Anim Behav. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Chimpanzees deceive a human competitor by hiding. Cognition. 2006;101:495–514. doi: 10.1016/j.cognition.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Hauser MD. Sources of acoustic variation in rhesus macaque vocalizations. Ethology. 1991;89:29–46. [Google Scholar]
- Janik VM. Whistle matching in wild bottlenose dolphins (Tursiops truncatus) Science. 2000;289:1355–1357. doi: 10.1126/science.289.5483.1355. [DOI] [PubMed] [Google Scholar]
- Macedonia JM. The vocal repertoire of the ringtailed lemur (Lemur catta) Fol Prim. 1993;61:186–217. doi: 10.1159/000156749. [DOI] [PubMed] [Google Scholar]
- Miller CT, Cohen YE. Vocalizations as auditory objects: behavior and neurophysiology. In: Platt M, Ghazanfar AA, editors. Primate Neuroethology. Oxford University Press; New York, NY: 2009. [Google Scholar]
- Miller CT, Ghazanfar AA. Meaningful acoustic units in nonhuman primate vocal behavior. In: Bekoff M, Allen C, Burghardt G, editors. The cognitive animal. MIT Press; Cambridge, MA: 2002. pp. 265–274. [Google Scholar]
- Miller CT, Hauser MD. Multiple acoustic features underlie vocal signal recognition in tamarins: antiphonal calling experiments. J of Comp Phys A. 2004;190:7–19. doi: 10.1007/s00359-003-0468-1. [DOI] [PubMed] [Google Scholar]
- Miller CT, Iguina C, Hauser MD. Processing vocal signals for recognition during antiphonal calling. Anim Behav. 2005;69:1387–1398. [Google Scholar]
- Miller CT, Mandel K, Wang X. The communicative content of the common marmoset phee call during antiphonal calling. 2009. Submitted for Publication. [DOI] [PMC free article] [PubMed]
- Miller CT, Scarl JS, Hauser MD. Sensory biases underlie sex differences in tamarin long call structure. Anim Behav. 2004;68:713–720. [Google Scholar]
- Miller CT, Wang X. Sensory-motor interactions modulate a primate vocal behavior: antiphonal calling in common marmosets. J of Comp Phys A. 2006;192:27–38. doi: 10.1007/s00359-005-0043-z. [DOI] [PubMed] [Google Scholar]
- Mitani J. Sexual selection and adult male orangutan long calls. Anim Behav. 1985;33:272–283. [Google Scholar]
- Mitani J, Gros-Louis J. Chorusing and convergence in chimpanzees: tests of three hypotheses. Behaviour. 1998;135:1041–1064. [Google Scholar]
- Moore SW, Lewis ER, Narins PM, Lopez PT. The call-timing algorithm of the white lipped frog, Leptodactylus albilabris. J of Comp Phys A. 1989;164:1432–1351. [Google Scholar]
- Norcross JL, Newman JD. Context and gender specific differences in the acoustic structure of common marmoset (Callithrix jacchus) phee calls. Am J Primatol. 1993;30:37–54. doi: 10.1002/ajp.1350300104. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Social context affects phee call production by nonreproductive common marmosets (Callithrix jacchus) Am J Primatol. 1997;43:135–146. doi: 10.1002/(SICI)1098-2345(1997)43:2<135::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Owren MJ, Bernacki R. The acoustic features of vervet monkey (Cercopithecus aethiops) alarm calls. J Acoust Soc Am. 1988;83:1927–1935. doi: 10.1121/1.396477. [DOI] [PubMed] [Google Scholar]
- Pistorio A, Vintch B, Wang X. Acoustic analyses of vocal development in a New World primate, the common marmoset (Callithrix jacchus) J Acoust Soc Am. 2006;120:1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Pola Y, Snowdon CT. The vocalizations of pygmy marmosets (Cebuella pygmaea) Anim Behav. 1975;23:826–842. doi: 10.1016/0003-3472(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Rendall D, Rodman PS, Edmond RE. Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim Behav. 1996;51:1007–1015. [Google Scholar]
- Rylands AB. Marmosets and Tamarins: Systematics, Behaviour, and Ecology. Oxford University Press; Oxford, UK: 1993. [Google Scholar]
- Schwartz JH. Call monitoring and interactive playback systems in the study of acoustic interactions among male anurans. In: Ryan MJ, editor. Anuran Communication. Smithsonian Institution Press; Washington, D.C.: 2001. [Google Scholar]
- Seyfarth RM, Cheney DL, Marler P. Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim Behav. 1980;28:1070–1094. [Google Scholar]
- Struhsaker TT. Auditory communication among vervet monkeys (Cercopithecus aethiops) In: Altmann SA, editor. Social communication among primates. Chicago University Press; Chicago, IL: 1967. pp. 281–324. [Google Scholar]
- Suguira H. Matching of acoustic features during the vocal exchange of coo calls by Japanese macaques. Anim Behav. 1998;55:673–687. doi: 10.1006/anbe.1997.0602. [DOI] [PubMed] [Google Scholar]
- Todt D, Naguib M. Vocal Interactions in Birds: The Use of Song as a Model in Communication. In: Slater PJB, editor. Advances in The Study of Behavior. Academic Press; New York: 2000. pp. 247–287. [Google Scholar]
- Wang X, Kadia SC. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. J Neurophys. 2001;86:2616–2620. doi: 10.1152/jn.2001.86.5.2616. [DOI] [PubMed] [Google Scholar]
- Waser PM. Experimental playbacks show vocal mediation of avoidance on a forest monkey. Nature. 1975;255:56–58. [Google Scholar]
- Waser PM, Brown CH. Habitat acoustics and primate communication. Am J Primatol. 1986;10:135–154. doi: 10.1002/ajp.1350100205. [DOI] [PubMed] [Google Scholar]
- Wilson ML, Hauser MD, Wrangham RW. Does participation in cooperative intergroup conflict depend on numerical assessment, range, location or rank for wild chimpanzees? Anim Behav. 2001;61:1203–1216. [Google Scholar]
- Zuberbuhler K, Cheney DL, Seyfarth RM. Conceptual semantics in a nonhuman primate. J Comp Psych. 1999;113:33–42. [Google Scholar]