Abstract
Adipogenesis is tightly regulated by altering gene expression, and TNF-α is a multifunctional cytokine that plays an important role in regulating lipogenesis. MicroRNAs are strong post-transcriptional regulators of cell differentiation. In our previous work, we found high expression of miR-181a in a fat-rich pig breed. Using bioinformatic analysis, miR-181a was identified as a potential regulator of TNF-α. Here, we validated TNF-α as the target of miR-181a by a dual luciferase assay. In response to adipogenesis, a mimic or inhibitor was used to overexpress or reduce miR-181a expression in porcine pre-adipocytes, which were then induced into mature adipocytes. Overexpression of miR-181a accelerated accumulation of lipid droplets, increased the amount of triglycerides, and repressed TNF-α protein expression, while the inhibitor had the opposite effect. At the same time, TNF-alpha rescued the increased lipogenesis by miR181a mimics. Additionally, miR-181a suppression decreased the expression of fatty synthesis associated genes PDE3B (phosphodiesterase 3B), LPL (lipoprotein lipase), PPARγ (proliferator-activated receptor-γ), GLUT1(glucose transporter), GLUT4, adiponectin and FASN (fatty acid synthase), as well as key lipolytic genes HSL (hormone-sensitive lipase) and ATGL (adipose triglyceride lipase) as revealed by quantitative real-time PCR. Our study provides the first evidence of the role of miR-181a in adipocyte differentiation by regulation of TNF-α, which may became a new therapeutic target for anti-obesity drugs.
Introduction
Adipogenesis is a key process in adipocyte development and fat metabolism. Dysfunctions in adipocyte tissues may cause health problems such as obesity and coronary artery disease, both in humans [1] and companion animals [2]. On the other hand, adipose tissues are highly related to important aspects such as meat quality and animal productivity in farm animals [3]. Therefore, understanding the mechanisms regulating adipose tissue formation would be highly beneficial to both human and animal health.
Development from progenitor mesenchymal cells into adipocytes involves dramatic changes in gene expression programs. Adipogenesis in mammals is regulated both genetically and hormonally. It has been demosntrated that adipogenic transcription factors, such as proliferator-activated receptor-γ (PPARγ), CCAAT/enhancer-binding proteins (C/EBPs), Krüppel-like factors (KLFs) and sterol regulatory element-binding protein (SREBP) are involved in the differentiation of adipocytes [4–6].
Interestingly, microRNA (miR), a class of small non-coding RNA with multiple functions in regulating gene expression by targeting mRNA associated with the RNA-induced silencing complex (RISC) [7], are increasingly recognized for their involvement in adipogenesis regulation. Some miRs differentially expressed during adipogenesis have been identified, including miR-24 [8,9], miR-31 [9] and the miR-17-92 cluster which alter cell proliferation [10]; miR-8 which represses Wnt signaling [11]; and miR-27 [12–14] and miR-130 which target PPARγ [15]. Details on miRs regulating adipogenesis have been reviewed by Romao et al. [16].
TNF-α inhibits adipocyte differentiation from pre-adipocytes and mesenchymal stem cells [17,18] by downregulating the expression of key transcription factors for adipogenesis, such as C/EBPα and PPARγ, in pre-adipocytes [19,20]. It has been suggested that TNF-α triggers activation of NF-κB through the TAK1/TAB1/NIK axis, leading to a physical association between PPAR-γ and NF-κB, thereby inhibiting the ligand-dependent PPAR-γ transactivation [21]. It is also believed that TNF-α enhances the Wnt/b-catenin signaling pathway by inducing Msx2 expression, which in turn suppresses adipocytic differentiation [22]. MiRs, such as miR-19a and miR-181 [23], have been shown to negatively regulate human TNF-α, but the regulation of TNF-α by miRs in adipocytes is still unclear. In our former study, miR-181a was shown to be significantly up-regulated in fat-rich pigs (Lantang, a local breed in China), relative to those with relatively less fat (Landrace), either in adipose tissue (Figure S1) or skeletal muscle. The results led to a hypothesis that miR-181a might play an important role in adipogenesis or adipocyte development. By using Targetscan and miRanda software, TNF-α was predicted to be a potential target for miR-181a in pigs and humans. In this study, we demonstrated the ability miR-181a to inhibit TNF-α expression by targeting the 3’ UTR of its mRNA, thus affecting adipogenesis.
Materials and Methods
Sample collection and culture of porcine primary pre-adipocytes
Subcutaneous fat tissue from a 7-day-old piglet was isolated aseptically and transferred to Dulbecco’s modified essential medium–F12 nutrient mixture, (DMEM/F12, GIBCO, New York, CA). After removing the visible connective tissues, the adipose tissue was cut into small pieces of about 1 mm3, and the subcutaneous pre-adipocytes were obtained as described in previous reports [24]. Minced tissue was transferred into a Carlsberg’s flask, digested in 0.2% type-II collagenase (1 mg/mL, GIBCO) for 2 h at 37°C and then filtered through a 150 µm mesh. Cells in the filtrate were centrifuged at 500 × g for 10 min, and erythrocytes were lysed using erythrocyte lysis buffer (0.154 MNH 4Cl, 10 mM KHCO3 and 0.1 mM EDTA). After filtering through a 40 µm mesh, cells were rinsed with F12 and centrifuged at 1500 × g for 5 min. The pre-adipocytes were collected and plated in growth medium.
Ethics Statement
All of the animal experiments were conducted in accordance with the guidelines of Guangdong Province on the Review of Welfare and Ethics of Laboratory Animals approved by the Guangdong Province Administration Office of Laboratory Animals (GPAOLA). All animal procedures were conducted under the protocol (SCAU-AEC-2010-0416) approved by the Animal Ethics Committee of South China Agricultural University.
Target prediction and plasmid construction
Using Targetscan (www.targetscan.org) and miRanda (www.microrna.org), human TNF-α was predicted to be the target of miR-181a. By homology comparison, the pig TNF-α cDNA sequence was shown to have 82% identity with the human sequence. Importantly, the predicted target site is also conserved. The sequence of porcine TNF-α 3’ UTR of about 1140 bp was amplified from the pig genome using two primers, 5’- TCTAGACAGAGTGGGTATGCCAATGC-3’ and 5’- GTTAACACCAGTAGGGCGGTTACAGAC-3’. The PCR product was digested with XbaI and HpaI and ligated to pGL3-control (Promega Co., Madison, WI) at the corresponding sites to obtain the plasmid pGL3-TNF-α-UTR. The mutant plasmid pGL3-TNF-α-UTR-muta was generated using the QuikChange Lightning site-directed mutagenesis kit (Stratagene, Santa Clara, USA) per the manufacturer’s instruction. In brief, the following primers for mutating the miR-181a binding site on the TNF-α UTR were designed (mutated nucleotides are underlined): 5’-TATTTATTTACTAGGACCGGTATTTATTCAGGAGGGCGAGG-3’ (forward) and 5’- CCTCGCCCTCCTGAATAAATACCGGTCCTAGTAAATAAATA-3’ (reverse). With pGL3-TNF-α-UTR as the template, the generated PCR product was then digested with DpnI, transformed and amplified in Escherichia coli DH5α.
Leuciferase reporter assay
CHO cells were maintained in RPMI 1640 (GIBCO) and supplemented with 10% fetal bovine serum (FBS, GIBCO). Cells were seeded in a 24-well plate one day before transfection with pGL3-TNF-α-UTR / pGL3-TNF-α-UTR-muta (500 ng), pRL-TK (Renilla luciferase normalization control, 50 ng, Promega) and miR-181a mimic (75 pM, GenePharma, Shanghai, China) using Lipofectamine 2000 ( and (Invitrogen Co., Carlsbad, CA), while a scrambled sequence as used as a negative control (NC). Cells were collected 48 h after transfection, and luciferase activity was measured using a dual luciferase reporter assay system (Promega).
Cell culture and transfections
Porcine primary pre-adipocytes or Hela cells were maintained in DMEM-F12 and DMEM, respectively, with 10% FBS and cultured in a humidified 5% CO2 incubator with a constant temperature of 37 °C. Cells were seeded in 6-well plates the day before transfection and transfected with miR-181a mimic/inhibitor (100 pM) using Lipofectamine 2000, and the scrambled sequence was used as a negative control (NC group). The cells were harvested 48 h after transfection for Western blot analysis, and the pre-adipocytes were induced to differentiate 24 h after transfection as previously reported [24]. Cells were stimulated with insulin, dexamethasone, octoic acid and octoic acid (50 nM, Sigma, USA) for eight days and collected for quantitative realtime PCR (qRT-PCR), Oil Red O staining, triglyceride (TG) assay and Western blot.
TNF-α siRNA design and transfections
SiTNF-α1(AGATTGAGGTGAAATCTTC), siTNF-α2(CTCAGATCATCGTCTCAAA), and siTNF-α3(GCCCAAGGACTCAGATCAT) were designed using siDesigner (http://sidirect2.rnai.jp/) [25] and synthesize by GenePharma(Shanghai, China). Pre-adipocytes were transfected with TNF-α siRNAs and the NC respectively, then cells was induced to differentiate as above described and collected at day eight for Oil Red O staining and TG assay.
TNF-α inhibitor and TNF-α performance
Pre-adipocytes were added TNF-α inhibitor (Santa Cruz, California), TNF-α (Pepro Tech) or DMSO(NC), respectively, then induced to differentiate. Cells were cultured with such mixture medium for six days and collected for Oil Red O staining and TG assay.
miR181a mimics and TNF-α performance
Pre-adipocytes were added miR181a mimics (100pM), TNF-α (Pepro Tech) and miR181a mimics + TNF-α, respectively, then induced to differentiate. Cells were cultured with such mixture medium for eight days and collected for Oil Red O staining and TG assay.
RNA analysis and qRT-PCR
Total RNA was extracted from differentiated adipocytes using Trizol reagent (Invitrogen), and RNA density was determined with a radiometer (Eppendorf, Hamburg, Germany). Total RNA (1 µg) was reverse-transcribed into cDNA using the M-MLV reverse transcriptase (Promega) with OligodT18. After 1 h of incubation at 42°C and 10 min of deactivation at 75°C, the reaction mixes were used as the templates for PCR. qRT-PCR was performed with standard protocols on a STRATAGENE Mx3005P sequence detection system. The PCR mixture contained 1 µL of cDNA, 10 µL of 2× SYBR Green PCR Master Mix, 1.5 µM of each primer and water to make up the final volume to 20 µL. The reaction was performed in a 96-well optical plate at 95°C for 1 min, followed by 35 cycles of 95°C for 15 s, optimal reannealing temperature for 15 s, and 72°C for 40 s. All reactions were run in duplicate, and a negative control without template was included for each gene. Primers were designed based on the sequence of each gene by using Premier 5.0 (Table S1).
MiR-181a qRT-PCR detection
Stem-loop qRT-PCR was performed as previously described [26]. A looped antisense primer (GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAACTCACC) was used for reverse transcription. The RT reaction mix was diluted to one tenth for use as the template for real-time PCR. The reaction was carried out in a 96-well optical plate at 95°C for 1 min, followed by 35 cycles of 95°C for 15 s, 56 °C for 15 s and 72°C for 25 s. The cycle threshold (Ct) was recorded, and the amount of miR-181a relative to that of U6 RNA was calculated using the expression 2-(CtmiRNA2CtU6RNA).
Oil Red O staining
Cells were rinsed with Ca2+, Mg2+-free phosphate-buffered saline (PBS) twice and fixed in 4% polyoxymethylene in PBS (w/v) for 30 min at room temperature. Oil Red O (0.5 g, Amresco Inc, solon, OH) was dissolved in isopropanol (100 ml, w/v), diluted with water (6:4, v/v) and filtered. The fixed cells were then stained with the filtered Oil Red O solution for 1 h at room temperature, washed in water and photographed.
TG assay
Cells were washed with PBS and scraped from the plates in 100 µL lysis buffer per well. After being placed on ice for 5 min, the lysate was centrifuged at 8000 × g, 4°C for 1 min. The supernatant was analyzed in a TG assay using the Food Triglyceride Assay Kit (APLLYGEN, Beijin, China) according to the manufacturer’s protocol, with a series of diluted glycerol as a standard. Total protein detected by a bicinchoninic acid (BCA) assay (Bioteke, Beijin, China) was used for normalization of TG concentration.
Western blot analysis
Cells were lysed by radio immunoprecipitation assay (RIPA) buffer with protease inhibitors. Total soluble protein was quantified using a BCA protein assay. Total protein (50 µg) was loaded onto a 10% SDS page gel, separated by electrophoresis and transferred onto a polyvinylidene difluoride (PVDF) membrane. Blots were blocked with 5% skim milk and incubated with the primary antibody overnight at 4°C, followed by incubation with the secondary antibody for 1 h at room temperature and measured with an Infrared Imaging System (LI-COR CO, Lincoln, NE). Protein expression was normalized by β-actin (Abcam, Cambridge, UK).
Statistical analysis
Results are presented as means ± SEM, and all experiments included at least six replicates per group. Data were evaluated using Student’s t-test, and differences between groups were considered statistically significant at P < 0.05. All statistical analysis was performed with SPSS 17.0 software.
Results
Target verification of miR-181a against 3’ UTR of TNF-α mRNA using a luciferase report assay
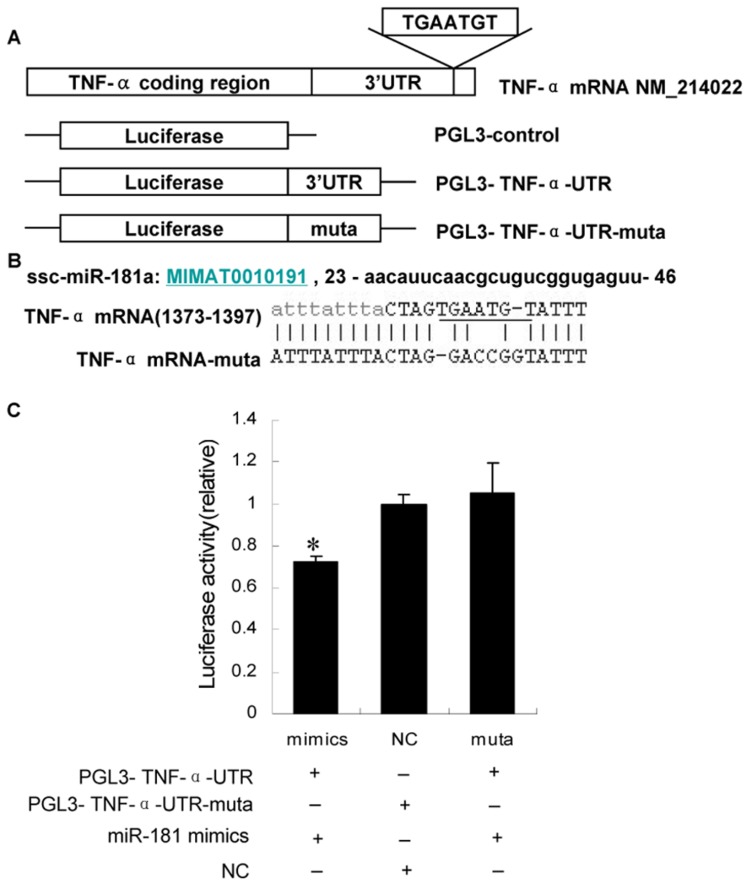
To investigate whether miR-181a (Figure 1A) has an effect on adipogenesis or adipocyte development, target genes were predicted, and miR-181a was found to directly target TNF-α through its 3’-UTR sequence. The full-length 3’ UTR of TNF-α mRNA was inserted downstream of the luciferase gene in the pGL3 reporter plasmid, and the seed sequence was also mutated to disrupt miR-181a binding (Figure 1B). The wild-type (pGL3-TNF-α-UTR) or mutated (pGL3- TNF-α-UTR-muta) plasmid was co-transfected with the miR-181a mimic into CHO cells, together with the Renilla control pRL-TK for normalization. Forty eight hours after transfection, the luciferase activity of the miR-181a group was significantly lower than that of the NC group (P < 0,05), and the reduction was rescued in the mutation group (Figure 1C). Thus, TNF-α was initially confirmed as the target of miR-181a.
Figure 1. MiR-181a directly regulates TNF-α expression via 3’ UTR sites.
(A) Schematic of TNF-α mRNA and the luciferase reporter plasmids containing the miR-181a binding sites of TNF-α mRNA. The 3’ UTR sites were inserted downstream of the luciferase reporter, as indicated. TGAATGT was the predicted target site of miR-181a. (B) MiR-181a sequences and predicted binding site between miR-181a and TNF-α mRNA. Sequence of miR-181a (www.mirbase.org) is shown. TNF-α mRNA has one putative binding site for miR-181a on the 3’ UTR. Seven nucleotides of TNF-α 3’ UTR (underlined) were replaced with GACCGGT using site-directed mutagenesis in order to disrupt the binding with miR-181a seed regions. (C) CHO cells were transfected with each of the constructed plasmids, together with miR-181a and Renilla luciferase reporter plasmid (*P < 0.05, n = 8).
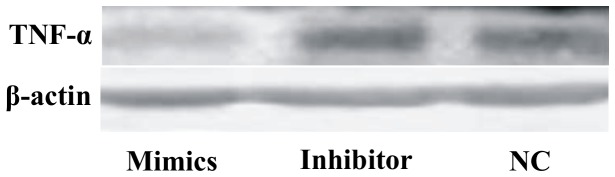
MiR-181a Suppresses TNF-α Protein Levels in Hela Cells
To further verify TNF-α as the target of miR-181a, we chose the TNF-α high expressing Hela cell line to detect whether overexpression of miR-181a could suppress the endogenous expression of TNF-α. MiR-181a mimic, inhibitor or NC was transfected into Hela cells and harvested 48 h after transfection. Western blot was performed to detect TNF-α protein levels. The miR-181a mimic suppressed the protein level of TNF-α as expected, while the inhibitor had the reverse effect (Figure 2), indicating that miR-181a indeedly targets TNF-α and regulates its endogenous expression in human cells.
Figure 2. MiR-181aa suppresses TNF-α protein level in Hela cells detected by Western blot.
Porcine adipocyte differentiation model
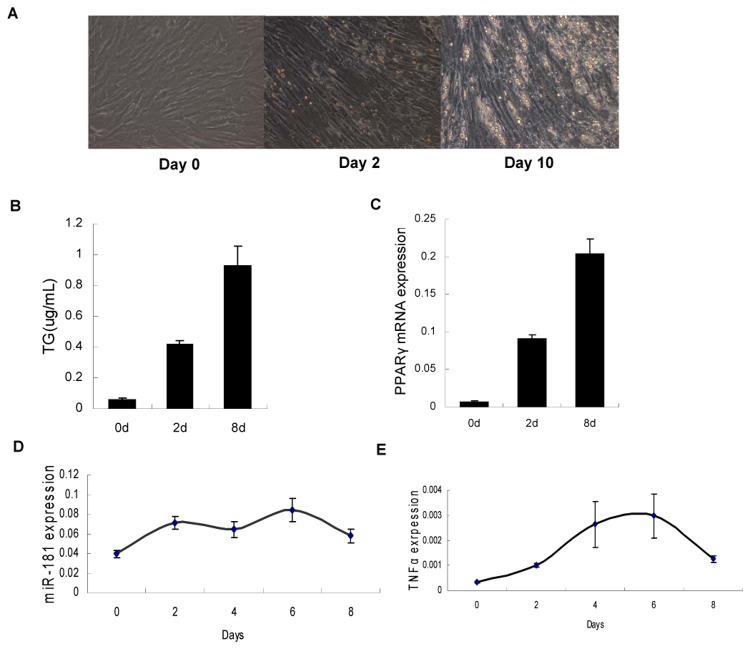
To determine the variations in miR-181a and TNF-α during the process of porcine adipogenesis, pre-adipocytes were obtained from a 7-day-old piglet and stimulated into mature adipocytes for about eight days. During this process, lipid droplets gradually accumulated (Figure 3A), while the TG concentration dramatically increased (Figure 3B). The expression of PPARγ, the classic marker of adipogenesis, also progressively increased (Figure 3C).
Figure 3. Porcine adipocyte differentiation model.
During the process of differentiation, (A) lipid droplets gradually accumulated, (B) TG concentration increased dramatically on day 2 and day 8, measured as described in Materials and Methods. (B) PPARγ expression also increased during adipocyte differentiation, measured using qRT-PCR (n = 6). (D, E) Expression profile of TNF and miR-181a during differentiation of porcine adipocytes from day 0 to day 8, also measured using qRT-PCR (n = 6).
We measured the time-dependent decline in the expression levels of miR-181a (Figure 3D) and TNF-α (Figure 3E). TNF-α expression increased in the first few days, reached a peak value on day 6 and then decreased thereafter. The expression of miR-181a showed a trend similar to that of TNF-α, indicating that it may vary according to changes in TNF-α.
Durability of miR-181a mimic in transfected porcine adipocytes
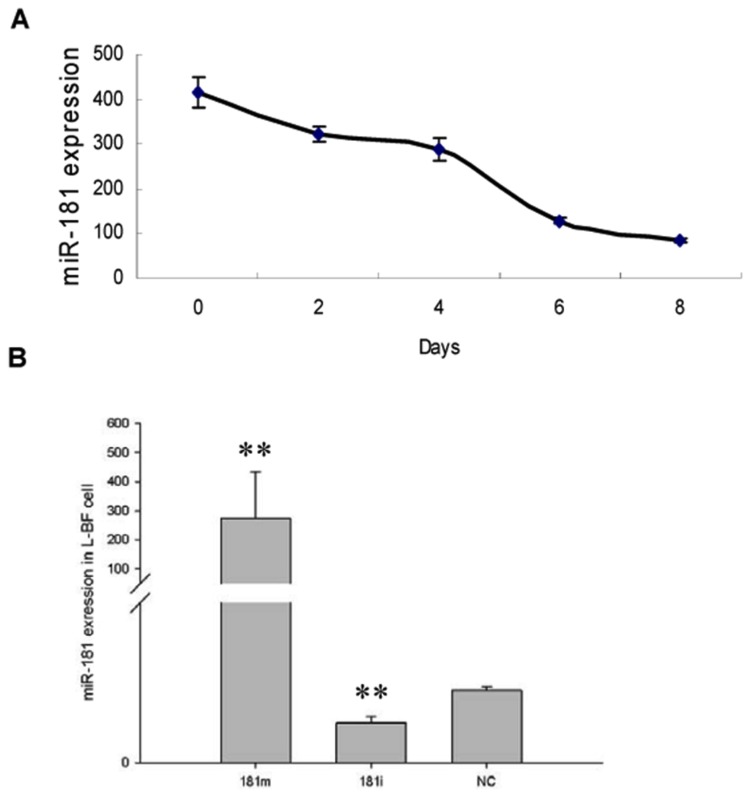
In order to ensure effectiveness of the miR-181a mimic throughout the differentiation process, we verified its persistence after transfection. MiR-181a mimic, inhibitor or NC was transfected into pre-adipocytes, which were then induced to differentiate into mature adipocytes for 8 days (Figure 4A). Cells transfected with the mimic were harvested in 2-day intervals, while the inhibitor and NC groups were collected on day 8. Although the level of miR-181a gradually decreased during differentiation (Figure 4B), it was still dramatically higher than that of the NC group (P < 0.01), and the cells transfected with the inhibitor showed a lower miR-181a level than that of the NC group (P < 0.01, Figure 4C). These results suggest that use of the miR-181a mimic is feasible and suitable for further study.
Figure 4. Level of miR-181a in porcine adipocytes after transfection of its mimic.
The miR-181a mimic was transfected using Lipofectmine 2000, and endogenous miR-181a and mimics were quantified using qRT-PCR (n = 6). (A) Concentration of measured miR-181a in porcine adipocytes declined from day 0 to day 8. (B) At day 8, the measured miR-181a concentration in the miR-181a mimic transfected group remained significantly higher vs. control (P < 0.01), while transfection of the miR-181a inhibitor resulted in a significantly lower miR-181a level vs. the control (P < 0.01), n = 6.
MiR-181a regulates adipocyte differentiation
To ascertain whether miR-181a has a direct effect on porcine adipocyte differentiation, the miR-181a mimic, inhibitor or NC was transfected into pre-adipocytes, taking TNF-α siRNA /inhibitor and TNF-α as the reference effects, and then stimulated to differentiate. After 8 days, formation of lipid droplets was observed by staining with Oil Red O. The miR-181a mimic obviously increased lipid droplets in porcine adipocytes (Figure 5A, 5C), same with the effects of TNF-α siRNA and TNF-α inhibitor (Figure 5B, 5C) and this regulation was rescued by the miR-181a inhibitor (Figure 5A) and TNF-α treatment (Figure 5B). But the inhibitor did not led to significant change of lipogenesis (Figure 5A) and TG content (Figure 5E) relative to control group. More interestingly, increased lipogenesis and TG content by miR-181a mimic dramatically diminished by TNF-α addition (Figure 5C, 5F). The degree of differentiation was also determined by measuring TG concentrations. Similar to the results of Oil Red O staining, the miR-181a mimic, TNF-α siRNA and TNF-α inhibitor could significantly increase the amount of TG (Figure 5E, 5F; *P < 0.05, **P < 0.01), and this effect was also rescued by transfection of the inhibitor and also TNF-α treatment (Figure 5E). By Western blot analysis on day 8, the miR-181a mimic decreased while the miR-181a inhibitor promoted TNF-α protein levels (Figure 5D).
Figure 5. Changes in miR-181a levels modulate adipocyte differentiation.
After transfection of protected miR-181a mimic or inhibitor, pre-adipocytes were stimulated to differentiate, taking TNF-α siRNA, TNF-α inhibitor and TNF-α as the reference or cotransfected controls. After 8 days, cells were harvested for analysis. (A) Formation of lipid droplets in the cells that transfected with miR-181a mimics and inhibitor were observed by staining with Oil Red O. (B) Formation of lipid droplets in the cells that treated with TNF-α siRNA, TNF-α inhibitor and TNF-α were observed as miR-181a treatment. (C) Formation of lipid droplets in the cells that contransfection with TNF-α were observed as miR-181a mimics treatment. (D) TNF-α protein abundance 8 days post-induction was assessed by Western blot analysis and quantified using gray scale scanning. (E,F) The degrees of differentiation in concorresponding to Figure 5B,5C treatment, respectively, were also determined by measuring the TG level, represented as the means SD, and each sample was assayed in triplicate (*P < 0.05, **P < 0.01, n=8).
Transfection of miR-181a alters expression of fat metabolism related genes
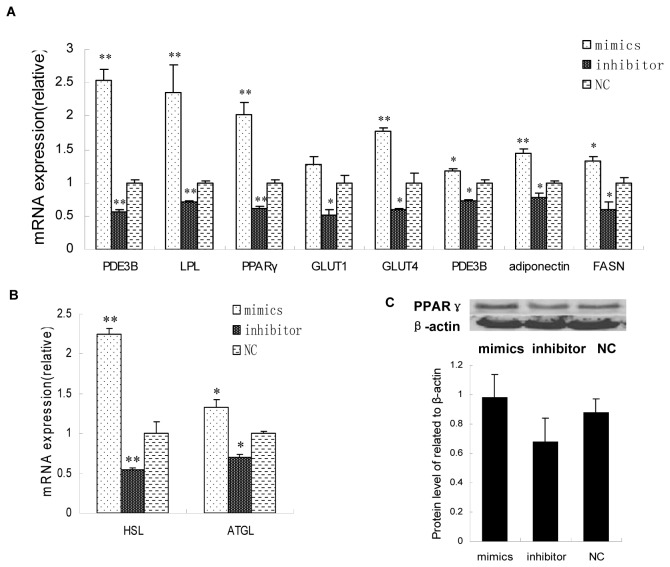
To detect alterations in expression of fat metabolism related genes after miR-181a transfection, pre-adipocytes were prepared as described above and stimulated to differentiate for 8 days. Cells were collected to detect expression of the genes and proteins using qRT-PCR and Western blot, respectively. Expression levels of the fatty synthesis related genes (PDE3B, LPL, PPARγ, GLUT1, GLUT4, adiponectin and FASN) were dramatically increased in cells after transfection of the miR-181a mimic relative to control, while they decreased significantly after transfection of the inhibitor relative to the control (Figure 6A, *P < 0.05, **P < 0.01). Interestingly, mRNA levels of the lipolysis associated genes, HSL and ATGL, also increased after miR-181a transfection, and decreased after inhibitor transfection (Figure 6B, *P < 0.05, **P < 0.01). Western blotting showed that the miR-181a increased the protein levels of PPARγ (Figure 6C). We thus demonstrated that miR-181a promoted adipogenesis by targeting TNF-α and consequently altering the expression of genes regulated by TNF-α.
Figure 6. Fat metabolism related gene expression after transfection with miR-181a mimic and inhibitor.
(A) Expression levels of PDE3B, LPL, PPARγ, GLUT1, GLUT4, adiponectin and FASN genes measured by qRT-PCR dramatically increased in cells after transfection of the miR-181a mimic vs. control, while they obviously decreased after transfection of inhibitor vs. control. Interestingly, (B) mRNA levels of HSL and ATGL increased after transfection with miR-181a and decreased after transfection with the inhibitor (*P < 0.05, **P < 0.01, n = 6). (C) Western blotting showed that the miR-181a may have increased protein levels of PPARγ.
Discussion
Adipocyte development and fat metabolism are important topics of research as dysfunctions of adipocyte tissue are associated with health problems. Some miRNAs have been reported to suppress adipocyte differentiation. For example, the miR-27 family negatively regulates adipogenesis by repressing PPARγ protein levels [12,14]. By binding to the same target, miR-130 is also a repressor as it inhibits induction of pre-adipocytes into mature adipocytes [15]. Likewise, miR-448 and miR-15a were reported as potential inhibitors of adipogenesis, by suppressing Kruppel-like factor 5 (KLF5) [27] and promoting proliferation [28], respectively, while other miRNAs can accelerate adipocyte differentiate, such as miR-103 and miR-143 [29]. Thus, a greater understanding of miRNAs involved in adipogenesis will be beneficial for the exploration of new mechanisms in adiopcyte development and its regulation.
Apart from key regulators such as PPARγ, in vivo studies have suggested that TNF-α is relevant to adipocyte metabolism, including its effects on glucose homeostasis [30], promotion of lipolysis [31] and potent inhibition of adipocyte differentiation [32,33]. The variation in TNF-α expression, peaking on day 6, during primary porcine adipocyte development suggests a role in lipogenesis.
This study demonstrates that miR-181a is a novel regulator of TNF-α in porcine adipocytes. Most miRNAs function by targeting the 3’ UTR of mRNA, although a few miRNAs have been demonstrated to regulate gene expression through the coding region of target mRNAs [8,34]. Results of our bioinformatic analysis and luciferase reporter assay showed that miR-181a also functions by targeting the 3’ UTR of TNF-α mRNA with its seed region (Figure 1C). In both Hela cells and primary porcine adipocytes tested in our study, the miR-181a mimic decreased the TNF-α protein level, which was rescued by the miR-181a inhibitor. These results provide strong evidence that miR-181a inhibits TNF-α both in human and porcine cells. Since TNF-α is believed to inhibit adipocyte differentiation (27), it follows that suppression of TNF-α should promote this process. Here we showed forced expression of miR-181a via transfection of its mimic in cultured pocine primary adipocytes resulted in increased lipid droplets and TG levels. Moreover, pre-adipocytes transfected with TNF-α siRNA or deal with TNF-α inhibitor showed the similar result of miR-181a transfection, increasing lipid droplets and TG levels (Figure 5B, 5E, 5C, 5F). Moreover, TNF-α neutralized the effect of lipogenesis caused by miR181a mimics (Figure 5C, 5F). The combined results above strongly suggested that miR181a possibally regualtes porcine lipogenesis by targeting TNF-α.
Though the miR181a inhibitor rescued the increased lipogenesis by addition of miR181a mimics, the inhibitor did not cause significant change of lipogenesis relative to control group. Similarly, the miR181a inhibitor rescued the increased lipogenesis by addition of siRNA-TNF-alpha, but the inhibitor had no statistical effect on the lipogenesis (Data not shown), There may be a risk of off-target effects because the inhibitor can sequester the miRNA without causing degradation [35], which was described by previous studies [36,37]. Therefore, measuring miRNA levels is sometimes not a reliable measure of miRNA inhibition [38].
Although several targets of miR-181a have been verified, such as the homeobox protein Hox-A11, a repressor of muscle differentiation [39], p300/CBP-associated factor (PCAF) [40] and GRP78 [41], targets related to adipocyte differentiation have not yet been reported. For the first time we demonstrated that miR-181a targeted the TNF-α genes in association with porcine adipogensis. During the preparation of this manuscript, Río et al. also verified TNF-α as the target of human miR-181c in hematopoietic progenitor cells [42].
Data on the effect of miRNAs on TNF-α during adipgenesis is limited. It has been reported that TNF-α downregulates the expression of miR-103 and miR-143 in differentiated adipocytes [29], but the direct relationships between these two miRNA and TNF-α remain unclear. Liu et al. indicated that human TNF-α is a novel target of miR-19a [23], which is consistent with our result (S2). The present study is the first to indicate that miR-181a promotes adipogenesis via targeting TNF-α.
TNF-α has been demonstrated to regulate adipocyte genes such as by downregulating expression of genes involved in the uptake and storage of FFA and glucose associated genes PPARγ, GLUT4, LPL and FASN [43], the lipolytic genes HSL [44] and ATGL [45], and the TNF-α signaling gene PDE3B. By qRT-PCR analysis, this study showed that miR-181a increased the expression of those genes listed above (Figure 6A), potentially by reducing TNF-α levels.
PPARγ was the first transcriptional factor found to target TNF-α signaling in adipocytes and plays a key role in promoting adipogenesis. It was proposed that TNF-α inhibits PPARγ by activating NFκB through the NIK-TAK1/TAB1-mediated cascade [21]. Since PPARγ mRNA dramatically increased and its protein level also increased after miR-181a transfection, it is conceivable that the observed changes in expression of genes such as GLUT4 may be, at least partly, due to alteration of PPARγ expression. As the GLUT4 promoter includes response elements for PPARγ and is regulated by PPARγ [46], it is likely that miR-181 increased the expression of GLUT4 via a TNF-α targeting and PPARγ dependent mechanism. LPL is a triglyceride hydrolase which hydrolyses plasma lipoproteins to provide FFA for adipocyte storage and lipogenesis. It was reported that TNF-α suppresses LPL in 3T3-L1 adipocytes [44]. In this study, miR-181a was shown to increase LPL mRNA expression by inhibiting TNF-α. Nevertheless, LPL is also regulated by PPARγ, and therefore the influence of miR-181 on LPL expression by repressing TNF-α may be mediated primarily through elevation of PPARγ expression [47].
Although HSL is a regulator of stimulus-induced lipolysis [47], a previous study showed that TNF-α represses HSL mRNA expression [43]. Thus it is reasonable that miR-181a elevated HSL mRNA expression by inhibiting TNF-α in our study. ATGL, a rate-limiting enzyme that mediates basal lipolysis, is also negatively regulated by TNF-α (44), and its mRNA level was shown in our study to be upregulated by miR-181a. In addition, our results showed that inhibition of miR-181a decreased PDE3B mRNA expression by increasing TNF-α expression, which is in line with a former report that TNF-α promotes perilipin phosphorylation by decreasing the expression of PDE3B, resulting in accelerating TG hydrolysis [48].
Obesity is a worldwide health problem and is a major risk factor for chronic diseases. It is characterized by the increased number and expanded size of adipocytes [49]. It is known that obesity is associated with elevated infiltration of macrophages into adipose tissue [50,51], which are principally responsible for the increased production of adipose tissue-derived TNF-α [52]. The pig is a useful model organism for comparative studies [53]. Since we demonstrated miR-181a as a new regulator of TNF-α in porcine adipocytes, suppression of miR-181a expresssion may lead to inhibition of adipocyte differentiation. Therefore, miR-181a may be a new biomarker or new potent therapeutic target for obesity.
In summary, miR-181a regulates adipogenesis by affecting expression of TNF-α, as well as genes involved in adipogenesis. This paper contributes to our understanding of adipogenesis regulation via a miRNA and TNF-α mediated pathway.
Supporting Information
Expression of miR-181a in different breed of pigs. Total RNA were extracted from adipose tissues of Landrace and Lantang pigs (Fat-rich pigs) and subjected to miRNA sequencing and microarray.
(PDF)
The primers of fat metabolism related genes.
(DOC)
Acknowledgments
The authors are grateful to swine breeding farm of South China Agricultural University in Guangdong Province for providing collection of tissue samples.
Funding Statement
This work was supported by grants from the Chinese Key Basic Plan (No. 2009CB941600), Chinese 973 Plan (2011CB944202); the Chinese Transgenic breeding Key Project, (2014ZX08009-048B); and the National Natural Science Foundation of China (31072105). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Poulos SP, Hausman DB, Hausman GJ (2010) The development and endocrine functions of adipose tissue. Mol Cell Endocrinol 323: 20-34. doi:10.1016/j.mce.2009.12.011. PubMed: 20025936. [DOI] [PubMed] [Google Scholar]
- 2. German AJ, Ryan VH, German AC, Wood IS, Trayhurn P (2010) Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J 185: 4-9. doi:10.1016/j.tvjl.2010.04.004. PubMed: 20472476. [DOI] [PubMed] [Google Scholar]
- 3. Dodson MV, Jiang Z, Chen J, Hausman GJ, Guan le L et al. (2010) Allied industry approaches to alter intramuscular fat content and composition in beef animals. J Food Sci 75: R1-R8. doi:10.1111/j.1750-3841.2009.01382.x. PubMed: 20492190. [DOI] [PubMed] [Google Scholar]
- 4. Shao D, Lazar MA (1997) Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J Biol Chem 272: 21473-21478. doi:10.1074/jbc.272.34.21473. PubMed: 9261165. [DOI] [PubMed] [Google Scholar]
- 5. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM (2000) Transcriptional regulation of adipogenesis. Genes Dev 14: 1293-1307. PubMed: 10837022. [PubMed] [Google Scholar]
- 6. White UA, JM S (2010) Transcriptional factors that promote formation of white adipose tissue. Mol Endocrinol: Cell and Publishing House; 318: 10-14 [Google Scholar]
- 7. Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215-233. doi:10.1016/j.cell.2009.01.002. PubMed: 19167326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lal A, Kim HH, Abdelmohsen K, Kuwano Y, Pullmann R Jr. et al. (2008) p16(INK4a) translation suppressed by miR-24. PLOS ONE 3: e1864. doi:10.1371/journal.pone.0001864. PubMed: 18365017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun F, Wang J, Pan Q, Yu Y, Zhang Y et al. (2009) Characterization of function and regulation of miR-24-1 and miR-31. Biochem Biophys Res Commun 380: 660-665. doi:10.1016/j.bbrc.2009.01.161. PubMed: 19285018. [DOI] [PubMed] [Google Scholar]
- 10. Wang Q, Li YC, Wang J, Kong J, Qi Y et al. (2008) miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci U S A 105: 2889-2894. doi:10.1073/pnas.0800178105. PubMed: 18287052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kennell JA, Gerin I, MacDougald OA, Cadigan KM (2008) The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc Natl Acad Sci USA 105: 15417-15422. doi:10.1073/pnas.0807763105. PubMed: 18824696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C et al. (2009) microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun 390: 247-251. doi:10.1016/j.bbrc.2009.09.098. PubMed: 19800867. [DOI] [PubMed] [Google Scholar]
- 13. Kim SY, Kim AY, Lee HW, Son YH, Lee GY et al. (2010) miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun 392: 323-328. doi:10.1016/j.bbrc.2010.01.012. PubMed: 20060380. [DOI] [PubMed] [Google Scholar]
- 14. Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z (2009) A role of miR-27 in the regulation of adipogenesis. FEBS J 276: 2348-2358. doi:10.1111/j.1742-4658.2009.06967.x. PubMed: 19348006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM et al. (2010) miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol 31: 626-638. PubMed: 21135128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romao JM, Jin W, Dodson MV, Hausman GJ, Moore SS et al. (2011) MicroRNA regulation in mammalian adipogenesis. Exp Biol Med (Maywood) 236: 997-1004. doi:10.1258/ebm.2011.011101. PubMed: 21844119. [DOI] [PubMed] [Google Scholar]
- 17. Xu H, Sethi JK, Hotamisligil GS (1999) Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J Biol Chem 274: 26287-26295. doi:10.1074/jbc.274.37.26287. PubMed: 10473584. [DOI] [PubMed] [Google Scholar]
- 18. Takada I, Kouzmenko AP, Kato S (2009) Molecular switching of osteoblastogenesis versus adipogenesis: implications for targeted therapies. Expert Opin Ther Targets 13: 593-603. doi:10.1517/14728220902915310. PubMed: 19397478. [DOI] [PubMed] [Google Scholar]
- 19. Xing H, Northrop JP, Grove JR, Kilpatrick KE, Su JL et al. (1997) TNF alpha-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARgamma without effects on Pref-1 expression. Endocrinology 138: 2776-2783. doi:10.1210/en.138.7.2776. PubMed: 9202217. [DOI] [PubMed] [Google Scholar]
- 20. Loftus TM, Lane MD (1997) Modulating the transcriptional control of adipogenesis. Curr Opin Genet Dev 7: 603-608. doi:10.1016/S0959-437X(97)80006-8. PubMed: 9388775. [DOI] [PubMed] [Google Scholar]
- 21. Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S et al. (2003) Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol 5: 224-230. doi:10.1038/ncb942. PubMed: 12598905. [DOI] [PubMed] [Google Scholar]
- 22. Qadir AS, Lee HL, Baek KH, Park HJ, Woo KM et al. (2011) Msx2 is required for TNF-alpha-induced canonical Wnt signaling in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 408: 399-404. doi:10.1016/j.bbrc.2011.04.029. PubMed: 21514273. [DOI] [PubMed] [Google Scholar]
- 23. Liu M, Wang Z, Yang S, Zhang W, He S et al. (2011) TNF-α is a novel target of miR-19a. Int J Oncol 38: 1013-1022. PubMed: 21271217. [DOI] [PubMed] [Google Scholar]
- 24. Zhou G, Wang S, Wang Z, Zhu X, Shu G et al. (2010) Global comparison of gene expression profiles between intramuscular and subcutaneous adipocytes of neonatal landrace pig using microarray. Meat Sci 86: 440-450. doi:10.1016/j.meatsci.2010.05.031. PubMed: 20573458. [DOI] [PubMed] [Google Scholar]
- 25. Naito Y, Yamada T, Ui-Tei K, Morishita S, Saigo K (2004) siDirect: highly effective, target-specific siRNA design software for mammalian RNA interference. Nucleic Acids Res 32: W124-W129. doi:10.1093/nar/gkh442. PubMed: 15215364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH et al. (2005) Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33: e179. doi:10.1093/nar/gni178. PubMed: 16314309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinoshita M, Ono K, Horie T, Nagao K, Nishi H et al. (2010) Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol Endocrinol 24: 1978-1987. doi:10.1210/me.2010-0054. PubMed: 20719859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andersen DC, Jensen CH, Schneider M, Nossent AY, Eskildsen T et al. (2010) MicroRNA-15a fine-tunes the level of Delta-like 1 homolog (DLK1) in proliferating 3T3-L1 preadipocytes. Exp Cell Res 316: 1681-1691. doi:10.1016/j.yexcr.2010.04.002. PubMed: 20385127. [DOI] [PubMed] [Google Scholar]
- 29. Xie H, Lim B, Lodish HF (2009) MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 58: 1050-1057. doi:10.2337/db08-1299. PubMed: 19188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stephens JM, Pekala PH (1991) Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem 266: 21839-21845. PubMed: 1939208. [PubMed] [Google Scholar]
- 31. Kawakami M, Murase T, Ogawa H, Ishibashi S, Mori N et al. (1987) Human recombinant TNF suppresses lipoprotein lipase activity and stimulates lipolysis in 3T3-L1 cells. J Biochem 101: 331-338. PubMed: 3495531. [DOI] [PubMed] [Google Scholar]
- 32. Beutler B, Greenwald D, Hulmes JD, Chang M, Pan YC et al. (1985) Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 316: 552-554. doi:10.1038/316552a0. PubMed: 2993897. [DOI] [PubMed] [Google Scholar]
- 33. Torti FM, Dieckmann B, Beutler B, Cerami A, Ringold GM (1985) A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science 229: 867-869. doi:10.1126/science.3839597. PubMed: 3839597. [DOI] [PubMed] [Google Scholar]
- 34. Eulalio A, Huntzinger E, Izaurralde E (2008) Getting to the root of miRNA-mediated gene silencing. Cell 132: 9-14. doi:10.1016/j.cell.2007.12.024. PubMed: 18191211. [DOI] [PubMed] [Google Scholar]
- 35. Esau C, Davis S, Murray SF, Yu XX, Pandey SK et al. (2006) miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 3: 87-98. doi:10.1016/j.cmet.2006.01.005. PubMed: 16459310. [DOI] [PubMed] [Google Scholar]
- 36. Xie YF, Shu R, Jiang SY, Liu DL, Ni J et al. (2013) MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J Inflamm (Lond) 10: 20. doi:10.1186/1476-9255-10-20. PubMed: 23680172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S (2012). nhib of MicroRNA Funct By antimiR Oligonucleotides Silence 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Z (2009) MicroRNA interference technologies: 7 Anti-miRNA Antisense Oligonucleotides Technology 460 Springer Verlag; pp. 127-143. [Google Scholar]
- 39. Naguibneva I, Ameyar-Zazoua M, Polesskaya A, Ait-Si-Ali S, Groisman R, et al (2006) The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat Cell Biol 8: 278-284. doi:10.1038/ncb1373. PubMed: 16489342. [DOI] [PubMed] [Google Scholar]
- 40. Zhao J, Gong AY, Zhou R, Liu J, Eischeid AN et al. (2012) Downregulation of PCAF by miR-181a/b provides feedback regulation to TNF-alpha-induced transcription of proinflammatory genes in liver epithelial cells. J Immunol 188: 1266-1274. doi:10.4049/jimmunol.1101976. PubMed: 22219331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX et al. (2012) miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis 45: 555-563. doi:10.1016/j.nbd.2011.09.012. PubMed: 21983159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rio P, Agirre X, Garate L, Banos R, Alvarez L et al. (2012) Downregulated expression of hsa-miR-181c in Fanconi anemia patients: implications in TNFalpha regulation and proliferation of hematopoietic progenitor cells. Blood. [DOI] [PubMed] [Google Scholar]
- 43. Ruan H, Miles PD, Ladd CM, Ross K, Golub TR et al. (2002) Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 51: 3176-3188. doi:10.2337/diabetes.51.11.3176. PubMed: 12401708. [DOI] [PubMed] [Google Scholar]
- 44. Ruan H (2003) Troglitazone Antagonizes Tumor Necrosis Factor- -induced Reprogramming of Adipocyte Gene Expression by Inhibiting the Transcriptional Regulatory Functions of NF-B. J Biol Chem 278: 28181-28192. doi:10.1074/jbc.M303141200. PubMed: 12732648. [DOI] [PubMed] [Google Scholar]
- 45. Kralisch S, Klein J, Lossner U, Bluher M, Paschke R et al. (2005) Isoproterenol, TNFalpha, and insulin downregulate adipose triglyceride lipase in 3T3-L1 adipocytes. Mol Cell Endocrinol 240: 43-49. doi:10.1016/j.mce.2005.06.002. PubMed: 16009485. [DOI] [PubMed] [Google Scholar]
- 46. Jain RG, Phelps KD, Pekala PH (1999) Tumor necrosis factor-alpha initiated signal transduction in 3T3-L1 adipocytes. J Cell Physiol 179: 58-66. doi:10.1002/(SICI)1097-4652(199904)179:1. PubMed: 10082133. [DOI] [PubMed] [Google Scholar]
- 47. Cawthorn WP, Sethi JK (2008) TNF-α and adipocyte biology. FEBS Lett 582: 117-131. doi:10.1016/j.febslet.2007.11.051. PubMed: 18037376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS (2002) Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 51: 2929-2935. doi:10.2337/diabetes.51.10.2929. PubMed: 12351429. [DOI] [PubMed] [Google Scholar]
- 49. Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885-896. doi:10.1038/nrm2066. PubMed: 17139329. [DOI] [PubMed] [Google Scholar]
- 50. Coenen KR, Gruen ML, Chait A, Hasty AH (2007) Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes 56: 564-573. doi:10.2337/db06-1375. PubMed: 17327423. [DOI] [PubMed] [Google Scholar]
- 51. Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175-184. doi:10.1172/JCI29881. PubMed: 17200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL et al. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796-1808. doi:10.1172/JCI19246. PubMed: 14679176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lunney JK (2007) Advances in swine biomedical model genomics. Int J Biol Sciences 3: 179-184. PubMed: 17384736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of miR-181a in different breed of pigs. Total RNA were extracted from adipose tissues of Landrace and Lantang pigs (Fat-rich pigs) and subjected to miRNA sequencing and microarray.
(PDF)
The primers of fat metabolism related genes.
(DOC)