Abstract
Background:
Neuroendocrine cell hyperplasia of infancy (NEHI) is a childhood diffuse lung disease of unknown etiology. We investigated the mechanism for lung disease in a subject whose clinical, imaging, and lung biopsy specimen findings were consistent with NEHI; the subject’s extended family and eight other unrelated patients with NEHI were also investigated.
Methods:
The proband’s lung biopsy specimen (at age 7 months) and serial CT scans were diagnostic of NEHI. Her mother, an aunt, an uncle, and two first cousins had failure to thrive in infancy and chronic respiratory symptoms that improved with age. Genes associated with autosomal-dominant forms of childhood interstitial lung disease were sequenced.
Results:
A heterozygous NKX2.1 mutation was identified in the proband and the four other adult family members with histories of childhood lung disease. The mutation results in a nonconservative amino acid substitution in the homeodomain in a codon extensively conserved through evolution. None of these individuals have thyroid disease or movement disorders. NKX2.1 mutations were not identified by sequence analysis in eight other unrelated subjects with NEHI.
Conclusions:
The nature of the mutation and its segregation with disease support that it is disease-causing. Previously reported NKX2.1 mutations have been associated with “brain-thyroid-lung” syndrome and a spectrum of more severe pulmonary phenotypes. We conclude that genetic mechanisms may cause NEHI and that NKX2.1 mutations may result in, but are not the predominant cause of, this phenotype. We speculate that altered expression of NKX2.1 target genes other than those in the surfactant system may be responsible for the pulmonary pathophysiology of NEHI.
Neuroendocrine cell hyperplasia of infancy (NEHI) is a recently characterized distinct form of childhood interstitial lung disease (ILD). Affected subjects typically present with profound tachypnea, retractions, crackles on lung auscultation, hypoxemia, and failure to thrive in the first few months to year of life.1,2 Consistent lung histopathology and a highly specific radiographic pattern have also been recognized. Minimal to no pathologic alterations are characteristically observed on lung biopsy specimens, and the diagnosis is based on the presence of increased numbers of bombesin-immunopositive neuroendocrine cells within distal airways and as clusters in the alveolar ducts termed neuroepithelial bodies.2,3 Distinct geographic ground-glass opacities centrally and in the right middle lobe and lingula are observed on CT scans of affected individuals, and such findings have been shown to be specific for diagnosis when compared with lung biopsy findings.4 No particular therapies beyond supportive care are available, but the clinical course for children with NEHI is usually one of gradual improvement over years, although the long-term consequences have not been determined.2,3,5,6
The etiology of NEHI is unknown. It is currently unclear whether the observed neuroendocrine cell prominence represents a primary causative mechanism or reflects a secondary reaction to some other process, as increased neuroendocrine cells occur in a variety of other pulmonary conditions associated with hypoxemia and lung injury, including bronchopulmonary dysplasia, sudden infant death syndrome, pulmonary hypertension, and cystic fibrosis.7‐13 However, it has been shown that airway injury does not account for the extent and distribution of neuroendocrine cells in the lungs of children with NEHI.3 The report by Popler et al,14 which identified four families with affected siblings with NEHI, suggests a genetic basis for NEHI. Genetic mechanisms are well recognized as the cause of a different group of Childhood ILD disorders resulting from mutations in genes important in surfactant function and metabolism. These include the genes encoding surfactant proteins B and C (SP-B, SP-C), member A3 of the ATP-binding cassette family of transporters (ABCA3), and thyroid transcription factor 1 (TTF-1), the latter of which is important for the expression of surfactant-related genes as well as multiple other genes in the lung, thyroid, and brain.15,16 Although the clinical features in children with surfactant dysfunction disorders are variable, in general, the course is much more severe than that reported for NEHI, with significant mortality observed in surfactant dysfunction disorders but none reported in NEHI. Lung histopathology findings in children with surfactant dysfunction disorders are also quite distinct from those of NEHI, and include prominent alveolar type 2 cell hyperplasia, intraalveolar accumulations of proteinaceous material and macrophages, mesenchymal thickening, and interstitial fibrosis.5,17
We used a candidate gene approach to investigate the mechanism for lung disease in a subject whose clinical, imaging, and lung biopsy specimen findings were consistent with NEHI; the subject’s extended family was investigated as well. We identified a heterozygous substitution in the gene encoding TTF-1, NKX2.1, in the proband and four other adult family members with histories of childhood lung disease that improved with age.
Materials and Methods
Case History
The proband was born at 39 weeks’ gestation with birth weight 3,120 g. There were no neonatal respiratory or other health concerns. Family members noted that she had rapid breathing in the first weeks of life, and she had trouble breastfeeding. Medical evaluations were prompted at 4 months of age due to failure to thrive. At that time, tachypnea and hypoxemia were prominent, and supplemental oxygen was initiated. There were no symptoms of cough, fever, wheezing, or acute infection. Results of sweat chloride testing, immunologic evaluations, and an echocardiogram were all normal. A chest CT scan and lung biopsy were performed at 7 months of age (Fig 1). Although initially interpreted as nonspecific findings of uncertain etiology, subsequent re-review of the lung biopsy specimen and bombesin immunostaining prompted by the 2005 description of NEHI2 led to confirmation of this diagnosis.
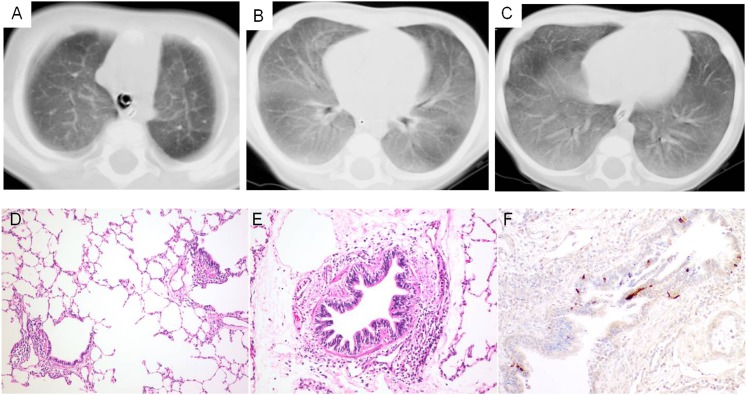
Figure 1.
Proband with neuroendocrine cell hyperplasia of infancy (NEHI). Chest CT scan and lung biopsy specimen from infancy. A-C, Chest CT scan performed at age 7 months shows relatively diffuse ground-glass opacities, with some central prominence in the upper lobes. D-F, Specimen from a lung biopsy performed at age 7 months shows near normal architecture with mild peribronchiolar lymphocytic aggregates (D, hematoxylin and eosin [H&E], original magnification ×10; E, original magnification ×20). F, Bombesin immunostaining (original magnification ×20) demonstrates increased neuroendocrine cells within a bronchiole.
Symptoms of tachypnea, retractions, and hypoxemia persisted for this subject. She remained on continuous supplemental oxygen until 4 years of age, and nighttime supplemental oxygen until 17 years of age. Although she was physically active, symptoms of exercise intolerance and desaturation with 6-min walk testing continued into adolescence. Serial pulmonary function testing (Table 1) since the age of 8 years shows a mixed ventilatory defect, with proportionate reduction in FEV1 and FVC, elevated functional residual capacity, and markedly elevated residual volume that have partially improved over time. Serial chest CT scans (Fig 2) have shown remarkably consistent persistence of geographic ground-glass opacities centrally and most prominent in the right middle lobe and lingula. Thyroid function testing has been normal, and there is no history of movement disorders, developmental delay, or recurrent or atypical infections.
Table 1.
—Pulmonary Function Test Results Over Time in the Proband
| Age, y | FEV1 (%)a | FVC (%)a | FEV1/FVC | TLC (%)a | FRCpleth (%)a | RV (%)a | Dlco, mL/min/mmHg (%) |
| 7.5 | 0.54 (48) | 0.66 (43) | 0.82 | … | … | … | … |
| 8.4 | 0.60 (46) | 0.68 (41) | 0.88 | … | … | … | 16.1 (79) |
| 8.9 | 0.75 (51) | 0.84 (47) | 0.90 | … | … | … | … |
| 9.9 | 0.65 (38) | 0.74 (37) | 0.89 | 2.59 (105) | … | 1.85 (366) | 10.8 (62) |
| 10.3 | 0.71 (41) | 0.87 (44) | 0.81 | 2.56 (102) | … | 1.60 (310) | … |
| 10.6 | 0.88 (49) | 0.91 (44) | 0.97 | 2.60 (102) | … | 1.69 (320) | … |
| 10.9 | 0.92 (49) | 1.11 (52) | 0.83 | 2.72 (102) | … | 1.59 (289) | … |
| 11.9 | 0.89 (48) | 0.94 (45) | 0.89 | 2.93 (97) | 2.11 (142) | 1.90 (243) | … |
| 12.9 | 0.98 (48) | 1.04 (45) | 0.94 | 3.12 (94) | 2.14 (131) | 2.05 (242) | 19.8 (103) |
| 13.9 | 1.16 (50) | 1.24 (48) | 0.93 | 3.17 (89) | 2.12 (121) | 1.87 (207) | 17.7 (88) |
| 14.9 | 1.39 (56) | 1.47 (53) | 0.95 | 3.30 (91) | 2.38 (134) | 1.83 (200) | 18.9 (92) |
| 15.9 | 1.34 (48) | 1.45 (47) | 0.93 | 3.79 (94) | 2.50 (126) | 2.34 (233) | 18.2 (82) |
| 16.9 | 1.55 (55) | 1.63 (52) | 0.96 | 3.97 (99) | 2.38 (120) | 2.26 (225) | 23.2 (105) |
| 17.5 | 1.52 (53) | 1.63 (51) | 0.93 | 4.55 (112) | 2.78 (138) | 2.52 (248) | … |
| 17.8 | 1.66 (58) | 1.81 (56) | 0.92 | 3.98 (97) | 2.41 (120) | 2.01 (197) | 23.2 (103) |
| 17.9 | 1.62 (56) | 1.77 (55) | 0.91 | 4.01 (98) | 2.47 (123) | 2.20 (216) | … |
| 18.7 | 1.45 (50) | 1.57 (49) | 0.92 | 3.93 (96) | 2.53 (126) | 2.28 (224) | 25.3 (113) |
| 18.9 | 1.53 (53) | 1.60 (50) | 0.95 | 3.82 (94) | 2.31 (115) | 2.13 (209) | … |
| 19.3 | 1.69 (54) | 1.87 (53) | 0.90 | 3.76 (80) | 2.42 (96) | 1.89 (179) | 21.5 (84) |
| 19.9 | 1.50 (48) | 1.57 (45) | 0.96 | 4.05 (85) | 2.56 (101) | 2.40 (227) | 23.2 (91) |
| 20.3 | 1.53 (49) | 1.66 (47) | 0.93 | 4.22 (89) | 2.54 (100) | 2.40 (223) | 18.7 (74) |
| 20.8 | 1.56 (50) | 1.64 (47) | 0.95 | 3.83 (81) | 2.20 (87) | 2.09 (194) | 21.3 (84) |
A significant bronchodilator response was demonstrated on two occasions (ages 15.9 y and 17.5 y) but was not present on testing at age 17.9 y (not shown). Dlco = diffusing capacity of the lung for carbon monoxide; FRCpleth = functional residual capacity by plethysmography; RV = residual volume; TLC = total lung capacity.
FEV1, FVC, TLC, FRCpleth, and RV in liters and the percentage of predicted.
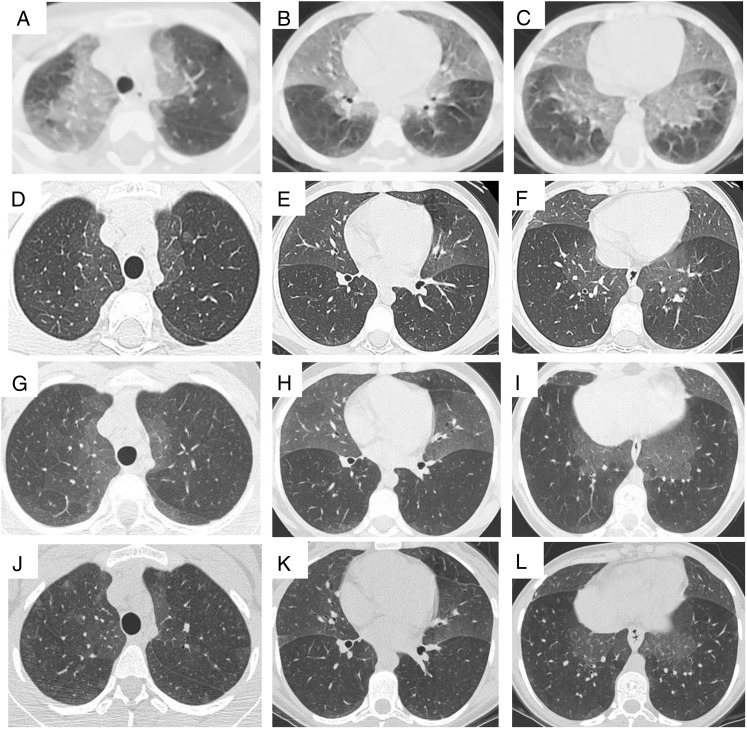
Figure 2.
A-L, Serial chest CT scans from the proband with NEHI. Serial chest CT scans (A-C, age 3 y; D-F, 8 y; G-I, 12 y; and J-L, 17 y) show persistence of characteristic geographic ground-glass opacities centrally and most prominently in the right middle lobe and lingula, highly consistent with NEHI. No architectural distortion or other abnormalities are present. See Figure 1 legend for expansion of abbreviation.
The proband’s family history (Fig 3) was notable for extensive family history of unexplained childhood lung disease which improved over time. The proband’s mother had history of recurrent and prolonged hospitalizations in the first 2 years of life for pneumonia and chronic respiratory symptoms, with severe failure to thrive, weighing > 4.0 kg at birth but only 6.8 kg at 1 year of age. One of her siblings had a history of tachypnea and retractions in infancy and early childhood, and another had unexplained chronic lung disease throughout childhood. Two other siblings of the mother who were born in the 1940s died in early infancy from what was believed to be a respiratory cause, but specific details on their condition are not available, nor were samples for genetic studies. Two first cousins of the proband have history of significant unexplained lung disease and failure to thrive: One required supplemental oxygen until 12 years of age and had a gastrostomy tube, and the other had a lung biopsy at 11 years of age. This biopsy specimen showed significant acute and chronic bronchiolitis, with focally increased neuroendocrine cell numbers (Fig 4). The proband’s father and sibling have no history of chronic lung disease.
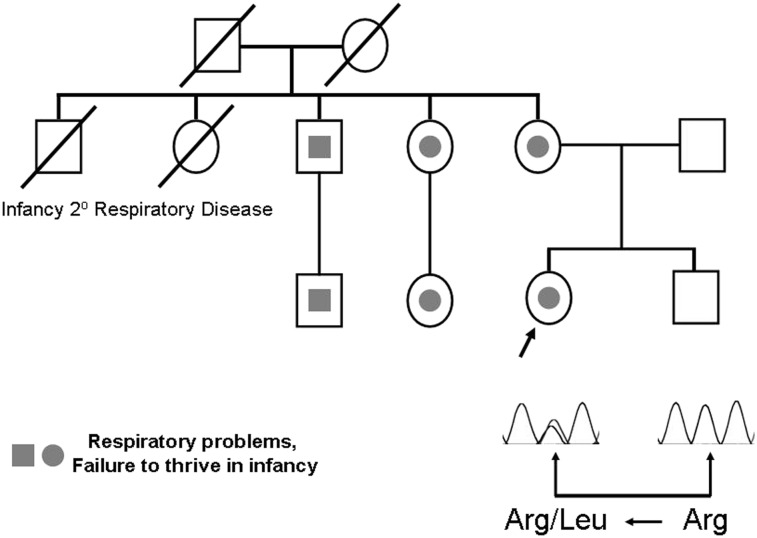
Figure 3.
Pedigree demonstrating NEHI and lung disease in association with an NKX2.1 mutation. The proband (arrow) was diagnosed with NEHI based upon lung biopsy performed in infancy. Multiple other family members, including the proband’s mother, had nonspecific pulmonary symptoms and failure to thrive as infants but have either resolved their pulmonary disease or improved significantly as they have aged. The proband and other family members (gray) are heterozygous for a missense mutation in codon 191 that is predicted to result in the substitution of leucine for arginine. All family members with a history of lung disease carry the mutation; three others (not shown) who are heterozygous were not known to have lung disease as infants but their pulmonary status has not been formally evaluated. See Figure 1 legend for expansion of abbreviation.
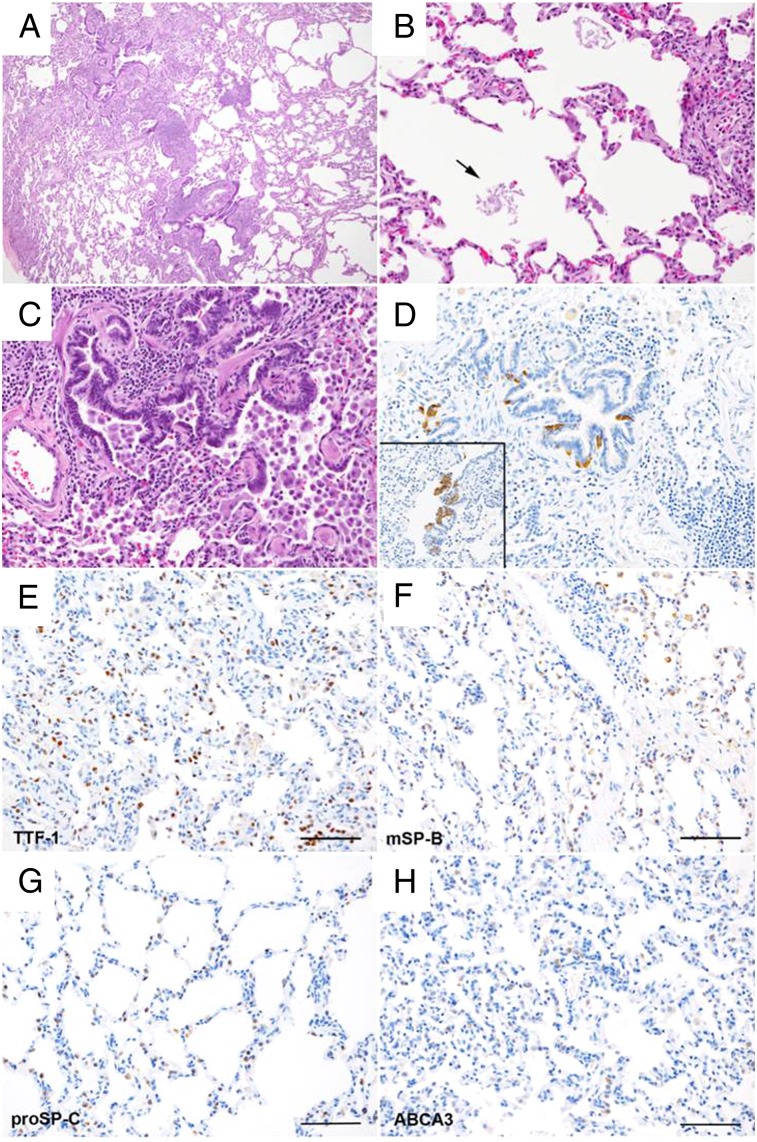
Figure 4.
A-H, Lung biopsy specimen from another affected family member. This lung biopsy was performed at age 11 years in the proband’s cousin, who had history of chronic respiratory symptoms and failure to thrive since infancy. A, C, The biopsy specimen shows patchy chronic bronchiolitis, with prominent lymphocytic inflammation, airway injury and remodeling, and dense collections of alveolar macrophages (A, H&E, original magnification ×4; C, H&E, original magnification ×20). B, Scattered patchy lymphoid aggregates are seen, without reactive germinal center formation. Scant proteinaceous material was seen within few alveoli (arrow) (H&E, original magnification ×20). D, Bombesin immunostaining (original magnification ×20) demonstrates focally increased neuroendocrine cells within a subset of airways and prominent neuroendocrine bodies (inset). E-H, Immunostaining patterns for TTF-1, SP-B, pro-SP-C, and ABCA3 were all normal (scale bar, 100 μm). ABCA3 = member A3 of the ATP-binding cassette family; mSP-B = mature SP-B protein; proSP-C = SP-C proprotein; TTF-1 = thyroid transcription factor-1. See Figure 1 legend for expansion of other abbreviation.
Genetic Studies
All subjects were enrolled in a prospective study aimed at identifying genetic mechanisms for lung disease that was approved by the institutional review board at Johns Hopkins University (IRB number NA_00045539); written informed consent was obtained from all subjects or one of their parents. DNA was extracted from peripheral blood or saliva using commercially available kits (Gentra Puregene Blood Kit, QIAGEN; Oragene, DNA Genotek Inc) according to the manufacturers’ directions. SFTPC, ABCA3, and NKX2.1 were sequenced as previously reported.18‐20 We also sequenced NKX2.1 in eight other unrelated subjects with sporadic or familial NEHI.
Immunohistochemistry
Neuroendocrine cells were delineated by immunohistochemistry for bombesin (ImmunoStar), performed on formalin-fixed 5-mm paraffin sections as previously described.2 To evaluate SP expression, additional immunostaining was performed on available lung tissue from the proband’s cousin, including for SP-B, pro-SP-C (both 1:2,000; EMD Millipore Corporation), TTF-1 (1:50, Lab Vision; Thermo Fisher Scientific Inc), and ABCA3 (1:800, Seven Hills Bioreagents; Cincinnati Children’s Hospital Medical Center). Additional lung tissue was not available from the proband for any additional studies beyond the bombesin immunostaining for confirmation of the diagnosis of NEHI.
Results
Genetic Investigations
Based on the apparent autosomal-dominant pattern of disease in this family and the known variability of the lung disease associated with SFTPC mutations, this gene was first sequenced in the proband and her mother, with no mutations identified. With the recent recognition of the potential role of NKX2.1 mutations in causing diffuse lung disease in children, we subsequently sequenced NKX2.1 in the proband and found that she is heterozygous for a G to T transversion in codon 191 of NKX2.1 that is predicted to result in the substitution of leucine for arginine (c.572 G>T, p.Arg191Leu). This mutation is located in the homeodomain, which has been extremely conserved during evolution, and is predicted to disrupt TTF-1 structure and/or function. The proband’s mother is also heterozygous for the mutation, but it was not found in the proband’s father or sibling, both of whom had no history of lung disease. NKX2.1 was sequenced from all family members with a history of lung disease and all were found to carry the mutation (Fig 3). None of these individuals have thyroid disease or movement disorders. Three other family members were also found to be heterozygous for the mutation (not shown); these individuals were not known to have lung disease as infants but their pulmonary status has not been formally evaluated. Because heterozygous ABCA3 mutations have been reported to be associated with an increased risk of neonatal respiratory disease and to potentially modify the course of patients with SFTPC mutations, we also sequenced ABCA3 from the proband, but did not identify any ABCA3 coding variants.21,22
We investigated whether NKX2.1 mutations might be responsible for NEHI in other cases. NKX2.1 mutations were not identified by sequence analysis in eight other unrelated subjects with sporadic and familial NEHI.
Immunohistochemistry
We used available lung tissue from the proband’s cousin to investigate possible alteration in SP expression. Although some scant proteinosis was observed, the alveolar architecture was normal, without interstitial expansion or alveolar type 2 cell hyperplasia; immunostaining confirmed normal expression patterns of SP-B, pro-SP-C, ABCA3, and TTF-1 (Fig 4E‐H).
Discussion
NEHI is a distinct entity with well-described clinical, physiologic, radiographic, and histologic features. The incidence and prevalence of NEHI are unknown, although the disorder is felt to be rare. Although mortality due to NEHI has not been reported, it results in significant morbidity in young children.2,3,6 Most children require supplemental oxygen for years, and many need additional nutritional support, including via gastrostomy tube in some cases. Hospitalization and overall health-care utilization are high, as many children undergo extensive diagnostic testing for other more common causes of respiratory disease in this age group. There is no known therapy for NEHI, bronchodilators and corticosteroids are not helpful in most cases, and management largely consists of supportive care.2 To date, the etiology and pathogenesis have been unknown.
We identified a heterozygous NKX2.1 mutation in a subject with a definitive diagnosis of NEHI. This subject was a term infant who had a classic presentation for NEHI in the first months of life, with indolent persistent tachypnea, retractions, crackles, hypoxemia, and failure to thrive that were otherwise unexplained. The patterns found on the chest CT scan and the lung biopsy specimen demonstrate the prototypical findings which have been well described in this disorder.4 The mutation strongly segregated with lung disease in this family, as all family members with a history of lung disease were found to carry the mutation, whereas the mutation was not found in the proband’s father and sibling, both of whom had no history of lung disease. Although three members of the extended family were heterozygous for the mutation and did not have a history of childhood lung disease, variable or incomplete penetrance is well recognized in many autosomal-dominant disorders, including those involving the lung. For example, in families with BMPR2 mutations, only 20% of individuals with such mutations develop the severe phenotype of pulmonary hypertension.23 Similarly, hereditary hemorrhagic telangiectasia due to ENG or ACVRL1 (ALK1) mutations, and pulmonary fibrosis due to SFTPC or telomerase mutations exhibit either highly variable or incomplete penetrance.19,24‐26
NKX2.1 encodes TTF-1, which is expressed in the thyroid gland, brain, and lung, and haploinsufficiency for NKX2.1 results in “brain-thyroid-lung” syndrome (MIM no. 610978) and benign familial chorea (MIM no. 118700).27‐29 Affected individuals have variable degrees of pulmonary disease, thyroid dysfunction, and neurologic abnormalities.20,29‐33 None of the affected individuals in this family had a history of thyroid disease or chorea. TTF-1 is a critical regulator of early lung development and cellular differentiation, and specifically regulates the expression of SP-B, SP-C, and ABCA3 as well as many other genes.15,16,34‐36 A spectrum of pulmonary phenotypes has been described due to NKX2.1 mutations and deletions, with presentations ranging from severe neonatal respiratory distress syndrome, ILD in older children and adults, and recurrent pulmonary infections.20 Lung pathology findings have included deficient lung alveolarization and changes consistent with surfactant dysfunction, and alterations in surfactant protein expression have been observed when suitable samples were available for analysis.20,31,32,37 NEHI, thus, represents a novel clinical and histologic phenotype not previously described in association with NKX2.1 mutations. However, the absence of NKX2.1 mutations in eight other individuals with NEHI suggests that NKX2.1 mutations are probably not the mechanism underlying most NEHI cases. We speculate that, instead, the gene or genes primarily responsible for NEHI are regulated by TTF-1.
The identified NXK2.1 mutation has not been reported previously but is highly likely to be deleterious. It results in a nonconservative amino acid substitution in a region of the protein that has been extensively conserved in evolution, and is important for DNA binding and possibly nuclear localization.38 Three different informatic tools predict that the mutation is likely to be damaging or deleterious (SIFT [http://sift.bii.a-star.edu.sg]; Polyphen 2 [http://genetics.bwh.harvard.edu/pph2/]; PROVEAN [http://provean.jcvi.org/index.php]).39‐41 This sequence variant is also not listed in either the Exome Variant Sequencing Project database or the 1000 Genomes Project, indicating that it is not a common polymorphism.42,43 The mechanism(s) whereby this mutation results in the phenotype of NEHI and whether this phenotypic association is specific for the p.Arg191Leu mutation are unknown. In three individuals from an unrelated family who were heterozygous for a different missense mutation located in close proximity in the homeodomain region, p.Phe198Leu, surfactant protein expression, particularly SP-C, was noticeably decreased.20,44 However, although these individuals each also had isolated pulmonary disease, the clinical and lung histology phenotypes were quite distinct from NEHI. Another homeodomain missense mutation, p.Arg195Trp, identified in a child with brain-thyroid-lung syndrome who died of respiratory failure at the age of 18 months, caused increased SFTPC but decreased SFTPB transcription in vitro.37 Collectively, these observations suggest that different NKX2-1 mutations may have distinct effects on expression of different target genes, thereby resulting in varying phenotypic manifestations. A limitation of our studies is that we have not yet performed studies to characterize the specific effects of the mutation in vitro. Such studies will be important to determine exactly how this mutation results in a NEHI phenotype, but as the pulmonary phenotype is not one of disrupted surfactant function and none of the subjects exhibited findings of hypothyroidism, the predicted effects of the mutation on expression of surfactant- or thyroid-related TTF-1 target genes are unclear. Notably, the lung histology findings in the cousin’s biopsy specimen were clearly not those characteristically observed in surfactant dysfunction disorders, and expression of ABCA3, SP-B, and pro-SP-C were normal as determined by immunohistochemistry.
The lung disease reported in other members of the proband’s family includes some features that are consistent with a diagnosis of NEHI, but the available data do not allow us to conclude whether these individuals truly had typical NEHI phenotypes. Specifically, the cousin’s lung biopsy at age 11 years showed acute and chronic bronchiolitis with significant airway injury and some limited lymphoid hyperplasia. It is unknown whether this individual’s histologic phenotype could have evolved over time, as our current understanding of NEHI histology derives from lung biopsies performed in infants and children in the first few years of life.2,3,5 However, as phenotypic and histologic variability has also been observed in familial interstitial pneumonia and ILD associated with SFTPC mutations,45,46 we speculate that these findings suggest a possible relationship between NEHI and a spectrum of airway injury disorders including chronic bronchiolitis and follicular bronchiolitis.
All affected individuals presented with chronic respiratory symptoms and poor growth in infancy, and all have gradually improved over time, though some have experienced persistence of their respiratory symptoms into adulthood. Interestingly, the proband continued to have strikingly consistent abnormalities on chest CT scan and pulmonary function testing into adolescence. This extent of disease persistence seen in this case has not been previously described. While prior reports of NEHI have emphasized that children demonstrate gradual improvement over several years,2,3,5,6,47 emerging data suggest persistence of exercise tolerance and air-trapping in at least a subset of patients.48 Therefore, we caution that further studies are needed to determine the long-term outcomes of this disorder.
In summary, we conclude that genetic mechanisms may cause NEHI and that NKX2.1 mutations may result in this phenotype, but are not the predominant cause. We speculate that the pulmonary pathophysiology of NEHI may result from altered expression of genes that are regulated by NKX2.1 other than those in the surfactant system. If this is the case, this information may be helpful in interpreting data from studies designed to identify genetic causes of NEHI with family based, agnostic approaches using whole-exome or whole-genome sequencing. Genetic discovery in NEHI will facilitate a better understanding of the epidemiology of this disorder, as well as improved and noninvasive diagnostic tests, and will help elucidate disease pathogenesis thereby facilitating development of targeted therapeutic strategies.
Acknowledgments
Author contributions: Dr Nogee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Young: contributed to study inception and data acquisition and analysis; drafted the manuscript; provided critical input and helped revise the final version of the manuscript; and approved the final version of the manuscript.
Dr Deutsch: contributed to data acquisition and analysis; provided critical input and helped revise the final version of the manuscript; and approved the final version of the manuscript.
Dr Bokulic: contributed to data acquisition and analysis; provided critical input and helped revise the final version of the manuscript; and approved the final version of the manuscript.
Dr Brody: contributed to data acquisition and analysis; provided critical input and helped revise the final version of the manuscript; and approved the final version of the manuscript.
Dr Nogee: contributed to study inception and data acquisition and analysis; drafted the manuscript; provided critical input and helped revise the final version of the manuscript; and approved the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Abbreviations
- ABCA3
member A3 of the ATP-binding cassette family of transporters
- ILD
interstitial lung disease
- NEHI
neuroendocrine cell hyperplasia of infancy
- SP
surfactant protein
- TTF-1
thyroid transcription factor-1
Footnotes
Funding/Support: This work was supported by grants through the National Institutes of Health [HL54703, Dr Nogee]; American Thoracic Society/chILD Foundation/chILD (Lung) Foundation-UK (Dr Young); and the Eudowood Foundation (Dr Nogee).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Deterding RR, Fan LL, Morton R, Hay TC, Langston C. Persistent tachypnea of infancy (PTI)—a new entity. Pediatr Pulmonol. 2001;suppl 23:72-73 [PubMed] [Google Scholar]
- 2.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40(2):157-165 [DOI] [PubMed] [Google Scholar]
- 3.Young LR, Brody AS, Inge TH, et al. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest. 2011;139(5):1060-1071 [DOI] [PubMed] [Google Scholar]
- 4.Brody AS, Guillerman RP, Hay TC, et al. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am J Roentgenol. 2010;194(1):238-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutsch GH, Young LR, Deterding RR, et al. ; Pathology Cooperative Group; ChILD Research Co-operative Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176(11):1120-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerby GS, Wagner BD, Popler J, et al. Abnormal infant pulmonary function in young children with neuroendocrine cell hyperplasia of infancy [published online ahead of print November 20, 2012]. Pediatr Pulmonol. doi: 10.1002/ppul.22718 [DOI] [PubMed] [Google Scholar]
- 7.Johnson DE, Anderson WR, Burke BA. Pulmonary neuroendocrine cells in pediatric lung disease: alterations in airway structure in infants with bronchopulmonary dysplasia. Anat Rec. 1993;236:115-119, 172-173 [DOI] [PubMed] [Google Scholar]
- 8.Johnson DE, Lock JE, Elde RP, Thompson TR. Pulmonary neuroendocrine cells in hyaline membrane disease and bronchopulmonary dysplasia. Pediatr Res. 1982;16(6):446-454 [DOI] [PubMed] [Google Scholar]
- 9.Johnson DE, Wobken JD, Landrum BG. Changes in bombesin, calcitonin, and serotonin immunoreactive pulmonary neuroendocrine cells in cystic fibrosis and after prolonged mechanical ventilation. Am Rev Respir Dis. 1988;137(1):123-131 [DOI] [PubMed] [Google Scholar]
- 10.Cutz E, Perrin DG, Pan J, Haas EA, Krous HF. Pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome: potential markers of airway chemoreceptor dysfunction. Pediatr Dev Pathol. 2007;10(2):106-116 [DOI] [PubMed] [Google Scholar]
- 11.Perrin DG, McDonald TJ, Cutz E. Hyperplasia of bombesin-immunoreactive pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome. Pediatr Pathol. 1991;11(3):431-447 [DOI] [PubMed] [Google Scholar]
- 12.Schindler MB, Bohn DJ, Bryan AC, Cutz E, Rabinovitch M. Increased respiratory system resistance and bronchial smooth muscle hypertrophy in children with acute postoperative pulmonary hypertension. Am J Respir Crit Care Med. 1995;152(4 pt 1):1347-1352 [DOI] [PubMed] [Google Scholar]
- 13.Sunday ME, Kaplan LM, Motoyama E, Chin WW, Spindel ER. Gastrin-releasing peptide (mammalian bombesin) gene expression in health and disease. Lab Invest. 1988;59(1):5-24 [PubMed] [Google Scholar]
- 14.Popler J, Gower WA, Mogayzel PJ, Jr, et al. Familial neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2010;45(8):749-755 [DOI] [PubMed] [Google Scholar]
- 15.DeFelice M, Silberschmidt D, DiLauro R, et al. TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J Biol Chem. 2003;278(37):35574-35583 [DOI] [PubMed] [Google Scholar]
- 16.Kolla V, Gonzales LW, Gonzales J, et al. Thyroid transcription factor in differentiating type II cells: regulation, isoforms, and target genes. Am J Respir Cell Mol Biol. 2007;36(2):213-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatr Dev Pathol. 2009;12(4):253-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullard JE, Wert SE, Whitsett JA, Dean M, Nogee LM. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172(8):1026-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameron HS, Somaschini M, Carrera P, et al. A common mutation in the surfactant protein C gene associated with lung disease. J Pediatr. 2005;146(3):370-375 [DOI] [PubMed] [Google Scholar]
- 20.Hamvas A, Deterding RR, Wert SE, et al. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene NKX2-1. Chest. 2013;144(3):794-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bullard JE, Nogee LM. Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatr Res. 2007;62(2):176-179 [DOI] [PubMed] [Google Scholar]
- 22.Wambach JA, Wegner DJ, Depass K, et al. Single ABCA3 mutations increase risk for neonatal respiratory distress syndrome. Pediatrics. 2012;130(6):e1575-e1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin ED, Loyd JE, Phillips JA., 3rd Genetics of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009;30(4):386-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loyd JE. Pulmonary arterial hypertension: insights from genetic studies. Proc Am Thorac Soc. 2011;8(2):154-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottin V, Dupuis-Girod S, Lesca G, Cordier JF. Pulmonary vascular manifestations of hereditary hemorrhagic telangiectasia (rendu-osler disease). Respiration. 2007;74(4):361-378 [DOI] [PubMed] [Google Scholar]
- 26.Garcia CK. Idiopathic pulmonary fibrosis: update on genetic discoveries. Proc Am Thorac Soc. 2011;8(2):158-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krude H, Schütz B, Biebermann H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109(4):475-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pohlenz J, Dumitrescu A, Zundel D, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109(4):469-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breedveld GJ, van Dongen JW, Danesino C, et al. Mutations in TITF-1 are associated with benign hereditary chorea. Hum Mol Genet. 2002;11(8):971-979 [DOI] [PubMed] [Google Scholar]
- 30.Devriendt K, Vanhole C, Matthijs G, de Zegher F. Deletion of thyroid transcription factor-1 gene in an infant with neonatal thyroid dysfunction and respiratory failure. N Engl J Med. 1998;338(18):1317-1318 [DOI] [PubMed] [Google Scholar]
- 31.Galambos C, Levy H, Cannon CL, et al. Pulmonary pathology in thyroid transcription factor-1 deficiency syndrome. Am J Respir Crit Care Med. 2010;182(4):549-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinlein B, Griese M, Liebisch G, et al. Fatal neonatal respiratory failure in an infant with congenital hypothyroidism due to haploinsufficiency of the NKX2-1 gene: alteration of pulmonary surfactant homeostasis. Arch Dis Child Fetal Neonatal Ed. 2011;96(6):F453-F456 [DOI] [PubMed] [Google Scholar]
- 33.Teissier R, Guillot L, Carré A, et al. Multiplex Ligation-dependent Probe Amplification improves the detection rate of NKX2.1 mutations in patients affected by brain-lung-thyroid syndrome. Horm Res Paediatr. 2012;77(3):146-151 [DOI] [PubMed] [Google Scholar]
- 34.Stahlman MT, Gray ME, Whitsett JA. Expression of thyroid transcription factor-1(TTF-1) in fetal and neonatal human lung. J Histochem Cytochem. 1996;44(7):673-678 [DOI] [PubMed] [Google Scholar]
- 35.Kimura J, Deutsch GH. Key mechanisms of early lung development. Pediatr Dev Pathol. 2007;10(5):335-347 [DOI] [PubMed] [Google Scholar]
- 36.Tagne JB, Gupta S, Gower AC, et al. Genome-wide analyses of Nkx2-1 binding to transcriptional target genes uncover novel regulatory patterns conserved in lung development and tumors. PLoS ONE. 2012;7(1):e29907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guillot L, Carré A, Szinnai G, et al. NKX2-1 mutations leading to surfactant protein promoter dysregulation cause interstitial lung disease in “Brain-Lung-Thyroid Syndrome.” Hum Mutat. 2010;31(2):E1146-E1162 [DOI] [PubMed] [Google Scholar]
- 38.Christophe-Hobertus C, Duquesne V, Pichon B, Roger PP, Christophe D. Critical residues of the homeodomain involved in contacting DNA bases also specify the nuclear accumulation of thyroid transcription factor-1. Eur J Biochem. 1999;265(1):491-497 [DOI] [PubMed] [Google Scholar]
- 39.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11(5):863-874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE. 2012;7(10):e46688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.NHLBI Exome Sequencing Project (ESP) Exome variant server. University of Washington website. http://snp.gs.washington.edu/EVS. Accessed March 20, 2013
- 43.1000 Genomes Project Consortium; Abecasis GR, Altshuler D, Auton A, et al. A map of human genome variation from population-scale sequencing [published correction appears in Nature. 2011;473(7348):544]. Nature. 2010;467(7319):1061-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amin RS, Wert SE, Baughman RP, et al. Surfactant protein deficiency in familial interstitial lung disease. J Pediatr. 2001;139(1):85-92 [DOI] [PubMed] [Google Scholar]
- 45.Thomas AQ, Lane K, Phillips J, III, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165(9):1322-1328 [DOI] [PubMed] [Google Scholar]
- 46.Steele MP, Speer MC, Loyd JE, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukkarinen H, Pelkonen A, Lohi J, et al. Neuroendocrine cell hyperplasia of infancy: a prospective follow-up of nine children. Arch Dis Child. 2013;98(2):141-144 [DOI] [PubMed] [Google Scholar]
- 48.Popler J, Young LR, Deterding RR. Beyond infancy: persistence of chronic lung disease in neuroendocrine cell hyperplasia of infancy (NEHI). Am J Respir Crit Care Med. 2010;181(Meeting Abstracts):A6721 [Google Scholar]