Abstract
Background:
Alterations in respiratory mechanics predispose healthy obese individuals to low lung volume breathing, which places them at risk of developing expiratory flow limitation (EFL). The high ventilatory demand in endurance-trained obese adults further increases their risk of developing EFL and increases their work of breathing. The objective of this study was to investigate the prevalence and magnitude of EFL in fit obese (FO) adults via measurements of breathing mechanics and ventilatory dynamics during exercise.
Methods:
Ten (seven women and three men) FO (mean ± SD, 38 ± 5 years, 38% ± 5% body fat) and 10 (seven women and three men) control obese (CO) (38 ± 5 years, 39% ± 5% body fat) subjects underwent hydrostatic weighing, pulmonary function testing, cycle exercise testing, and the determination of the oxygen cost of breathing during eucapnic voluntary hyperpnea.
Results:
There were no differences in functional residual capacity (43% ± 6% vs 40% ± 9% total lung capacity [TLC]), residual volume (21% ± 4% vs 21% ± 4% TLC), or FVC (111% ± 13% vs 104% ± 15% predicted) between FO and CO subjects, respectively. FO subjects had higher FEV1 (111% ± 13% vs 99% ± 11% predicted), TLC (106% ± 14% vs 94% ± 7% predicted), peak expiratory flow (123% ± 14% vs 106% ± 13% predicted), and maximal voluntary ventilation (128% ± 15% vs 106% ± 13% predicted) than did CO subjects. Peak oxygen uptake (129% ± 16% vs 86% ± 15% predicted), minute ventilation (128 ± 35 L/min vs 92 ± 25 L/min), and work rate (229 ± 54 W vs 166 ± 55 W) were higher in FO subjects. Mean inspiratory (4.65 ± 1.09 L/s vs 3.06 ± 1.21 L/s) and expiratory (4.15 ± 0.95 L/s vs 2.98 ± 0.76L/s) flows were greater in FO subjects, which yielded a greater breathing frequency (51 ± 8 breaths/min vs 41 ± 10 breaths/min) at peak exercise in FO subjects. Mechanical ventilatory constraints in FO subjects were similar to those in CO subjects despite the greater ventilatory demand in FO subjects.
Conclusion:
FO individuals achieve high ventilations by increasing breathing frequency, matching the elevated metabolic demand associated with high fitness. They do this without developing meaningful ventilatory constraints. Therefore, endurance-trained obese individuals with higher lung function are not limited by breathing mechanics during peak exercise, which may allow healthy obese adults to participate in vigorous exercise training.
In contrast to the widespread belief that all obese adults are inactive, there are many obese individuals who exercise rigorously, compete in endurance races, and potentially have high levels of cardiorespiratory fitness (CRF). However, it is unclear whether this group of endurance-trained obese individuals encounters obesity-related respiratory limitations.1‐4
End-expiratory lung volume (EELV) increases in obese adults at or near maximal exercise,5‐7 which may be in response to the presence of expiratory flow limitation (EFL).8 The high ventilatory demand in trained athletes may exacerbate their work of breathing, as well as their risk of developing EFL.9‐11 However, little is known about CRF in “fit and obese” individuals; in addition, the mechanical mechanism by which they are able to generate the high ventilatory demand associated with increased physical fitness is unclear. Understanding these physiologic changes in endurance-trained obese individuals may provide valuable new insights for prescribing exercise training in obese adults.
We sought to investigate CRF, lung function, respiratory, and ventilatory dynamics during submaximal and maximal exercise, and the oxygen cost of breathing in endurance-trained obese individuals. We hypothesized that endurance-trained obese subjects would experience considerable mechanical ventilatory constraints, which may cause them to hyperinflate especially during intense exercise. Although their oxygen cost of breathing would be similar to healthy sedentary obese adults, their work of breathing during peak exercise would be increased in proportion to their increase in peak ventilation.
Materials and Methods
Subjects
In accordance with the institutional review board (University of Texas Southwestern Medical Center, approval number 122010-108), all details of the experiments were discussed with the volunteers, and informed consent was obtained before participation. All subjects were obese based on their percentage of body fat (body fat ≥ 30%) and had the same exclusion criteria: history of asthma, cardiovascular disease, or musculoskeletal abnormalities. We used percent body fat to determine obesity rather than BMI because BMI is a general measure of the relationship of weight to height and can underestimate the true level of obesity. For example, some of the subjects had a BMI that was < 30 kg/m2, but their percent body fat was > 30%, which qualified them for inclusion in the study.
Fit Obese:
A total of 19 fit obese (FO) participants were recruited for the study. However, nine were disqualified for different reasons: asthma (two), high BP (one), and body fat < 30% (six). Therefore, seven women and three men completed the study. Candidates for this study were screened carefully based on their exercise training history within the preceding 12 months. The subjects exercised aerobically at least four times per week, and their training sessions lasted 1 to 4 h. Moreover, they had recently (ie, within the preceding 12 months) competed in endurance events such as marathons, Ironmans, and road races, or were training to compete in future races.
Control Obese:
Seven women and three men were randomly selected from our large database of prior and ongoing studies to serve as a control group (control obese [CO]) for comparison purposes. These subjects had the same exclusion criteria as the FO subjects. However, they had not engaged in any regular exercise activities for 6 months prior to enrollment in the study.
Body Composition and Pulmonary Function
Hydrostatic weighing, with the measurement of residual volume, was performed to determine percent body fat, lean body mass, and total body fat mass. Participants underwent standard spirometry, lung volume, airway resistance, maximal inspiratory pressure, and maximal expiratory pressure determinations (model V62W body plethysmograph, SensorMedics).12 Predicted values were based on published norms.13‐16
Cardiorespiratory Responses and Rating of Perceived Breathlessness During Submaximal Exercise
A submaximal exercise test was performed to further evaluate and compare fitness levels between the groups. Testing began with the subjects seated on the cycle ergometer for 3 min; then the subjects performed a 6-min constant-load exercise cycling test at 60 W (women), or 105 W (men).17 The three CO men exercised at 90 W, rather than 105 W, as dictated by the requirements of a prior study. Physiologic data averaged from the last 2 min of the exercise stage were used in the analyses. Rating of perceived breathlessness and rating of perceived exertion were measured every 2 min of the test, and the last value recorded was used for analyses.
Peak Cardiorespiratory Exercise Capacity and Breathing Mechanics
Peak aerobic power, peak oxygen uptake (o2peak) (open circuit spirometry), was determined by graded cycle ergometer exercise (model CPE 2000, Medical Graphics Corporation) to exhaustion as described previously.17 Expiratory and inspiratory flows were measured continuously at rest and during exercise as described previously.18 EELV was estimated from measurement of inspiratory capacity (IC) during each of the protocol stages, and total lung capacity (TLC) during body plethysmography (EELV = TLC − IC).5,6 All subjects performed an FVC maneuver before and 2 min after exercise, with the largest loop accepted. EFL was computed as the percentage of the expiratory tidal flow-volume loop that met or exceeded the expiratory boundary of the maximal flow-volume loop.
The Oxygen Cost of Breathing
The oxygen cost of breathing was determined from 6-min measurements of oxygen uptake (o2) and minute ventilation (e) at rest and 4-min measurements of o2 and e during eucapnic voluntary hyperpnea at 40 L/min and 60 L/min (women), or 60 L/min and 90 L/min (men), as described previously.17 To maintain eucapnia during the voluntary hyperpnea maneuver, the subjects breathed from a 1,000-L inspiratory reservoir bag containing 4% or 5% CO2 (21% oxygen and balance nitrogen).19 The oxygen cost of breathing was assessed by calculating the slope of the o2 (mL/min) vs e (L/min) relationship at rest and during eucapnic voluntary hyperpnea. Physiologic data were averaged from the 6-min measurements at rest and the 4-min measurements during the hyperventilation maneuvers.
Data Analyses
Differences between groups were determined by an independent Student t test. Values are reported as mean ± SD. A P value of < .05 was considered significant.
Results
Subjects and Body Composition
All subjects were obese (body fat ≥ 30%). There were no differences (P > .05) between groups in body composition, age, height, or body size parameters (Table 1).
Table 1.
—Subject Characteristics and Pulmonary Function
| Characteristic | Control Obese (n = 10 [7 Female]) | Fit Obese (n = 10 [7 Female]) |
| Age, y | 37.6 ± 5.0 (28-45) | 37.6 ± 5.3 (30-47) |
| Height, cm | 170 ± 9 (156-188) | 169 ± 10 (152-183) |
| Weight, kg | 94.9 ± 11.6 (84.6-116.8.0) | 93.9 ± 16.2 (73.5-112.0) |
| BMI, kg/m2 | 33.1 ± 3.2 (28.4-36.8) | 32.6 ± 3.6 (26.9-38.0) |
| Body fat, % | 38.9 ± 4.7 (32.5-45.7) | 37.7 ± 4.9 (29.7-43.9) |
| Fat mass, kg | 36.6 ± 3.5 (30.4-41.5) | 35.3 ± 7.9 (24.4-48.8) |
| Lean body mass, kg | 58.3 ± 11.0 (49.3-78.6) | 58.6 ± 10.8 (43.4-74.7) |
| TLC | ||
| L | 5.48 ± 1.08 (4.09-7.89) | 6.24 ± 1.72 (3.97-9.44) |
| % predicted | 94 ± 7 (84-101) | 106 ± 14a (88-129) |
| FRC | ||
| L | 2.20 ± 0.62 (1.25-3.03) | 2.65 ± 0.71 (1.89-3.91) |
| % TLC | 40 ± 9 (27-51) | 43 ± 6 (35-57) |
| RV | ||
| L | 1.19 ± 0.37 (0.79-1.94) | 1.32 ± 0.38 (0.74-2.05) |
| % TLC | 21 ± 4 (15-25) | 21 ± 4 (16-27) |
| FVC | ||
| L | 4.15 ± 0.75 (3.20-5.75) | 4.80 ± 1.39 (3.23-7.23) |
| % predicted | 104 ± 15 (82-127) | 111 ± 13 (94-131) |
| FEV1 | ||
| L | 3.24 ± 0.50 (2.56-4.16) | 3.94 ± 1.09 (2.84-5.94) |
| % predicted | 99 ± 11 (81-112) | 111 ± 13a (96-138) |
| PEF | ||
| L/s | 8.39 ± 1.36 (6.76-10.81) | 9.84 ± 1.80 (8.41-14.03) |
| % predicted | 106 ± 13 (87-123) | 123 ± 14a (106-147) |
| MVV | ||
| L/min | 133 ± 23 (100-173) | 161 ± 36 (123-230) |
| % predicted | 106 ± 13 (89-126) | 128 ± 15a (104-151) |
Data are presented as means ± SD (range). Predicted values for spirometry and lung volumes were based on the norms of Knudson et al,13,14 and Goldman and Becklake,16 respectively. FRC = functional residual capacity; MVV = measured maximal voluntary ventilation; PEF = peak expiratory flow; RV = residual volume; TLC = total lung capacity.
P < .05.
Pulmonary Function
Pulmonary function values are shown in Table 1 and Figure 1. The FO group had larger TLC than did the CO group (106% ± 14% vs 94% ± 7% predicted, P < .05) (Fig 1A). However, there were no differences between the FO and CO groups in functional residual capacity (43% ± 6% vs 40% ± 9% TLC) or residual volume (21% ± 4% vs 21% ± 4% TLC).
Figure 1.
A, Lung volumes. B, Spirometry. Data are presented as mean and SD. Predicted values for spirometry and lung volumes were based on the norms of Knudson et al,13,14 and Goldman and Becklake,16 respectively. Dotted lines represent the normal range.20 FRC = functional residual capacity (reported as %TLC); MVV = maximal voluntary ventilation; PEF = peak expiratory flow; %pred = percent predicted; RV = residual volume (reported as % total lung capacity); TLC = total lung capacity. *P < .05.
FVC (111% ± 13% vs 104% ± 15% predicted) was not different between the FO and CO groups, respectively (Fig 1B). However, the FO group had higher FEV1 (111% ± 13% vs 99% ± 11% predicted, P < .05), peak expiratory flow (PEF) (123% ± 14% vs 106% ± 13% predicted, P < .01), and maximal voluntary ventilation (MVV) (128% ± 15% vs 106% ± 13% predicted, P < .01) than did the CO group. There were no differences between the FO and CO groups in maximal inspiratory pressure (134% ± 26% vs 122% ± 20% predicted), maximal expiratory pressure (109% ± 21% vs 104% ± 28% predicted), or airway resistance (129% ± 30% vs 129% ± 45% predicted).
Cardiorespiratory Responses and Rating of Perceived Breathlessness During Submaximal Exercise
The FO group exercised at lower relative exercise intensities than did the CO group (Table 2). The FO group had significantly (P < .05) lower relative o2 (50% ± 7% vs 64% ± 8% o2peak), respiratory exchange ratio (0.91 ± 0.08 vs 1.02 ± 0.09), relative heart rate (66% ± 6% vs 77% ± 9% maximal heart rate), blood lactate (2.0 ± 1.2 mmol/L vs 4.5 ± 2.2 mmol/L), rating of perceived breathlessness (2.0 ± 1.6 vs 4.0 ± 1.6), and rating of perceived exertion (9.8 ± 2.1 vs 12.7 ± 2.3), respectively. In addition, the ventilatory response to exercise (e/o2 slope) was significantly lower in the FO group compared with the CO group (26 ± 3 vs 31 ± 6). These observations strongly suggest that the individuals in the FO group had higher fitness levels than those in the CO group.
Table 2.
—Cardiorespiratory Responses to Submaximal (6-Min) Exercise
| Parameters | Control Obese (n = 10 [7 Female]) | Fit Obese (n = 10 [7 Female]) |
| Work rate,a W | 69 ± 14 (60-90) | 74 ± 22 (60-105) |
| o2, L/min | 1.31 ± 0.24 (1.08-1.81) | 1.54 ± 0.38 (1.14-2.17) |
| o2, % peak | 64 ± 8 (47-76) | 50 ± 7a (40-61) |
| co2, L/min | 1.30 ± 0.21 (1.09-1.65) | 1.41 ± 0.41 (1.05-2.07) |
| RER | 1.02 ± 0.09 (0.91-1.19) | 0.91 ± 0.08a (0.77-1.04) |
| e, L/min | 42 ± 7 (32-53) | 39 ± 10 (28-54) |
| e/co2, slope | 31 ± 6 (24-42) | 26 ± 3a (23-33) |
| Petco2, torr | 41 ± 6 (32-51) | 44 ± 3 (39-49) |
| Spo2, % | 99 ± 1 (98-100) | 99 ± 1 (96-100) |
| Heart rate, bpm | 139 ± 15 (108-155) | 118 ± 13a (99-143) |
| Heart rate, % max | 77 ± 9 (61-91) | 66 ± 6a (58-75) |
| Lactate, mmol/L | 4.5 ± 2.2 (2.3-8.8) | 2.0 ± 1.2a (0.9-4.9) |
| RPB, 0-10 scale | 4.0 ± 1.6 (2-6) | 2.0 ± 1.6a (0-5) |
| RPE, 6-20 scale | 12.7 ± 2.3 (9-15) | 9.8 ± 2.1a (7-13) |
Data are presented as mean ± SD (range). The three fit obese men exercised at 105 W, whereas the three control obese men exercised at 90 W, rather than 105 W. All fit obese and control obese women exercised at 60 W. bpm = beats per min; Petco2 = end-tidal CO2; RER = respiratory exchange ratio; RPB = rating of perceived breathlessness; RPE = rating of perceived exertion; Spo2 = oxygen saturation; co2 = CO2 uptake; e = minute ventilation; o2 = oxygen uptake.
P < .05.
Peak Cardiorespiratory Exercise Capacity
The FO group had increased CRF and exercise capacity (about 40%, as indicated by exercise time to exhaustion, peak work rate, and o2peak) compared with the CO group (Table 3). The FO subjects further increased their e by approximately 40% compared with the CO subjects (128 ± 35 L/min vs 92 ± 25 L/min, P < .05). There were no differences in peak heart rate, oxygen saturation, end-tidal Pco2, respiratory exchange ratio, or peak lactate concentration between groups. FO individuals had shorter inspiratory time (0.562 ± 0.090 s vs 0.764 ± 0.185 s, P < .01) and expiratory time (0.631 ± 0.114 s vs 0.759 ± 0.172 s; P = .07) than did CO subjects. The FO group had significantly higher (P < .01) mean inspiratory (tidal volume [Vt]/inspiratory time) (4.65 ± 1.09 L/s vs 3.06 ± 1.21 L/s) and mean expiratory flows (Vt/expiratory time) (4.15 ± 0.95 L/s vs 2.98 ± 0.76 L/s) at peak exercise.
Table 3.
—Peak Exercise Data
| Parameters | Control Obese (n = 10 [7 Female]) | Fit Obese (n = 10 [7 Female]) |
| Exercise time, min | 7.0 ± 1.1 (5.0-8.4) | 10.0 ± 1.5a (8.0-12.0) |
| Work rate, W | 166 ± 55 (100-270) | 229 ± 54a (160-330) |
| Work rate, % pred | 100 ± 17 (67-129) | 140 ± 16a (125-171) |
| o2 , L/min | 2.10 ± 0.69 (1.39-3.29) | 3.10 ± 0.87a (2.13-4.80) |
| o2 , % predictedb | 86 ± 15 (64-109) | 129 ± 16a (110-152) |
| o2, mL/kg PWT/min | 34 ± 8 (23-47) | 50 ± 8a (40-68) |
| o2, mL/kg LBM/min | 35 ± 6 (28-42) | 52 ± 6a (43-64) |
| co2, L/min | 2.55 ± 0.85 (1.66-4.04) | 3.73 ± 1.03a (2.62-5.67) |
| RER | 1.22 ± 0.07 (1.10-1.31) | 1.21 ± 0.04 (1.13-1.26) |
| e, L/min | 92 ± 25 (70-146) | 128 ± 35a (90-200) |
| e, % MVV | 69 ± 12 (54-85) | 79 ± 11a (60-93) |
| e/co2 | 37 ± 5 (30-47) | 35 ± 5 (30-43) |
| Petco2, torr | 33 ± 5 (26-42) | 33 ± 4 (25-38) |
| O2 saturation, % | 99 ± 1 (97-100) | 98 ± 1 (96-99) |
| Heart rate, bpm | 181 ± 12 (164-200) | 177 ± 7 (164-189) |
| Heart rate, % predicted | 100 ± 6 (90-106) | 96 ± 5 (87-105) |
| Lactate, mmol/L | 7.9 ± 1.6 (6.1-11.0) | 9.4 ± 2.0 (6.7-12.1) |
| RPB, 0-10 scale | 7.5 ± 2.3 (4-10) | 8.0 ± 1.6 (6-10) |
| RPE, 6-20 scale | 17.6 ± 2.3 (13-20) | 18.4 ± 1.3 (17-20) |
| Ti, s | 0.764 ± 0.185 (0.472-1.113) | 0.562 ± 0.090a (0.453-0.744) |
| Te, s | 0.759 ± 0.172 (0.502-1.053) | 0.631 ± 0.114a (0.502-0.862) |
| Vt/Ti, L/s | 3.06 ± 1.21 (1.81-5.46) | 4.65 ± 1.09a (3.48-6.74) |
| Vt/Te, L/s | 2.98 ± 0.76 (1.91-4.38) | 4.15 ± 0.95a (2.94-6.10) |
Data are presented as mean ± SD (range). Exercise time = exercise time to peak; LBM = lean body mass; O2 = oxygen; PWT = predicted weight21; Te = expiratory time; Ti = inspiratory time; Vt = tidal volume; Vt/Ti = mean inspiratory flow. See Table 1 and 2 legends for expansion of other abbreviations.
P < .05.
Although interpretation of peak o2 in determining cardiovascular conditioning in obesity is a complex issue,22 the recommendation is to use a method whereby peak o2 is compared with an age, sex, and weight-corrected predicted peak o2.21 Thus, we used the following equation adapted from Wasserman et al,23 Hansen et al,24 and Wasserman and Whipp25: predicted peak o2 = (predicted peak o2 in mL/min/kg × predicted weight) + [(actual weight – predicted weight) × 6 mL/kg]23‐25 to predict peak o2.
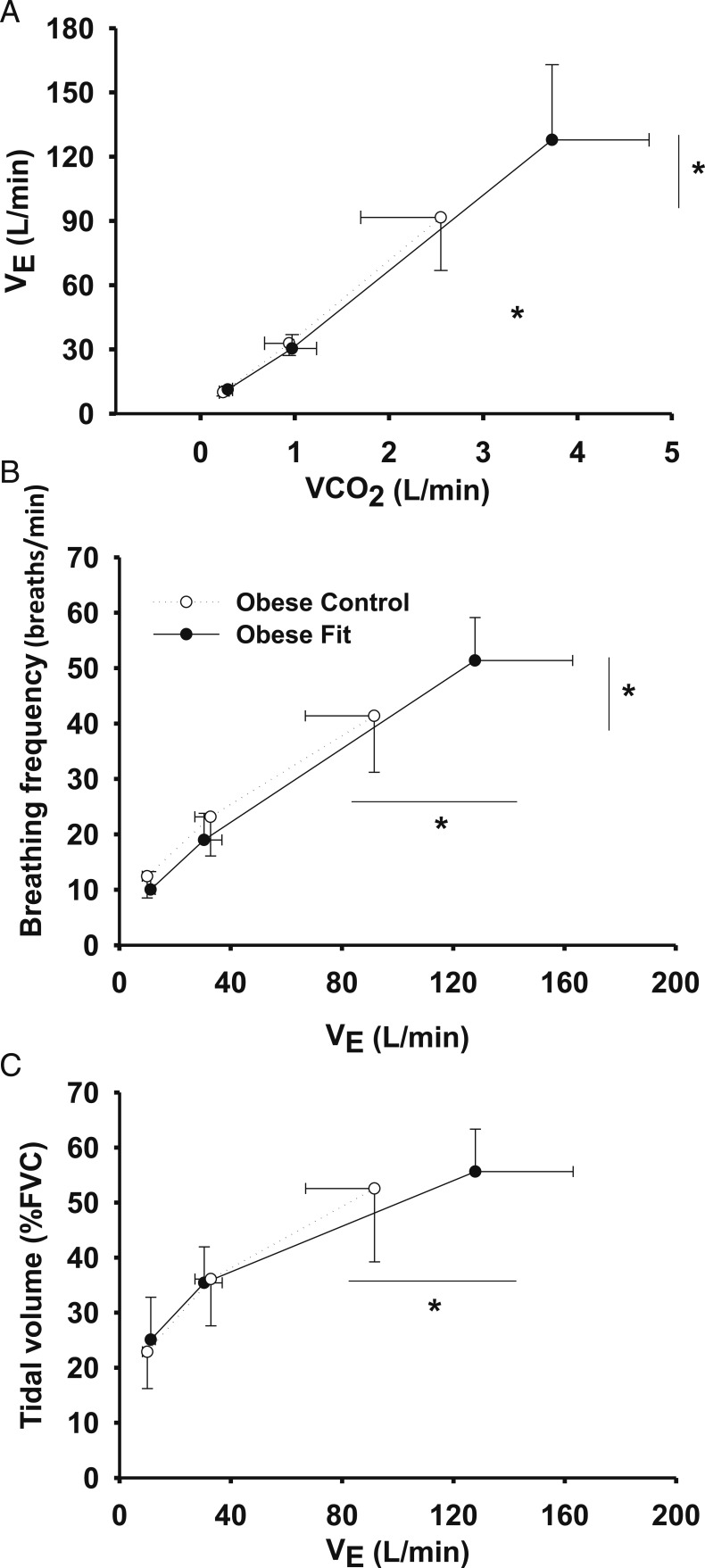
Figure 2 illustrates the ventilatory response (e vs CO2 output) (Fig 2A) and breathing pattern (breathing frequency [Fig 2B], Vt [Fig 2C]) during the peak exercise test. e (128 ± 35 L/min vs 92 ± 25 L/min) and breathing frequency (51 ± 8 breaths/min vs 41 ± 10 breaths/min) at peak exercise were higher in the FO group (P < .05).
Figure 2.
A-C, Ventilatory response and breathing pattern (B, breathing frequency; C, tidal volume) during the peak exercise test (rest, submaximal exercise, and peak exercise). VCO2 = CO2 uptake; Ve = minute ventilation.
Breathing Mechanics
CO individuals with EFL and without EFL at peak exercise are shown in Figures 3A and 3B, respectively. FO subjects with EFL and without EFL at peak exercise are shown in Figures 3C and 3D. EFL was observed in four FO subjects (three women and one man) and in five CO subjects (3 women and two men) at peak exercise. However, the degree of flow limitation was mild (< 20% Vt) and was not different between the FO and CO groups (13% ± 6% Vt vs 16% ± 7% Vt, respectively). Figure 3E illustrates that there were no significant differences between the CO and FO groups regarding EELV and end-inspiratory lung volume at rest, submaximal, or peak exercise.
Figure 3.
Sample flow volume loops from individuals at peak exercise. A, Control obese (CO) subjects with EFL. B, CO subjects without EFL. C, Fit obese (FO) subjects with EFL. D, FO subjects without EFL. EFL was observed in four (three women and one man) FO and five (three women and two men) CO subjects; however, the degree of flow limitation was mild (EFL < 25% Vt) and was not different between the FO and CO groups (13% ± 6% Vt vs 16% ± 7% Vt, respectively). E, Dynamic lung volumes (end-inspiratory lung volume and end-expiratory lung volume) during the peak exercise test (rest, submaximal exercise, and peak exercise). EELV = end-expiratory lung volume; EFL = expiratory flow limitation; EILV = end-inspiratory lung volume; FB = breathing frequency; F/V = flow volume; O2sat = oxygen saturation; PetCO2 = end tidal CO; VO2 = oxygen uptake; Vt = tidal volume; WR = work rate. See Figure 1 and 2 legends for expansion of other abbreviations.
The Oxygen Cost of Breathing
The oxygen cost of breathing measured during eucapnic voluntary hyperpnea was not significantly different between the FO and CO groups (1.64 ± 0.63 mL O2/L e vs 2.14 ± 0.57 mL O2/L e, respectively; P = .08). In addition, the o2 of the respiratory muscles (estimated from the unit-corrected product of the oxygen cost slope and e) at peak exercise was not different between the FO and CO groups (206 ± 89 mL/min vs 201 ± 83 mL/min, P = .89; 6.9% ± 2.8% vs 9.6% ± 3.1% o2peak, P = .05). This was despite the 40% increase in e in the FO group.
Discussion
The results of this study demonstrate several important findings. Obese individuals are capable of achieving high CRF levels. Only 40% to 50% of the FO or CO subjects experienced EFL (and then only minimal) at peak exercise, and operational lung volumes were not different between groups. Nevertheless, FO individuals were able to increase ventilation by about 40% at peak exercise without a considerable increase in mechanical ventilatory constraints compared with CO subjects. This increase in e resulted from the FO subjects having higher mean inspiratory and expiratory flows, which allowed them to shorten their breathing cycle time and increase e by further increasing breathing frequency. In this sample of FO subjects, their pulmonary function was in the upper limits of normal. Finally, the oxygen cost of breathing in FO subjects was not higher, even at peak exercise, because of the increase in frequency strategy vs increasing Vt.
Cardiorespiratory Fitness
This is the first study, to our knowledge, that compared CRF between sedentary and endurance-trained obese adults, and it showed that high CRF can be achieved in this population despite the presence of obesity. The FO group had a 43% increase in exercise time to exhaustion, a 38% increase in peak work rate, and a 48% increase in o2peak compared with their control counterparts. Although quantifying and interpreting CRF in obesity is a complex issue,22 we strongly believe that predicted o2peak values allow for a normalized evaluation of CRF in the obese population. o2peak and peak power output in the FO group were about 130% of their predicted values. Moreover, o2peak relative to their predicted body weight was about 50 mL/kg/min, which is above the 90th percentile according to the American College of Sports Medicine.26 Moreover, the data from the submaximal exercise test further confirm that the FO group indeed had higher CRF than did the CO group. This was reflected by the 28% lower relative o2, 12% lower respiratory exchange ratio, 18% drop in heart rate, reduction in blood lactate levels by more than one-half, and 19% reduction in the ventilatory response to exercise, despite working at a higher absolute work rate. Collectively, these observations strongly suggest that the individuals in the FO group had higher fitness levels than those in the CO group. In fact, the increased CRF in this FO group was comparable to, if not higher than, values reported after exercise training studies in healthy obese individuals.27‐31 Finally, several studies on normal-weight and fit individuals have reported fitness levels comparable to this FO group.8,10,32,33
Mechanical Ventilatory Constraints
We did not observe increased EFL in this FO group at peak exercise even though the FO group maximal e was approximately 40% higher than in the CO group. This degree of flow limitation (< 20% of Vt) is considered to be a mild constraint according to Johnson et al.34 The proportion of subjects who developed EFL in the current investigation is very similar to that in prior studies in sedentary obese individuals.5‐7,35,36 For instance, Ofir et al7 reported that approximately 55% of sedentary obese subjects who underwent an incremental exercise test experienced a moderate degree of flow limitation near peak exercise (about 38% of Vt). Babb et al5 reported that about 45% of sedentary obese women experienced mild EFL (about 12% of Vt) at peak exercise. Likewise, DeLorey et al6 reported mild EFL at peak exercise in six of 10 obese sedentary adults (about 12% of Vt). However, these comparisons should be done with care because of differences in the methodologies, the degrees of exertion, and the protocols used. The FO subjects had reduced EELV at rest. EELV also decreased during the early stages of exercise but returned to resting levels at peak exercise.5‐7 This dynamic hyperinflation is also an index of ventilatory constraint34 and allows subjects to further increase expiratory flow rates and minimize EFL.8,37 At peak exercise, end-inspiratory lung volume approached 90% of TLC and e was approximately 79% of MVV, which may also indicate slight mechanical ventilatory constraint.38 Despite some degree of mechanical ventilatory constraint observed, neither end-tidal CO2 nor oxygen saturation revealed relative hypoventilation. Moreover, they do not appear to have exacerbated mechanical ventilatory constraints that could considerably alter breathing mechanics and ventilatory dynamics during heavy exercise. Part of this lack of increase in mechanical ventilatory constraints in the FO group despite the large increase in ventilatory demand at peak efforts could be due to the overall greater lung function in the fit group compared with the control group. The increase in TLC, FEV1, PEF, and MVV may have masked some of the expected ventilatory constraints associated with a 40% increase in ventilatory demand. Furthermore, our findings also show that our FO group did not have increased mechanical ventilatory constraints compared with normal-weight subjects of similar CRF.32,39
Breathing Strategy
Increasing inspiratory and expiratory flows can shorten the breathing cycle for a given Vt and can, thus, further increase their e by increasing breathing frequency. In fact, the FO group was able to achieve higher e at maximal efforts by further increasing breathing frequency. Finally, others have also reported similar breathing frequency at peak exercise in nonobese adults with CRF similar to that of the FO group.10,40
From a practical point of view of minimizing the work of breathing, increasing e by increasing breathing frequency near peak exercise is a good strategy, despite the inevitable increase in the dead space ventilation. There are strong data to suggest that over the ventilatory range from 30 to 130 L/min, increasing ventilation by changing breathing frequency has little impact on the work of breathing.41,42 Subjects were able to maintain a normal Vt, limit EFL, and limit their encroachment on TLC. All these changes allowed the FO group to increase e by increasing breathing frequency, which kept the increase in the work of breathing to a minimum.
Pulmonary Function
Data from the pulmonary function tests revealed that the “obesity effect” was present in the FO group, just as it was in the CO group. There are conflicting reports on the effects of endurance training on pulmonary function and lung volumes.43‐45 Some reports suggest pulmonary volumes in endurance-trained adults are greater than in sedentary control subjects,46,47 whereas other investigators have found no consistent differences in lung volumes with endurance training.43,44 Our results suggest improved overall lung function with increased CRF and agree with some reports,44,46,47 but not with others.32,48 The overall consensus, however, is that increased CRF may have a stronger influence in “effort-dependent” lung function variables such as PEF, MVV, and FEV1,21,49 while having less of an effect on “effort-independent” variables such as FVC and TLC.27,43,44,47 From our data, we cannot determine if the improved lung function in the FO group was a result of their training, or if they were able to achieve such a high CRF because of their already enhanced lung function. Further interventional research studies to answer this very important question are warranted.
Oxygen Cost of Breathing
Obesity reduces chest wall and total respiratory system compliance, which increases the work of breathing.1,4 Compared with our data on normal-weight subjects,48 both the FO and CO subjects had an increase in the work of breathing. Our data from the FO group suggest that their oxygen cost of breathing is about 40% higher than those reported in nonobese individuals.48 The data from the FO group was somewhat lower compared with the CO group, but failed to reach significance (P = .08). Regardless, the FO subjects were able to increase e at peak exercise by 40% without a significant tax on the work of breathing.
Conclusions
These novel data suggest that young, otherwise healthy, obese adults can participate in vigorous physical activity without being ventilatory compromised, even at peak efforts. Therefore, these findings are good news for those who carry extra weight but want to participate in physical activity, because many reports suggest that increased fitness is associated with lower risk of mortality regardless of the degree of obesity.49‐51 Although the results of the current investigation are encouraging for healthy individuals with mild-to-moderate obesity, more research is warranted to investigate these responses in more extreme stages of obesity.
Acknowledgments
Author contributions: Dr Babb had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Lorenzo: contributed to the data collection, data processing and analysis, critical input, and the writing of the manuscript.
Dr Babb: contributed to the planning of the project, supervising and assisting in data collection, directing data processing and analysis, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: The authors appreciate the considerable time and effort of the subjects who participated in this project. The authors also express their appreciation for the assistance of Raksa Moran, RN; Todd Bassett, MS; Sarah Haller, MS; Jessica Pineda, BS; Vipa Bernhardt, PhD; and Keri Shafer, MD, on this project.
Abbreviations
- CO
control obese
- CRF
cardiorespiratory fitness
- EELV
end-expiratory lung volume
- EFL
expiratory flow limitation
- FO
fit obese
- IC
inspiratory capacity
- MVV
maximal voluntary ventilation
- PEF
peak expiratory flow
- TLC
total lung capacity
- e
minute ventilation
- o2
oxygen uptake
- o2peak
peak oxygen uptake
- Vt
tidal volume
Footnotes
This study was presented in abstract form at the American College of Sports Medicine Annual Meeting, San Francisco, CA, May 2012. (Lorenzo S, Bassett JT, Moran RB, Pineda J, and Babb TG.)
Funding/Support: This work was supported by the American Heart Association [11POST4920002], the National Institutes of Health [HL096782 to Dr Babb], the King Charitable Foundation Trust, an American Lung Association Career Investigator Award, an American Heart Association Grant in Aid, The Research and Education Institute at Texas Health Resources, the Cain Foundation, and Texas Health Presbyterian Hospital Dallas.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Cherniack RM. Respiratory effects of obesity. Can Med Assoc J. 1958;80(8):613-616 [PMC free article] [PubMed] [Google Scholar]
- 2.Yap JCH, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. 1995;79(4):1199-1205 [DOI] [PubMed] [Google Scholar]
- 3.Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109(1):144-151 [DOI] [PubMed] [Google Scholar]
- 4.Gilbert R, Sipple JH, Auchincloss JH., Jr Respiratory control and work of breathing in obese subjects. J Appl Physiol. 1961;16:21-26 [DOI] [PubMed] [Google Scholar]
- 5.Babb TG, DeLorey DS, Wyrick BL, Gardner PP. Mild obesity does not limit change in end-expiratory lung volume during cycling in young women. J Appl Physiol. 2002;92(6):2483-2490 [DOI] [PubMed] [Google Scholar]
- 6.DeLorey DS, Wyrick BL, Babb TG. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int J Obes (Lond). 2005;29(9):1039-1047 [DOI] [PubMed] [Google Scholar]
- 7.Ofir D, Laveneziana P, Webb KA, O’Donnell DE. Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol. 2007;102(6):2217-2226 [DOI] [PubMed] [Google Scholar]
- 8.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol. 2007;581(pt 3):1309-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol. 1992;73(3):874-886 [DOI] [PubMed] [Google Scholar]
- 10.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol. 1998;84(6):1872-1881 [DOI] [PubMed] [Google Scholar]
- 11.Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol. 2002;93(1):201-206 [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society Standardization of spirometry (1994 update). Am J Respir Crit Care Med. 1995;152(3):1107-1136 [DOI] [PubMed] [Google Scholar]
- 13.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-734 [DOI] [PubMed] [Google Scholar]
- 14.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587-600 [DOI] [PubMed] [Google Scholar]
- 15.Burrows B, Kasik JE, Niden AH, Barclay WR. Clinical usefulness of the single-breath pulmonucy diffusing capacity test. Am Rev Respir Dis. 1961;84:789-806 [DOI] [PubMed] [Google Scholar]
- 16.Goldman HI, Becklake MR. Respiratory function tests; normal values at median altitudes and the prediction of normal results. Am Rev Tuberc. 1959;79(4):457-467 [DOI] [PubMed] [Google Scholar]
- 17.Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008;178(2):116-123 [DOI] [PubMed] [Google Scholar]
- 18.Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol. 1997;82(3):746-754 [DOI] [PubMed] [Google Scholar]
- 19.Rundell KW, Anderson SD, Spiering BA, Judelson DA. Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. Chest. 2004;125(3):909-915 [DOI] [PubMed] [Google Scholar]
- 20.Wasserman K, Hansen JE, Sue DY, et al. Normal values. In: DeStefano FR, ed. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 4th ed Philadelphia, PA: Lippincott Williams and Wilkins; 2005:160-182 [Google Scholar]
- 21.Riddle W, Younes M, Remmers JE, et al. Graphical analysis of patient performance in the pulmonary function laboratory. IEEE Trans Biomed Eng. 1980;1:282-290 [Google Scholar]
- 22.Lorenzo S, Babb TG. Quantification of cardiorespiratory fitness in healthy nonobese and obese men and women. Chest. 2012;141(4):1031-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasserman K, Hansen JE, Sue DY, et al. Measurements during integrative cardiopulmonary exercise testing. In: DeStefano FR, ed. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2005:76-110 [Google Scholar]
- 24.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 pt 2):S49-S55 [DOI] [PubMed] [Google Scholar]
- 25.Wasserman K, Whipp BJ. Excercise physiology in health and disease. Am Rev Respir Dis. 1975;112(2):219-249 [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 3rd ed. Philadelphia, PA: Lea & Febiger, 1986; 1-179 [Google Scholar]
- 27.Kollias J, Boileau RA, Barlett HL, Buskirk ER. Pulmonary function and physical conditioning in lean and obese subjects. Arch Environ Health. 1972;25(2):146-150 [DOI] [PubMed] [Google Scholar]
- 28.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274(24):1915-1921 [DOI] [PubMed] [Google Scholar]
- 29.Ozcelik O, Dogan H, Kelestimur H. Effects of eight weeks of exercise training and orlistat therapy on body composition and maximal exercise capacity in obese females. Public Health. 2006;120(1):76-82 [DOI] [PubMed] [Google Scholar]
- 30.Tremblay A, Simoneau JA, Bouchard C. Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism. 1994;43(7):814-818 [DOI] [PubMed] [Google Scholar]
- 31.Björntorp P, De Jounge K, Sjöström L, Sullivan L. The effect of physical training on insulin production in obesity. Metabolism. 1970;19(8):631-638 [DOI] [PubMed] [Google Scholar]
- 32.Babcock MA, Pegelow DF, Johnson BD, Dempsey JA. Aerobic fitness effects on exercise-induced low-frequency diaphragm fatigue. J Appl Physiol. 1996;81(5):2156-2164 [DOI] [PubMed] [Google Scholar]
- 33.Mota S, Casan P, Drobnic F, et al. Expiratory flow limitation during exercise in competition cyclists. J Appl Physiol. 1999;86(2):611-616 [DOI] [PubMed] [Google Scholar]
- 34.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest. 1999;116(2):488-503 [DOI] [PubMed] [Google Scholar]
- 35.Ferretti A, Giampiccolo P, Cavalli A, Milic-Emili J, Tantucci C. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119(5):1401-1408 [DOI] [PubMed] [Google Scholar]
- 36.Pankow W, Podszus T, Gutheil T, Penzel T, Peter J, Von Wichert P. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol. 1998;85(4):1236-1243 [DOI] [PubMed] [Google Scholar]
- 37.Pellegrino R, Violante B, Nava S, Rampulla C, Brusasco V, Rodarte JR. Expiratory airflow limitation and hyperinflation during methacholine-induced bronchoconstriction. J Appl Physiol. 1993;75(4):1720-1727 [DOI] [PubMed] [Google Scholar]
- 38.Babb TG. Mechanical ventilatory constraints in aging, lung disease, and obesity: perspectives and brief review. Med Sci Sports Exerc. 1999;31(suppl 1):S12-S22 [DOI] [PubMed] [Google Scholar]
- 39.McClaran SR, Wetter TJ, Pegelow DF, Dempsey JA. Role of expiratory flow limitation in determining lung volumes and ventilation during exercise. J Appl Physiol. 1999;86(4):1357-1366 [DOI] [PubMed] [Google Scholar]
- 40.Dominelli PB, Guenette JA, Wilkie SS, Foster GE, Sheel AW. Determinants of expiratory flow limitation in healthy women during exercise. Med Sci Sports Exerc. 2011;43(9):1666-1674 [DOI] [PubMed] [Google Scholar]
- 41.Otis AB. The Work of Breathing. Handbook of Physiology - The Respiratory System III. Washington, DC: American Physiological Society; 1964:463-476 [Google Scholar]
- 42.Milic-Emili G, Petit JM, Deroanne R. The effects of respiratory rate on the mechanical work of breathing during muscular exercise. Int Z Angew Physiol. 1960;18:330-340 [DOI] [PubMed] [Google Scholar]
- 43.Hagberg JM, Yerg JE, II, Seals DR. Pulmonary function in young and older athletes and untrained men. J Appl Physiol. 1988;65(1):101-105 [DOI] [PubMed] [Google Scholar]
- 44.Robinson EP, Kjeldgaard JM. Improvement in ventilatory muscle function with running. J Appl Physiol. 1982;52(6):1400-1406 [DOI] [PubMed] [Google Scholar]
- 45.Saltin B, Hartley LH, Kilbom A, Astrand I. Physical training in sedentary middle-aged and older men. II. Oxygen uptake, heart rate, and blood lactate concentration at submaximal and maximal exercise. Scand J Clin Lab Invest. 1969;24(4):323-334 [DOI] [PubMed] [Google Scholar]
- 46.Raven PB. Pulmonary function of elite distance runners. Ann N Y Acad Sci. 1977;301:371-381 [DOI] [PubMed] [Google Scholar]
- 47.Grimby G, Saltin B. Physiological analysis of physically well-trained middle-aged and old athletes. Acta Med Scand. 1966;179(5):513-526 [DOI] [PubMed] [Google Scholar]
- 48.Lorenzo S, Babb TG. Oxygen cost of breathing and breathlessness during exercise in nonobese women and men. Med Sci Sports Exerc. 2012;44(6):1043-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69(3):373-380 [DOI] [PubMed] [Google Scholar]
- 50.Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;31(suppl 11):S646-S662 [DOI] [PubMed] [Google Scholar]
- 51.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282(16):1547-1553 [DOI] [PubMed] [Google Scholar]