Abstract
Mass spectrometry (MS) has the unique ability to profile, in an easily accessible body tissue (peripheral blood/serum,) the sizes and relative amounts of a wide variety of biomolecules in a single platform setting. Using electrospray ionization (ESI)-MS, we distinguished individual serum from wild-type control mice from serum of mice containing an oncogenic Kras mutation, which leads to development of pancreatic ductal adenocarcinoma (PDAC) similar to that observed in humans. Identification of differences in significant ESI-MS sera mass peaks between Kras-activated mice and control mice was performed using t tests and a “nested leave one out” cross validation procedure. Peak distributions in serum of control mice from mice with Kras-mutant dependent PDAC were distinguished from those of pancreatic intraepithelial neoplasia (PanIN) lesions (p value = 0.00024). In addition, Kras mutant mice with PDAC were distinguished from Kras mutant mice with PanIN alone (p value = 0.0057). Test specificity, a measure of the false positives, was greater for the control versus Kras mutated mice, and the test sensitivity, a measure of false negatives, was greater for the PDAC versus PanIN containing mice. Receiver operator characteristic (ROC) curve discriminatory values were 0.85 for both comparisons. These studies indicate ESI-MS serum mass profiling can detect physiological changes associated with pancreatic cancer initiation and development in a GEM (genetic engineered mouse) model that mimics pancreatic cancer development in humans. Such technology has the potential to aid in early detection of pancreatic cancer, and in developing therapeutic drug interventions.
Keywords: pancreatic cancer, serum profiling, electrospray mass spectrometry, Kras mutation, genetically engineered mouse, early detection
Introduction
Approximately 35,000 individuals in the United States died of pancreatic cancer in 2011.1 Pancreatic cancer ranks 4th in mortality and 1st in 5-year mortality rate (95%).1 The high mortality rate is likely due to the lack of overt symptoms during the early more curable stages of this disease and lack of early detection procedures. Early detection of cancers, especially pancreatic cancer, is an important aspect of cancer treatment because early clinical stages (I, II) are easier to cure than later stages (III, IV).1 There is a need for robust, accurate and non-invasive detection methodology, e.g. from blood, for the early stages of pancreatic cancer.1,2 Serum protein CA-19.9, a biomarker found in peripheral blood, is used to monitor existing pancreatic cancer but is not useful in diagnosis.3 Multiple micro (mi) RNAs from plasma were shown to be indicators for pancreatic cancer, and mi-155 is possibly predictive for early-stage pancreatic neoplasia.4 However there are still some discrepancies among micro RNA technologies.6–8 The use of mass spectrometry (MS) to identify biomolecule differences and biomarkers between sera of disease-free and diseased individuals is useful in cancer diagnostics and therapeutic development.7–10
Serum profiling is the analysis of biomolecules/biomarkers and their patterns in serum which correlate with a particular disease process. The underlying hypothesis of serum mass profiling is that sera contain ample numbers and kinds of low molecular weight peptides and other biomolecules (e.g., proteins, nucleic acids, glycoconjugates, lipids), and this complexity will vary between disease states.7–10 The basis for some of this complexity involves exoprotease degradation of proteins11 and cellular signaling mechanisms,12 and is hypothesized to reflect homeostatic as well as defense/stress mechanisms which change with physiological state.13 Consequently organs/tissues shed/secret varying amounts and different kinds of biomolecules into the peripheral blood.
Liquid electrospray ionization (ESI)-MS is the simplest biomarker platform available requiring a serum dilution and injection into the mass spectrometer. Liquid MS analyzes disease-related profiles in sera, as opposed to indirect genotypic/nucleic acid classifications. ESI-MS serum mass profiling examines all biomolecules in sera, whereas other biomarker platforms (DNA, RNA and various antibody methods) focus on a single component or small groups of similar components and can require a significant amount of preparation prior to analysis. To improve specificity in disease detection, the more biomolecules analyzed at once, the greater disease discriminatory powers of the platform.13,14 Importantly, MS analysis meets the accuracy, robustness, and reproducibility guidelines for stringent clinical laboratory testing.15–17 Standard statistical approaches, like those used in this study, are better suited than novel algorithms.15,18 Mass profiling of sera and other biological fluids is presently used to catalog disease states and identify biomarker patterns in different cancers.19–21 Previously, we utilized a large number of electrospray ionization mass spectrometry (ESI-MS) peaks to distinguish sera from early-stage ovarian, lung, and pancreatic cancer patients from healthy disease-free individuals.13,22,23 We have also used ESI-MS technology to distinguish sera differences in rodents infected with Listeria pathogens or xenobiotic toxicants.24,25 MS is also useful for elucidation of companion biomarkers for drug development against various cancers.26
Rodent models for pancreatic cancer are useful for developing important early detection technology as well as aiding in identifying novel biomarkers and designing/testing therapeutics.27,28 Pancreatic cancer animal models fall into two categories, those cancers induced by chemicals29 and those induced by oncogenic mutation changes.30,31 The genetic models have advantages with respect to better mimicking human pancreatic cancer development. Most human pancreatic tumors are characterized by Kras mutations which are the most frequent and earliest genetic events of this disease. Mice harboring the Kras mutant allele (LSL-KrasG12D/+) develop the full range of premalignant lesions in the pancreas (pancreatic intraepithelial neoplasia-PanIN) and pancreatic ductal adenocarcinoma (PDAC).30,31 These mice are an excellent model mimics of human pancreatic cancer development and are useful for understanding pathobiology, the development of biomarkers of cancer inception and progression, and for development of chemo preventives and therapeutic agents to suppress the progression of PanINs to PDAC. In the present study we distinguished sera in individual mice with various stages of pancreatic cancer using this mouse-Kras mutation model. Such straight forward high resolution analyses from an accessible body fluid holds promise for future diagnostics as well as for monitoring therapeutic interventions for this deadly cancer.
Materials and Methods
Mouse handling and diet
The generation of wild type C57Bl/6 and p48Cre/+-LSL-KrasG12D/+ mice expressing activated KrasG12D oncogene is described below. All genetically engineered mouse (GEM) research was performed using animal protocols approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee. Animals were housed in ventilated cages under standardized conditions (21 °C, 60% humidity, 12-h light/12-dark cycle, 20 air changes/hour) in the University rodent barrier facility. Semi-purified modified AIN-76A diet ingredients were purchased from Bioserv, Inc., NJ. AIN-76A diets were prepared weekly and stored in the cold room. Mice were allowed ad libitum access to the diets and to automated tap water purified by reverse osmosis.
Breeding and Genotyping analysis
LSL-KrasG12D/+ and p48Cre/+ mice were maintained in a C57BL/6 heterozygous genetic background. LSL-KrasG12D/+ and p48Cre/+ mice were bred and the offspring of male activated p48Cre/+-LSL-KrasG12D/+ (25%) along with C57BL/6 mice were generated at required quantities. The genotype of each pup was confirmed by tail DNA extraction and PCR.27 Genotype-identified five week-old male mice were selected and randomized for this study so that average body weights in each group were equal (n=12/group, C57BL/6 wild-type, n=20/group p48Cre/+-LSL-KrasG12D/+ mice) and were fed with AIN-76A diet for 36 weeks until termination of the study. Mice were routinely checked for signs of weight loss or any signs of toxicity or any abnormalities. Food intake and body weight of each animal were measured once weekly for the first 6 weeks and then once a month until termination.
Histology of PanIN lesions and PDAC
At 41 weeks of age, blood samples and serum were collected by standard procedures at the time of sacrifice. Mice were euthanized by CO2 asphyxiation and necropsied; pancreata were collected and weighed. Pancreata (head to tail) required for histopathologic and IHC evaluations, to identify PanIN lesions and PDAC (Fig. 1), were fixed in neutral-buffered formalin. Normal and tumor pancreatic tissues were fixed in 10% neutral buffered formalin for 24–48 h and then processed and embedded in paraffin according to standard protocols. Tissue sections (4 µm) of each pancreas stained with H&E (Hematoxylin & Eosin) were histologically evaluated by a pathologist blinded to the experimental groups. PanIN lesions and carcinoma were classified according to histopathologic criteria.30,31 To quantify the progression of PanIN lesions, the total number of ductal lesions and their grade were determined. The relative proportion of each PanIN lesion grade to the overall number of analyzed ducts was recorded for each animal. Pancreatic carcinoma and normal appearing pancreatic tissue were evaluated for all the animals (Figs. 1,2).
Figure 1.
Gross morphology and histology of pancreas tissue from control and Kras GEM animals. Panels A and B, mouse control pancreas and pancreas with ductal adenocarcinoma (PDAC); panels C and D, mouse pancreas control and PDAC histology; panels E and F, mouse PanIN 1a and PanIN 3, range of PanIN observed in this study.
Figure 2.
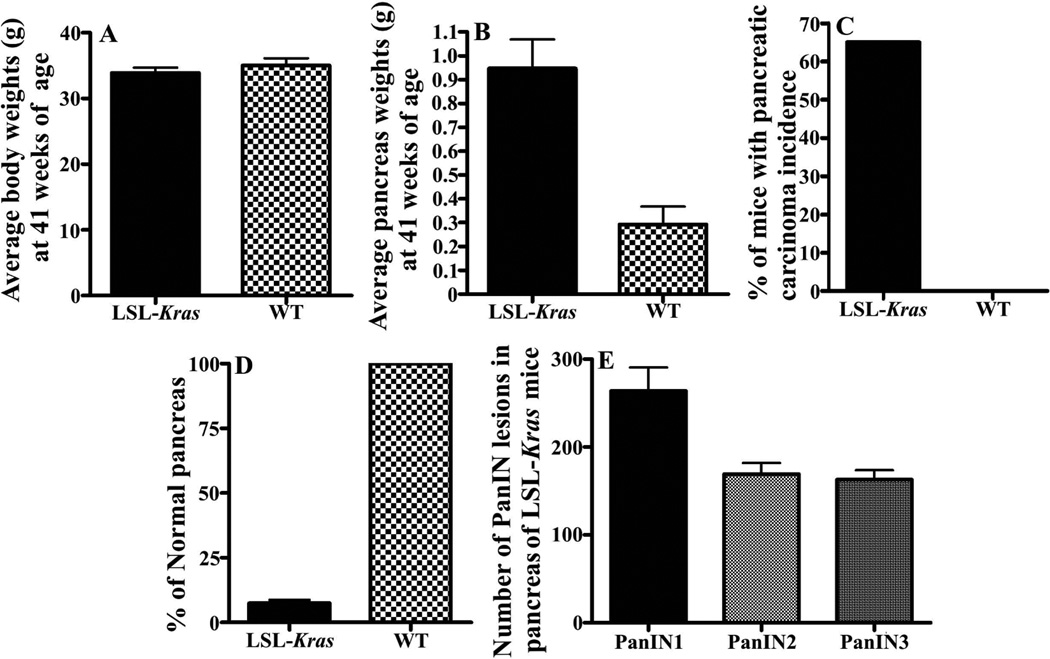
Physiological parameters associated with the Kras GEM model. Panels A and B, average body and pancreata weights for control (wild-type) and Kras mice; Panel C, % of mice with pancreatic ductal adenocarcinoma; Panel D, % normal pancreata present; Panel E, numbers of PanIN lesions by grade in Kras mice.
Electrospray mass spectrometry of sera from GEM model KrasG12D animals and wild-type controls
Serum from a mouse control or GEM KrasG12D aliquot was thawed only once, gently mixed and diluted 1 to 300 into a solution of 50% methanol and 2% formic acid. The samples were loop injected, utilizing a 20 µl stainless steel loop, into the nano source of a LCQ Advantage ion trap mass spectrometer (ThermoScientific), fitted with a 20 micron inner diameter (100 micron outer diameter) fused silica (Polymicro Technologies) tip at a flow rate of 0.5 µl/min using an Eldex MicroPro series 1000 pumping system.13 High-resolution triplicate mass spectra were collected from disease and disease-free sera in random fashion per day. The spectra were sampled at an m/Z (mass divided by charge) resolution of two hundredths over an m/Z range of 150 to 2000. Positive ion mode spectra were collected with 3 microscans utilizing a maximum inject time of 425 microseconds over 30 min for each injection. Raw spectral data from the Advantage LCQ instrument were extracted using the manufacturer's software "Qual Browser" version 1.4SR1. Spectral data were exported in a format providing rounded unit m/Z and intensity values. Data were then normalized to the highest m/Z sum intensity value in segments of 25 m/Z from 150–2000. MS spectral peak assignments were calculated as centroid m/Z¬ peak area values (valley to valley) using Mariner Data Explorer 4.0.0.1 software (Applied BioSystems). Centroid area is defined as the area of the peak calculated from its geometrical m/Z center.
Statistical and quantitative analysis
Statistical significances of individual m/Z peaks were determined between groups (mouse controls versus GEM) using Student's t-tests (one-tailed distribution, two-sample unequal variance) within a standard nested LOOCV procedure32–34. P values were considered significant if <0.05. Triplicate ESI-MS centroid m/Z peak areas from normalized MS spectra were averaged and placed into two pathological groups (e.g. PDAC or control) such that each tested sample does not play a role in the peak selection process. A peak list was created from the significant peak areas within the spectral range of 150–2000 m/Z. Pathological group assignment of each peak was dependent on the group with the greater group mean. If the group peak mean of the pancreatic cancer group was higher than the group peak mean of the control group then the m/Z peak was assigned to the pancreatic cancer group, otherwise the m/Z peak was assigned to the control group. Each m/Z peak (small m/Z range exhibited in Fig. 3A) was then tested and retained in its nested LOOCV databases to prevent possible "over-fitting" due to data complexity.35, 36 Simple filtering of m/Z peaks by t-test p-values removed non-significant peak m/Z from further comparison. Any peak m/Z with both groups having no signal was excluded from further consideration. Each m/Z peak in each “left-out” or “blinded” sample was then compared, or scored, with its respective LOOCV database of m/Z peak areas.
Figure 3.
Electrospray MS m/Z peaks distinguishing sera from mouse controls versus sera from GEM animals and model peak analysis. ESI-MS was performed as described in Materials and Methods. Panel A, mass peaks are averages from 5 individual serum samples per category. Panel B, model of electrospray MS methodology used to identify, quantify, and classify significant sera m/Z peaks into mouse pancreatic cancer (PDAC or PanIN) or control descriptors.
Scoring of each m/Z peak was accomplished by comparison of the observed triplicate mean peak area with the expected peak areas of both groups. Peak group membership assignment was accomplished by assigning the observed peak (from the left out sample) the same membership as the closest expected peak mean (simplified nearest neighbor group assignment) as in Fig.3B. That is, each tested peak is described by its group membership (PDAC: for a PDAC peak) or (control: for a control peak). Alternatively, a peak with the control mean higher than the PDAC mean would switch the locations of the labeled group membership in Fig.3B. The total number of peaks assigned to each group was then determined and % peaks identified with each specific group membership was plotted for each left-out sample. This identification ratio (%) is calculated as the percentage of identified peaks for sera from PDAC animals compared to the known maximum number of peaks in the database that could be identified for PDAC. This resulted in a percentage of total identifications for the test series which provides a pathology identity for each sample tested. Means and standard deviations (SD) of resulting LOOCV group percentage peak identifications were calculated using Excel 2010.
Test metrics
The diagnostic value of a test/procedure is defined by its sensitivity, specificity, predictive value, and efficiency.37,38 Test sensitivity was determined from TP/(TP+FN) where TP was the number of true positives for disease presence, and FN was the number of false negatives for disease presence. Specificity was calculated from TN/(TN+FP) where TN is the number of true negatives and FP is the number of false positives. Mouse PDAC disease and control TP, FP, TN, and FN values were determined using cutoffs of the average % GEM disease peaks identified minus 1.4–2 SD (Figs. 4,5).
Figure 4.
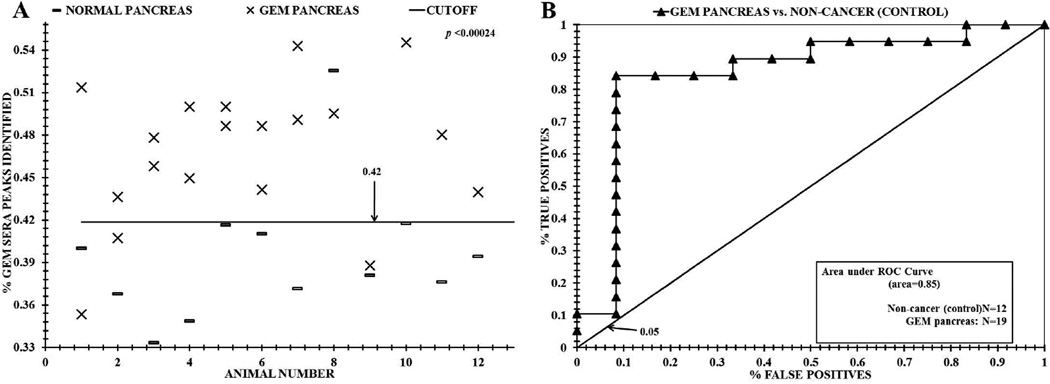
Distinguishing sera between GEM KrasG12D animals and wild-type (non-disease) mice using ESI-MS profiling. Panel A, distinguishing sera from GEM animals from control mice based on significant "% GEM sera peaks identified" using the mass peak analyses described in the Materials and Methods and Fig. 3B. Panel B, ROC area under the curve discriminating sera from wild-type mice versus GEM animals.
Figure 5.
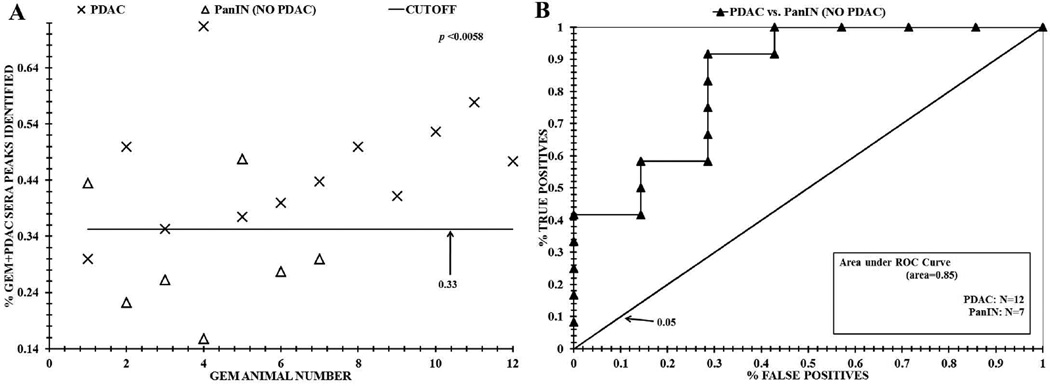
ESI-MS analysis distinguishing sera from GEM animals with PDAC and without PDAC. Panel A, distribution differences based on "% GEM+PDAC sera peaks identified" between PDAC mice and GEM animals without PDAC using the mass peak analyses described in the Materials and Methods and Fig. 3. Panel B, ROC area under the curve discriminating sera from these two cohorts.
Results
Tumor Development in GEM model of PDAC
We analyzed PDAC GEM model p48Cre/+-LSL-KrasG12D/+ rodents in which Cre recombinase activates the KrasG12D oncogene specifically in the pancreas versus the C57BL/6 mice as wild type controls. All wild type and transgenic mice had steady body weight gains. At 41 weeks of age, all mice were euthanized and evaluated for pancreas weights. We did not notice any difference in the amount of water or food consumption among wild type and transgenic mice. None of the animals exhibited any observable toxicity or any gross morphologic changes in liver, spleen, kidney or lung despite notable and significant differences in the body weights (Fig. 2A). The average pancreas of wild-type mice weighed about 0.29g, whereas the pancreas (pancreatic tumor) of transgenic mice at 41 weeks age weighed about 0.94 g (Fig. 2B, also see histology, Fig. 1, A, B ). All of the transgenic p48Cre/+-LSL-KrasG12D/+ mice developed PanIN lesions that can progress to PDAC by 41 weeks age with 100% penetrance (Fig. 1, panels E-F, PanIN 1a, 3). In this study, by 41 weeks all 20 disease GEM animals possessed extensive PanIN lesions with 12 mice developing overt PDAC versus the controls (Fig. 2C). We observed 263 PanIN 1, 169 PanIN 2 and 162 PanIN 3 lesions in male transgenic mice respectively (Fig. 2E). Only 7% of the total pancreatic tissues in the p48Cre/+-LSL-KrasG12D/+ mice appeared normal compared to 100% in the C57BL/6 wild-type mice (2D).
Distinguishing sera between GEM KrasG12D animals and wild-type (non-disease) mice using ESI MS profiling
Initial studies were performed to test the extent to which ESI-MS mass analysis could distinguish sera from GEM KrasG12D mice versus control mice. Fig. 3A exhibits an example of significant ESI-MS m/Z peaks (p < 0.05) in the 600 through 700 m/Z range (mean of 5 area values at each peak value with all spectra normalized together) that were found to differ in the sera of wild-type and GEM animals. All m/Z peaks are in whole number Dalton/charge units because of the round down method used in their compression. To obtain these significant m/Z peak data, pair-wise t-test comparisons were performed between sera from control and GEM animals. Peak differences include m/Z values 617, 640, 679 for pancreatic cancer, and 624, 631, 660 for PanIN, and 642, 683, 688 for control. Illustrated in Fig. 3B is how significant m/Z peaks like those exhibited in Fig. 3A are identified in mouse sera for construction of disease peak databases, as well as for "% PDAC peaks identified" values and assignments. Such peak identifications are used in discriminating mouse sera in Figs. 4 and 5 and in Table 1. MS peak areas in the m/Z range of 500–2000 (triplicate averaged) were identified to differ significantly between GEM/pancreatic cancer mice and control mice by comparing each of N mouse sera by t-tests. By convention (see Methods), peaks in sera of GEM/pancreatic cancer animals having higher mass peak areas than control peaks were designated as GEM peaks. Whereas, peaks in sera of control animals having higher mass peak areas than GEM/pancreatic cancer peaks were designated as control peaks. All the significant m/Z peaks in an individual serum sample were assigned a group peak identifier by examining whether a particular peak area meets the 50% threshold cutoff value or above necessary for the specific peak identification. The % peaks identified values represents the decimal percentage of peaks identified as a particular group for the tested sample.
Table-1.
| Test metrics of mouse pancreatic tumor analysis | I | ||||||
|---|---|---|---|---|---|---|---|
| Mouse Pancreas Data | Avg* (SD**) GEM |
Avg* (SD**) Normal |
TP | FP | TN | FN | N |
| LOOCV Normal vs. GEM pancreas ROC(area=0.85) |
0.47 (0.05) |
0.40 (0.05) |
16/19 (84%) |
1/12 (8%) |
11/12 (92%) |
3/19 (16%) |
GEM pancreas(N=19) Control(N=12) |
| PDAC | PanIN | ||||||
| LOOCV PDAC vs. PanIN (no PDAC) ROC(area=0.85) |
0.46 (0.11) |
0.30 (0.11) |
11/12 (92%) |
2/7 (29%) |
5/7 (71%) |
1/12 (8%) |
PDAC (N=12) PanIN(N=7) |
| II | ||||||
|---|---|---|---|---|---|---|
| Test data | Efficiency {accuracy} |
Specificity | Sensitivity | Positive Predictive Value |
Negative Predictive Value |
False Discovery Rate |
| LOOCV Normal vs. GEM pancreas | 0.87 | 0.92 | 0.84 | 0.94 | 0.79 | 0.06 |
| LOOCV PDAC vs. PanIN (no PDAC) | 0.84 | 0.71 | 0.92 | 0.85 | 0.83 | 0.15 |
Mean % peaks identified,
standard deviation
In a straight-forward discrimination problem, our ESI-MS methodology described in the Methods and in Fig. 3, readily separated mouse control serum samples from human control serum samples. Human sera samples used for this analysis contained 10 individuals (3 male, average age 53 years; 7 female, average age 52 years) with unremarkable medical histories. These two groups are separated on an individual basis by using LOOCV generated "% mouse peaks identified" present from the total number of significant peak differences in the mouse m/Z database. The individual human serum samples averaged having 24% ± 3% of the total peaks in this mouse database versus 62% ± 2% of the mouse peak database being present in the individual mouse serum samples. Significant peak numbers ranged from 195 to 215 since the LOOCV analysis yielded variable numbers due to non-identical databases being tested against peaks from the "left out serum sample". The presence of "mouse m/Z peaks" in a human serum sample is derived from those individual human peaks which happened to have an area value above the 50% threshold cutoff (Fig. 3B), which would give them the "mouse" designation.
Fig. 4A exhibits data from the ESI-MS "% GEM sera peaks identified" analysis described in Fig. 3 to distinguished sera of individual healthy control mice from individual diseased GEM animals. The data were generated using the "at least two replicates per run, two runs per day" rule of the National Committee for Clinical Laboratory Standards,39 and “nested leave one out cross validation” procedures.32–36 The mean % total of significant GEM sera peaks (Xs, Fig. 4A) from 19 mouse sera samples (54 triplicate injections averaged) was 47% of the total significant GEM sera MS peaks found in all the GEM sera samples (also exhibited in Table 1). This mean is larger than the mean of 40% of the GEM peaks (36 triplicate injections averaged for 12 control mice) found in the disease-free sera (dashes) (p value = 0.00024). Data exhibited in Fig. 4 were obtained from 99 to 120 significant ESI-MS sera m/Z peaks in the 500–2000 m/Z range. All MS data was collected and processed blindly before attempting to distinguish the data into "GEM" or "control" groups. A "cutoff” value of 0.42 assigned to separate the GEM sera % peak identified distribution from the non-diseased mouse control sera distribution. This value was obtained by subtracting two SD from the "% GEM sera peaks identified" mean, and was assigned to quantify the test metric values exhibited in Table 1. Fig. 4, panel B exhibits the ROC (receiver operator characteristic) curve analysis of these data. ROC curve analysis measures test discrimination and was performed as described.29 The area under a ROC curve (AUC) is an assessment of the discriminatory power of a comparison test, with 1.0 being perfect discrimination and 0.5 being random discrimination (indicated by the diagonal in Fig 4B & 5B). From Fig. 4B the discriminatory ROC AUC for distinguishing sera from the GEM animals from non-disease mouse sera is 0.85.
ESI-MS analysis distinguishing sera from GEM animals with PDAC and without PDAC
Further experiments were performed to test the extent to which ESI-MS analysis of sera could distinguish PDAC and non-PDAC mice. The mouse N values used in this study are 12 controls and 19 GEM animals of which 12 had PDAC and 7 did not. Fig. 4 indicated that we are able to distinguish the sera of the control versus GEM animals using our ESI-MS methodology. To possibly increase the utility of this assay, we determined whether we could distinguish sera from GEM animals with and without PDAC. Fig. 5A exhibits the % sera peaks identified from GEM/PDAC animals plotted against total number of GEM sera samples analyzed. Of note a distribution difference is evident between GEM animals with PDAC (Xs) and GEM animals without PDAC (triangles) (p value = 0.0057). This analysis was performed using “nested leave one out cross validation” (LOOCV) to remove “overfitting” of complex MS data. This resulted in a range of 14 to 23 significant peaks being used to attain this sera distinction. A "cutoff” value of 0.33 was calculated to separate the “% sera peaks identified in GEM PDAC animals” from the mouse control sera peak distribution. This value was obtained by subtracting two SD from the "% GEM sera peaks identified" mean, and was assigned to quantify the test metric values exhibited in Table 1. Fig. 5, panel B exhibits the ROC curve analysis of these data. From Fig. 5B the discriminatory ROC AUC for distinguishing sera from the GEM animals with PDAC versus animals without PDAC is 0.85.
Test metrics and predictive values for ESI-MS sera analysis of Kras G12D GEM animals and mouse controls
Table 1 summarizes the test metric values for this study, the means of the “GEM sera peaks identified” values ,standard deviations, test values (true positives, false positives, true negatives, false negatives), test specificities, sensitivities, positive/negative predictive values, ROC values, that ESI-MS analysis discriminated sera between GEM and mouse controls (Figs. 4 and 5). Such metrics use nomenclature from predictive value theory.37,38 The standard deviations (panel I) for the sera analysis for PDAC animals are smaller than those for the non-PDAC GEM animals, which was indicative of greater scatter in trying to distinguish the PDAC animals from the non-PDAC animals, and thus greater difficulty of the ESI-technology to discriminate PDAC and non-PDAC animals. This could be caused by the fewer numbers of non-PDAC animals. All these test values would likely improve if larger sample sizes (N) were analyzed for all classes. The ROC AUC values are essentially the same for discriminating controls from GEM animals and discriminating GEM PDAC animals from GEM non-PDAC animals. With respect to the test metric values in panel II, specificity and sensitivity values reflect the false positive (FP) and false negative (FN) numbers, and false discovery rate was much higher for distinguishing PDAC from non-PDAC animals versus distinguishing controls from GEM animals. This likely reflects the greater ease the ESI-MS technology has in distinguishing control animals from diseased ones versus the finer sera distinctions between PDAC and non-PDAC GEM animals.
Discussion
Early detection and prevention of pancreatic cancer is of utmost interest.1,23,27 Serum mass profiling is a promising technology for identifying biomarkers and their patterns relevant to the diagnosis, monitoring, and the understanding of a variety of disease states and their progressions at the individual level. In this study we applied our ESI-MS serum profiling technology to a GEM animal model mimicking pancreatic cancer development in humans. Mice harboring the Kras mutant allele (LSL-KrasG12D/+) develop the full range of premalignant lesions in the pancreas (pancreatic intraepithelial neoplasia-PanIN) and pancreatic ductal adenocarcinoma (PDAC) observed in humans.27,28 So far, few studies have reported on detection and prevention of pancreatic cancer progression using appropriate animal models that mimic human disease progression.30,31 Significant sera mass peak profiles from wild-type and Kras mutated mice were analyzed for differences from each other as well as differences between PDAC and non-PDAC GEM animals. We were able to distinguish these sera peak profiles from controls from PanIN lesions and PDAC using this mouse-Kras mutation model using a “nested leave one out cross validation” (LOOCV) procedure. Such straight forward analyses at acceptable resolution and accuracy from an available bodily fluid holds promise for future diagnostics as well as for monitoring therapeutic interventions for this deadly cancer in both animal models and human patients. Serum profiling of biomolecules associated with disease states like pancreatic cancer by ESI MS has several advantages over other biomarker platforms. For example, ESI-MS allows direct injection analysis of a disease phenotype in blood without potential artifacts from in vitro manipulations involving nucleic acid and enzyme amplifications. The most commonly used MS methodology for serum profiling is solid-state SELDI/MALDI (surface enhanced laser desorption ionization/matrix assisted laser desorption ionization)-MS.15,19–21 The ESI-MS approach used here has potential advantages over SELDI/MALDI-MS, because of the all-liquid handling, reduced sample manipulation, absence of grid washings, reduced chemical additives, and no involvement of random crystallization processes.
To our knowledge our ESI-MS methodology presents a new paradigm to distinguish sera from individual disease-free animals versus individual GEM animals with and without pancreatic cancer. This paradigm involves comparing mass peaks at individual m/Z values which differed significantly between two distinct sera groups, e.g., from control or pancreatic disease mice. During this process one serum sample, from either a control or a GEM animal, was “left out” of the analysis (LOOCV). Subsequently, peaks which have greater areas among the disease group were by convention designated “disease" peaks. Peaks having greater areas among control samples were designated “control” peaks. All mass peaks from the “left out” serum samples were then tested against this "disease" and “control” mass peak LOOCV database in blinded fashion. We used an arbitrary 50% area cut-off value for each significant mass peak in the "disease" and “control” database that we used to assign a particular classification. We identified each “left out” peak as either a control peak (non-pancreatic disease) if that peak area value was equal to or below the 50% value, or a GEM peak if that particular m/Z peak area value was above the 50% value. In this way, we built up a "% GEM pancreatic disease peaks identified" fraction for each sera sample (control or GEM) out of the total number of group peaks in that GEM disease peak database. Therefore, a certain % of control peaks was assigned a GEM peak classification. We then plotted this % GEM value versus sample number as exhibited in Figs. 4 and 5, and arrived at distributions for control and GEM disease which were distinguishable by ROC curves and t tests (Figures and Table 1).
Figs. 4 and 5 exhibit the distribution differences between sera analysis of the control versus GEM animals and the GEM PDAC animals versus the GEM PanIN animals, respectively. The analyses were complicated by the fact that 12 of the 19 GEM animals had PDAC and PanIN lesions and 7 GEM animals had only PanIN lesions with no observable PDAC. Thus in Fig. 4, in looking at sera from 12 control animals versus 19 GEM animals, we were not distinguishing the PDAC from the PanIN animals, just GEM from controls. For this distinction, which has a p value of 0.00024, we used the m/Z ranges of 500–550, 600– 700, and 1200–2000. At these mass ranges the PanIN animals are generally mixed in with the PDAC animals with no “% GEM peak identification” differences (not shown). However, by eliminating the 1200–2000 m/Z mass range and using a specific GEM vs. PDAC comparison we were able to distinguish the PDAC GEM animals from the GEM animals without PDAC (Fig. 5). The p value for this distinction was 0.0057 which is considerably less than for the control versus GEM animal distinction in Fig. 4. This could be due to the fewer animals used in this comparison (12 versus 7) and/or to the possibly fewer physiological changes between PDAC GEM animals and PanIN only GEM animals. It is noted that this PDAC-GEM distinction is basically between a very early-stage PDAC as only 1 in 12 PDAC animals had a lymph node metastasis.
The changes in serum mass profiles we do see here, with which we are able to distinguish, with reasonable accuracy, animals individually into their proper groups, are in accord with the basic principles of serum profiling. Namely, that disease and disease progression can be distinguished in steady state because these physiological differences cause measurable biomolecule changes in the peripheral blood due to host systemic responses, homeostasis and defense, as well as stress mechanisms. In addition, direct inputs from diseased tissues like tumors are also possible. These biomolecular changes and differences are evidenced in this study by our ability to distinguish their mass peaks and mass profile/patterns in the control, GEM, GEM+PDAC, or GEM-PDAC cohorts. At the m/Z data values being mined here, from 500 to 700 and 1200 to 2000, these ranges likely encompass the lower mass peptide “serome” which result from differential host tissue/organ exoprotease activities.11 Other biomolecules besides peptides and proteins may also be involved in the responses we observe.12 Since we are seeing fairly evident biomolecule changes as reflected in our serum profiles from apparent small inputs, for example normal pancreatic ductal cells becoming altered to PanINs, one would need to hypothesize a mechanism(s) to account for such apparent amplification of small signal(s) from minute starting inputs. Possibilities could involve “alarmin”-like molecules believed to be shed/secreted by cells which have been damaged/altered in some fashion which in turn bind to signal transduction pathway receptors to activate in a synergistic and cooperative manner more extensive innate defense responses.12 The purpose of the present study was to establish the ability of this ESI-MS technology to make these distinctions at the individual level in a valuable animal model which mimics human pancreatic cancer development. At this point in the studies we do not know the structural identities of these peaks which are yielding the discriminations presented herein. Identification of these mass peaks, at least for the ones present in significant amounts, is a logical next step utilizing tandem mass (MS/MS).8,25 The structural identification by tandem MS/MS of such distinguishing MS peaks will aid in understanding pancreatic carcinogenesis mechanisms in this animal model and in humans, and help in the identification of potential biomarkers and aid in future therapeutic applications.
With respect to the current Kras GEM animal model used in this study, the ability of our ESI-MS technology to individually discriminate the early stage progression from control to PanIN to early-stage PDAC will bode well for the use of this technology in following and monitoring pancreatic cancer drug treatments. For example, this model was previously used to demonstrate that certain drugs actually delay progression of pancreatic PanIN lesions to PDAC.27,28,30 The EGFR inhibitor gefitinib was able to inhibit the progression of PanIN lesions into PDAC as was the cholesterol lowering drug atorvastatin and nitric oxide releasing aspirin. These are important studies with clinical implications for the chemoprevention of pancreatic cancer in humans. The ability of our ESI-MS technology in the present study to be able to distinguish the important steps in the change from control pancreas to PanIN lesions to PDAC will have utility in monitoring and analyzing such therapeutic effects in both this Kras GEM animal model and, potentially, in humans, such as those at risk for pancreatic cancer. In addition, the utility of this technology for the early detection of pancreatic cancer and those neoplastic lesions with the ability to progress to pancreatic cancer is evident.
Novelty and impact.
Using a novel mass spectrometry platform, we demonstrated the ability to distinguish, in individual mice, serum changes associated with pancreatic cancer development in a mouse model dependent on a specific Kras mutation. This model mimics early-stage pancreatic cancer development in humans. We distinguished individual serum of control mice from mice with PanIN lesions and pancreatic ductal adenocarcinoma. Such technology can aid in early detection of pancreatic cancer, and in development of therapeutic interventions.
Acknowledgements
Authors thank the University of Oklahoma Health Sciences Center Rodent Barrier Facility staff, and Dr. Howard Crawford for providing Kras mice breeding pairs. The authors also thank Dr. Jane Hanas for editing the manuscript.
Grant sponsors: Oklahoma Center for the Advancement of Technology (OCAST AR11-001) to J.S.H., and National Institutes of Health/National Cancer Institute (NIH/NCI-N01-CN-53300) to C.V.R.
Abbreviations
- PDAC
pancreatic ductal adenocarcinoma
- PanIN
pancreatic intraepithelial neoplasia
- ESI-MS
electrospray ionization mass spectrometry
- GEM
genetically engineered mouse
- LOOCV
nested leave one out cross validation
- ROC
receiver operator characteristic
- SELDI/MALDI
surface enhanced laser desorption ionization/matrix assisted laser desorption ionization
- H&E
hematoxylin & eosin
Footnotes
Authors report no financial disclosure or conflict of interest.
References
- 1.American Cancer Society. Cancer facts & figures. Atlanta (GA): American Cancer Society; 2011. [Google Scholar]
- 2.Jones S, Zhang X, Parsons DW, Lin JCH, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Donahue CI, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattani AM, Mandeli J, Bruckner HW. Tumor markers in patients with pancreatic carcinoma. Cancer. 1996;78:57–62. doi: 10.1002/(SICI)1097-0142(19960701)78:1<57::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prevent Res. 2009;2:807–813. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker M. MicroRNA profiling: separating signal from noise. Nat Methods. 2010;7:687–692. doi: 10.1038/nmeth0910-687. [DOI] [PubMed] [Google Scholar]
- 6.Jayaprakash AD, Jabado O, Brown BD, Sachidanandam R. Identification and remediation of biases in the activity of RNA ligases in small-RNA deep sequencing. Nucl Acids Res. 2011;39:e141. doi: 10.1093/nar/gkr693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richter R, Knappe PS, Schrader M, Ständker L, Jürgens M, Tammen H, Forssmann WG. Composition of the peptide fraction in human blood plasma: database of circulating human peptides. J Chromatog B Biomed Sci Appl. 1999;726:25–35. doi: 10.1016/s0378-4347(99)00012-2. [DOI] [PubMed] [Google Scholar]
- 8.Hanas JS, Hocker JR, Cheung JY, Larabee JL, Lerner MR, Lightfoot SA, Morgan DL, Denson KD, Prejeant KC, Gusev Y, Smith BJ, Hanas RJ, Postier RG, Brackett DJ. Biomarker Identification in Human Pancreatic Cancer Sera. Pancreas. 2008;36:61–69. doi: 10.1097/mpa.0b013e3180d0a738. [DOI] [PubMed] [Google Scholar]
- 9.Chambers G, Lawrie L, Cash P, Murray GL. Proteomics: a new approach to the study of disease. J Pathol. 2000;192:280–288. doi: 10.1002/1096-9896(200011)192:3<280::AID-PATH748>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Verma M, Wright GL, Jr, Hanash SM, Gopal-Srivastava R, Srivastava S. Proteomic approaches within the NCI early detection research network for the discovery and identification of cancer biomarkers. Annal NY Acad Sci. 2001;945:103–115. doi: 10.1111/j.1749-6632.2001.tb03870.x. [DOI] [PubMed] [Google Scholar]
- 11.Villanueva J, Shaffer DR, Philip J, Chaparro CA, Erdjument-Bromage H, Olshen AB, Fleisher M, Lilja H, Brogi E, Boyd J, Sanchez-Carbayo M, Holland EC, Cordon-Cardo C, Scher HI, Tempst P. Differential exoprotease activities confer tumor-specific serum peptidome patterns. J Clin Invest. 2006;116:271–284. doi: 10.1172/JCI26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi ME. DAMPs, PAMPs, and alarmins: all we need to know about danger. J Leukocyte Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 13.Hocker JR, Bishop EA, Lightfoot SA, Lerner MR, Peyton MD, Brackett DJ, Hanas RJ, McMeekin DS, Walker JL, Hanas JS. Serum profiling to distinguish early-stage and late-stage ovarian cancer patients from disease-free individuals. Cancer Invest. 2012;30:189–197. doi: 10.3109/07357907.2011.636115. [DOI] [PubMed] [Google Scholar]
- 14.Hocker JR, Peyton MD, Lerner MR, Lightfoot SA, Hanas RJ, Brackett DJ, Hanas JS. Distinguishing non-small cell lung adenocarcinoma patients from squamous cell carcinoma patients and from control individuals using serum profiling. Cancer Invest. 2012;30:180–188. doi: 10.3109/07357907.2011.633294. [DOI] [PubMed] [Google Scholar]
- 15.Semmes OJ, Feng Z, Adam BL, Banez LL, Bigbee WL, Campos D, Cazares LH, Chan DW, Grizzle WE, Izbicka E, Kagan J, Malik G, McLerran D, Moul JW, Partin A, Prasanna P, Rosenzweig J, Sokoll LJ, Srivastava S, Srivastava S, Thompson I, Welsh MJ, White N, Winget M, Yasui Y, Zhang Z, Zhu L. Evaluation of serum protein profiling by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry for the detection of prostate cancer: I. Assessment of platform reproducibility. Clin Chem. 2005;51:102–112. doi: 10.1373/clinchem.2004.038950. [DOI] [PubMed] [Google Scholar]
- 16.Hortin GL. Can mass spectrometric protein profiling meet desired standards of clinical laboratory practice? Clin Chem. 2005;51:3–5. doi: 10.1373/clinchem.2004.043281. [DOI] [PubMed] [Google Scholar]
- 17.West-Nørager M, Bro R, Marini F, Høgdall EV, Høgdall CK, Nedergaard L, Heegaard NH. Feasibility of Serodiagnosis of Ovarian Cancer by Mass Spectrometry. Anal Chem. 2009;81:1907–1913. doi: 10.1021/ac802293g. [DOI] [PubMed] [Google Scholar]
- 18.Sorace JM, Zhan M. A data review and re-assessment of ovarian cancer serum proteomic profiling. BMC Bioinformat. 2003;4:24. doi: 10.1186/1471-2105-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang SY, Xiao XY, Zhang WG, Zhang LJ, Zhang W, Zhou B, Chen G and He DC. Application of serum SELDI proteomic patterns in diagnosis of lung cancer. BMC Cancer. 2005;5:1–7. doi: 10.1186/1471-2407-5-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Zhang Z, Rosenzweig J, Wang YY, Chan DW. Proteomics and bioinformatics approaches for identification of serum biomarkers to detect breast cancer. Clin Chem. 2002;481:296–304. [PubMed] [Google Scholar]
- 21.Yu Y, Chen S, Wang LS, Chen WL, Guo WJ, Yan H, Zhang WH, Peng CH, Zhang SD, Li HW, Chen GQ. Prediction of pancreatic cancer by serum biomarkers using surface-enhanced laser desorption/ionization-based decision tree classification. Oncol. 2005;68:79–86. doi: 10.1159/000084824. [DOI] [PubMed] [Google Scholar]
- 22.Hocker JR, Peyton MD, Lerner MR, Mitchell SL, Lightfoot SA, Lander TJ, Bates-Albers LM, Vu NT, Hanas RJ, Kupiec TC, Brackett DJ, Hanas JS. Serum profiling of stage I/II lung cancer patients using electrospray-ionization mass spectrometry. Lung Cancer. 2011;74:206–211. doi: 10.1016/j.lungcan.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Hocker JR, Lerner MR, Mitchell SL, Lightfoot SA, Lander TJ, Quillet A, Hanas RJ, Peyton MD, Postier RG, Brackett DJ, Hanas JS. Distinguishing early-stage pancreatic cancer patients from disease-free individuals using serum profiling. Cancer Invest. 2011;29:173–179. doi: 10.3109/07357907.2010.543214. [DOI] [PubMed] [Google Scholar]
- 24.Hocker JR, Dillon M, Drevets DD, Hanas JS. Discriminating experimental Listeria monocytogenes infections in mice using serum profiling. App Micro Biotech. 2012;96:1049–1058. doi: 10.1007/s00253-012-4392-6. [DOI] [PubMed] [Google Scholar]
- 25.Larabee JL, Hocker JR, Cheung JY, Gallucci RM, Hanas JS. Serum profiling of rat dermal exposure to JP-8 fuel reveals an acute-phase response. Toxicol Mech Meth. 2008;18:41–51. doi: 10.1080/15376510701697072. [DOI] [PubMed] [Google Scholar]
- 26.Troiani S, Uggeri M, Moll J, Isacchi A, Kalisz HM, Rusconi L, Valsasina B. Searching for biomarkers of Aurora-A kinase activity: identification of in vitro substrates through a modified KESTREL approach. J Proteome Res. 2005;4:1296–1303. doi: 10.1021/pr050018e. [DOI] [PubMed] [Google Scholar]
- 27.Mohammed A, Janakiram NB, Li Q, Madka V, Ely M, Lightfoot S, Crawford H, Steele VE, Rao CV. The epidermal growth factor receptor inhibitor gefitinib prevents the progression of pancreatic lesions to carcinoma in a conditional LSL-KrasG12D/+ transgenic mouse model. Cancer Prevent Res. 2011;3:1417–1426. doi: 10.1158/1940-6207.CAPR-10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed A, Janakiram NB, Lightfoot S, Gali H, Awasthi V, Rao CV. Early Detection and Prevention of Pancreatic Cancer: Use of Genetically Engineered Mouse Models and advanced Imaging Technologies. Current Medicinal Chemistry. 2012;19(12):3701–3713. doi: 10.2174/092986712801661095. [DOI] [PubMed] [Google Scholar]
- 29.Postier RG, Lerner MR, Lightfoot SA, Vannarath R, Wilkerson KB, Lane MM, Hanas JS, Brackett DJ. DNA ploidy and Markovian analysis of Neoplastic progression in experimental pancreatic cancer. Journal of Histochemistry and Cytochemistry. 2003;51:303–309. doi: 10.1177/002215540305100305. [DOI] [PubMed] [Google Scholar]
- 30.Mohammed A, Qian L, Janakiram NB, Lightfoot S, Steele VE, Rao CV. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48(Cre/+) LSL-Kras(G12D/+) mice. Internat J Cancer. 2012;131:1951–1962. doi: 10.1002/ijc.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao CV, Mohammed A, Janakiram NB, Li Q, Ritchie RL, Lightfoot S, Vibhudutta A, Steele VE. Inhibition of Pancreatic Intraepithelial Neoplasia Progression to Carcinoma by Nitric Oxide–Releasing Aspirin in p48Cre/+–LSL-KrasG12D/+ Mice. Neoplasia. 2012;14:778–787. doi: 10.1593/neo.121026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vapnik VN. Statistical learning theory. Ch. 10. New York: John Wiley and Sons, Inc.; 1998. [Google Scholar]
- 33.Varma S, Simon R. Bias in error estimation when using cross-validation for model selection. BMC Bioinformatics. 2006;7:91. doi: 10.1186/1471-2105-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambroise C, McLachlan GJ. Selection bias in gene extraction on the basis of microarray gene-expression data. Proc Natl Acad Sci U S A. 2002;99:6562–6566. doi: 10.1073/pnas.102102699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan W, Zhou M, Hampton CY, Benigno BB, Walker LD, Gray A, McDonald JF, Fernández FM. Ovarian cancer detection from metabolomic liquid chromatography/mass spectrometry data by support vector machines. BMC Bioinformatics. 2009;10:259–274. doi: 10.1186/1471-2105-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ransohoff DF. Rules of evidence for cancer molecular-marker discovery and validation. Nat Rev Cancer. 2004;4:309–314. doi: 10.1038/nrc1322. [DOI] [PubMed] [Google Scholar]
- 37.Altman DG, Bland M. Statistics notes: diagnostic tests 2: predictive values. Brit Med J. 1994;309:102. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altman DG, Bland M. Statistics notes: diagnostic tests 1: sensitivity and specificity. Brit Med J. 1994;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Committee for Clinical Laboratory Standards. User evaluation of precision performance of clinical chemistry devices. NCCLS Tentative Guideline EP5-T 2004 [Google Scholar]