Abstract
The development of targeted agents in oncology has rapidly expanded over the past 2 decades and has led to clinically significant improvements in the treatment of numerous cancers. Unfortunately, not all success at the bench in preclinical experiments has translated to success at the bedside. As preclinical studies shift toward defining proof of mechanism, patient selection, and rational drug combinations, it is critical to understand the lessons learned from prior translational studies to gain an understanding of prior drug development successes and failures. By learning from prior drug development, future translational studies will provide more clinically relevant data, and the underlying hope is that the clinical success rate will improve and the treatment of patients with ineffective targeted therapy will be limited.
Because standard chemotherapy demonstrates limited efficacy against a range of adult solid tumor malignancies, there has been an impetus toward the development of targeted agents in oncology. Likewise, there has been a shift of translational research away from simple screening studies of activity in preclinical models toward studies that define proof of mechanism, patient selection, and rational drug combinations. These strategies are substantially changing the preclinical rationale used to drive clinical development. Although these more robust preclinical studies have successfully guided the development of targeted agents in several tumor types, not all success at the “bench” has translated to success at the “bedside.” As preclinical models become more sophisticated, translational studies of targeted agents will have the potential to produce more clinically relevant data not only to guide “go/no-go” decisions but also to investigate resistance pathways and rational drug combinations. This review will provide examples of lessons learned from prior preclinical studies used in the development of targeted agents and addresses strategies moving forward.
Epidermal Growth Factor Receptor Targeted Agents
One of the most broadly active classes of targeted agents for solid malignancies has been the development of small molecule tyrosine kinase inhibitors (TKIs) and monoclonal antibodies against the epidermal growth factor receptor (EGFR). EGFR overexpression and activation is common in epithelial cancers (1,2), and the efficacy of targeting this pathway was initially demonstrated preclinically in vitro by blocking epidermal growth factor–stimulated phosphorylation of membrane receptors, leading to inhibition of tumor cell proliferation among a range of cancer types (3–6). These results were then recapitulated in a diverse array of xenograft models, leading some to speculate whether this would be the first example of “pathway targeting” ves “disease targeting” as a strategy for clinical development (7–12). Interestingly, early research suggested that the number of EGFRs was not an important determinant in the efficacy of antibody-mediated EGFR blockade because efficacy against T222 (non–small cell lung cancer [NSCLC], squamous) or A431 (vulvar squamous carcinoma) cells was comparable despite an approximately 100-fold higher number of EGFRs in the A431 cells (8). In colorectal cancer (CRC), preclinical studies indicated that antibodies directed against EGFR would be effective and that the addition of cetuximab to irinotecan-refractory CRC tumors could “resensitize” them to irinotecan, resulting in greater efficacy with the combination over cetuximab alone (13–15). These studies were largely reiterated clinically in CRC, where single-agent treatment with cetuximab or panitumumab resulted in improved overall and progression-free survival and a randomized phase III study of cetuximab in combination with irinotecan vs cetuximab monotherapy revealed improvements in these same measures in patients receiving the combination (Figure 1) (16–18). Interestingly, when cetuximab was initially approved for the treatment of CRC, it was only indicated for patients with tumors exhibiting overexpression of the EGFR. However, when investigators retrospectively analyzed the tumors of patients receiving cetuximab monotherapy or cetuximab in combination with irinotecan with EGFR-negative CRC, major objective responses were observed, suggesting that these patients had the potential to respond to EGFR-based antibody therapy (19). Similar results were observed with panitumumab, with no statistically significant difference seen in overall response rate, progression-free survival, or overall survival between patients with low/negative EGFR and patients with high EGFR (20). The lack of correlation between EGFR overexpression and response to EGFR antibodies was supported by scant data in preclinical models but suggested the opposite of what was considered to be common sense, indicating that the application of patient-selection biomarkers should be more comprehensively studied in preclinical models and/or that clinical trials should incorporate adaptive trial designs that include biomarker-negative subsets (21–23). However, as discussed below, such rules may be less stringent when targeting pathways that appear to be critical drivers in disease subtypes.
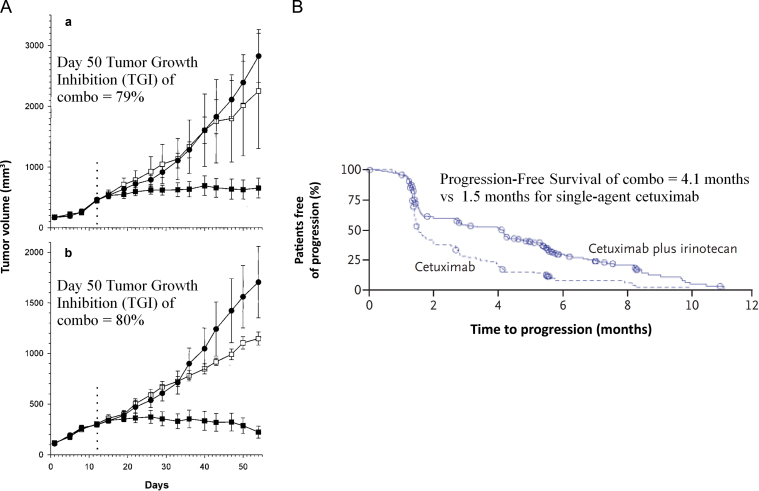
Figure 1.
Preclinical studies investigating epidermal growth factor receptor inhibition in colorectal xenografts correlated with improved patient outcomes. A) Growth inhibition of a preclinical study of CPT-11 refractory colorectal tumor xenografts in nude mice. Mice with established DLD-1 (a) or HT-29 (b) tumors were treated with two cycles of CPT-11 therapy (100mg/kg) on days 0 and 7. Mice with tumors that did not respond to CPT-11 therapy (defined as >2× initial tumor volume at day 12; shown as dotted vertical line) were selected, randomized, and then treated with IMC-C225 at 1mg/dose/every 3 days (•), continued CPT-11 at 100mg/kg/week (□), or received combination therapy (▪). Bars represent standard error (14). B) Time to disease progression in two study groups on a phase III clinical trial investigating cetuximab as monotherapy or in combination with irinotecan, in patients refractory to irinotecan (18). Reprinted with permission from the American Association for Cancer Research and the New England Journal of Medicine.
Unfortunately, the erroneous application of EGFR immunohistochemistry was not the only mistake made in EGFR antibody development in CRC. After the publication of large randomized trials, several retrospective studies revealed that patients whose tumors had a mutation in KRAS derived no benefit from therapy with cetuximab or panitumumab, leading to subsequent revision of the marketing label for both agents (24–28). Although there was a strong scientific rationale for activation of the RAS/RAF/MEK pathway to mediate resistance to upstream EGFR blockade, the limited preclinical studies investigating this were suggestive but not conclusive before the launch of large trials in unselected patients (29–33). More recently, a preclinical phase II trial of patient-derived human tumor xenograft models treated with cetuximab confirmed the key role of KRAS mutation in cetuximab resistance, suggesting that more extensive evaluation in relevant preclinical models may have prevented (or perhaps limited) the treatment of patients unlikely to attain benefit from EGFR inhibitors (Figure 2) (34).
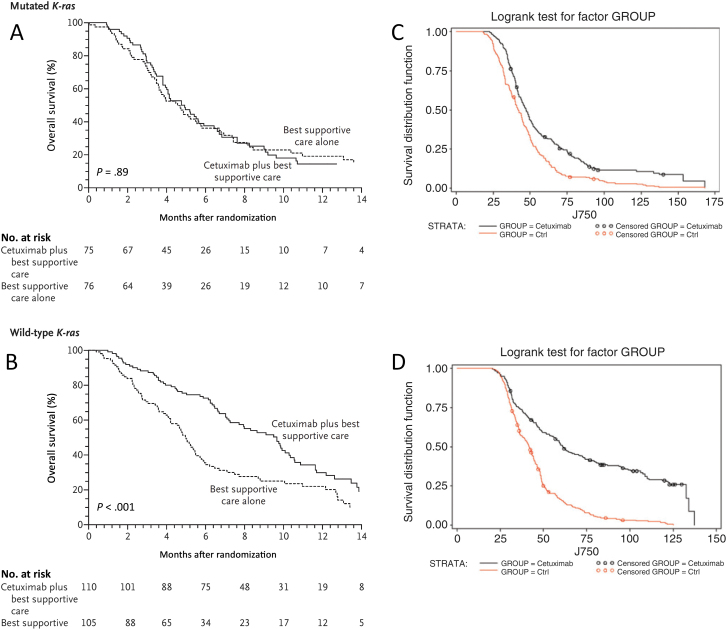
Figure 2.
Preclinical studies in patient-derived human tumor xenograft (PDTX) models correctly predict lack of response to epidermal growth factor receptor (EGFR) antibodies in KRAS-mutant colorectal cancer (CRC). Results from a retrospective analysis evaluating the effect of KRAS mutational status on overall survival in patients treated with cetuximab (A, B) and results from a PDTX trial evaluating the effect of KRAS mutational status on overall survival in PDTX patients treated with cetuximab (C, D) (24). KRAS-mutant PDTX patients treated with cetuximab had similar survival curves compared with control (CTRL) PDTX patients (C). KRAS wild-type PDTX patients treated with cetuximab had statistically significantly improved survival compared with control PDTX patients (D). The results from the xenopatient trial mirror the results seen clinically, suggesting that the lack of efficacy of EGFR monoclonal antibodies in KRAS-mutant CRC could have been predicted preclinically (34). Reprinted with permission from the New England Journal of Medicine and the American Association for Cancer Research.
Similarly, the discovery that EGFR mutation predicts clinical benefit from EGFR TKIs in patients with NSCLC was again initially observed retrospectively from clinical samples obtained from patients with gefitinib-responsive lung cancer and then subsequently corroborated in preclinical models (35,36). Conse quently, substantial advancements in EGFR targeting of NSCLC have been made by acquiring tissue from patients developing resistance, developing preclinical models that mimic clinical resistance mechanisms, and using these models to develop new inhibitors, a true “bedside-to-bench-and back” approach (37–41). For example, in the approximately 50% of patients with EGFR-mutant lung cancers who develop acquired resistance through mutation of the T790M gatekeeper threonine residue, second-generation irreversible EGFR inhibitors have been shown in preclinical models to be more potent in targeting the T790M mutant than either gefitinib or erlotinib, whereas further studies of one of these, BIBW-2992, in combination with cetuximab, demonstrated that only the combination induced dramatic shrin kage of erlotinib-resistant tumors harboring the T790M mutation through depletion of phosphorylated and total EGFR (42). Given the promising preclinical results, this work is now translating back to the bedside, with an ongoing clinical trial of the combination of BIBW-2992 and cetuximab in patients with unresectable NSCLC (Figure 3).
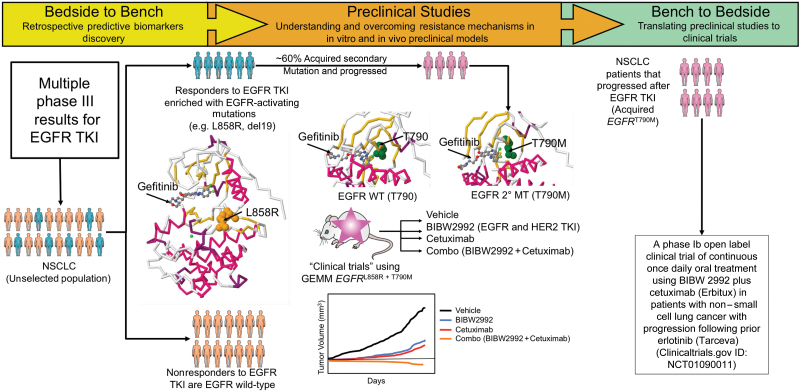
Figure 3.
An example of bedside-to-bench-and-back approach with an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) in non–small cell lung cancer (NSCLC). Bedside to Bench: Retrospective analysis of multiple phase III studies of an EGFR TKI in NSCLC revealed that responders to the EGFR TKI were enriched with activating EGFR mutations (MT) (eg, L858R, deletion in exon 19), whereas nonresponders were enriched for EGFR wild-type (WT). The protein structure of EGFR with activating mutation L858R illustrates the binding of gefitinib in the ATP-pocket (protein databank code: 2ITZ). More than 60% of the responders eventually acquired secondary mutations and progressed on EGFR TKI therapy. One of the most common secondary acquired mutations was the “gate-keeper” mutation of EGFR T790M. Protein structures of the wild-type T790 (PDB code: 2ITY) and acquired mutation T790M (PDB code: 3UG2) are shown. Multiple preclinical studies have been conducted to understand and overcome this resistance mechanism. One of these studies conducted by William Pao and colleagues (32) employed a genetically engineered mouse model that harbors this mutation. They observed that tumors regressed in the combination arm of a second generation EGFR TKI (BIBW2992) that inhibits both EGFR and HER2 with the monoclonal antibody EGFR cetuximab; the activity of this combination was statistically superior compared with the individual treatment arms (BIBW2992 or cetuximab). Bench to Bedside: A phase I clinical trial is currently open to investigate the combination of BIBW2992 and cetuximab in NSCLC patients that progressed after EGFR TKI (ClinicalTrials.gov ID: NCT01090011).
A very surprising clinical result that was not accurately predicted in preclinical models was the combination of EGFR and vascular endothelial growth factor (VEGF) inhibitors. Among preclinical studies suggesting promising activity were those in CRC and NSCLC, where the combination of cetuximab or gefitinib with the anti-VEGF receptor antibody DC101 led to striking supra-additive–to–synergistic tumor growth inhibition in CRC and NSCLC models, respectively (43,44). Despite these and numerous other studies of similar combinations (45–48), the clinical results were disappointing, and in a randomized phase III study in advanced CRC of chemotherapy and bevacizumab vs the same with panitumumab, the combination of the two targeted agents resulted in increased toxicity and decreased progression-free survival, with similar results when cetuximab was added to bevacizumab and chemotherapy, despite a promising randomized phase II trial (27,49). In NSCLC, vandetanib, a small molecule TKI of VEGFR2, EGFR, and RET induced sustained tumor regressions in an EGFR TKI-resistant mouse model in vivo, and the combination of bevacizumab and erlotinib demonstrated similar efficacy (50). However, a clinical study of vandetanib failed to meet its primary objective of demonstrating an overall survival benefit, and another phase III clinical trial of bevacizumab plus erlotinib vs erlotinib alone revealed no difference in overall survival (51,52). The lack of concordance between the preclinical and clinical studies is likely multifactorial and due to issues such as tumor heterogeneity, inability to accurately predict clinical toxicity in murine models, pharmacokinetic interactions, and overestimation of angiogenesis dependence in preclinical models, among others.
In advanced pancreatic cancer, for which erlotinib is approved in conjunction with gemcitabine, data from preclinical studies were underwhelming with regard to preclinical study endpoints and the number of models tested. In one preclinical study, an EGFR TKI (PKI166) was studied in the L3.6pl pancreatic orthotopic cell line xenograft model, and the results demonstrated a 45% and 59% reduction in tumor volume by PKI166 or gemcitabine, respectively, but an 85% reduction with the combination (53). Perhaps more relevant to the clinical trials, in a study of erlotinib as monotherapy or in combination with gemcitabine and wortmannin against patient-derived pancreatic cancer xenografts, a twofold increase in apoptosis in tumors treated with gemcitabine, wortmannin, and erlotinib was observed relative to the vehicle control (54). It should be noted that only two patient-derived xenografts were evaluated, tumor growth inhibition was not measured, and the combination of gemcitabine and erlotinib in one of the xenografts did not increase apoptosis in the absence of wortmannin. Despite the lack of robust preclinical data, a phase III clinical trial of the combination of gemcitabine and erlotinib vs gemcitabine alone in patients with advanced pancreas cancer resulted in an increase in overall survival of just 0.33 months, or roughly 10 days (55). The scientific literature is replete with negative phase III trials of novel agents in combination with gemcitabine in pancreatic cancer, the majority of which were preceded by preclinical studies (56–60). Numerous reviews have been written proposing biological mechanisms for this discordance, including poor tumor penetration in patients, inability to adequately recapitulate stromal effects in preclinical models, and tumor heterogeneity (61–63). Future preclinical studies will need to address these issues to improve the preclinical rationale needed for future clinical trials in pancreatic cancer.
Finally, an important question that has been investigated preclinically and clinically is whether monoclonal antibodies and small molecule TKIs directed toward EGFR share similar mechanisms of action in preventing ligand-induced receptor activation and blockade of EGFR-dependent pathways that could make them interchangeable in multiple disease types. Preclinical studies have shown that some of the mechanisms of action and their antitumor effects are not completely overlapping. Anti-EGFR monoclonal antibodies, but not EGFR TKIs, have the capacity to form receptor-containing complexes that result in receptor internalization, an important mechanism for attenuating receptor signaling (64). In addition, anti-EGFR monoclonal antibodies can elicit antibody-dependent cellular cytotoxicity, which increases antitumor efficacy (65). In contrast with anti-EGFR monoclonal antibodies, low-molecular-weight EGFR TKIs can induce the formation of inactive EGFR homodimers and EGFR/HER2 heterodimers, which impair EGFR-mediated transactivation of the HER2 tyrosine kinase (66–68). Clinically, EGFR monoclonal antibodies and EGFR TKIs have had different activity profiles, particularly with the lack of efficacy seen with EGFR TKIs in CRC, which is most likely due to the low incidence of mutations in the ATP site of the EGFR tyrosine kinase domain (0.34%) (69–71). These data highlight the importance of fully understanding mechanisms of action, even when drugs target similar pathways, because different classes of drugs may lead to clinical development in different tumor types.
Lessons Learned From EGFR Inhibition
Overall, preclinical studies of EGFR inhibitors have largely predicted clinical benefit in the malignancies for which they are approved—CRC, NSCLC, head and neck cancer, and pancreatic cancer—although patient selection strategies have been discovered retrospectively, thereby exposing thousands of patients to ineffective and potentially toxic treatments. Current studies suggest that patient-derived human tumor xenografts would have been able to accurately predict the lack of efficacy of EGFR monoclonal antibodies in KRAS-mutant CRC, and increasingly these models are being used to test molecular hypotheses of response, although clearly they have their own limitations (72–77). The lack of correlation between EGFR overexpression and response to EGFR inhibitors also highlights the importance of comprehensively evaluating patient selection biomarkers in the preclinical setting and not overinterpreting scant preclinical data before clinical implementation. The disparate results from preclinical and clinical studies of EGFR inhibition in pancreatic cancer have also confirmed the long-held observation that preclinical activity overestimates clinical benefit, which can be because of multiple factors, including tumor heterogeneity, microenvironmental factors, and unrealistic approximations of drug exposure (8,53,78,79). Lastly, two common sense assumptions have been refuted in preclinical and clinical models—that of the ability to select patients for EGFR pathway-based therapy independent of disease subtype and the notion that blockade by either small molecule EGFR TKI or antibody targeting is equivalent in diseases where one or the other has been clinically benchmarked.
Targeted Therapies Against Drivers of Oncogenesis
A highly successful strategy in targeted drug development in oncology has been with agents that target critical mediators of oncogenesis (80–83). One of the earliest examples was trastuzumab, a monoclonal antibody directed against the Her2/neu receptor that is currently used for the treatment of breast and gastric cancer. Early data in cell lines revealed that the Her2/neu pathway was important in tumorigenesis, and amplification of the Her2/neu oncogene was associated with neoplastic transformation and a high rate of tumor cell proliferation (84–86). Clinical data also supported the importance of amplification of Her2/neu because its presence was inversely correlated with the median overall survival of breast cancer patients (87). These observations led to the subsequent development of antibodies directed against the receptor, with in vitro and in vivo studies conducted more than a decade ago revealing activity both as a single agent and in combination with chemotherapy (86,88–91). Importantly, these early preclinical studies demonstrated a direct association between Her2/neu overexpression/amplification and reversal of the malignant phenotype by antibody blockade (91–93). Many years after the publication of these studies, a phase III trial of trastuzumab with chemotherapy vs chemotherapy alone in patients with metastatic breast cancer overexpressing Her2/neu revealed a longer time to disease progression and overall survival in patients receiving trastuzumab (94). Despite the ensuing controversies surrounding the optimal methodology for documenting Her2/neu overexpression (95–97), as pointed out in the analysis by Simon and colleagues, if this study had been performed in an unselected population, it is very likely that a survival benefit would not have been detected (98). In some respects, the development of trastuzumab is an exemplar of preclinical-to-clinical translation, although interestingly, its use in other diseases where Her2/neu is overexpressed has only been proven clinically effective in gastric cancer, indicating the disease-specific context of such targets (80).
In most gastrointestinal stromal tumors (GIST), the proto-oncogene c-kit has an activating mutation, and in preclinical studies in human GIST cell lines, the receptor TKI imatinib was shown to rapidly and completely eliminate GIST cells containing a c-kit mutation (99). These results were concordant with clinical activity in GIST, as early trials demonstrated promising efficacy in this chemotherapy–refractory disease and a phase III study confirmed a statistically significant survival benefit of imatinib in patients with unresectable CD117-positive GIST (81,100). Bedside-back-to-bench research used patient samples from a phase II clinical trial of imatinib to correlate mutations to clinical outcome and guide therapy, whereas preclinical studies conducted in GIST cell lines revealed that most mutations in the c-kit activation loop were resistant to clinically achievable doses of imatinib (101). Interestingly, it was in studies of chronic myeloid leukemia (CML) that analysis of the crystal structure of dasatinib-bound abelson leukemia gene (ABL) kinase suggested that the increased binding affinity over imatinib was at least partially due to its ability to recognize multiple states of breakpoint-cluster gene, abelson leukemia gene (BCR-ABL), including BCR-ABL–activating loop mutants (102). Given this finding in CML, further preclinical studies of dasatinib in GIST cell lines were performed, revealing induction of apoptosis in the imatinib-resistant, c-kit–activating loop mutant cell lines (102). These results were translated into ongoing clinical trials evaluating the efficacy of dasatinib in imatinib-resistant unresectable GIST, reflecting, in this case, the relevance of transdisciplinary preclinical research into resistance mechanisms of specific classes of agents (103). Perhaps such basic resistance processes that alter receptor structural biology and drug binding affinity are more readily adaptable from one disease to another, indicating the importance of not only detailed characterization in vitro and in vivo but also multidisciplinary collaboration.
The rapid clinical development of vemurafenib, an inhibitor of BRAF in BRAF-mutated melanoma, although obviously an example of success, has illustrated the importance of preclinical models in assessing the disease-specific activity of targeting mutations as well as resistance mechanisms. Initially, vemurafenib was shown to potently inhibit melanoma cell lines bearing the BRAF V600E mutation but not in cells lacking oncogenic BRAF, in vitro and in vivo (104–106). These preclinical results were recapitulated clinically, as vemurafenib was found to statistically significantly increase overall survival in patients with BRAF V600E mutant metastatic melanoma when compared with standard chemotherapy (82). Although scant preclinical data existed on the activity of vemurafenib in BRAF-mutant CRC, single-agent vemurafenib was administered in a phase I extension trial of patients with previously treated metastatic CRC with only one confirmed partial response among 19 evaluable patients, clearly indicating de novo resistance in this disease (107). Subsequently, preclinical studies investigating the activity of vemurafenib in BRAF-mutant CRC have revealed inherent resistance mechanisms involving the c-met, EGFR, and PI3K pathways, among others, suggesting that up-front rational combinations will be required in CRC (108–110).
Another powerful application of preclinical research has been the investigation into the acquired resistance mechanisms that occur fairly rapidly in patients with BRAF V600E–mutant metastatic melanoma receiving single-agent vemurafenib. Although dramatic pictures of a patient initially responding and then developing dramatic resistance to vemurafenib have been widely disseminated, importantly, this devastating outcome has been investigated comprehensively in preclinical models using patient tissue, cell lines, and next-generation sequencing technology (111–119). One of the most “actionable” resistance mechanisms has been the discovery of MAP kinase pathway reactivation, leading to the preclinical and clinical combination of RAF and MEK inhibitors for advanced melanoma (111,120). In preclinical models, rapid recovery of MAPK pathway signaling was associated with BRAF inhibitor resistance, and complete inhibition of the MAPK pathway induced cell death in BRAF V600E melanoma (111). When the combination of a BRAF inhibitor and a MEK inhibitor was studied in a clinical trial, progression-free survival was statistically significantly improved (121). An interesting dilemma that may be more easily tested in preclinical models is whether dual targeting up-front vs sequential targeting is more effective in combating resistance mechanisms because the clinical trials have focused on the combination vs monotherapy in untreated patients. The differential biological impact of targeting BRAF in melanoma and CRC is a clear example of an instance where more extensive and unbiased preclinical studies could have defined mechanisms of de novo and acquired resistance earlier and hastened the development of effective rational combinations. As it is, groups working in this area rapidly exploited clinical data to investigate resistance mechanisms in preclinical studies, setting the standard for comprehensive translational drug development strategies in oncology (109,112,121,122).
Inhibition of the anaplastic lymphoma kinase (ALK) in lung cancer, although clearly an example of the therapeutic benefit of targeting driver mutations, also illustrates the iterative and nonlinear nature of translational studies in drug development. During its initial preclinical development, crizotinib was identified as an ATP-competitive small-molecule inhibitor of the catalytic activity of c-met kinases that also inhibited ALK at pharmacologically relevant concentrations in lymphoma cell lines expressing the NPM-ALK oncogenic fusion protein (123). Concurrent with phase I trials of the agent, a transforming fusion gene comprising portions of the echinoderm microtubule-associated protein-like 4 (EML4) gene and ALK gene was described in 6.7% of NSCLC tumors (124). This finding led investigators to screen and select patients in the latter stages of the phase I trial for the ALK fusions by fluorescence in situ hybridization (FISH), resulting in dramatic tumor regression and disease stabilization when these patients were treated with crizotinib, a result that was ultimately confirmed in a phase II registration trial and a recently published phase III trial (83,125,126). These clinical results were recapitulated mechanistically in preclinical models, whereas experiments in ALK knockout mice performed years earlier corroborated the relative lack of toxicity observed in the clinical trials (127,128).
Finally, the subsequent identification of resistance mechanisms to crizotinib has been an elegant example of the value of “bench-to-bedside-and-back” translational research. Repeat FISH analysis and DNA sequencing characterized the amplification of the ALK fusion gene and mutations in the kinase domain of ALK from patients progressing on crizotinib as the potential molecular mechanisms of resistance (129–131). In vitro NSCLC cell lines selected for resistance to crizotinib also show amplification of the ALK fusion gene as well as kinase domain mutations such as L1196M (130). Data from both resistant patient tumor samples and cell lines have led to rationales for novel second-generation ALK TKIs that inhibit these mechanisms of resistance to now be tested in the clinic.
Lessons Learned in Targeted Therapies Against Drivers of Oncogenesis
The last decade has witnessed an impressive growth of agents directed at drivers of the oncogenic process. By definition, these agents may be easier to study in preclinical models and to develop clinically, although clinical and scientific observations must be exchanged as seamlessly as possible between patients and preclinical models, respectively, to derive the greatest benefit. An important concept is that although acquired treatment-associated mutations may be effectively targeted across tumor types, such as use of dasatinib after imatinib failure in CML and GIST, the drivers of oncogenesis to be targeted may differ among disease sites because of inherent resistance mechanisms. This is clearly illustrated in the striking difference in efficacy of vemurafenib in BRAF V600E–mutant melanoma and BRAF V600E–mutant CRC, with preclinical studies highlighting the differences in resistance mechanisms. This provides an opportunity for investigators to interrogate disease-specific banks of cell lines and patient-derived tissues for the presence of drivers and the functional impact of targeting them and to assess resistance mechanisms and develop rational targeting strategies. In fact, this shift of preclinical research away from drug efficacy screening and toward molecular and functional studies of responsiveness or resistance is paving the way for the future of translational research. The ALK story with crizotinib also highlights the importance of early identification and rapid translation of novel targets, so that even in phase I trials patients can be screened for rare genetic drivers.
Targeting Angiogenesis
An important obstacle to the development of antiangiogenic agents has been the lack of suitable preclinical models for assessing their efficacy. In fact, although there have been clear clinical successes (132–135), these agents have been tested in numerous phase III trials with negative results, often based upon either nonexistent or misleading preclinical data (136–138). Clearly, in vitro assays, although perhaps useful for screening, do not recapitulate angiogenesis in vivo because of the absence of critical factors such as host- and tumor-derived microenvironmental factors (139). In vivo, one of the earliest metrics for assessing antiangiogenic effects preclinically was the use of microvessel density in murine models (140–142). After about a decade of attempts at clinical translation with poor results (Figure 4), the assessment of microvessel density in cancer patients as a pharmacodynamic marker of angiogenesis inhibition was finally abandoned, and its demise was the subject of an elegant review by Dr Judah Folkman (143). More recently, functional imaging studies dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) have been assessed in murine models and in patients and appear to be a valid translatable pharmacodynamic endpoint for assessing the effects of VEGF blockade, although it is less clear for other angiogenic targets (144–146). Figure 5 depicts the results of a bench-to-bedside study of DCE-MRI after treatment of mice and patients with G6-31 (see below) or bevacizumab, respectively (147).
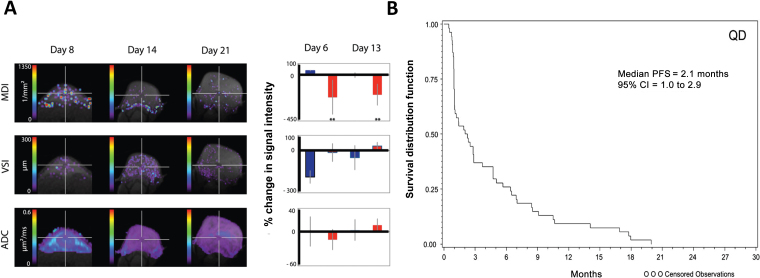
Figure 4.
Preclinical microvessel density studies have not accurately predicted clinical efficacy with angiogenesis inhibitors. A) A preclinical study of vatalanib (PTK787) in H1975 non–small cell lungt cancer (NSCLC) xenografts exhibits statistically significant changes in tumor microvessel density index (MDI), tumor vessel size (VSI), and apparent diffusion coefficient (ADC). The blue bars represent vehicle-treated tumors, and the red bars represent vatalanib-treated tumors (224). B) Kaplan–Meier plots for progression-free survival (PFS) in patients with NSCLC treated with vatalanib in a phase II clinical trial demonstrating a median PFS of 2.1 months (225). Reprinted with permission from PLoS One and Oxford University Press.
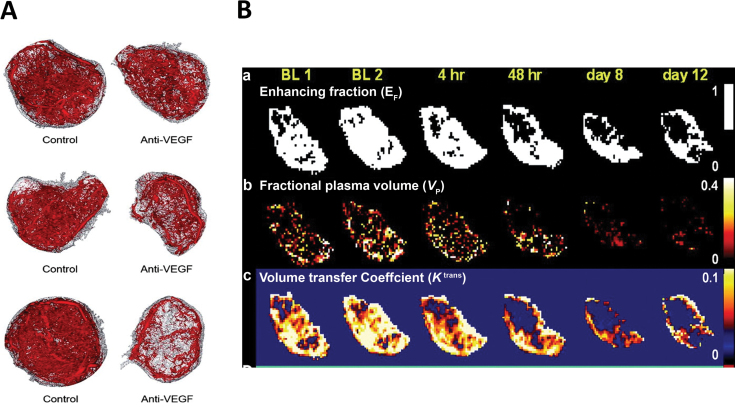
Figure 5.
Results of a bench-to-bedside study of dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) after treatment of mice and patients with G6-31. A) Ex vivo evidence for rapid antivascular effects of G6-31. Representative micro–computed tomography angiographic data for each treatment group is shown at 90 minutes, 24 hours, and 48 hours. The extracted vascular network (red) and the entire tumor (gray) are shown. B) Representative MRI parameter maps from one patient. a) Map of enhancing voxels demonstrates a statistically significant decrease in blood flow beginning at 48 hours that persists through day 12. b) Map of fractional blood plasma volume (v p) also demonstrates a statistically significant decrease, beginning at 4 hours that persists through day 12. c) Map of the K trans exhibit reduction in blood flow within 4 hours that persist at 48 hours and day 8, but returns to baseline levels by day 12. These changes are consistent with reduction in either vessel permeability and/or blood flow (147). Reprinted with permission from the American Association for Cancer Research.
A limitation to the preclinical testing of antibodies that target angiogenic growth factors in murine models has been the presence of both murine and human tumor–associated angiogenic growth factors. Thus, bispecific murine/human antibodies must be developed, aprocess that has often lagged behind the clinical development of these agents. For example, Genentech developed their bispecific murine/human monoclonal antibody against VEGF A/B, G6-31, available to investigators in 2006 for preclinical studies, after bevacizumab had completed positive randomized phase II and phase III trials in CRC (132,148). Before that, most preclinical studies were conducted with either bevacizumab (ignoring the murine stromal VEGF) or DC101, an antibody against the murine VEGFR2 receptor. Nonetheless, the activity of antibodies targeting the VEGF pathway was quite robust in preclinical models of CRC and breast cancer, among others, despite the fact that single-agent activity is very limited in patients (149,150). This has prompted closer scrutiny of human xenograft models, leading to the conclusion that the exaggerated antitumor efficacy is most likely due to rapid tumor and vascular growth in these models that results in greater dependence on limited angiogenesis growth factors and thus responsiveness to antiangiogenic therapy (151–153).
An interesting transgenic model that has been clinically benchmarked recently is the rat insulin promoter (RIP)–T-antigen transgene (Tag) model developed by Hanahan and colleagues in which the RIP directs expression of the SV40 large Tag to beta cells of the pancreatic islets (154,155). This model was designed to avoid the confounding biases of neutralizing antibody responses by developing an immunodeficient variant, rendering it completely deficient in adaptive immunity. This allowed the investigators to demonstrate that B and T lymphocytes were not factors in pancreatic islet tumorigenesis. In this study, the inhibition of VEGFR2, but not VEGFR1, markedly disrupted the angiogenic switch, angiogenesis, and initial tumor growth (156). In late-stage tumors, phenotypic resistance to VEGFR2 blockade emerged, and potential resistant mechanisms were discovered, including induction of the proangiogenic fibroblast growth factor. Further investigation with sunitinib, a TKI of primarily VEGFR2, VEGFR3, c-kit, and PDGFR, was also shown to be effective in inhibiting growth in the RIP-Tag model, and a phase III clinical trial of sunitinib in patients with advanced, well-differentiated pancreatic neuroendocrine tumors demonstrated an improvement in overall response rate, progression-free survival, and overall survival (157,158). It is also important to note that efficacy of sunitinib was not observed across multiple tumor types, despite the elegant work performed in the RIP-Tag model. A phase II trial of sunitinib in patients with metastatic refractory CRC was completed despite a relative lack of preclinical evaluation, and the trial failed to demonstrate a meaningful single-agent objective response in patients (159).
Another limitation of preclinical evaluation is the inability to accurately predict the toxicity of a particular agent, especially in combination with other chemotherapeutic agents. Preclinical evaluation of the TKI vatalanib, an inhibitor of VEGFR-1/Flt-1, VEGFR-2/KDR, VEGFR-3/Flt-4, PDGF-β, c-Fms, and c-kit (but with greater potency for VEGFR-1 and VEGFR-2), revealed tumor growth and angiogenesis inhibition in cell line-derived xenografts, but phase III evaluation of the combination of FOLFOX4 and vatalanib resulted in no statistically significant improvement in response rate, progression-free survival, or overall survival (160,161). Of note, there were clinically significant adverse events associated with this combination, reflected by an 18.1% study discontinuation rate because of toxicity (161).
Extensive reviews have described the transitory nature of the clinical benefit from angiogenesis inhibitors due to evasive resistance and adaptation to circumvent angiogenic blockade (151,152). Bedside-to-bench investigation is ongoing in an effort to target known resistance mechanisms, identify predictive markers of response, and develop combination regimens that synergize with VEGF inhibitors (136,162–164). Recently, evaluation of the role of placental-growth factor (PlGF) has led to a potential improvement in angiogenic therapy in CRC. PlGF has been shown to be upregulated in response to anti-VEGF therapy in both preclinical models and prospective evaluation in patients treated with anti-VEGF therapy (162,163,165). Results from a recent phase III trial of a dual-inhibitor of PlGF and VEGF-A in patients with refractory metastatic CRC demonstrated that continued blockade of VEGF family members confers a modest clinical benefit in patients with acquired resistance to anti-VEGF therapy, but because of the study design it is currently unclear whether patients were benefitting from continued VEGF inhibition, inhibition of PlGF, or both (166).
Lessons Learned From Angiogenesis
The clinical benchmarking of the first angiogenic agents followed decades of elegant preclinical studies that likely overstated their single-agent activity and led to numerous failed clinical trials. Preclinical studies investigating the combination of chemotherapy and antiangiogenic agents were also lacking before clinical trials. Since then, much has been learned about the process of angiogenesis and the inherent bias that resides in preclinical models. Nonetheless, progress has been made with the development of more robust and translational pharmacodynamic markers of activity and with murine models that are designed to mimic specific disease subtypes. Going forward, there will continue to be challenges with effectively assessing the activity of these agents in humans using preclinical models, and this will continue to be complicated by the lack of predictive biomarkers for selecting patients. However, these obstacles should not hinder development of angiogenesis inhibitors but should stimulate more relevant model development and further research into predictive and pharmacodynamic biomarkers.
Targeting Basic Cellular Processes
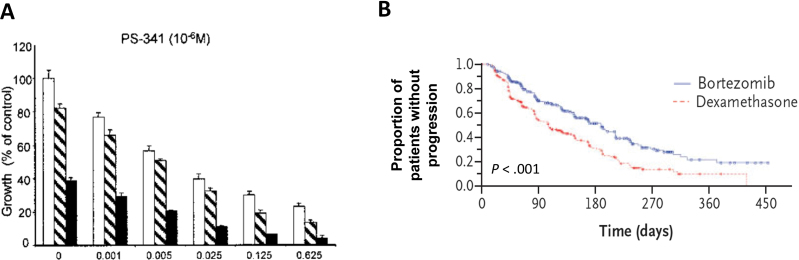
Another focus in targeted cancer therapy research has been the inhibition of basic cellular processes that appear to be particularly critical for maintenance of the malignant phenotype, such as protein processing and epigenetic gene regulation (167–170). Many investigators have focused on the ubiquitin–proteasome pathway, where inhibition of the degradation of cell cycle regulatory proteins by proteasome inhibition can induce apoptosis (167,171,172). Bortezomib, the only proteasome inhibitor approved for therapy, demonstrated activity against preclinical models of multiple myeloma in vitro and in vivo and in patient-derived multiple myeloma cells (172–175). The mechanism of its activity in multiple myeloma is thought to be partially due to blocking of NF-κB–mediated transcription of interleukin 6 and insulin-like growth factor 1 by inhibition of the proteasomal degradation of I κβ (172,173). The preclinical antitumor and mechanistic studies of bortezomib in multiple myeloma were successfully translated clinically, as reflected in the phase I trial of bortezomib in patients with refractory hematologic malignancies, which demonstrated clinical activity in nine patients with multiple myeloma comprised of one complete response and eight patients with a reduction in paraprotein levels and/or marrow plasmacytosis, as well as in a phase III clinical trial of bortezomib vs dexamethasone (Figure 6) (176,177). Likewise, subsequent studies in pediatric tumors demonstrated preferential activity against pediatric acute lymphocytic leukemia models vs solid tumors, which was subsequently recapitulated clinically, where the combination of bortezomib and induction chemotherapy resulted in striking activity in pediatric patients with relapsed B-precursor acute lymphoblastic leukemia (178,179).
Figure 6.
Preclinical studies of bortezomib in multiple myeloma cell lines accurately predicted benefit in patients with multiple myeloma. A) Preclinical study of bortezomib vs dexamethasone against multiple myeloma cell lines. Dexamethasone in control media (□) and with bortezomib 0.0025 (□) or 0.005 (■) × 10−6 M. The preclinical study demonstrates statistically significant activity of bortezomib compared with dexamethasone (172). B) Progression-free survival in a phase III clinical trial of bortezomib vs dexamethasone in patients with multiple myeloma. A statistically significant increase in progression-free survival was seen in patients receiving bortezomib compared with patients receiving dexamethasone (177). Reprinted with permission from the American Association for Cancer Research and the New England Journal of Medicine.
Surprisingly, despite the marked preclinical in vivo activity documented against an array of solid tumors, the clinical activity of bortezomib has been unremarkable both as a single agent and in combination (168,180–184). One major disappointment was in pancreatic cancer, for which, in preclinical studies, bortezomib demonstrated activity both in vitro and in vivo and was hypothesized to reverse NF-κB–mediated chemotherapy resistance (168,181,185,186). After the failure of bortezomib in a phase II trial in patients with metastatic pancreatic cancer, a repeat investigation of bortezomib and gemcitabine in an orthotopic pancreatic adenocarcinoma mouse model revealed tumor inhibition with gemcitabine but tumor promotion with bortezomib by induction of angiogenesis (187). Additionally, other studies have highlighted the difficulties of achieving adequate and consistent exposure with bortezomib in xenograft models, whereas in patients with solid tumors, there is concern that the agent does not achieve sustained exposure to modulate proteasomal targets (188–190). The challenges of bortezomib in solid tumors have illustrated the importance of pharmacology and accurate approximation of human exposure in preclinical models, particularly when transitioning from hematologic to solid malignancies, as well as the difficulty of precisely establishing target modulation with agents that exhibit variable context-dependent effects (187–189).
A somewhat similar scenario in nonhematological malignancies has been observed with the development of the histone deacetylase (HDAC) inhibitors, epigenetic modulators that have protean effects on cells but in cancer are thought to potentially de-repress cell cycle regulatory genes and nuclear receptors such as estrogen receptor α (191–193). The first HDAC inhibitor approved for clinical use, vorinostat, exhibited in preclinical models a broad spectrum of epigenetic activity as well as efficacy against a range of solid tumors in vitro and in vivo, particularly in combination with other agents (194–196). However, in this case, the activity in cutaneous T-cell lymphoma, for which both vorinostat and romidepsin are approved, was identified serendipitously in a phase I trial, with subsequent confirmation in single-arm phase II trials with 30% and 34% objective response rates, respectively (197,198). Although investigations into the mechanisms of actions are ongoing, preclinical investigations in cutaneous T-cell lymphoma cell lines have shown that vorinostat induces an accumulation of acetylated histones, an increase of p21WAF1 and bax, a decrease of STAT6, and activation of caspase 3 (199). Similar findings have been seen with romidepsin (200). Similar to bortezomib, a large number of clinical studies of vorinostat with a variety of agents were launched, often with proposed mechanisms that were worked out both in vitro and in vivo (201–207). Additive or synergistic effects against tumor cell growth have been reported in numerous preclinical studies in multiple solid tumors where vorinostat was combined with chemotherapeutic or targeted agents, but several clinical studies have failed to successfully build upon the preclinical results (205,208,209). Hypotheses for the lack of efficacy seen in certain tumor types include the difficulty of achieving intratumoral therapeutic effect of vorinostat in CRC (208), inability to exert synergistic cytotoxicity within the central nervous system in recurrent glioblastoma multiforme (205), and limited drug exposure because of poor tolerability (210). More recent studies have investigated improved patient selection and predictive markers using a bedside-back-to-bench approach, highlighted by a phase II study of vorinostat combined with tamoxifen that demonstrated that histone hyperacetylation and HDAC2 expression may be viable pharmacodynamic and predictive biomarkers, respectively (211). A recent randomized phase II study investigating the HDAC inhibitor entinostat in combination with the EGFR TKI erlotinib in NSCLC revealed no additional efficacy, whereas a preplanned biomarker analysis revealed that the subset of patients with EGFR wild-type tumors and high e-cadherin protein expression exhibited statistically significantly longer overall survival (12.2 months vs 5.4 months; P = 0.03) (212).
Lessons Learned From Targeting Basic Cellular Processes
Somewhat surprising has been the clinical approval and robust activity of agents impacting a range of targets in normal and malignant cells in a rather nonselective manner, providing support for continued preclinical work to identify and characterize such challenging targets. The ability to clinically translate the success of these agents from hematologic malignancies and preclinical models has been hampered by pharmacologic limitations and difficulties in adequately predicting drug exposure and toxicity. Therefore for success in preclinical models to be translated, comprehensive pharmacokinetic/pharmacodynamic analyses indexed to human exposure need to be included, with the huge caveat that toxicity may be poorly recapitulated in these models. Future clinical trials with these agents will be dependent on bedside-back-to-bench studies that will improve patient selection and biomarker development that will be critical for successful clinical development.
Preclinical Models and Prediction of Pharmacokinetics and Toxicity
Lack of efficacy and clinically significant toxicity are major reasons for targeted therapy failure, and therapeutics with favorable pharmacokinetics are more likely to be efficacious and safe. Thorough preclinical pharmacokinetic evaluation may guide dose selection in the phase I clinical trial setting. Pharmacokinetic endpoints that are shown to correlate with biological activity in preclinical studies may enable safer dose selection of molecularly targeted agents in phase I clinical trials (213). However, the predictive value of preclinical models may be limited by individual human genetic sensitivities, immunologically mediated phenomena, and idiosyncratic reactions (214). Preclinical data may also help formulate clinical trial design. If the preclinical data suggest a wide therapeutic window with little toxicity, an aggressive dose titration may be reasonable. Imatinib is an example of a targeted agent that demonstrated dramatic efficacy as well as a favorable pharmacokinetic profile before any dose-limiting toxicity was encountered (215). However, if preclinical data suggest a narrow therapeutic window, more conservative phase I clinical trial design would be warranted. There are several approaches by which human pharmacokinetic data can be predicted from preclinical data, and although a comprehensive review of the prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data is beyond the scope of this review, comprehensive reviews of this topic are available in the literature (216).
Limitations in Preclinical Models and Future Directions
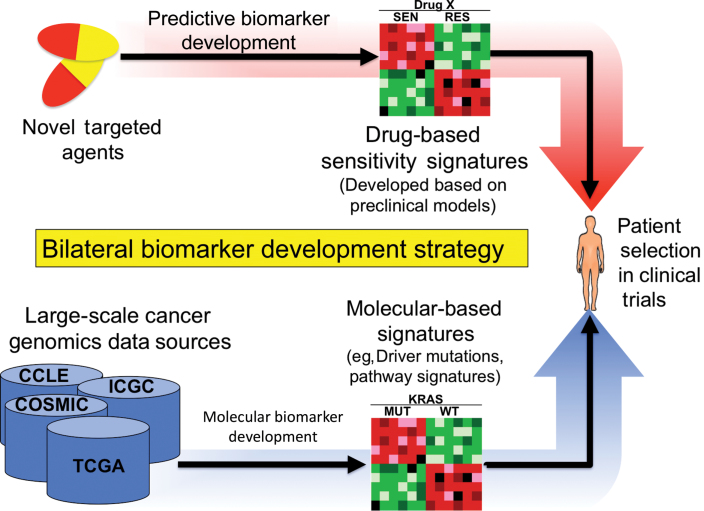
As new molecular targets for cancer therapy are discovered and characterized, the ability to efficiently and effectively translate these findings to patients has become rate limiting. In fact, results from internationally collaborative tumor sequencing efforts are yielding an unprecedented array of aberrations, many of which appear to be tractable targets for drug development (217–219). The push toward personalized medicine has now added another facet to preclinical drug development, beyond the usual demonstration of efficacy. Thus, preclinical studies increasingly rely upon larger banks of disease-specific cell lines and tissues that have undergone extensive genetic annotation. The future of drug development likely will take on a bilateral approach, in which drug responsiveness signatures are developed in cell lines and patient-derived xenograft models, concurrent with the molecular categorization of patient tissues (Figure 7). Convergence will occur at the patient exhibiting a response profile that is indexed to a molecular bin within a specific disease context. Nonetheless, challenges will remain in categorizing models as responsive or nonresponsive to improve the accuracy of preclinical models in predicting clinical benefit. Rather than discarding the process of preclinical drug development as being hopelessly flawed, the drug development community should strive for establishing appropriate model scenarios, greater stringency, and more guidance for what criteria constitute a sufficient body of data for clinical testing. For agents where a particular disease subtype has been clinically benchmarked, consideration should be given to incorporating a positive control (the tumor type known to respond in a clinical setting) into screens where an agent is being tested for use against other malignancies as an internal standard to gauge relative activity. Additionally, real-time retrotranslation should be conducted using tolerable drug exposures achieved in early clinical trials to determine whether the exposures achieved in humans are sufficient for preclinical efficacy to help guide development decisions because drug exposures in preclinical studies have typically surpassed achievable levels in humans (220–223). Patient-derived tumor xenografts, although arguably a more relevant in vivo model for testing than cell line xenografts, are still subject to the same limitations in terms of the tumor microenvironment and therefore should not be regarded as positive when only modest tumor growth inhibition, not regression, is observed (76). Likewise, combination therapies, where much of drug development is focused, for good reason, will need to excel beyond the standard assessments of antiproliferative synergy toward assays that detect the potentiation of cell death with the combination. This will become paramount as resistance pathways are identified clinically, along with use of tools such as gene set enrichment analysis and synthetic lethality that increasingly are revealing strategies for rational combinations. Although not the subject of this review, another important component of preclinical translation is the ability to design early clinical trials where feasibility testing of efficacy and patient selection strategies can occur (22). This has been the subject of numerous reviews, but suffice it to say that we need to overhaul the design of phase I trials to routinely accommodate disease-specific expanded cohorts, tissue acquisition upon disease progression, and early testing of rational combinations. The lessons learned thus far in preclinical translation of targeted agents in oncology should be viewed as facilitating a roadmap that will avoid the obstacles of the past and provide a way forward in the future.
Figure 7.
A potential future bilateral biomarker development strategy. The future of drug development likely will take on a bilateral approach, in which drug responsiveness signatures are developed in cell lines and patient-derived xenograft models in concert with the comprehensive molecular categorization of patient tissues. Thus, drug responsive signatures can then be directly indexed against clinical samples, facilitating patient selection. COSMIC = Catalog of Somatic Mutations in Cancer; ICGC = International Cancer Genome Consortium; MUT = mutant; TCGA = The Cancer Genome Atlas; RES = resistant; SEN = sensitive; WT = wild-type;
Funding
This work was supported by the National Cancer Institute K12 (5K12CA132783-03) Paul Calabresi Career Development Award for Clinical Oncology.
Note
The study sponsor had no role in the writing or decision to submit the review article for publication.
References
- 1. Ozanne B, Richards CS, Hendler F, et al. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J Pathol. 1986;149(1):9–14 [DOI] [PubMed] [Google Scholar]
- 2. Sozzi G, Miozzo M, Tagliabue E, et al. Cytogenetic abnormalities and overexpression of receptors for growth factors in normal bronchial epithelium and tumor samples of lung cancer patients. Cancer Res. 1991;51(1):400–404 [PubMed] [Google Scholar]
- 3. Sato J, Kawamoto T, Le A, et al. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1(5):511–529 [PubMed] [Google Scholar]
- 4. Gill GN, Kawamoto T, Cochet C, et al. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Bio Chem. 1984;259(12):7755–7760 [PubMed] [Google Scholar]
- 5. Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62(20):5749–5754 [PubMed] [Google Scholar]
- 6. Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by cp-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57(21):4838–4848 [PubMed] [Google Scholar]
- 7. Fan Z, Baselga J, Masui H, et al. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53(19):4637–4742 [PubMed] [Google Scholar]
- 8. Masui H, Kawamoto T, Sato JD, et al. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1984;44(3):1002–1007 [PubMed] [Google Scholar]
- 9. Matar P, Rojo F, Cassia R, et al. combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer res. 2004;10(19):6487–6501 [DOI] [PubMed] [Google Scholar]
- 10. Judde J-G, Rebucci M, Vogt N, et al. Gefitinib and chemotherapy combination studies in five novel human non small cell lung cancer xenografts. Evidence linking EGFR signaling to gefitinib antitumor response. In t J Cancer. 2007;120(7):1579–1590 [DOI] [PubMed] [Google Scholar]
- 11. Yang X-D, Jia X-C, Corvalan JRF, et al. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38(1):17–23 [DOI] [PubMed] [Google Scholar]
- 12. Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19(56):6550–6565 [DOI] [PubMed] [Google Scholar]
- 13. Fan Z, Masui H, Altas I, et al. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53(18):4322–4328 [PubMed] [Google Scholar]
- 14. Prewett MC, Hooper AT, Bassi R, et al. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody imc-c225 in combination with irinotecan (cpt-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8(5):994–1003 [PubMed] [Google Scholar]
- 15. Yang X-D, Jia X-C, Corvalan JRF, et al. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant hemotherapy. Cancer Res. 1999;59(6):1236–1243 [PubMed] [Google Scholar]
- 16. Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. New Engl J Med. 2007;357(20):2040–2048 [DOI] [PubMed] [Google Scholar]
- 17. Gibson TB, Ranganathan A, Grothey A. Randomized phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6 (1):29–31 [DOI] [PubMed] [Google Scholar]
- 18. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345 [DOI] [PubMed] [Google Scholar]
- 19. Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23(9):1803–1810 [DOI] [PubMed] [Google Scholar]
- 20. Hecht JR, Mitchell E, Neubauer MA, et al. Lack of correlation between epidermal growth factor receptor status and response to panitumumab monotherapy in metastatic colorectal cancer. Clin Cancer Res. 2010;16(7):2205–2213 [DOI] [PubMed] [Google Scholar]
- 21. Phillips K, Bebber SV, Issa A. Diagnostics and biomarker development: priming the pipeline. Nat Rev Drug Discov. 2006;5(6):463–469 [DOI] [PubMed] [Google Scholar]
- 22. An M-W, Mandrekar SJ, Sargent DJ. A 2-stage phase ii design with direct assignment option in stage II for initial marker validation. Clin Cancer Res. 2012;18(16):4225–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dancey JE, Dobbin KK, Groshen S, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16(6):1745–1755 [DOI] [PubMed] [Google Scholar]
- 24. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. New Engl J Med. 2008;359(17):1757–1765 [DOI] [PubMed] [Google Scholar]
- 25. Lièvre A, Bachet J-B, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995 [DOI] [PubMed] [Google Scholar]
- 26. Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the ras/raf signaling pathway impairs the response of metastatic colorectal cancers to anti–epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643–2648 [DOI] [PubMed] [Google Scholar]
- 27. Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. New Engl J Med. 2009;360(6):563–572 [DOI] [PubMed] [Google Scholar]
- 28. Amado RG, Wolf M, Peeters M, et al. Wild-type kras is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–1634 [DOI] [PubMed] [Google Scholar]
- 29. Shields J, Pruitt K, McFall A, et al. Understanding Ras: ‘it ain’t over ‘til it’s over’. Trends Cell Biol. 2000;10(4):147–154 [DOI] [PubMed] [Google Scholar]
- 30. McCarthy SA, Samuels ML, Pritchard CA, et al. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Gene Devel. 1995;9(16):1953–1964 [DOI] [PubMed] [Google Scholar]
- 31. Schulze A, Lehmann K, Jefferies HBJ, et al. Analysis of the transcriptional program induced by Raf in epithelial cells. Gene Devel. 2001;15(8):981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Camp ER, Summy J, Bauer TW, et al. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res. 2005;11(1):397–405 [PubMed] [Google Scholar]
- 34. Julien S, Merino-Trigo A, Lacroix L, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18(19):5314–5328 [DOI] [PubMed] [Google Scholar]
- 35. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. New Engl J Med. 2004;350(21):2129–2139 [DOI] [PubMed] [Google Scholar]
- 36. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500 [DOI] [PubMed] [Google Scholar]
- 37. Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating erbb3 signaling. Science. 2007;316(5827):1039–1043 [DOI] [PubMed] [Google Scholar]
- 38. Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non–small-cell lung cancer to gefitinib. New Engl J Med. 2005;352(8):786–792 [DOI] [PubMed] [Google Scholar]
- 39. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Engelman JA, Zejnullahu K, Gale C-M, et al. PF00299804, an irreversible pan-ERBB Inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67(24):11924–11932 [DOI] [PubMed] [Google Scholar]
- 41. Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102(21):7665–7670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Regales L, Gong Y, Shen R, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119(10):3000–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tonra JR, Deevi DS, Corcoran E, et al. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res. 2006;12(7):2197–2207 [DOI] [PubMed] [Google Scholar]
- 44. Guy S, Ashton S, Hughes G, et al. Gefitinib (Iressa, ZD1839) enhances the activity of the novel vascular-targeting agent ZD6126 in human colorectal cancer and non-small cell lung cancer (NSCLC) xenograft models [abstract]. Clin Cancer Res. 2003;9(Suppl):B7 [Google Scholar]
- 45. Jung YD, Mansfield PF, Akagi M, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38(8):1133–1140 [DOI] [PubMed] [Google Scholar]
- 46. Ciardiello F, Caputo R, Damiano V, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9(4):1546–1556 [PubMed] [Google Scholar]
- 47. Ciardiello F, Bianco R, Damiano V, et al. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res. 2000;6(9):3739–3747 [PubMed] [Google Scholar]
- 48. Shaheen RM, Ahmad SA, Liu W, et al. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer. 2001;85(4):584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–680 [DOI] [PubMed] [Google Scholar]
- 50. Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15(10):3484–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non–small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase iii trial (ZEPHYR). J Clin Oncol. 2012;30(10):1114–1121 [DOI] [PubMed] [Google Scholar]
- 52. Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377(9780):1846–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bruns CJ, Solorzano CC, Harbison MT, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60(11):2926–2935 [PubMed] [Google Scholar]
- 54. Ng SSW, Tsao M-S, Nicklee T, et al. Effects of the epidermal growth factor receptor inhibitor OSI-774, tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma 1. Mol Cancer Ther. 2002;1(10):777–783 [PubMed] [Google Scholar]
- 55. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966 [DOI] [PubMed] [Google Scholar]
- 56. Gatto C, Rieppi M, Borsotti P, et al. BAY 12–9566, a novel inhibitor of matrix metalloproteinases with antiangiogenic activity. Clin Cancer Res. 1999;5(11):3603–3607 [PubMed] [Google Scholar]
- 57. Moore MJ, Hamm J, Dancey J, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12–9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III Trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21(17):3296–3302 [DOI] [PubMed] [Google Scholar]
- 58. End DW, Smets G, Todd AV, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61(1):131–137 [PubMed] [Google Scholar]
- 59. Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22(8):1430–1438 [DOI] [PubMed] [Google Scholar]
- 60. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Philip PA, Mooney M, Jaffe D, et al. Consensus report of the National Cancer Institute Clinical Trials Planning Meeting on pancreas cancer treatment. J Clin Oncol. 2009;27(33):5660–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gutierrez-Barrera AM, Menter DG, Abbruzzese JL, et al. Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochem Biophys Res Comm. 2007;358(3):698–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Azmi A, Mohammad M, Kaseb A, et al. Utility of animal models in pancreatic cancer research. In: Lowy A, Leach S, Philip P, eds. Utility of Animal Models in Pancreatic Cancer Research. New York: Springer; 2008:577–599 [Google Scholar]
- 64. Fan Z, Lu Y, Wu X, et al. Antibody-induced epidermal growth factor receptor dimerization mediates inhibition of autocrine proliferation of A431 squamous carcinoma cells. J Biol Chem. 1994;269(44):27595–27602 [PubMed] [Google Scholar]
- 65. Naramura M, Gillies S, Mendelsohn J, et al. Therapeutic potential of chimeric and murine anti-(epidermal growth factor receptor) antibodies in a metastasis model for human melanoma. Cancer Immunol Immunother. 1993;37(5):343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anido J, Matar P, Albanell J, et al. ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER2-overexpressing breast cancer cells. Clin Cancer Res. 2003;9(4):1274–1283 [PubMed] [Google Scholar]
- 67. Moulder SL, Yakes FM, Muthuswamy SK, et al. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61(24):8887–8895 [PubMed] [Google Scholar]
- 68. Moasser MM, Basso A, Averbuch SD, et al. The Tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61(19):7184–7188 [PubMed] [Google Scholar]
- 69. Herbst RS, Maddox A-M, Rothenberg ML, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non–small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002;20(18):3815–3825 [DOI] [PubMed] [Google Scholar]
- 70. Barber TD, Vogelstein B, Kinzler KW, et al. Somatic mutations of EGFR in colorectal cancers and glioblastomas. New Engl J Med. 2004;351(27):2883–2883 [DOI] [PubMed] [Google Scholar]
- 71. Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20(21):4292–4302 [DOI] [PubMed] [Google Scholar]
- 72. Fichtner I, Rolff J, Soong R, et al. Establishment of patient-derived non–small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res. 2008;14(20):6456–6468 [DOI] [PubMed] [Google Scholar]
- 73. Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1(6):508–523 [DOI] [PubMed] [Google Scholar]
- 74. Krumbach R, Schüler J, Hofmann M, et al. Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance. Eur J Cancer. 2011;47(8):1231–1243 [DOI] [PubMed] [Google Scholar]
- 75. Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kopetz S, Lemos R, Powis G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res. 2012;18(19):5160–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ellis LM, Fidler IJ. Finding the tumor copycat: Therapy fails, patients don’t. Nat Med. 2010;16(9):974–975 [DOI] [PubMed] [Google Scholar]
- 78. Higgins B, Kolinsky K, Smith M, et al. Antitumor activity of erlotinib (OSI-774, Tarceva) alone or in combination in human non-small cell lung cancer tumor xenograft models. Anti-Cancer Drugs. 2004;15(5):503–512 [DOI] [PubMed] [Google Scholar]
- 79. Shin DM, Donato NJ, Perez-Soler R, et al. Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clin Cancer Res. 2001;7(5):1204–1213 [PubMed] [Google Scholar]
- 80. Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697 [DOI] [PubMed] [Google Scholar]
- 81. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–632 [DOI] [PubMed] [Google Scholar]
- 82. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vvemurafenib in melanoma with BRAF V600E mutation. New Engl J Med. 2011;364(26):2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Crinò L, Kim D, Riely G, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29(2011 Suppl):abstract 7514. [Google Scholar]
- 84. Aboud-Pirak E, Hurwitz E, Pirak ME, et al. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst. 1988;80(20):1605–1611 [DOI] [PubMed] [Google Scholar]
- 85. Hancock MC, Langton BC, Chan T, et al. A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res. 1991;51(17):4575–4580 [PubMed] [Google Scholar]
- 86. Pegram M, Finn R, Arzoo K, et al. The effect of HER-2/neu overexpression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene. 1997;15(5):537–547 [DOI] [PubMed] [Google Scholar]
- 87. Slamon D, Clark G, Wong S, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182 [DOI] [PubMed] [Google Scholar]
- 88. Hudziak RM, Lewis GD, Winget M, et al. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9(3):1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89(10):4285–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Arteaga CL, Winnier AR, Poirier MC, et al. p185c-erbB-2 signaling enhances cisplatin-induced cytotoxicity in human breast carcinoma cells: association between an oncogenic receptor tyrosine kinase and drug-induced DNA repair. Cancer Res. 1994;54(14):3758–3765 [PubMed] [Google Scholar]
- 91. Pegram M, Hsu S, Lewis G, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18(13):2241–2251 [DOI] [PubMed] [Google Scholar]
- 92. Pietras R, Fendly B, Chazin V, et al. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9(7):1829–1838 [PubMed] [Google Scholar]
- 93. Pietras R, Pegram M, Finn R, et al. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17(17):2235–49 [DOI] [PubMed] [Google Scholar]
- 94. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med. 2001;344(11):783–792 [DOI] [PubMed] [Google Scholar]
- 95. Penault-Llorca F, Adelaïde J, Houvenaeghel G, et al. Optimization of immunohistochemical detection of ERBB2 in human breast cancer: impact of fixation. J Pathol. 1994;173(1):65–75 [DOI] [PubMed] [Google Scholar]
- 96. Farabegoli F, Ceccarelli C, Santini D, et al. c-erbB-2 over-expression in amplified and non-amplified breast carcinoma samples. Int J Cancer. 1999;84(3):273–277 [DOI] [PubMed] [Google Scholar]
- 97. Dowsett M, Cooke T, Ellis I, et al. Assessment of HER2 status in breast cancer: why, when and how? Eur J Cancer. 2000;36(2):170–176 [DOI] [PubMed] [Google Scholar]
- 98. Simon R, Maitournam A. Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res. 2004;10(20):6759–6763 [DOI] [PubMed] [Google Scholar]
- 99. Tuveson D, Willis N, Jacks T, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20(36):5054–5058 [DOI] [PubMed] [Google Scholar]
- 100. Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. New Engl J Med. 2001;344(14):1052–1056 [DOI] [PubMed] [Google Scholar]
- 101. Frost MJ, Ferrao PT, Hughes TP, et al. Juxtamembrane mutant V560GKit is more sensitive to imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816VKit is resistant. Mol Cancer Ther. 2002;1(12):1115–1124 [PubMed] [Google Scholar]
- 102. Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399–401 [DOI] [PubMed] [Google Scholar]
- 103. Trent J, Wathen K, Mehren Mv, et al. A phase II study of dasatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST). J Clin Oncol. 2011;29(Suppl):abstract 10006. [Google Scholar]
- 104. Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105(8):3041–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Joseph EW, Pratilas CA, Poulikakos PI, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107(33):14903–14908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70(13):5518–5527 [DOI] [PubMed] [Google Scholar]
- 107. Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol. 2010;28(Suppl):abstract 3534. [Google Scholar]
- 108. Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated reactivation of MAPK signaling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7388):100–103 [DOI] [PubMed] [Google Scholar]
- 111. Paraiso KHT, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71(7):2750–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18(6):683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF–targeted melanoma cells. Cancer Res. 2010;70(16):6670–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shi H, Kong X, Ribas A, et al. Combinatorial treatments that overcome PDGFRβ-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res. 2011;71(15):5067–5074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kaplan FM, Shao Y, Mayberry MM, et al. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene. 2011;30(3):366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106(48):20411–20416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature. 2011;480(7377):387–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sturm OE, Orton R, Grindlay J, et al. The mammalian MAPK/ERK pathway exhibits properties of a negative feedback amplifier. Sci Signal. 2010;3(153):ra90. [DOI] [PubMed] [Google Scholar]
- 121. Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. New Engl J Med. 2012;367(18):1694–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Smalley KSM, Flaherty KT. Integrating BRAF/MEK inhibitors into combination therapy for melanoma. Br J Cancer .2009;100(3):431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67(9):4408–4417 [DOI] [PubMed] [Google Scholar]
- 124. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566 [DOI] [PubMed] [Google Scholar]
- 125. Kwak EL, Bang Y-J, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. New Engl J Med. 2010;363(18):1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shaw A, Kim D, Nakagawa K. Phase III Study of Crizotinib Versus Pemetrexed Or Docetaxel Chemotherapy Ii Patients With Advanced ALK-Positive Non-Small Cell Lung Cancer (PROFILE 1007). Vienna: ESMO Congress; 2012 [Google Scholar]
- 127. Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A. 2008;105(50):19893–19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chen Z, Sasaki T, Tan X, et al. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 2010;70(23):9827–9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. New Engl J Med. 2010;363(18):1734–1739 [DOI] [PubMed] [Google Scholar]
- 130. Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non–small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342 [DOI] [PubMed] [Google Scholar]
- 133. Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti–vascular endothelial growth factor antibody, for metastatic renal cancer. New Engl J Med. 2003;349(5):427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. New Engl J Med. 2006;355(24):2542–2550 [DOI] [PubMed] [Google Scholar]
- 135. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740 [DOI] [PubMed] [Google Scholar]
- 136. Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lee C-G, Heijn M, di Tomaso E, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60(19):5565–5570 [PubMed] [Google Scholar]
- 138. Yao JC, Hoff PM. Molecular targeted therapy for neuroendocrine tumors. Hematol Oncol Clin North America. 2007;21(3):575–581 [DOI] [PubMed] [Google Scholar]
- 139. Auerbach R, Akhtar N, Lewis RL, et al. Angiogenesis assays: problems and pitfalls. Cancer Metastasis Rev. 2000;19(1):167–172 [DOI] [PubMed] [Google Scholar]
- 140. Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis — correlation in invasive breast carcinoma. New Engl J Med. 1991;324(1):1–8 [DOI] [PubMed] [Google Scholar]
- 141. De J, Van D, Baak A. Hot spot microvessel density and the mitotic activity index are strong additional prognostic indicators in invasive breast cancer. Histopathology. 2000;36(4):306–312 [DOI] [PubMed] [Google Scholar]
- 142. Yano T, Tanikawa S, Fujie T, et al. Vascular endothelial growth factor expression and neovascularisation in non-small cell lung cancer. Eur J Cancer. 2000;36(5):601–609 [DOI] [PubMed] [Google Scholar]
- 143. Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst. 2002;94(12):883–893 [DOI] [PubMed] [Google Scholar]
- 144. Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24(20):3293–3298 [DOI] [PubMed] [Google Scholar]
- 145. Miller JC, Pien HH, Sahani D, et al. Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst. 2005;97(3):172–187 [DOI] [PubMed] [Google Scholar]
- 146. Rehman S, Jayson GC. Molecular imaging of antiangiogenic agents. Oncologist. 2005;10(2):92–103 [DOI] [PubMed] [Google Scholar]
- 147. O’Connor JPB, Carano RAD, Clamp AR, et al. Quantifying antivascular effects of monoclonal antibodies to vascular endothelial growth factor: insights from imaging. Clin Cancer Res. 2009;15(21):6674–6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liang W-C, Wu X, Peale FV, et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281(2):951–961 [DOI] [PubMed] [Google Scholar]
- 149. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544 [DOI] [PubMed] [Google Scholar]
- 150. Cobleigh MA, Langmuir VK, Sledge GW, et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Sem Oncol. 2003;30(Suppl 16):117–124 [DOI] [PubMed] [Google Scholar]
- 151. Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14(20):6371–6375 [DOI] [PubMed] [Google Scholar]
- 152. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Verheul HMW, Voest EE, Schlingemann RO. Are tumours angiogenesis-dependent? J Pathol. 2004;202(1):5–13 [DOI] [PubMed] [Google Scholar]
- 154. Hanahan D. Heritable formation of pancreatic [beta]-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315(6015):115–122 [DOI] [PubMed] [Google Scholar]
- 155. Adams TE, Alpert S, Hanahan D. Non-tolerance and autoantibodies to a transgenic self antigen expressed in pancreatic [beta] cells. Nature. 1987;325(6101):223–228 [DOI] [PubMed] [Google Scholar]
- 156. Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309 [DOI] [PubMed] [Google Scholar]
- 157. Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23(5):939–952 [DOI] [PubMed] [Google Scholar]
- 158. Raymond E, Dahan L, Raoul J-L, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. New Engl J Med. 2011;364(6):501–513 [DOI] [PubMed] [Google Scholar]
- 159. Saltz LB, Rosen LS, Marshall JL, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25(30):4793–4799 [DOI] [PubMed] [Google Scholar]
- 160. Drevs J, Hofmann I, Hugenschmidt H, et al. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res. 2000;60(17):4819–4824 [PubMed] [Google Scholar]
- 161. Hecht JR, Trarbach T, Hainsworth JD, et al. Randomized, placebo-controlled, phase iii study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29(15):1997–2003 [DOI] [PubMed] [Google Scholar]
- 162. Kopetz S, Hoff PM, Morris JS, et al. Phase II Trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2009;28(3):453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583 [DOI] [PubMed] [Google Scholar]
- 164. Ronzoni M, Manzoni M, Mariucci S, et al. Circulating endothelial cells and endothelial progenitors as predictive markers of clinical response to bevacizumab-based first-line treatment in advanced colorectal cancer patients. Ann Oncol. 2010;21(12):2382–2389 [DOI] [PubMed] [Google Scholar]
- 165. Hindryckx P, Waeytens A, Laukens D, et al. Absence of placental growth factor blocks dextran sodium sulfate-induced colonic mucosal angiogenesis, increases mucosal hypoxia and aggravates acute colonic injury. Lab Invest. 2010;90(4):566–576 [DOI] [PubMed] [Google Scholar]
- 166. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase iii randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506 [DOI] [PubMed] [Google Scholar]
- 167. Palombella VJ, Rando OJ, Goldberg AL, et al. The ubiquitinproteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78(5):773–785 [DOI] [PubMed] [Google Scholar]
- 168. Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–2622 [PubMed] [Google Scholar]
- 169. Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45 [DOI] [PubMed] [Google Scholar]
- 170. Alland L, Muhle R, Hou H, et al. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387(6628): 49–55 [DOI] [PubMed] [Google Scholar]
- 171. Loda M, Cukor B, Tam S, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3 (2):231–234 [DOI] [PubMed] [Google Scholar]
- 172. Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–3076 [PubMed] [Google Scholar]
- 173. Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell. 2005;8(5):407–419 [DOI] [PubMed] [Google Scholar]
- 174. An WG, Hwang SG, Trepel JB, et al. Protease inhibitor-induced apoptosis: accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition. Leukemia. 2000;14(7):1276–1283 [DOI] [PubMed] [Google Scholar]
- 175. Pérez-Galán P, Roué G, Villamor N, et al. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107(1):257–264 [DOI] [PubMed] [Google Scholar]
- 176. Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20(22):4420–4427 [DOI] [PubMed] [Google Scholar]
- 177. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. New Engl J Med. 2005;352(24):2487–2498 [DOI] [PubMed] [Google Scholar]
- 178. Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program. Ped Blood Cancer. 2008;50(1):37–45 [DOI] [PubMed] [Google Scholar]
- 179. Messinger YH, Gaynon PS, Sposto R, et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood. 2012;120(2):285–290 [DOI] [PubMed] [Google Scholar]
- 180. Orlowski RZ, Eswara JR, Lafond-Walker A, et al. Tumor growth inhibition induced in a murine model of human burkitt’s lymphoma by a proteasome inhibitor. Cancer Res. 1998;58(19):4342–4348 [PubMed] [Google Scholar]
- 181. Teicher BA, Ara G, Herbst R, et al. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5(9):2638–2645 [PubMed] [Google Scholar]
- 182. Harbison M, Bruns C, Bold R, et al. Proteasome inhibitor PS-341 is effective as an anti-angiogenic agent in the treatment of human pancreatic carcinoma via the inhibition of NF-κB and subsequent inhibition of vascular endothelial growth factor production. Proc Am Assoc Cancer Res. 2000;41(71):2000 [Google Scholar]
- 183. Morris M, Beekman K, Kelly W, et al. Phase II study of bortezomib for castrate metastatic prostate cancer (PC). J Clin Oncol. 2005;23(16S):4633 [Google Scholar]
- 184. Alberts SR, Foster NR, Morton RF, et al. PS-341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group (NCCTG) randomized phase II study. Ann Oncol. 2005;16(10):1654–1661 [DOI] [PubMed] [Google Scholar]
- 185. Nawrocki ST, Carew JS, Pino MS, et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65(24):11658–11666 [DOI] [PubMed] [Google Scholar]
- 186. Nawrocki ST, Bruns CJ, Harbison MT, et al. Effects of the proteasome inhibitor PS-341 on apoptosis and angiogenesis in orthotopic human pancreatic tumor xenografts. Mol Cancer Ther. 2002;1(14):1243–1253 [PubMed] [Google Scholar]
- 187. Märten A, Zeiss N, Serba S, et al. Bortezomib is ineffective in an orthotopic mouse model of pancreatic adenocarcinoma. Mol Cancer Ther. 2008;7(11):3624–3631 [DOI] [PubMed] [Google Scholar]
- 188. Williamson MJ, Silva MD, Terkelsen J, et al. The relationship among tumor architecture, pharmacokinetics, pharmacodynamics, and efficacy of bortezomib in mouse xenograft models. Mol Cancer Ther. 2009;8(12):3234–3243 [DOI] [PubMed] [Google Scholar]
- 189. Ruiz S, Krupnik Y, Keating M, et al. The proteasome inhibitor NPI-0052 is a more effective inducer of apoptosis than bortezomib in lymphocytes from patients with chronic lymphocytic leukemia. Mol Cancer Ther. 2006;5(7):1836–1843 [DOI] [PubMed] [Google Scholar]
- 190. Papandreou CN, Daliani DD, Nix D, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22(11):2108–2121 [DOI] [PubMed] [Google Scholar]
- 191. Kawai H, Li H, Avraham S, et al. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor α. Int J Cancer. 2003;107(3):353–358 [DOI] [PubMed] [Google Scholar]
- 192. Gui C-Y, Ngo L, Xu WS, et al. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A. 2004;101(5):1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1(4):287–299 [DOI] [PubMed] [Google Scholar]
- 194. Marks PA, Rifkind RA, Richon VM, et al. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202 [DOI] [PubMed] [Google Scholar]
- 195. Coffey DC, Kutko MC, Glick RD, et al. the histone deacetylase inhibitor, cbha, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Cancer Res. 2001;61(9):3591–3594 [PubMed] [Google Scholar]
- 196. Butler LM, Agus DB, Scher HI, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60(18):5165–5170 [PubMed] [Google Scholar]
- 197. Mann BS, Johnson JR, Cohen MH, et al. FDA approval summary: vorinostat for treatment of advanced primary cutaneous t-cell lymphoma. Oncologist. 2007;12(10):1247–1252 [DOI] [PubMed] [Google Scholar]
- 198. Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous t-cell lymphoma. J Clin Oncol. 2009;27(32):5410–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zhang C, Richon V, Ni X, et al. Selective induction of apoptosis by histone deacetylase inhibitor saha in cutaneous t-cell lymphoma cells: relevance to mechanism of therapeutic action. J Investig Dermatol. 2005;125(5):1045–1052 [DOI] [PubMed] [Google Scholar]
- 200. Piekarz RL, Robey RW, Zhan Z, et al. T-cell lymphoma as a model for the use of histone deacetylase inhibitors in cancer therapy: impact of depsipeptide on molecular markers, therapeutic targets, and mechanisms of resistance. Blood. 2004;103(12):4636–4643 [DOI] [PubMed] [Google Scholar]
- 201. Haritunians T, Mori A, O’Kelly J, et al. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2006;21(2):333–339 [DOI] [PubMed] [Google Scholar]
- 202. Catley L, Weisberg E, Tai Y-T, et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003;102(7):2615–2622 [DOI] [PubMed] [Google Scholar]
- 203. Mahalingam D, Medina EC, Esquivel JA, et al. Vorinostat enhances the activity of temsirolimus in renal cell carcinoma through suppression of survivin levels. Clin Cancer Res. 2010;16(1):141–153 [DOI] [PubMed] [Google Scholar]
- 204. Yin D, Ong JM, Hu J, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13(3):1045–1052 [DOI] [PubMed] [Google Scholar]
- 205. Friday BB, Anderson SK, Buckner J, et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro-Oncology. 2012;14(2):215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Hodges-Gallagher L, Valentine C, Bader S, et al. Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat. 2007;105(3):297–309 [DOI] [PubMed] [Google Scholar]
- 207. Owonikoko TK, Ramalingam SS, Kanterewicz B, et al. Vorinostat increases carboplatin and paclitaxel activity in non-small cell lung cancer cells. Int J Cancer. 2010;126(3):743–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wilson P, El-Khoueiry A, Iqbal S, et al. A phase I/II trial of vorinostat in combination with 5-fluorouracil in patients with metastatic colorectal cancer who previously failed 5-FU-based chemotherapy. Cancer Chemother Pharmacol. 2010;65(5):979–988 [DOI] [PubMed] [Google Scholar]
- 209. Belani C, Ramalingam S, Kalemkerian G, et al. Randomized, double-blind phase II/III study of first-line paclitaxel (P) plus carboplatin (C) in combination with vorinostat or placebo in patients with advanced non-small-cell lung cancer (NSCLC). Eur J Cancer. 2009;7(Suppl 507):abstract O-9007. [Google Scholar]
- 210. Vansteenkiste J, Van Cutsem E, Dumez H, et al. Early phase II trial of oral vorinostat in relapsed or refractory breast, colorectal, or non-small cell lung cancer. Invest New Drug. 2008;26(5):483–488 [DOI] [PubMed] [Google Scholar]
- 211. Munster PN, Thurn KT, Thomas S, et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer. 2011;104(12):1828–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Witta SE, Jotte RM, Konduri K, et al. Randomized phase II Trial of erlotinib with and without entinostat in patients with advanced non–small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol. 2012;30(18):2248–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Peters TS. Do preclinical testing strategies help predict human hepatotoxic potentials? Toxicol Pathol. 2005;33(1):146–154 [DOI] [PubMed] [Google Scholar]
- 215. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. New Engl J Med. 2001;344(14):1031–1037 [DOI] [PubMed] [Google Scholar]
- 216. Obach RS, Baxter JG, Liston TE, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exper Ther. 1997;283(1):46–58 [PubMed] [Google Scholar]
- 217. Lee E, Iskow R, Yang L, et al. Landscape of somatic retrotransposition in human cancers. Science. 2012;337(6097):967–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417): 519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Deng R, Iyer S, Theil F-P, et al. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? mAbs. 2011;3(1):61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Pratz KW, Sato T, Murphy KM, et al. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115(7):1425–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Wilson P, LaBonte M, Martin S, et al. Sustained inhibition of deacetylases is required for the antitumor activity of the histone deactylase inhibitors panobinostat and vorinostat in models of colorectal cancer. Invest New Drug. 2013;31(4):1–13 [DOI] [PubMed] [Google Scholar]
- 223. Smith MA, Houghton PJ. A proposal regarding reporting of in vitro testing results. Clin Cancer Res. 2013;19(11):2828–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Ullrich RT, Jikeli JF, Diedenhofen M, et al. In-vivo visualization of tumor microvessel density and response to anti-angiogenic treatment by high resolution MRI in mice. PLoS One. 2011;6(5):e19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Gauler TC, Besse B, Mauguen A, et al. Phase II trial of PTK787/ZK 222584 (vatalanib) administered orally once-daily or in two divided daily doses as second-line monotherapy in relapsed or progressing patients with stage IIIB/IV non-small-cell lung cancer (NSCLC). Ann Oncol. 2012;23(3):678–687 [DOI] [PubMed] [Google Scholar]