Abstract
Pulmonary arterial hypertension (PAH) is the leading cause of death in systemic sclerosis (SSc) and affects up to 12% of all patients with SSc, with a 50% mortality rate within 3 years of PAH diagnosis. Compared with the idiopathic form of PAH (IPAH), patients with SSc-associated PAH (SSc-PAH) have a threefold increased risk of death and may receive a diagnosis late in the course of disease because of insidious onset and the high prevalence of cardiac, musculoskeletal, and pulmonary parenchymal comorbidities. Treatment with conventional forms of PAH therapy often yield poor results compared with IPAH cohorts; unfortunately, the exact reasons behind this remain poorly understood but likely include variations in the pathologic mechanisms, differences in cardiovascular response to increasing afterload, and inadequate strategies to detect and treat SSc-PAH early in its course. Current methods for screening and longitudinal evaluation of SSc-PAH, such as the 6-min walk test, transthoracic echocardiography, and MRI, each have notable advantages and disadvantages. We provide an up-to-date, focused review of SSc-PAH and how it differs from IPAH, including pathogenesis, appropriate screening for disease onset, and new approaches to treatment and longitudinal assessment of this disease.
Systemic sclerosis (SSc) is a complex, multisystem disease characterized by fibrosis and excessive collagen deposition within the skin and internal organs, chronic inflammation, autoimmune dysregulation, and microvascular endothelial dysfunction. There are two forms of SSc that are characterized by the extent of skin involvement. Limited cutaneous SSc predominantly involves the skin of the hands, arms, and face, and diffuse SSc involves large areas of skin and multiple organs.1 Both forms are systemic diseases associated with significant morbidity and mortality. Historically, mortality was largely caused by renal disease. With the advent of angiotensin-converting enzyme inhibitors to treat SSc renal crisis, SSc-associated pulmonary arterial hypertension (SSc-PAH) has emerged as a leading cause of morbidity and mortality, accounting for up to 30% of premature deaths.2
Although SSc-PAH represents a significant proportion of the pulmonary arterial hypertension (PAH) population (15%-30%),3,4 much of what we know about PAH is derived from studies of idiopathic PAH (IPAH). It is clear, though, that SSc-PAH behaves differently. Patients with SSc-PAH have a threefold higher risk of death than patients with IPAH, despite similar hemodynamic indices, and are frequently less responsive to PAH therapy.5,6 This article highlights key features of SSc-PAH that distinguish it from IPAH and emerging strategies for the diagnosis and management of this disease.
Epidemiology
PAH is defined by an elevated mean pulmonary artery pressure (mPAP) of > 25 mm Hg, with a pulmonary capillary wedge pressure of < 15 mm Hg.7 Prevalence of SSc-PAH among patients with SSc varies but is between 10% and 12%.4,8‐10 Thus, the prevalence of SSc-PAH may be as high as four to five times that of IPAH.11 In contrast, the prevalence data from REVEAL (Registry to Evaluate Early and Long-term PAH Disease Management) suggest that IPAH is more than twice as common as SSC-PAH.12 This discrepancy highlights possible disparities in SSc-PAH screening and diagnosis compared with IPAH, which is usually associated with fewer comorbidities.
Single-center cohort studies suggest that male patients aged ≥ 47 years at the time of SSc diagnosis,13 who have had SSc for > 10 years,14 or who have a diffusing capacity of lung for carbon monoxide (Dlco) of < 55%15 are at highest risk for SSc-PAH. The PHAROS (Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma) study, an ongoing, multicenter, prospective observational study of SSc-PAH, published the baseline characteristics of a cohort of 237 patients at high risk for SSc-PAH or who had received a diagnosis of SSc-PAH16; these patients are being followed prospectively at 18 centers across North America. The study reported that patients with SSc-PAH are predominantly women (87%), white (67%), and have limited cutaneous SSc (57%). Seventy-nine percent of patients with SSc-PAH have a Dlco < 55% compared with 55% of those with SSc only. This multiyear study is in its initial stages but promises to offer valuable information about the epidemiology and clinical factors associated with SSc-PAH.
Pathogenesis
SSc-PAH occurs as a consequence of progressive remodeling of the small- to medium-sized pulmonary vasculature. The exact mechanisms of disease progression remain unclear, but it is believed that inflammation and endothelial injury are common precursors. Functionally, the inflammatory process creates a disequilibrium between vasoactive, proliferative mediators (eg, thromboxane A2 and endothelin-1) and antiproliferative vasodilators (eg, nitric oxide and prostacyclin) within the endothelium. Pulmonary artery vasoconstriction and cellular proliferation occur and may be exacerbated by increased levels of serotonin released from activated platelets.17 At the same time, sympathetic activity increases, hypoxemia occurs, and ischemia-reperfusion injury in the pulmonary vasculature promotes additional cytokine release, furthering vascular remodeling, fibrosis, and intraluminal microthrombosis.18 The end result is a progressive increase in pulmonary vascular resistance, pulmonary arterial pressure, and right ventricular (RV) pressure overload. Compensatory mechanisms in the right ventricle initially preserve the stroke volume (SV) and cardiac index, but as the limits of compensation are exceeded, cardiac failure and death follow.
Autoimmune Dysfunction
In SSc, the presence of autoantibodies (anticentromere, antitopoisomerase 1, and anti-RNA-polymerase III) is well described.19 In SSc-PAH, autoimmune dysregulation may also be preponderant.20 For example, a study by Riemekasten et al21 showed that angiotensin II type-1 receptor antibodies and endothelin-1 receptor type A antibodies were present in most patients with SSc. In vitro, these antibodies upregulated inflammatory mediators and increased cytotoxicity, suggesting a significant role in vascular remodeling. Patients who were antibody positive also had increased mortality compared with those who were antibody negative (P ≤ .003).
Antiendothelial cell antibodies, also seen in patients with SSc, enhance expression of adhesion molecules and endothelial cell apoptosis and are associated with severe digital ischemia and PAH.22,23 Similarly, antifibroblast antibodies, detected in up to 30% of patients with SSc-PAH,24 activate several mechanisms central to the vascular remodeling process, including the activation of platelet-derived growth factor receptors, which stimulate the release of reactive oxygen species, fibroblast proliferation, and collagen synthesis.18,25
In many autoimmune-mediated diseases (ie, systemic lupus erythematosus, mixed connective tissue disease [CTD]), PAH improves with aggressive immunosuppression. Although there is no current evidence that immunosuppression improves outcomes in SSc-PAH,26 new trials are underway to evaluate the potential benefits of novel immunomodulatory agents (discussed in the Investigational Therapies section of this review).
Genetic Factors
Unfortunately, little is known about the role of genetic polymorphisms in SSc-PAH. The most familiar genetic polymorphisms described in other forms of PAH, such as the bone morphogenic protein receptor-II gene (BMPRII), seen in > 80% of familial PAH cases are not seen in patients with SSc-PAH.27,28 However, examples of a possible genetic influence, including a 6-base insertion of intron 7 of endoglin, a part of the tissue growth factor β1 receptor complex, have been described by Wipff et al.29 The authors noted a negative association between the polymorphism and SSc-PAH. Although small and exploratory in nature, the study reinforced the need for clues that better elucidate the underlying mechanisms of SSc-PAH.
Clinical Features
Patients with SSc-PAH often present with few symptoms. They may experience progressive dyspnea, fatigue, or chest palpitations but are frequently unaware of their own symptoms and may unwittingly alter lifestyle habits to accommodate the symptoms. Symptoms that should prompt immediate consideration of SSc-PAH include syncope, lightheadedness or orthostasis, and angina or chest pain.
Physical examination findings may be absent early in the disease process because symptoms usually appear when RV function begins to decline. Notable findings include decreased pulse pressure, presence of a left parasternal heave, loud pulmonary portion of the second heart sound, prominent jugular a wave, and a pulmonic or tricuspid regurgitant (TR) murmur. Extracardiac findings indicative of increased right atrial pressures include hepatomegaly, hepatojugular reflex, increasing lower-extremity edema, and abdominal ascites.
SSc-PAH and Interstitial Lung Disease
In interstitial lung disease (ILD), the mechanisms for increased peripheral vascular resistance (PVR) are not well defined. Whereas chronic hypoxic vasoconstriction is a possible initial trigger, vascular remodeling may occur, even in the absence of hypoxemia, suggesting that other mechanisms such as inflammation, fibrosis, and cell proliferation involving all three components of the vascular wall as well as parenchymal loss because of fibrosis may independently contribute to pulmonary hypertension (PH) in these patients.30 PH frequently develops in patients with diffuse SSc and ILD. However, up to 25% of these patients have an mPAP > 35 mm Hg, suggesting that their PH is out of proportion with that expected from ILD alone7,31,32 and that there is a high likelihood of intrinsic vascular disease in addition to ILD. Although no trials currently support the use of PAH therapy in this population, observational trials suggest that the combination of PH and ILD may increase mortality risk up to fivefold over SSc-PAH.33,34 Therefore, we generally offer aggressive therapy to patients with SSc and ILD (FVC < 70% and presence of interstitial fibrosis on CT scan) when mPAP > 35 mm Hg.
Cardiac Involvement in SSc-PAH
Unlike IPAH, SSc also exerts a primary inflammatory effect on the heart, causing myocardial fibrosis and impaired microcirculatory function in 10% to 50% of patients.35 Although findings consistent with tamponade are uncommon,36 the presence of pericardial effusion occurs threefold more often in SSc-PAH than in IPAH.5 Additionally, SSc often causes left ventricular (LV) hypertrophy, nonsystolic LV dysfunction, and left atrial enlargement.37 Overall, the cumulative influence of SSc on the heart may impair the heart’s ability to compensate against increasing PVR in SSc-PAH. Our group has shown that despite similar hemodynamic indices, patients with SSc-PAH have higher mortality and are less responsive to therapy than patients with IPAH.5,38 These findings were recently reiterated in the PAH Quality Enhancement Research Initiative (QUERI), which showed that over a 3-year follow-up of 507 patients with SSc-PAH and IPAH, survival was lower in the SSc-PAH cohort (60% vs 77% P < .0001) despite similar baseline characteristics and lower mPAP, PVR, and pulmonary artery systolic pressure (PASP) compared with the IPAH cohort.6
Musculoskeletal Involvement in SSc-PAH
Musculoskeletal complications are common in SSc. Up to 66% of patients present with joint involvement, and 81% present with muscle involvement39 frequently occurring in concert with myocardial involvement. These features are more likely in men and patients with diffuse SSc.39 Musculoskeletal complications can also affect the patient’s ability to perform common tests, such as the 6-min walk test or cardiopulmonary exercise testing, frequently used to guide PAH therapy. There are no data linking these features to worse survival though, suggesting that although it can have a significant impact on common outcome measures, musculoskeletal involvement in SSc may not have a direct impact on the progression of SSc-PAH itself.
Diagnosis and Management
Screening for SSc-PAH
Unfortunately, there are no telltale pathognomonic features to easily identify the presence of SSc-PAH. In fact, the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) study showed that 22% of patients with SSc-PAH had minimal or no dyspnea.16 The American College of Cardiology Foundation and American Heart Association recommend annual transthoracic echocardiography (TTE) screening in all patients with SSc regardless of symptoms.40 Adherence to these recommendations is variable, though, and may be due to an absence of data that characterize a clear benefit to early treatment in asymptomatic patients with SSc-PAH.41 We screen patients in our SSc center annually for PAH, and as a result, nearly 50% of new diagnoses are World Health Organization (WHO) functional class (FC) I or II,42 a dramatic difference compared with other studies that reported that 70% of cases are diagnosed as WHO FC III or IV.43,44
Several methods of screening have been proposed to identify SSc-PAH. TTE estimation of TR jet velocity is currently the most widely used screening tool. With use of the modified Bernoulli equation, evaluation of the TR jet velocity offers an estimate of PASP. A meta-analysis suggested that TTE-derived TR jet velocity correlates reasonably well with right-sided heart catheterization (RHC)-derived PASP across populations (correlation coefficient, 0.70; 95% CI, 0.67-0.73).45 Practically speaking, TR jet velocity estimated by TTE suffers from considerable between-person variability, may be overestimated in patients without PH, and is often complicated by an inadequate Doppler signal.46 Furthermore, no consensus exists on a threshold beyond which PH should be suspected but below which PH is unlikely. On the basis of a graded scale, the European Society of Cardiology and European Respiratory Society suggested that PH is likely if the TR jet velocity is > 3.4 m/s (Table 1).46 This suggestion is reasonable since a prospective trial of 137 patients with SSc demonstrated a 98% positive predictive value for PH on RHC if the TR jet velocity on TTE was ≥ 3.4 m/s.47 One should be cautious not to assume that a TR jet velocity of < 3.4 m/s indicates the absence of SSc-PAH. Although the European Society of Cardiology/European Respiratory Society guidelines arbitrarily suggest that TR jet velocity be ≤ 2.8 m/s with an otherwise normal TTE makes PH unlikely,46 we believe that other screening modalities should be used before declaring the absence of PAH in high-risk patients with SSc. Alternative features of TTE that may hint at the presence of PH but are not well studied include dilation of the right ventricle or right atrium, presence of septal deformity, increased pulmonary valve regurgitation, and short acceleration time of the RV ejection into the pulmonary artery.46
Table 1.
—European Respiratory Society/European Society of Cardiology Criteria for Determining Probability of PH on TTE Evaluation
| Probability | Criteria |
| PH unlikely if | TR jet velocity ≤ 2.8 m/s, PASP ≤ 36 mm Hg, and otherwise normal TTE |
| PH possible if | TR jet velocity ≤ 2.8 m/s and PASP ≤ 36 mm Hg, but additional echocardiographic features suggest PH |
| PH possible if | TR jet velocity of 2.9-3.4 m/s, PASP of 37-50 mm Hg, and no additional features on TTE suggestive of PH |
| PH likely if | TR jet velocity > 3.4m/s and PASP > 50 mm Hg with or without the presence of additional features suggestive of PH on TTE |
Thresholds are based on expert consensus and were arbitrarily decided with the use of best available data at the time of publication.46 PASP = pulmonary artery systolic pressure; PH = pulmonary hypertension; TR = tricuspid regurgitant; TTE = transthoracic echocardiography.
Another approach to screening includes the measuring of N-terminal pro-brain natriuretic peptide (pro-BNP) levels. Pro-BNP levels correlate well with RHC hemodynamics, and although a normal pro-BNP level does not exclude PAH, an elevated pro-BNP > 240 pg/mL has a 90% specificity for detecting the presence of SSc-PAH.48 However, pro-BNP measurement should not be used without other screening modalities because an elevated pro-BNP level is not specific to SSc-PAH and can reflect other causes of cardiac dysfunction commonly seen in patients with SSc.
Evaluation of Dlco may also help to identify the presence of PAH. Although it does not correlate well with RHC-derived hemodynamics, less than one-sixth of patients with SSc-PAH have a Dlco > 60% predicted.47,49 In one study, a Dlco/alveolar volume (Va) of < 70% predicted suggested an 18-fold higher risk for developing SSc-PAH within 2.5 years compared with a Dlco/Va ≥ 70%.50 A Dlco > 80% is unusual in SSc-PAH.49
There may be some benefit to using multiple screening modalities to detect SSc-PAH. Schreiber et al51 developed a screening formula that is based on Dlco and oxygen saturation as measured by pulse oximetry (Spo2) (predicted mPAP [mm Hg] = 136 − Spo2 [%] − 0.25 × Dlco [% predicted]), which was validated in 129 patients with SSc. A predicted mPAP > 25 mm Hg revealed a positive and negative likelihood ratio for diagnosing PAH on RHC of 1.3 and 0.3, respectively. When an mPAP threshold of 35 mm Hg was used, the positive and negative likelihood ratios were 12.3 and 0.8, respectively.
The Cochin RPS (a PAH risk prediction score) described by Meune et al52 was derived from observation of age, FVC, and Dlco/Va in 1,165 patients evaluated for SSc-PAH and then validated in 443 separate patients over 3 years. A 35-fold higher risk for developing PAH was found in patients with the highest quintile Cochin RPS compared with the two lowest-scoring groups. With the use of a receiver operating characteristic curve, the study also established a threshold score that showed positive and negative likelihood ratios of 3.53 and 0.08, respectively, compared with diagnosis by RHC.
DETECT (Early, Simple and Reliable Detection of Pulmonary Arterial Hypertension in Systemic Sclerosis) is a recently completed observational, prospective, cohort study that evaluated several clinical aspects of screening, including demographic, imaging, serum biomarkers, and pulmonary function tests (PFTs), with a goal of improving noninvasive screening and detection of SSc-PAH. Detailed results have not been published, but a recent abstract reported that 466 patients with SSc were screened for SSc-PAH before receiving RHC. From clinical data, an algorithm was devised that decreased the rate of missed SSc-PAH with RHC to < 50% of expected on the basis of current screening guidelines.53 Specific parameters included in the algorithm were not reported. At our institution, we screen all patients with SSc annually with TTE and PFTs. We refer all patients with SSc and unexplained dyspnea and those with abnormal PFTs, a TR jet velocity of ≥ 3.2 m/s, or other TTE abnormalities for RHC, regardless of symptoms.
Vasodilator Testing
During RHC, it is standard to evaluate the response of the pulmonary arteries to the administration of acute vasodilators (epoprostenol, inhaled nitric oxide, or adenosine). In 10% to 15% of patients with IPAH, the mPAP decreases by ≥ 10 mm Hg to a value of 40 mm Hg. In these patients, consensus recommendations suggest initial treatment with high-dose calcium channel blockers (CCBs).40,46 In SSc-PAH, far fewer patients (about 1%) demonstrate vasodilator responsiveness.54,55 Additionally, in this population, CCBs carry some risk of untoward side effects (eg, exacerbating reflux, esophageal dysmotility). Therefore, current guidelines do not advocate vasodilator challenge during RHC or treatment of SSc-PAH with CCB.46,54
Exercise-Induced PAH
Before 2008, the classification of PAH included a provision for patients who had a resting mPAP < 25 mm Hg but demonstrated an increase in mPAP to > 30 mm Hg during exercise.56 In SSc, studies have suggested a high prevalence of exercise-induced PAH (EIPAH).57,58 Steen et al59 evaluated 54 patients with SSc at high risk for PAH and identified EIPAH by TTE in 24. Of these, 19% had resting PAH, and 62% had EIPAH on RHC. Nonetheless, few data suggest that early treatment improves outcomes, and almost no data conclusively link EIPAH to resting PAH. A recent pilot study by Kovacs et al60 supported the notion that in patients with SSc and EIPAH, clinical progression to resting PAH may occur and may be slowed by treatment. No large studies have corroborated these results. The debate surrounding EIPAH is further complicated by studies that show that even in healthy individuals, mPAP can exceed 30 mm Hg during exercise, especially in those aged ≥ 50 years.61 Given the absence of clear data on the pathologic implications of EIPAH and unclear thresholds beyond which EIPAH should be considered, EIPAH is not currently included as a diagnostic classification of PAH,7 and screening for EIPAH is not recommended in PAH guidelines.40,46
Current Therapy
Despite an improved understanding of SSc-PAH, little progress has been made in modifying outcomes with the available three main therapeutic modalities: prostacyclin analogs, endothelin receptor antagonists (ERAs), and phosphodiesterase inhibitors. Despite the frequent use of CCBs for relief of Raynaud phenomenon in SSc, they are not recommended for treatment of SSc-PAH.
Epoprostenol, a prostacyclin analog, remains the most effective PAH therapy known, and in IPAH, it is the only therapy that improved survival in a randomized controlled trial.62 In SSc-PAH, no therapy has ever demonstrated a survival advantage. Epoprostenol improves exercise capacity and hemodynamics in SSc-PAH63 but has drawbacks. IV formulations pose obvious problems from an infection control and lifestyle standpoint. New room temperature-stable epoprostenol alleviates previous requirements for ice packing, but patients with sclerodactyly often struggle with the manual dexterity required to administer it. Inhaled prostacyclin analogs offer an alternative route of administration but require frequent administration (up to nine times daily) and have not been studied in SSc-PAH or CTD as a cohort. Treprostinil offers the option of subcutaneous administration, but patients frequently experience infusion site skin irritation, limiting its tolerability. As a result, we reserve this therapy for patients who present with WHO FC IV symptoms at diagnosis or who fail to respond to oral therapies.
Phosphodiesterase type 5 inhibitors (PDE-5Is), such as sildenafil and tadalafil, offer a potential advantage over other therapies in that they are orally administered, well tolerated, and only require dosing one (tadalafil) to three times (sildenafil) daily. In addition, there is now a generic formulation of sildenafil, which provides a less costly alternative to other PAH-specific medications. Data on the effectiveness of PDE-5Is in SSc-PAH have been difficult to interpret because SSc-PAH is not well represented in the study cohorts in clinical trials. One large clinical trial of sildenafil suggested improvement in 6-min walk distance (6MWD) by 45 to 50 m over 12 weeks compared with placebo.64 A subgroup analysis of patients in this trial with CTD (45% of whom had SSc-PAH) demonstrated improved 6MWD by an average of 55 m with low-dose sildenafil compared with placebo.65 Statistically significant improvements in mPAP and PVR were also seen in the sildenafil group. Despite the limited data and lack of clear efficacy over other classes of PAH therapy, we believe that the low cost, ease of administration, and tolerability of PDE-5Is make this agent the most attractive first-line therapy for SSc-PAH.
Bosentan, an oral endothelin-A receptor antagonist, has also been associated with improvement in 6MWD, hemodynamics, and time to clinical worsening in patients with IPAH.66 A small retrospective study by our group suggested that patients with SSc-PAH did not fare as well because most patients reported a decline in FC over the 6-month follow-up period.38 In contrast, ambrisentan, a more selective ERA with a potential advantage of preserving the vasodilatory effect of nitric oxide and prostacyclin released by endothelial cell endothelin-B receptors while suppressing vasoconstriction and cellular proliferation activated by endothelin-A receptors, showed modest improvements in 6MWD (15-23 m) in patients with CTD. The representation of SSc-PAH in this cohort is not reported.67 In 2008, a meta-analysis of randomized placebo-controlled trials evaluating the effect of ERAs on exercise capacity suggested that although the effect size of ERAs was statistically significant when all patients with PAH were included (0.44; 95% CI, 0.29-0.58), a subgroup analysis of patients with CTD-PAH (mostly SSc-PAH) did not show a statistically significant effect size (0.27; 95% CI, −0.01 to 0.54).68 Although treatment guidelines support the use of ERAs or PDE-5Is as first-line therapy in patients with PAH in general, for the treatment of SSc-PAH, our personal preference is to reserve ERAs for patients who do not tolerate PDE-5Is and for those who do not respond to PDE-5I monotherapy.40,46
Because single agents rarely result in substantial and sustained clinical improvement in SSc-PAH, many clinicians now offer a combination of PAH-specific therapies under the assumption that they will work in concert through independent pathways. Six randomized trials and > 25 observational trials or case series have been published, with virtually all showing improvement in outcomes compared with monotherapy.69 Unfortunately, few of these studies involved significant numbers of patients with SSc-PAH. Nonetheless, a systematic review by Johnson et al69 concluded that although insufficient data exist to determine whether one combination is superior to others, combination therapy should be considered in the following situations: as an alternative to parenteral therapy if parenteral therapy is not a viable option, as a bridge to lung transplantation, or as a mechanism to facilitate weaning from parenteral therapy.
Consensus guidelines also support adjunct therapies for the management of SSc-PAH and IPAH, despite a lack of clear evidence of benefit or disease modification. Patients with IPAH and patients with CTD who have advanced PAH are recommended to receive anticoagulation, despite equipoise over the risk/benefit profile.46 In practice, less than one-half of the patients with SSc-PAH tolerate long-term anticoagulation, given a high incidence of ulcerative esophagitis and gastric antral vascular ectasias in SSc. In addition, supplemental oxygen for saturation < 90% at rest or with exercise, diuretics for volume management, and digoxin for management of arrhythmias in the setting of refractory RV failure are rational and guideline driven but are recommended largely on the basis of conventional wisdom and expert opinion.40,46
Investigational Therapies
Recently, investigators have been evaluating the ability of antineoplastic agents to slow aberrant proliferation of vascular smooth muscle and endothelium in PAH. Imatinib mesylate is a tyrosine kinase inhibitor initially designed to interfere with the Bcr-Abl kinase pathway in chronic myelogenous leukemia.70 In a phase 2 study of PAH, imatinib was associated with improvement in PVR and cardiac output, although the 6MWD was not altered. A phase 3 randomized placebo-controlled study of imatinib is currently underway in patients with PAH. Initial reports have shown that at 24 weeks, 6MWD, and hemodynamics (mPAP, PVR, and cardiac output) but not time to clinical worsening were significantly improved.71 The specific effect of imatinib in SSc-PAH and concern regarding potential increased incidence of subdural hematomas in patients with PAH taking imatinib are yet unresolved. Rituximab, an anti-CD20 medication that targets B-cell populations and may lower platelet-derived growth factor-specific antibodies, is also being studied within an SSc-PAH population (phase 2 trial) as a result of case reports of improved outcomes in patients with advanced SSc-PAH.72,73
As in many progressive pulmonary diseases, lung transplantation is a therapy of last resort for many patients. Unfortunately, patients with SSc are generally considered poor candidates for lung transplantation because of an increased risk of aspiration from esophageal dysmotility and renal disease from nephrotoxic immunosuppressants. Nonetheless, carefully selected patients may tolerate transplant well.74,75 Additionally, atrial septostomy may provide temporary symptomatic relief in patients who have not responded to other therapies. By creating a right-to-left intraatrial shunt, the intention of atrial septostomy is to decrease RV filling pressures to improve cardiac output and overall systemic oxygen delivery. The procedure has reported intraoperative mortality rates of 5% to 50% and creates one problem (shunt) to alleviate another (elevated filling pressures).40 The long-term benefits of atrial septostomy remain unclear because these data often are skewed by the fact that the procedure is usually used as a bridge to transplant or as a palliative measure.
Assessing Disease Progression and Clinical Efficacy
Once therapy is initiated, careful follow-up is essential. Unfortunately, guidelines in this area are lacking. Clinically, assessment of WHO FC is probably the easiest measure of therapeutic success. In randomized trials of epoprostenol, patients with PAH who remained in WHO FC III and IV had poor prognosis compared with those who improved to a lower FC.76,77 Thus, in IPAH, recommendations suggest treating patients with a goal to improve FC to I or II.40 We have adopted this practice at our institution for all PH cohorts.
Another popular method of measuring therapeutic success is the 6-min walk test. European and American consensus guidelines suggest obtaining a 6MWD at every clinic visit, and virtually all PAH trials use this as a primary end point of therapeutic success.40,46 Mathai et al78 recently evaluated 405 patients from a 16-week randomized clinical trial of taldalafil and determined that the minimal clinically important improvement in 6MWD following therapy is 33 m. Another study suggested 41 m as the threshold.79 However, as previously noted, in SSc-PAH, 6MWD as a surrogate marker for RV function may be confounded by the high prevalence of myopathy, arthropathy, and ILD.80‐83
Pro-BNP also offers some value as a longitudinal management tool. In SSc-PAH, baseline and longitudinal changes in pro-BNP levels are predictive of therapeutic response and survival outcomes and correlate well with hemodynamic, echocardiographic, and functional status measurements.84‐86 A retrospective study of almost 200 patients with PAH showed that a decrease in pro-BNP > 15% per year was associated with improved survival.87 Likewise, several studies support the notion that higher pro-BNP levels generally reflect worse outcomes.88 Conversely, a normal pro-BNP level does not exclude SSc-PAH,48 and significant within- and between-person variability in published studies limits clinical applicability of this test when used alone.
Regarding TTE, several alternative TTE-derived outcome measures have been studied, including tricuspid annular plane systolic excursion (TAPSE), the presence of pericardial effusion, the Tei index, and the diastolic eccentricity index.89‐92 With the exception of TAPSE, their utility in SSc-PAH populations remains unknown. We showed that TAPSE is a robust outcome measure for assessing SSc-PAH disease severity, with TAPSE < 1.7 cm reflecting a nearly fourfold increased risk of death compared with higher values.93 Unfortunately, almost no data exist regarding TAPSE as a longitudinal metric in SSc-PAH.
Cardiac MRI offers a clear advantage over TTE given its ability to easily visualize RV morphology, detect myocardial inflammation or scarring, and measure RV volume and ejection fractions. Its major disadvantages are cost, access, and patient tolerability. As a diagnostic technique, its role is yet undefined, but as a prognostic tool following initiation of therapy, one study showed that after 1 year of therapy in 64 patients with IPAH, differences in SV, RV volume, and LV filling patterns among patients independently predicted mortality.94 Another study by the same authors suggested that compared with 6MWD, changes in SV of at least 10 mL were clinically important after 1 year of PAH therapy.95 These results have not been evaluated in SSc-PAH. Members of our group demonstrated significant decreases in myocardial perfusion reserve affecting the right ventricle as well as the left ventricle in a population of patients with PAH largely represented by SSc-PAH. Whether decreased myocardial perfusion reserve is characteristic of SSc-PAH or, rather, associated with PAH in general needs further evaluation.96
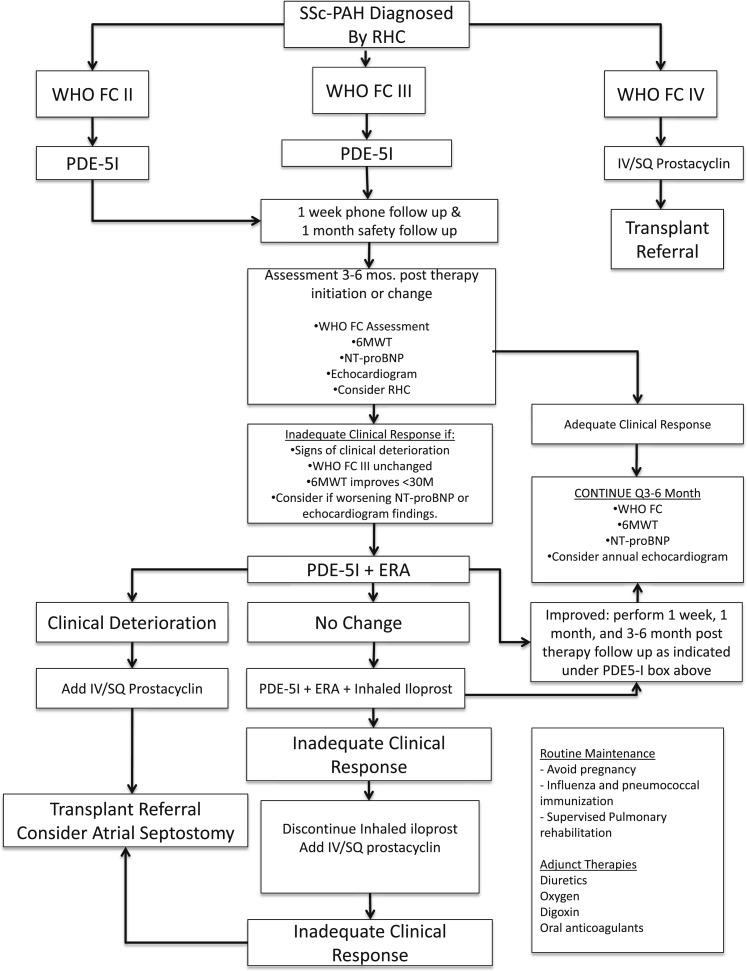
There is no single outcome measure sufficiently powerful to generate an accurate assessment of prognosis or therapeutic success. We use several outcome measures in our clinic and assess all patients at baseline, 3 to 4 months following a change in therapy, and every 6 months thereafter in stable patients. Patients who show clinical deterioration may, on an individualized basis, require more frequent assessment (Fig 1).
Figure 1.
Treatment algorithm for SSc-PAH used in the Johns Hopkins pulmonary hypertension program. 6MWT = 6-min walk test; ERA = endothelin receptor antagonist; FC = functional class; NT-proBNP = N-terminal pro-brain natriuretic peptide; PDE-5I = phosphodiesterase-5 inhibitor; RHC = right-sided heart catheterization; SQ = subcutaneous; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension; WHO = World Health Organization.
Conclusion
SSc-PAH remains a disease with high morbidity and mortality and is unique from IPAH in several ways. Despite recent advances in understanding the epidemiology, pathology, treatment, and outcomes, there remains a deep chasm between where we are and where we need to be. Incremental steps are being made. A comprehensive approach to this disease will afford a better understanding of relevant areas for further study.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Hassoun has been on scientific advisory boards for Gilead; Pfizer, Inc; Novartis Corporation; and Merck Sharp & Dohme Corp and has received research funding (REVEAL registry of patients with PAH) from Actelion Pharmaceuticals US, Inc. Dr Chaisson has reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- 6MWD
6-min walk distance
- CCB
calcium channel blocker
- CTD
connective tissue disease
- Dlco
diffusing capacity of lung for carbon monoxide
- EIPAH
exercise-induced pulmonary arterial hypertension
- ERA
endothelin receptor antagonist
- FC
functional class
- ILD
interstitial lung disease
- IPAH
idiopathic pulmonary arterial hypertension
- LV
left ventricular
- mPAP
mean pulmonary artery pressure
- PAH
pulmonary arterial hypertension
- PASP
pulmonary artery systolic pressure
- PDE-5I
phosphodiesterase type 5 inhibitor
- PFT
pulmonary function test
- PH
pulmonary hypertension
- pro-BNP
pro-brain natriuretic peptide
- PVR
peripheral vascular resistance
- RHC
right-sided heart catheterization
- RV
right ventricular
- SSc
systemic sclerosis
- SSc-PAH
systemic sclerosis-associated pulmonary arterial hypertension
- SV
stroke volume
- TAPSE
tricuspid annular plane systolic excursion
- TR
tricuspid regurgitant
- TTE
transthoracic echocardiography
- Va
alveolar volume
- WHO
World Health Organization
Footnotes
Funding/Support: Dr Chaisson is supported by the National Institutes of Health [Grant 5T32HL007534-30]. Dr Hassoun is supported by the National Institutes of Health/National Heart, Lung, and Blood Institute [Grants P50 HL084946 and R01 HL114910].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140(1):37-50 [PubMed] [Google Scholar]
- 2.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Respir J. 2007;30(6):1103-1110 [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023-1030 [DOI] [PubMed] [Google Scholar]
- 5.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54(9):3043-3050 [DOI] [PubMed] [Google Scholar]
- 6.Clements PJ, Tan M, McLaughlin VV, et al. ; Pulmonary Arterial Hypertension Quality Enhancement Research Initiative (PAH-QuERI) Investigators The pulmonary arterial hypertension quality enhancement research initiative: comparison of patients with idiopathic PAH to patients with systemic sclerosis-associated PAH. Ann Rheum Dis. 2012;71(2):249-252 [DOI] [PubMed] [Google Scholar]
- 7.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(suppl 1):S43-S54 [DOI] [PubMed] [Google Scholar]
- 8.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29(2):239-254 [DOI] [PubMed] [Google Scholar]
- 9.Allcock RJ, Forrest I, Corris PA, Crook PR, Griffiths ID. A study of the prevalence of systemic sclerosis in northeast England. Rheumatology (Oxford). 2004;43(5):596-602 [DOI] [PubMed] [Google Scholar]
- 10.Avouac J, Airò P, Meune C, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol. 2010;37(11):2290-2298 [DOI] [PubMed] [Google Scholar]
- 11.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352(9129):719-725 [DOI] [PubMed] [Google Scholar]
- 12.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376-387 [DOI] [PubMed] [Google Scholar]
- 13.Chang B, Schachna L, White B, Wigley FM, Wise RA. Natural history of mild-moderate pulmonary hypertension and the risk factors for severe pulmonary hypertension in scleroderma. J Rheumatol. 2006;33(2):269-274 [PubMed] [Google Scholar]
- 14.Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J. 2005;35(1):28-33 [DOI] [PubMed] [Google Scholar]
- 15.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48(2):516-522 [DOI] [PubMed] [Google Scholar]
- 16.Hinchcliff M, Fischer A, Schiopu E, Steen VD; PHAROS Investigators Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS): baseline characteristics and description of study population. J Rheumatol. 2011;38(10):2172-2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655-1665 [DOI] [PubMed] [Google Scholar]
- 18.Kherbeck N, Tamby MC, Bussone G, et al. The role of inflammation and autoimmunity in the pathophysiology of pulmonary arterial hypertension. Clin Rev Allergy Immunol. 2013;44(1):31-38 [DOI] [PubMed] [Google Scholar]
- 19.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989-2003 [DOI] [PubMed] [Google Scholar]
- 20.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J. 2005;26(6):1110-1118 [DOI] [PubMed] [Google Scholar]
- 21.Riemekasten G, Philippe A, Näther M, et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70(3):530-536 [DOI] [PubMed] [Google Scholar]
- 22.Negi VS, Tripathy NK, Misra R, Nityanand S. Antiendothelial cell antibodies in scleroderma correlate with severe digital ischemia and pulmonary arterial hypertension. J Rheumatol. 1998;25(3):462-466 [PubMed] [Google Scholar]
- 23.Bordron A, Dueymes M, Levy Y, et al. The binding of some human antiendothelial cell antibodies induces endothelial cell apoptosis. J Clin Invest. 1998;101(10):2029-2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamby MC, Humbert M, Guilpain P, et al. Antibodies to fibroblasts in idiopathic and scleroderma-associated pulmonary hypertension. Eur Respir J. 2006;28(4):799-807 [DOI] [PubMed] [Google Scholar]
- 25.Tamby MC, Servettaz A, Tamas N, et al. IgG from patients with systemic sclerosis bind to DNA antitopoisomerase 1 in normal human fibroblasts extracts. Biologics. 2008;2(3):583-591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez O, Sitbon O, Jaïs X, Simonneau G, Humbert M. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest. 2006;130(1):182-189 [DOI] [PubMed] [Google Scholar]
- 27.Morse J, Barst R, Horn E, Cuervo N, Deng Z, Knowles J. Pulmonary hypertension in scleroderma spectrum of disease: lack of bone morphogenetic protein receptor 2 mutations. J Rheumatol. 2002;29(11):2379-2381 [PubMed] [Google Scholar]
- 28.Austin ED, Loyd JE. Genetics and mediators in pulmonary arterial hypertension. Clin Chest Med. 2007;28(1):43-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wipff J, Kahan A, Hachulla E, et al. Association between an endoglin gene polymorphism and systemic sclerosis-related pulmonary arterial hypertension. Rheumatology (Oxford). 2007;46(4):622-625 [DOI] [PubMed] [Google Scholar]
- 30.Ryu JH, Krowka MJ, Pellikka PA, Swanson KL, McGoon MD. Pulmonary hypertension in patients with interstitial lung diseases .Mayo Clinic Proc. 2007;82(3):342-350 [DOI] [PubMed] [Google Scholar]
- 31.MacGregor AJ, Canavan R, Knight C, et al. Pulmonary hypertension in systemic sclerosis: risk factors for progression and consequences for survival. Rheumatology (Oxford). 2001;40(4):453-459 [DOI] [PubMed] [Google Scholar]
- 32.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62(11):1088-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathai SC, Hummers LK, Champion HC, et al. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum. 2009;60(2):569-577 [DOI] [PubMed] [Google Scholar]
- 34.Le Pavec J, Girgis RE, Lechtzin N, et al. Systemic sclerosis-related pulmonary hypertension associated with interstitial lung disease: impact of pulmonary arterial hypertension therapies. Arthritis Rheum. 2011;63(8):2456-2464 [DOI] [PubMed] [Google Scholar]
- 35.Boueiz A, Mathai SC, Hummers LK, Hassoun PM. Cardiac complications of systemic sclerosis: recent progress in diagnosis. Curr Opin Rheumatol. 2010;22(6):696-703 [DOI] [PubMed] [Google Scholar]
- 36.Thompson AE, Pope JE. A study of the frequency of pericardial and pleural effusions in scleroderma. Br J Rheumatol. 1998;37(12):1320-1323 [DOI] [PubMed] [Google Scholar]
- 37.de Groote P, Gressin V, Hachulla E, et al. ; ItinerAIR-Scleroderma Investigators Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis. 2008;67(1):31-36 [DOI] [PubMed] [Google Scholar]
- 38.Girgis RE, Mathai SC, Krishnan JA, Wigley FM, Hassoun PM. Long-term outcome of bosentan treatment in idiopathic pulmonary arterial hypertension and pulmonary arterial hypertension associated with the scleroderma spectrum of diseases. J Hearth Lung Transplant. 2005;24(10):1626-1631 [DOI] [PubMed] [Google Scholar]
- 39.Randone SB, Guiducci S, Cerinic MM. Musculoskeletal involvement in systemic sclerosis. Best Pract Res Clin Rheumatol. 2008;22(2):339-350 [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin VV, Archer SL, Badesch DB, et al. ; ACCF/AHA ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294 [DOI] [PubMed] [Google Scholar]
- 41.Mathai SC, Hassoun PM. Pulmonary arterial hypertension associated with systemic sclerosis. Expert Rev Respir Med. 2011;5(2):267-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campo A, Mathai SC, Le Pavec J, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182(2):252-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hachulla E, de Groote P, Gressin V, et al. ; Itinér AIR-Sclérodermie Study Group The three-year incidence of pulmonary arterial hypertension associated with systemic sclerosis in a multicenter nationwide longitudinal study in France. Arthritis Rheum. 2009;60(6):1831-1839 [DOI] [PubMed] [Google Scholar]
- 44.Hachulla E, Carpentier P, Gressin V, et al. ; ItinérAIR-Sclérodermie Study Investigators Risk factors for death and the 3-year survival of patients with systemic sclerosis: the French ItinérAIR-Sclérodermie study. Rheumatology (Oxford). 2009;48(3):304-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97(8):612-622 [DOI] [PubMed] [Google Scholar]
- 46.Gailiè N, Hoeper MM, Humbert M, et al. ; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):1219-1263 [DOI] [PubMed] [Google Scholar]
- 47.Mukerjee D, St George D, Knight C, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford). 2004;43(4):461-466 [DOI] [PubMed] [Google Scholar]
- 48.Cavagna L, Caporali R, Klersy C, et al. Comparison of brain natriuretic peptide (BNP) and NT-proBNP in screening for pulmonary arterial hypertension in patients with systemic sclerosis. J Rheumatol. 2010;37(10):2064-2070 [DOI] [PubMed] [Google Scholar]
- 49.York M, Farber HW. Pulmonary hypertension: screening and evaluation in scleroderma. Curr Opin Rheumatol. 2011;23(6):536-544 [DOI] [PubMed] [Google Scholar]
- 50.Allanore Y, Borderie D, Avouac J, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum. 2008;58(1):284-291 [DOI] [PubMed] [Google Scholar]
- 51.Schreiber BE, Valerio CJ, Keir GJ, et al. Improving the detection of pulmonary hypertension in systemic sclerosis using pulmonary function tests. Arthritis Rheum. 2011;63(11):3531-3539 [DOI] [PubMed] [Google Scholar]
- 52.Meune C, Avouac J, Airò P, et al. Prediction of pulmonary hypertension related to systemic sclerosis by an index based on simple clinical observations. Arthritis Rheum. 2011;63(9):2790-2796 [DOI] [PubMed] [Google Scholar]
- 53.McLaughlin V, Coghlan G, Denton C, et al. An evidence-based screening algorithm for pulmonary arterial hypertension in systemic sclerosis: the DETECT study [abstract]. Chest. 2012;142(4_MeetingAbstracts):809A22948592 [Google Scholar]
- 54.Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum. 2011;41(1):19-37 [DOI] [PubMed] [Google Scholar]
- 55.Alpert MA, Pressly TA, Mukerji V, et al. Acute and long-term effects of nifedipine on pulmonary and systemic hemodynamics in patients with pulmonary hypertension associated with diffuse systemic sclerosis, the CREST syndrome and mixed connective tissue disease. Am J Cardiol. 1991;68(17):1687-1691 [DOI] [PubMed] [Google Scholar]
- 56.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107(2):216-223 [DOI] [PubMed] [Google Scholar]
- 57.Mininni S, Diricatti G, Vono MC, et al. Noninvasive evaluation of right ventricle systolic pressure during dynamic exercise by saline-enhanced Doppler echocardiography in progressive systemic sclerosis. Angiology. 1996;47(5):467-474 [DOI] [PubMed] [Google Scholar]
- 58.Alkotob ML, Soltani P, Sheatt MA, et al. Reduced exercise capacity and stress-induced pulmonary hypertension in patients with scleroderma. Chest. 2006;130(1):176-181 [DOI] [PubMed] [Google Scholar]
- 59.Steen V, Chou M, Shanmugam V, Mathias M, Kuru T, Morrissey R. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest. 2008;134(1):146-151 [DOI] [PubMed] [Google Scholar]
- 60.Kovacs G, Maier R, Aberer E, et al. Pulmonary arterial hypertension therapy may be safe and effective in patients with systemic sclerosis and borderline pulmonary artery pressure. Arthritis Rheum. 2012;64(4):1257-1262 [DOI] [PubMed] [Google Scholar]
- 61.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888-894 [DOI] [PubMed] [Google Scholar]
- 62.Barst RJ, Rubin LJ, Long WA, et al. ; Primary Pulmonary Hypertension Study Group A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296-301 [DOI] [PubMed] [Google Scholar]
- 63.Badesch DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132(6):425-434 [DOI] [PubMed] [Google Scholar]
- 64.Galiè N, Ghofrani HA, Torbicki A, et al. ; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148-2157 [DOI] [PubMed] [Google Scholar]
- 65.Badesch DB, Hill NS, Burgess G, et al. ; SUPER Study Group Sildenafil for pulmonary arterial hypertension associated with connective tissue disease. J Rheumatol. 2007;34(12):2417-2422 [PubMed] [Google Scholar]
- 66.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896-903 [DOI] [PubMed] [Google Scholar]
- 67.Galié N, Badesch D, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46(3):529-535 [DOI] [PubMed] [Google Scholar]
- 68.Avouac J, Wipff J, Kahan A, Allanore Y. Effects of oral treatments on exercise capacity in systemic sclerosis related pulmonary arterial hypertension: a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67(6):808-814 [DOI] [PubMed] [Google Scholar]
- 69.Johnson SR, Brode SK, Mielniczuk LM, Granton JT. Dual therapy in IPAH and SSc-PAH. A qualitative systematic review. Respir Med. 2012;106(5):730-739 [DOI] [PubMed] [Google Scholar]
- 70.National Institutes of Health Clinical Center A 24-week randomized placebo-controlled, double-blind multi-center clinical trial evaluating the efficacy and safety of oral qti571 as an add-on therapy in the treatment of severe pulmonary arterial hypertension: Imatinib in pulmonary arterial hypertension, a randomized, efficacy study (IMPRES). NCT00902174. Bethesda, MD: National Institutes of Health; 2012. http://clinicaltrials.gov/ct2/show/NCT00902174. Accessed August 1, 2012
- 71.Hoeper M, Barst R, Galie N, et al. Imatinib in pulmonary arterial hypertension, a randomized efficacy study (IMPRES) [abstract]. Eur Respir J. 2011;38(suppl):A413 [Google Scholar]
- 72.McGonagle D, Tan AL, Madden J, et al. Successful treatment of resistant scleroderma-associated interstitial lung disease with rituximab. Rheumatology (Oxford). 2008;47(4):552-553 [DOI] [PubMed] [Google Scholar]
- 73.National Institutes of Health Clinical Center A randomized, double-blind, placebo-controlled, phase II multicenter trial of a monoclonal antibody to CD20 (rituximab) for the treatment of systemic sclerosis-associated pulmonary arterial hypertension (SSc-PAH). NCT01086540. Bethesda, MD: National Institutes of Health; 2012. http://clinicaltrials.gov/ct2/show/record/NCT01086540. Accessed December 12, 2012
- 74.Shitrit D, Amital A, Peled N, et al. Lung transplantation in patients with scleroderma: case series, review of the literature, and criteria for transplantation. Clin Transplant. 2009;23(2):178-183 [DOI] [PubMed] [Google Scholar]
- 75.Schachna L, Medsger TA, Jr, Dauber JH, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54(12):3954-3961 [DOI] [PubMed] [Google Scholar]
- 76.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780-788 [DOI] [PubMed] [Google Scholar]
- 77.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477-1482 [DOI] [PubMed] [Google Scholar]
- 78.Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(5):428-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilbert C, Brown MC, Cappelleri JC, Carlsson M, McKenna SP. Estimating a minimally important difference in pulmonary arterial hypertension following treatment with sildenafil. Chest. 2009;135(1):137-142 [DOI] [PubMed] [Google Scholar]
- 80.Impens AJ, Wangkaew S, Seibold JR. The 6-minute walk test in scleroderma—how measuring everything measures nothing. Rheumatology (Oxford). 2008;47(suppl 5):v68-v69 [DOI] [PubMed] [Google Scholar]
- 81.Buch MH, Denton CP, Furst DE, et al. Submaximal exercise testing in the assessment of interstitial lung disease secondary to systemic sclerosis: reproducibility and correlations of the 6-min walk test. Ann Rheum Dis. 2007;66(2):169-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garin MC, Highland KB, Silver RM, Strange C. Limitations to the 6-minute walk test in interstitial lung disease and pulmonary hypertension in scleroderma. J Rheumatol. 2009;36(2):330-336 [DOI] [PubMed] [Google Scholar]
- 83.Schoindre Y, Meune C, Dinh-Xuan AT, Avouac J, Kahan A, Allanore Y. Lack of specificity of the 6-minute walk test as an outcome measure for patients with systemic sclerosis. J Rheumatol. 2009;36(7):1481-1485 [DOI] [PubMed] [Google Scholar]
- 84.Mathai SC, Bueso M, Hummers LK, et al. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35(1):95-104 [DOI] [PubMed] [Google Scholar]
- 85.Williams MH, Handler CE, Akram R, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27(12):1485-1494 [DOI] [PubMed] [Google Scholar]
- 86.Simeoni S, Lippi G, Puccetti A, et al. N-terminal pro-BNP in sclerodermic patients on bosentan therapy for PAH. Rheumatol Int. 2008;28(7):657-660 [DOI] [PubMed] [Google Scholar]
- 87.Mauritz GJ, Rizopoulos D, Groepenhoff H, et al. Usefulness of serial N-terminal pro-B-type natriuretic peptide measurements for determining prognosis in patients with pulmonary arterial hypertension. Am J Cardiol. 2011;108(11):1645-1650 [DOI] [PubMed] [Google Scholar]
- 88.Denton CP, Hachulla E. Risk factors associated with pulmonary arterial hypertension in patients with systemic sclerosis and implications for screening. Eur Respir Rev. 2011;20(122):270-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yeo TC, Dujardin KS, Tei C, Mahoney DW, McGoon MD, Seward JB. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol. 1998;81(9):1157-1161 [DOI] [PubMed] [Google Scholar]
- 90.Eysmann SB, Palevsky HI, Reichek N, Hackney K, Douglas PS. Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation. 1989;80(2):353-360 [DOI] [PubMed] [Google Scholar]
- 91.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034-1041 [DOI] [PubMed] [Google Scholar]
- 92.Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39(7):1214-1219 [DOI] [PubMed] [Google Scholar]
- 93.Mathai SC, Sibley CT, Forfia PR, et al. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis-associated pulmonary arterial hypertension. J Rheumatol. 2011;38(11):2410-2418 [DOI] [PubMed] [Google Scholar]
- 94.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28(10):1250-1257 [DOI] [PubMed] [Google Scholar]
- 95.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest. 2011;139(5):1003-1009 [DOI] [PubMed] [Google Scholar]
- 96.Vogel-Claussen J, Skrok J, Shehata ML, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology. 2011;258(1):119-127 [DOI] [PMC free article] [PubMed] [Google Scholar]