Abstract
Aims
The aim of this study was to test the growth inhibition activity of isothiocyanates (ITC), defense compounds of plants, against common human microbial pathogens.
Methods and Results
In this study we have tested the growth inhibitory activity of a diverse collection of new and previously known representative ITC of various structural classes against pathogenic bacteria, fungi and molds by a serial dilution method. Generally, the compounds were more active against Gram-positive bacteria and fungi exhibiting species-specific bacteriostatic or bactericidal effect. The most active compounds inhibited the growth of both drug-susceptible and multi drug resistant (MDR) pathogens at micromolar concentrations. In the case of Mycobacterium tuberculosis some compounds were more active against MDR, rather than against susceptible strains. The average anti-microbial activity for some of new derivatives was significantly higher than previously reported for the most active ITC compounds. The structure-activity relationship (SAR) established for various classes of ITC with Bacillus cereus (model organism for B. anthracis) followed a distinct pattern, thereby enabling prediction of new more efficient inhibitors. Remarkably, tested bacteria failed to develop resistance to ITC. While effectively inhibiting microbial growth, ITCs displayed moderate toxicity towards eukaryotic cells.
Conclusions
High antimicrobial activity coupled with moderate toxicity grants further thorough studies of the ITC compounds aimed at elucidation of their cellular targets and inhibitory mechanism.
Significance and impact of the study
This systematic study identified new ITC compounds highly active against common human microbial pathogens at the concentrations comparable with those for currently used antimicrobial drugs (e.g. rifampicin, fluconazole). Tested representative pathogens do not develop resistance to the inhibitors. These properties justify further evaluation of ITC compounds as potential antimicrobial agents for medicinal use and for industrial applications.
Keywords: Isothiocyanate, natural, synthetic, bacteria, fungi, growth, inhibition, bacteriostatic, bactericidal
INTRODUCTION
Despite availability of numerous diverse collections of synthetic products and high throughput approaches for their biological testing, natural compounds remain a major source for antimicrobial drugs development. These compounds are especially valuable, since they have undergone natural selection overtime. In our study we concentrated on isothiocyanates (ITC), which are secondary metabolites, used by certain plants for the defense against microbial pathogens (Drobnica et al. 1957; Fenwick, Heaney and Mullin 1983; Delaquis and Mazza 1995; Osborn 1996; Fahey, Zalcmann and Talalay 2001; Tierens et al. 2001). ITCs are stored in the plant cells as inactive precursors, glucosinolates, which can be converted to active compounds by the enzyme myrosinase, normally separated from ITC precursors by a cellular compartment. Destruction of the compartment upon plant damage (which greatly increases the incidence of infection) brings the enzyme in contact with the pro-drug, causing glucose release from the substrate followed by an intramolecular reaction of the liberated aglicone yielding ITC (Fahey, Zalcmann and Talalay (2001) and references therein)). ITC compounds subsequently kill opportunistic pathogenic bacteria, or fungi that enter a plant through the damaged site (Osborn 1996; Mewis 2006). These defense compounds are typical for the family Cruciferae and have been known for a long time for their antimicrobial, nematocidal, and allelopathic activities (Fahey, Zalcmann and Talalay (2001) and references therein). Some of ITC-precursors-enriched plants have been used in folk medicine (Swart, Van Dyk and Malan 2002), while synthetic compound, 4-bromophenyl ITC constitutes an active ingredient of a formulation for dermatomycises treatment (Drobnica et al. 1967).
ITCs have gained recognition following the discovery of their anti-cancer chemoprotective properties (Zhang and Talalay 1994; Michaud et al. 1999; Hecht 2000; Talalay and Fahey 2001; Conaway et al 2002) and selective apoptotic activity against major types of cancer cells (Zhang et al 2003; Srivastava et al 2003; Xiao et al 2003). Many representatives of cruciferous plants naturally enriched with ITC precursors (broccoli, cabbage, watercress, Brussels sprouts, etc.), are widely consumed by humans. Epidemiological studies established reliable correlation between the dietary intake of ITCs and decreased risk of cancer (Michaud et al. 1999; Lam et al. 2009; Herr and Buchier 2010). Remarkably, many ITCs possess both anticancer and antimicrobial activity. Thus ITC sulforaphan has been suggested as a means for treatment of stomach ulcer caused by Helicobacter pylori and for prophylactics of stomach cancer (Fahey et al. 2002).
High antimicrobial activity of many ITCs revealed in previous studies merits search for more active representatives of this class and thorough investigation of their action mechanism in order to use the compounds to combat infections. The studies of ITCs as potential anti-microbial agents are yet at the starting stage (Johns et al. 1982; Chmel et al. 1958; Drobnica et al 1968; Tajima et al 1998; Tajima, Kimoto and Taketo 2001; Fahey et al 2002; Tajima, Kimoto and Taketo 2003; Johansson, Pavia and Chiao 2008; Jung et al 2010, Dufour et al 2012; Conrad et al 2013). Very limited set of ITC have been used in these studies. To this end, in the present research we have tested a diverse collection of known and newly synthesized ITC compounds against common classes of human pathogens including a variety of clinical strains of bacteria, yeasts and fungi.
MATERIALS AND METHODS
All chemicals were from Sigma, Aldrich, Acros Organics, Fluka or TCI America. All solvents used in the synthesis were anhydrous. The structures of tested ITC compounds are presented in Table 1. The synthetic protocols for each compound is indicated in Table 1 and described below. The compounds identity was confirmed by UV, NMR, and mass spectroscopy (see supplemental materials). Gas chromatography with MS detection was performed on 6890GC/5973MS chromatograph (Hewelett Packard). Thin layer chromatography (TLC) was performed on Sigma-Aldrich TLC plates, column chromatography (20 × 2.5 cm) – on Kieselgel 60 (Fluka). UV spectra were recorded on UV160U spectrophotometer (Shimadzu).
Table 1.
Structure and growth-inhibitory activity of ITC and related compounds against B. cereus.
| # | Structure | MIC, μg ml−1
|
Synthetic protocol | |
|---|---|---|---|---|
| E. coli | B. cereus | |||
| Aliphatic ITC derivatives | ||||
| 8. | CH3-NCS | >128 | 128 | * |
| 22. | CH2=CH-CH2 –NCS | >128 | 128 | * |
| 7. | CH3CH2CH2CH2-NCS | >128 | 128 | * |
| Hydroxy- ITC derivatives | ||||
| 18. | HO -(CH2)4–NCS | >128 | 16 | * |
| 5. | HO -(CH2)5–NCS | >128 | 16 | * |
| 21. | HO -(CH2)6–NCS | >128 | 16 | * |
| 44. | HO -(CH2)7–NCS | >128 | 16 | * |
| 45. | HO -(CH2)8–NCS | >128 | 8 | I |
| Charged ITC derivative | ||||
| 6. | −2O3PO -(CH2)5 -NCS | 128 | >128 | III |
| Bifunctional ITC derivatives | ||||
| 38. | SCN -(CH2)4 –NCS | >64 | 2 | I |
| 39. | SCN -(CH2)5 –NCS | >64 | 2 | I |
| 40. | SCN -(CH2)6 –NCS | >64 | 2 | I |
| 41. | SCN -(CH2)7 -NCS | >64 | 2 | I |
| 42. | SCN -(CH2)8 -NCS | >64 | 2 | I |
| 43. | SCN-[(CH2)2 -O-]2 -(CH2)2 -NCS | >64 | 4 | I |
| 37. |
|
8 | 0.5 | I |
| 16. |
|
64 | 2 | I |
| Aromatic and heterocyclic ITC derivatives | ||||
| 4. |
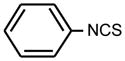
|
>128 | >128 | * |
| 1. |
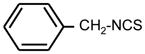
|
64 | 16 | * |
| 17. |
|
>128 | 16 | * |
| 3. |
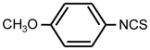
|
>128 | 64 | * |
| 2. |
|
64 | 8 | * |
| 20. |
|
128 | 8 | * |
| 27. |
|
>128 | 16 | I |
| 19. |
|
>128 | 8 | I |
| 34. |
|
>128 | 8 | II |
| 29. |
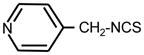
|
>128 | >128 | II |
| 30. |
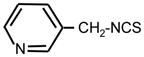
|
>128 | 8 | II |
| 31. |
|
>128 | 4 | II |
| 32. |
|
128 | 8 | II |
| 35. |
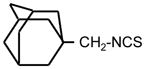
|
>128 | 8 | I |
| 14. |
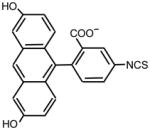
|
>128 | 64 | ** |
| 33. |
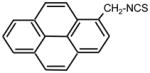
|
>128 | 0.5 | I |
| Control compounds | ||||
| 23. |
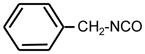
|
>128 | >128 | ** |
| 25. |
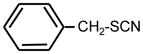
|
>128 | >128 | ** |
Known compounds
Synthesis of ITC
Protocol I (Dyson and George 1937)
Solution of 1 mmol of amino compound in 2 ml of chloroform was added dropwise to the mixture of 1.1 mmol of thiophosgene and 3 ml of chloroform under rigorous agitation in the ice bath. In the case of diamino compounds the amount of thiophosgene was doubled. After 5 min anhydrous triethylamine (two molar equivalents per each amino group) in 2 ml of chloroform was added dropwise under continuous agitation. The mixture was brought to room temperature and supplemented with water (40 ml). After partitioning organic phase was collected, washed by another 40 ml of water and evaporated in vacuo. The products were purified by silicagel column chromatography using hexane/acetone mixture as eluent. Fractions, containing the product were detected by UV light, combined and evaporated under reduced pressure. The products were collected and stored at −20 °C.
Protocol II
One mmol of amino compound was dissolved in 0.5 ml of methylene chloride and added dropwise to the solution of 1.1 mmol of thiocarbonyldiimidazole in 2 ml of the same solvent, followed by addition of 2 eq (to each amino group) of trifluoroacetic acid. After 2 h incubation at 20 °C 2.2 eq of triethylamine was added, the mixture extracted twice with 3 ml of water, organic phase collected, dried over anhydrous Na2SO4, evaporated under reduced pressure and the product purified described above.
Protocol III
Solution of 1 mmol of ITC 5 in 0.2 ml of trimethylphosphate was treated with 1.1 molar excess of POCl3 at room temperature for 1h followed by addition of 0.1 ml of water and rigorous agitation for 20 min. The product was subjected to preparative TLC in acetonitrile-water (5:1) developing system and visualized using UV monitor. After elution with 50 % aqueous methanol the product was concentrated by evaporation under reduced pressure.
Microbial strains and growth conditions
All strains of bacteria and fungi (Tables 2–4) were from the collection of Public Health Research Institute Tuberculosis Center, UMDNJ. Growth conditions for microorganisms are specified below. Components for growth media preparation were purchased from Difco and Sigma.
Table 2.
Activity (MIC, μg ml−1) of selected ITC against representative bacterial species.
| Bacteria | Gram type | ITC compound
|
|||||
|---|---|---|---|---|---|---|---|
| 2 | 20 | 37 | 41 | 42 | 44 | ||
| Acinetobacter sp., BK12786 | − | >64 | >64 | >64 | 32 | >64 | 64 |
| Acinetobacter sp., BK12787 | − | >64 | >64 | >64 | 32 | >64 | 64 |
| Enterobacter cloacii BK12313 | − | >64 | >64 | >64 | >64 | 64 | >64 |
| Enterobacter cloacii BK12315 | − | >64 | >64 | >64 | >64 | 64 | >64 |
| Klebsiella pneumonii BK12260 | − | >64 | >64 | >64 | >64 | >64 | >64 |
| Klebsiella pneumonii BK12261 | − | >64 | >64 | >64 | >64 | >64 | >64 |
| Pseudomonas aeruginosa BK12347 | − | >64 | >64 | >64 | 64 | >64 | >64 |
| Pseudomonas aeruginosa BK12348 | − | >64 | >64 | >64 | 64 | >64 | >64 |
| Serattia marcescens BK12421 | − | >64 | >64 | 64 | >64 | 64 | >64 |
| Serattia marcescens BK12426 | − | >64 | >64 | 64 | >64 | 64 | >64 |
| Shigella sp., BK 12440 | − | 64 | 32–64 | 16 | 64 | >64 | 64 |
| Shigella sp., BK 12441 | − | 32–64 | 32–64 | 16 | 64 | >64 | 64 |
| Bacillus subtilis BK11590 | + | 32–64 | 16–32 | 4 | 2 | 2 | 4 |
| Enterococcus sp., BK11936 | + | 64 | 32 | 16 | 8 | 8 | 32 |
| Enterococcus sp., BK11940 | + | 64 | 32 | 16 | 8 | 8 | 32 |
| MRSA (Staphylococcus aureus) BK12060 | + | 32–64 | 32 | 8 | 4 | 2–4 | 8 |
| MRSA (Staphylococcus aureus) BK12064 | + | 64 | 32 | 4 | 4 | 4 | 16 |
| Staphylococcus epidermidis BK12798 | + | 32 | 16–32 | 0.5 | 4 | 2 | 8 |
| Staphylococcus epidermidis BK12799 | + | 32 | 16–32 | 0.5 | 1 | 2 | 8 |
| Streptococcus sp., BK12233* | + | 4 | 2 | <0.5 | 1–2 | 1–2 | ND |
| Streptococcus sp., BK12235* | + | 8 | 4 | <0.5 | 1–2 | 2 | ND |
Determined on Tryptic Soy agar medium due to the poor growth on Muller-Hinton medium
BK# indicates the number in PHRI TB Center Bacterial Strains Collection
ND – not determined
Table 4.
Activity (MIC, μg ml−1) of selected ITC on pathogenic yeast and fungi.
| Fungus, strain | Compound
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 20 | 30 | 33 | 37 | 41 | 45 | Fluconazole | |
| Candida albicans 5108mdr | 2 | 4 | 0.5 | >64 | 0.5 | 8 | 4 | 32 |
| Candida crusei 6258mdr | 2 | ND | 0.5 | >64 | 0.5 | 2 | 4 | 32 |
| Candida parapsilosis 22019 | 4 | ND | 1 | 32 | 0.5 | 2 | 8 | 2 |
| Candida albicans 90028 | 4 | ND | 2 | 32 | 0.5 | 8 | 8 | 0.25 |
| Candida albicans 90030 | 4 | ND | 1 | 8 | 0.5 | <1 | 4 | 4 |
| Candida dlabrata 250mdr | 1** | ND | 2** | 64 | 0.125** | <1 | 4** | >64 |
| Cryptococcus neoformans CNH99 | <1 | ND | 1 | 4 | <1 | <1 | <1 | 4 |
| Aspergillus fumigatus Rit 1 | 8 | 4 | 4 | >64 | 0.5 | 4 | 8 | >64 |
| Aspergillus fumigatus Rit 11 | 8 | 4 | 4 | >64 | 0.5 | 4 | 8 | >64 |
| Aspergillus fumigatus Rit 21 | 8 | 4 | 4 | >64 | 0.5 | 4 | 8 | >64 |
MICs after 48 hours of growth
ND – not determined
Determination of MIC
Consecutive two-fold dilutions of ITCs were prepared in liquid growth media (specified below) in 96 well plates and immediately mixed with equal volume of a microorganism suspension (~2 × 104 CFU ml−1 as determined by counting the colonies after plating of an aliquot of the bacterial suspension with known turbidity at 600 nm). Visible microbial growth was examined after 24 h incubation at 37 °C. In the case of mycobacteria the growth was determined after 3 weeks incubation at 37 °C. In another version the MIC was determined by plating of the bacteria suspension on agar containing a testing compound (Anon 2000). MICs for bacteria were determined using Muller-Hinton broth or agar; in case of mycobacteria 7H10 Middlebrook culture medium was used. MICs for fungi and yeast were determined using RPMI 1640 liquid medium.
The effect of pre-incubation of the ITC 37 in the culture medium on its growth-inhibitory activity
To determine the ITC 37 stability in culture medium at 37 °C, we incubated the compound (4 μg ml−1) in Muller Hinton broth for various periods of time indicated in Fig. S3 prior to the inoculation with equal volume of Bacillus cereus suspension. After incubation for 20 h at 37 °C the bacterial growth was assessed by measuring the turbidity at 600 nm using microtiter plate reader.
Determination of the growth-inhibitory mode for ITC 37
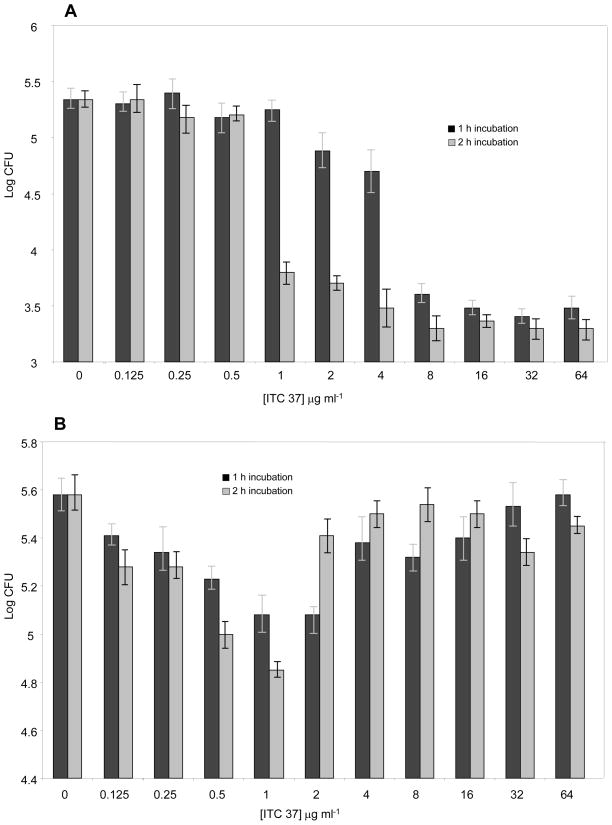
To determine the action mode for the compound on B. cereus or S. aureus, the ITC was added to the bacterial cultures in MHB medium containing 2×104 CFU ml−1 at the concentrations indicated in Fig. 1. After incubation for 1 and 2 h at 37 °C, the number of surviving cells was determined by plating the serial dilutions of the culture medium onto ITC-free Muller Hinton agar and subsequent incubation for 24h.
Fig. 1.
Effect of incubation of B. cereus (A) and S. aureus (B) with various concentrations of ITC 37 on cell viability. Solid bars correspond to 1 h incubation, shaded bars – 2 h incubation.
Toxicity studies
The toxicity assays of the compounds were performed on human macrophages kindly provided by Dr. R. Shepard (PHRI) using cell proliferation kit (Promega). To assess the toxicity of ITC compounds, we used macrophages isolated from donor human blood and immobilized in 96-well plates employing an established protocol (http://www.promega.com/resources/protocols/technical-bulletins/0/celltiter-96-aqueous-nonradioactive-cell-proliferation-assay-protocol/). The assay takes advantage of the ability of life cells to generate colorized compound, which was detected by light absorption.
Attempt to select spontaneous ITC-resistant mutants of B. cereus, Staphylococcus aureus, Mycobacterium bovis, and Candida albicans
Microbial culture suspension of about 109 – 1011 CFU (for various species) was plated onto the Muller-Hinton agar medium containing ITC 37 at a concentration of 10 μg ml−1. The plating density was 107 – 108 CFU per cm2.
RESULTS
SAR for ITC in B. cereus and Escherichia coli growth inhibition assay
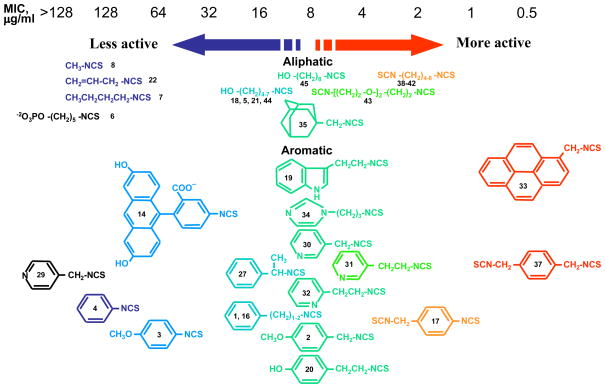
First we assessed growth inhibitory activity of newly synthesized and known ITC of various classes on B. cereus and E. coli as representative of Gram-positive (G+) and Gram-negative (G−) bacterial species correspondingly. Table 1 (see also Fig. 2) shows the MIC values for tested compounds. As reference compounds in this testing we included previously described ITCs 2 and 20, possessing high antibacterial activity (Tajima 1998; Swart 2002). It is seen that with B. cereus ITCs with short hydrocarbon chains (ITC 8, 22, 7) displayed poor activity (MIC = 128 μg ml−1). Remarkably, addition of a hydroxyl group to the terminal carbon of ITC 7 (creating ITC 18) strongly increased the potency (8-fold). In this subset of compounds variation of the spacer between the hydroxyl and ITC groups from 4 to 7 carbons (ITC 18, 5, 21, 44) did not change the ITC activity, while 8-carbon compound (45) displayed lower MIC. Adamantane-based ITC 35 displayed moderate activity (MIC = 8 μg ml−1).
Fig. 2.
Growth inhibitory activity of ITC compounds against B. cereus. The compounds are numbered as in Table 1. The ITCs are colored and positioned according to their growth inhibitory potency indicated on the top.
Bifunctional compounds containing two ITC groups in the molecule (ITC 38–42) possessed high activity (MIC 2 μg ml−1) irrespectively on the linker size. Notably, the substitution of two carbons in the spacer with oxygens in ITC 42 creating ITC 43, reduced the activity. Aromatic ITC efficiently inhibit the growth of various microorganisms (Drobnica et al 1968; Tajima 1998; Swart 2002, Sofrata et al 2011). In SAR studies as reference compound for this group we have chosen benzyl-ITC (ITC 1) possessing moderate activity (MIC = 16 μg ml−1). In control experiments we established a crucial role of the isothiocyano group of ITC 1 in bacterial growth inhibition. Indeed, neither of the parent chemically related derivatives - isocyanate 23 or thiocyanate 25 - displayed antibacterial activity (Table 1).
The deletion of a methylene group in ITC 1 (leading to ITC 4) dramatically decreased the activity, while insertion of an extra methylene group (producing ITC 17), or introduction of an additional methyl substitution into the methylene group adjacent to ITC group (ITC 27) had no effect (Table 1). The insertion of hydroxy- or methoxy groups in the para- position on the phenyl ring (compare ITC 1 – ITC 2, ITC 4 –ITC 3, and ITC 17 – ITC 20) increased growth-inhibitory potency. Similarly to the trend seen in aliphatic compounds bifunctional aromatic ITCs possessed enhanced activity. Thus MIC for ITC 16 and 37 was 2 and 0.5 μg ml−1, correspondingly.
Heterocyclic ITC compounds displayed various levels of activity. Derivatives of indole (ITC 19), pyridine (ITC 30–32) and imidazole (ITC 34) showed comparable potency (MIC = 8–16 μg ml−1). The growth inhibitory efficiency of pyridine derivatives strictly depended on the position of a nitrogen atom in the ring and on the size of methylene spacer between the ITC group and the ring. Thus placing a nitrogen in the para-position (ITC 29) strongly decreased the activity (MIC > 128 μg ml−1) compared to the reference ITC 1, while meta-substitution (ITC 30) yielded better inhibitor (MIC = 8 μg ml−1). ITC derivative with a bulky side chain, fluorescein had low activity, while ITC compounds with more hydrophobic pyrene ring (ITC 33) was exceptionally active (MIC = 0.5 μg ml−1). ITC 33 was also highly potent against Staphylococcus epidermidis, and methicillin-resistant Staphylococcus aureus strains (MIC = 0.5 μg ml−1) (not shown). As seen from Tab. 1, the ITC compounds were much less active against E. coli, a gram-negative bacterium. The most efficient was ITC 37 (MIC = 8 μg ml−1). The MIC for ITC 1, 16, and 32 were in the range of 64–128 μg ml−1, while for the rest of the compounds it was beyond the detection range (>128 μg ml−1).
Activity of the representative ITCs against gram positive and gram-negative bacteria
Next we assessed growth-inhibitory potency of some representative ITC compounds with wide selection of (G−) and (G−) bacterial species listed in Tab. 2. For these testing we have chosen ITCs from various structural groups possessing significant growth inhibitory activity towards B. cereus. Notably, ITC compounds were much more active against G+ than against G− bacteria (Tab. 2). The most susceptible genera were Staphylococcus, Streptococcus, and Bacillus (MIC from <0.5 to 2 μg ml−1). Bifunctional ITC 37, 41 and 42 were highly active against G+ strains. ITC 37 was also moderately effective against some G− strains while ITC 42 and 43 were highly discriminative, affecting only G+ species. Generally, the compounds synthesized in the present study were 2–32 times more active than the reference ITC 2 and 20.
Activity against mycobacteria
Table 3 represents the results of the growth inhibition of clinical isolates of tuberculosis and non-tuberculosis mycobacteria by the same ITC compounds. The ITCs were generally more efficient against M. bovis and M. tuberculosis, than against non-tuberculosis mycobacteria. The most effective were ITC 37, 40–41, and 45, inhibiting the growth of all tested clinical isolates of M. tuberculosis including multi drug resistant (MDR) strains, at concentrations comparable to that of common TB drug rifampicin. Again, the average efficiency of new ITCs was 2–32 times higher than that for the known ITCs.
Table 3.
Activity (MIC, μg ml−1) of selected ITC against mycobacteria
| Mycobacterium | Compound
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 20 | 30 | 37 | 40 | 41 | 42 | 45 | Rif | |
| M. intracellularie | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 4 | 0.5 |
| M. avium | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 4 | 0.5 |
| M. scrofulaceum | 32 | 32 | 32 | 16 | 32 | 32 | 32 | 8 | 0.5 |
| M. cansassi | 32 | 32 | 32 | 4 | 8 | 8 | 4 | 1 | 0.5 |
| M. fortuitum | 32 | 16 | 16 | 32 | 16 | 32 | 32 | 4 | >4 |
| M. tuberculosis W4 | ND | ND | ND | 2 | 2 | 2 | 4 | 1 | 0.5 |
| M. tuberculosis 210 | ND | ND | ND | 2 | 2 | 4 | 4 | 1 | 0.5 |
| M. tuberculosis W * | 8 | 4 | 4 | 1 | 0.5 | 2 | 4 | 1 | >4 |
| M. tuberculosis H37Rv | 32 | 16 | 32 | 2 | 2 | 2 | 4 | 1 | 0.5 |
| M. tuberculosis CDC1561 | 32 | 16 | 16 | 4 | 4 | 2 | 8 | 2 | 0.5 |
| M. tuberculosis P* | 8 | 4 | 4 | 2 | 2 | 4 | 4 | 2 | >4 |
| M. tuberculosis AB | ND | ND | ND | 2 | 4 | 2 | 4 | 2 | 0.5 |
| M. bovis BCG | ND | ND | ND | 2 | 2 | 2 | 4 | 0.5 | 0.5 |
MDR strains
ND – not determined
Activity against pathogenic fungi and molds
The testing results for anti-fungal and anti-yeast activity of ITC compounds are presented in Table 4. It is seen that synthesized ITC compounds efficiently inhibited the growth of the pathogens, being generally more active compared not only to the reference ITC 2 and 20 but also in comparison to the common antifungal drug fluconazol. The most active were ITC 30, 37, and 41, while ITC 33 highly effective against B. cereus, displayed only moderate species-specific antifungal activity.
Testing of the growth-inhibitory mode of ITCs
As previously reported, ITC compounds displayed bactericidal activity against Helicobacter pylori (Fahey et al 2002) and Aggregibacter actinomycetemcomitans (Sofrata et al 2011), while having mostly bacteriostatic effect on E. coli (Tajima et al 2001). Both of these bacterial species belong to G− class. In the present research we investigated the growth inhibitory mechanism of one of the most potent ITC 37 on G+ bacterial pathogens B. cereus and S. aureus (which are both highly and equally sensitive to ITC 37) by determining the number of the surviving cells after the pre-incubation with the ITC. In the case of B. cereus (Fig. 1A), increasing ITC concentration (up to 4 μg ml−1) caused progressive decrease in cell viability so that no CFUs were detected after subsequent 20 h incubation on the same media without ITC. Some colonies emerged only after 4 days of plating subculturing greatly impaired fitness of the survived cells. Notably, neither increase of the drug concentration, nor incubation duration reduced the number of the residual bacterial colonies. The effect of the ITC 37 on S. aureus was more complex (Fig. 1B). Increasing the concentration of the drug up to 1 μg/ ml−1 and incubation time with the ITC from 1 to 2 h, progressively reduced the number of survived cells. However, further increase in the inhibitor’s concentration restored the viability “paradoxic effect”, so that at ITC concentration 64 μg ml−1 the bactericidal effect completely disappeared.
As seen from Fig. S2, the action of ITC 37 was fast, so that at the concentration equal to MIC complete inhibition of the bacterial growth was observed after 30 min exposure to the compound.
Effect of ITC pre-incubation in culture medium on its growth inhibitory activity
It was suggested that ITC can decay due to reaction with culture media components (Adesida et al 1996). To examine the stability of ITC in a culture medium we investigated the effect of ITC 37 pre-incubation in the MHB medium on the ITC growth-inhibitory activity against B. cereus. No growth of bacteria was detected upon immediate addition of the bacterial culture to the inhibitor at concentration equal to MIC and subsequent incubation for 24 h (Fig. S3). However, progressive increase in growth rate was observed if the bacteria were added to the ITC 37 pre-incubated for various periods of time at 37 °C, indicating gradual decrease of inhibition potency and therefore, ITC decay.
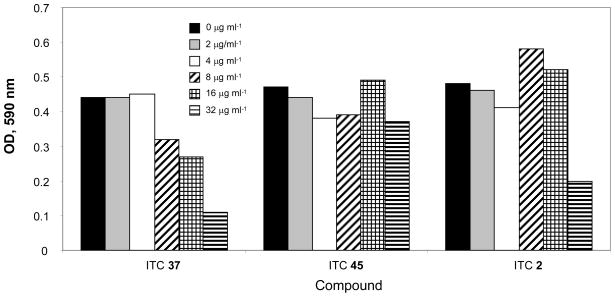
Cytotoxicity of selected ITC compounds on human monocytes
The results of this testing (Fig. 3) indicate that the toxic effect for all of the ITC compounds became detectable at the concentration 16–32 μg/ ml−1, which is much higher than MIC values for the ITC-susceptible bacteria. Also, SAR for cytotoxicity was less sharp then for bacterial growth inhibition (compare Fig. 3 with Tables 1–4). An example could be MICs for B. subtilis (8, 0.5, and 8 μg/ ml−1 for ITC 2, 37, and 45 μg/ ml−1 respectively) and the concentrations of the same compounds reducing 2-fold the number of survived blood monocytes (32, ~22, and >32 μg/ ml−1).
Fig. 3.
Cytotoxicity of ITC on human monocytes. The cells were incubated with an ITC at various concentrations for 24 h and their viability was tested using Cell Proliferation kit (Promega). The optical density reflects the degree of cell viability.
An attempt to select ITC-resistant mutants
Developing resistance to growth inhibitors is an inherent property of most microorganisms. Usually resistance is developed through selection of bacteria that have pre-existing mutations abolishing the binding of a drug, or the enhanced expression of the efflux systems that removes a drug from the cell. The frequency of resistant mutations is specific to both the tested microorganism and a particular drug. To the best of our knowledge, no studies have been conducted so far on resistance to ITCs. We attempted to select pre-existing resistance mutants to one of the most potent ITC 37 by plating B. cereus, S. aureus, M. bovis and C. albicans suspension on agar in the presence of the ITC compound. Optimistically, the frequency of resistance mutations was below the detection limit (less than 10−9 – 10−11). Notably, we were able to see the resistant mutants as countable separate colonies at the same growth conditions when rifampicin was used as control drug at MIC concentrations.
DISCUSSION
SAR for ITCs in microbial growth inhibition
As follows from the testing, ITC growth-inhibitory activity was much higher against G+, than towards G− bacterial species (Tables 1 and 2). SAR for B. cereus has identified the general structural determinants of ITC compounds that strongly affect antimicrobial activity (Table 1 and Fig. 2). They include: i) the size of the organic radical attached to ITC group, which is evident from the poor performance of ITC derivatives with small-size substituents (ITC 8, 22 and 7) compared to those of bigger size; ii) the presence of a methylene group adjacent to ITC group in aromatic compounds, which follows from the comparison of the activity of ITC 1 and 4, as well as 3 and 4. We also showed the importance of presence (in some types of compounds) of a polar uncharged group at distance 3 – 8 carbons from ITC group as follows from comparing the activities of the ITC 1 and 2; 7 and 18; 1 and 30; 17 and 32; 17 and 20. This is also supported by high antimicrobial activity of sulforaphane (Fahey et al., 2002), which can be viewed as derivative of ITC 7 with methylsulfoxide substitution at terminal methyl group. The presence of negatively charged group strongly reduced the inhibitory activity (compare ITC 5 and 6), most likely affecting the cellular uptake.
Bi-functional compounds with two ITC groups show more than 2-fold increase of the activity compared to corresponding mono-functional compounds (compare ITC 1 and 37; 7 and 38; 4 and 16; 1 and 16), displaying strong synergistic effect. In this class of compounds general lipophylicity of the linker seems to be a factor contributing to their activity. Indeed, the substitution of two methylene groups by polar oxygen atoms in ITC 42, while preserving the size and geometry of the molecule, created less active ITC 43. Notably, significant difference of ITC SAR was observed for various microorganisms. Indeed, with B. cereus ITC 37 was much more active than ITC 45, but the activity of the ITCs was reversed in the case of mycobacteria (compare Table 1 and Table 3). Also, ITC 33 was highly active against B. cereus, while displaying poor performance with fungi and mold (compare Tables 1 and 4). Finally, ITC 30 was much more efficient against fungi and molds than against bacteria in relation to ITC 37 (which displayed comparable activity with both bacterial and fungal pathogens) as seen from Tables 1, 3, and 4. These species-specific effects can be explained by the different efficiency of ITC cellular uptake in the tested microorganisms or by different cellular target(s) for the ITCs.
Notably, in case of Mycobacterium tuberculosis some compounds were more active against MDR, than against drug susceptible clinical isolates. This can be explained by reduced fitness of the most of MDR strains, since resistance mutations in many cases affect normal functioning of a targeted enzyme or protein (Comas, 2011).
Establishment of SAR for ITC provides guidance for the future design of more efficient inhibitors. For example, it can be predicted that highly active compound ITC 37 can be further improved by introducing a nitrogen atom into the aromatic ring, since this modification strongly augments the general antimicrobial activity of corresponding mono-functional ITC (compound 1). Also, further enhancement of the activity can be expected for hyrdoxy-ITC derivatives with longer carbon spacer.
Generally, this study yielded efficient antimicrobials active against pathogenic bacteria, fungi, and yeast. The activity of the best of new compounds was 4–32 fold higher than that for previously described ITCs.
The mechanism of ITC inhibitory activity
The actual mechanism by which ITCs inhibit the growth of microbial pathogens remains unknown. The presence of an ITC group is the most crucial factor determining the compounds activity, since related cyanate, or thiocyante derivatives (compounds 23 and 25, respectively) do not inhibit bacterial growth. It has been suggested that ITC group covalently cross-links to a cellular target (Tajima et al 2003). ITC compounds can target a unique protein or limited number of proteins (that possess a vital function) by modifying a group crucial for the activity of the targeted protein. As such, it can be envisioned that a group adjacent to the reactive ITC function can also contribute to inhibition through establishing additional interactions, thereby facilitating the binding of the compound in the vicinity of the targeted aminoacid residue and increasing the crosslinking efficiency. In this way the process can be considered as a case of affinity modification. This mechanism is supported by: i) deleterious effect of ITC group inactivation by pre-incubation of ITCs with sulfhydryl compounds (Adesida et al 1996; present study) or just in culture medium, on growth-inhibitory potency; ii) strong influence of the structure and size of the substituent adjacent to ITC group on antimicrobial activity of the compounds. Indeed, ITC with small carrier groups display poor activity (since they might not provide substantial additional interaction with the targets), while ITCs with larger groups are much more potent. Additional studies are required to confirm this mechanism.
The SAR for ITC compounds does not allow for simple interpretation, as it could reflect the interaction of an ITC with a target, involvement of multiple targets, the efficiency of cellular uptake, or all of the above. G− bacteria (generally having less permeable membranes) are more resistant to ITC inhibitors than G+ type, suggesting intracellular localization of a target. This is consistent with elimination of the growth-inhibitory activity of ITC 18 by phosphorylation at hydroxyl group leading to ITC 6, which renders the compound less permeable due to acquisition of two negative charges.
Strong positive effect of uncharged polar groups (hydroxyls, methoxy-, or heterocyclic nitrogens) on ITC activity detected for ITC 2, 3, 18, 20, 30, and 32, could be explained by favorable interactions with the target. Alternatively, these groups could maintain an optimal solubility of ITC in the intracellular matrix, since highly lipophlic compounds would be retained by cellular membrane, preventing ITC diffusion inside the cell.
To test whether microbial species develop resistance to ITC we attempted to select pre-existing resistance mutants in large population of microbial species. However, none of the tested three bacterial and one fungal cell populations contained mutants resistant to ITC 37. This could be explained either by high evolutional conservation of the ITC binding site on the cellular target(s), or by presence of multiple targets, so that the incidence of organisms having simultaneous resistance mutations in all targets, is very low. Given high dependence of microbial resistance on various conditions (e.g. concentration of compound and microorganisms, time, species, growth phase of organisms, etc.), additional studies with various ITCs and microbial species are required to generalize the above finding. High antimicrobial activity of some ITC synthesized in this study and their moderate toxicity grants further evaluation of these compounds as potential medicinal drugs as well as candidates for industrial applications (e.g. food and wood preservation).
The next challenge in developing more active and specific ITC compounds is the identification of cellular targets in various microbial pathogens, followed by co-crystallization of the targeted molecules with ITC compounds. These data can provide the structural information about the interactions of the ITC with the target, necessary for rational drug design.
Supplementary Material
Acknowledgments
This work was supported by NIH grant RO1 GM-30717-21 for AM. We are grateful to Dr. Krasnoperov (NJIT) for introduction to gas chromatography and MS analysis.
Footnotes
Supporting information includes: i) the analytical data on ITC compounds; ii) the diagrams for proliferation rate of B. cereus in the presence of various concentrations of ITC 37, and iii) the dependence of ITC 37 growth inhibitory potency against B. cereus on the compound pre-incubation time in LB culture medium.
References
- Adesida A, Edwards LG, Thornalley PJ. Inhibition of human leukaemia 60 cell growth by mercapturic acid metabolites of phenylethyl isothiocyanate. Food Chem Toxicol. 1996;34:385–392. doi: 10.1016/0278-6915(96)00124-x. [DOI] [PubMed] [Google Scholar]
- Chmel L, Drobnica L, Kuzelova K. Experimental study on the use of isothiocyanates in the therapy of dermatomycoses. Cesk Dermatol. 1958;33:361–367. [Google Scholar]
- Comas I, Borrell S, Roetzer A, Rose G, Malla B, Kato-Maeda M, Galagan J, Nienmann S, Gagneux S. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nature genetics. 2011;44:106–10. doi: 10.1038/ng.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad A, Biehler D, Nobis T, Richter H, Engels I, Biehler K, Frank U. Broad Spectrum Antibacterial Activity of a Mixture of Isothiocyanates from Nasturtium (Tropaeoli majoris herba) and Horseradish (Armoraciae rusticanae radix) Drug Res (Stuttg) 2013;63:65–68. doi: 10.1055/s-0032-1331754. [DOI] [PubMed] [Google Scholar]
- Drobnica L, Zemanova M, Nemec P, Antos K, Kristian P, Stullerova A, Knoppova V, Nemec P., Jr Antifungal activity of isothiocyanate and related compounds. I. Naturally occurring isothiocyanates and their analogues. Appl Microbiol. 1967;15:701–709. doi: 10.1128/am.15.4.701-709.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnica L, Zemanova M, Nemec P, Antos K, Kristian P, Martvon A, Zavodska E. Antifungal activity of isothiocyanates and related compounds. III. Derivatives of biphenyl, stilbene, azobenzene, and several polycondensed aromatic hydrocarbons. Applied Microbiol. 1968;16:582–587. doi: 10.1128/am.16.4.582-587.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour V, Alazzam B, Ermal G, Thepaut M, Rossero A, Tresse O, Baysse C. Antimicrobial activities of isothiocyanates against Campylobacter jejuni isolates. Cell Infect Microbiol. 2012;2:1–13. doi: 10.3389/fcimb.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson GM, George HJ. The reactions of thiocarbonyl chloride. Part I. Reaction with aromatic primary aminocompounds. J Chem Soc Trans. 1924;125:1709–1713. [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[α]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18:123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- Herr I, Buchier MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;55:377–383. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Johansson NL, Pavia CS, Chiao JW. Growth inhibition of a spectrum of bacterial and fungal pathogens by sulforaphane, an isothiocyanate product found in broccoli and other cruciferous vegetables. Planta Med. 2008;74:747–750. doi: 10.1055/s-2008-1074520. [DOI] [PubMed] [Google Scholar]
- Johns T, Kitts WD, Newsome F, Towers GH. Anti-reproductive and other medicinal effects of Tropaeolum tuberosum. J Ethnopharmacol. 1982;5:149–161. doi: 10.1016/0378-8741(82)90040-x. [DOI] [PubMed] [Google Scholar]
- Jung M, Hong E, Kim GH. Evaluation of antibacterial activity of 3-butenyl, 4-phenethyl, and benzyl isothiocyanate in Brassica vegetables. J Food Science. 2010;75:412–416. doi: 10.1111/j.1750-3841.2010.01725.x. [DOI] [PubMed] [Google Scholar]
- Lam TK, Lindsley K, Gallicchio L, Shields M, Hammond E, Tao XG, Chen L, Robinson KA, Caulfield LE, Herman JG, Guallar E, Alberg AJ. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18:184–195. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I, Tokuhisa JG, Jack C, Schultz JC, Heidi M, Appel HM, Ulrichs C, Gershenzon J. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry. 2006;67:2450–2462. doi: 10.1016/j.phytochem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mi L, Pasqua AJD, Chung FL. Proteins as binding targets in cancer prevention. Carcinogenesis. 2011;32:1405–1413. doi: 10.1093/carcin/bgr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999;91:605–613. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- Osbourn AE. Preformed Antimicrobial Compounds and Plant Defense against Fungal Attack. Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofrata A, Santangelo EM, Azeem M, Borg-Karlson AK, Gustaffson A, Putser K. Benzyl Isothiocyanate, a major Component from the roots of Salvadora Persica Is Highly Active Against Gram-Negative Bacteria. PloS ONE. 2011;6:e23045. doi: 10.1371/journal.pone.0023045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SK, Xiao D, Lew KL, Hershberger P, Kokkinakis DM, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- Swart H, Van Dyk S, Malan SF. The activity of p-methoxybenzylisothiocyanate against Neisseria gonorrhoeae, Haemophilus ducreyi, and other microorganisms. Bioorg Med Chem Lett. 2002;12:2435–2437. doi: 10.1016/s0960-894x(02)00437-7. [DOI] [PubMed] [Google Scholar]
- Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- Tajima H, Kimoto H, Taketo Y, Taketo A. Effects of synthetic hydroxy isothiocyanates on microbial systems. Biosci Biotechnol Biochem. 1998;62:491–495. doi: 10.1271/bbb.62.491. [DOI] [PubMed] [Google Scholar]
- Tajima H, Kimoto H, Taketo A. Specific antimicrobial synergism of synthetic hydroxy isothiocyanates with aminoglycoside antibiotics. Biosci Biotechnol Biochem. 2001;65:1886–1888. doi: 10.1271/bbb.65.1886. [DOI] [PubMed] [Google Scholar]
- Tajima H, Kimoto H, Taketo A. Paradoxical effect of synthetic hydroxy isothiocyanates on antimicrobial action of aminoglycosides. Biosci Biotechnol Biochem. 2003;67:1844–1846. doi: 10.1271/bbb.67.1844. [DOI] [PubMed] [Google Scholar]
- Tierens KF, Thomma BP, Brouwer M, Schmidt J, Kistner K, Porzel A, Mauch-Mani B, Cammue BP, Broekaert WF. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol. 2001;125:1688–1699. doi: 10.1104/pp.125.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994;54:1976s–1981s. [PubMed] [Google Scholar]
- Zhang Y, Tang L, Lonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–1052. [PubMed] [Google Scholar]
- Anon 1. National Committee for Clinical Laboratory Standards. NCCLS document M7\A5. 5. National Committee for Clinical Laboratory Standards; Wayne, Pa: 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.