Abstract
The ecology of highly pathogenic avian influenza (HPAI) H5N1 has significantly changed from sporadic outbreaks in terrestrial poultry to persistent circulation in terrestrial and aquatic poultry and potentially in wild waterfowl. A novel genotype of HPAI H5N1 arose in 1996 in southern China and through ongoing mutation, reassortment, and natural selection, has diverged into distinct lineages and expanded into multiple reservoir hosts. The evolution of Goose/Guangdong-lineage highly pathogenic H5N1 viruses is ongoing: while stable interactions exist with some reservoir hosts, these viruses are continuing to evolve and adapt to others, and pose an un-calculable risk to sporadic hosts, including humans.
Keywords: virus evolution, reservoir, wild bird, clade, lineage
1. Introduction
It has been speculated that influenza viruses have infected humans and animals since ancient times (Morens and Taubenberger, 2010). Influenza A, B, and C viruses are thought to have diverged from a common ancestor at an indeterminate time, and have formed multiple lineages, particularly influenza A viruses (Buonagurio et al., 1985; Palese and Shaw, 2007; Xu et al., 2011; Tong et al., 2012). Influenza A viruses exist in many different environments and utilize a variety of different host species (Webster et al., 1992; Reperant et al., 2009; Taubenberger and Kash, 2010; Tong et al., 2012)(Figure 1). The ecology of these viruses is highly variable with respect to their interactions with each other (reassortment, competition), with their hosts (immunity, receptor availability, host temperature), and with their environment (ambient temperature, humidity, composition of sediment, salinity of water, pH)(Watanabe et al., 2012b; Webster et al., 1992).
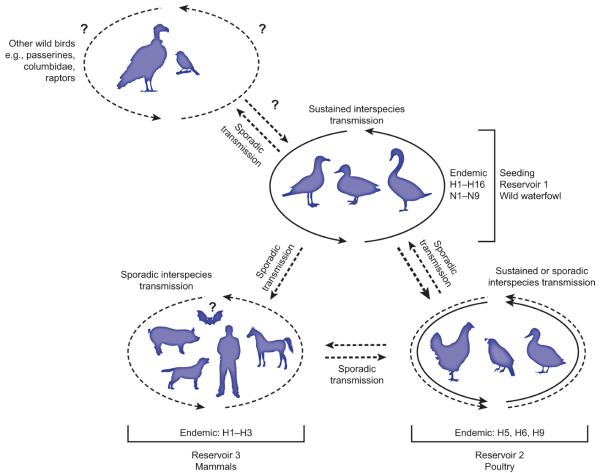
Figure 1. Wild waterfowl represent the seeding reservoir of influenza A viruses.
Three reservoir groups of influenza A viruses are shown: wild waterfowl, poultry, and mammals. Arrows indicate transmission of influenza A viruses between different host reservoir groups and circulation among species within each group. Dotted lines represent sporadic transmission, solid lines represent sustained transmission between different species. Question marks indicate unknown or unresolved transmission patterns. The hemagglutinin subtypes endemic in one or more species in each group are indicated. For wild waterfowl, endemic neuraminidase types are also indicated.
Here we focus on the ecology of highly pathogenic avian influenza (HPAI) Goose/Guangdong (Gs/Gd)-lineage H5N1 by examining (1) the general characteristics of avian influenza viruses; (2) the adaptation of Gs/Gd-lineage H5N1 to domestic geese as reservoir host; (3) unstable transition periods associated with changes in host range, virus genotype, and geographical range; and (4) ongoing adaptation of Gs/Gd-lineage H5N1 to wild waterfowl as host reservoir.
2. General Characteristics of influenza A viruses
Influenza viruses of the Orthomyxoviridae family contain a negative-sense single stranded and segmented RNA genome and are differentiated into influenza A, B, and C. Influenza B viruses have historically caused yearly epidemic outbreaks in humans, while influenza C viruses have been largely associated with young children and pigs. Influenza A viruses are associated with yearly epidemics as well as sporadic pandemics and have a large animal reservoir amongst the wild aquatic birds of the world (Kimura et al., 1997; Webster et al., 2002; Palese and Shaw, 2007; Wright et al., 2007).
Influenza A viruses contain eight gene segments and encode at least ten proteins: polymerase basic 1 (PB1), PB2, polymerase acid (PA), hemagglutinin (HA), nucleoprotein (NP), neuraminidase (NA), matrix 1 (M1), M2, nonstructural 1 (NS1) and 2 (NS2, also nuclear export protein - NEP) (as reviewed in Webster, et al., 1992; and in Palese and Shaw, 2007). Additional to the ten core proteins, several other viral proteins have been recently described with strain-specific or as yet unknown functions: PB1-F2 (Zamarin et al., 2006; McAuley, et al., 2007; Chen, et al., 2001; Chen, et al., 2010; Krumbholz, et al., 2011), PB1-N40 (Wise, et al., 2009), PA-X (Jagger et al., 2012), and PA-N155 and PA-N182 (Muramoto et al., 2013).
HA, responsible for virus attachment to the host cell, is the major target of the humoral immune response. HA-specific immune pressure of a host population against influenza A is associated with ongoing antigenic changes and immune escape of virus subtypes (Smith et al., 2004; Wright et al., 2007).
The primary natural host reservoir of avian influenza A viruses (AIV) are wild waterfowl, particularly anatidae (i.e. ducks, geese, swans) in the order Anseriformes and scolopacidae (shorebirds/waders) and laridae (gulls, terns) in the order Charadriiformes (Stallknecht and Shane, 1988; Webster et al., 1992; Krauss et al., 2004; Olsen et al., 2006; Stallknecht and Brown, 2008; Krauss et al., 2010) (Figure 1). Sixteen HA types and nine NA types have been detected in viruses isolated from wild waterfowl. AIV are considered the ancestors of influenza A viruses of mammals (Scholtissek et al., 1978; Webster et al., 1992; Krauss et al., 2004; Fouchier et al., 2005; Krauss et al., 2007; Wright et al., 2007; Garten et al., 2009; Guan et al., 2010; Hoye et al., 2010; Krauss et al., 2010; Taubenberger and Kash, 2010; Vandegrift et al., 2010).
The vast majority of AIV cause little to no disease in birds and are characterized by a HA cleavage site which relies on proteases of intestinal and respiratory tract mucosa (Bertram et al., 2010; Klenk et al., 1975, Lazarowitz et al., 1973). Low pathogenic avian influenza (LPAI) viruses replicate primarily in the intestinal tract of birds and it is generally agreed that wild waterfowl contract LPAI viruses mainly via fecal-oral inoculation (Krauss et al., 2004; Fouchier et al., 2005; Hoye et al., 2010; Krauss et al., 2010; Vandegrift et al., 2010). Virus shedding is highly species- and virus strain- specific and also includes the avian respiratory tract (Webster et al., 1978; Webster et al., 1992; Stallknecht and Brown, 2008; Costa et al., 2011). Virus shedding from the respiratory tract tends to be more pronounced in infections with highly pathogenic avian influenza (HPAI) viruses and is also associated with adaptation to terrestrial poultry (chicken, quail, pheasant)(Makarova et al., 2003; Humberd et al., 2006; Alexander, 2007a; Sorrell and Perez, 2007)
In wild waterfowl the highest attack rates of LPAI have been found in immunologically naïve juvenile (1st season) birds (Hinshaw et al., 1985; Webster et al., 1992; Krauss et al., 2004; Fouchier and Munster, 2009). Long-term North American wild bird surveillance has demonstrated that the relative abundance of particular subtypes undergo cyclic changes between years, presumably due to herd immunity of adult birds (Sharp et al., 1993; Krauss et al., 2004). Wild waterfowl have been shown to transmit LPAI viruses across flyways, which overlap considerably in Eurasia and in the Americas (Duan et al., 2007; Widjaja et al., 2004; Spackman et al., 2005; Munster et al., 2007; Hill et al., 2012). Major bird breeding and stopover/refueling sites are shared by bird species heading to vastly different areas during their yearly migrations (Carboneras, 1992; Berthold, 1993; Wetlands International, 2012). These areas are of particular importance for the ecology of AIV, as bird densities are high, multiple species are present, and due to climate, stresses of migration or of molting, birds may be more susceptible to infection (Reed et al., 2003; Krauss et al., 2004; Weber and Stilianakis, 2007; Krauss et al., 2010)
Highly pathogenic avian influenza (HPAI) viruses cause high levels of morbidity and mortality in chickens and other terrestrial poultry and strain-dependent in ducks and other waterfowl (Alexander, 2000; Alexander 2007b; Brown, 2010; Sturm-Ramirez et al., 2004; Munster et al., 2005; Capua and Alexander, 2007; Vandegrift et al., 2010). HPAI viruses contain a multibasic cleavage site (MBCS), which allows HA processing by ubiquitously expressed proteases and in the some hosts such as chickens enables the systemic spread of the virus (Bertram et al., 2010; Horimoto et al., 1995; Klenk et al., 1975). Two subtypes of low pathogenic avian influenza (LPAI), H5 and H7, can naturally switch to a highly pathogenic phenotype by the spontaneous acquisition of a MBCS during circulation in poultry (Capua et al., 2000; Garcia et al., 1996; Lebarbenchon and Stallknecht, 2011; Medina and Garcia-Sastre, 2011; Perdue et al., 1997; Rohm et al., 1995; Gohrbandt et al., 2011; Munster et al., 2010; Schrauwen et al., 2011; Veits et al., 2012) (Figure 2). Only one HPAI virus lineage has become established in aquatic poultry as a stable reservoir: the Gs/Gd-lineage of HPAI H5N1 that arose in the early 1990s in Southern China (Rohm et al., 1995; Xu et al., 1999; Alexander, 2007b; Capua and Alexander, 2007; Vijaykrishna et al., 2008; Brown, 2010; Lebarbenchon and Stallknecht, 2011) (Figure 2B).
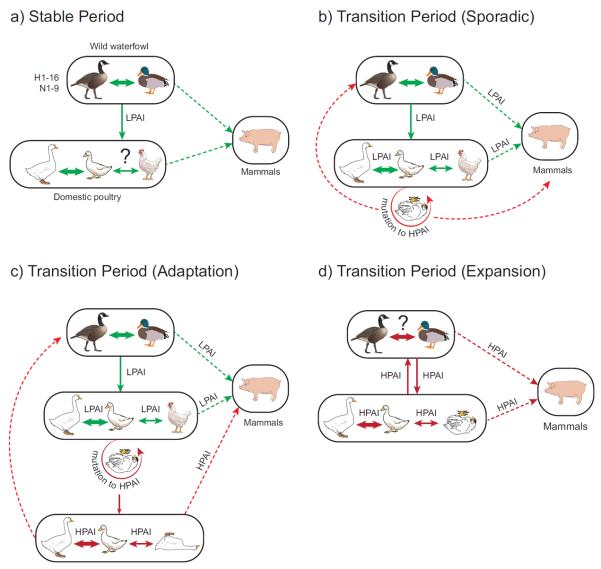
Figure 2. Changing ecology of avian influenza viruses.
a) stable period of LPAI-host interaction. Arrows indicate transmission of avian influenza virus (AIV) between different host reservoir groups and circulation among species within each group. Dotted lines represent sporadic transmission, thin solid lines represent frequent transmission, thick solid lines represent sustained transmission. Question marks indicate unknown or unresolved transmission patterns. Green color indicates transmission of LPAI. b) sporadic transition of H5 and H7 subtypes from LPAI to HPAI. Red color indicates transmission of HPAI. Inverted chicken or goose indicates commonly observed mortality in terrestrial poultry or aquatic poultry, respectively. c) transient adaptation period of HPAI H5N1 in aquatic poultry. d) transient expansion period of HPAI in wild waterfowl.
3. Adaptation of early Gs/Gd-lineage HPAI H5N1 to geese (1996–1999)
A highly pathogenic Gs/Gd/1/96-like virus is the ancestor of the currently circulating Asian HPAI H5N1 viruses and distinct from sporadically emerging HPAI viruses of the H5 subtype (Vijaykrishna et al., 2008; Xu et al., 1999). Gs/Gd-lineage HPAI H5N1 viruses have acquired the capacity to persist in several avian host species since the early 1990s: the low pathogenic ancestor of Gs/Gd/1/96 was likely introduced into poultry from migratory waterfowl, where it had been generated by multiple reassortment events between LPAI viruses of several subtypes (Duan et al., 2007; Vijaykrishna et al., 2008). The transmission of this LPAI H5N1 ancestor to poultry is thought to have led to limited circulation in the new reservoir host characteristic of sporadic introductions of a pathogen into a non-reservoir host species (Figure 2 B, C). During the initial circulation in poultry the LPAI ancestor of Gs/Gd/1/96 is thought to have acquired a MBCS and to have become pathogenic to domestic waterfowl and terrestrial poultry (Duan et al., 2007; Vijaykrishna et al., 2008; Xu et al., 1999)(Figure 2C). Gs/Gd/1/96 H5N1 caused an outbreak in domestic geese in Guangdong province, Southern China, with an unusual high morbidity for AIV infection of aquatic poultry of 40% (Xu et al., 1999). Experimentally inoculation of domestic geese with Gs/Gd/1/96-like isolates from 1999 lead to high morbidity and mortality as well as robust viral shedding from both the trachea and cloaca. High viral titers were detected in internal organs demonstrating the systemic spread of the virus (Webster et al., 2002). Under experimental conditions, transmission of a Gs/Gd/1/96-like isolate from geese to domestic chickens occurred via fecal material but not via the aerosol route, while transmission to quails occurred via either route (Webster et al., 2002). The infectious dose 50% and lethal dose 50% in quail (QID50 and QLD50) and chicken (CID50 and CLD50) were determined: Quails were highly susceptible with a QID50 of 50 EID50 and a QLD50 of 316 EID50. The CID50 was 6309 EID50 and the CLD50 10 000 EID50. In contrast to the morbidity and mortality observed in geese, ducks showed no clinical signs upon experimental inoculation but shed from trachea and cloaca, with contact transmission occurring between some of the ducks (Figure 2C) (Webster et al., 2002). The high virulence of Gs/Gd/1/96 in geese combined with phylogenetic analysis of the virus origins indicated that this pathogen had only recently emerged in this host (Guan et al., 2002b; Vijaykrishna et al., 2008; Xu et al., 1999). During the outbreak in of Gs/Gd/1/96 in geese in Guangdong province, another HPAI H5N1 virus, Gs/Gd/2/96, was isolated from the same flock. This virus, differing in only five residues from Gs/Gd/1/96, showed distinct virulence in birds: it was phenotypically low pathogenic in both waterfowl (geese, ducks) and chickens despite the presence of a MBCS (Li et al., 2006a). The genetically close relationship between these two viruses, their co-circulation in the same flock of geese, and their significant phenotypic differences, indicate that Gs/Gd/2/96 may be ancestral to Gs/Gd/1/96-like viruses. Whether these viruses were co-introduced into geese, or whether one or both of these viruses arose in geese is unknown.
3.1 Outbreak of highly pathogenic H5N1 in Hong Kong (1997)
The outbreak of highly pathogenic H5N1 viruses in Hong Kong poultry in 1997 and the concomitant infection of 18 individuals with 6 deaths heralded the appearance of a starkly different AIV subtype (Shortridge et al., 1998). Reassortment events between a Gs/Gd/1/96-like HPAI H5N1 and low pathogenic H9N2 (A/quail/HK/G1/97-like) and H6N1 (A/teal/HK/W312/97-like) viruses generated a virus lineage, represented by A/Hong Kong/156/97, that caused the chicken outbreaks and human cases (Duan et al., 2007). The Gs/Gd/1/96-like ancestor virus was likely introduced into Hong Kong from southern China where this virus lineage was circulating in domestic geese. This introduction was followed by circulation and reassortment in Hong Kong poultry, possibly in the highly susceptible quail (Duan et al., 2007; Guan et al., 1999; Hoffmann et al., 2000; Webster et al., 2002; Xu et al., 1999). Outbreaks in poultry and sporadic human cases occurred until the culling of the entire poultry population of Hong Kong effectively eradicated this H5N1 lineage (WHO OIE FAO H5N1 Evolution Working Group, 2012; Shortridge et al., 1998). HK/156/97-like viruses have not been reported in wild or domestic birds since the Hong Kong outbreaks of 1997 with the exception of an identification in Vietnamese waterfowl samples of 2004 and 2005 (Duan et al., 2008; WHO OIE FAO H5N1 Evolution Working Group, 2012; Li et al., 2010; Li et al., 2006b).
Pekin ducks (Anas platyrhynchos domestica) inoculated with A/Hong Kong/156/97-like viruses isolated from humans, chickens, and ducks showed no morbidity and limited replication with low titers in the trachea and cloaca (Shortridge et al., 1998). Inoculation of geese (Embden (Anser anser domesticus); Chinese (Anser cygnoides)) with 106 EID50 of A/Hong Kong/156/97-like viruses (trachea, nares, eyes, oral) led to more robust shedding than that observed in ducks, both from the trachea and intestinal tract. A/Hong Kong/156/97 therefore appeared to be more adapted to replication in domestic geese than in ducks. Morbidity and mortality occurred only in some of the geese consistent with field observations of A/Hong Kong/156/97-like H5N1 detected in healthy geese during the outbreak in Hong Kong in 1997 and was lower than the mortality induced by parental virus Gs/Gd/1/96 (Shortridge et al., 1998; Xu et al., 1999). Experimentally inoculated pigs shed virus from nasal passages, with no morbidity and no transmission between pen-mates observed (Shortridge et al., 1998). During the outbreak in Hong Kong, transmission from infected birds to humans occurred sporadically via direct contact (Buxton Bridges et al., 2000; Claas et al., 1998a; CDC, 1998). In conclusion the novel reassortant HK/156/97-like viruses were able to utilize aquatic poultry as host reservoir, with transmission to and within the terrestrial poultry population of Hong Kong, and with sporadic transmission to humans. We can speculate that the rapid depopulation of all poultry in Hong Kong prevented this lineage of HPAI H5N1 becoming established in the region.
4. Diversification of Gs/Gd-lineage H5N1 and hosts range expansion (1999–2002)
Domestic geese served as reservoir for early Gs/Gd-lineage HPAI H5N1 viruses from 1996 to 2000, with the viruses endemic in geese in Southern China by 1999 (Figure 2C, Figure 3) (Cauthen et al., 2000; Guan et al., 2002b; Webster et al., 2002). In 2000, Gs/Gd/1/96-like viruses were repeatedly detected in domestic ducks (Guan et al., 2002b). The duck-isolate Gs/Gd-lineage viruses were antigenically distinct from isolates from geese, and from HK/156/97-like viruses, indicating divergence into a sublineage adapted to ducks as additional reservoir hosts (Guan et al., 2002b). Concurrently with expansion into domestic ducks reassortment events with LPAI viruses were detected during active surveillance in mainland China, Hong Kong and Gs/Gd/1/96-like H5N1 was detected in live bird markets in Hanoi, Vietnam (Chen et al., 2006a; Nguyen et al., 2005) (Guan et al., 2002a). Gs/Gd/1/96-like internal gene segments were also detected in H9N2 and H6N1 viruses in domestic waterfowl, further demonstrating the genetic compatibility of Gs/Gd-lineage H5N1 with co-circulating LPAI viruses (Cheung et al., 2007; Guan et al., 2000; Vijaykrishna et al., 2008; Xu et al., 2007).
Figure 3. Timeline of major events of goose/Guangdong-lineage H5N1 evolution.
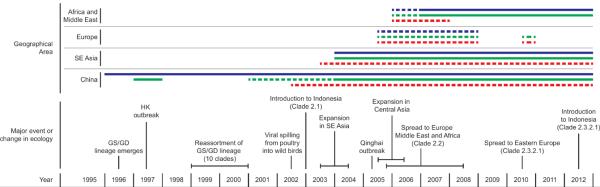
Shown are times of major changes in the evolution of highly pathogenic H5N1, goose/Guangdong-lineage. Expansion into different geographical areas is depicted, as is status in various hosts in different locations: solid lines depict stable interactions between virus and hosts and dashed lines depict transient interactions. Blue lines represent aquatic poultry hosts, green lines terrestrial poultry hosts, and red lines wild birds hosts. SE Asia – South East Asia, Gs/GD - goose/Guangdong, HK – Hong Kong.
In Hong Kong five different genotypes (A – E) of Gs/Gd-variants were detected in poultry in 2001, with closely related viruses circulating in mainland China at the time (Chen et al., 2004; Duan et al., 2008; Guan et al., 2002a). These viruses were highly pathogenic in experimentally inoculated chickens and quail, though severity of disease varied between the five genotypes (Guan et al., 2002a). Initially, these Gs/Gd-variants were detected in apparently healthy terrestrial poultry in Hong Kong's retail markets (chicken, silky chicken, quail, pheasant) (Guan et al., 2002a), but eventually several retail markets reported significant increases in poultry mortality leading to the preemptive depopulation of Hong Kong poultry (Guan et al., 2002a; Shortridge et al., 1998).
In Southern China, HPAI H5N1 isolates of the Gs/Gd/1/96-like viruses and their reassortants (Gs/Gd-variants) were isolated from apparently healthy domestic ducks between 2000 and 2002 (Chen et al., 2004). These Gs/Gd-variants were found to be pathogenic to experimentally inoculated chickens but not to Sheldrake ducks (Chen et al., 2004). In chickens, both Gs/Gd/1/96-like viruses and Gs/Gd-variants met the criteria for highly pathogenic phenotype (intravenous pathogenicity index (IVPI) > 1.2), though the severity of infection was varied: chickens inoculated intravenously or intranasally with earlier isolates (1999, 2000) tended to exhibit less severe symptoms and slower progression of disease then chickens inoculated with later isolates (2001, 2002) (Chen et al., 2004). A local (northern Chinese) strain of three-week old Sheldrake/shelducks were inoculated intranasally with 107.85 – 108.5 EID50 of Gs/Gd-variants and remained asymptomatic for the duration of the experiment. Several of the inoculated ducks shed 102 – 104.3 EID50/mL of virus, with shedding generally higher from the trachea than cloaca. No contact transmission was observed between the ducks (Chen et al., 2004).
One of the five genotypes detected in 2001 (genotype A) contained a deletion in the stalk region of the N1 protein. A similar deletion had been noted in the NA of the HK/97-lineage outbreak viruses from Hong Kong 1997 (Claas et al., 1998b; Guo et al., 2000; Li et al., 2011; Matrosovich et al., 1999). NA-stalk deletions have been reported to naturally occur in LPAI viruses of aquatic birds and to undergo positive selection in terrestrial poultry (Croville et al., 2012; Hoffmann et al., 2012). Furthermore, deletions in the NA stalk region have also been associated with a switch from predominantly intestinal to predominantly respiratory shedding and increased virulence in chickens (Banks et al., 2001; Hossain et al., 2008; Matrosovich et al., 1999; Munier et al., 2010; Zhou et al., 2009).
Late in 2002 two outbreaks of Gs/Gd-variants occurred in wild birds in Hong Kong (Ellis et al., 2004). During these outbreaks varying levels of severity were observed in wild waterfowl ranging from no morbidity to fatal disease (Ellis et al., 2004). Follow-up surveillance failed to detected circulation of HPAI H5N1 in wild waterfowl in Hong Kong suggesting that sustained transmission of Gs/Gd-variants in wild birds did not occur (Figure 2C, Figure 3) (Ellis et al., 2004). These wild bird H5N1 viruses were classified as genotypes Z and Z+ and replicated better in experimentally inoculated mallard ducks than HPAI H5N1 isolates from 1997 to 2001 (Guan et al., 2004; Sturm-Ramirez et al., 2004; Chen et al., 2004). High viral burden was detected in drinking water and virus shedding was generally higher from the trachea than from the cloaca. In experimentally inoculated mallards, the 2002 wild bird isolates were highly pathogenic while H5N1 isolates from chicken and pheasant obtained between 1997 and 2001 were not (Sturm-Ramirez et al., 2004; Chen et al., 2004). Transmission to direct contact mallards was consistently observed for the wild bird isolates from 2002, but sporadically for the poultry isolates of 1997–2001. The authors speculated that aerosol and oral-to-oral transmission through the common water source was enhanced in the highly pathogenic viruses. Long-lasting (10 days) tracheal shedding was detected in one surviving animal (Sturm-Ramirez et al., 2004). Guan et al. (2004) argued that the unusually high pathogenicity of the 2002 H5N1 viruses in wild waterfowl indicated previous adaptation of the viruses to terrestrial birds, followed by re-introduction into wild waterfowl (Guan et al., 2004).
Instability in a viral genome is a hallmark of adaptation of a pathogen to a new host environment (Domingo and Holland, 1997). In the case of HPAI H5N1, the inherent mutability of the RNA genome, the large genetic reservoir of LPAI viruses circulating in poultry, and selection pressures encountered by novel strains or in novel hosts or sites of replication (respiratory system rather than intestinal system) were drivers of the diversification of the virus population and the adaptation to new hosts (Alexander, 2007a; Drake and Holland, 1999; Taubenberger and Kash, 2010; Webster et al., 1992)
5. Expansion of genotype Z in South East Asia (2003–2004)
Early in 2003 two human cases of HPAI H5N1 occurred in Hong Kong while closely related viruses were detected in poultry in Southern China (Duan et al., 2008; Li et al., 2004; Peiris et al., 2004). The human virus isolates (A/Hong Kong/213/03-like) were almost identical to genotype Z+ isolates from wild bird outbreaks (A/egret/Hong Kong/757.3/02-like) in Hong Kong in late 2002 (Guan et al., 2004). It is possible that the patients, both of the same family, and another family member who died of pneumonia in Fujian province, were infected in mainland China (Li et al., 2004; Peiris et al., 2004). Later in 2003 and 2004, poultry outbreaks of HPAI H5N1 were reported from Vietnam, Thailand, Cambodia, Laos, Indonesia, and Malaysia, along with sporadic human infections (Aubin et al., 2005; Li et al., 2004; Organization, 2012; Tiensin et al., 2005; Viseshakul et al., 2004; Webster et al., 2005). Genotype Z outcompeted most other reassortants between its first detection in 2002 in Hong Kong and the winter of 2003/2004. This genotype was responsible for the outbreaks in southern China and the successful expansion of HPAI H5N1 into South East Asia (Li et al., 2004). HPAI H5N1 of clade 1 expanded to most of South-East Asia (Vietnam, Thailand, Malaysia (VTM) – lineage) and clade 2.1 viruses to Indonesia (Figure 3).
5.1 Indonesian-lineage H5N1: Introduction to endemicity of clade 2.1
Poultry outbreaks of Gs/Gd-lineage H5N1 have occurred in Indonesian from mid-2003 onwards. Evidence suggests that a single introduction event into Java around November 2002 introduced clade 2.1 into Indonesia. The introduction may have occurred via wild migratory birds or via poultry trade (Kilpatrick et al., 2006; Smith et al., 2006b; Vijaykrishna et al., 2008; Wang et al., 2008). The early Indonesian-lineage H5N1 viruses were most closely related to an H5N1 isolate from Hunan province, Southern China, and developed into several sublineages geographically restricted to the Indonesian islands. Additional introductions of Gs/Gd-lineage H5N1 viruses into Indonesia were not reported until late 2012 with the appearance of clade 2.3.2.1 in Java and several outbreaks in chickens and ducks (Smith et al., 2006b; WHO OIE FAO H5N1 Evolution Working Group, 2012; GISAID, 2013; EMPRES-I, 2013).
Indonesia maintains a large poultry sector of both terrestrial and aquatic birds (Wint and Robinson, 2007; Food and Agriculture Organization of the United Nations, 2012). Migratory waterfowl may have introduced HPAI H5N1 to Java, but the virus was likely perpetuated within Indonesia by agricultural practices and poultry trade although some involvement by wild bird movements cannot be ruled out in the expansion of H5N1 across the Indonesian archipelago (Stoops et al., 2009). Indonesia-lineage HPAI H5N1 viruses have been isolated from chicken, quail, turkey, and duck since 2004 and outbreaks of H5N1 in poultry have led to sporadic human infections predominately associated with direct contact to sick animals (Aditama et al., 2012; Sedyaningsih et al., 2007; Webster et al., 2005).
Sustained circulation of H5N1 in terrestrial poultry and concurrent selection pressures, such as large-scale vaccination of poultry have led to the genetic and antigenic divergence of this lineage into several subgroups (clades 2.1.1, 2.1.2, and 2.1.3) (Cattoli et al., 2011; WHO OIE FAO H5N1 Evolution Working Group, 2012; Smith et al., 2006b; Wang et al., 2012). Java harbored ancestors to all three 2.1 sublineages in 2004, further indicating that the viral introduction had occurred here (WHO OIE FAO H5N1 Evolution Working Group, 2012; Lam et al., 2012; Smith et al., 2006b).
Experimental inoculation (oral, ocular, nasal) of Pekin ducks with 106 EID50 of A/Duck/Indramayu/BBVW/109/06 (clade 2.1.3) resulted in no morbidity or mortality, though the virus replicated in internal organs and shedding was observed from the respiratory and digestive tract of some of the ducks (Bingham et al., 2009). The presence of ducks in retail markets was a risk factor for the presence of HPAI H5N1, but backyard duck densities were not. The presence of ducks as an associated risk in rice-rotation settings could not be excluded, but taken together ducks are thought to have a lesser impact on the circulation of HPAI H5N1 in Indonesia than in other countries in South East Asia (Gilbert et al., 2006a; Indriani et al., 2010; Loth et al., 2011; Tiensin et al., 2007).
HPAI H5N1 was declared endemic in Indonesia in 2006 (Brown, 2010) and the Indonesian-lineage viruses' ongoing evolution has included reassortment between sub-lineages (Lam et al., 2008) (Nidom et al., 2012). Analysis of Indonesian-lineage H5N1 sequences from 2003 – 2007 demonstrated considerable co-circulation of different sub-clades and subgroups within Indonesia, with limited geographical separation. During active surveillance in retail markets and farms in 2009/2010, apparently healthy chickens harbored, at low prevalence, several distinct groups of clade 2.1.3 H5N1 viruses and in 2011, subclades 2.1.3 and 2.1.2 were reported (WHO OIE FAO H5N1 Evolution Working Group, 2012; Nidom et al., 2012; Webby, personal communication). In late 2012 outbreaks of HPAI H5N1 of clade 2.3.2.1 were reported, but reassortants with endemic Indonesian-lineage viruses have not been identified to date (GISAID, 2013; EMPRES-I, 2013). The extensive use of vaccination in the management of the endemic Indonesian-lineage viruses may confer a temporary advantage to the antigenically distinct clade 2.3.2.1 virus.
5.2 Expansion of clade 1 from Southern China into South East Asia
Outbreaks of HPAI H5N1 occurred frequently in South East Asia during 2003–2005 as different viruses expanded their geographical range from Southern China to Indonesia (clade 2.1) and most of remaining South East Asia (clade 1). The outbreak viruses of the Vietnam/Thailand/Malaysia (VTM)-lineage belonged to clade 1, genotype Z, (Chen et al., 2006a; Guan et al., 2004; Li et al., 2004; Smith et al., 2006b; Tiensin et al., 2005). VTM-lineage H5N1 viruses were likely generated in poultry in Yunnan province, southern China, in 2002–2003 (Vijaykrishna et al., 2008; Wang et al., 2008). Poultry is traded at large volumes across the region, including across borders, with live bird markets bringing together different sub-populations of poultry and implicated in previous outbreaks of HPAI H5N1 as well as in its dispersal (Martin et al., 2011; Soares Magalhaes et al., 2010; Van Kerkhove et al., 2009). The presence of free-grazing and backyard ducks, or of low-biosecurity duck farms, was identified as a major risk factor for HPAI H5N1 outbreaks in Vietnam and Thailand (Gilbert et al., 2006a; Loth et al., 2011; Songserm et al., 2006; Tiensin et al., 2007). Pekin ducks experimentally inoculated via the nasal, ocular, and oral route with A/Muscovy duck/Vietnam/453/2004 (clade 1) displayed variable levels of morbidity and mortality with virus replication in multiple organs, and oropharyngeal and cloacal shedding depending on viral dose (Bingham et al., 2009; Middleton et al., 2007). The duck infectious dose 50% was determined as 103.2 EID50 (Middleton et al., 2007).
A significant number of wild bird species including little grebe (Tachybaptus ruficollis), open-bill stork (Anastomus oscitans), tree sparrow (Passer montanus), and brown-headed gull (Larus brunnicephalus) were also found infected with H5N1 during the expansion of VTM-lineage H5N1 in South East Asia (Keawcharoen et al., 2011; Uchida et al., 2008a). Even though multiple wild bird species were susceptible to infection with this H5N1 lineage, the contribution of migratory birds to the spread of VTM-lineage H5N1 remains unresolved (Keawcharoen et al., 2011; Songserm et al., 2006; Uchida et al., 2008a). Keawcharoen et al. (2011) found a positive association between the presence of wild birds infected with H5N1 and poultry outbreaks principally in central Thailand. This association could reflect the overall higher density of poultry in central Thailand and/or low biosecurity measures and spillover of H5N1 into wild birds from poultry outbreaks. The authors also report the presence of infected wild birds preceding outbreaks, indicating that wild bird movement may have contributed to the expansion of H5N1 in this region (Keawcharoen et al., 2011).
6. Transient adaptation of highly pathogenic H5N1 to migratory waterfowl
The contribution of wild birds, including migratory waterfowl, to the dissemination of Gs/Gd-lineage H5N1 viruses throughout East and South East Asia in 2003–2005 remains unclear. Early (1996–2001) HPAI H5N1 viruses were almost exclusively associated with terrestrial and aquatic poultry, while wild waterfowl and passerine species (songbirds, crows, sparrows) appear to have been sporadic hosts (Alexander, 2007b; Keawcharoen et al., 2011; Uchida et al., 2008a; Webster et al., 2005). Since 2002, Gs/Gd-lineage HPAI H5N1 have been increasingly isolated from wild birds, including various species of migratory ducks and geese (Chen et al., 2006a; Sturm-Ramirez et al., 2005). Migratory bird movement has been proposed as a contributing factor in the expansion of HPAI H5N1 in South East Asia and East Asia (Gaidet et al., 2008; Gilbert et al., 2006b; Liang et al., 2010; Si et al., 2009; Webster et al., 2005).
6.1 H5N1 outbreaks in South Korea and Japan 2003–2004
It has been noted during previous outbreaks in Hong Kong and during active surveillance in southern China that HPAI H5N1 prevalence was highest during the winter months (Li et al., 2004). Studies primarily with LPAI viruses have shown greatest stability and environmental persistence in cooler conditions, among other factors (Stallknecht et al., 1990a; Stallknecht et al., 1990b; Webster et al., 1978).
The Korean peninsula and Japan are visited by various interconnected migratory bird populations including ducks that breed in Siberia, Mongolia, and western China and winter in eastern China, the Korean peninsula and Japan (Carboneras, 1992; Partnership for the Ease Asian-Australasian Flyway, 2012; Wetlands International, 2012). During the winter of 2003/2004 several outbreaks of Gs/Gd-lineage H5N1 (clade 2.5) occurred in chickens and ducks in South Korea and in chickens in Japan (Lee et al., 2005; Mase et al., 2005). These H5N1 viruses were closely related to each other and to viruses from southern China (WHO OIE FAO H5N1 Evolution Working Group, 2012; Lee et al., 2005; Mase et al., 2005). Pathogenic to chicken and quail, they replicated systemically including robustly in the respiratory tract of infected animals. Pekin ducks exhibited low mortality when experimentally inoculated via the intranasal route, with robust viral shedding and transmission to direct contact ducks (WHO OIE FAO H5N1 Evolution Working Group, 2012; Lee et al., 2005; Mase et al., 2005). In Japan, dead wild crows in close vicinity to several outbreak sites were found to be H5N1 infected, likely through scavenging on dead chickens (Mase et al., 2005; Tanimura et al., 2006). Poultry depopulation was used to control these outbreaks and the virus did not persist in South Korea or in Japan. The route of introduction of these viruses into either country could not be determined. H5N1 virus was not detected in migratory waterfowl during active surveillance following the outbreaks in South Korea, and a connection between migratory birds and the outbreaks in Japan could not be verified (Lee et al., 2005; Mase et al., 2005; Yamane, 2006).
6.2 Outbreak of HPAI H5N1 in wild waterfowl at Qinghai Lake in 2005
A significant shift in the ecology of Gs/Gd-lineage HPAI H5N1 was recognized in April 2005 when a large-scale outbreak was detected in several wild bird species at Qinghai Lake, China (Chen et al., 2005; Liu et al., 2005). The Qinghai Lake ecosystem is a major breeding site for migratory waterfowl in the Central Asian Flyway and the East Asia-Australasia Flyway (Olsen et al., 2006; Wetlands International, 2012). The Qinghai nature reserve hosts approximately 150 000 birds of greater than 180 species in spring and summer (Chen et al., 2005; Miyabayashi and Mundkur, 1999). Bar-headed geese (Anser indicus), a major bird species utilizing this site, are known to migrate to South East Asia and over the Himalayas to the Indian subcontinent in fall (September) and to return to Qinghai Lake in April for the summer (Carboneras, 1992; Wetlands International, 2012). Introduction of H5N1 to Qinghai Lake possibly occurred by migratory birds, though whether this reflects a previously established and persistent circulation of HPAI H5N1 in (any) wild bird species or a transient occurrence is also under ongoing debate (Figure 2C, D)(Chen et al., 2006a; Prosser et al., 2011). Additionally, captive-bred bar-headed geese were reared close to the nature reserve (Butler, 2006; Feare et al., 2010). A time of most recent ancestor (TMRCA) analysis indicated that the Qinghai- lineage of H5N1 arose in South East China around December 2004 through reassortment between clade 2.2. (genotype V) and clade 1 (genotype Z) (Chen et al., 2005; Vijaykrishna et al., 2008).
Poyang Lake in Jiangxi province, 1700 km southeast of Qinghai Lake, is an important overwintering site for migratory waterfowl (Global Nature Fund, 2012; Takekawa et al., 2010). Apparently healthy migratory ducks sampled at Poyang Lake in January and March 2005 shed HPAI H5N1 viruses of genotypes Z and V including reassortants containing different combinations of all gene segments of the Qinghai-lineage H5N1 (Chen et al., 2006a).
The initial outbreak at Qinghai Lake was detected in late April/early May 2005 and was initially restricted to one location where bar-headed geese had close contact with brown-headed gulls and great black-headed gulls (Larus ichthyaetus). Bar-headed geese were found to be symptomatically infected first and represented the majority of fatal cases, with mortality also observed in the gull species (Chen et al., 2005; Chen et al., 2006b; Liu et al., 2005). Later, as the outbreak spread to more locations on the lake, great cormorants (Phalacrocorax carbo) as well as Ruddy Shelducks (Tadorna ferruginea) were infected, though these species represented a small proportion of the birds symptomatically affected in the Qinghai outbreak. Additionally, several dead whooper swans (Cygnus cygnus), common pochards (Aythya ferina), and black-necked cranes (Grus nigricollis) were recovered (Chen et al., 2006b). During a study from 2007 to 2008, Takekawa et al (2010) did not detect migration of waterfowl (five duck species, 33 individuals) between Poyang Lake and Qinghai Lake using satellite tracking. However, due to the sample size limitations and species selected the possibility of waterfowl migration between the sites could not be excluded (Takekawa et al., 2010).
Bar-headed geese have been raised in captivity in Tibet and Chinese provinces, including in the vicinity of Qinghai Lake (Butler, 2006; Feare et al., 2010). Captive bred and reared bar-headed geese are likely to come into close contact with both poultry and wild birds, and the release of captive bar-headed geese back into the wild has also been reported (Butler, 2006; Feare et al., 2010). These practices may have contributed to HPAI H5N1 virus transmission into wild bird populations (Butler, 2006; Feare et al., 2010).
A subset of the Qinghai Lake H5N1 viruses at the outbreak site in 2005 carried the PB2 E627K mutation, a marker for mammalian adaptation (Chen et al., 2006b; Liu et al., 2005; Hatta et al., 2001; Subbarao et al., 1993). Feare et al. (2010) suggested that Qinghai-lineage H5N1 may have been generated in a mammalian host and were then transmitted to wild birds (Feare et al., 2010). Later Qinghai-lineage H5N1, including avian isolates from Europe and Africa, predominantly carried the PB2 E627K mutation, indicating a lack of negative selection of this position in birds (Cattoli, et al., 2009; Kamal et al., 2007). Overall a significant proportion of Gs/Gd-lineage H5N1 viruses have carried PB2 627K (Russell et al., 2012), and this marker could be enriched in ratite cell culture during serial passaging (Shinya et al., 2009). PB2 627K has been associated with viral replication at lower temperatures (Massin et al., 2001). The avian respiratory tract is critical for temperature regulation of birds and correspondingly the respiratory tract of birds is at a comparatively lower temperature than the intestinal tract (Schmidt-Nielsen et al., 1969). The higher level of respiratory replication observed in HPAI compared to LPAI viruses could have an association with PB2 627K.
At Qinghai Lake, one dominant genotype and three minor genotypes of HPAI H5N1 were detected and found to be highly pathogenic to experimentally inoculated domestic chickens (Chen et al., 2005; Chen et al., 2006b; Liu et al., 2005). Serological evidence suggests that a number of wild bird species can be naturally infected with Gs/Gd-lineage HPAI H5N1 and recover (Siengsanan-Lamont et al., 2011; Niqueux et al., 2010). Experimental infection of various duck species (mallard, gadwall (Anas strepera), common teal (Anas crecca), Eurasian wigeon (Anas penelope), tufted duck (Aythya fuligula), common pochard) with a Qinghai-lineage H5N1 virus isolated in Europe showed differing levels of host morbidity and mortality, and of the magnitude and duration of virus shedding (Keawcharoen et al., 2008). Virus was shed overwhelmingly from trachea rather than cloaca. Mallard ducks were identified as high-risk vectors of virus dissemination due to robust virus shedding in the absence of discernable symptoms (Keawcharoen et al., 2008).
7. Expansion of Qinghai-lineage H5N1 to Europe and Africa (2005–2008)
In the months following the Qinghai lake outbreak, Qinghai-lineage H5N1 was detected in Mongolia, Inner Mongolia, Russia, and Liaoning province (North East China) in wild bird species as well as chickens (Chen et al., 2006b; Lipatov et al., 2007; Prosser et al., 2011; Shestopalov et al., 2006). Mongolia and central-eastern Siberia are vast, sparsely populated territories that contain major breeding, staging, and stopover areas for migratory waterfowl including bar-headed geese, and swan and duck species (Carboneras, 1992; Miyabayashi and Mundkur, 1999; Wetlands International, 2012). Mongolia has a minimal commercial poultry sector and little backyard poultry (Wint and Robinson, 2007; FAO, 2012). Three flyways intersect in Mongolia and central-eastern Siberia: the East Asia/ Australia flyway, the Central Asia flyway, and the East Africa/ West Asia flyway (Wetlands International, 2012). Eastern Siberia as a major breeding area for migratory birds has been identified as a possible key location for HPAI H5N1 virus amplification in wild birds and for its subsequent dispersal along overlapping flyways (Liang et al., 2010; Si et al., 2009).
In late 2005 and throughout 2006, Qinghai-lineage H5N1 expanded westward across Eurasia, into the Indian subcontinent, and northern and central Africa (OIE 2012c; WHO, 2012). The most commonly identified hosts that signaled the westward expansion of the Qinghai lineage were various species of swan, duck, goose, gull and grebe usually symptomatic or dead at time of sampling (Feare, 2007; Happold et al., 2008; Kou et al., 2009). The geographic spread of Qinghai-lineage H5N1 from China to Siberia, India, Europe and Africa was largely correlated in time and space with annual bird migration movements along the overlapping flyways spanning Eurasia (Artois et al., 2009; Gaidet et al., 2008; Gilbert et al., 2006b; Liang et al., 2010; Si et al., 2009). In Western Europe and Scandinavia, Qinghai-lineage HPAI H5N1 viruses were detected mostly in migratory species such as dead whooper swans (Cygnus olor), common pochard, tufted duck, grey heron (Ardea cinerea) and great crested grebe (Podiceps cristatus), as well as in mute swans, which are predominantly sedentary in this region, and predators and scavengers (Carboneras, 1992; (Delany, 2006; Feare, 2007). Indicator (symptomatic) bird species may not have functioned as vectors of HPAI H5N1 even though disease severity is highly variable in an outbred population exposed to different doses of virus. Indicator species may have merely come into close contact with birds able to carry HPAI H5N1 across at least intermediate distances. A particularly cold winter around the Black Sea area had caused unusual migration of several bird species into Western Europe further suggesting an introduction of HPAI H5N1 by wild migratory birds (Gilbert et al., 2006b; Reperant et al., 2010).
Experimental inoculation of four different swan species (mute swans, whooper swans, black swans (Cygnus atratus), and trumpeter swans (Cygnus buccinator)) with a Qinghai-lineage H5N1 originally isolated from a dead whooper swan, showed the four swan species to be highly susceptible to infection (Brown et al., 2008). Contact transmission was observed, as was robust viral shedding from trachea and cloaca. Interestingly, bar-headed geese inoculated with this H5N1 isolate at the same time showed lower sensitivity to the virus than mute swans, with several animals recovering despite robust virus shedding of up to eight days' duration (Brown et al., 2008).
The introduction of HPAI H5N1 into the African continent occurred at least three times as indicated by the presence of three distinct sublineages (A, B, C in Ducatez et al., (2007) or II, IV, I in Cattoli et al., (2009)) that had previously been detected in Europe and Ural Russia (Ducatez et al., 2007; Cattoli et al., 2009; Salzberg et al., 2007). In 2006, eight African countries reported outbreaks of HPAI H5N1 (Burkina Faso, Cameroon, Cote d'Ivoire, Djibouti, Egypt, Niger, Nigeria, and Sudan) and in 2007 an additional three countries reported outbreaks (Ghana, Benin, Togo). Sublineage A (II) was detected in Nigeria, Niger, and Togo; sublineage B (IV) in Egypt, Djibouti, and Nigeria; and sublineage C (I) in Burkina Faso, Cameroon, Cote d'Ivoire, Nigeria, Sudan, Benin and Ghana (Ducatez et al., 2007; Cattoli et al., 2009).
In Africa HPAI H5N1 was detected almost exclusively in domestic chicken with few reports of H5N1 in duck, goose, turkey, guinea fowl, ostrich, and domestic pigs, as well as in wild birds (vultures, wild duck, sparrow, egret) (EMPRES-I, 2013; Ducatez et al., 2007). In most affected African countries the presence of HPAI H5N1 was transient, with the notable exception of Egypt (continued persistence of the virus) and Nigeria, where HPAI H5N1 circulated for approximately 18 months (OIE, 2012b). All three sublineages of clade 2.2 that were detected in Africa in 2006–2007 were present in Nigeria and here reassortment between the sublineages occurred before the virus disappeared by late 2007 (EMPRES-I, 2013; OIE, 2012a; Owoade et al., 2008). Human infections in Egypt, Djibouti, and Nigeria were associated with close contact with sick poultry (Ducatez et al., 2007; Cattoli et al., 2009).
The underlying causes of HPAI H5N1 persistence in Egypt, but absence from the remainder of initially affected African countries remain unclear. Differences in population density of ducks and in overall poultry densities, as well as in poultry production systems, and climate may have prevented sustainable transmission in all initially affected countries save Egypt (Couacy-Hymann et al., 2012).
Though much evidence points to a contribution of wild bird migration in the dissemination of Qinghai-lineage HPAI H5N1, poultry movement (trade of live birds) has also been shown to be involved (Kilpatrick et al., 2006). Taken together, HPAI H5N1 expansion from Asia to Siberia, India, Europe and Africa appears to have occurred both via wild and through live bird (poultry) trade.
7.1 Egyptian-lineage H5N1: Introduction to endemicity of clade 2.2
During the westward expansion of Qinghai-lineage H5N1 poultry outbreaks and human cases were reported from the Middle East and particularly Egypt (Aly et al., 2008; WHO, 2012). The introduction route into Egypt remains unclear, however, a migratory common teal, sampled in the Nile Delta region several months prior to the first reported outbreak of HPAI H5N1 in poultry, tested positive for H5N1 with the HA gene closely related to subsequent outbreak viruses in Egypt (Saad et al., 2007). Regardless of the manner of introduction, dissemination of Qinghai-lineage H5N1 throughout Egypt relied on poultry movement (live bird trade) and on low-level biosecurity in mid-size commercial farms and backyard flocks (Aly et al., 2008). Since its introduction into Egypt in 2006, Qinghai-lineage HPAI H5N1 has become established in both backyard poultry and the commercial poultry sector with sporadic transmission to humans (Kayali et al., 2011a; Kayali et al., 2011b; Alexander, 2007b; Brown, 2010) (Figure 3). During 2007–2008, active surveillance identified backyard flocks as exhibiting the highest prevalence of H5N1, with a relatively lower incidence in commercial farms. HPAI H5N1 was readily identified in previously vaccinated birds consistent with vaccine failures recognized in late 2007 (Hafez et al., 2010). In 2008, HPAI H5N1 clade 2.2 was declared endemic in Egypt (Brown, 2010). Active surveillance in 2009–2010 determined H5N1 prevalence in healthy animals to be 4.5% with no other AIV subtype identified. Routine vaccination of farm poultry did not prevent a H5N1 prevalence of 7% in this sector, clearly demonstrating vaccine escape by circulating H5N1 viruses (Kayali et al., 2011a). In Egypt, seasonality has been observed for human HPAI H5N1 infections with a peak from December to March, but not for poultry outbreaks (Kayali et al., 2011b)(Murray and Morse, 2011).
Egyptian lineage H5N1 has diversified into several sub-lineages of clade 2.2 since its introduction 2006 with varying degrees of cross-reactivity. Geographic separation from other reservoirs of HPAI H5N1, a high rate of virus activity in chickens, and immune pressure due to poultry vaccination seem to have promoted this diversification (Abdelwhab and Hafez, 2011; Arafa et al., 2012; Kayali et al., 2011b; Watanabe et al., 2012a). Recently, H9N2 viruses have been reported from chicken and quail in Egypt, raising the possibility of further, rapid changes in H5N1 phenotype through reassortment with these LPAI viruses (El-Zoghby et al., 2012; Monne et al., 2012). Furthermore, sero-surveillance also identified the presence of H7 AIV in broiler, breeder, and layer chickens in flocks with chickens showing clinical signs in the absence of mass mortality (Afifi et al., 2013).
7.2 Additional activity of Qinghai-lineage H5N1 in Asia (2006–2007)
The Fujian sub-lineage (clade 2.3.4, genotype V) of Gs/Gd-lineage H5N1 became dominant in China in late 2005 and expanded to other countries in South-East Asia in a new wave of H5N1 outbreaks. Multiple clades of HPAI H5N1 continued to co-circulate in China during 2006–2007 including clade 2.3.2, 2.3.4, 2.5, 4, 7, and Qinghai-lineage clade 2.2 (Duan et al., 2008; Smith et al., 2006a; WHO OIE FAO H5N1 Evolution Working Group, 2012).
South Korea experienced several outbreaks of H5N1 in chickens, quails and ducks during the winter of 2006–2007 with Qinghai-lineage viruses closely related to Russian and Mongolian isolates of 2006 (Lee et al., 2008). Subsequent surveillance in migratory waterfowl in South Korea yielded two H5N1 viruses closely related to the viruses causing the outbreaks in poultry (Lee et al., 2008). In early January 2007 a sick wild hawk in Japan harbored H5N1 of the Qinghai-lineage closely related to outbreak viruses reported from South Korea in late 2006, followed by several poultry outbreaks with closely related H5N1 viruses in Japan (Lee et al., 2008; Shivakoti et al., 2010).
8. HPAI H5N1 in migratory waterfowl and other wild birds in Asia (2008–2012) and expansion of clade 2.3.2.1
Active and passive surveillance of wild birds was carried out in fourteen provinces of China between 2004 and 2007 (Kou et al., 2009). Widely varying levels of H5N1 prevalence were detected in different bird species, with Anseriformes, particularly mallard ducks, exhibiting the highest incidence of 4.37% (Kou et al., 2009).
Fujian-lineage H5N1 (clade 2.3.4) became dominant in poultry in China followed by much of South East Asia from late 2005 onwards, though these viruses were rarely associated with wild birds (Smith et al., 2006a) (WHO OIE FAO H5N1 Evolution Working Group, 2012). Multiple clades and sub-clades of Gs/Gd-lineage H5N1 continue to co-circulate in China and South East Asia, with clade 2.3.2 the dominant sub-clade since 2007/2008 (EMPRES, 2012). Clade 2.3.2.1 H5N1 viruses have been particularly associated with wild waterfowl infections (WHO OIE FAO H5N1 Evolution Working Group, 2012).
In South Korea, several outbreaks of H5N1 (clade 2.3.2.1) occurred in April 2008 in chicken and duck farms. The outbreak virus was closely related to isolates from dead whooper swans in Japan and to isolates obtained during chicken outbreaks in Russia in 2008 (Kim et al., 2010; Uchida et al., 2008b; Usui et al., 2009). The South Korean H5N1 virus was highly pathogenic to both chickens and domestic ducks and was dispersed within South Korea through poultry trade (Kim et al., 2010). Kim et al (2010) speculated that the initial introduction may have occurred via regular migration of wild ducks from China and South East Asia to South Korea during March and April (WHO OIE FAO H5N1 Evolution Working Group, 2012; Kim et al., 2010; Smith et al., 2009b). Surveillance of migratory birds was carried out at different sites in Mongolia in spring/summer (2005 – 2010) and fall (2001 – 2009). HPAI H5N1 viruses of clade 2.2 (2005, 2006) and 2.3.2 (2009, 2010) were detected in migratory birds in Mongolia during their northward migration in spring, but not during their return southward in fall indicating a lack of H5N1 virus amplification in the Siberian breeding areas (Sakoda et al., 2010). During the autumn sampling, ten other LPAI virus subtypes were isolated, demonstrating that AIV circulate in this population during fall migration. In July 2009, HPAI H5N1 viruses of clade 2.3.2.1 were detected in dead and sick migratory waterfowl in Mongolia (whooper swans, bar-headed geese, ruddy shelduck and common goldeneye) and in neighboring Siberia (black-headed gull, spoon-bill, great crested grebe, little grebe) (Kang et al., 2011; Sharshov et al., 2010; Sakoda et al., 2010). The absence of concurrent poultry outbreaks in Russia indicated an introduction by migratory birds (Sharshov et al., 2010). These viruses were closely related to each other and formed a discrete subgroup within subclade 2.3.2.1, distinct from the South Korean/ Japanese subgroup of 2008 (WHO OIE FAO H5N1 Evolution Working Group, 2012; Kang et al., 2011; Prosser et al., 2011; Sharshov et al., 2010). Following outbreaks in wild waterfowl in Mongolia and Tyva (eastern Russia) in 2009/2010, and chicken outbreaks in Nepal in February of 2010, the same group of viruses was detected during outbreaks in chickens in Romania in March (WHO OIE FAO H5N1 Evolution Working Group, 2012; Nagarajan et al., 2009; Reid et al., 2011) and in a dead common buzzard in neighboring Bulgaria (Reid et al., 2011; Marinova-Petkova et al., 2012).
In fall of 2010 H5N1 viruses (clade 2.3.2.1) were detected during active surveillance in Hokkaido, Japan, in healthy migratory waterfowl returning from Siberian breeding areas (Kajihara et al., 2011). The introduced H5N1 viruses represented three different subgroups indicating at least three separate introduction events and were closely related to virus isolates from China, Mongolia, Russia, Romania and Bulgaria (2009 – 2010) (Kajihara et al., 2011; Sakoda et al., 2012; Marinova-Petkova et al., 2012). Closely related clade 2.3.2.1 H5N1 viruses were detected in South Korea in wild birds (including various duck species, whooper swan, white-fronted goose, and predators) and during domestic poultry outbreaks (chicken, quail, turkey, duck) from December 2010 until spring 2011 (Kim et al., 2012; Kwon et al., 2011; Sakoda et al., 2010; Yamamoto et al., 2011). Throughout Japan several outbreaks of HPAI H5N1 occurred in wild birds (migratory duck, swan, grebe species; predators) and poultry (chicken) farms from late 2010 until March 2011 (Sakoda et al., 2012).
Sporadic wild bird infections with HPAI H5N1 were reported in Hong Kong in 2011 (clade 2.3.2.1) (large-billed crow (Corvus macrorhynchus), oriental magpie robin (Copsychus saularis), black-headed gull (Chroicocephalus ridibundus)) and during 2012 infected (dead) wild birds included both residential species (large-billed crow, oriental magpie robin, little egret (Egretta garzetta), scaly-breasted munia (Lonchura punctulata), crested myna (Acridotheres tristis)) and migratory birds (black-headed gull, peregrine falcon (Falco peregrinus), grey heron (Ardea cinerea)) (OIE, 2012a, 2012b; GISAID, 2013). Also in 2012 Vietnam reported multiple outbreaks of clade 2.3.2.1 H5N1 viruses, which were closely related to isolates from Mongolia 2009 – Japan 2011, as well as to viruses from Bangladesh and India (both 2011–2012) and to the H5N1 virus of clade 2.3.2.1 recently introduced to Indonesia by unknown route(s) (GISAID, 2013; EMPRES-I, 2013).
9. Conclusions
The high mutation rates of AIV together with frequent reassortment translate into a large and diverse viral reservoir. Gs/Gd-lineage HPAI H5N1 viruses represent an extensive population of virus strains and quasi-species, with great diversity of phenotype in various hosts. The ecology of Gs/Gd-lineage H5N1 viruses continues to evolve as viruses mutate and reassort.
In summary, (1) HPAI H5N1 viruses of the Gs/Gd-lineage arose in the early 1990s with the ability to persist in geese. (2) Gs/Gd-lineage HPAI H5N1 diversified into sub-lineages and adapted to domestic ducks as host reservoir. (3) Diverse populations of LPAI and HPAI viruses co-circulate in poultry and/or wild waterfowl of Southern China and South East Asia. (4) Gs/Gd-lineage HPAI H5N1 viruses have expanded geographically from China to South East Asia, East Asia, Europe, the Middle East and Africa. (5) Gs/Gd-lineage HPAI H5N1 viruses should be considered endemic in poultry in China, Vietnam, Cambodia, Indonesia, Bangladesh, and Egypt. (6) Gs/Gd-lineage HPAI H5N1 viruses have infected multiple species of wild birds and are likely to be in a transition state towards utilizing wild waterfowl as reservoir hosts.
The potential consequences of the ongoing adaptation of HPAI H5N1 to wild waterfowl are numerous: (a) geographic expansion of Gs/Gd-lineage H5N1 viruses could increase in frequency. (b) HPAI H5N1 and LPAI viruses remain available for reassortment with each other. The phenotype of AIV reassortants (virulence, host range) is currently not predictable. (c) Sporadic transmission could occur to any species with at least some susceptibility for avian H5N1 viruses: poultry, non-reservoir wild birds, and mammals. (d) At any time virus phenotypes may arise with enhanced ability to transmit to and among mammals.
Consequently, it is critical to understand (1) the fitness landscape of LPAI viruses and of HPAI H5N1 viruses in various hosts, including that of reassortants; (2) the restrictions that guide AIV reassortment; and (3) the determinants of host susceptibility, virus transmission, and immune response against HPAI H5N1 and other AIV.
Highlights
The ecology of highly pathogenic (HP) H5N1 is diverse, specific to strain and to host species
over time Asian HP H5N1 viruses have expanded their host range from geese to poultry
Mutation and reassortment have given rise to multiple virus lineages
Asian HP H5N1 has utilized wild waterfowl in long-distance expansion
Wild waterfowl are in a transient state towards becoming a reservoir of HP H5N1
Acknowledgements
The authors wish to thank Julie Groff (Biomedical Communications) and Sharon Naron (Scientific Editing, both St. Jude Children's Research Hospital) for excellent assistance with the figures and manuscript preparation. This work was supported by the American Lebanese Syrian Associated Charities (ALSAC) and NIAID/NIH contract No. HHSN266200700005C (Centers of Excellence for Influenza Research and Surveillance (CEIRS)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelwhab EM, Hafez HM. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: epidemiology and control challenges. Epidemiol Infect. 2011;139:647–657. doi: 10.1017/S0950268810003122. [DOI] [PubMed] [Google Scholar]
- Aditama TY, Samaan G, Kusriastuti R, Sampurno OD, Purba W, Misriyah, Santoso H, Bratasena A, Maruf A, Sariwati E, Setiawaty V, Glass K, Lokuge K, Kelly PM, Kandun IN. Avian influenza H5N1 transmission in households, Indonesia. PLoS One. 2012;7:e29971. doi: 10.1371/journal.pone.0029971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifi MA, El-Kady MF, Zoelfakar SA, Abdel-Moneim AS. Serological surveillance reveals widespread influenza A H7 and H9 subtypes among chicken flocks in Egypt. Trop Animal Health and Prod. 2013;45:687–690. doi: 10.1007/s11250-012-0243-9. [DOI] [PubMed] [Google Scholar]
- Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol. 2000;74:3–13. doi: 10.1016/s0378-1135(00)00160-7. [DOI] [PubMed] [Google Scholar]
- Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine. 2007a;25:5637–5644. doi: 10.1016/j.vaccine.2006.10.051. [DOI] [PubMed] [Google Scholar]
- Alexander DJ. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis. 2007b;51:161–166. doi: 10.1637/7602-041306R.1. [DOI] [PubMed] [Google Scholar]
- Aly MM, Arafa A, Hassan MK. Epidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 2006. Avian Dis. 2008;52:269–277. doi: 10.1637/8166-103007-Reg.1. [DOI] [PubMed] [Google Scholar]
- Arafa A, Suarez D, Kholosy SG, Hassan MK, Nasef S, Selim A, Dauphin G, Kim M, Yilma J, Swayne D, Aly MM. Evolution of highly pathogenic avian influenza H5N1 viruses in Egypt indicating progressive adaptation. Arch Virol. 2012 doi: 10.1007/s00705-012-1385-9. [DOI] [PubMed] [Google Scholar]
- Arias CF, Escalera-Zamudio M, Soto-Del Rio Mde L, Cobian-Guemes AG, Isa P, Lopez S. Molecular anatomy of 2009 influenza virus A (H1N1) Arch Med Res. 2009;40:643–654. doi: 10.1016/j.arcmed.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Arsnoe DM, Ip HS, Owen JC. Influence of body condition on influenza A virus infection in mallard ducks: experimental infection data. PLoS One. 2011;6:e22633. doi: 10.1371/journal.pone.0022633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artois M, Bicout D, Doctrinal D, Fouchier R, Gavier-Widen D, Globig A, Hagemeijer W, Mundkur T, Munster V, Olsen B. Outbreaks of highly pathogenic avian influenza in Europe: the risks associated with wild birds. Revue scientifique et technique. 2009;28:69–92. doi: 10.20506/rst.28.1.1854. [DOI] [PubMed] [Google Scholar]
- Aubin JT, Azebi S, Balish A, Banks J, Bhat N, Bright RA, Brown I, Buchy P, Burguiere AM, Chen HI, Cheng P, Cox NJ, Crosier A, Curns A, Cuvelier F, Deng GH, Desheva J, Desvaux S, Diep NH, Donis RO, Douglas A, Dowell SF, Dung NT, Edwards L, Fukuda K, Garten R, Govorkova E, Gregory V, Hampson A, Hanh NTH, Harper S, Hay A, Hoffmann E, Hulse D, Imai M, Itamura S, Jadhao S, Jeannin P, Kang C, Katz J, Kim JH, Klimov A, Kwon YK, Lee CW, Lien PS, Li YB, Lim W, Lin YP, Lindstom S, Loftin L, Mabry J, Mai LQ, Maines T, Manuguerra JC, Mase M, Matsuoka Y, McCarron M, Medina MJ, Nguyen D, Ninomiya A, Obuchi M, Odagiri T, Peiris M, Perdue ML, Reynes JM, Robertson J, Rousseaux C, Saito T, Sangkitporn S, Shaw M, Simmerman JM, Slomka M, Smith C, Sorn S, Spackman E, Stohr K, Suarez DL, Sung HW, Swayne DE, Tardy-Panit M, Tashiro M, Thawatsupha P, Tumpey T, Uyeki T, Van Tu P, van der Werf S, Vong S, Webby R, Webster R, Wood J, Xu XY, Yi G, Zhang WQ, World Hlth Org Global Influenza. P. Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005;11:1515–1521. doi: 10.3201/eid1110.050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl J, Vijaykrishna D, Holmes EC, Smith GJ, Guan Y. Gene flow and competitive exclusion of avian influenza A virus in natural reservoir hosts. Virology. 2009;390:289–297. doi: 10.1016/j.virol.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J, Speidel ES, Moore E, Plowright L, Piccirillo A, Capua I, Cordioli P, Fioretti A, Alexander DJ. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of highly pathogenic H7N1 avian influenza viruses in Italy. Arch Virol. 2001;146:963–973. doi: 10.1007/s007050170128. [DOI] [PubMed] [Google Scholar]
- Barber MR, Aldridge JR, Jr., Webster RG, Magor KE. Association of RIG-I with innate immunity of ducks to influenza. Proc Natl Acad Sci U S A. 2010;107:5913–5918. doi: 10.1073/pnas.1001755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold P. Bird Migration, A General Survey. 2 ed Oxford University Perss; 1993. The phenomena of bird migration; pp. 35–85. [Google Scholar]
- Bertram S, Glowacka I, Steffen I, Kuhl A, Pohlmann S. Novel insights into proteolytic cleavage of influenza virus hemagglutinin. Rev Med Virol. 2010;20:298–310. doi: 10.1002/rmv.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham J, Green DJ, Lowther S, Klippel J, Burggraaf S, Anderson DE, Wibawa H, Hoa DM, Long NT, Vu PP, Middleton DJ, Daniels PW. Infection studies with two highly pathogenic avian influenza strains (Vietnamese and Indonesian) in Pekin ducks (Anas platyrhynchos), with particular reference to clinical disease, tissue tropism and viral shedding. Avian Pathol. 2009;38:267–278. doi: 10.1080/03079450903055371. [DOI] [PubMed] [Google Scholar]
- Brown IH. Summary of avian influenza activity in Europe, Asia, and Africa, 2006–2009. Avian Dis. 2010;54:187–193. doi: 10.1637/8949-053109-Reg.1. [DOI] [PubMed] [Google Scholar]
- Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet Microbiol. 2009;136:20–26. doi: 10.1016/j.vetmic.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE, Swayne DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis. 2008;14:136–142. doi: 10.3201/eid1401.070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 2007;51:285–289. doi: 10.1637/7636-042806R.1. [DOI] [PubMed] [Google Scholar]
- Buonagurio Deborah A., Nakada Susumu, Desselberger Ulrich, Krystal Mark, Palese Peter. Noncumulative sequence changes in the hemagglutinin genes of influenza C virus isolates. Virology. 1985;146:221–232. doi: 10.1016/0042-6822(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Butler D. Blogger reveals China's migratory goose farms near site of flu outbreak. Nature. 2006;441:263. doi: 10.1038/441263a. [DOI] [PubMed] [Google Scholar]
- Buxton Bridges C, Katz JM, Seto WH, Chan PK, Tsang D, Ho W, Mak KH, Lim W, Tam JS, Clarke M, Williams SG, Mounts AW, Bresee JS, Conn LA, Rowe T, Hu-Primmer J, Abernathy RA, Lu X, Cox NJ, Fukuda K. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181:344–348. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- Capua I, Alexander DJ. Animal and human health implications of avian influenza infections. Bioscience Reports. 2007;27:359–372. doi: 10.1007/s10540-007-9057-9. [DOI] [PubMed] [Google Scholar]
- Capua I, Mutinelli F, Marangon S, Alexander DJ. H7N1 avian influenza in Italy (1999 to 2000) in intensively reared chickens and turkeys. Avian Pathol. 2000;29:537–543. doi: 10.1080/03079450020016779. [DOI] [PubMed] [Google Scholar]
- Carboneras C. Order Anseriformes. In: Del Hoyo J, Elliott A, Sargatal J, editors. Handbook of the Birds of the World, vol. 1 Ostrich to Ducks. Lynx Edicions; Barcelonas, Spain: 1992. [Google Scholar]
- Cattoli G, Fusaro A, Monne I, Coven F, Joannis T, El-Hamid HS, Hussein AA, Cornelius C, Amarin NM, Mancin M, Holmes EC, Capua I. Evidence for differing evolutionary dynamics of A/H5N1 viruses among countries applying or not applying avian influenza vaccination in poultry. Vaccine. 2011;29:9368–9375. doi: 10.1016/j.vaccine.2011.09.127. [DOI] [PubMed] [Google Scholar]
- Cattoli G, Monne I, Fusaro A, Joannis TM, Lombin LH, Aly MM, Arafa AS, Sturm-Ramirez KM, Couacy-Hymann E, Awuni JA, Batawui KB, Awoume KA, Aplogan GL, Sow A, Ngangnou AC, El Nasri Hamza IM, Gamatie D, Dauphin G, Domenech JM, Capua I. Highly pathogenic avian influenza virus subtype H5N1 in Africa: a comprehensive phylogenetic analysis and molecular characterization of isolates. PLoS One. 2009;4:e4842. doi: 10.1371/journal.pone.0004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauthen AN, Swayne DE, Schultz-Cherry S, Perdue ML, Suarez DL. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J Virol. 2000;74:6592–6599. doi: 10.1128/jvi.74.14.6592-6599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, Basta S, O'Neill R, Schickli J, Palese P, Henklein P, Bennink JR, Yewdell JW. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7:1306–1312. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Chen GW, Wang CH, Huang CH, Wang YC, Shih SR. Differential localization and function of PB1-F2 derived from different strains of influenza A virus. J Virol. 2010;84:10051–10062. doi: 10.1128/JVI.00592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Deng G, Li Z, Tian G, Li Y, Jiao P, Zhang L, Liu Z, Webster RG, Yu K. The evolution of H5N1 influenza viruses in ducks in southern China. Proc Natl Acad Sci U S A. 2004;101:10452–10457. doi: 10.1073/pnas.0403212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matsuoka Y, Swayne D, Chen Q, Cox NJ, Murphy BR, Subbarao K. Generation and characterization of a cold-adapted influenza A H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine. 2003;21:4430–4436. doi: 10.1016/s0264-410x(03)00430-4. [DOI] [PubMed] [Google Scholar]
- Chen H, Smith GJ, Li KS, Wang J, Fan XH, Rayner JM, Vijaykrishna D, Zhang JX, Zhang LJ, Guo CT, Cheung CL, Xu KM, Duan L, Huang K, Qin K, Leung YH, Wu WL, Lu HR, Chen Y, Xia NS, Naipospos TS, Yuen KY, Hassan SS, Bahri S, Nguyen TD, Webster RG, Peiris JS, Guan Y. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci U S A. 2006a;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Smith GJD, Zhang SY, Qin K, Wang J, Li KS, Webster RG, Peiris JSM, Guan Y. H5N1 virus outbreak in migratory waterfowl. Nature. 2005;436:191–192. doi: 10.1038/nature03974. [DOI] [PubMed] [Google Scholar]
- Chen HL, Li YB, Li ZJ, Shi JZ, Shinya K, Deng GH, Qi QL, Tian GB, Fan SF, Zhao HD, Sun YX, Kawaoka Y. Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China. J Virol. 2006b;80:5976–5983. doi: 10.1128/JVI.00110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention Update: Isolation of avian influenza A (H5N1) viruses from humans-Hong Kong, 1997–98. MMWR Morb Mortal Wkly Rep. 1998;46:1245–1247. [PubMed] [Google Scholar]
- Cheung CL, Vijaykrishna D, Smith GJ, Fan XH, Zhang JX, Bahl J, Duan L, Huang K, Tai H, Wang J, Poon LL, Peiris JS, Chen H, Guan Y. Establishment of influenza A virus (H6N1) in minor poultry species in southern China. J Virol. 2007;81:10402–10412. doi: 10.1128/JVI.01157-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas EC, de Jong JC, van Beek R, Rimmelzwaan GF, Osterhaus AD. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine. 1998a;16:977–978. doi: 10.1016/s0264-410x(98)00005-x. [DOI] [PubMed] [Google Scholar]
- Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998b;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- Costa TP, Brown JD, Howerth EW, Stallknecht DE. Variation in viral shedding patterns between different wild bird species infected experimentally with low-pathogenicity avian influenza viruses that originated from wild birds. Avian Pathol. 2011;40:119–124. doi: 10.1080/03079457.2010.540002. [DOI] [PubMed] [Google Scholar]
- Couacy-Hymann E, Kouakou VA, Aplogan GL, Awoume F, Kouakou CK, Kakpo L, Sharp BR, McClenaghan L, McKenzie P, Webster RG, Webby RJ, Croville MF, Soubies G, Barbieri SM, Klopp J, Mariette C, Bouchez J, Camus-Bouclainville O, Guerin C, J.L. Field monitoring of avian influenza viruses: whole-genome sequencing and tracking of neuraminidase evolution using 454 pyrosequencing. J Clin Microbiol. 2012;50:2881–2887. doi: 10.1128/JCM.01142-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JM, MacRae S, Newton JR, Wattrang E, Elton DM. Equine influenza: a review of an unpredictable virus. Vet J. 2011;189:7–14. doi: 10.1016/j.tvjl.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Domingo E, Holland JJ. RNA virus mutations and fitness for survival. Annu Rev Microbiol. 1997;51:151–178. doi: 10.1146/annurev.micro.51.1.151. [DOI] [PubMed] [Google Scholar]
- Delaney S. The Mute Swan in Europe – A preliminary assessment of numbers, distribution and potential risks in dissemination of HPAI – H5N1. [accessed 03.16.2013];Wetlands International. 2006 http://www.wetlands.org/LinkClick.aspx?fileticket=0hONijRYbSs%3D&tabid=56;
- Drake JW, Holland JJ. Mutation rates among RNA viruses. Proc Natl Acad Sci U S A. 1999;96:13910–13913. doi: 10.1073/pnas.96.24.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatez MF, Olinger CM, Owoade AA, Tarnagda Z, Tahita MC, Sow A, De Landtsheer S, Ammerlaan W, Ouedraogo JB, Osterhaus AD, Fouchier RA, Muller CP. Molecular and antigenic evolution and geographical spread of H5N1 highly pathogenic avian influenza viruses in western Africa. J Gen Virol. 2007;88:2297–2306. doi: 10.1099/vir.0.82939-0. [DOI] [PubMed] [Google Scholar]
- Duan L, Bahl J, Smith GJ, Wang J, Vijaykrishna D, Zhang LJ, Zhang JX, Li KS, Fan XH, Cheung CL, Huang K, Poon LL, Shortridge KF, Webster RG, Peiris JS, Chen H, Guan Y. The development and genetic diversity of H5N1 influenza virus in China, 1996–2006. Virology. 2008;380:243–254. doi: 10.1016/j.virol.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Campitelli L, Fan XH, Leung YH, Vijaykrishna D, Zhang JX, Donatelli I, Delogu M, Li KS, Foni E, Chiapponi C, Wu WL, Kai H, Webster RG, Shortridge KF, Peiris JS, Smith GJ, Chen H, Guan Y. Characterization of low-pathogenic H5 subtype influenza viruses from Eurasia: implications for the origin of highly pathogenic H5N1 viruses. J Virol. 2007;81:7529–7539. doi: 10.1128/JVI.00327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zoghby EF, Arafa AS, Hassan MK, Aly MM, Selim A, Kilany WH, Selim U, Nasef S, Aggor MG, Abdelwhab EM, Hafez HM. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch Virol. 2012;157:1167–1172. doi: 10.1007/s00705-012-1269-z. [DOI] [PubMed] [Google Scholar]
- Ellis TM, Bousfield RB, Bissett LA, Dyrting KC, Luk GS, Tsim ST, Sturm-Ramirez K, Webster RG, Guan Y, Malik Peiris JS. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004;33:492–505. doi: 10.1080/03079450400003601. [DOI] [PubMed] [Google Scholar]
- Elton D, Bryant N. Facing the threat of equine influenza. Equine Vet J. 2011;43:250–258. doi: 10.1111/j.2042-3306.2010.00357.x. [DOI] [PubMed] [Google Scholar]
- Empres-i database [accessed 03.06.2013]; http://empres-i.fao.org/eipws3g/;
- [accessed 04.30.2013];Empres HPAI H5N1 Global overview. 2012 Apr-Jun; http://www.fao.org/docrep/016/ap387e/ap387e.pdf;
- Feare CJ. The role of wild birds in the spread of HPAI H5N1. Avian Dis. 2007;51:440–447. doi: 10.1637/7575-040106R1.1. [DOI] [PubMed] [Google Scholar]
- Feare CJ, Kato T, Thomas R. Captive rearing and release of Bar-headed Geese (Anser indicus) in China: a possible HPAI H5N1 virus infection route to wild birds. J Wildl Dis. 2010;46:1340–1342. doi: 10.7589/0090-3558-46.4.1340. [DOI] [PubMed] [Google Scholar]
- Flint PL, Franson JC. Does influenza A affect body condition of wild mallard ducks, or vice versa? Proc Biol Sci. 2009;276:2345–2346. doi: 10.1098/rspb.2008.1962. discussion 2347–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations . Global Livestock Production and Health Atlas. Rome, Italy: 2012. [accessed 09.10.2012]. kids.fao.org/glipha; [Google Scholar]
- Fouchier RA, Munster VJ. Epidemiology of low pathogenic avian influenza viruses in wild birds. Rev Sci Tech. 2009;28:49–58. doi: 10.20506/rst.28.1.1863. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79:2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidet N, Newman SH, Hagemeijer W, Dodman T, Cappelle J, Hammoumi S, De Simone L, Takekawa JY. Duck migration and past influenza A (H5N1) outbreak areas. Emerg Infect Dis. 2008;14:1164–1166. doi: 10.3201/eid1407.071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Crawford JM, Latimer JW, Rivera-Cruz E, Perdue ML. Heterogeneity in the haemagglutinin gene and emergence of the highly pathogenic phenotype among recent H5N2 avian influenza viruses from Mexico. J Gen Virol. 1996;77(Pt 7):1493–1504. doi: 10.1099/0022-1317-77-7-1493. [DOI] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr., Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Chaitaweesub P, Parakarnawongsa T, Premashthira S, Tiensin T, Kalpravidh W, Wagner H, Slingenbergh J. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg Infect Dis. 2006a;12:227–234. doi: 10.3201/eid1202.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Xiao X, Domenech J, Lubroth J, Martin V, Slingenbergh J. Anatidae migration in the western palearctic and spread of highly pathogenic avian influenza H5N1 virus. Emerg Infect Dis. 2006b;12:1650–1656. doi: 10.3201/eid1211.060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISAID - Global Initiative on Sharing Avian Influenza Data [Accessed 05.02.2013];EpiFlu™. [Google Scholar]
- Global Nature Fund. International Stiftung fuer Umwelt und Natur [Accessed 09.17.2012]; http://www.globalnature.org/docs/02_vorlage.asp?id=15793&sp=E&m1=11089& m2=11093&m3=11178&m4=15621&m5=15793&m6=&domid=1011.
- Gohrbandt S, Veits J, Breithaupt A, Hundt J, Teifke JP, Stech O, Mettenleiter TC, Stech J. H9 avian influenza reassortant with engineered polybasic cleavage site displays a highly pathogenic phenotype in chicken. JGV. 2011;92:1843–1853. doi: 10.1099/vir.0.031591-0. [DOI] [PubMed] [Google Scholar]
- Guan Y, Peiris JS, Lipatov AS, Ellis TM, Dyrting KC, Krauss S, Zhang LJ, Webster RG, Shortridge KF. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc Natl Acad Sci U S A. 2002a;99:8950–8955. doi: 10.1073/pnas.132268999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Peiris M, Kong KF, Dyrting KC, Ellis TM, Sit T, Zhang LJ, Shortridge KF. H5N1 influenza viruses isolated from geese in Southeastern China: evidence for genetic reassortment and interspecies transmission to ducks. Virology. 2002b;292:16–23. doi: 10.1006/viro.2001.1207. [DOI] [PubMed] [Google Scholar]
- Guan Y, Poon LL, Cheung CY, Ellis TM, Lim W, Lipatov AS, Chan KH, Sturm-Ramirez KM, Cheung CL, Leung YH, Yuen KY, Webster RG, Peiris JS. H5N1 influenza: a protean pandemic threat. Proc Natl Acad Sci U S A. 2004;101:8156–8161. doi: 10.1073/pnas.0402443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol. 2000;74:9372–9380. doi: 10.1128/jvi.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, de Jong MD, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007;20:243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Shortridge KF, Krauss S, Webster RG. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc Natl Acad Sci U S A. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YJ, Krauss S, Senne DA, Mo IP, Lo KS, Xiong XP, Norwood M, Shortridge KF, Webster RG, Guan Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267:279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- Hafez MH, Arafa A, Abdelwhab EM, Selim A, Khoulosy SG, Hassan MK, Aly MM. Avian influenza H5N1 virus infections in vaccinated commercial and backyard poultry in Egypt. Poult Sci. 2010;89:1609–1613. doi: 10.3382/ps.2010-00708. [DOI] [PubMed] [Google Scholar]
- Happold JR, Brunhart I, Schwermer H, Stark KD. Surveillance of H5 avian influenza virus in wild birds found dead. Avian Dis. 2008;52:100–105. doi: 10.1637/8021-051407-Reg. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Lindstrom A, Jenni-Eiermann S, Koolhaas A, Piersma T. Long flights do not influence immune responses of a long-distance migrant bird: a wind-tunnel experiment. Journal of Experimental Biology. 2007;210:1123–1131. doi: 10.1242/jeb.02712. [DOI] [PubMed] [Google Scholar]
- Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science. 2001;293:1840–1842. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- Hill NJ, Takekawa JY, Cardona CJ, Meixell BW, Ackerman JT, Runstadler JA, Boyce WM. Cross-seasonal patterns of avian influenza virus in breeding and wintering migratory birds: a flyway perspective. Vector Borne Zoonotic Dis. 2012;12:243–253. doi: 10.1089/vbz.2010.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw VS, Webster RG, Bean WJ, Sriram G. THE ECOLOGY OF INFLUENZA-VIRUSES IN DUCKS AND ANALYSIS OF INFLUENZA-VIRUSES WITH MONOCLONAL-ANTIBODIES. Comparative Immunology Microbiology and Infectious Diseases. 1980a;3:155–164. doi: 10.1016/0147-9571(80)90051-x. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Webster RG, Turner B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can J Microbiol. 1980b;26:622–629. doi: 10.1139/m80-108. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Wood JM, Webster RG, Deibel R, Turner B. Circulation of influenza viruses and paramyxoviruses in waterfowl originating from two different areas of North America. Bull World Health Organ. 1985;63:711–719. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Stech J, Leneva I, Krauss S, Scholtissek C, Chin PS, Peiris M, Shortridge KF, Webster RG. Characterization of the influenza A virus gene pool in avian species in southern China: was H6N1 a derivative or a precursor of H5N1? J Virol. 2000;74:6309–6315. doi: 10.1128/jvi.74.14.6309-6315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TW, Munier S, Larcher T, Soubieux D, Ledevin M, Esnault E, Tourdes A, Croville G, Guerin JL, Quere P, Volmer R, Naffakh N, Marc D. Length variations in the NA stalk of an H7N1 influenza virus have opposite effects on viral excretion in chickens and ducks. J Virol. 2012;86:584–588. doi: 10.1128/JVI.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T, Rivera E, Pearson J, Senne D, Krauss S, Kawaoka Y, Webster RG. Origin and molecular changes associated with emergence of a highly pathogenic H5N2 influenza virus in Mexico. Virology. 1995;213:223–230. doi: 10.1006/viro.1995.1562. [DOI] [PubMed] [Google Scholar]
- Hossain MJ, Hickman D, Perez DR. Evidence of expanded host range and mammalian-associated genetic changes in a duck H9N2 influenza virus following adaptation in quail and chickens. PLoS One. 2008;3:e3170. doi: 10.1371/journal.pone.0003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden KJ, Brockhoff EJ, Caya FD, McLeod LJ, Lavoie M, Ing JD, Bystrom JM, Alexandersen S, Pasick JM, Berhane Y, Morrison ME, Keenliside JM, Laurendeau S, Rohonczy EB. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can Vet J. 2009;50:1153–1161. [PMC free article] [PubMed] [Google Scholar]
- Hoye BJ, Munster VJ, Nishiura H, Klaassen M, Fouchier RA. Surveillance of wild birds for avian influenza virus. Emerg Infect Dis. 2010;16:1827–1834. doi: 10.3201/eid1612.100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humberd J, Guan Y, Webster RG. Comparison of the replication of influenza A viruses in Chinese ring-necked pheasants and chukar partridges. J Virol. 2006;80:2151–2161. doi: 10.1128/JVI.80.5.2151-2161.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriani R, Samaan G, Gultom A, Loth L, Indryani S, Adjid R, Dharmayanti NL, Weaver J, Mumford E, Lokuge K, Kelly PM, Darminto Environmental sampling for avian influenza virus A (H5N1) in live-bird markets, Indonesia. Emerg Infect Dis. 2010;16:1889–1895. doi: 10.3201/eid1612.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabayashi Y, Mundkur T. Atlas of Key Sites for Anatidae in the East Asian Flyway. [accessed 09.12.2012];Wetlands International. 1999 http://www.wetlands.org/WatchRead/Currentpublications/tabid/56/mod/1570/articleType/ArticleView/articleId/1518/Default.aspx,;
- Wetlands International [accessed 09.10.2012];Flyways for Waterbirds. 2012 http://www.wetlands.org;
- Ito T, Okazaki K, Kawaoka Y, Takada A, Webster RG, Kida H. Perpetuation of influenza A viruses in Alaskan waterfowl reservoirs. Arch Virol. 1995;140:1163–1172. doi: 10.1007/BF01322743. [DOI] [PubMed] [Google Scholar]
- Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337:199–204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara M, Matsuno K, Simulundu E, Muramatsu M, Noyori O, Manzoor R, Nakayama E, Igarashi M, Tomabechi D, Yoshida R, Okamatsu M, Sakoda Y, Ito K, Kida H, Takada A. An H5N1 highly pathogenic avian influenza virus that invaded Japan through waterfowl migration. Jpn J Vet Res. 2011;59:89–100. [PubMed] [Google Scholar]
- Kamal RP, Tosh C, Pattnaik B, Behera P, Nagarajan S, Gounalan S, Shrivastava N, Shankar BP, Pradhan HK. Analysis of the PB2 gene reveals that Indian H5N1 influenza virus belongs to a mixed-migratory bird sub-lineage possessing the amino acid lysine at position 627 of the PB2 protein. Arch Virol. 2007;152:1637–1644. doi: 10.1007/s00705-007-1002-5. [DOI] [PubMed] [Google Scholar]
- Kang HM, Batchuluun D, Kim MC, Choi JG, Erdene-Ochir TO, Paek MR, Sugir T, Sodnomdarjaa R, Kwon JH, Lee YJ. Genetic analyses of H5N1 avian influenza virus in Mongolia, 2009 and its relationship with those of eastern Asia. Vet Microbiol. 2011;147:170–175. doi: 10.1016/j.vetmic.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Kayali G, El-Shesheny R, Kutkat MA, Kandeil AM, Mostafa A, Ducatez MF, McKenzie PP, Govorkova EA, Nasraa MH, Webster RG, Webby RJ, Ali MA. Continuing threat of influenza (H5N1) virus circulation in Egypt. Emerg Infect Dis. 2011a;17:2306–2308. doi: 10.3201/eid1712.110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayali G, Webby RJ, Ducatez MF, El Shesheny RA, Kandeil AM, Govorkova EA, Mostafa A, Ali MA. The epidemiological and molecular aspects of influenza H5N1 viruses at the human-animal interface in Egypt. PLoS One. 2011b;6:e17730. doi: 10.1371/journal.pone.0017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keawcharoen J, van den Broek J, Bouma A, Tiensin T, Osterhaus AD, Heesterbeek H. Wild birds and increased transmission of highly pathogenic avian influenza (H5N1) among poultry, Thailand. Emerg Infect Dis. 2011;17:1016–1022. doi: 10.3201/eid1706.100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keawcharoen J, van Riel D, van Amerongen G, Bestebroer T, Beyer WE, van Lavieren R, Osterhaus AD, Fouchier RA, Kuiken T. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1) Emerg Infect Dis. 2008;14:600–607. doi: 10.3201/eid1404.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Chmura AA, Gibbons DW, Fleischer RC, Marra PP, Daszak P. Predicting the global spread of H5N1 avian influenza. Proc Natl Acad Sci U S A. 2006;103:19368–19373. doi: 10.1073/pnas.0609227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Lee YJ, Park CK, Oem JK, Lee OS, Kang HM, Choi JG, Bae YC. Highly pathogenic avian influenza (H5N1) outbreaks in wild birds and poultry, South Korea. Emerg Infect Dis. 2012;18:480–483. doi: 10.3201/eid1803.111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Park CK, Lee YJ, Woo GH, Lee KK, Oem JK, Kim SH, Jean YH, Bae YC, Yoon SS, Roh IS, Jeong OM, Kim HY, Choi JS, Byun JW, Song YK, Kwon JH, Joo YS. An outbreak of highly pathogenic H5N1 avian influenza in Korea, 2008. Vet Microbiol. 2010;141:362–366. doi: 10.1016/j.vetmic.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Kimura H, Abiko C, Peng G, Muraki Y, Sugawara K, Hongo S, Kitame F, Mizuta K, Numazaki Y, Suzuki H, Nakamura K. Interspecies transmission of influenza C virus between humans and pigs. Virus Res. 1997;48:71–79. doi: 10.1016/s0168-1702(96)01427-x. [DOI] [PubMed] [Google Scholar]
- Klenk HD, Rott R, Orlich M, Blodorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975;68:426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Kou Z, Li Y, Yin Z, Guo S, Wang M, Gao X, Li P, Tang L, Jiang P, Luo Z, Xin Z, Ding C, He Y, Ren Z, Cui P, Zhao H, Zhang Z, Tang S, Yan B, Lei F, Li T. The survey of H5N1 flu virus in wild birds in 14 Provinces of China from 2004 to 2007. PLoS One. 2009;4:e6926. doi: 10.1371/journal.pone.0006926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Obert CA, Franks J, Walker D, Jones K, Seiler P, Niles L, Pryor SP, Obenauer JC, Naeve CW, Widjaja L, Webby RJ, Webster RG. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog. 2007;3:e167. doi: 10.1371/journal.ppat.0030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Stallknecht DE, Negovetich NJ, Niles LJ, Webby RJ, Webster RG. Coincident ruddy turnstone migration and horseshoe crab spawning creates an ecological 'hot spot' for influenza viruses. Proc Biol Sci. 2010;277:3373–3379. doi: 10.1098/rspb.2010.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Walker D, Pryor SP, Niles L, Chenghong L, Hinshaw VS, Webster RG. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis. 2004;4:177–189. doi: 10.1089/vbz.2004.4.177. [DOI] [PubMed] [Google Scholar]
- Krumbholz A, Philipps A, Oehring H, Schwarzer K, Eitner A, Wutzler P, Zell R. Current knowledge on PB1-F2 of influenza A viruses. Medical Microbiology and Immunology. 2011;200:69–75. doi: 10.1007/s00430-010-0176-8. [DOI] [PubMed] [Google Scholar]
- Kwon HI, Song MS, Pascua PN, Baek YH, Lee JH, Hong SP, Rho JB, Kim JK, Poo H, Kim CJ, Choi YK. Genetic characterization and pathogenicity assessment of highly pathogenic H5N1 avian influenza viruses isolated from migratory wild birds in 2011, South Korea. Virus Res. 2011;160:305–315. doi: 10.1016/j.virusres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Lam TT, Hon CC, Lemey P, Pybus OG, Shi M, Tun HM, Li J, Jiang J, Holmes EC, Leung FC. Phylodynamics of H5N1 avian influenza virus in Indonesia. Mol Ecol. 2012;21:3062–3077. doi: 10.1111/j.1365-294X.2012.05577.x. [DOI] [PubMed] [Google Scholar]
- Lam TT, Hon CC, Pybus OG, Kosakovsky Pond SL, Wong RT, Yip CW, Zeng F, Leung FC. Evolutionary and transmission dynamics of reassortant H5N1 influenza virus in Indonesia. PLoS Pathog. 2008;4:e1000130. doi: 10.1371/journal.ppat.1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre-Margalef N, Gunnarsson G, Munster VJ, Fouchier RA, Osterhaus AD, Elmberg J, Olsen B, Wallensten A, Haemig PD, Fransson T, Brudin L, Waldenstrom J. Effects of influenza A virus infection on migrating mallard ducks. Proc Biol Sci. 2009;276:1029–1036. doi: 10.1098/rspb.2008.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz SG, Compans RW, Choppin PW. Proteolytic cleavage of the hemagglutinin polypeptide of influenza virus. Function of the uncleaved polypeptide HA. Virology. 1973;52:199–212. doi: 10.1016/0042-6822(73)90409-1. [DOI] [PubMed] [Google Scholar]
- Lebarbenchon C, Stallknecht DE. Host shifts and molecular evolution of H7 avian influenza virus hemagglutinin. Virol J. 2011;8 doi: 10.1186/1743-422X-8-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Suarez DL, Tumpey TM, Sung HW, Kwon YK, Lee YJ, Choi JG, Joh SJ, Kim MC, Lee EK, Park JM, Lu X, Katz JM, Spackman E, Swayne DE, Kim JH. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol. 2005;79:3692–3702. doi: 10.1128/JVI.79.6.3692-3702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Choi YK, Kim YJ, Song MS, Jeong OM, Lee EK, Jeon WJ, Jeong W, Joh SJ, Choi KS, Her M, Kim MC, Kim A, Kim MJ, Ho Lee E, Oh TG, Moon HJ, Yoo DW, Kim JH, Sung MH, Poo H, Kwon JH, Kim CJ. Highly pathogenic avian influenza virus (H5N1) in domestic poultry and relationship with migratory birds, South Korea. Emerg Infect Dis. 2008;14:487–490. doi: 10.3201/eid1403.070767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jiang Y, Jiao P, Wang A, Zhao F, Tian G, Wang X, Yu K, Bu Z, Chen H. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J Virol. 2006a;80:11115–1123. doi: 10.1128/JVI.00993-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zu Dohna H, Cardona CJ, Miller J, Carpenter TE. Emergence and genetic variation of neuraminidase stalk deletions in avian influenza viruses. PLoS One. 2011;6:e14722. doi: 10.1371/journal.pone.0014722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, zu Dohna H, Miller J, Cardona CJ, Carpenter TE. Identifying errors in avian influenza virus gene sequences and implications for data usage of public databases. Genomics. 2010;95:29–36. doi: 10.1016/j.ygeno.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie AT, Chaisingh A, Auewarakul P, Long HT, Hanh NT, Webby RJ, Poon LL, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JS. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- Li Y, Lin Z, Shi J, Qi Q, Deng G, Li Z, Wang X, Tian G, Chen H. Detection of Hong Kong 97-like H5N1 influenza viruses from eggs of Vietnamese waterfowl. Arch Virol. 2006b;151:1615–1624. doi: 10.1007/s00705-006-0738-7. [DOI] [PubMed] [Google Scholar]
- Liang L, Xu B, Chen Y, Liu Y, Cao W, Fang L, Feng L, Goodchild MF, Gong P. Combining spatial-temporal and phylogenetic analysis approaches for improved understanding on global H5N1 transmission. PLoS One. 2010;5:e13575. doi: 10.1371/journal.pone.0013575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, Subbarao K, Guan Y, Krauss S, Shortridge K, Webster R, Cox N, Hay A. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc Natl Acad Sci U S A. 2000;97:9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liniger M, Summerfield A, Zimmer G, McCullough KC, Ruggli N. Chicken cells sense influenza A virus infection through MDA5 and CARDIF signaling involving LGP2. J Virol. 2012;86:705–717. doi: 10.1128/JVI.00742-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatov AS, Evseenko VA, Yen HL, Zaykovskaya AV, Durimanov AG, Zolotykh SI, Netesov SV, Drozdov IG, Onishchenko GG, Webster RG, Shestopalov AM. Influenza (H5N1) viruses in poultry, Russian Federation, 2005–2006. Emerg Infect Dis. 2007;13:539–546. doi: 10.3201/eid1304.061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xiao H, Lei F, Zhu Q, Qin K, Zhang XW, Zhang XL, Zhao D, Wang G, Feng Y, Ma J, Liu W, Wang J, Gao GF. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science. 2005;309:1206. doi: 10.1126/science.1115273. [DOI] [PubMed] [Google Scholar]
- Loth L, Gilbert M, Wu J, Czarnecki C, Hidayat M, Xiao X. Identifying risk factors of highly pathogenic avian influenza (H5N1 subtype) in Indonesia. Prev Vet Med. 2011;102:50–58. doi: 10.1016/j.prevetmed.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova NV, Ozaki H, Kida H, Webster RG, Perez DR. Replication and transmission of influenza viruses in Japanese quail. Virology. 2003;310:8–15. doi: 10.1016/s0042-6822(03)00094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler J, Gorman OT, Ludwig S, Schroeder E, Fitch WM, Webster RG, Scholtissek C. Derivation of the nucleoproteins (NP) of influenza A viruses isolated from marine mammals. Virology. 1990;176:255–261. doi: 10.1016/0042-6822(90)90250-u. [DOI] [PubMed] [Google Scholar]
- Marinova-Petkova A, Georgiev G, Seiler P, Darnell D, Franks J, Krauss S, Webby RJ, Webster RG. Spread of influenza virus A (H5N1) clade 2.3.2.1 to Bulgaria in common buzzards. [[accessed 09.19.2012]];Emerg Infect Dis [Internet] 2012 Oct; doi: 10.3201/eid1810.120357. 2012. http://dx.doi.org/10.3201/eid1810.120357. [DOI] [PMC free article] [PubMed]
- Martin V, Zhou X, Marshall E, Jia B, Fusheng G, FrancoDixon MA, DeHaan N, Pfeiffer DU, Soares Magalhaes RJ, Gilbert M. Risk-based surveillance for avian influenza control along poultry market chains in South China: The value of social network analysis. Prev Vet Med. 2011;102:196–205. doi: 10.1016/j.prevetmed.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mase M, Tsukamoto K, Imada T, Imai K, Tanimura N, Nakamura K, Yamamoto Y, Hitomi T, Kira T, Nakai T, Kiso M, Horimoto T, Kawaoka Y, Yamaguchi S. Characterization of H5N1 influenza A viruses isolated during the 2003–2004 influenza outbreaks in Japan. Virology. 2005;332:167–176. doi: 10.1016/j.virol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Massin P, van der Werf S, Naffakh N. Residue 627 of PB2 is a determinant of cold sensitivity in RNA replication of avian influenza viruses. J Virol. 2001;75:5398–5404. doi: 10.1128/JVI.75.11.5398-5404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley JL, Hornung F, Boyd KL, Smith AM, McKeon R, Bennink J, Yewdell JW, McCullers JA. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe. 2007;2:240–249. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina RA, Garcia-Sastre A. Influenza A viruses: new research developments. Nature Reviews Microbiology. 2011;9:590–603. doi: 10.1038/nrmicro2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton D, Bingham J, Selleck P, Lowther S, Gleeson L, Lehrbach P, Robinson S, Rodenberg J, Kumar M, Andrew M. Efficacy of inactivated vaccines against H5N1 avian influenza infection in ducks. Virology. 2007;359:66–71. doi: 10.1016/j.virol.2006.08.046. [DOI] [PubMed] [Google Scholar]
- Monne I, Hussein HA, Fusaro A, Valastro V, Hamoud MM, Khalefa RA, Dardir SN, Radwan MI, Capua I, Cattoli G. H9N2 influenza A virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respi Viruses. 2012 Jul 2; doi: 10.1111/j.1750-2659.2012.00399.x. doi: 10.1111/j.1750-2659.2012.00399.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens DM, Taubenberger JL. Historical thoughts on influenza viral ecosystems, or behold a pale horse, dead dogs, failing fowl, and sick swine. Influenza Other Respi Viruses. 2010;4:327–337. doi: 10.1111/j.1750-2659.2010.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munier S, Larcher T, Cormier-Aline F, Soubieux D, Su B, Guigand L, Labrosse B, Cherel Y, Quere P, Marc D, Naffakh N. A genetically engineered waterfowl influenza virus with a deletion in the stalk of the neuraminidase has increased virulence for chickens. J Virol. 2010;84:940–952. doi: 10.1128/JVI.01581-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Baas C, Lexmond P, Waldenstrom J, Wallensten A, Fransson T, Rimmelzwaan GF, Beyer WE, Schutten M, Olsen B, Osterhaus AD, Fouchier RA. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007;3:e61. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Schrauwen EJ, de Wit E, van den Brand JM, Bestebroer TM, Herfst S, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Insertion of a multibasic cleavage motif into the hemagglutinin of a low-pathogenic avian influenza H6N1 virus induces a highly pathogenic phenotype. J Virol. 2010;84:7953–7960. doi: 10.1128/JVI.00449-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Wallensten A, Baas C, Rimmelzwaan GF, Schutten M, Olsen B, Osterhaus AD, Fouchier RA. Mallards and highly pathogenic avian influenza ancestral viruses, northern Europe. Emerg Infect Dis. 2005;11:1545–1551. doi: 10.3201/eid1110.050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto Y, Noda T, Kawakami E, Akkina R, Kawaoka Y. Identification of a novel influenza A virus proteins translated from PA mRNA. J Virol. 2013;87:2455–2462. doi: 10.1128/JVI.02656-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EJ, Morse SS. Seasonal oscillation of human infection with influenza A/H5N1 in Egypt and Indonesia. PLoS One. 2011;6:e24042. doi: 10.1371/journal.pone.0024042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan S, Murugkar HV, Tosh C, Behera P, Jain R, Tripathi S, Khandia R, Gupta V, Kulkarni DD, Dubey SC. Avian influenza virus (H5N1) in chickens in India. Vet Rec. 2009;164:128. doi: 10.1136/vr.164.4.128. [DOI] [PubMed] [Google Scholar]
- Nguyen DC, Uyeki TM, Jadhao S, Maines T, Shaw M, Matsuoka Y, Smith C, Rowe T, Lu X, Hall H, Xu X, Balish A, Klimov A, Tumpey TM, Swayne DE, Huynh LP, Nghiem HK, Nguyen HH, Hoang LT, Cox NJ, Katz JM. Isolation and characterization of avian influenza viruses, including highly pathogenic H5N1, from poultry in live bird markets in Hanoi, Vietnam, in 2001. J Virol. 2005;79:4201–4212. doi: 10.1128/JVI.79.7.4201-4212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidom CA, Yamada S, Nidom RV, Rahmawati K, Alamudi MY, Kholik, Indrasari S, Hayati RS, Iwatsuki Horimoto K, Kawaoka Y. Genetic characterization of H5N1 influenza viruses isolated from chickens in Indonesia in 2010. Virus Genes. 2012;44:459–465. doi: 10.1007/s11262-012-0722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niqueux E, Guionie O, Schmitz A, Hars J, Jestin V. Presence of serum antibodies to influenza A subtypes H5 and N1 in swans and ibises in French wetlands, irrespective of highly pathogenic H5N1 natural infection. Avian Dis. 2010;54:502–508. doi: 10.1637/8804-040109-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- Olsen CW, Carey S, Hinshaw L, Karasin AI. Virologic and serologic surveillance for human, swine and avian influenza virus infections among pigs in the north-central United States. Arch Virol. 2000;145:1399–1419. doi: 10.1007/s007050070098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owoade AA, Gerloff NA, Ducatez MF, Taiwo JO, Kremer JR, Muller CP. Replacement of sublineages of avian influenza (H5N1) by reassortments, sub-Saharan Africa. Emerg Infect Dis. 2008;14:1731–1735. doi: 10.3201/eid1411.080555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P, Shaw ML. Orthomyxoviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5 ed. Lippincott Williams & Wilkins; 2007. pp. 1647–1689. [Google Scholar]
- Partnership for the East Asian-Australasian Flyway [accessed 09.20.2012];Clin Microbiol Rev. 20:243–267. http://www.eaaflyway.net/index.php. human health. [Google Scholar]
- Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, Ng TK, Chan KH, Lai ST, Lim WL, Yuen KY, Guan Y. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue ML, Garcia M, Senne D, Fraire M. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res. 1997;49:173–186. doi: 10.1016/s0168-1702(97)01468-8. [DOI] [PubMed] [Google Scholar]
- Prosser DJ, Cui P, Takekawa JY, Tang M, Hou Y, Collins BM, Yan B, Hill NJ, Li T, Li Y, Lei F, Guo S, Xing Z, He Y, Zhou Y, Douglas DC, Perry WM, Newman SH. Wild bird migration across the Qinghai-Tibetan plateau: a transmission route for highly pathogenic H5N1. PLoS One. 2011;6:e17622. doi: 10.1371/journal.pone.0017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KD, Meece JK, Henkel JS, Shukla SK. Birds, migration and emerging zoonoses: west nile virus, lyme disease, influenza A and enteropathogens. Clin Med Res. 2003;1:5–12. doi: 10.3121/cmr.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SM, Shell WM, Barboi G, Onita I, Turcitu M, Cioranu R, Marinova-Petkova A, Goujgoulova G, Webby RJ, Webster RG, Russell C, Slomka MJ, Hanna A, Banks J, Alton B, Barrass L, Irvine RM, Brown IH. First reported incursion of highly pathogenic notifiable avian influenza A H5N1 viruses from clade 2.3.2 into European poultry. Transboundary and Emerging Diseases. 2011;58:76–78. doi: 10.1111/j.1865-1682.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reperant LA, Fuckar NS, Osterhaus AD, Dobson AP, Kuiken T. Spatial and temporal association of outbreaks of H5N1 influenza virus infection in wild birds with the 0 degrees C isotherm. PLoS Pathog. 2010;6:e1000854. doi: 10.1371/journal.ppat.1000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reperant LA, Kuiken T, Osterhaus AD. Adaptive pathways of zoonotic influenza viruses: from exposure to establishment in humans. Vaccine. 2012;30:4419–4434. doi: 10.1016/j.vaccine.2012.04.049. [DOI] [PubMed] [Google Scholar]
- Reperant LA, Rimmelzwaan GF, Kuiken T. Avian influenza viruses in mammals. Rev Sci Tech. 2009;28:137–159. doi: 10.20506/rst.28.1.1876. [DOI] [PubMed] [Google Scholar]
- Rohm C, Horimoto T, Kawaoka Y, Suss J, Webster RG. Do hemagglutinin genes of highly pathogenic avian influenza viruses constitute unique phylogenetic lineages? Virology. 1995;209:664–670. doi: 10.1006/viro.1995.1301. [DOI] [PubMed] [Google Scholar]
- Rott R. The pathogenic determinant of influenza virus. Vet Microbiol. 1992;33:303–310. doi: 10.1016/0378-1135(92)90058-2. [DOI] [PubMed] [Google Scholar]
- Saad MD, Ahmed LS, Gamal-Eldein MA, Fouda MK, Khalil F, Yingst SL, Parker MA, Montevillel MR. Possible avian influenza (H5N1) from migratory bird, Egypt. Emerg Infect Dis. 2007;13:1120–1121. doi: 10.3201/eid1307.061222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda Y, Ito H, Uchida Y, Okamatsu M, Yamamoto N, Soda K, Nomura N, Kuribayashi S, Shichinohe S, Sunden Y, Umemura T, Usui T, Ozaki H, Yamaguchi T, Murase T, Ito T, Saito T, Takada A, Kida H. Reintroduction of H5N1 highly pathogenic avian influenza virus by migratory water birds, causing poultry outbreaks in the 2010–2011 winter season in Japan. J Gen Virol. 2012;93:541–550. doi: 10.1099/vir.0.037572-0. [DOI] [PubMed] [Google Scholar]
- Sakoda Y, Sugar S, Batchluun D, Erdene-Ochir TO, Okamatsu M, Isoda N, Soda K, Takakuwa H, Tsuda Y, Yamamoto N, Kishida N, Matsuno K, Nakayama E, Kajihara M, Yokoyama A, Takada A, Sodnomdarjaa R, Kida H. Characterization of H5N1 highly pathogenic avian influenza virus strains isolated from migratory waterfowl in Mongolia on the way back from the southern Asia to their northern territory. Virology. 2010;406:88–94. doi: 10.1016/j.virol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Salzberg SL, Kingsford C, Cattoli G, Spiro DJ, Janies DA, Aly MM, Brown IH, Couacy-Hymann E, De Mia GM, Dung do H, Guercio A, Joannis T, Maken Ali AS, Osmani A, Padalino I, Saad MD, Savic V, Sengamalay NA, Yingst S, Zaborsky J, Zorman-Rojs O, Ghedin E, Capua I. Genome analysis linking recent European and African influenza (H5N1) viruses. Emerg Infect Dis. 2007;13:713–718. doi: 10.3201/eid1305.070013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato SB, Kawasaki K, Ohnishi S. Hemolytic activity of influenza virus hemagglutinin glycoproteins activated in mildly acidic environments. Proc Natl Acad Sci U S A. 1983;80:3153–3157. doi: 10.1073/pnas.80.11.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, Lasiewsk RC, Bretz WL, Kanwishe J, Cohn JE. Temperature Regulation and Respiration in Ostrich. The COndor. 1969;71:341–352. [Google Scholar]
- Scholtissek C, Rohde W, Von Hoyningen V, Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978;87:13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- Schrauwen EJ, Bestebroer TM, Munster VJ, de Wit E, Herfst S, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Insertion of a multibasic cleavage site in the haemagglutinin of human influenza H3N2 virus does not increase pathogenicity in ferrets. J Gen Virol. 2011;92:1410–1415. doi: 10.1099/vir.0.030379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedyaningsih ER, Isfandari S, Setiawaty V, Rifati L, Harun S, Purba W, Imari S, Giriputra S, Blair PJ, Putnam SD, Uyeki TM, Soendoro T. Epidemiology of cases of H5N1 virus infection in Indonesia, July 2005–June 2006. J Infect Dis. 2007;196:522–527. doi: 10.1086/519692. [DOI] [PubMed] [Google Scholar]
- Sharp GB, Kawaoka Y, Wright SM, Turner B, Hinshaw V, Webster RG. Wild ducks are the reservoir for only a limited number of influenza A subtypes. Epidemiol Infect. 1993;110:161–176. doi: 10.1017/s0950268800050780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharshov K, Silko N, Sousloparov I, Zaykovskaya A, Shestopalov A, Drozdov I. Avian influenza (H5N1) outbreak among wild birds, Russia, 2009. Emerg Infect Dis. 2010;16:349–351. doi: 10.3201/eid1602.090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov AM, Durimanov AG, Evseenko VA, Ternovoi VA, Rassadkin YN, Razumova YV, Zaykovskaya AV, Zolotykh SI, Netesov SV. H5N1 Influenza Virus, Domestic Birds, Western Siberia, Russia. Emerg Infect Dis. 2006;12:1167–1169. doi: 10.3201/eid1207.051338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya K, Makino A, Ozawa M, Kim JH, Sakai-Tagawa Y, Ito M, Le QM, Kawaoka Y. Ostrich involvement in the selection of H5N1 influenza virus possessing mammalian-type amino acids in the PB2 protein. J Virol. 2009;83:13015–13018. doi: 10.1128/JVI.01714-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakoti S, Ito H, Otsuki K, Ito T. Characterization of H5N1 highly pathogenic avian influenza virus isolated from a mountain hawk eagle in Japan. J Vet Med Sci. 2010;72:459–463. doi: 10.1292/jvms.09-0478. [DOI] [PubMed] [Google Scholar]
- Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti KG, Norwood M, Senne D, Sims L, Takada A, Webster RG. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- Si Y, Skidmore AK, Wang T, de Boer WF, Debba P, Toxopeus AG, Li L, Prins HH. Spatio-temporal dynamics of global H5N1 outbreaks match bird migration patterns. Geospatial Health. 2009;4:65–78. doi: 10.4081/gh.2009.211. [DOI] [PubMed] [Google Scholar]
- Siengsanan-Lamont J, Robertson I, Blacksell SD, Ellis T, Fenwick S, Saengchoowong S, Suwanpukdee S, Yongyuttawichai P, Sariya L, Prompiram P, Chaichoun K, Wiriyarat W, Pothieng D, Ratanakorn P. Virological and molecular epidemiological investigations into the role of wild birds in the epidemiology of influenza A/H5N1 in central Thailand. Vet Microbiol. 2011;148:213–218. doi: 10.1016/j.vetmic.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305:371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Bahl J, Vijaykrishna D, Zhang J, Poon LL, Chen H, Webster RG, Peiris JS, Guan Y. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci U S A. 2009a;106:11709–11712. doi: 10.1073/pnas.0904991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GJ, Fan XH, Wang J, Li KS, Qin K, Zhang JX, Vijaykrishna D, Cheung CL, Huang K, Rayner JM, Peiris JS, Chen H, Webster RG, Guan Y. Emergence and predominance of an H5N1 influenza variant in China. Proc Natl Acad Sci U S A. 2006a;103:16936–16941. doi: 10.1073/pnas.0608157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GJ, Naipospos TS, Nguyen TD, de Jong MD, Vijaykrishna D, Usman TB, Hassan SS, Nguyen TV, Dao TV, Bui NA, Leung YH, Cheung CL, Rayner JM, Zhang JX, Zhang LJ, Poon LL, Li KS, Nguyen VC, Hien TT, Farrar J, Webster RG, Chen H, Peiris JS, Guan Y. Evolution and adaptation of H5N1 influenza virus in avian and human hosts in Indonesia and Vietnam. Virology. 2006b;350:258–268. doi: 10.1016/j.virol.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Ellis TM, Dyrting KC, Leung YH, Bahl J, Wong CW, Kai H, Chow MK, Duan L, Chan AS, Zhang LJ, Chen H, Luk GS, Peiris JS, Guan Y. Characterization of avian influenza viruses A (H5N1) from wild birds, Hong Kong, 2004–2008. Emerg Infect Dis. 2009b;15:402–407. doi: 10.3201/eid1503.081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009c;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Soares Magalhaes RJ, Ortiz-Pelaez A, Thi KL, Dinh QH, Otte J, Pfeiffer DU. Associations between attributes of live poultry trade and HPAI H5N1 outbreaks: a descriptive and network analysis study in northern Vietnam. BMC Vet Res. 2010;6:10. doi: 10.1186/1746-6148-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T, Jam-on R, Sae-Heng N, Meemak N, Hulse-Post DJ, Sturm-Ramirez KM, Webster RG. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerg Infect Dis. 2006;12:575–581. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell EM, Perez DR. Adaptation of influenza A/Mallard/Potsdam/178-4/83 H2N2 virus in Japanese quail leads to infection and transmission in chickens. Avian Dis. 2007;51:264–268. doi: 10.1637/7538-032906R.1. [DOI] [PubMed] [Google Scholar]
- Spackman E, Gelb J, Jr., Preskenis LA, Ladman BS, Pope CR, Pantin-Jackwood MJ, McKinley ET. The pathogenesis of low pathogenicity H7 avian influenza viruses in chickens, ducks and turkeys. Virol J. 2010;7:331. doi: 10.1186/1743-422X-7-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, McCracken KG, Winker K, Swayne DE. H7N3 avian influenza virus found in a South American wild duck is related to the Chilean 2002 poultry outbreak, contains genes from equine and North American wild bird lineages, and is adapted to domestic turkeys. J Virol. 2006;80:7760–7764. doi: 10.1128/JVI.00445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, McCracken KG, Winker K, Swayne DE. An avian influenza virus from waterfowl in South America contains genes from North American avian and equine lineages. Avian Dis. 2007a;51:273–274. doi: 10.1637/7529-032106R.1. [DOI] [PubMed] [Google Scholar]
- Spackman E, Swayne DE, Suarez DL, Senne DA, Pedersen JC, Killian ML, Pasick J, Handel K, Pillai SP, Lee CW, Stallknecht D, Slemons R, Ip HS, Deliberto T. Characterization of low-pathogenicity H5N1 avian influenza viruses from North America. J Virol. 2007b;81:11612–11619. doi: 10.1128/JVI.01368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, Stallknecht DE, Slemons RD, Winker K, Suarez DL, Scott M, Swayne DE. Phylogenetic analyses of type A influenza genes in natural reservoir species in North America reveals genetic variation. Virus Res. 2005;114:89–100. doi: 10.1016/j.virusres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Brown JD. Ecology of Avian Influenza in Wild Birds. In: Swayne D, editor. Avian Influenza. 1 ed. Blackwell PUblishing; 2008. pp. 43–58. [Google Scholar]
- Stallknecht DE, Brown JD. Tenacity of avian influenza viruses. Rev Sci Tech. 2009;28:59–67. doi: 10.20506/rst.28.1.1880. [DOI] [PubMed] [Google Scholar]
- Stallknecht DE, Kearney MT, Shane SM, Zwank PJ. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Dis. 1990a;34:412–418. [PubMed] [Google Scholar]
- Stallknecht DE, Shane SM. Host range of avian influenza virus in free-living birds. Vet Res Commun. 1988;12:125–141. doi: 10.1007/BF00362792. [DOI] [PubMed] [Google Scholar]
- Stallknecht DE, Shane SM, Kearney MT, Zwank PJ. Persistence of avian influenza viruses in water. Avian Dis. 1990b;34:406–411. [PubMed] [Google Scholar]
- Stegmann T, Booy FP, Wilschut J. Effects of low pH on influenza virus. Activation and inactivation of the membrane fusion capacity of the hemagglutinin. J Biol Chem. 1987;262:17744–17749. [PubMed] [Google Scholar]
- Stoops AC, Barbara KA, Indrawan M, Ibrahim IN, Petrus WB, Wijaya S, Farzeli A, Antonjaya U, Sin LW, Hidayatullah N, Kristanto I, Tampubolon AM, Purnama S, Supriatna A, Burgess TH, Williams M, Putnam SD, Tobias S, Blair PJ. H5N1 surveillance in migratory birds in Java, Indonesia. Vector Borne Zoonotic Dis. 2009;9:695–702. doi: 10.1089/vbz.2008.0183. [DOI] [PubMed] [Google Scholar]
- Sturm-Ramirez KM, Ellis T, Bousfield B, Bissett L, Dyrting K, Rehg JE, Poon L, Guan Y, Peiris M, Webster RG. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J Virol. 2004;78:4892–4901. doi: 10.1128/JVI.78.9.4892-4901.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm-Ramirez KM, Hulse-Post DJ, Govorkova EA, Humberd J, Seiler P, Puthavathana P, Buranathai C, Nguyen TD, Chaisingh A, Long HT, Naipospos TSP, Chen H, Ellis TM, Guan Y, Peiris JSM, Webster RG. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79:11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez DL. Avian influenza: our current understanding. Anim Health Res Rev. 2010;11:19–33. doi: 10.1017/S1466252310000095. [DOI] [PubMed] [Google Scholar]
- Subbarao EK, London W, Murphy BR. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne DE. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis. 2007;51:242–249. doi: 10.1637/7763-110706-REGR.1. [DOI] [PubMed] [Google Scholar]
- Swayne DE, Beck JR, Perdue ML, Brugh M, Slemons RD. Assessment of the ability of ratite-origin influenza viruses to infect and produce disease in rheas and chickens. Avian Dis. 1996;40:438–447. [PubMed] [Google Scholar]
- Swayne DE, Slemons RD. Using mean infectious dose of high- and low-pathogenicity avian influenza viruses originating from wild duck and poultry as one measure of infectivity and adaptation to poultry. Avian Dis. 2008;52:455–460. doi: 10.1637/8229-012508-Reg.1. [DOI] [PubMed] [Google Scholar]
- Takekawa JY, Newman SH, Xiao X, Prosser DJ, Spragens KA, Palm EC, Yan B, Li T, Lei F, Zhao D, Douglas DC, Muzaffar SB, Ji W. Migration of waterfowl in the East Asian flyway and spatial relationship to HPAI H5N1 outbreaks. Avian Dis. 2010;54:466–476. doi: 10.1637/8914-043009-Reg.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura N, Tsukamoto K, Okamatsu M, Mase M, Imada T, Nakamura K, Kubo M, Yamaguchi S, Irishio W, Hayashi M, Nakai T, Yamauchi A, Nishimura M, Imai K. Pathology of fatal highly pathogenic H5N1 avian influenza virus infection in large-billed crows (Corvus macrorhynchos) during the 2004 outbreak in Japan. Vet Pathol. 2006;43:500–509. doi: 10.1354/vp.43-4-500. [DOI] [PubMed] [Google Scholar]
- Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010;7:440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiensin T, Chaitaweesub P, Songserm T, Chaisingh A, Hoonsuwan W, Buranathai C, Parakamawongsa T, Premashthira S, Amonsin A, Gilbert M, Nielen M, Stegeman A. Highly pathogenic avian influenza H5N1, Thailand, 2004. Emerg Infect Dis. 2005;11:1664–1672. doi: 10.3201/eid1111.050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiensin T, Nielen M, Songserm T, Kalpravidh W, Chaitaweesub P, Amonsin A, Chotiprasatintara S, Chaisingh A, Damrongwatanapokin S, Wongkasemjit S, Antarasena C, Songkitti V, Chanachai K, Thanapongtham W, Stegeman JA. Geographic and temporal distribution of highly pathogenic avian influenza A virus (H5N1) in Thailand, 2004–2005: an overview. Avian Dis. 2007;51:182–188. doi: 10.1637/7635-042806R.1. [DOI] [PubMed] [Google Scholar]
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torremorell M, Allerson M, Corzo C, Diaz A, Gramer M. Transmission of Influenza A Virus in Pigs. Transboundary and Emerging Diseases. 2012 doi: 10.1111/j.1865-1682.2011.01300.x. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Kapczynski DR, Swayne DE. Comparative susceptibility of chickens and turkeys to avian influenza A H7N2 virus infection and protective efficacy of a commercial avian influenza H7N2 virus vaccine. Avian Dis. 2004;48:167–176. doi: 10.1637/7103. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Chaichoune K, Wiriyarat W, Watanabe C, Hayashi T, Patchimasiri T, Nuansrichay B, Parchariyanon S, Okamatsu M, Tsukamoto K, Takemae N, Ratanakorn P, Yamaguchi S, Saito T. Molecular epidemiological analysis of highly pathogenic avian influenza H5N1 subtype isolated from poultry and wild bird in Thailand. Virus Res. 2008a;138:70–80. doi: 10.1016/j.virusres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Mase M, Yoneda K, Kimura A, Obara T, Kumagai S, Saito T, Yamamoto Y, Nakamura K, Tsukamoto K, Yamaguchi S. Highly pathogenic avian influenza virus (H5N1) isolated from whooper swans, Japan. Emerg Infect Dis. 2008b;14:1427–1429. doi: 10.3201/eid1409.080655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Yamaguchi T, Ito H, Ozaki H, Murase T, Ito T. Evolutionary genetics of highly pathogenic H5N1 avian influenza viruses isolated from whooper swans in northern Japan in 2008. Virus Genes. 2009;39:319–323. doi: 10.1007/s11262-009-0403-9. [DOI] [PubMed] [Google Scholar]
- van Gils JA, Munster VJ, Radersma R, Liefhebber D, Fouchier RA, Klaassen M. Hampered foraging and migratory performance in swans infected with low-pathogenic avian influenza A virus. PLoS One. 2007;2:e184. doi: 10.1371/journal.pone.0000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhove MD, Vong S, Guitian J, Holl D, Mangtani P, San S, Ghani AC. Poultry movement networks in Cambodia: implications for surveillance and control of highly pathogenic avian influenza (HPAI/H5N1) Vaccine. 2009;27:6345–6352. doi: 10.1016/j.vaccine.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Vandegrift KJ, Sokolow SH, Daszak P, Kilpatrick AM. Ecology of avian influenza viruses in a changing world. In: Ostfeld RS, Schlesinger WH, editors. Year in Ecology and Conservation Biology 2010. 2010. pp. 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veits J, Weber S, Stech O, Breithaupt A, Graber M, Gohrbandt S, Bogs J, Hundt J, Teifke JP, Mettenleiter TC, Stech J. Avian influenza virus hemagglutinins H2, H4, H8, and H14 support a highly pathogenic phenotype. PNAS. 2012;109:2579–2584. doi: 10.1073/pnas.1109397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaykrishna D, Bahl J, Riley S, Duan L, Zhang JX, Chen H, Peiris JS, Smith GJ, Guan Y. Evolutionary dynamics and emergence of panzootic H5N1 influenza viruses. PLoS Pathog. 2008;4:e1000161. doi: 10.1371/journal.ppat.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viseshakul N, Thanawongnuwech R, Amonsin A, Suradhat S, Payungporn S, Keawchareon J, Oraveerakul K, Wongyanin P, Plitkul S, Theamboonlers A, Poovorawan Y. The genome sequence analysis of H5N1 avian influenza A virus isolated from the outbreak among poultry populations in Thailand. Virology. 2004;328:169–176. doi: 10.1016/j.virol.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. 2002;12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- Wint W, Robinson T. Gridded livestock of the world, 2007. Food and Agriculture Organization of the United Nations; Rome, Italy: 2007. http://www.fao.org/docrep/010/a1259e/a1259e00.htm. [PubMed] [Google Scholar]
- WHO, World Health Organization H5N1 avian influenza: Timeline of major event. 2012 [Google Scholar]
- WHO OIE FAO H5N1 Evolution Working Group Continued evolution of highly pathogenic avian influenza A (H5N1): updated nomenclature. Influenza Other Respi Viruses. 2012;6:1–5. doi: 10.1111/j.1750-2659.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Organization for Animal Health (Office International des Epizooties, OIE) World Animal Health Information Database (WAHID) Interface. Paris, France: 2012a. [accessed 09.15.2012]. http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home; [Google Scholar]
- World Organization for Animal Health (Office International des Epizooties, OIE) UPDATE ON HIGHLY PATHOGENIC AVIAN INFLUENZA IN ANIMALS (TYPE H5 and H7) Paris, France: 2012b. [accessed 09.15.2012]. http://www.oie.int/animal-health-in-the-world/update-on-avian-influenza/2011; [Google Scholar]
- World Organization of Animal Health (Office International des Epizooties, OIE) UPDATE ON HIGHLY PATHOGENIC AVIAN INFLUENZA IN ANIMALS (TYPE H5 and H7) 2012c [Google Scholar]
- Wallensten A, Munster VJ, Latorre-Margalef N, Brytting M, Elmberg J, Fouchier RA, Fransson T, Haemig PD, Karlsson M, Lundkvist A, Osterhaus AD, Stervander M, Waldenstrom J, Bjorn O. Surveillance of influenza A virus in migratory waterfowl in northern Europe. Emerg Infect Dis. 2007;13:404–411. doi: 10.3201/eid1303.061130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Vijaykrishna D, Duan L, Bahl J, Zhang JX, Webster RG, Peiris JS, Chen H, Smith GJ, Guan Y. Identification of the progenitors of Indonesian and Vietnamese avian influenza A (H5N1) viruses from southern China. J Virol. 2008;82:3405–3414. doi: 10.1128/JVI.02468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZG, Jiang WM, Liu S, Hou GY, Li JP, Wang ZY, Chen JM. Increased substitution rate in H5N1 avian influenza viruses during mass vaccination of poultry. Chin. Sci. Bull. 2012;57:2419–2424. [Google Scholar]
- Watanabe Y, Ibrahim MS, Ellakany HF, Kawashita N, Daidoji T, Takagi T, Yasunaga T, Nakaya T, Ikuta K. Antigenic Analysis of Highly Pathogenic Avian Influenza Virus H5N1 Sublineages Co-Circulating in Egypt. J Gen Virol. 2012a doi: 10.1099/vir.0.044032-0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Ibrahim MS, Suzuki Y, Ikuta K. The changing nature of avian influenza A virus (H5N1) TIM. 2012b;20:11–20. doi: 10.1016/j.tim.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Weber TP, Stilianakis NI. Ecologic immunology of avian influenza (H5N1) in migratory birds. Emerg Infect Dis. 2007;13:1139–1143. doi: 10.3201/eid1308.070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Guan Y, Peiris M, Walker D, Krauss S, Zhou NN, Govorkova EA, Ellis TM, Dyrting KC, Sit T, Perez DR, Shortridge KF. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J Virol. 2002;76:118–126. doi: 10.1128/JVI.76.1.118-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Guan Y, Poon L, Krauss S, Webby R, Govorkova E, Peiris M. The spread of the H5N1 bird flu epidemic in Asia in 2004. Arch Virol. 2005:117–129. doi: 10.1007/3-211-29981-5_10. [DOI] [PubMed] [Google Scholar]
- Webster RG, Yakhno M, Hinshaw VS, Bean WJ, Murti KG. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology. 1978;84:268–278. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby R. WHO information meeting on influenza vaccine composition for Northern Hemisphere 2011–2012. Geneva, Switzerland: Feb 17, 2012. Personnel Communication. [Google Scholar]
- Widjaja L, Krauss SL, Webby RJ, Xie T, Webster RG. Matrix gene of influenza a viruses isolated from wild aquatic birds: ecology and emergence of influenza a viruses. J Virol. 2004;78:8771–8779. doi: 10.1128/JVI.78.16.8771-8779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille M, Robertson GJ, Whitney H, Bishop MA, Runstadler JA, Lang AS. Extensive geographic mosaicism in avian influenza viruses from gulls in the northern hemisphere. PLoS One. 2011;6:e20664. doi: 10.1371/journal.pone.0020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise HM, Foeglein A, Sun J, Dalton RM, Patel S, Howard W, Anderson EC, Barclay WS, Digard P. A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA. J Virol. 2009;83:8021–8031. doi: 10.1128/JVI.00826-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; 2007. pp. 1691–1740. [Google Scholar]
- Xu J, Christman MC, Donis RO, Lu G. Evolutionary dynamics of influenza A nucleoprotein (NP) lineages revealed by large-scale sequence analyses. Infect Genet and Evol. 2011;11:2125–2132. doi: 10.1016/j.meegid.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KM, Li KS, Smith GJ, Li JW, Tai H, Zhang JX, Webster RG, Peiris JS, Chen H, Guan Y. Evolution and molecular epidemiology of H9N2 influenza A viruses from quail in southern China, 2000 to 2005. J Virol. 2007;81:2635–2645. doi: 10.1128/JVI.02316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Subbarao, Cox NJ, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology. 1999;261:15–19. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Sakoda Y, Motoshima M, Yoshino F, Soda K, Okamatsu M, Kida H. Characterization of a non-pathogenic H5N1 influenza virus isolated from a migratory duck flying from Siberia in Hokkaido, Japan, in October 2009. Virol J. 2011;8:65. doi: 10.1186/1743-422X-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane I. Epidemics of emerging animal diseases and food-borne infection problems over the last 5 years in Japan. Annals of the New York Academy of Sciences. 2006;1081:30–38. doi: 10.1196/annals.1373.003. [DOI] [PubMed] [Google Scholar]
- Zamarin D, Ortigoza MB, Palese P. Influenza A virus PB1-F2 contributes to viral pathogenesis in mice. J Virol. 2006;80:7976–7983. doi: 10.1128/JVI.00415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Yu Z, Hu Y, Tu J, Zou W, Peng Y, Zhu J, Li Y, Zhang A, Ye Z, Chen H, Jin M. The special neuraminidase stalk-motif responsible for increased virulence and pathogenesis of H5N1 influenza A virus. PLoS One. 2009;4:e6277. doi: 10.1371/journal.pone.0006277. [DOI] [PMC free article] [PubMed] [Google Scholar]