Abstract
Objective
To compare the efficacy of wheelchair tilt-in-space and recline on enhancing muscle and skin perfusion over the ischial tuberosities in people with spinal cord injury (SCI).
Design
Repeated measures and before-after trial design.
Setting
University research laboratory.
Participants
Power wheelchair users with SCI (N=20).
Interventions
Six combinations of wheelchair tilt-in-space and recline angles were presented to participants in a random order. The testing protocol consisted of a baseline 5 min sitting with no tilt/recline and 5 min positioned in tilted and reclined position at each of 6 conditions, including: (1) 15° tilt-in-space and 100° recline, (2) 25° tilt-in-space and 100° recline, (3) 35° tilt-in-space and 100° recline, (4) 15° tilt-in-space and 120° recline, (5) 25° tilt-in-space and 120° recline, and (6) 35° tilt-in-space and 120° recline.
Main Outcome Measures
Muscle and skin perfusion was assessed by near-infrared spectroscopy and laser Doppler flowmetry, respectively.
Results
Muscle perfusion was significantly increased at 25° and 35° tilt-in-space when combined with 120° recline and skin perfusion was significantly increased at 3 tilt-in-space angles (15°, 25°, 35°) when combined with 120° recline and at 35° tilt-in-space when combined with 100° recline (P<.05). Even in the positions of increased muscle perfusion and skin perfusion (25° and 35° of tilt-in-space combined with 120° of recline), the amount of muscle perfusion change was significantly lower than the amount of skin perfusion change (P<.05).
Conclusions
Our results indicate that a larger angle of tilt-in-space and recline is needed to improve muscle perfusion compared to skin perfusion. A position of 25° tilt-in-space combined with 120° recline is effective in enhancing muscle and skin perfusion of weight-bearing soft tissues at the ischial tuberosities.
Keywords: deep tissue injury, laser Doppler flowmetry, near-infrared spectroscopy, pressure ulcer prevention, wheelchair seating
Pressure ulcers have remained a significant healthcare issue in the United States ever since Ambroise Paré described one of the earliest pressure ulcers in the literature indicating that “the bedsore on the buttock has come from having been too long a time lying on it, without moving himself” in the sixteenth century.1, 2 People with spinal cord injury (SCI) who lose mobility and sensation are particularly at risk of pressure ulcers on gluteal muscles.3, 4 The National Pressure Ulcer Advisory Panel (NPUAP) states that most pressure ulcers are avoidable, thus, prevention remains a mainstay of pressure ulcer treatment.5
Clinically, an essential component of a pressure ulcer prevention program is to periodically reduce the magnitude of pressure on weight bearing soft tissues.2, 3 During pressure relieving, ischemic soft tissues are able to improve blood flow to meet the metabolic needs of local cells. Such practice is based on the ischemia theory of pressure ulcer etiology.3, 6, 7 Wheelchair tilt-in-space and recline repositioning systems are commonly recommended for preventing sitting-induced pressure ulcers in people with SCI, especially in people with tetraplegia.3, 8, 9 Previous research studies have established the efficacy of tilt-in-space and recline on reducing the magnitude of seating interface pressure and enhancing skin blood flow.3, 10–12 However, the efficacy of performing wheelchair tilt-in-space and recline on enhancing muscle blood flow and reducing the risk of deep tissue injury (DTI) is largely unknown.3, 11
DTI was a recently recognized form of pressure ulcers by the NPUAP.13–15 Pressure ulcers are classified as superficial pressure ulcers (localized tissue damage originates from the skin and proceeds to the deep tissues) and DTI (presence of muscle damage with an intact skin caused by prolonged compression of muscle tissues around the bony prominences). Shea16 was the first person to report a wound underneath intact skin. Because muscle tissues are more susceptible to ischemic damage than the skin,17–19 necrosis of deep muscle tissues may occur before skin damage occurs. Furthermore, computational modeling has demonstrated that larger deformation and stresses act on muscle tissues adjacent to the ischial tuberosities.20–22 Research studies are needed to examine the effectiveness of current clinical guidelines (e.g. use of wheelchair tilt-in-space and recline) on preventing DTI.23, 24
Near-infrared spectroscopy (NIRS) is a relatively new technology that can measure the saturation of tissue oxygen (StO2), which is expressed as a percentage of the ratio of oxygenated hemoglobin to total hemoglobin primarily of the microvasculature of muscles.25 StO2 represents a dynamic balance between oxygen supply and consumption by local cells and has been used to study muscle perfusion in various diseases and under physiological stimuli.26–28 Such information may be useful to assess muscle perfusion of weight-bearing tissues and its response to wheelchair tilt-in-space and recline in people with SCI. A similar technology to NIRS uses laser Doppler to measure blood flow velocity within the measuring volume.4, 29
Laser Doppler flowmetry has been extensively used in quantifying skin perfusion in pressure ulcer prevention research.30–33 Using laser Doppler technology, we demonstrated that performing wheelchair tilt-in-space and recline needs to exceed a certain angle for effectively enhancing skin perfusion,3 needs to be maintained for at least 3 min for a significant increase in skin perfusion at the ischial tuberosities,11 and does not cause a decrease in skin perfusion over the sacrum.34 In this study, we examined the efficacy of tilt-in-space and recline on enhancing muscle perfusion for reducing ischemia. The purpose of this study was to compare muscle and skin perfusion over the ischial tuberosities in response to six combinations of wheelchair tilt-in-space and recline angles in people with SCI. We hypothesized that a larger angle of tilt-in-space and recline is needed to enhance muscle perfusion when compared to skin perfusion.
METHODS
A repeated measures and before-after trial design was used in this study.
Participants
We recruited 20 power wheelchair users with SCI to participate in this study. The inclusion criteria included: traumatic SCI at the level of C4 through T5 with an American Spinal Injury Association (ASIA) impairment scale of A, B or C; at least 6 months post spinal injury; use of a power wheelchair as a primary means of mobility; and wheelchair seat width of 43 cm (17 inches) to 53 cm (21 inches). The exclusion criteria included: cardiorespiratory or other diseases that may affect cardiovascular or circulatory function; diagnosed skeletal deformities (scoliosis, pelvic obliquity, hip and knee contracture); and active pressure ulcers. The rationale of the inclusion and exclusion criteria was to recruit a relatively homogenous group for this study. All participants gave informed consent to participate in this study, which was approved by a university institutional review board. The 20 participants included 18 males and 2 females: 15 Caucasians, 2 African Americans, and 3 Hispanic Americans. The descriptive data of the participants were: age, 41.4 ± 12.6y (mean ± standard deviation); body mass index, 25.4 ± 3.7kg/m2; and spinal injury duration, 7.8 ± 6.1y.
Apparatus
NIRSa was used to continuously measure muscle perfusion (in % unit) over the right ischial tuberosity with a sampling frequency of 0.5 Hz. The NIRS probea (38 × 15 × 10 mm) used in this study has a configuration of 15 mm distance between illumination and detection fibers/sensors that can detect oxygenation to a depth of 0 to 14 mm. The device uses a spectrometer to derive the muscle oxygenation measurement from the absorbance differences in the near-infrared spectrum at wavelengths between 680 and 800 nm. The NIRS device used in this study has been shown to be reliable on measuring StO2 in skeletal muscles,35 and is different from transcutaneous oxygen pressure (TcpO2), which measures the partial pressure of oxygen in the skin.4
A thin (1–2 mm thickness) laser Doppler flowmetry (LDF)b probe was used simultaneously to measure skin perfusion over the left ischial tuberosity with a sampling rate of 32 Hz. The LDF device utilizes a 780 nm laser light with a separation of 0.25 mm between emitting and receiving sensors that leads to a measurement depth of 1 to 2 mm. The output value is reported in blood perfusion units (BPU). LDF has demonstrated reliability in various medical applications.4, 29
A power wheelchairc with tilt-in-space and recline seat positioning functions was used in this study. The seat width was 48 cm (19 inches). Both tilt-in-space and recline provide pressure relief while tilt-in-space works best for users with high muscle tone and limited range of motion of lower extremity joints and recline is better for users who require a full pressure relief.11 A standard high-density pre-contoured foam seat cushionc provided by the same manufacturer was used in this study. We adapted the wheelchair seat cushion by cutting out a 10 cm diameter by 1 cm depth circular portion of foam in the region of the right ischial tuberosity. This was done to accommodate the thickness of the NIRS probe and prevent the probe from affecting the seat interface pressure. A hole larger than the size of the NIRS probe was needed to accommodate the variations of the ischial tuberosity location of each participant when seated on the cushion. Two angle gaugesd were used to measure wheelchair tilt-in-space and recline angles.
Wheelchair Tilt-in-space and Recline Protocol
Six protocols of various wheelchair tilt-in-space and recline angles were tested in a random order in this study. A wheelchair tilt-in-space feature provides a change in angle of the seat and back support relative to the vertical without changing the angle between the seat and back support. The recline feature changes the angle of the back support relative to vertical without a change in the seat angle, therefore, changing the seat to back support angle. For this study, both of these features were manipulated. The starting position for tilt was a fully vertical back support (this would be 0 degrees) and the seating system is always tilted posteriorly. The starting position of recline is with a 90 degree seat to back support angle and the recline angle indicated is the resulting seat to back support angle after the back support was reclined. Each protocol consisted of a baseline 5 min sitting at no tilt/recline and a 5 min tilted and reclined position at each of 6 combinations of tilt-in-space and recline angles, including (1) 15° tilt-in-space and 100° recline, (2) 25° tilt-in-space and 100° recline, (3) 35° tilt-in-space and 100° recline, (4) 15° tilt-in-space and 120° recline, (5) 25° tilt-in-space and 120° recline, and (6) 35° tilt-in-space and 120° recline. A 5 min washout period was provided between protocols. See Table 1 for a sample protocol from one of the participants in this study. The randomization of 6 conditions assigned to participants and rationale of selection of these settings is provided in our previous publication.3
Table 1.
A repeated measures before-after trial design was used to examine the efficacy of wheelchair tilt-in-space and recline angles on enhancing muscle and skin perfusion in people with spinal cord injury. Muscle and skin perfusion was simultaneously measured at the right and left ischial tuberosities, respectively. The example only lists muscle perfusion as an example of calculating normalized muscle perfusion. Tilt-in-space (T): 15°, 25,° 35° and recline (R): 100°, 120°. An example of a complete protocol for a research participant.
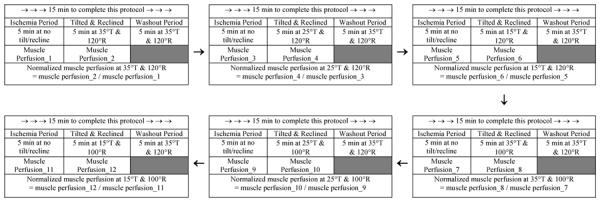
|
Procedure
Room temperature was maintained at 24°C ± 2°C. The participants stayed in the laboratory for at least 30 min prior to testing to accommodate to the room temperature. During the acclimation period, the participants were asked to empty their bladders. Then the participants were transferred to a standard mat to position them in a side-lying posture with their hips and knees flexed to 90°. The LDF probe was taped onto the skin over the left ischial tuberosity and the NIRS probe was taped onto the skin over the right ischial tuberosity. Then, the participant was transferred back to the wheelchair for data collection. The locations of LDF and NIRS were confirmed by palpation with a participant leaning laterally and forward. Wheelchair tilt-in-space and recline angles were always adjusted by the same researcher. The range of acceptable angles was ±2° of the pre-determined angle. The adjustment of angles was completed within 30 sec of initiating each tilt-in-space and recline condition. Each participant required approximately 2 hours to complete testing all 6 positions. Figure 1 shows examples of muscle and skin perfusion responses to 15° tilt-in-space combined with 100° recline and 35° tilt-in-space combined with 120° recline.
Fig 1.
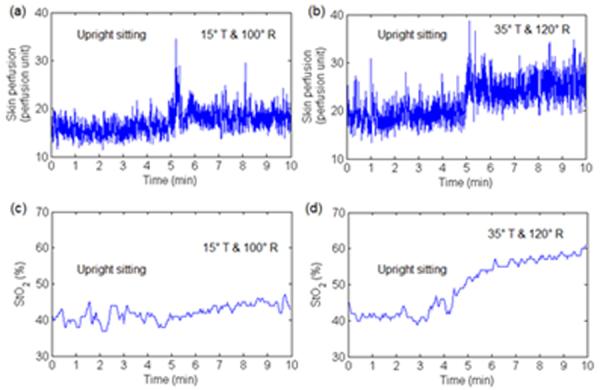
An example of skin and muscle perfusion in response to 15° tilt-in-space combined with 100° recline and 35° tilt-in-space combined with 120° recline. The 5 min upright sitting was used to induce soft tissue ischemia and the 5 min tilted and reclined period was used to improve muscle and skin perfusion. Muscle and skin perfusion was simultaneously measured at the right and left ischial tuberosities, respectively. (a) Skin perfusion response to 15° tilt-in-space combined with 100° recline. (b) Skin perfusion response to 35° tilt-in-space combined with 120° recline. (c) Muscle perfusion (StO2) response to 15° tilt-in-space combined with 100° recline. (d) Muscle perfusion response to 35° tilt-in-space combined with 120° recline.
Statistical Analysis
The independent variables included the 6 combinations of wheelchair tilt-in-space and recline angles and the dependent variables were muscle and skin perfusion responses. Our previous study demonstrated that of the selected tilt-in-space and recline angles were reasonable to assess skin perfusion response,3, 11 and we assumed that such protocols are also reasonable for examining muscle perfusion responses. Muscle and skin perfusion during the 5-min tilted and reclined position was normalized to that of its baseline 5-min upright sitting period (Table 1).31, 36 Because 20–30 sec was needed to complete the adjustment of tilt-in-space and recline angles, muscle and skin perfusion obtained during the first 30 sec of each 5 min tilted and reclined period was excluded from analysis.3, 11 A one-way analysis of variance with repeated-measures was used to examine the efficacy of wheelchair tilt-in-space and recline on enhancing muscle and skin perfusion over the ischial tuberosities. We also used paired samples t-tests for post-hoc analyses for the before-after trial comparisons of perfusion between the 5 min baseline sitting and each 5 min tilted and recline position. For each condition of tilt-in-space and recline angles, normalized muscle and skin perfusion were compared, also using paired t-tests. We considered using Bonferroni corrections in analysis of data, however due to the high number of measures (12) and the low number of participants (20) in this pilot study, as well as the nature of the pilot study, we decided not to use these corrections as they would increase likelihood committing a Type II error, which was not acceptable for this exploratory study.37 All statistical tests were performed using SPSSe at an alpha level of .05.
RESULTS
Normalized muscle perfusion during the tilted and reclined positions is shown in Fig 2. Muscle perfusion during 2 protocols: 25° and 35° tilt-in-space combined with 120° recline, showed a significant increase compared to baseline sitting (P<.05); however the remaining 4 protocols did not show a significant difference in muscle perfusion compared to baseline sitting (N.S.).
Fig 2.
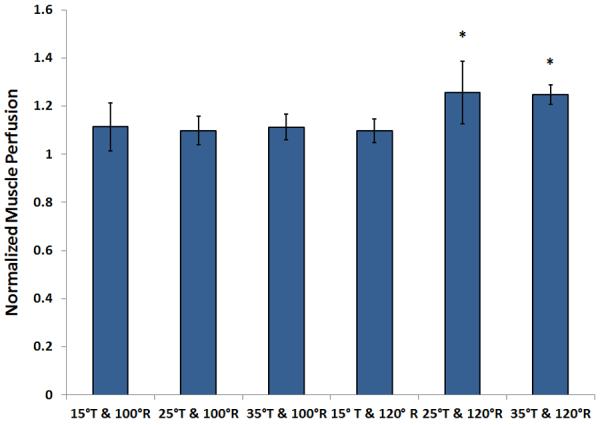
Comparison of normalized muscle perfusion in response to wheelchair tilt-in-space (T: 15°, 25°, 35°) in combination with recline (R: 100°, 120°). During tilted and reclined positions, 2 testing positions showed a significant increase in muscle perfusion compared with the upright seated position (P<.05), and the remaining 4 conditions did not show a significant increase in skin perfusion. Data shown as mean ± SE. Abbreviation: BPU, blood perfusion unit.
Normalized skin perfusion during the tilted and reclined positions is shown in Fig 3. Skin perfusion during 4 protocols: 35° tilt-in-space combined with 100° recline and all 3 tilt-in-space angles combined with 120° recline, showed a significant increase compared to baseline sitting (P<.05). The remaining 2 angle combinations: 15° and 25° tilt-in-space combined with 100° recline, did not show a significant increase compared to its baseline sitting (N.S) (Fig. 3).
Fig 3.
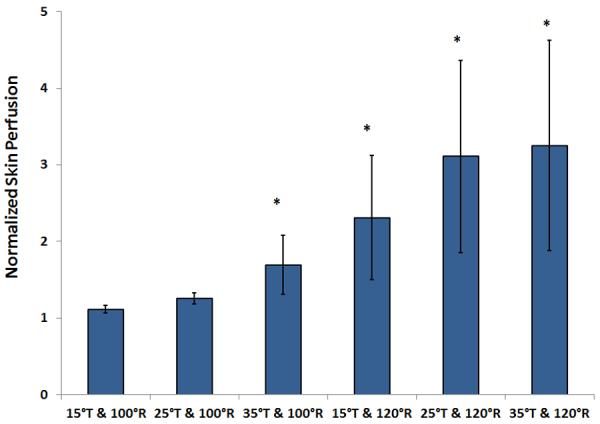
Comparison of normalized skin perfusion in response to wheelchair tilt-in-space (T: 15°, 25°, 35°) in combination with recline (R: 100°, 120°). During tilted and reclined positions, 4 testing positions showed a significant increase in skin perfusion compared with the upright seated position (P<.05), whereas positions at 15° tilt-in-space combined with 100° recline and 25° tilt-in-space combined with 100° recline did not show a significant increase in skin perfusion. Data shown as mean ± SE. Abbreviation: BPU, blood perfusion unit.
Normalized skin perfusion during 3 angle combinations: all 3 tilt-in-space angles combined with 120° recline, showed a significant increase compared to normalized muscle perfusion (P<.05); however the remaining 3 angle combinations did not show a significant difference between normalized muscle and skin perfusion (N.S).
DISCUSSION
We demonstrated that a larger angle of wheelchair tilt-in-space and recline is required to improve muscle perfusion than that required to improve skin perfusion in adults with SCI. This is particularly important to complement the research findings about the efficacy of tilt-in-space and recline on reducing the magnitude of seating interface pressure10 and enhancing skin perfusion3, 11 for the development of a specific guideline for use of wheelchair tilt-in-space and recline to prevent sitting-induced pressure ulcers in people with SCI. Our findings also support computational modeling on the understanding of deformation and mechanical stresses of gluteal muscles in the sitting position. By comparing muscle and skin perfusion responses at the same angle of tilt-in-space and recline, our findings suggest that muscle tissues may be at higher risk of tissue injury than the skin in people with SCI.
The main finding of this study is that increasing muscle perfusion of the weight-bearing tissues may require different pressure relief maneuvers than those required to improve skin perfusion in wheelchair users with SCI. Research studies have demonstrated that smaller angles of tilt-in-space and recline can reduce peak seating pressure (e.g. 15° tilt-in-space combined with 100° recline).38 However, using LDF to assess skin perfusion over the ischial tuberosity, we previously showed that this angle did not increase skin perfusion and a relatively larger angle is needed to improve skin perfusion to the ischemic weight-bearing tissues.3, 39 In this study, we further demonstrated that a combined tilt-in-space and recline angle may noticeably enhance skin perfusion over the ischial tuberosity but may not increase muscle perfusion (e.g. 35° tilt-in-space and 100° recline). Our findings are particularly useful for clinicians to educate wheelchair users to perform tilt-in-space and recline by showing the efficacy on reducing muscle and skin ischemia. This evidence may encourage wheelchair users to perform pressure relief maneuvers involving proper angles and durations of tilt-in-space and recline for reducing the risk of pressure ulcers, including DTI.
Muscle and skin perfusion of the weight-bearing tissues in response to combined wheelchair tilt-in-space and recline is dependent on many factors, including intrinsic physiological and extrinsic mechanical factors. Physiological factors regulating microvascular responses include local metabolic and myogenic controls and central sympathovagal function over the cardiovascular system.31, 40–42 The impaired microvascular reactivity in people with SCI is largely due to the sympathetic dysfunction over the cardiovascular system.31, 42 To overcome sitting-induced tissue ischemia, local metabolic and/or myogenic controls need to cause a vasodilatory response to increase blood flow. Both metabolic and myogenic controls require adequate removal of occlusion pressure surrounding arterioles and capillaries to initiate a vasodilatory response. When mechanical stresses and deformation are removed through performance of wheelchair tilt-in-space and recline, metabolic controls increase release of vasodilators to blood flow and myogenic controls enhance amplitudes of vasomotion for reducing blood flow resistance, thus quickly increasing blood flow to the ischemic muscles and skin.43, 44
The difference in muscle and skin perfusion in response to wheelchair tilt-in-space and recline is mainly due to the difference in the degree of pressure reduction between the muscles and skin governed by mechanical factors. Mechanical factors include the composition, structure and viscoelastic properties of the skin, fat and muscles and the geometric shape of bony prominences and support surface.20, 22–24 Modeling studies have shown that mechanical stresses are concentrated on muscles adjacent to bony prominences rather than on the skin, fat and muscles far from the bony prominences.19, 45 This implies that a larger occlusion pressure is acting on arterioles and capillaries of muscles than the skin, which may have led to the smaller response of increased muscle perfusion seen in this study.
The technical advantage of LDF is to quantify skin blood flow oscillations associated with vasomotion, however the disadvantage is the limited measurement of the depth of up to 1–2 mm. Skin blood flow oscillations provide a great opportunity to study underlying physiological mechanisms (e.g. metabolic, neurogenic and myogenic) and their response to various causative factors of pressure ulcers.40, 41, 46 The advantage of using NIRS is the ability to detect perfusion at a deeper level, involving skeletal muscles. However, most NIRS requires a much longer time to quantify changes in muscle perfusion compared to LDF. When assessing physiological signals such as muscle and skin perfusion, spatial and temporal variations in these measurements should be expected.31, 36, 41, 47 Several methods have been established to overcome these variations, including expressing blood flow as a percentage of thermally induced maximal blood flow, measuring blood flow at a controlled temperature (e.g. 37°C), and normalization of blood flow response to a baseline value.36 Without using these methods, poor reproducibility of NIRS measurements may be observed.48
In several previous studies, researchers8, 49, 50 monitored the usage patterns of wheelchair tilt-in-space and recline and found that although the majority of wheelchair users used tilt-in-space and recline features every day, they performed angles of tilt-in-space and recline that were relatively small. According to our results, these pressure redistribution/relieving activities may not be effective on reducing muscle and skin ischemia. Our studies have shown that clinicians should be recommending both tilt-in-space and recline features, along with specific angles of tilt-in-space combined with recline, to wheelchair users to allow effective reduction of ischemia in both muscle and skin tissues.
Study Limitations
The major limitations of this study are grounded in the current state of science on the NIRS technology. Various configurations of NIRS probes have been used to measure muscle perfusion to the different depths from the skin surface. The configuration of the illumination and detection sensors in this study allowed us to detect muscle perfusion at a depth up to 14 mm. It is unclear whether this depth was adequate to include the entire muscle thickness between the ischial tuberosity and the skin surface. For the detected muscle tissues, our results demonstrated that a larger angle of tilt-in-space and recline was needed in muscles than in the skin. If the depth was not enough to include the entire thickness of gluteal muscles, based on the computational modeling research,20–22 a larger angle will be needed to improve muscle perfusion because of relatively higher stresses immediately surrounding the bony prominence. However, a larger NIRS probe, needed for deeper detection, may significantly affect the normal pressure distribution between the buttock and support surface. Secondly, the recovery period was 5 min in this study. Muscle perfusion of weight bearing tissues may require a longer time for a full vasodilatory response. Future work may examine the muscle perfusion response at different recovery times. A larger sample size using this protocol should be used to verify our results. In addition to this, we studied a sample with a specific body size for this research. The results may not generalize well to participants with substantially different anthropometric characteristics.
CONCLUSION
In this study and our previous study, we have demonstrated that wheelchair tilt-in-space and recline can enhance muscle and skin perfusion in adult wheelchair users with SCI. Although smaller angles of wheelchair tilt-in-space and recline are preferred by wheelchair users for functional purposes, our results indicate that 25° tilt-in-space combined with 120° recline or larger angles are required to reduce muscle and skin tissue ischemia of weight-bearing soft tissues. Our findings support the use of combined wheelchair tilt-in-space and recline to reduce risk of pressure ulcers in wheelchair users with SCI.
ACKNOWLEDGMENT
We appreciate Permobil, Inc. for providing the power wheelchair for the experiments.
Supported by the National Institute of Health (grant no. R03HD060751)
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
List of Abbreviations
- ASIA
American Spinal Injury Association
- BPU
Blood Perfusion Unit
- DTI
Deep Tissue Injury
- LDF
Laser Doppler Flowmetry
- NIRS
Near-Infrared Spectroscopy
- NPUAP
National Pressure Ulcer Advisory Panel
- SCI
Spinal Cord Injury
- StO2
Saturation of Tissue Oxygen
- TcpO2
Transcutaneous Oxygen Pressure
Footnotes
Suppliers InSpectra 650, Hutchinson Technology Inc., Hutchinson, MN 55350.
PeriFlux System 5000 and Probe PR 415, Perimed Inc., Ardmore, PA 19003.
Corpus C300 and seating system, Permobil Inc., Lebanon, TN 37090.
Digital angle gauge, Wixey, available at: http://www.wixey.com.
SPSS 20, SPSS Inc., Chicago, IL 60606.
REFERENCES
- 1.Levine JM. Historical notes on pressure ulcers: the cure of Ambrose Pare. Decubitus. 1992;5(2):23–4. 6. [PubMed] [Google Scholar]
- 2.Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974–84. doi: 10.1001/jama.296.8.974. [DOI] [PubMed] [Google Scholar]
- 3.Jan YK, Jones MA, Rabadi MH, Foreman RD, Thiessen A. Effect of wheelchair tilt-in-space and recline angles on skin perfusion over the ischial tuberosity in people with spinal cord injury. Arch Phys Med Rehabil. 2010;91(11):1758–64. doi: 10.1016/j.apmr.2010.07.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jan YK, Brienza DM. Technology for pressure ulcer prevention. Topics in Spinal Cord Injury Rehabilitation. 2006;11(4):30–41. [Google Scholar]
- 5.Black JM, Edsberg LE, Baharestani MM, Langemo D, Goldberg M, McNichol L, et al. Pressure ulcers: avoidable or unavoidable? Results of the National Pressure Ulcer Advisory Panel Consensus Conference. Ostomy Wound Manage. 2011;57(2):24–37. [PubMed] [Google Scholar]
- 6.Kosiak M. Etiology of decubitus ulcers. Arch Phys Med Rehabil. 1961;42:19–29. [PubMed] [Google Scholar]
- 7.Olesen CG, de Zee M, Rasmussen J. Missing links in pressure ulcer research--an interdisciplinary overview. J Appl Physiol. 2010;108(6):1458–64. doi: 10.1152/japplphysiol.01006.2009. [DOI] [PubMed] [Google Scholar]
- 8.Lacoste M, Weiss-Lambrou R, Allard M, Dansereau J. Powered tilt/recline systems: Why and how are they used? Assist Technol. 2003;15(1):58–68. doi: 10.1080/10400435.2003.10131890. [DOI] [PubMed] [Google Scholar]
- 9.Michael SM, Porter D, Pountney TE. Tilted seat position for non-ambulant individuals with neurological and neuromuscular impairment: a systematic review. Clinical Rehabilitation. 2007;21(12):1063–74. doi: 10.1177/0269215507082338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobson DA. Comparative effects of posture on pressure and shear at the body-seat interface. J Rehabil Res Dev. 1992;29(4):21–31. doi: 10.1682/jrrd.1992.10.0021. [DOI] [PubMed] [Google Scholar]
- 11.Jan YK, Liao F, Jones MA, Rice LA, Tisdell T. Effect of durations of wheelchair tilt-in-space and recline on skin perfusion over the ischial tuberosity in people with spinal cord injury. Arch Phys Med Rehabil. 2013;94 doi: 10.1016/j.apmr.2012.11.019. DOI: 10.1016/j.apmr.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonenblum SE, Sprigle SH. The impact of tilting on blood flow and localized tissue loading. J Tissue Viability. 2011;20(1):3–13. doi: 10.1016/j.jtv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Ankrom MA, Bennett RG, Sprigle S, Langemo D, Black JM, Berlowitz DR, et al. Pressure-related deep tissue injury under intact skin and the current pressure ulcer staging systems. Adv Skin Wound Care. 2005;18(1):35–42. doi: 10.1097/00129334-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Black JM, National Pressure Ulcer Advisory P Moving toward consensus on deep tissue injury and pressure ulcer staging. Adv Skin Wound Care. 2005;18(8):415–6. 8, 20–1. doi: 10.1097/00129334-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Black J. Deep tissue injury: an evolving science. Ostomy Wound Manage. 2009;55(2):4. [PubMed] [Google Scholar]
- 16.Shea JD. Pressure sores: classification and management. Clinical Orthopaedics & Related Research. 1975;112:89–100. [PubMed] [Google Scholar]
- 17.Daniel RK, Priest DL, Wheatley DC. Etiologic factors in pressure sores: an experimental model. Arch Phys Med Rehabil. 1981;62(10):492–8. [PubMed] [Google Scholar]
- 18.Nola GT, Vistnes LM. Differential response of skin and muscle in the experimental production of pressure sores. Plast Reconstr Surg. 1980;66(5):728–33. doi: 10.1097/00006534-198011000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Linder-Ganz E, Gefen A. Mechanical compression-induced pressure sores in rat hindlimb: muscle stiffness, histology, and computational models. J Appl Physiol. 2004;96(6):2034–49. doi: 10.1152/japplphysiol.00888.2003. [DOI] [PubMed] [Google Scholar]
- 20.Makhsous M, Lim D, Hendrix R, Bankard J, Rymer WZ, Lin F. Finite element analysis for evaluation of pressure ulcer on the buttock: Development and validation. Ieee T Neur Sys Reh. 2007;15(4):517–25. doi: 10.1109/TNSRE.2007.906967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linder-Ganz E, Shabshin N, Itzchak Y, Gefen A. Assessment of mechanical conditions in sub-dermal tissues during sitting: a combined experimental-MRI and finite element approach. J Biomech. 2007;40(7):1443–54. doi: 10.1016/j.jbiomech.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Linder-Ganz E, Shabshin N, Itzchak Y, Yizhar Z, Siev-Ner I, Gefen A. Strains and stresses in sub-dermal tissues of the buttocks are greater in paraplegics than in healthy during sitting. J Biomech. 2008;41(3):567–80. doi: 10.1016/j.jbiomech.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Mak AF, Zhang M, Tam EW. Biomechanics of pressure ulcer in body tissues interacting with external forces during locomotion. Annu Rev Biomed Eng. 2010;12:29–53. doi: 10.1146/annurev-bioeng-070909-105223. [DOI] [PubMed] [Google Scholar]
- 24.Bouten CV, Oomens CW, Baaijens FP, Bader DL. The etiology of pressure ulcers: skin deep or muscle bound? Arch Phys Med Rehabil. 2003;84(4):616–9. doi: 10.1053/apmr.2003.50038. [DOI] [PubMed] [Google Scholar]
- 25.Rolfe P. In vivo near-infrared spectroscopy. Annu Rev Biomed Eng. 2000;2:715–54. doi: 10.1146/annurev.bioeng.2.1.715. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29(4):463–87. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 27.Hamaoka T, McCully KK, Niwayama M, Chance B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: recent developments. Philosophical transactions Series A, Mathematical, physical, and engineering sciences. 2011;369(1955):4591–604. doi: 10.1098/rsta.2011.0298. [DOI] [PubMed] [Google Scholar]
- 28.Owen-Reece H, Smith M, Elwell CE, Goldstone JC. Near infrared spectroscopy. Br J Anaesth. 1999;82(3):418–26. doi: 10.1093/bja/82.3.418. [DOI] [PubMed] [Google Scholar]
- 29.Oberg PA. Laser Doppler flowmetry. Crit Rev Biomed Eng. 1990;18(2):125–63. [PubMed] [Google Scholar]
- 30.Schubert V, Fagrell B. Postocclusive reactive hyperemia and thermal response in the skin microcirculation of subjects with spinal cord injury. Scand J Rehabil Med. 1991;23(1):33–40. [PubMed] [Google Scholar]
- 31.Jan YK, Brienza DM, Boninger ML, Brenes G. Comparison of skin perfusion response with alternating and constant pressures in people with spinal cord injury. Spinal Cord. 2011;49(1):136–41. doi: 10.1038/sc.2010.58. [DOI] [PubMed] [Google Scholar]
- 32.Mayrovitz HN, Smith JR. Adaptive skin blood flow increases during hip-down lying in elderly women. Adv Wound Care. 1999;12(6):295–301. [PubMed] [Google Scholar]
- 33.Li Z, Tam EW, Kwan MP, Mak AF, Lo SC, Leung MC. Effects of prolonged surface pressure on the skin blood flowmotions in anaesthetized rats - an assessment by spectral analysis of laser Doppler flowmetry signals. Phys Med Biol. 2006;51:2681–94. doi: 10.1088/0031-9155/51/10/020. [DOI] [PubMed] [Google Scholar]
- 34.Jan YK, Crane BC. Wheelchair tilt-in-space and recline does not reduce sacral skin perfusion as changing from the upright to the reclined and tilted position in people with spinal cord injury. Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2013.01.004. DOI: 10.1016/J.APMR.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner AW, Parker DE, Webb N, Montgomery PS, Scott KJ, Blevins SM. Calf muscle hemoglobin oxygen saturation characteristics and exercise performance in patients with intermittent claudication. J Vasc Surg. 2008;48(3):644–9. doi: 10.1016/j.jvs.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jan YK, Brienza DM, Geyer MJ. Analysis of week-to-week variability in skin blood flow measurements using wavelet transforms. Clinical Physiology and Functional Imaging. 2005;25(5):253–62. doi: 10.1111/j.1475-097X.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 37.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aissaoui R, Lacoste M, Dansereau J. Analysis of sliding and pressure distribution during a repositioning of persons in a simulator chair. IEEE Trans Neural Syst Rehabil Eng. 2001;9(2):215–24. doi: 10.1109/7333.928581. [DOI] [PubMed] [Google Scholar]
- 39.Sprigle S, Sonenblum S. Assessing evidence supporting redistribution of pressure for pressure ulcer prevention: A review. J Rehabil Res Dev. 2011;48(3):203–13. doi: 10.1682/jrrd.2010.05.0102. [DOI] [PubMed] [Google Scholar]
- 40.Jan YK, Brienza DM, Geyer MJ, Karg P. Wavelet-based spectrum analysis of sacral skin blood flow response to alternating pressure. Arch Phys Med Rehabil. 2008;89(1):137–45. doi: 10.1016/j.apmr.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 41.Jan YK, Lee B, Liao F, Foreman RD. Local cooling reduces skin ischemia under surface pressure in rats: an assessment by wavelet analysis of laser Doppler blood flow oscillatioins. Physiol Meas. 2012;33:1733–45. doi: 10.1088/0967-3334/33/10/1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jan YK, Anderson M, Soltani J, Burns S, Foreman RD. Comparison of changes in heart rate variability and sacral skin perfusion in response to postural changes in people with spinal cord injury. J Rehabil Res Dev. 2013;50(2) doi: 10.1682/jrrd.2011.08.0138. DOI:10.1682/JRRD.2011.08.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clifford PS. Local control of blood flow. Advances in Physiology Education. 2011;35(1):5–15. doi: 10.1152/advan.00074.2010. [DOI] [PubMed] [Google Scholar]
- 44.Addor G, Delachaux A, Dischl B, Hayoz D, Liaudet L, Waeber B, et al. A Comparative Study of Reactive Hyperemia in Human Forearm Skin and Muscle. Physiol Res. 2008;57(5):685–92. doi: 10.33549/physiolres.931225. [DOI] [PubMed] [Google Scholar]
- 45.Gefen A, Gefen N, Linder-Ganz E, Margulies SS. In vivo muscle stiffening under bone compression promotes deep pressure sores. J Biomech Eng. 2005;127(3):512–24. doi: 10.1115/1.1894386. [DOI] [PubMed] [Google Scholar]
- 46.Liao FY, Jan YK. Using multifractal detrended fluctuation analysis to assess sacral skin blood flow oscillations in people with spinal cord injury. J Rehabil Res Dev. 2011;48(7):787–99. doi: 10.1682/jrrd.2010.08.0145. [DOI] [PubMed] [Google Scholar]
- 47.Geyer MJ, Jan YK, Brienza DM, Boninger ML. Using wavelet analysis to characterize the thermoregulatory mechanisms of sacral skin blood flow. J Rehabil Res Dev. 2004;41(6):797–806. doi: 10.1682/jrrd.2003.10.0159. [DOI] [PubMed] [Google Scholar]
- 48.Keller BP, Schuurman JP, van der Werken C. Can near infrared spectroscopy measure the effect of pressure on oxygenation of sacral soft tissue? J Wound Care. 2006;15(5):213–7. doi: 10.12968/jowc.2006.15.5.26908. [DOI] [PubMed] [Google Scholar]
- 49.Ding D, Leister E, Cooper RA, Cooper R, Kelleher A, Fitzgerald SG, et al. Usage of tilt-in-space, recline, and elevation seating functions in natural environment of wheelchair users. J Rehabil Res Dev. 2008;45(7):973–83. doi: 10.1682/jrrd.2007.11.0178. [DOI] [PubMed] [Google Scholar]
- 50.Sonenblum SE, Sprigle S, Maurer CL. Use of power tilt systems in everyday life. Disability and rehabilitation Assistive technology. 2009;4(1):24–30. doi: 10.1080/17483100802542744. [DOI] [PubMed] [Google Scholar]


