Abstract
Most calcineurin inhibitor (CNI) based protocols reduce blood trough goals approximately 2–3 months post-transplant in clinically stable kidney transplant recipients. The CNI target trough level to prevent rejection, after reduction, is unknown. Using a multivariate Cox proportional hazards model we determined the association of time-varying tacrolimus (TAC) trough levels with acute rejection (AR) occurring in the first 6 months post-transplant, but specifically we assessed this association after 3 months. 1,930 patients received TAC based immunosuppression prior to AR in a prospective study. Of the 151 (7.8%) who developed AR, 47 developed AR after 3 months post-transplant. In an adjusted time varying multivariate model, each 1 ng/mL decrease in TAC trough levels was associated with a 7.2% increased risk of AR [HR=1.07, 95% CI (1.01, 1.14) P=0.03] in the first 6 months. There was an additional 23% increased risk of AR with each 1 ng/mL decrease in the TAC trough levels in months 3–6 [HR=1.23, 95% CI (1.06, 1.43) P=0.008]. In conclusion, lower TAC trough levels were significantly associated with increased risk of AR in the first 6 months post-transplant with additional risk of AR between months 3–6 post-transplant. The timing and practice of TAC dose reduction should be personalized based on the individual’s risk factors.
Keywords: tacrolimus reduction, acute rejection, kidney transplant, trough levels
Introduction
Calcineurin inhibitors (CNIs) such as tacrolimus (TAC) are the back bone of immunosuppressant regimens and are used in greater than 80% of all kidney transplants in the United States.1 Despite the wide use of these agents, the optimal TAC trough blood target after 3 months post-tranplant to prevent rejection of transplanted allografts is unclear. Most institutions reduce TAC exposure between 2–3 months post-transplant in rejection free kidney transplant recipients. According to international guidelines, the evidence behind this practice is of low quality.2 The majority of acute rejections (AR) episodes take place in the first 6 months, a period during which this reduction takes place.3,4
While the concept of dose reduction was introduced to limit the long term undesirable side effect of CNIs on the renal allograft known as “chronic CNI nephrotoxicity” as well as other CNI related side effects3–5, there are now data to support that most late graft loss is due to an immunologic insult.6,7 Currently, the existence of chronic CNI nephrotoxicity is being debated by many investigators in the field.8–11 The purpose of this work herein is to delineate the association between lower TAC trough levels and early AR within 6 months and to assess this relationship particularly after 3 months, a time most centers lower the TAC trough goal. We hypothesized that after 3 months there is an increased risk of AR in association with lower TAC trough level. Therefore, we used the prospective cohort of the Genomics of Deterioration of Kidney Allograft Function (DeKAF Genomics) study to test this hypothesis.
Materials and Methods
Patients
3,402 kidney or simultaneous kidney-pancreas transplant (SPK) recipients in 5 U.S. and 2 Canadian transplant centers were consented and enrolled at the time of transplantation in the DeKAF Genomics, a prospective observational, study between 2006 and 2011.12 The present study uses 1,930 recipients from this study who received TAC within the first 6 months post-transplant. The study was approved by the Institutional Review Boards and informed consent was obtained from all participants. This trial is registered at www.clinicaltrials.gov (NCT00270712).
Immunosuppression & Clinical Data
Induction and maintenance immunosuppression regimens were center specific. All centers reduced TAC trough levels per their center specific protocol. (Table I) Clinical data were collected at the time of transplantation and regularly thereafter until allograft failure and maintained in a central database. All biopsies were obtained for cause. AR was diagnosed by the treating physicians.
Table I.
Goal trough levels during the first six months post-transplant for all centers.
| Center # | Target Tacrolimus trough level | Time post-transplant |
|---|---|---|
| 1 | 10–12 ng/mL | 0–2 months |
| 8–10 ng/mL | 3–6 months | |
| 2 | 10–14 ng/mL | 1–3 months |
| 6–10ng/m L | 4–6 months | |
| 3 | 8–12 ng/mL | 0–3 months |
| 6–8 ng/mL | 4–6 months | |
| 4 | 10–15 ng/mL | 0–2 months |
| 5–10 ng/mL | 3–6 months | |
| 5 | 10–12 ng/mL | 0–1 months |
| 8–10 ng/mL | 2–4 months | |
| 6 to 8 ng/mL | 5 months and beyond | |
| 6 | 8–12 ng/mL | 0–2 months |
| 6–10 ng/mL | 3–6 months | |
| 7 | 6–10 ng/mL | 0–2 months |
| 6–8 ng/mL | 3–6 months |
Statistical Analyses
Cox proportional hazards models were used to investigate the association between time varying TAC trough levels between day 8 and 6 months post-transplant and time to first AR event occurring between 8 days and 6 months post-transplant. We created an interaction variable, TAC trough levels at 3 months, in order to assess the interaction between TAC trough levels and time after 3 months. This variable describes the effect of lower trough levels after 3 months. Individuals were considered at risk for AR beginning on the later of 8 days post-transplant or first TAC use. Censoring occurred at the earliest of permanent TAC discontinuation, 6 months post-transplant, last date of follow up, graft failure or death. Participants who temporarily stopped TAC for reasons other than AR were excluded from the risk set until restarting TAC.
A multivariate model was created by performing backwards selection on potential clinical covariates, using a retention p-value of 0.10 and stratifying by transplant center. The potential clinical covariates eligible for backwards selection were: TAC trough levels from day 8 to 6 months, TAC trough levels 3–6 months, recipient gender, race, age, smoking status [never, past or current], body mass index, blood type, cause of end-stage renal disease, SPK transplant, preemptive transplant, prior kidney transplant, prior non-kidney transplant, number of HLA mismatches, T or B cell cross-match positive, PRA status, dialysis prior to transplant, CMV sero-status, type of antibody induction, steroid use at day 7 post-transplant, and donor factors (age, gender and donor status [living or deceased]).
The intra-individual variability of TAC levels was measured using the coefficient of variation (CV) for each subject. The CV was compared between subjects with no rejection in the first six months post-transplant, and subjects with AR before and after 3 months post-transplant using a t-test. Comparison of creatinine levels at 12 months post-transplant, were conducted using a two sample t-test. All analyses were conducted using SAS/Genetics v9.2 (The SAS Institute, Cary, NC, USA, http://www.sas.com).
Results
The characteristics of the study patients are described in Table II. All centers undertook reduction of TAC trough levels at 2–3 months post-transplant as per their protocols described in Table I. Figure 1 shows the decline in levels after 3 months post-transplant.. In general, TAC trough levels of 8–15 ng/mL were targeted in months 0–3 post-transplant and targets of 5–10 ng/mL in months 4–6, as per the center’s protocol. TAC trough levels measured prior to an oral dose, were obtained as part of clinical care, and used in this analysis. Two measurements, if available, were obtained in each of weeks 1–8 and in each of months 3, 4, 5 and 6 post-transplant, for a maximum of 24 measurements per patient. There was mean of 16.3 trough levels per patient [Interquartile range, (IQR) 16–22). The median TAC trough levels during the entire six months post-transplant was 8.2 ng/mL (IQR 6.4–10.2 ng/mL).
Table II.
Characteristics of all study patients and stratified by presence of acute rejection (AR) in the first six months post-transplant.
| ALL (N=1930) | Subjects AR free during first 6 months (N=1779) | Subjects with AR during first 6 months (N=151) | |
|---|---|---|---|
| Age of recipient in years mean (SD) | 50(13.3) | 50(13.3) | 48.4(13.7) |
| Male recipient | 1217(63.1%) | 1111(62.4%) | 106(70.2%) |
| African American recipient | 353(18.3%) | 335(18.8%) | 18(11.9%) |
| BMI mean (SD) | 28 (5.5) | 28.2(5.5) | 27.9(6.0) |
| Simultaneous kidney-pancreas transplant | 157(8.1%) | 134(7.5%) | 23 (15.2%) |
| Diabetes pre-transplant | 753 (39%) | 696 (39%) | 57 (38%) |
| Immune related factors | |||
| HLA mismatch | |||
| None | 224(11.6%) | 218(12.3%) | 6(3.97%) |
| 1 or 2 | 297(15.4%) | 277(15.6%) | 20(13.3%) |
| 3 or 4 | 754(39.1%) | 695(39.1%) | 59(39.1%) |
| 5 or 6 | 653(33.9%) | 587(33.0%) | 66(43.7%) |
| Panel reactive antibody positive | 952(49.5%) | 859(48.4%) | 93(61.6%) |
| Positive T or B cell cross match | 138(7.2%) | 113(6.4%) | 25(16.7%) |
| Prior solid organ transplant | 504 (26.1%) | 444 (25.0%) | 60 (39.7%) |
| Prior kidney transplant | 302 (15.6%) | 265 (14.9%) | 37 (24.5%) |
| Donor related factors | |||
| Age of donor in years mean (SD) | 40.8(14) | 40.6(14) | 42.8(13.7) |
| Deceased donor | 816(42.%) | 754(42.4%) | 62(41.1%) |
| Immunosuppression | |||
| Induction regimen | |||
| Monoclonal antibody | 782(40.5%) | 747(42.0%) | 35(23.2%) |
| Polyclonal antibody | 1026(53.2%) | 928(52.2%) | 98(64.9%) |
| Combination | 49(2.5%) | 39(2.2%) | 10(6.6%) |
| None | 73(3.8%) | 65(3.7%) | 8(5.3%) |
| Steroid free by 7 days post-transplant | 722(37.45%) | 668(37.6%) | 54(36.0%) |
| Use of mycophenolate in first 6 monthsl | 1597(99.3%) | 1463(99.2%) | 134(100.0%) |
| Use of mTOR inhibitors | 3(0.2%) | 2(0.1%) | 1(0.8%) |
| Median TAC trough, ng/mL immediately proximal to AR event median (Interquartile range) | N/A | N/A | 7.6(4.8–9.9) |
Figure 1.
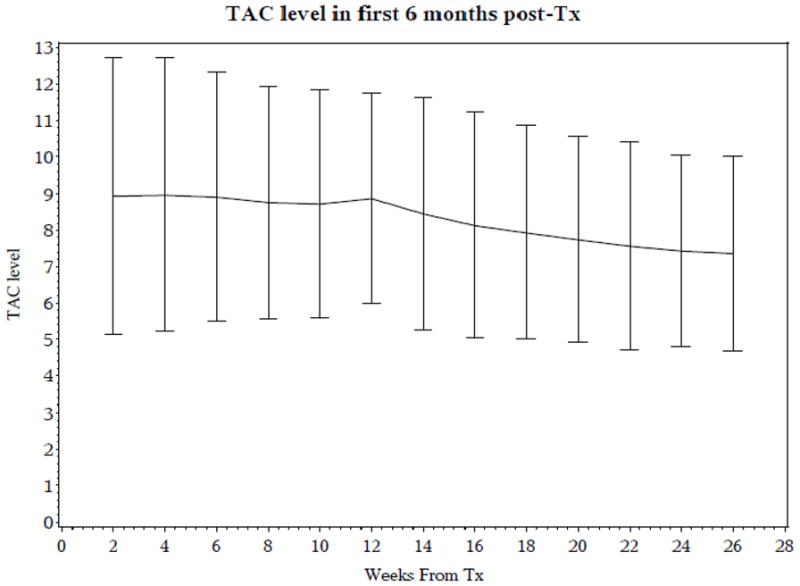
Mean TAC trough levels with 95% Confidence Intervals, during the first 6 months post- transplant in all subjects.
Acute rejection occurred in 151 of 1930 patients by 6 months. Acute rejection occurred in 104 of these patients in the first 3 months [median (IQR) of time to first AR: 20 days (15–40)] and in 47 of these patients after 3 months post-transplant [126 days (113 – 164)]. Most of the AR events (92.7%) were biopsy confirmed (Tables III and IV). The acute changes, as reflected by i and t scores greater than or equal to 2, are more common in AR biopsies after 3 months, compared to AR before 3 months post-transplant. Also chronic changes, as reflected by ci and ct scores greater than or equal to 2, are more common in AR biopsies after 3 months, compared to AR before 3 months post-transplant. (Table III) The group with AR, had a median TAC trough level of 7.6 ng/mL (IQR 4.8 – 9.9) immediately proximal to the AR event with the lower quartile being below the target TAC trough goal of 5.0 ng/ml for all study sites. The lower IQR of 4.8 ng/mL means that 25% of these subjects had a level below 4.8 ng/mL. These levels were obtained at a median of 3 days prior to acute rejection (IQR 2–5 days). The CV for subjects with AR between 3 and 6 months post-transplant was 0.31 (±0.13) and not statistically different from CV of subjects with rejection before 3 months post-transplant 0.32 (±0.15) (p=0.87) and from CV of subjects with no rejection within the first six months post-transplant 0.33 (±0.13) (p=0.49).
Table III.
Diagnosis and treatment of acute rejection biopsies (n=151), as determined by local pathologist at transplant center
| All (N=151) | AR before 3 months post-transplant (N=104) | AR after 3 months and before 6 months post-transplant (N=47) | |
|---|---|---|---|
| Diagnosis | |||
| Cellular | 100 (66.2%) | 60 (57.7%) | 40 (85.1%) |
| Antibody-mediated | 30 (19.9%) | 29 (27.9%) | 1 (2.1%) |
| Both | 10 (6.6%) | 9 (8.65%) | 1 (2.1%) |
| No biopsy or Indeterminate biopsy | 11 (7.3%)* | 6 (5.77%) | 5 (10.6%) |
| Drug Treatment | |||
| Steroids only | 84 (55.6%) | 48 (46.1%) | 36 (76.6%) |
| Antibodies only | 4 (2.65%) | 4 (3.85%) | 0 (0.0%) |
| Steroids and Antibodies | 33 (21.85%) | 28 (26.9%) | 5 (10.64%) |
| Steroids followed by antibodies | 15 (9.9%) | 12 (11.54%) | 3 (6.38%) |
| Other | 15 (9.9%) | 12 (11.5%) | 3 (6.38%) |
5 of these subjects had no biopsies and 6 had indeterminate biopsies.
Table IV.
Pathology scores for Acute Rejection, as determined by local pathologist at transplant center
| Banff Score ≥2 | All (N=151) | AR before 3 months post-transplant (N=104) | AR after 3 months and before 6 months post-transplant (N=47) |
|---|---|---|---|
| i (interstitial inflammation) * | 50 (42.7%) | 31 (34.8.0%) | 19 (67.9%) |
| t (tubulitis) * | 60 (50.8%) | 37 (41.1%) | 23 (82.1%) |
| ci (interstitial fibrosis) ** | 7 (6%) | 3 (3.4%) | 4 (14.3%) |
| ct (tubular atrophy) ** | 6(5.1%) | 3 (3.4%) | 3 (10.7%) |
| v (intimal artertitis) | 8 (6.9%) | 6 (6.9%) | 2 (7.14%) |
| g (glomerulitis) | 1 (0.9%) | 1 (1.1%) | 0 (0.0%) |
| cv (vascular fibrosis) | 4 (3.4%) | 2 (2.3%) | 2 (7.14%) |
| cg (glomerulopathy) | 1 (0.9%) | 1 (1.1%) | 0 (0.0%) |
| ah (arteriolar hyaline thickening) | 4 (3.4%) | 3 (3.4%) | 1 (3.6%) |
| Missing scores | 34 (22.5%) | 15 (14.4 %) | 19 (40.4 %) |
i and t-scores (range 0–3) correlated with Spearman correlation coefficient (95% CI) of 0.76 (0.67–0.86). The p-value is <0.0001
ci and ct-scores (range 0–3) correlated with Spearman correlation coefficient (95% CI) between local ci-score and ct-score is 0.73 (0.60–0.86). The p-value is <0.0001
In a multivariate Cox proportional model stratified by transplant center, time varying TAC trough level was an independent predictor of AR, after adjusting for HLA mismatches, positive T- or B-cell crossmatch, PRA status, donor age, gender, body mass index (BMI) and steroid use at day 7 post-transplant. For each 1 ng/mL reduction in the TAC trough level there was a 7.2 % increased risk of AR [HR=1.072, 95% CI (1.01, 1.14) P=0.03]. Using an interaction term, we assessed that there was an additional risk of AR of 23% with each 1 ng/mL reduction in the TAC trough level after 3 months [HR=1.23, 95% CI (1.06, 1.43) P=0.008] (Table V). These models were not adjusted for use of mycophenolate since over 99% of the patients were on this medication concomitantly. Since the median TAC trough levels immediately proximal to the AR event was 7.6 ng/ml (IQR 4.8–9.9), at least 25% of patients have less than the stated goal trough level of 5ng/ml. In a subgroup analysis excluding patients with positive T or B cell crossmatch, 1 ng/mL lower TAC trough level after 3 months was associated with 29% increased risk of AR [HR=1.29, 95% CI (1.09, 1.52) P=0.002]. In another multivariate model with the specific antibody induction agent forced into the model, the association of TAC trough levels with AR did not change in significance and direction. (data not shown)
Table V.
Adjusted Hazard Ratios of Acute Rejection occurring before 6 months post-transplant
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| TAC trough levels (all troughs 0–6 months)* | 1.07(1.01,1.14) | 0.035 |
| TAC trough levels (troughs after 3 months)† | 1.23(1.06,1.43) | 0.008 |
| Male recipient | 1.44(0.99,2.08) | 0.051 |
| Donor age in years | 1.02(1.00,1.03) | 0.011 |
| Simultaneous kidney pancreas | 1.66(0.99,2.75) | 0.051 |
| Number of HLA Mismatch | ||
| 0 | Reference | |
| 1 or 2 | 3.01(1.19,7.58) | 0.02 |
| 3 or 4 | 3.36(1.44,7.87) | 0.005 |
| 5 or 6 | 4.87(2.08,11.4) | 0.0002 |
| Panel reactive antibody present | 1.50(1.05,2.15) | 0.03 |
| T or B cell cross match positive | 3.87(2.40,6.22) | <0.001 |
| Steroid free at 7 days post-transplant | 0.59(0.38,0.89) | 0.012 |
| BMI at baseline (linear) | 0.99(0.96,1.02) | 0.66 |
| BMI at baseline (squared) | 1.003(1.0,1.01) | 0.052 |
Hazard is increased for each reduction of TAC trough level by 1ng/mL anytime in the first 6 months post-transplant
Additional hazard for each reduction of TAC trough level by 1ng/mL after 3 months. This variable assesses the interaction between TAC trough levels and time after 3 months in order to determine the additional effect of lower trough levels particularly after 3 months.
BMI, body mass index
The long-term impact of the AR events after 3 months post-transplant was assessed by creatinine levels at 12 months post-transplant. Patients with AR after 3 months post-transplant had higher creatinine levels at 12 months compared to patients without AR and those with AR before 3 months post-transplant. (Table VI)
Table VI.
Creatinine levels at 12 months post-transplant for patients based on acute rejection status during the first six months post-transplant.
Discussion
This study found that lower TAC trough levels in the first 6 months post-transplant were associated with an increased risk of AR. This is not a new finding as it is well known that TAC exposure and AR are well correlated in the first 3 months post-transplant.13–15 The optimal timing of dose reduction or the TAC trough levels after 3 months is less clear although it is common practice that TAC is reduced at this time. The novel aspect of this study is that we showed that troughs achieved at 3 months post-transplant were equally important and lower trough levels were significantly associated with higher risk of AR. For every 1 ng/ml reduction in TAC trough levels after 3 months, the additional risk of AR increased by 23%. AR after 3 months post-transplant was associated with higher creatinine levels at 12 months post-transplant. These findings question the merits of systematic TAC exposure reduction at 3 months and/or the degree of exposure reduction. TAC trough levels remained important even after accounting for traditional risk factors such as higher number of HLA mismatches, cross match and PRA status.
According to recent evidence based guidelines, the current practice of reducing TAC trough goals is not justified by strong scientific evidence. 2 The initial trials employing TAC to prevent AR used much higher trough goals of 10–20 ng/mL in the first 3 months and 5–15 ng/mL thereafter. 3,16 Based on this, it became standard practice to reduce TAC troughs at around 3 months post-transplant. The rationale for reducing TAC troughs even further both before and after 3 months is primarily based on the controversial concept of CNI related nephrotoxicity and the desire to prevent long term allograft dysfunction.17 However, the existence of chronic CNI nephrotoxicity is debatable8–11 whereas early rejection is a well-established risk factor for allograft loss.18 TAC trough levels that are too low or reduced too early may negate any potential gains made by reduction in chronic CNI related nephrotoxicity. A growing body of evidence now shows that the most late graft loss is due to immunologic insults i.e. late rejection and not necessarily related chronic CNI nephrotoxicity. For example, Gaston et. al showed that 35% of 171 patients had biopsies for cause diagnosed as CNI related chronic nephrotoxicity.6 However, this chronic CNI nephrotoxicity did not impact late all-cause allograft failure in their study. 6
Our findings are consistent with that of previously published studies. Initial randomized trials published in 1996–97 of 3 different doses of TAC13,14 showed that a therapeutic trough range of 5–15 ng/mL during the first year post-transplant had the maximum benefit and least side effects, compared to higher trough levels. Using the same trial data, Kershner et al15 showed that the higher the TAC trough levels (range 26–40 ng/ml) the lower the incidence of rejection post-transplant at the expense of elevated creatinine and non-renal CNI side effects. In contrast, currently TAC trough goals are 8–15 ng/mL in months 0–3 post-transplant are generally well tolerated. Our study shows that further dose reduction at 3 months post-transplant, may not be the optimal time for all patients due to the increased risk of AR. Naesens et al. also noted that low mean TAC levels between 3 and 12 months post-transplant were independently associated with higher increase in chronicity scores on protocol biopsies at 12 months post-transplant.19 In our study, the rejections that occurred between 3 and 6 months post-transplant had higher chronicity scores compared to rejections before 3 months post-transplant.(Table IV)
Mycophenolate is a key component of CNI based regimens today. Previous studies using TAC and mycophenolate reduced the TAC trough levels around 3 months post-transplant with higher 20,21 or similar rates of AR. 22,23,24 ,25 However, none of these trials were large enough to study the association of TAC trough level reduction after 3 months post-transplant on risk of AR.
The finding of the ELITE Symphony trial may not be generalizable to the present study population. In the ELITE Symphony trial26, low TAC trough levels were targeted (3–7 ng/mL) during the first year post-transplant; however the study patients achieved higher average trough level of 6–8 ng/mL. Moreover, the TAC trough levels did not vary significantly through the duration of the study because levels were not lowered at 3 months; and 30 to 40% of patients remained above the target.27 This may partially explain why the TAC group in ELITE trial was not associated with increased risk of AR which suggested that medium TAC trough goal (7ng/mL), without reduction by 3 months, was acceptable. Also, the ELITE Symphony trial, did not include any U.S. transplants and excluded moderate to high risk patients such as those with high PRA and positive crossmatch. Therefore the ELITE Symphony trial may not be applicable to the moderate risk patients enrolled in the present study.28 In the ELITE Symphony trial, all subjects were required to have at least nine trough levels checked during their 12 month study. 28 In contrast, the present study was an observational study that did not require subjects to have trough levels checked at specific time-points and had an average of 17 trough levels over the first six months post-transplant.29 Therefore, the more frequent trough level monitoring suggests more closer follow-up in the present study consistent with the moderate risk patient population enrolled in the present study than in the ELITE Symphony trial.
Our study found that conventional risk factors such as more HLA mismatches, positive T- or B-cell crossmatch, PRA positive status, older donor age, were associated with increased risk of AR. Being steroid free by 7 days post-transplant varied by center, but was associated with lower risk of AR in the multivariate model. It is likely that lower immunologic risk patient were selected to be steroid free by 7 days post-transplant. Only after adjusting for other risk factors for AR, being off steroids appeared to be associated with lower risk of AR. This is consistent with the finding that the unadjusted association of steroid free by 7 days was not associated with reduced risk of AR [HR=0.93, 95% C.I. (0.67–1.30), p=0.07]
The present study has several limitations. It is an observational study and not a randomized controlled trial with pre-specified trough levels. Several different induction regimens were used in this study and steroid withdrawal was transplant center dependent. Therefore, the impact of selection bias and unmeasured confounders, such as doses of steroids and mycophenolate, may not be accounted for in our study. The present study did not collect detailed information on hypertension, lipid profiles; therefore the impact of TAC reduction on these outcomes could not be described. The present study only collected information about the use of steroid and mycophenolate, but not the exact doses of these immunosuppressants. The TAC troughs were not measured in a single central laboratory. However, the majority (97.1%) of TAC whole blood concentrations were obtained from centers using liquid chromatography-mass spectroscopy to measure trough concentrations. All troughs were measured using CLIA certified assays or CLIA quality assays.29 Another limitation is that we did not collect information on adherence to immunosuppressive medications, therefore cannot rule out the role of poor adherence on acute rejections after dose reduction.
We conclude that low TAC trough levels are significantly associated with AR with an additional risk of AR with a reduction in trough levels at 3 months. These AR events after 3 months post-transplant have a detrimental impact on kidney function at 12 months post-transplant. This study questions the practice at most transplant centers of reducing TAC trough goal at 2–3 months post- transplant for fear of chronic nephrotoxicity and other side-effects. Given the ongoing debate about the existence of chronic nephrotoxicity, transplant centers may be reducing TAC at the expense of AR and potentially poor long term allograft survival. Based on the results of this study a personalized approach is needed to determine the ideal timing and degree of TAC dose reduction after assessing risks and benefits for each patients, particularly high risk patients.
Acknowledgments
Funding Source
This work was supported by the National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119) and DeKAF (5U01-AI058013)
We acknowledge the dedication and hard work of our coordinators at each of the seven clinical sites: University of Alberta, Nicoleta Bobocea, Tina Wong, Adrian Geambasu and Alyssa Sader; University of Manitoba, Myrna Ross and Kathy Peters; University of Minnesota, Mandi DeGrote and Jill Nagorski; Hennepin County Medical Center, Lisa Berndt; Mayo Clinic, Tom DeLeeuw; University of Iowa, Wendy Wallace and Tammy Lowe; University of Alabama, Jacquelin Vaughn and Tena Hilario. We also acknowledge the dedicated work of our research assistants: Marcia Brott, Brian Kasel and Winston Wildebush.
Abbreviations
- AR
Acute Rejection
- BMI
Body Mass index
- CNI
Calcineurin Inhibitor
- CI
Confidence Interval
- DEKAF
Deterioration in Kidney Allograft Function
- HLA
Human Leukocyte Antigen
- HR
Hazard Ratio
- PRA
Panel Reactive Antibody
- SPK
Simultaneous Kidney Pancreas
- TAC
Tacrolimus
DeKAF Genomics Investigators
J. Michael Cecka, M.D. UCLA Immunogenetics Center, Los Angeles, CA 90095, Email: mcecka@ucla.edu
Fernando G. Cosio, M.D. Division of Nephrology, Mayo Clinic, Rochester, MN 55905, Email: Cosio.Fernando@mayo.edu
Robert Gaston, M.D. University of Alabama, Division of Nephrology, Birmingham, AL 35294-0006, Email: rgaston@uab.edu
Sita Gourishankar M.D. Division of Nephrology and Immunology, University of Alberta, Edmonton, Alberta, Canada, Email: sitag@ualberta.ca
Lawrence Hunsicker, M.D. Nephrology Division, Iowa City, IA 52242-1082, Email: lawrence-hunsicker@uiowa.edu
Bertram Kasiske, M.D. Department of Medicine, Hennepin County Medical Center and the University of Minnesota, Minneapolis, MN 55415, Email: kasis001@umn.edu
Rosalyn Mannon, University of Alabama, Division of Nephrology, Birmingham, AL 35294-0006, Email: rmannon@uab.edu
David Rush, M.D. Health Sciences Center, Winnipeg MB, Canada, Email: drush@exchange.hsc.mb.ca
Footnotes
Conflict of Interest
None to report
Disclosure:
Authors have no conflicts to report
Accepted for oral presentations at the June 2012 American Transplant Congress in Boston.
Contributor Information
Ajay K. Israni, Department of Medicine, Nephrology Division, Hennepin County Medical Center, University of Minnesota; Department of Epidemiology & Community Health, University of Minnesota, Minneapolis, MN.
Samy M. Riad, Department of Medicine, Nephrology, University of Medicine, Minneapolis, MN.
Robert Leduc, Division of Biostatistics, University of Minnesota, Minneapolis, MN.
William S. Oetting, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN.
Weihua Guan, Division of Biostatistics, University of Minnesota, Minneapolis, MN.
David Schladt, Division of Biostatistics, University of Minnesota, Minneapolis, MN.
Arthur J. Matas, Department of Surgery, University of Minnesota, Minneapolis, MN.
Pamala A. Jacobson, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN.
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2010 Annual Data Report. Rockville, M.D: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2010. [Google Scholar]
- 2.Kidney Disease Improving Global Outcomes (KDIGO) KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation. 2009;9:S14–5. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 3.Pirsch JDMJ, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63:977–83. doi: 10.1097/00007890-199704150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Spencer CMGK, Gillis JC. Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs. 1997;54:925–75. doi: 10.2165/00003495-199754060-00009. [DOI] [PubMed] [Google Scholar]
- 5.Pallardo LMOF, Guirado L, Conesa J, Hortal LJ, Romero R, et al. Calcineurin inhibitor reduction based on maintenance immunosuppression with mycophenolate mofetil in renal transplant patients: POP study. Transplant Proc. 2007;39:2187–9. doi: 10.1016/j.transproceed.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 7.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am J Transplant. 2012;12:388–99. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 8.Kandaswamy RHA, Casingal V, Gillingham KJ, Ibrahim H, Matas AJ. Stable kidney function in the second decade after kidney transplantation while on cyclosporine-based immunosuppression. Transplantation. 2007;83:722–6. doi: 10.1097/01.tp.0000256179.14038.e2. [DOI] [PubMed] [Google Scholar]
- 9.Matas AJ. Chronic progressive calcineurin nephrotoxicity: an overstated concept. Am J Transplant. 2011;11:687–92. doi: 10.1111/j.1600-6143.2011.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matas AJ, Leduc R, Rush D, et al. Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: preliminary data from the DeKAF study. American Journal of Transplantation. 2010;10:315–23. doi: 10.1111/j.1600-6143.2009.02943.x. [DOI] [PubMed] [Google Scholar]
- 11.Snanoudj RRV, Elie C, Rabant M, Giradin C, Morelon E, Kreis H, Fournet J-C, Noel L-H, Legendre C. Specificity of histological markers of long-term CNI nephrotoxicity in kidney-transplant recipients under low-dose cyclosporine therapy. Am J Transplant. 2011;11:2635–46. doi: 10.1111/j.1600-6143.2011.03718.x. [DOI] [PubMed] [Google Scholar]
- 12.Israni A, Leduc R, Holmes J, et al. Single Nucleotide Polymorphisms, Acute Rejection and Severity of Tubulitis in Kidney Transplantation, Accounting for Center-to-Center Variation. Transplantation. 2010;90:1401–8. doi: 10.1097/TP.0b013e3182000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laskow DAVF, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900–5. doi: 10.1097/00007890-199610150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Vincenti FLD, Neylan JF, Mendez R, Matas AJ. One-year follow-up of an open-label trial of FK506 for primary kidney transplantation. A report of the U.S. Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;61:1576–81. doi: 10.1097/00007890-199606150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kershner RP, Fitzsimmons WE. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation. 1996;62:920–6. doi: 10.1097/00007890-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Mayer ADDJ, Squifflet JP, Besse T, Grabensee B, Klein B, et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997;64:436–43. doi: 10.1097/00007890-199708150-00012. [DOI] [PubMed] [Google Scholar]
- 17.Golshayan DPM. Minimization of calcineurin inhibitors to improve long-term outcomes in kidney transplantation. Transpl Immunol. 2008;20:21–8. doi: 10.1016/j.trim.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Kidney Disease Improving Global Outcomes (KDIGO) KDIGO clinical practice guideline for the care of kidney transplant recipients: Treatment of acute rejection. American Journal of Transplantation. 2009;9:S21–2. [Google Scholar]
- 19.Naesens M, Lerut E, Damme BV, Vanrenterghem Y, Kuypers DRJ. Tacrolimus exposure and evolution of renal allograft histology in the first year after transplantation. American Journal of Transplantation. 2007;7:2114–23. doi: 10.1111/j.1600-6143.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 20.Ciancio G, Burke G, Gaynor JJ, Roth D, Kupin W, Rosen A, et al. A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow-up. Clinical Transplantation. 2008;22:200–10. doi: 10.1111/j.1399-0012.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 21.Filler GWN, Milford DV, Watson AR, Gellermann J, Tyden G, et al. Four-year data after pediatric renal transplantation: A randomized trial of tacrolimus vs. cyclosporin microemulsion. Pediatr Transplant. 2005;9:498–503. doi: 10.1111/j.1399-3046.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 22.Ciancio GBG, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A Randomized Trial of Three Renal Transplant Induction Antibodies: Early Comparison of Tacrolimus, Mycophenolate Mofetil, and Steroid Dosing, and Newer Immune-Monitoring. Transplantation. 2005;80:457–65. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- 23.Grenda RWA, Vondrak K, Webb NJA, Beattie J, Fitzpatrick M, et al. A Prospective, Randomized, Multicenter Trial of Tacrolimus-Based Therapy with or without Basiliximab in Pediatric Renal Transplantation. American Journal of Transplantation. 2006;6:1666–72. doi: 10.1111/j.1600-6143.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 24.Heilman RLCH, Mazur M, Petrides S, Moss A, Mekeel K, et al. Outcomes of Simultaneous Kidney-Pancreas Transplantation With Positive Cross-Match. Transplant Proc. 2009;41:303–6. doi: 10.1016/j.transproceed.2008.08.154. [DOI] [PubMed] [Google Scholar]
- 25.Gaston RSKB, Shah T, Cibrik D, Shaw LM, Angelis M, Mulgaonkar S, Meier-Kriesche H-U, Patel D, Bloom RD. Fixed- or Controlled-Dose Mycophenolate Mofetil with Standard- or Reduced-Dose Calcineurin Inhibitors: The Opticept Trial. Am J Transplant. 2009;9:1607–19. doi: 10.1111/j.1600-6143.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- 26.Ekberg HT-SH, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–75. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 27.Ekberg HMR, Pearson TC, Vincenti F, Tedesco-Silva H, Daloze P. The Challenge of Achieving Target Drug Concentrations in Clinical Trials: Experience From the Symphony Study. Transplantation. 2009;87:1360–6. doi: 10.1097/TP.0b013e3181a23cb2. [DOI] [PubMed] [Google Scholar]
- 28.Leichtman AB. Balancing efficacy and toxicity in kidney-transplant immunosuppression. New England Journal of Medicine. 2007;357:2625–7. doi: 10.1056/NEJMe078181. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson P, Oetting WS, Brearley AM, Leduc R, Guan W, Schladt D, Matas AJ, Lamba V, Julian BA, Mannon RB, Israni A for DeKAF investigators. Novel Polymorphisms Associated with Tacrolimus Trough Concentrations: Results from a Multicenter Kidney Transplant Consortium. Transplantation. 2011;91:300–8. doi: 10.1097/TP.0b013e318200e991. [DOI] [PMC free article] [PubMed] [Google Scholar]


