Abstract
Intervertebral disc (IVD) degeneration is closely associated with low back pain (LBP), which is a major health concern in the U.S. Cellular biosynthesis of extracellular matrix (ECM), which is important for maintaining tissue integrity and preventing tissue degeneration, is an energy demanding process. Due to impaired nutrient support in avascular IVD, adenosine triphosphate (ATP) supply could be a limiting factor for maintaining normal ECM synthesis. Therefore, the objective of this study was to investigate the energy metabolism in the annulus fibrosus (AF) and nucleus pulposus (NP) of porcine IVD under static and dynamic compressions. Under compression, pH decreased and the contents of lactate and ATP increased significantly in both AF and NP regions, suggesting that compression can promote ATP production via glycolysis and reduce pH by increasing lactate accumulation. A high level of extracellular ATP content was detected in the NP region and regulated by compressive loading. Since ATP can serve not only as an intra-cellular energy currency, but also as a regulator of a variety of cellular activities extracellularly through the purinergic signaling pathway, our findings suggest that compression-mediated ATP metabolism could be a novel mechanobiological pathway for regulating IVD metabolism.
Keywords: IVD, mechanical loading, ATP, lactate, energy metabolism
Low back pain (LBP) is a major health concern and economic burden in the U.S.1,2 While the exact causes of LBP remain unknown, LBP is closely associated with intervertebral disc (IVD) degeneration.3,4 Aging, nutrition supply, mechanical factors, and other aspects contribute to disc degeneration. However, the pathology of disc degeneration is not yet determined.5–7
The IVD is a strong yet deformable tissue that lies between vertebrae and is important for the whole spine structure. Cells only form 1% of the disc volume, but they play crucial roles in maintaining disc integrity and health by producing extracellular matrix (ECM) components and the chemicals responsible for breaking down the matrix.8 These anabolic and catabolic processes are highly energy demanding.9–11 Most cellular activities are driven by adenosine triphosphate (ATP) as an energy source that is generated via glycolysis and mitochondrial respiration with the consumption of glucose and oxygen. Since the IVD is the largest avascular cartilaginous tissue in the human body, its nutrient supply relies mainly on diffusion, an inefficient transport mechanism that can be hindered by factors12 such as endplate calcification and change of proteoglycan content.13 Therefore, poor nutrient supply is a potential mechanism for disc degeneration. It also suggests that energy production may be a limiting factor for ECM biosynthesis in the IVD.
The IVD is subjected to a variety of mechanical loads in vivo, especially compression. Compressive loading can alter oxygen and glucose transport in the IVD and ATP production and ECM synthesis of IVD cells in vitro.14–18 ATP is also constantly released from cells, and such release is promoted by mechanical loading, including IVD cells.14,15 Extracellular ATP can regulate cell metabolism, survival, and growth through purinergic signaling pathways.19 Hydrolysis of extracellular ATP produces adenosine diphosphate (ADP) and adenosine, which can modulate diverse cellular actions via purinergic P2Y and P1 receptors, respectively.19,20 It also releases inorganic pyrophosphate (PPi) and phosphate (Pi), which are strongly associated with mineral crystal formation or tissue calcification.21,22 Due to the avascular nature of the IVD, extracellular accumulation of ATP and its derivate can be expected within the disc and influence the biological function of the IVD.
Our recent studies showed that compressive loading can promote ATP production and release of IVD cells in a 3D agarose gel model, suggesting compression intrinsically affects energy metabolism of IVD cells.14,15 However, the in vivo environment involves the cell-ECM interaction, a variety of mechanical events (e.g., tensile/compressive stress and hydrostatic pressure), compression-dependent solute transport, and inhomogeneous distribution of nutrients.17,23 Therefore, the objective of this study was to investigate the in situ energy metabolism of the annulus fibrosus (AF) and nucleus pulposus (NP) in porcine IVD under static and dynamic compression.
MATERIALS AND METHODS
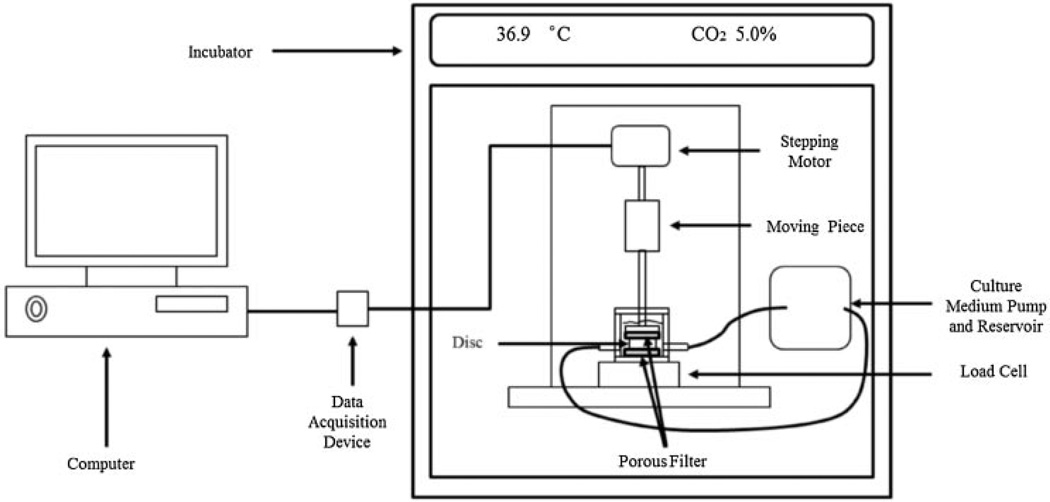
Lumbar spines of 4- to 8-month-old mature pigs (~250 lbs) were obtained within 2 h of euthanasia. Functional spinal units (FSUs) were isolated by making parallel transverse cuts through the vertebrae. The bone part was removed until the cartilaginous endplate was exposed. The FSUs were placed in custom made compression chambers (Fig. 1) and cultured in high glucose (4.5 g/L) Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen Corp., Carlsbad, CA) containing 10% fetal bovine serum (FBS, Invitrogen Corp., Carlsbad, CA) and 1% antibiotic–antimycotic (Invitrogen Corp.) in an incubator at 37°C overnight. The medium was continuously circulated at 0.37 ml/min, which is about twice the rate of blood flow to the disc in vivo.24 For whole disc compression, the disc height was measured at three locations around the disc. The discs were divided into three groups: control, static compression, and dynamic compression. A loading system consisting of a stepper motor (Moog Animatics, Santa Clara, CA) and a high accuracy low profile load cell (OMEGA Engineering, Inc., Stamford, CT) was developed to apply compression to the IVD (Fig. 1). For static compression, a 10% compressive strain was applied to the disc for either 1 or 2 h. For dynamic compression, the disc was subjected to a sinusoidal compression with a magnitude of 10% strain at the frequency of either 0.1 or 1 Hz for 1 h. One hour static compression group was considered 1 h 0 Hz group when the effect of loading frequency was analyzed. The control group was left undisturbed in the incubator for the duration of the experiment.
Figure 1.
Bioreactor system for whole disc loading experiments.
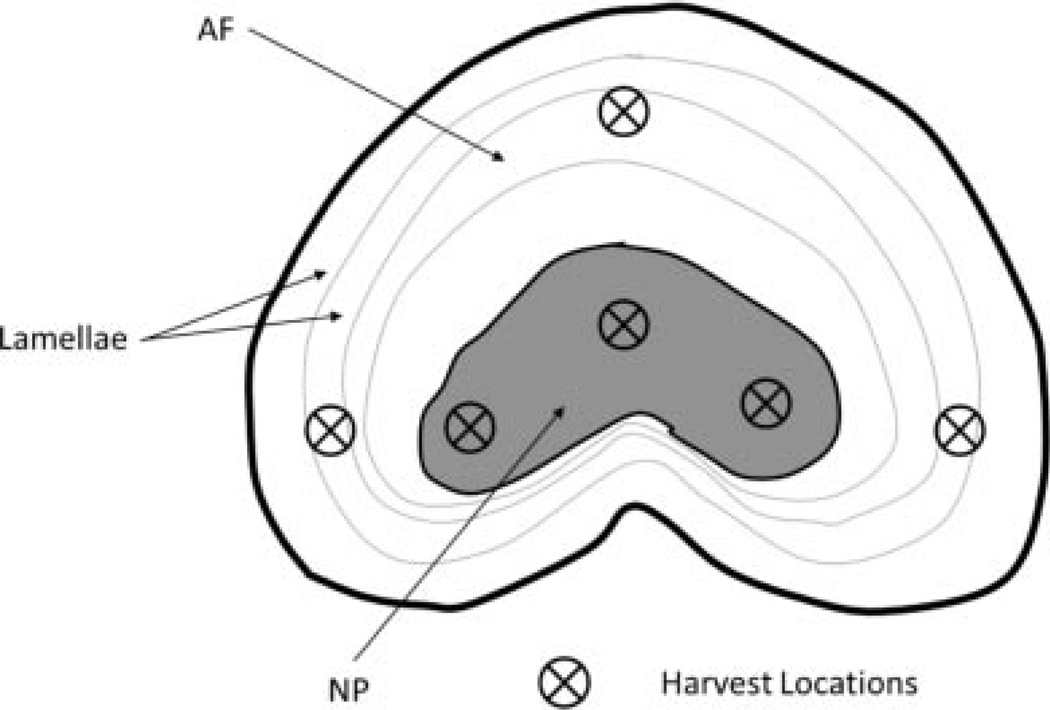
After the experiment, a transverse cut was made on each unit to expose the AF and NP. Extracellular ATP concentration and pH were measured using a custom-made optical ATP sensor25 and a pH probe (Cole-Palmar Co.; Vernon Hills, IL), respectively, at six locations (Fig. 2). The tissue samples (~10 mm3) were then harvested from the locations. The samples obtained from the AF and NP regions were used to determine the contents of lactate, ATP, and DNA. ATP and lactate were released from the tissue samples using perchloric acid treatment26 and then determined using the Luciferin-luciferase method (Sigma, St. Louis, MO) and an enzymatic assay (Sigma), respectively. The DNA content was measured based on the protocol described in our previous study.15 Cell viability was also examined using LIVE/DEAD® Cell Viability Assay (Invitrogen). Briefly, small samples obtained from AF and NP regions were incubated in PBS containing 1 µmol/L ethidium homodimer-1 and 1 µmol/ L calcein AM. Staining was visualized on an inverted fluorescent microscope with 495/515 nm excitation/emission for calcein (detecting live cells) and 495/635 nm excitation/ emission for ethidium homodimer (detecting dead cells). Image analysis was conducted to determine the number of live and dead cells. The lactate and ATP contents were normalized by DNA content. A one-way ANOVA followed by a Tukey test was performed to examine differences in ATP and lactate contents among experimental groups of each region using SPSS software (IBM, Armonk, NY). A Student’s t-test was used to examine the difference between AF and NP regions.
Figure 2.
Harvest locations of AF and NP tissue samples.
RESULTS
Effects of Static Compression
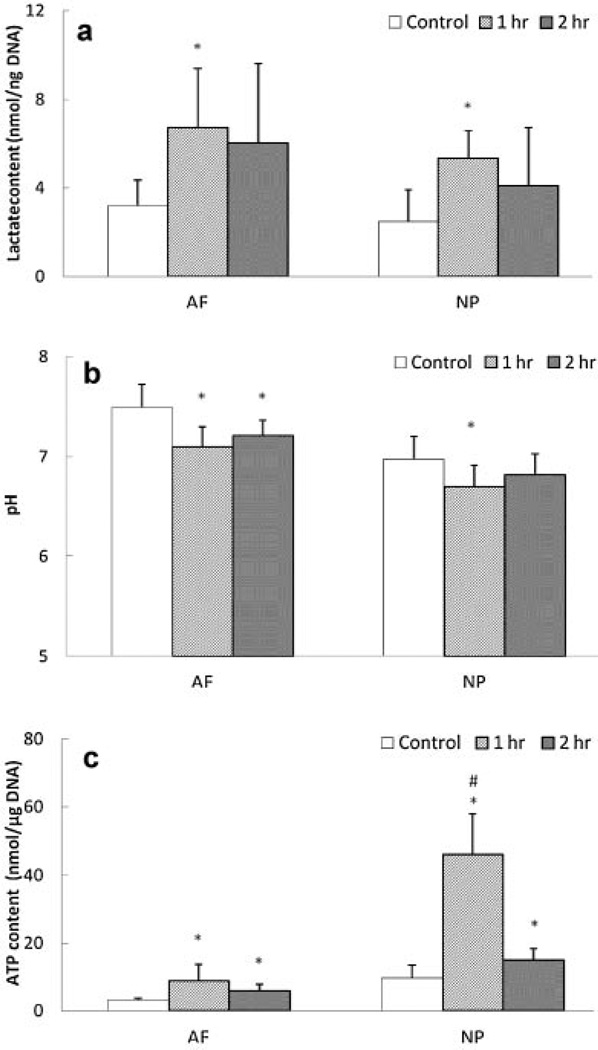
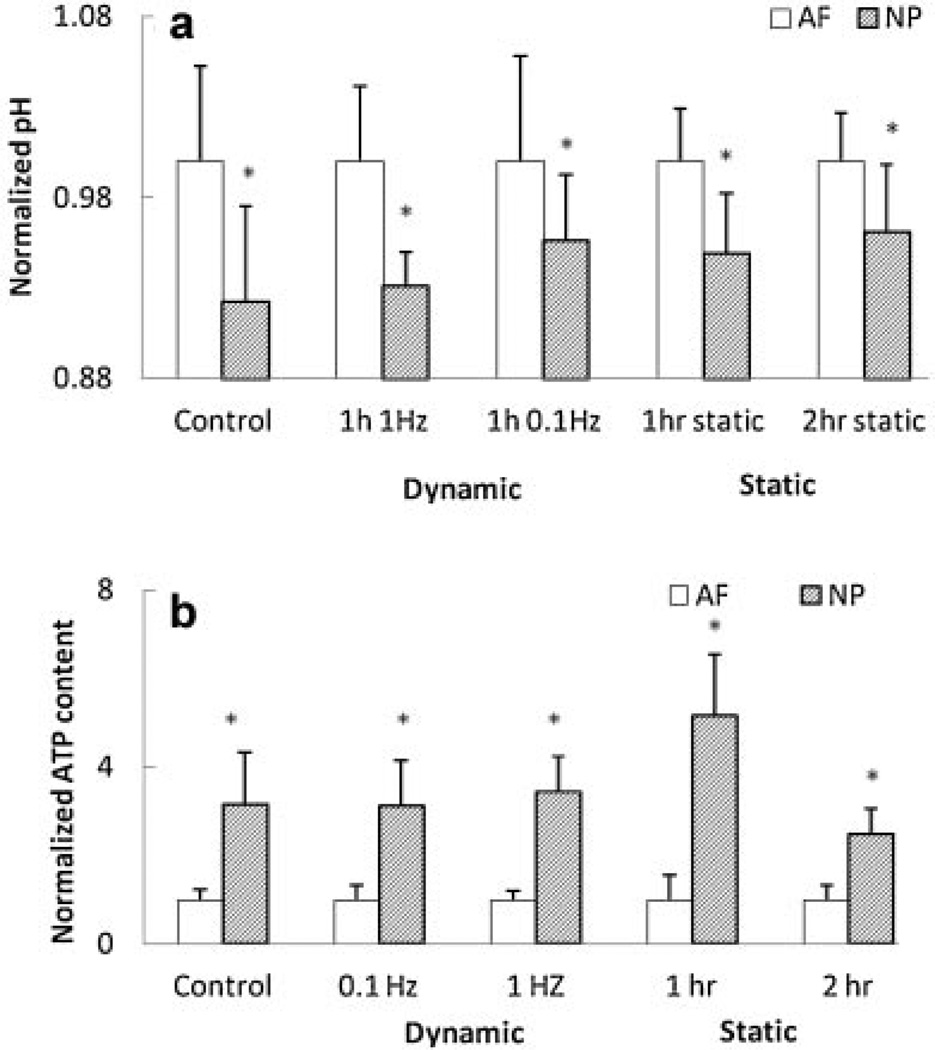
Static compression significantly increased lactate accumulation in both AF and NP regions after 1 h, whereas lactate accumulation after 2 h of static compression was reduced to a non-significant level in both regions compared to the non-loading group (Fig. 3a). Accordingly, pH was significantly decreased in AF and NP regions 1 h after static compression, whereas after 2 h of compression, a significant difference in pH was only seen in the AF region (Fig. 3b). Total ATP content in all static compression groups was significantly increased (Fig. 3c). In addition, the 1 h compression group exhibited higher ATP content than the 2 h compression group for the NP region (Fig. 3c).
Figure 3.
Effects of static compression on (a) lactate content, (b) pH, and (c) total ATP content in the AF and NP regions (lactate content: n = 6; pH: n = 7; total ATP content; n = 8). *Significantly different from the control group (p < 0.05);#significantly different from the other groups (p < 0.05).
Effects of Dynamic Compression
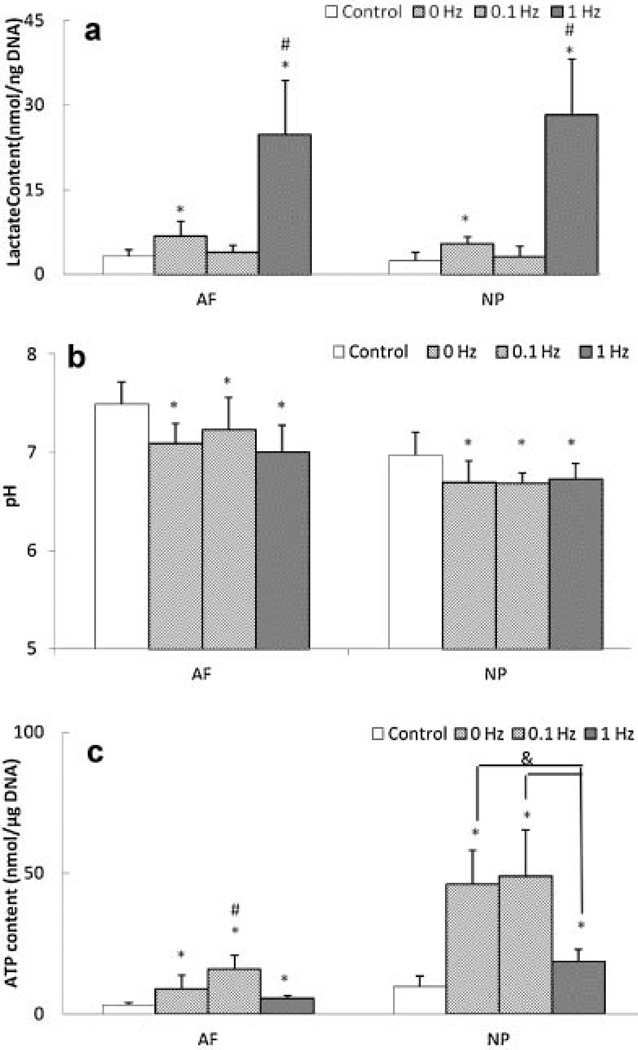
Only 1 Hz dynamic compression significantly increased lactate accumulation (Fig. 4a). However, all dynamic loading conditions significantly decreased pH and increased total ATP content in AF and NP regions (Fig. 4b and c). Also, frequency-dependent differences were found in lactate accumulation and total ATP content. Latacte accumulation was significantly higher in the 1Hz compression group than the 0.1 Hz and static (0 Hz) compression groups (Fig. 4a) for AF and NP regions. The total ATP content was significantly higher in the 0.1 Hz compression group than the 1 Hz and static (0 Hz) compression groups for the AF region, while the 1 Hz compression group exhibited significantly lower ATP content than the 0.1 Hz and static (0 Hz) compression groups for the NP region (Fig. 4c).
Figure 4.
Effects of dynamic compression on (a) lactate content, (b) pH, and (c) total ATP content in the AF and NP regions (lactate content: n = 6; pH: n = 7; total ATP content; n = 8). Static compression represents as 0 Hz. *Significantly different from the control group (p < 0.05);#significantly different from the other groups (p < 0.05);&significant difference between two groups (p < 0.05).
Comparison Between AF and NP Regions
Under all loading conditions, the NP region exhibited significantly lower pH values and higher total ATP content than the AF region (Fig. 5a and b). No significant differences were found in lactate accumulation between the AF and NP regions for all loading conditions. The live/dead cell staining showed a high cell vialibity in the IVD after compression, with AF and NP regions having cell viabilities of 88.0 ± 3.3% and 84.5 ± 2.5%, respectively.
Figure 5.
Comparison of (a) pH and (b) total ATP content between the AF and NP regions (lactate content: n = 6; pH: n = 7; total ATP content; n = 8). The measurement of the NP group was normalized by that of the AF group. *Significantly different from the AF group (p < 0.05).
Excellular ATP Content in the NP Region
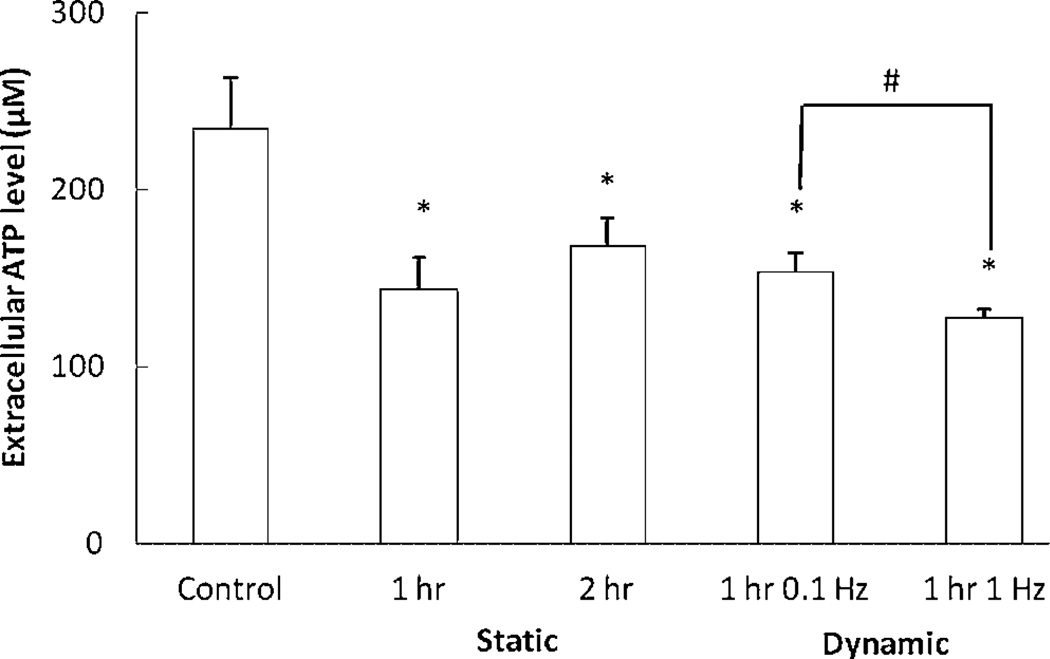
A high level of extracellular ATP content was meseared at the NP region (165.3 ± 40.8 µM). Under both static and dynamic compression, extracellular ATP content was significantly reduced in the NP region (Fig. 6). However, the extracellular ATP level in the AF region was <1 µM, the detection limit of the optical sensor.
Figure 6.
Effects of static and dynamic compression on extracellular ATP content in the NP regions (n = 5). *Significantly different from the control group (p < 0.05);#significant difference between two groups (p < 0.05).
DISCUSSION
ECM synthesis is an ATP demanding process, especially proteoglycan synthesis in which ATP serves not only as an energy source, but also as a building block in the formation of UDP-sugars and 3′-phosphoadenosine 5′-phosphosulphate (PAPS).9,11 Because of limited nutrient supply to the IVD, ATP production could be a restrictive factor for maintaining normal ECM synthesis. Furthermore, the IVD is subjected to a variety of mechanical loads. Compressive loading up-regulates ECM gene expression of IVD cells.16,27,28 However, whether up-regulation can increase ECM production may still depend on adequate ATP supply. Although our recent studies showed that static and dynamic compression promote energy production of IVD cells in an in vitro 3D agarose gel model,14,15 some in vivo factors were not taken into account, such as cell-ECM interaction and complex mechanical stimuli. Therefore, we investigated energy metabolism inside the IVD under compression. To our knowledge, this is the first study to demonstrate that mechanical compression affects in situ energy metabolism in the IVD.
Static compression siginificantly increased total ATP content and lactate accumulation in both the AF and NP regions (Fig. 3), suggesting that static compression promotes glycolysis and consequently increases ATP production in NP and AF cells. This finding is supported by our previous study in which static compression promoted ATP production of AF cells in agarose culture.15 A decrease in solute diffusivity (or tissue permeability) caused by static compression17 may also facilate extracellular lactate accumulation. Furthermore, a decrease in pH caused by static compression (Fig. 3) may result from the increase in lactate accumulation.
Similarly, 1 Hz dynamic compression significantly increased total ATP content and lactate accumulation in both the AF and NP regions (Fig. 4), concurring with our previous study and suggesting that dynamic compression can promote ATP production of AF and NP cells via glycolysis.15 Another factor for increasing ATP production could be an increase in transport of nutrients by dynamic compression.17 Higher lactate accumulation caused by dynamic compression may also result in reduction of pH (Fig. 4). Furthermore, compared to the other compression groups, significantly higher lactate accumulation found in the 1 Hz compression group (Fig. 4) suggests that more ATP may be produced in this group. However, significantly lower total ATP content found in 1 Hz dynamic compression group suggests that this group may exhibit higher cellular ATP consumption. These findings also indicate that dynamic compression of 1 Hz promotes energy-demanding cellular activities, such as ECM production.16
Many mechanical and chemical events take place in the IVD under compression. For example, fluid pressurization is one of major mechancial events, especially in the NP region, when the IVD is subjected to compressive loading.23 Hydrostatic pressure modulates cellular ECM biosythesis,29,30 and thus may affect cellular ATP metabolism. With static compression, when a constant displacement is applied to the IVD, hydrostatic pressure reaches a peak and then gradually reduces to a lower equilibrium level.23 The significant difference in total ATP content between the 1- and 2-h static compression groups in the NP region could be due to time-dependent hydrostatic pressure. The finding that static compression significantly increased total ATP content in the NP region is inconsistent with our previous study,15 which found static compression exhibited no significant effect on ATP production of NP cells in the 3D agarose model. Since little fluid pressurization occurs in agarose gel under compression due to its high hydraulic permeability,31 hydrostatic pressure induced in the IVD under static compression is a possible factor causing changes in the total ATP content. Furthermore, solute transport and hydrostatic pressure in cartilaginous tissue under dynamic loading depend on loading frequency.32 This could be the reason for the frequency-dependent differences seen in this study. Moreover, the effects of loading frequency on ATP and lactate contents found in the current study were inconsistent with that found in our previous study,15 which showed almost no effects of loading frequency on ATP and lactate contents in IVD cells. Different mechancial and chemical events induced in the agarose and tissue cultures by mechancial loading may contribute to thsese differences. Therefore, the effects of hydrostatic pressure and other potential factors on ATP metabolism of IVD cells need to be further investigated.
Since both static and dynamic compressions can promote ATP release from IVD cells,14,15 extracellular ATP accumulation should occur in the IVD under compression, especially static compression, which can reduce transport rates of solutes.17 However, we found that both static and dynamic compression significantly reduced extracellular ATP content in the NP region, suggesting that compressive loading promotes hydrolysis of ATP. In addition, high extracellular ATP concentration was detected in the NP region (~165 µM). This high extracellular level could be due to the avascular nature of the IVD and the high content of proteoglycans that can inhibit ATP hydrolysis.33 Since extracellular ATP can mediate a variety of biological responses via purinergic receptors,19 a high extracellular ATP level may influence NP metabolism. Furthermore, compressive loading may promote extracellular ATP hydrolysis, which may result in increases in the extracellular contents of adenine derivatives (i.e., ADP, AMP, and adenosine), PPi, and Pi. Since ADP and adenosine can regulate different cellular activities via P2 and P1 purinergic receptors,19,20 increases in their extracellular concentrations may also have biological effects on NP cells. Due to the capability of PPi and Pi to regulate crystal formation and tissue calcification,21,22 increases in the extracellular concentrations of PPi and Pi in the NP region may contribute to the endplate calcification that occurs in aged discs and reduces nutrient supply to the IVD.13 Taken together, our findings suggest that ATP metabolism plays an important role in maintaining normal biological function of the IVD, while compression-regulated ATP (intracellular and extracellular) metabolism may be a novel mechanobiological pathway for regulating the biological activities of the IVD.
A significantly higher ATP content found in NP than in AF cells suggests cellular metabolism in NP cells is more active. This result is consistent with our previous studies.14,15 In addition, since the concentrations of glucose and oxygen are lower in the NP region than the AF region,17 similar lactate accumulation found in the NP and AF regions suggests that NP cells maintain high ATP content by producing ATP via mitochondrial respiration, which is more efficient in ATP production than glycolysis.
In summary, we demonstrated that static and dynamic loading increased the total ATP and lactate contents in the IVD, suggesting that compression can cause an increase in ATP production by promoting glycolysis. Compression can also reduce pH in the IVD due to high lactate accumulation. Due to the ability of ATP to regulate a variety of cellular activities, high extracellular ATP content in the NP region exhibited and regulated by compression may influence the normal biological function of the IVD. Our findings suggest that compression-mediated ATP metabolism could be a novel mechanobiological pathway for regulating the biological activities of the IVD.
ACKNOWLEDGMENT
The authors thank Dr. Tai-Yi Yuan for his assistance in the IVD compression experiment.
Grant sponsor: NIH; Grant numbers: AR056101, EB008653.
Footnotes
The authors have no conflict of interests with any of the materials presented in this study.
REFERENCES
- 1.The burden of musculoskeletal diseases in the United States, AA.o.O. Surgeons. 2008:21–53. [Google Scholar]
- 2.Willis WTCDR. Estimating cost of care for patients with acute low back pain: a retrospective review of patient records. J Am Osteopath Assoc. 2009;109:229–233. [PubMed] [Google Scholar]
- 3.Luoma K, et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JPUS. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams MA, Dolan P, McNally DS. The internal mechanical functioning of intervertebral discs and articular cartilage, and its relevance to matrix biology. Matrix Biol. 2009;28:384–389. doi: 10.1016/j.matbio.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Hadjipavlou AG, et al. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. 2008;90:1261–1270. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- 7.Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. Spine J. 2008;17:480–491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibby SR, et al. The pathophysiology of the intervertebral disc. Joint Bone Spine. 2001;68:537–542. doi: 10.1016/s1297-319x(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 9.Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Im MJC, Freshwater MF, Hoopes JE. Enzyme-activities in granulation tissue—energy for collagen-synthesis. J Surg Res. 1976;20:121–125. doi: 10.1016/0022-4804(76)90108-6. [DOI] [PubMed] [Google Scholar]
- 11.Prydz K, Dalen KT. Synthesis and sorting of proteoglycans— commentary. J Cell Sci. 2000;113:193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- 12.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 13.Grunhagen T, et al. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465–477. vii. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Salvatierra JC, et al. Difference in energy metabolism of annulus fibrosus and nucleus pulposus cells of the intervertebral disc. Cell Mol Bioeng. 2011;4:302–310. doi: 10.1007/s12195-011-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernando HN, et al. Mechanical loading affects the energy metabolism of intervertebral disc cells. J Orthop Res. 2011;29:1634–1641. doi: 10.1002/jor.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korecki CL, et al. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2009;27:800–806. doi: 10.1002/jor.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CY, Gu WY. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech. 2008;41:1184–1196. doi: 10.1016/j.jbiomech.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohshima H, Urban JP, Bergel DH. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. J Orthop Res. 1995;13:22–29. doi: 10.1002/jor.1100130106. [DOI] [PubMed] [Google Scholar]
- 19.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan LM, et al. ATP-induced chondrocalcinosis. Arthritis Rheum. 1992;35:1520–1525. doi: 10.1002/art.1780351216. [DOI] [PubMed] [Google Scholar]
- 22.Johnson K, Terkeltaub R. Inorganic pyrophosphate (PPI) in pathologic calcification of articular cartilage. Front Biosci. 2005;10:988–997. doi: 10.2741/1593. [DOI] [PubMed] [Google Scholar]
- 23.Yao H, Gu WY. Three-dimensional inhomogeneous triphasic finite-element analysis of physical signals and solute transport in human intervertebral disc under axial compression. J Biomech. 2007;40:2071–2077. doi: 10.1016/j.jbiomech.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano N, et al. Analysis of rabbit intervertebral disc physiology based on water metabolism. I. Factors influencing metabolism of the normal intervertebral discs. Spine (Phila Pa 1976) 1988;13:1291–1296. doi: 10.1097/00007632-198811000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Huang CYC, Lin WC. Optical ATP biosensor for extracellular ATP measurement. Biosens Bioelectron. 2013;43:355–361. doi: 10.1016/j.bios.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leach FR. ATP determination with firefly luciferase. J Appl Biochem. 1981;3:7. [Google Scholar]
- 27.Chen J, Yan W, Setton LA. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573–583. doi: 10.1016/j.matbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 28.MacLean JL, et al. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Handa T, et al. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine (Phila Pa 1976) 1997;22:1085–1091. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara H, et al. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol. 1996;80:839–846. doi: 10.1152/jappl.1996.80.3.839. [DOI] [PubMed] [Google Scholar]
- 31.Huang CY, et al. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22:313–323. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- 32.Yao H, Gu WY. Physical signals and solute transport in cartilage under dynamic unconfined compression: finite element analysis. Ann Biomed Eng. 2004;32:380–390. doi: 10.1023/b:abme.0000017540.84764.6f. [DOI] [PubMed] [Google Scholar]
- 33.Vieira VP, et al. Heparin and chondroitin sulfate inhibit adenine nucleotide hydrolysis in liver and kidney membrane enriched fractions. Int J Biochem Cell Biol. 2001;33:1193–1201. doi: 10.1016/s1357-2725(01)00083-8. [DOI] [PubMed] [Google Scholar]