Abstract
Background:
COPD and heart failure with preserved ejection fraction overlap clinically, and impaired left ventricular (LV) filling is commonly reported in COPD. The mechanism underlying these observations is uncertain, but may include upstream pulmonary dysfunction causing low LV preload or intrinsic LV dysfunction causing high LV preload. The objective of this study is to determine if COPD and emphysema are associated with reduced pulmonary vein dimensions suggestive of low LV preload.
Methods:
The population-based Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study recruited smokers aged 50 to 79 years who were free of clinical cardiovascular disease. COPD was defined by spirometry. Percent emphysema was defined as regions < −910 Hounsfield units on full-lung CT scan. Ostial pulmonary vein cross-sectional area was measured by contrast-enhanced cardiac magnetic resonance and expressed as the sum of all pulmonary vein areas. Linear regression was used to adjust for age, sex, race/ethnicity, body size, and smoking.
Results:
Among 165 participants, the mean (± SD) total pulmonary vein area was 558 ± 159 mm2 in patients with COPD and 623 ± 145 mm2 in control subjects. Total pulmonary vein area was smaller in patients with COPD (−57 mm2; 95% CI, −106 to −7 mm2; P = .03) and inversely associated with percent emphysema (P < .001) in fully adjusted models. Significant decrements in total pulmonary vein area were observed among participants with COPD alone, COPD with emphysema on CT scan, and emphysema without spirometrically defined COPD.
Conclusions:
Pulmonary vein dimensions were reduced in COPD and emphysema. These findings support a mechanism of upstream pulmonary causes of underfilling of the LV in COPD and in patients with emphysema on CT scan.
Chronic lower respiratory disease is the third-leading cause of death in the United States.1 The most morbid components of chronic lower respiratory disease are COPD, defined by spirometry as airflow obstruction that is not fully reversible, and pulmonary emphysema, defined by morphology as permanent enlargement of airspaces accompanied by destruction of their walls.2,3 Emphysema on CT imaging is present in approximately one-half of patients with COPD,4‐7 and an estimated 2% of the general population aged > 50 years has emphysema without spirometry-defined COPD.8
The number of Americans with a diagnosis of heart failure was 5.7 million in 2008, and approximately one-half of prevalent heart failure cases are characterized by preserved ejection fraction.9,10 By comparison, 12 million Americans have a diagnosis of COPD and an additional 12 million may have undiagnosed COPD and emphysema.11
Heart failure with preserved ejection fraction and COPD overlap clinically: 41% of patients hospitalized for exacerbations of heart failure with preserved ejection fraction had COPD when tested systematically, and approximately 20% of patients hospitalized for COPD exacerbations have heart failure.12,13 Studies using echocardiography demonstrate a high prevalence of left ventricular (LV) diastolic dysfunction in COPD.14‐16 Standard echocardiographic signs of diastolic dysfunction (eg, reversed ratio of peak filling rates during early-phase diastole and atrial contraction [E/A ratio]) are often interpreted as suggestive of high LV preload pressure; however, states of reduced LV filling pressure can also mimic these changes.17‐19 Older articles in the literature, mostly using invasive methods, suggested reduced cardiac output in COPD with generally preserved LV ejection fraction.13,20,21 Whether chronic lower respiratory disease is associated with intrinsic LV dysfunction or impaired LV filling due to upstream causes remains poorly understood.17
Assessment of LV preload pressure typically requires cardiac catheterization; however, pulmonary vein dimensions have been shown to correlate with left atrial dimensions,22,23 which, in turn, correlate with measures of LV filling pressure.24,25 Therefore, we measured pulmonary vein dimensions using contrast-enhanced MRI in a study of patients with predominantly mild to moderate COPD and of control subjects. Reduced pulmonary vein cross-sectional area would favor a process resulting in reduced LV filling pressures, such as elevated pulmonary vascular resistance (PVR), whereas increased pulmonary vein area would favor a mechanism of intrinsic dysfunction of the LV. We also assessed the relationship of pulmonary vein dimensions to emphysema on CT scans, given that we previously observed a stronger relationship of LV filling to emphysema than to lung function in the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study.26
Materials and Methods
See e-Appendix 1 (793.4KB, pdf) for a complete description of the methods. The MESA COPD Study recruited patients with COPD and control subjects predominantly from MESA, a population-based, prospective cohort study of subclinical atherosclerosis,27 and the Emphysema and Cancer Action Project, a separate, nonoverlapping lung cancer screening study,28 and also from the outpatient community at Columbia University Medical Center. Included participants were 50 to 79 years of age with a ≥ 10 pack-year smoking history. Exclusion criteria were clinical cardiovascular disease, stage 3B to 5 chronic kidney disease, asthma prior to age 45 years, prior lung resection, contraindication to MRI, and pregnancy. The current report includes participants from one site where three-dimensional, contrast-enhanced MRI of the pulmonary veins was performed.
Protocols for this study were approved by the institutional review board of Columbia University Medical Center and by the National Heart, Lung, and Blood institute (approval numbers AAAA7791 and AAAD6395). Written informed consent was obtained from all participants.
MRI
The cardiac MRI protocol was from the fifth examination of MESA modified to include assessment of pulmonary vasculature.29 Images were obtained using a 1.5 Tesla, whole-body magnetic resonance (MR) system (Signa LX; GE Healthcare LLC) with phased-array coil for signal reception. Pulmonary veins were assessed by MR angiography with a three-dimensional, vascular, spoiled gradient-recalled imaging sequence with 5-mm slice thickness interpolated to 2.5 mm to obtain an anatomic view of the entire thorax in the coronal plane.
Ventricular Assessment:
To measure LV function, the heart was imaged in short-axis orientation with ≥ 12 slices. Cine images were reconstructed at intervals of 20 to 35 milliseconds over the cardiac cycle with 40 phases. In a subset of participants, phase-contrast images were obtained using a segmented fast gradient-echo sequence without breathhold. The mitral inflow pattern was assessed using phase-contrast images to estimate blood flow across the mitral valve. All images were analyzed quantitatively using dedicated software for LV function (Cardiac Image Modeler; Auckland MRI Research Group), in addition to right ventricular (RV) parameters and phase-contrast images using QFLOW 7.2 (Medis Medical Imaging Systems BV).30
Pulmonary Vein Assessment:
Pulmonary vein area was assessed by a single reader using multiplanar reformation software (Volume Viewer Plus Suite 15.10.4; General Electric Co). Pulmonary vein ostia were defined by the signal intensity reflection between the planes of the left atrium and pulmonary vein.31 Cross-sectional area was determined by manual edge tracing on the reformatted orthogonal image. Total cross-sectional pulmonary vein area was reported as the sum of all pulmonary veins. The coefficient of variation of vein area measurements on blinded duplicate reading was 3.7%.
COPD Case Status
We used the standard clinical definition of COPD to define case status: a postbronchodilator FEV1/FVC ratio < 0.70.2,32 See e-Appendix 1 (793.4KB, pdf) for more details.
Chest CT Scan and Quantitative Assessment of Emphysema
Participants underwent full-lung thoracic CT scan on a General Electric 64-slice helical scanner (120 kVp, 200 mA at 0.5 s; General Electric Co) with 0.75-mm slice thickness. Image attenuation was assessed at a single reading center by trained readers without knowledge of other participant information (VIDA Diagnostics Inc). Percent of emphysema-like lung (also known as percent low-attenuation area and hereafter referred to as percent emphysema) was defined as the percentage of total voxels within the lung field that was < −910 Hounsfield units (HU).33
Anthropometry, Smoking Status, and Other Covariates
Age, sex, race/ethnicity, smoking status, and number of pack-years smoked were self reported. Study participants who reported smoking at least one cigarette in the 30 days prior to assessment were classified as current smokers. Height, weight, and seated BP were measured using standard techniques.34
Statistical Analysis
The sample was stratified by COPD status with dichotomous variables presented as proportions and continuous variables as means with SD, unless otherwise indicated. Multiple linear regression models were used to adjust for participant age, sex, race/ethnicity, height, weight, cohort, smoking status, and pack-years smoked. Alternate adjustments for body size were performed using body surface area and height squared. Additional sensitivity analyses included adjustments for variant vein anatomy, ventricular function, and systolic BP. A two-tailed P value < .05 was considered to indicate statistical significance. Analyses were performed using SAS version 9.3 (SAS Institute Inc) statistical software.
Results
Of 201 participants enrolled at one site where pulmonary vein quantification was performed, 165 completed spirometry, thoracic CT scans, and cardiac MRI with pulmonary vein quantification (e-Fig 1 (793.4KB, pdf) ). Participants enrolled but not completing all components of the pulmonary vein study had lower lung function, smaller LV dimensions, and were less likely to report current smoking (e-Table 1 (793.4KB, pdf) ).
The mean age of the study patients was 68 ± 7 years, and 60% were men. Fifty-three percent had COPD, and 32% were current smokers. Table 1 summarizes the clinical characteristics according to COPD status. Height and Hispanic ethnicity differed by COPD status. The extent of emphysema on CT scan was greater among participants with COPD (Table 1). LV end-diastolic volume (EDV) was smaller in patients with COPD than in control subjects when adjusted for age, sex, race/ethnicity, and body size (P = .04) (e-Table 2 (793.4KB, pdf) ).
Table 1.
—Characteristics of Participants With Pulmonary Vein Measurements in the MESA COPD Study
| Characteristic | COPD (n = 88) | No COPD (n = 77) |
| Age, y | 67 ± 8 | 69 ± 6 |
| Male patients, % | 64 | 56 |
| Race/ethnicity, % | ||
| White | 63 | 53 |
| Black | 28 | 26 |
| Hispanic | 8 | 17 |
| Asian | 1 | 4 |
| Height, cm | 170 ± 9 | 167 ± 9 |
| Weight, kg | 77 ± 17 | 80 ± 16 |
| BMI, kg/m2 | 26 ± 4 | 29 ± 5 |
| Current smoker, % | 35 | 27 |
| Pack-y smoked, median (IQR) | 40 (30) | 32 (27) |
| FEV1 % predicted | 72 ± 21 | 100 ± 18 |
| FEV1/FVC, % | 56 ± 12 | 77 ± 4 |
| COPD severity, % | ||
| FEV1 ≥ 80% predicted | 38 | … |
| 50% ≥ FEV1 < 80% predicted | 45 | … |
| FEV1 < 50% predicted | 17 | … |
| Total lung capacity,a L | 6.0 ± 1.2 | 5.2 ± 1.0 |
| % predicted total lung capacitya | 105 ± 13 | 98 ± 13 |
| Emphysema−910 HU, median (IQR) | 24 (22) | 12 (14) |
| Emphysema−950 HU, median (IQR) | 3.0 (6.7) | 1.0 (1.1) |
| LV EDV, mL | 117 ± 31 | 121 ± 27 |
| LV end-systolic volume, mL | 48 ± 17 | 48 ± 18 |
| LV stroke volume, mL | 69 ± 18 | 73 ± 13 |
| LV EDV index, mL/m2 | 61 ± 15 | 63 ± 12 |
| LV end-systolic volume index, mL/m2 | 25 ± 9 | 25 ± 8 |
| LV stroke-volume index, mL/m2 | 36 ± 9 | 38 ± 7 |
| LV ejection fraction, % | 60 ± 8 | 61 ± 7 |
Data are given as mean ± SD unless otherwise indicated. EDV = end-diastolic volume; HU = Hounsfield units; IQR = interquartile range; LV = left ventricle; MESA = Multi-Ethnic Study of Atherosclerosis.
Body plethysmography was attempted only on participants recruited from the Emphysema and Cancer Action Project and the local outpatient community (n = 106).
Pulmonary Vein Area in COPD
The mean total pulmonary vein cross-sectional area was 558 ± 159 mm2 in COPD cases compared with 623 ± 145 mm2 in control subjects (P = .006), for a mean difference of −65 mm2 (95% CI, −111 to −18 mm2), or about 10.4% smaller in patients with COPD compared with control subjects. This difference persisted with similar magnitude in the fully adjusted model (Table 2).
Table 2.
—Predicted Values and Mean Difference in Total Cross-Sectional Pulmonary Vein Area Among Patients With and Without COPD
| Pulmonary Vein Area | COPD (n = 88) | No COPD (n = 77) | Mean Difference in Total Pulmonary Vein Area (95% CI) | P Value |
| Total pulmonary vein area, mm2 | 558 | 623 | −65 (−111 to −18) | .006 |
| Predicted total pulmonary vein area, mm2 | ||||
| Base modela | 562 | 618 | −57 (−105 to −8) | .02 |
| Base model with smoking terms | 562 | 618 | −57 (−106 to −7) | .03 |
Predicted values and mean differences in the base model adjusted for age, sex, race/ethnicity, height, weight, and cohort. Smoking terms refer to smoking status (current vs former) and pack-y smoked.
Pulmonary Vein Area and Percent Emphysema
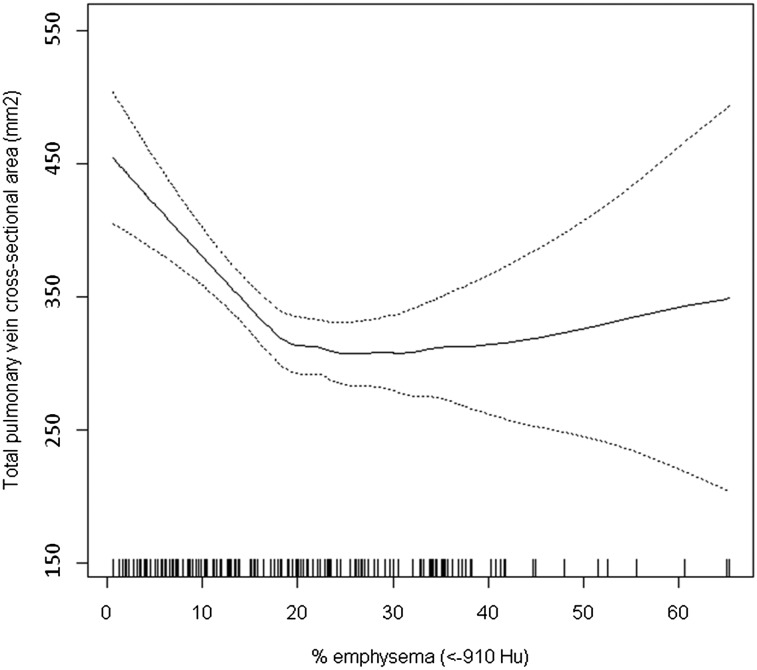
Percent emphysema on CT scan was associated with significant reduction in total pulmonary vein area (Table 3). The decrement was monotonic across quartiles of percent emphysema, with a 19% decrement between lowest and highest quartiles of percent emphysema in fully adjusted models. The continuous relationship of percent emphysema to total pulmonary vein area was nonlinear, with a flattening in the relationship at higher levels of percent emphysema (Fig 1).
Table 3.
—Predicted Values and Mean Difference in Total Cross-Sectional Pulmonary Vein Area According to Percent Emphysema on CT Scan
| Quartiles of Percent Emphysema −910 HU | Mean Difference in Total Pulmonary Vein Area per 10 Percentage-Point Increase in Emphysema−910 HU (95% CI) | |||||
| Pulmonary Vein Area | 4.6% (n = 41) | 13% (n = 41) | 23% (n = 42) | 40% (n = 41) | P Value | |
| Total pulmonary vein area, mm2 | 635 | 588 | 545 | 586 | … | .01 |
| Predicted total pulmonary vein area, mm2 | ||||||
| Base modela | 647 | 609 | 573 | 532 | −28 (−44 to −12) | < .001 |
| Base model with smoking terms | 652 | 610 | 571 | 526 | −30 (−47 to −14) | < .001 |
See Table 1 legend for expansion of abbreviations.
Predicted values and mean differences in the base model adjusted for age, sex, race/ethnicity, height, weight, and cohort. Mean differences were back-transformed to represent a 10 percentage-point increase in emphysema −910 HU above study population mean. Smoking terms refer to smoking status (current vs former) and pack-y smoked.
Figure 1.
Generalized additive model of total cross-sectional pulmonary vein area. Results of multivariate analysis of the relationship between total pulmonary vein cross-sectional area and percent emphysema (< −910 Hu on CT scan) are shown. Tick marks above the x axis represent observed emphysema measures. The predicted total pulmonary vein area is represented by the solid line and was obtained from a smoothed regression model adjusted for age, sex, height, weight, race/ethnicity, current smoking status, number of pack-y smoked, and cohort. The dashed lines represent the 95% confidence boundary. Test for nonlinearity of emphysema term: P = .001. Hu = Hounsfield units.
Pulmonary Vein Area in COPD and Emphysema
Examination of the joint relationship of COPD and percent emphysema to total pulmonary vein area revealed a significant, subadditive interaction term between COPD and percent emphysema (P = .03). Participants, therefore, were stratified into control subjects with neither COPD nor emphysema, COPD without emphysema, COPD with emphysema, and emphysema without COPD. The characteristics of participants according to these categories are shown in e-Table 3 (793.4KB, pdf) .
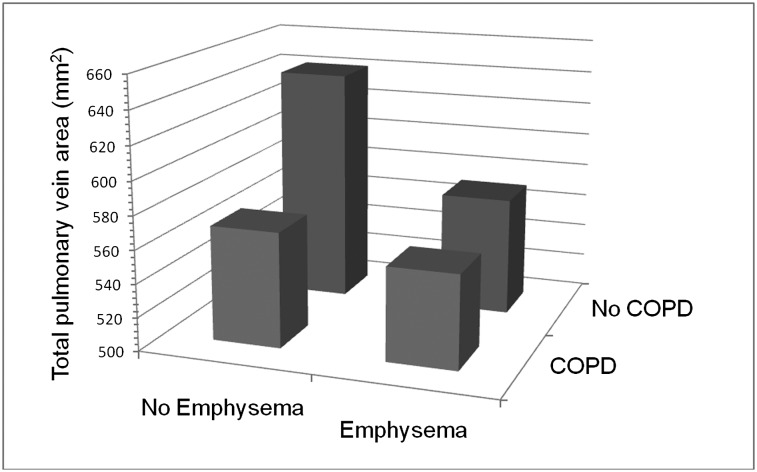
Participants with COPD with, and without, emphysema had large and statistically significant reductions in total pulmonary vein area compared with control subjects (Table 4).The magnitude of the decrement was similar in both groups without evidence of an additive effect. Participants with only emphysema also had a large and statistically significant decrement in total pulmonary vein area compared with control subjects. The magnitude of this decrement was similar to that observed with COPD (Fig 2).
Table 4.
—Predicted Values for and Mean Difference in Total Cross-Sectional Pulmonary Vein Area by Strata of COPD and Emphysema
| Pulmonary Vein Area | No COPD or Emphysema (n = 54) | COPD Without Emphysema (n = 29) | COPD With Emphysema (n = 59) | Emphysema Without COPD (n = 23) |
| Total pulmonary vein area, mm2 | 637 | 563 | 556 | 590 |
| Predicted total pulmonary vein area, mm2 | ||||
| Base modela | 641 | 571 | 556 | 570 |
| Mean difference (95% CI) | Reference | −70 (−135 to −5) | −85 (−145 to −25) | −71 (−140 to −1) |
| Base model with smoking terms | 641 | 569 | 556 | 570 |
| Mean difference (95% CI) | Reference | −72 (−138 to −6) | −85 (−146 to −25) | −71 (−140 to −1) |
Predicted values in the base model were adjusted for age, sex, race/ethnicity, height, weight, and cohort. Smoking terms refer to smoking status (current vs former) and pack-y smoked. No emphysema was defined as less than median percent emphysema−910 Hounsfield units in the entire study population. Predicted mean differences are relative to stratum with no COPD or emphysema.
Figure 2.
Predicted total cross-sectional pulmonary vein area by strata of COPD and emphysema. Predicted values in the model were adjusted for age, sex, race/ethnicity, height, weight, smoking status, and number of pack-y smoked. No emphysema was defined as less than median percent emphysema (< −910 Hounsfield units on CT scan) in the entire study population.
E/A RatioMR in COPD
Of 329 participants enrolled in the multicenter MESA COPD Study, E/A ratioMR measures were available for 194. Adjusting for age, sex, race/ethnicity, body size, LV mass, systolic BP, and diabetes, the presence of COPD was associated with increased odds of an abnormal E/A ratioMR (OR, 2.6; 95% CI, 1.3-5.4; P = .01) (e-Table 4 (793.4KB, pdf) ). Among 69 participants with both E/A ratioMR and pulmonary vein measures, pulmonary vein area was reduced among patients with COPD with and without an abnormal E/A ratioMR, but was elevated among control subjects without COPD who had an abnormal E/A ratioMR; however, none of these differences were statistically significant (e-Table 5 (793.4KB, pdf) ).
Sensitivity Analyses
Primary analysis was based on MR sequences acquired at full inspiration to minimize artifact from respiratory motion. To ensure the observed relationship between pulmonary vein area and chronic lower respiratory disease was not solely determined by lung volume attained at full inspiration, we analyzed a subset of scans at end expiration and found similar associations between COPD, percent emphysema, and reduced pulmonary vein area (e-Figs 2, 3 (793.4KB, pdf) ).
Sensitivity analyses using alternative metrics of body size and emphysema, as well as adjustment for oxygen saturation and physician-diagnosed sleep apnea, yielded consistent findings (e-Figs 2, 3 (793.4KB, pdf) ). The association between COPD, emphysema, and total pulmonary vein area also remained significant following addition of terms for LV or RV structure (e-Figs 2, 3 (793.4KB, pdf) ). In these models, both RV EDV and LV EDV were significantly associated with total pulmonary vein area (P < .01). Results of stratified analyses presented in Table 4 were similar when using alternate definitions of emphysema (e-Table 6 (793.4KB, pdf) ).
Plethysmography was performed in a subset of participants recruited from the Emphysema and Cancer Action Project and the local outpatient community (n = 106). Higher % predicted total lung capacity was associated with significantly smaller pulmonary vein area and a trend toward reduced LV EDV (e-Table 7 (793.4KB, pdf) ).
Discussion
Participants with predominantly mild to moderate COPD had reduced total pulmonary vein cross-sectional area as assessed by contrast-enhanced MRI compared with control subjects without COPD. In addition, pulmonary vein size was inversely associated with percent emphysema and was significantly reduced among participants with emphysema on CT scan who did not have clinical COPD. These findings suggest reduced LV preload in both clinical COPD and in patients with emphysema on CT scan.
To our knowledge, this study is the first to report pulmonary vein dimensions in COPD and emphysema. It is potentially clinically relevant for two reasons. First, among patients with COPD, LV diastolic dysfunction based on E/A ratio is common14‐16,26,35; however, these mitral inflow abnormalities can reflect increased stiffness of the LV or states of low LV filling pressure.17‐19 Our findings suggest low LV distending pressure resulting from upstream pulmonary causes rather than intrinsic cardiac dysfunction may contribute significantly to impaired LV filling in COPD. Second, since emphysema on CT scan is not usually assessed clinically, patients with emphysema but normal lung function presenting with dyspnea may appear to have diastolic dysfunction on echocardiography that may, in fact, be due to emphysema-related low LV preload. This underrecognized subgroup of chronic lower respiratory disease represented 13% of smokers in the present study.
Potential mechanisms of reduced pulmonary vein size in COPD and emphysema, while not the primary focus of this study, include altered intrathoracic pressure and PVR. Intrathoracic pressure may impair venous return to the right side of the heart, resulting in underfilling of the pulmonary veins.36,37 In support of this mechanism, smaller total pulmonary vein area was associated with reduced RV EDV. Prior studies have also demonstrated reduced RV dimensions in severe emphysema with COPD, as well as improvements in LV filling following lung volume reduction surgery.38‐40 Alternatively, hyperinflation-associated increase in intrathoracic pressure may act directly at the LV to impair filling. However, we believe this mechanism unlikely because upstream intrathoracic structures would also be exposed to this augmented pressure.41 Indeed, higher % predicted total lung capacity was associated with both smaller pulmonary veins and a trend toward smaller LV EDV.
Classic descriptions of cor pulmonale attribute increased PVR to emphysematous disruption of the vascular bed, hypoxic vasoconstriction, vascular remodeling, thrombosis, and left-sided heart dysfunction.15,42 In addition, hyperinflation-associated increases in PVR may result from compression, or lengthening and narrowing of alveolar vessels.43,44 While PVR and intrathoracic pressure were not assessed in the present study, these mechanisms may both contribute to impaired flow to the pulmonary veins and reductions in their dimensions. Given that the associations of COPD and emphysema to pulmonary vein area were independent of RV EDV, it is likely that, in addition to RV underfilling, an additional mechanism, such as increased PVR, likely contributes to reduced pulmonary vein dimensions.
Diastolic dysfunction is characterized by myocardial stiffening and fibrosis.45 An alternative explanation for our observation is that such fibrosis may extend to the left atrium and pulmonary veins. We think this explanation unlikely, however, given that the left atrium is typically enlarged in diastolic dysfunction with high, LV end-diastolic pressure.25,46‐48 RV dilation with impingement on the LV is also an important consideration in states of very high pulmonary artery pressure,15 which is unlikely in the present study of predominantly mild to moderate COPD. Smaller pulmonary vein dimensions were associated with reduced RV EDV. Furthermore, adjustment for RV dimensions in the present study did not alter the associations of COPD and emphysema with smaller total pulmonary vein area. Hence, our findings of reduced pulmonary vein dimensions in COPD and emphysema support an upstream mechanism that is independent of impaired LV relaxation.
Nonetheless, cardiopulmonary interactions in COPD and emphysema are complex.20 It is likely that intrinsic LV dysfunction is more common in patients with COPD,15 particularly since the present study excluded patients with clinical cardiovascular disease and atrial fibrillation. The significant increase in odds of an abnormal E/A ratioMR in the presence of COPD was associated with a nonsignificant reduction in total pulmonary vein area. While this may have been due to inadequate power or misclassification of pseudonormal mitral inflow velocities, residual confounding by unaccounted causes of intrinsic LV dysfunction is possible. Further, there is evidence to support accelerated aging in the pathogenesis of COPD and emphysema49,50 and similar mechanisms have been proposed for diastolic dysfunction.51,52 In addition, LV myocardial fibrosis has been demonstrated in patients with COPD and cor pulmonale.53
There are several limitations to the current study. We did not validate pulmonary vein area as a surrogate for LV end-diastolic pressure. However, in the context of chronic lower respiratory diseases, changes in lung mechanics alter juxtacardiac pressures.54 Whereas the volume (and cross-sectional area) of intrathoracic cardiovascular structures reflect their transmural pressure difference, direct quantification of transmural cardiac pressures would require invasive measurements of both intracardiac and pericardial/pleural pressures.54,55 Such procedures were too invasive to perform in 165 patients with mild to moderate lung disease who were free of clinical cardiac disease. MRI was performed instead of echocardiography to minimize potential bias from inadequate acoustic windows among patients with hyperinflation.17 Phase-contrast MR evaluation of E/A ratio, as was done in the present study, has been shown to correlate well with transthoracic echocardiography.56,57 These studies were small, however, and our secondary findings related to E/A ratioMR should be interpreted in light of this relatively novel method of assessing LV filling.
Vein measurements were performed on nongated MR images to reduce sequence acquisition time during breathhold. While this may have introduced motion artifact, only a minority of scans could not be quantified. To minimize respiratory artifact, pulmonary vein MR images were acquired at suspended inspiration, which may have affected pulmonary blood flow. Pulmonary veins were also assessed in a subset of participants with dedicated pulmonary vein sequences at end expiration and similar results were obtained.
COPD was defined by standard criteria2; however, there are currently no standard criteria to define emphysema on CT scan. We assessed emphysema quantitatively, which has much greater precision than radiologist assessment but for which a standard threshold of normality is currently lacking.58,59 Therefore, we used an arbitrary but reasonable threshold of the median value of percent emphysema. Sensitivity analyses showed that results were similar using a 25th percentile threshold and using a −950 HU definition (median or 25th percentile) to define percent emphysema.
The observational design of the study limits inference that COPD or emphysema reduced pulmonary vein area, although it seems unlikely that pulmonary vein size contributed to COPD and emphysema. Selection bias can affect the validity of case-control studies; however, the study was predominantly nested in two cohort studies and secondary analyses restricted to participants selected from those cohorts yielded consistent results, which makes selection bias an unlikely explanation for our results. Confounding is always of potential concern in observational studies; however, adjustment for multiple, precisely measured confounders had little impact on effect estimates. Finally, the study sample size was relatively small, particularly in stratified analyses, hence, effect estimates may be relatively imprecise.
In summary, COPD and emphysema on CT scan were associated with reduced pulmonary vein cross-sectional area. These findings suggest that impaired LV filling in COPD, and also in emphysema in the absence of clinical COPD, may be predominantly due to reduced LV preload from upstream pulmonary causes rather than intrinsic diastolic dysfunction, although cardiopulmonary interactions are likely complex.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Barr had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Smith: contributed to study concept and design; data analysis and interpretation; and drafting, revising, and final approval of the manuscript and served as principle author.
Dr Prince: contributed to study concept and design, data interpretation, and revision and final approval of the manuscript.
Dr Hoffman: contributed to study concept and design, data analysis, and revision and final approval of the manuscript.
Dr Bluemke: contributed to study concept and design, data interpretation, and revision and final approval of the manuscript.
Dr Liu: contributed to data analysis and revision and final approval of the manuscript.
Dr Rabinowitz: contributed to study design, data analysis, and revision and final approval of the manuscript.
Dr Hueper: contributed to data analysis and revision and final approval of the manuscript.
Ms Parikh: contributed to data analysis and interpretation and revision and final approval of the manuscript.
Dr Gomes: contributed to study concept and design, data interpretation, and revision and final approval of the manuscript.
Dr Michos: contributed to study concept and design and revision and final approval of the manuscript.
Dr Lima: contributed to study concept and design, data analysis, and revision and final approval of the manuscript.
Dr Barr: contributed to study concept and design, data analysis and interpretation, and revision and final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Hoffman has received grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI), the Alpha-1 Foundation, and the American Lung Association, and he is founder of and shareholder in VIDA Diagnostics Inc. Dr Gomes is a stockholder in St. Jude Medical Inc. Dr Barr has received grants and contracts from the NIH/NHLBI, US Environmental Protection Agency, and the Alpha-1 Foundation; in-kind donation for dietary supplement from Cenestra LLC; and reimbursement for travel from Boehringer Ingelheim GmbH. The other authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- E/A ratio
ratio of peak filling rates during early phase diastole and atrial contraction
- E/A ratioMR
ratio of peak filling rates during early phase diastole and atrial contraction estimated by magnetic resonance
- EDV
end-diastolic volume
- LV
left ventricle
- MESA
Multi-Ethnic Study of Atherosclerosis
- MR
magnetic resonance
- PVR
pulmonary vascular resistance
- RV
right ventricle
Footnotes
Funding/Support: This study was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute [Grants R01-HL093081, R01-HL077612, R01-HL075476, and N01-HC95159-HC95169]; and Fonds de la recherche en santé Québec.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary Data for 2010. National Vital Statistics Reports, vol. 60, No. 4. Hyattsville, MD: National Center for Health Statistics; 2012 [Google Scholar]
- 2.Roisin RR, Vestbo J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Global Initiative for Obstructive Lung Disease website. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf. Published 2011. Accessed August 20, 2012 [Google Scholar]
- 3.Meneely GR, Wyatt JP, Steele JD, Renzetti AD, Harris HW. Chronic bronchitis, asthma, and pulmonary emphysema-a statement by Committee on Diagnostic Standards for Nontuberculous Respiratory Diseases. Am Rev Respir Dis. 1962;85:762-768 [Google Scholar]
- 4.Auerbach O, Hammond EC, Garfinkel L, Benante C. Relation of smoking and age to emphysema. Whole-lung section study. N Engl J Med. 1972;286(16):853-857 [DOI] [PubMed] [Google Scholar]
- 5.Spain DM, Siegel H, Bradess VA. Emphysema in apparently healthy adults. Smoking, age, and sex. JAMA. 1973;224(3):322-325 [PubMed] [Google Scholar]
- 6.Burrows B, Earle RH. Course and prognosis of chronic obstructive lung disease. A prospective study of 200 patients. N Engl J Med. 1969;280(8):397-404 [DOI] [PubMed] [Google Scholar]
- 7.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55(8):635-642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh SE, Travers J, Weatherall M, et al. Proportional classifications of COPD phenotypes. Thorax. 2008;63(9):761-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259 [DOI] [PubMed] [Google Scholar]
- 10.Roger VL, Go AS, Lloyd-Jones DM, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188-197 [DOI] [PubMed] [Google Scholar]
- 11.Wilt TJ, Niewoehner D, Kim C, et al. Use of Spirometry for Case Finding, Diagnosis, and Management of Chronic Obstructive Pulmonary Disease (COPD). Rockville, MD: Agency for Healthcare Research and Quality; 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iversen KK, Kjaergaard J, Akkan D, et al. ; ECHOS-Lung Function Study Group Chronic obstructive pulmonary disease in patients admitted with heart failure. J Intern Med. 2008;264(4):361-369 [DOI] [PubMed] [Google Scholar]
- 13.Kachel RG. Left ventricular function in chronic obstructive pulmonary disease. Chest. 1978;74(3):286-290 [DOI] [PubMed] [Google Scholar]
- 14.Tutar E, Kaya A, Gulec S, et al. Echocardiographic evaluation of left ventricular diastolic function in chronic cor pulmonale. Am J Cardiol. 1999;83(9):1414-1417 [DOI] [PubMed] [Google Scholar]
- 15.Funk GC, Lang I, Schenk P, Valipour A, Hartl S, Burghuber OC. Left ventricular diastolic dysfunction in patients with COPD in the presence and absence of elevated pulmonary arterial pressure. Chest. 2008;133(6):1354-1359 [DOI] [PubMed] [Google Scholar]
- 16.Flu WJ, van Gestel YR, van Kuijk JP, et al. Co-existence of COPD and left ventricular dysfunction in vascular surgery patients. Respir Med. 2010;104(5):690-696 [DOI] [PubMed] [Google Scholar]
- 17.Boussuges A, Pinet C, Molenat F, et al. Left atrial and ventricular filling in chronic obstructive pulmonary disease. An echocardiographic and Doppler study. Am J Respir Crit Care Med. 2000;162(2 pt 1):670-675 [DOI] [PubMed] [Google Scholar]
- 18.Agmon Y, Oh JK, McCarthy JT, Khandheria BK, Bailey KR, Seward JB. Effect of volume reduction on mitral annular diastolic velocities in hemodialysis patients. Am J Cardiol. 2000;85(5):665-668 [DOI] [PubMed] [Google Scholar]
- 19.Gurudevan SV, Malouf PJ, Auger WR, et al. Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension: true diastolic dysfunction or left ventricular underfilling? J Am Coll Cardiol. 2007;49(12):1334-1339 [DOI] [PubMed] [Google Scholar]
- 20.Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener CF. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med. 1972;286(17):912-918 [DOI] [PubMed] [Google Scholar]
- 21.Gabinski C. Left ventricular function in chronic obstructive pulmonary disease. Cor Vasa. 1980;22(4):238-244 [PubMed] [Google Scholar]
- 22.Schwartzman D, Lacomis J, Wigginton WG. Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J Am Coll Cardiol. 2003;41(8):1349-1357 [DOI] [PubMed] [Google Scholar]
- 23.Knackstedt C, Visser L, Plisiene J, et al. Dilatation of the pulmonary veins in atrial fibrillation: a transesophageal echocardiographic evaluation. Pacing Clin Electrophysiol. 2003;26(6):1371-1378 [DOI] [PubMed] [Google Scholar]
- 24.Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22(7):1972-1982 [DOI] [PubMed] [Google Scholar]
- 25.Moya-Mur JL, García-Martín A, García-Lledó A, et al. Indexed left atrial volume is a more sensitive indicator of filling pressures and left heart function than is anteroposterior left atrial diameter. Echocardiography. 2010;27(9):1049-1055 [DOI] [PubMed] [Google Scholar]
- 26.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881 [DOI] [PubMed] [Google Scholar]
- 28.Mesia-Vela S, Yeh CC, Austin JH, et al. Plasma carbonyls do not correlate with lung function or computed tomography measures of lung density in older smokers. Biomarkers. 2008;13(4):422-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 suppl 2):S357-S365 [DOI] [PubMed] [Google Scholar]
- 30.Heckbert SR, Post W, Pearson GD, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48(11):2285-2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anselmino M, Blandino A, Beninati S, et al. Morphologic analysis of left atrial anatomy by magnetic resonance angiography in patients with atrial fibrillation: a large single center experience. J Cardiovasc Electrophysiol. 2011;22(1):1-7 [DOI] [PubMed] [Google Scholar]
- 32.Celli BR, MacNee W; ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946 [DOI] [PubMed] [Google Scholar]
- 33.Müller NL, Coxson H. Chronic obstructive pulmonary disease. 4: imaging the lungs in patients with chronic obstructive pulmonary disease. Thorax. 2002;57(11):982-985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Multi-Ethnic Study of Atherosclerosis Exam 5: field center procedures. Manual of operations. 2010. MESA–NHLBI website. http://www.mesa-nhlbi.org/publicdocs/2011/mesae5_mopjanuary2011.pdf. Accessed January 22, 2013
- 35.Watz H, Waschki B, Meyer T, et al. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest. 2010;138(1):32-38 [DOI] [PubMed] [Google Scholar]
- 36.Nakhjavan FK, Palmer WH, McGregor M. Influence of respiration on venous return in pulmonary emphysema. Circulation. 1966;33(1):8-16 [DOI] [PubMed] [Google Scholar]
- 37.Boerrigter B, Trip P, Bogaard HJ, et al. Right atrial pressure affects the interaction between lung mechanics and right ventricular function in spontaneously breathing COPD patients. PLoS ONE. 2012;7(1):e30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jörgensen K, Houltz E, Westfelt U, Ricksten SE. Left ventricular performance and dimensions in patients with severe emphysema. Anesth Analg. 2007;104(4):887-892 [DOI] [PubMed] [Google Scholar]
- 39.Jörgensen K, Müller MF, Nel J, Upton RN, Houltz E, Ricksten SE. Reduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema: an MRI study. Chest. 2007;131(4):1050-1057 [DOI] [PubMed] [Google Scholar]
- 40.Jörgensen K, Houltz E, Westfelt U, Nilsson F, Scherstén H, Ricksten SE. Effects of lung volume reduction surgery on left ventricular diastolic filling and dimensions in patients with severe emphysema. Chest. 2003;124(5):1863-1870 [DOI] [PubMed] [Google Scholar]
- 41.Pinsky MR. Cardiovascular issues in respiratory care. Chest. 2005;128(5)(suppl 2):592S-597S [DOI] [PubMed] [Google Scholar]
- 42.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part one. Am J Respir Crit Care Med. 1994;150(3):833-852 [DOI] [PubMed] [Google Scholar]
- 43.Whittenberger JL, McGregor M, Berglund E, Borst HG. Influence of state of inflation of the lung on pulmonary vascular resistance. J Appl Physiol. 1960;15:878-882 [DOI] [PubMed] [Google Scholar]
- 44.Butler J, Paley HW. Lung volume and pulmonary circulation. The effect of sustained changes in lung volume on pressure-flow relationships in the human pulmonary circulation. Med Thorac. 1962;19:261-267 [PubMed] [Google Scholar]
- 45.Wang JW, Nagueh SF. Current perspectives on cardiac function in patients with diastolic heart failure. Circulation. 2009;119(8):1146-1157 [DOI] [PubMed] [Google Scholar]
- 46.Matsuda M, Matsuda Y. Mechanism of left atrial enlargement related to ventricular diastolic impairment in hypertension. Clin Cardiol. 1996;19(12):954-959 [DOI] [PubMed] [Google Scholar]
- 47.Rossi A, Cicoira M, Florea VG, et al. Chronic heart failure with preserved left ventricular ejection fraction: diagnostic and prognostic value of left atrial size. Int J Cardiol. 2006;110(3):386-392 [DOI] [PubMed] [Google Scholar]
- 48.Murata M, Iwanaga S, Tamura Y, et al. A real-time three-dimensional echocardiographic quantitative analysis of left atrial function in left ventricular diastolic dysfunction. Am J Cardiol. 2008;102(8):1097-1102 [DOI] [PubMed] [Google Scholar]
- 49.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135(1):173-180 [DOI] [PubMed] [Google Scholar]
- 50.Karrasch S, Holz O, Jörres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med. 2008;102(9):1215-1230 [DOI] [PubMed] [Google Scholar]
- 51.Reed AL, Tanaka A, Sorescu D, et al. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol. 2011;301(3):H824-H831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: part II: causal mechanisms and treatment. Circulation. 2002;105(12):1503-1508 [DOI] [PubMed] [Google Scholar]
- 53.Kohama A, Tanouchi J, Hori M, Kitabatake A, Kamada T. Pathologic involvement of the left ventricle in chronic cor pulmonale. Chest. 1990;98(4):794-800 [DOI] [PubMed] [Google Scholar]
- 54.Butler J, Schrijen F, Henriquez A, Polu JM, Albert RK. Cause of the raised wedge pressure on exercise in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1988;138(2):350-354 [DOI] [PubMed] [Google Scholar]
- 55.Scharf SM, Pinsky MR, Magder S. Respiratory-Circulatory Interactions in Health and Disease. New York, NY: M. Dekker; 2001 [Google Scholar]
- 56.Rathi VK, Doyle M, Yamrozik J, et al. Routine evaluation of left ventricular diastolic function by cardiovascular magnetic resonance: a practical approach. J Cardiovasc Magn Reson. 2008;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubinshtein R, Glockner JF, Feng D, et al. Comparison of magnetic resonance imaging versus Doppler echocardiography for the evaluation of left ventricular diastolic function in patients with cardiac amyloidosis. Am J Cardiol. 2009;103(5):718-723 [DOI] [PubMed] [Google Scholar]
- 58.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT Scans—The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689-699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barr RG, Berkowitz EA, Bigazzi F, et al. ; COPDGene CT Workshop Group A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9(2):151-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement