Abstract
Background
Adjuvant endocrine therapy beyond 5 years reduces recurrence in patients with estrogen receptor–positive breast cancer. We have previously shown that immunohistochemical markers (IHC4) and two gene expression profile tests (recurrence score [RS] and PAM50 risk of recurrence [ROR]) are associated with time to distant recurrence, and we have now assessed the value of each of these scores and routine clinical variables for predicting outcome, specifically in years 5 to 10.
Methods
We used univariate and multivariable proportional hazards models to determine the prognostic value of all variables and scores (IHC4, RS, ROR) for distant recurrence, separately in years 0 to 5 and specifically for years 5 to 10 for all patients. All statistical tests were two-sided.
Results
Nodal status and tumor size were at least as strong in years 5 to 10 as in years 0 to 5 (nodal status, years 5–10: χ2 = 21.72 vs years 0–5: χ2 = 11.08, both P < .001; tumor size, years 5–10: χ2 = 10.52 vs years 0–5: χ2 = 10.82, both P = .001). Ki67 and the overall IHC4 score were the only statistically significant biomarkers related to distant recurrence univariablely in the 5 to 10 year period (χ2 = 8.67, χ2 = 13.22, respectively). The ROR score was the strongest molecular prognostic factor in the late follow-up period (χ2 = 16.29; P < .001), whereas IHC4 (χ2 = 7.41) and RS (χ2 = 5.55) were only weakly prognostic in this period. Similar results were seen for all subgroups and for all recurrences.
Conclusions
None of the IHC4 markers provided statistically significant prognostic information in years 5 to 10, except for nodal status and tumor size. ROR gave the strongest prognostic information in years 5 to 10. These results may help select patients who could benefit most from hormonal therapy beyond 5 years of treatment.
Adjuvant chemotherapy and endocrine therapy for early breast cancer have had a considerable impact on outcomes (1), but a substantial number of women, especially those with estrogen receptor (ER)–positive tumors, remain at risk for late recurrences. The annual rate is in excess of 2% for at least 15 years, even after 5 years of tamoxifen therapy (2), and currently it is not possible to identify a group of such women who can be considered as cured (3,4). This remains true for at least 10 years for women treated for 5 years with an aromatase inhibitor (5).
Most of the studies of prevention of late relapse have been performed in women receiving tamoxifen as initial adjuvant endocrine therapy for early ER-positive breast cancer. The MA17 trial clearly showed that extended adjuvant therapy with letrozole after 5 years of tamoxifen prolongs disease-free survival and overall survival, regardless of the patient’s nodal status involvement (6). Brewster et al. (7) found that ER positivity, nodal involvement, and grade were all associated with increased risk of late recurrence. It is of importance to determine to what extent the newer immunohistochemical and molecular scores can help in predicting late recurrence.
Recent publications from the transATAC (Anastrozole, Tamozifen, Alone or in Combination) cohort have demonstrated that the Oncotype DX recurrence score (RS) (8), the immunohistochemical (IHC4) score (9) and the PAM50-based risk of recurrence (ROR) score (10) all provide additional information beyond that available for clinical variables, summarized in the clinical treatment score (CTS), about the risk of distant recurrence in postmenopausal women with hormone receptor–positive breast cancer treated with anastrozole or tamoxifen, but it is unknown how much of this effect extends beyond 5 years. Here, we investigate the relationship between clinical variables, immunohistochemical markers, and these scores for the prediction of distant recurrence separately in years 0 to 5 and years 5 to 10 after diagnosis for postmenopausal women with early hormone receptor–positive breast cancer.
Methods
The ATAC trial evaluated the efficacy and safety of anastrozole vs tamoxifen given for 5 years in postmenopausal women with localized breast cancer (11). The transATAC study collected formalin-fixed, paraffin-embedded blocks from hormone receptor–positive breast cancers in a subset of women randomized to the monotherapy arms of the ATAC trial (12). This analysis includes all such patients from the United Kingdom, who have not received chemotherapy and for whom the RS, the IHC4, and the ROR (PAM50 without tumor size) scores were available (n = 940). The laboratory methods to derive the RS, IHC4, and ROR scores have been described in detail previously (9,10,13). Briefly, the CTS contains information on nodal status, tumor size, grade, age, and treatment received and was developed on the transATAC dataset and has been described in detail previously (9). The IHC4 score consists of a quantitative assessment of four variables (ER, progesterone receptor [PgR], HER2, and Ki67) and was also developed from the transATAC dataset in the presence of clinical variables (9). For ROR, expression profiles for 50 classifier genes and eight housekeeping genes were measured using the nCounter platform (Nanostring Technologies, Seattle, WA). After normalization, the expression profile for each sample plus tumor size was used to calculate a predefined ROR score (10). In this analysis, ROR is based on the ROR50 gene score without tumor size. The ATAC trial was performed in accordance with the Declaration of Helsinki (1996 revision), under the principles of good clinical practice and is registered as an International Standard Randomized Controlled Trial (number ISRCTN18233230).
Statistical Analyses
The time from randomization to first distant recurrence was the prospectively defined primary endpoint. Death before distant recurrence was treated as a censoring event. The association between baseline clinicopathological markers (nodal status, tumor size, grade), immunohistochemical markers (ER, PgR, HER2, Ki67), IHC4 score, ROR score, RS, and distant recurrence was assessed using hazard ratios derived from Cox proportional hazard models with associated 95% confidence intervals (CI). This was done separately for years 0 to 5 and years 5 to 10. For clinical and immunohistochemical variables, multivariable models included only those variables that were statistically significant in univariate analyses. For the comparisons of the IHC4, ROR, and RS scores, each score was added separately to CTS to determine the prognostic information in that score when added to CTS. Changes in likelihood ratio values (χ2) were used to measure and compare the relative amount of information of one variable/score compared with another. Because the IHC4 score was developed within the transATAC dataset, it was compared with the ROR and RS scores by sample splitting, in which the IHC4 score was generated using half the data and compared with the ROR and RS scores in the remaining half. This was done 100 times and averaged as previously described (9). Survival curves were estimated using the Kaplan–Meier method. P values were two-sided, based on normal approximation, and all confidence intervals were at the 95% level. The proportional hazards assumption was verified by Kaplan–Meier curves. Analyses were performed using STATA version 12.1 (STATA Corp., College Station, TX). All statistical tests were two-sided.
Results
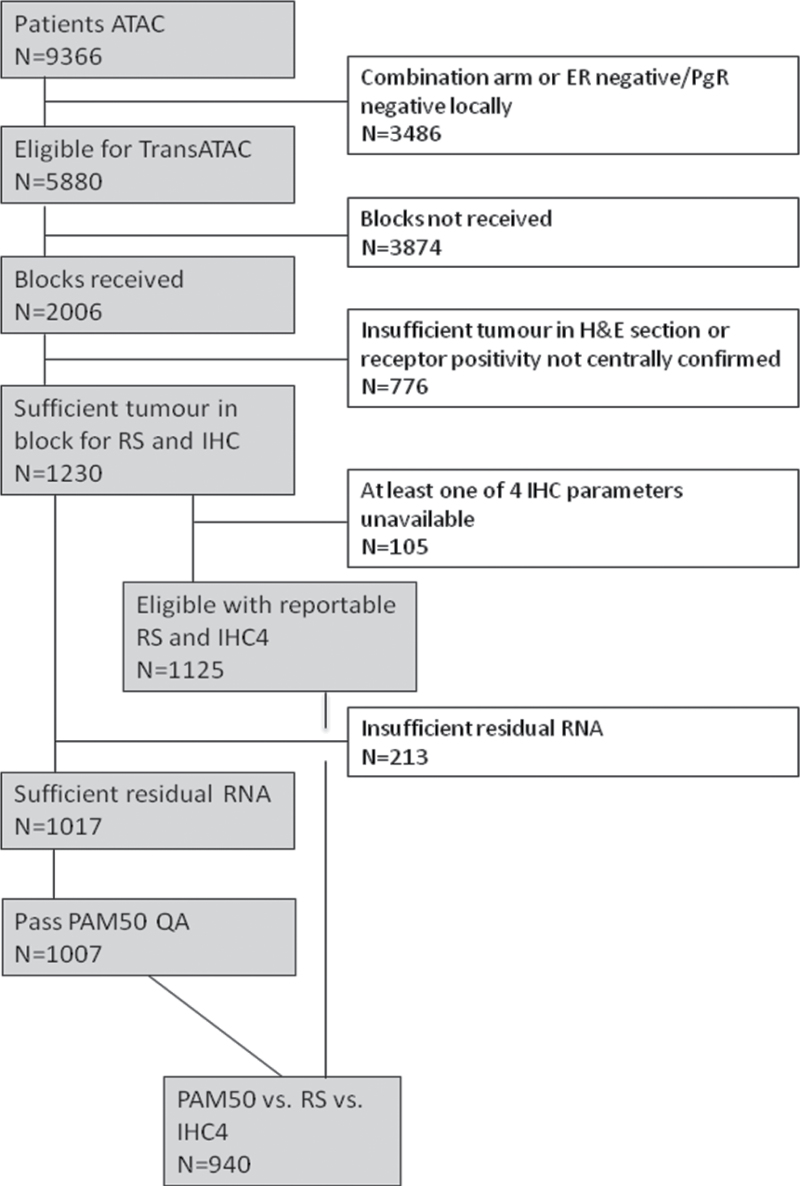
The median follow-up time for this analysis was 10 years (5). Values for RS, IHC4, and ROR were available for a total of 940 women in the monotherapy arms (ie, tamoxifen alone or anastrozole alone) for postmenopausal women who did not receive chemotherapy (Figure 1). There were 154 distant recurrences; 71 occurred in years 0 to 5 and 83 occurred in years 5 to 10 (for all recurrence: 83 in years 0 to 5, 107 in years 5 to 10). Baseline characteristics of this cohort have been published previously (9). This report focuses primarily on the prognostic discrimination during the 5-to-10-year follow-up period.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram for study numbers. ATAC = anastrozole, tamozifen, alone or in combination; ER = estrogen receptor; H&E = hematoxylin & eosin stain; IHC = immunohistochemical markers; PgR = progesterone receptor; QA = quality assurance; ROR = risk of recurrence; RS = recurrence score.
Years 0 to 5
Univariate Analyses.
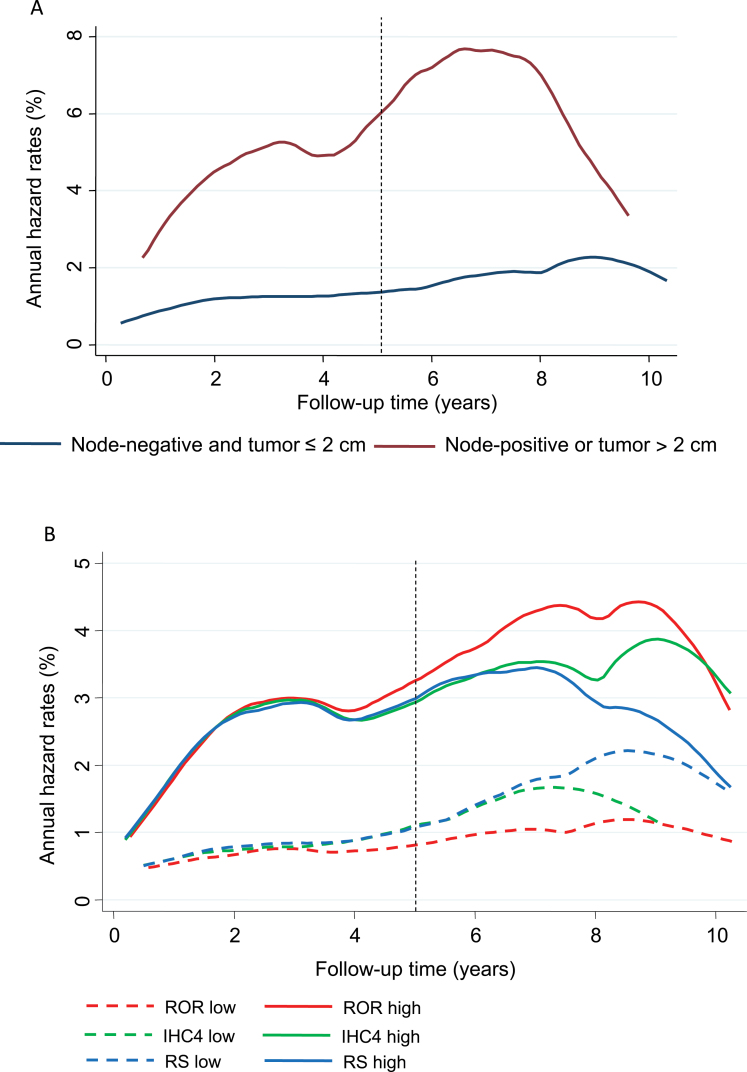
In a univariate analysis, tumor size and nodal status added the most prognostic information in years 0 to 5 (Table 1). Figure 2A shows annual hazard rates for low-risk women (node-negative, tumor size ≤ 2cm) vs high-risk women (node-positive, tumor size > 2cm). The difference in distant recurrence rates continues for at least 8 years. Patients with high ER scores developed statistically significantly fewer distant recurrences than those with lower scores, and similar results were seen for the PgR (Table 1). All IHC4 markers added statistically significant prognostic information, with Ki67 being the strongest immunohistochemical marker in this time period (Table 1). The CTS was the strongest score, whereas IHC4 and ROR provided similar amounts of information, and RS was less prognostic (Table 2). Figure 2B shows annual hazard rate curves for IHC4, RS, and ROR (cutoff point for all scores is the median). In univariate analyses, all scores had similar prognostic discrimination in years 0 to 5, and differences between scores are only seen in years 5 to 10 (Figure 2B).
Table 1.
Likelihood (χ2) for distant recurrence for all individual clinical variables*
| Clinical variables | 0 to 5 years | 5 to 10 years | ||
|---|---|---|---|---|
| (No. of distant recurrence = 71) | (No. of distant recurrence = 83) | |||
| Univariate | Multivariable | Univariate | Multivariable | |
| χ2 | χ2 | χ2 | χ2 | |
| (P) | (P) | (P) | (P) | |
| Nodal status, | 20.54 | 11.08 | 32.00 | 21.72 |
| negative vs positive | (<.001) | (<.001) | (<.001) | (<.001) |
| Tumor size, | 26.86 | 10.82 | 21.37 | 10.52 |
| ≤2cm vs >2 cm | (<.001) | (.001) | (<.001) | (.001) |
| Grade, | 17.69 | 3.20 | 3.73 | — |
| well/moderate vs poor | (<.001) | (.07) | (.05) | |
| ER | 6.27 | 2.96 | 2.34 | — |
| (.01) | (.09) | (.10) | ||
| PgR | 17.44 | 7.76 | 3.21 | — |
| (<.001) | (.005) | (.07) | ||
| Ki67 | 19.86 | 6.99 | 8.67 | 2.62 |
| (<.001) | (.008) | (.003) | (.10) | |
| HER2, | 8.42 | 0.45 | 2.82 | — |
| negative vs positive | (.004) | (.50) | (.09) | |
* Both univariate and multivariable analysis are presented for years 0 to 5 and years 5 to 10 separately. Likelihood ratio test based on Cox proportional hazard models for univariate and multivariable analyses. Changes in likelihood ratio values (χ2) were used. For multivariable analysis, the model is in addition to the model containing all other factors that were statistically significant in the univariate model. All statistical tests were two-sided. Nodal status, tumor size, grade, and HER2 are all categorical variables. ER,Ki67, and PgR are all continuous variables. ER = estrogen receptor; PgR = progesterone receptor.
Figure 2.
Annual hazard rate curves (%) for distant recurrence according to risk group and scores (all split at the median). A) Nodal status/tumor size. B) Immunohistochemical markers (IHC4), recurrence score (RS), and risk of recurrence (ROR) score.
Table 2.
Likelihood (χ2) for distant recurrence for all four scores*
| Scores | 0 to 5 years (No. of distant recurrence = 71) | 5 to 10 years (No. of distant recurrence = 83) | ||
|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |
| χ2 | χ2 | χ2 | χ2 | |
| (P) | (P) | (P) | (P) | |
| CTS | 73.29 | — | 74.25 | — |
| (<.001) | (<.001) | |||
| IHC4 | 36.89 | 24.89 | 13.22 | 7.41 |
| (<.001) | (<.001) | (<.001) | (.007) | |
| RS | 24.2 | 13.22 | 12.17 | 5.55 |
| (<.001) | (<.001) | (<.001) | (.02) | |
| ROR | 37.32 | 11.41 | 40.64 | 16.29 |
| (<.001) | (<.001) | (<.001) | (<.001) | |
* Both univariate and multivariable analysis are presented for years 0 to 5 and years 5 to 10 separately. Likelihood ratio test based on Cox proportional hazard models for univariate and multivariable analyses. Changes in likelihood ratio values (χ2) were used. Immunohistochemical markers (IHC4), recurrence score (RS), and risk of recurrence (ROR) were singly added to the clinical treatment score (CTS) for the multivariable models. LR = likelihood ratio; LR-X2 for all scores were estimated by sample splitting. All statistical tests were two-sided. All scores are continuous variables.
Multivariable Analyses.
The multivariable model included all variables that were statistically significant in the univariate analysis (Table 1). For the predefined scores, IHC4, RS, and ROR were singly added to the CTS to create three multivariable models (Table 2). Tumor size (χ2 = 10.82; P = .001) and nodal status (χ2 = 11.08; P < .001) were the strongest individual clinical factors in the multivariable analysis for years 0 to 5. Of the IHC markers, only PgR and Ki67 added clinically significant amounts of information in this period, with similar effect sizes seen (Table 1). When added to the CTS, the most prognostic information in years 0 to 5 was provided by IHC4, with the RS and ROR scores contributing similar prognostic information when added to CTS (Table 2).
Table 3 shows results for distant recurrence in five important subgroups (node-negative, node-positive, HER2-negative, HER2-negative/node-negative, and HER2-negative/node-positive) for the main four scores. For patients with node-negative disease, the IHC4 added the most prognostic information in the univariate and multivariable analysis in years 0 to 5 (Table 3), and RS and ROR provided less but a similar amount of added prognostic information in this time period (Table 3). For node-positive patients, none of the scores were statistically significant in the early follow-up period. For HER2-negative patients and HER2-negative/node-negative patients, IHC4 was the strongest score in years 0 to 5 in the multivariable analysis. None of the scores added statistically significant information in years 0 to 5 in the multivariable analysis for women with HER2-negative/node-positive tumors (Table 3).
Table 3.
Likelihood (χ2) for distant recurrence for all scores according to subgroup*
| Scores | 0 to 5 years | 5 to 10 years | 0 to 5 years | 5 to 10 years | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | |
| χ2 (P) | χ2 (P) | χ2 (P) | χ2 (P) | χ2 (P) | χ2 (P) | χ2 (P) | χ2 (P) | |
| Node-negative (n = 683) | Node-positive (N=257) | |||||||
| CTS | 28.47 (<.001) | — | 15.23 (<.001) | — | 29.11 (<.001) | — | 29.63 (<.001) | — |
| IHC4 | 41.50 (<.001) | 27.19 (<.001) | 5.85 (.02) | 1.98 (.20) | 3.51 (.06) | 1.38 (.20) | 5.98 (.01) | 6.05 (.01) |
| RS | 25.24 (<.001) | 14.52 (<.001) | 4.15 (.04) | 1.01 (.30) | 2.94 (.09) | 0.81 (.40) | 8.19 (.004) | 5.17 (.02) |
| ROR | 29.44 (<.001) | 10.41 (.001) | 19.91 (<.001) | 8.93 (.003) | 6.60 (.01) | 1.33 (.20) | 14.79 (<.001) | 8.37 (.004) |
| HER2-negative (n = 845) | ||||||||
| CTS | 70.03 (<.001) | — | 63.44 (<.001) | — | ||||
| IHC4 | 25.35 (<.001) | 14.61 (<.001) | 10.13 (.001) | 5.67 (.02) | ||||
| RS | 20.85 (<.001) | 10.35 (.001) | 6.98 (.008) | 2.81 (.09) | ||||
| ROR | 30.45 (<.001) | 8.69 (.003) | 39.21 (<.001) | 18.18 (<.001) | ||||
| HER2-negative/node-negative (n = 615) | HER2-negative/node-positive (n = 230) | |||||||
| CTS | 14.06 (<.001) | — | 20.12 (<.001) | — | 28.67 (<.001) | — | 24.15 (<.001) | — |
| IHC4 | 19.23 (<.001) | 12.06 (<.001) | 9.97 (.002) | 3.89 (.05) | 7.58 (.006) | 3.94 (.05) | 1.86 (.2) | 1.44 (.20) |
| RS | 13.52 (<.001) | 6.84 (.008) | 7.99 (.005) | 2.23 (.1) | 7.73 (.005) | 4.01 (.05) | 1.57 (.2) | 0.38 (.50) |
| ROR | 19.65 (<.001) | 8.61 (.008) | 28.73 (<.001) | 13.85 (<.001) | 7.82 (.005) | 1.96 (.20) | 8.28 (.004) | 4.78 (.03) |
* Both univariate and multivariable analyses are presented for years 0 to 5 and years 5 to 10 separately. Likelihood ratio test based on Cox proportional hazard models for univariate and multivariable analyses. Changes in likelihood ratio values (χ2) were used. Immunohistochemical markers (IHC4), recurrence score (RS), and risk of recurrence (ROR) are singly added to the clinical treatment score (CTS) for the multivariable models. LR = likelihood ratio; LR-X2 for all scores were done by sample splitting. All statistical tests were two-sided. All scores are continuous variables.
Years 5 to 10
Univariate Model.
In years 5 to 10, nodal status was the strongest prognostic factor of the individual clinical variables in the univariate analysis, followed by tumor size. Grade did not add statistically significant prognostic information in this time period (Table 1). In years 5 to 10, ER, PgR, and HER2 did not add any prognostic information, and Ki67 was the only statistically significant immunohistochemical variable adding prognostic information in the univariate analysis in years 5 to 10 (χ2 = 8.67; P = .003) (Table 1).
CTS was the strongest prognostic factor in years 5 to 10 in the univariate analysis and provided a very similar amount of prognostic information as for years 0 to 5 (Table 2). ROR added less but substantial prognostic information in this time period (χ2 = 40.64; P < .001), whereas IHC4 and RS added much less information (IHC4: χ2 = 13.22, P <.001; RS: χ2 = 12.17, P < .001) (Table 2). The annual hazard rates curves for all three scores (split at the median) are shown in Figure 2B. The difference in annual hazard rates for women with a low score was greater for the ROR score than for either IHC4 or RS (Figure 2B).
Multivariable Analyses.
Nodal status and tumor size were the only individual factors that added prognostic information in years 5 to 10 in the multivariable model (nodal status: χ2 = 21.72, P <.001; tumor size: χ2=10.52, P = .001) (Table 1). IHC4 (χ2 = 7.41; P = .007) and RS (χ2 = 5.55; P = .02) (Table 2) were weak prognostic factors in the 5-to-10-year follow-up period. In contrast, ROR added a substantial amount of prognostic information in a model including CTS (χ2 = 16.29; P < .001).
Table 3 shows results for distant recurrence in node-negative, node-positive, HER2-negative, HER2-negative/node-negative, and HER2-negative/node-positive populations only for the main four scores. For node-negative patients, IHC4 and RS did not add any prognostic information in this late time period (Table 3). In contrast, ROR added statistically significant prognostic information in this time period in both the univariate and multivariable models. ROR was the only molecular score that added statistically significant prognostic information in the late follow-up phase in the multivariable analysis in all five subgroups (Table 3). Although all of the three molecular markers added statistically significant information in years 5 to 10 (in contrast with years 0 to 5) in node-positive patients, IHC4 and RS lost their statistical significance for years 5 to 10 in node-positive/HER2-negative patients in the multivariable analysis.
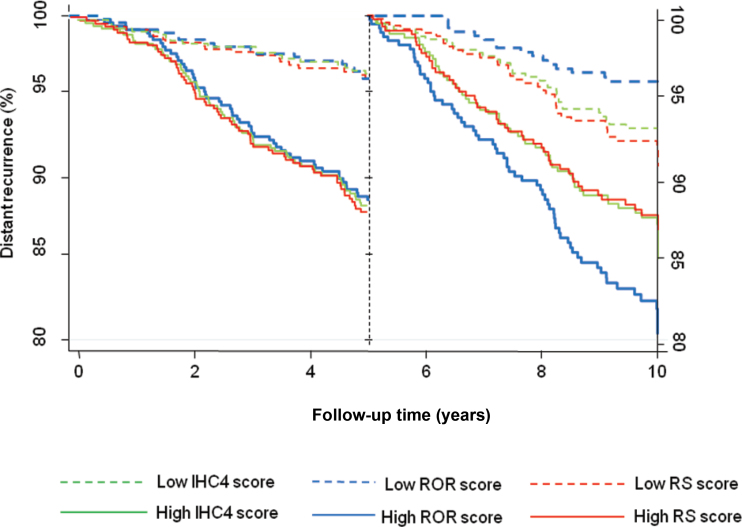
The effect on outcomes of the difference in prognostic discrimination between the three molecular scores is shown in Figure 3 using Kaplan–Meier curves for IHC4, RS, and ROR (split at the median) for each time period. It can be seen that the difference in distant recurrence rate between the low- and high-risk groups is approximately 7% for all three scores in years 0 to 5. However, in years 5 to 10 greater difference between low- and high-risk groups is seen for the ROR score (15.1%), compared with 5.4 % for RS and 9.8% for the IHC4 score.
Figure 3.
Kaplan–Meier estimates for distant recurrence according to immunohistochemical markers (IHC4), recurrence score (RS), and risk of recurrence (ROR) score group split at the median value.
All clinical and most immunohistochemical variables were strong prognostic factors for the development of distant recurrence in years 0 to 5. However, only nodal status and tumor size were strong prognostic factors in years 5 to 10 in the multivariable model, and none of the individual immunohistochemical markers added prognostic information in this time period. IHC4 and RS were also strong prognostic factors in years 0 to 5 but lost most of their prognostic value after 5 years of follow up. Nodal status and tumor size remained statistically significant in years 5 to 10, and this led to substantial prognostic information in CTS. The only strong molecular marker in years 5 to 10 was ROR, which retained its value in the presence of CTS, and this was seen for all subgroups. Similar results were seen when all recurrence was the endpoint (data not shown).
Discussion
The updated meta-analysis of individual patient data by the Early Breast Cancer Trialists’ Collaborative Group found that the risk of recurrence for ER-positive women after 5 years of tamoxifen is substantial in the adjuvant setting (1). Furthermore, the Adjuvant Tamoxifen: Longer Against Shorter trial showed that 10 years of tamoxifen use statistically significantly reduced the risk of recurrence as compared with 5 years of tamoxifen treatment (14). These results illustrate the need to address the importance of late recurrence in ER-positive breast cancer.
The transATAC cohort is a valuable resource for investigating factors that predict late recurrences because it is the only dataset for which data on IHC4, RS, and ROR are available. Furthermore, this dataset is ideal to investigate the importance of late recurrence because all participants have had 5 years of either anastrozole or tamoxifen. We have previously shown that RS and IHC4 are independent predictors of distant recurrence in postmenopausal hormone receptor–positive women (8,9). However, it is well known that recurrence risk extends for at least 20 years in these women (15), and therefore it is crucial to identify markers that predict late recurrence. Here, we have investigated the relationship between clinical variables, immunohistochemical markers, and four different scores (CTS, IHC4, RS, ROR) in predicting distant recurrence in years 0 to 5 and 5 to 10 separately.
There are few reports on late recurrence of breast cancer more than 5 years after diagnosis (7,15), and none of them have addressed immunohistochemical markers as prognostic factors, although there have been studies on mRNA expression profiles. Bianchini et al. (16) reported on risk stratification by mitotic kinase and an estrogen-related score and found that women with highly proliferative tumors and those with a high estrogen-related score were at greater risk of late recurrence and may benefit from additional hormonal therapy. Sgroi et al. (17) reported on the comparative performance of the Breast Cancer Index vs IHC4 and RS for late recurrence and found that the Breast Cancer Index is a strong prognostic score in predicting late recurrence. Dubsky et al. (18) reported on the EndoPredict test, which stratifies patients into low- and high-risk groups for late recurrence.
In our study, we found that clinical variables were strong prognostic factors in the initial 5 years of treatment, but only nodal status and tumor size remained prognostic in the multivariable analysis beyond 5 years of treatment. Although we have found that Ki67 has univariate prognostic value in years 5 to 10, it was not independent of classical variables in a multivariable analysis when all clinical factors were added to the model. None of the immunohistochemical markers added individually independent prognostic information for distant recurrence after 5 years of follow-up, although IHC4 returned some value.
The IHC4, RS, and ROR scores added statistically significant prognostic information in years 5 to 10 in the multivariable analyses, but of these, the ROR was the strongest score in this late follow-up period. These results were seen for all five subgroups in this study. Although IHC4, RS, and ROR added overall prognostic information in the late follow-up period, ROR was the best discriminator of patients into low-risk and high-risk groups for late distant recurrence. ROR has previously been shown to add prognostic information beyond that of standard markers (13,19), but this is the first time this score has been compared with other scores for late recurrence in women with hormone receptor–positive breast cancer. Although this analysis was based on the ROR50 gene score without tumor size, very similar results were found when the analysis was based on the ROR50 gene score with tumor size included.
Strengths of this analysis are the large sample size, a median long-term follow-up of 10 years, and use of an aromatase inhibitor or tamoxifen in all patients. Furthermore, clinical variables and immunohistochemical markers were available for all subjects, as well as data on RS and ROR. Limitations include the fact that the comparison with IHC4 was conducted in the same study for which the score was derived, but sample splitting for each of the four scores (CTS, IHC4, RS, and ROR) was performed to remove any overfitting. The transATAC cohort only includes participants from the United Kingdom for which enough tissue was available to perform laboratory analyses for IHC4 and PAM50 ROR (n = 940). Therefore, small tumors might be underrepresented in this cohort. This study includes women for which all four scores and clinical variables were available, which constitutes 84% of the transATAC collection. Only 21% of all ATAC participants overall had chemotherapy for their initial treatment, and only 18% with no initial chemotherapy treatment were included in the transATAC cohort where biopsy material was obtained. As a consequence, we were unable to analyze the value of ROR in determining prognosis for patients receiving chemotherapy or its ability to identify which patients might benefit from initial chemotherapy. The latter question is best addressed in a trial where chemotherapy is a randomized treatment option.
The prediction and treatment of late breast cancer recurrence is an important and largely unmet need and remains a major clinical problem. Although nodal status and tumor size added prognostic value 5 years after diagnosis, conventional immunohistochemical markers did not add information for late recurrence of those evaluated. The ROR score was the only molecular factor that showed promise in predicting late recurrence and to discriminate patients into low and high risk for late distant recurrence. These results help to identify women who are at high risk of late recurrence and who may benefit from either more intensive treatment (ie, chemotherapy) or extended endocrine treatment beyond 5 years. Validation of these results in other cohorts is needed.
Funding
This work was supported by Breakthrough Breast Cancer through the Mary-Jean Mitchell Green Foundation, Royal Marsden NIHR Biomedical Research Centre, Nanostring, and Cancer Research UK (CRUK) programme grant C569-10404.
I. Sestak, J. Cuzick, and M. Dowsatt contributed to the study design. I. Sestak did the statistical analysis. All authors contributed to data interpretation. I. Sestak and J. Cuzick drafted the report, and all authors approved the final manuscript.
References
- 1. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomized trials. Lancet. 2011;378(9793): 771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746 [DOI] [PubMed] [Google Scholar]
- 3. Tamoxifen for early breast cancer: an overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114): 1451–1467 [PubMed] [Google Scholar]
- 4. Tamoxifen for early breast cancer. Cochrane Database Syst Rev. 2001;CD000486 DOI:10.1002/14651858 [DOI] [PubMed] [Google Scholar]
- 5. Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–1141 [DOI] [PubMed] [Google Scholar]
- 6. Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364 (25):2381–2391 [DOI] [PubMed] [Google Scholar]
- 7. Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100(16):1179–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11): 1829–1834 [DOI] [PubMed] [Google Scholar]
- 9. Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011;29(32)4273–4278 [DOI] [PubMed] [Google Scholar]
- 10. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 Risk of Recurrence Score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013; 31(22):2783–2790 [DOI] [PubMed] [Google Scholar]
- 11. Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53 [DOI] [PubMed] [Google Scholar]
- 12. Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008;26(7):1059–1065 [DOI] [PubMed] [Google Scholar]
- 13. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of estrogen receptor-positive breast cancer: ATLAS, a randomized trial. Lancet. 2013;381(9869):805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esserman LJ, Moore DH, Tsing PJ, et al. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat. 2011;129(2):607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bianchini G, Pusztai L, Iwamoto T, et al. Molecular tumor characteristics influence adjuvant endocrine treatment outcome. Cancer Res. 2011;71(24 Supplemental):S1–S7 [Google Scholar]
- 17. Sgroi D, Sestak I, Cuzick J, et al. Comparative performance of Breast Cancer Index (BCI) vs. Oncotype Dx and IHC4 in the prediction of late recurrence in hormonal receptor-positive lymph node-negative breast cancer patients: a TransATAC study. Cancer Res. 2012;72(24 Supplemental): S1–S9 [Google Scholar]
- 18. Dubsky P, Brase JC, Fisch K, et al. The EndoPredict score identifies late distant metastases in ER+/HER2- breast cancer patients. Cancer Res. 2012;72(24 Supplemental):S4–S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16(21):5222–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]