Abstract
Background
Pulmonary vein (PV) isolation has disappointing results in patients with obesity, heart failure, obstructive sleep apnea (OSA) and enlarged left atria (LA), for unclear reasons. We hypothesized that these comorbidities cause higher numbers or non-PV locations of AF sources, where targeted source ablation (Focal Impulse and Rotor Modulation, FIRM) should improve the single-procedure success of ablation.
Methods
The CONFIRM trial prospectively enrolled 92 patients at 107 AF ablation procedures, in whom computational mapping identified AF rotors or focal sources. Patients underwent FIRM plus conventional ablation (FIRM-guided), or conventional ablation only, and were evaluated for recurrent AF quarterly with rigorous, often implanted, monitoring. We report the n=73 patients undergoing first ablation in whom demographic information was available (n= 52 conventional, n=21 FIRM-guided).
Results
Stable sources for AF were found in 97.1% of patients. The numbers of concurrent sources per patient (2.1±1.1) rose with LA diameter (p=0.021), lower LV ejection fraction (p=0.039), and the presence of obstructive sleep apnea (OSA, p=0.002) or hypomagnesemia (p=0.017). Right atrial sources were associated with obesity (BMI≥30 kg/m2, p=0.015). In patients with obesity, hypertension, OSA and LA diameter > 40 mm, single-procedure freedom from AF was >80% by FIRM-guided versus <50% by conventional ablation (all; p<0.05).
Conclusion
Patients with ‘difficult to treat’ AF exhibit more concurrent AF sources in more widespread biatrial distributions than other patients. These mechanisms explain the disappointing results of PV isolation, and how FIRM can identify patient-specific AF sources to enable successful ablation in this population.
Keywords: Atrium, Fibrillation, Rotors, FIRM ablation, Metabolic syndrome, Sleep apnea, Clinical Trials
The success of ablation for atrial fibrillation (AF), that conventionally targets triggers in the pulmonary veins (PV), is reduced in patients with specific comorbidities. In particular, the multi-procedure success rate from PV-isolation based AF ablation may be as low as 41% in patients with obstructive sleep apnea (OSA)1, 73% in patients with systolic heart failure2 and 61% in patients with metabolic syndrome3, in whom success falls as body mass index (BMI) rises 4. However, these disappointing results are mechanistically unexplained.
It has recently been shown that human AF may be caused by stable sources, in the form of localized spiral waves (rotors) or focal sources similar to those shown in elegant animal models5, 6, 7. The CONFIRM trial (CONventional ablation of AF with or without Focal Impulse and Rotor Modulation, FIRM) 8 recently showed stable sources in 97% of patients with paroxysmal and persistent AF, and is now validated in several laboratories9, 10. In CONFIRM, a single targeted source (FIRM) plus conventional ablation procedure increased single-procedure AF freedom to 82.4% on rigorous monitoring from 44.9% for conventional ablation alone. A recent on-treatment analysis showed that AF ablation success is highest when all sources are eliminated, either directly by FIRM or ‘coincidentally’ by PVI, intermediate if some sources are eliminated and lowest if all are missed11.
We hypothesized that AF in patients with high-risk demographics results from higher numbers of AF sources lying remote from the PVs, that are less easily ablated coincidentally. Accordingly, we hypothesized that FIRM may improve the success of conventional ablation even in ‘difficult-to-treat’ patients. We tested our hypothesis by studying the impact of pre-specified high-risk demographics on AF rotors and focal sources, and on long-term success after FIRM-guided versus conventional ablation, in the CONFIRM trial.
METHODS
Study Design and Enrollment
CONFIRM 8 prospectively enrolled 92 subjects at 107 consecutive AF ablation procedures for standard indications. Procedures were performed by 3 investigators at 3 sites. Subjects were ≥21 years of age, with AF despite one or more class I or III anti-arrhythmic drugs. Cases were prospectively assigned 2:1 to FIRM-blinded or FIRM-guided ablation. FIRM-Guided patients were treated with targeted source ablation (FIRM) followed by conventional ablation; FIRM-blinded patients received only conventional ablation. The only exclusion was an inability or refusal to provide specific written informed consent. This report includes the n=73 patients in whom demographic information was available at their first ablation procedure for paroxysmal or persistent AF 12.
Electrophysiology Study
Electrophysiology study was performed after discontinuing anti-arrhythmic medications for 5 half lives (for amiodarone, >60 days, median 230 days). After infusing intravenous heparin to maintain activated clotting time>350 seconds, a 64-pole basket catheter (Constellation™, Boston Scientific, MA) was advanced trans-septally to map the left atrium (LA) in all patients, and also to the right atrium (RA) in n=45 patients (including all FIRM-guided cases).
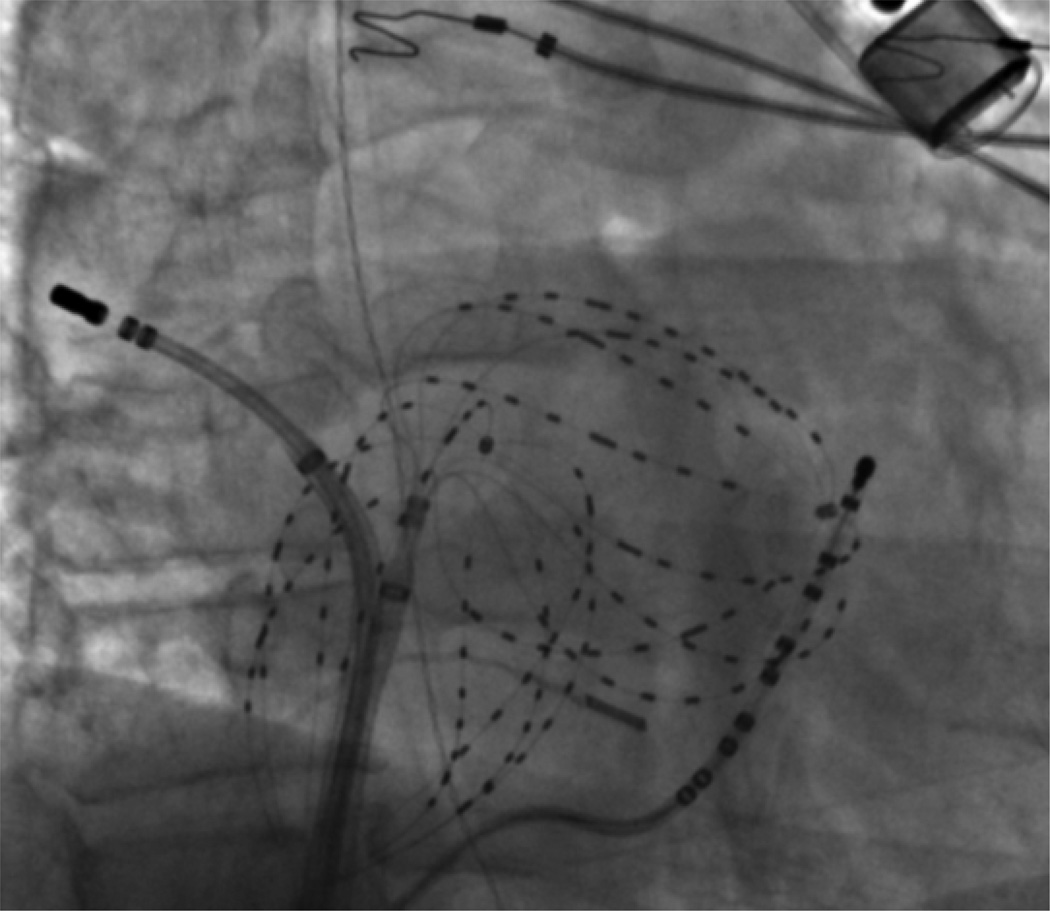
Figure 1 shows biatrial baskets. AF was observed in 69 patients (including all FIRM-guided cases) including AF induced by rapid pacing or isoproterenol when required 8. Recent studies show that induced and spontaneous AF in the same patient show very similar dominant frequency 13 and spatial patterns 14. AF electrograms were filtered at 0.05 – 500 Hz and exported at 1kHz temporal resolution (Bard LabPro, MA).
Figure 1. Focal Impulse and Rotor Mapping (FIRM) of Atrial Fibrillation, Using Contact Electrodes Widely Covering Both Atria.
Fluoroscopy also shows a coronary sinus catheter, an ablation catheter, an intracardiac ultrasound catheter and an esophageal temperature probe. A subcutaneous ECG monitor, to stringently document AF recurrence, is also visible.
FIRM Mapping of AF Sources
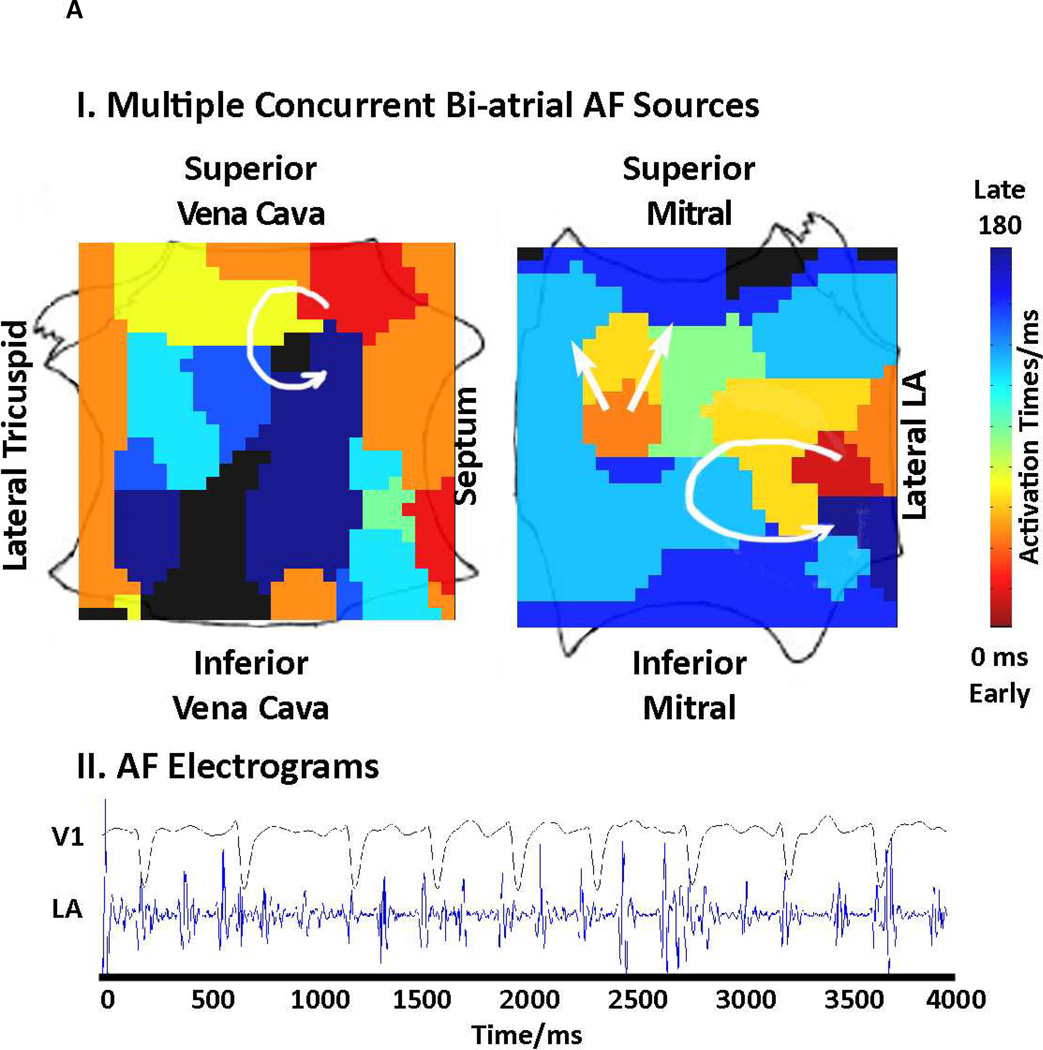
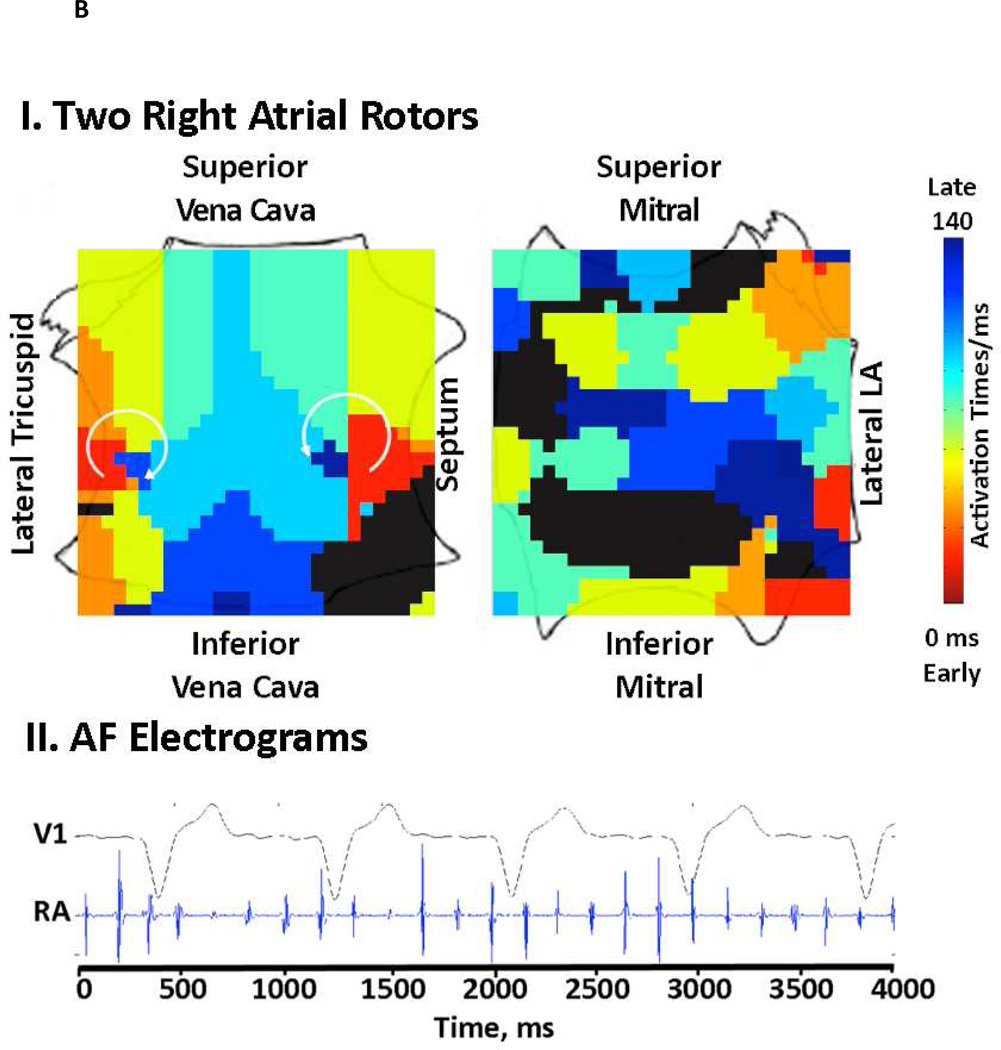
FIRM mapping has been described elsewhere 8, 14. Briefly, AF was recorded at wide field-of-view baskets for analysis with a novel mapping system (RhythmView™, Topera, Palo Alto, California) that projects these 3 dimensional data onto grids of AF propagation. Figure 2 shows FIRM maps of AF rotors (each indicated by a red-to-blue, early-to-late spiral wave).
Figure 2. Multiple AF Sources in “Difficult to Treat” AF Patients.
(A) Concurrent rotor and focal sources, including 2 in the LA, in a 49-year-old patient with OSA. (B) Right atrium showing 2 concurrent rotors in a 63 year old morbidly obese patient (BMI 41.9 kg/m2), that were successfully ablated by FIRM but would not have been targeted by conventional ablation near the PVs.
AF propagation (FIRM) maps were analyzed intra-procedurally to guide ablation in FIRM-Guided patients, and post-procedurally in FIRM-blinded patients. Electrical rotors (figure 2) were defined as sustained activation around a core, while focal impulses showed centrifugal activation from an origin. Rotors and focal impulses were considered mechanistic AF sources only if stable on repeated sampling over >30 minutes (i.e. thousands of cycles), unlike transient fibrillatory activity 15, 16.
Analysis of AF Source in Relation to Clinical Demographics
We related the numbers of AF sources to clinical variables in univariate and multivariate analyses. Being overweight was defined as 25≤BMI<30, obesity as 30≤BMI<40 and morbid obesity as BMI≥40 (in kg/m2). CKD stages were calculated from estimated glomerular filtration rate (GFR) as stated in the U.S. National Kidney Foundation guidelines 17. Systolic heart failure was defined as left ventricular ejection fraction ≤ 40%. LA enlargement was defined as diameter ≥ 40mm from the anteroposterior diameter on 2D transthoracic echocardiography. The presence of hypertension (HTN), diabetes mellitus (DM), OSA and other comorbidities were identified from each patient’s electronic medical record.
Ablation Approach
Radiofrequency energy was delivered with a 3.5 mm tip irrigated (Thermocool, Biosense-Webster, Diamond Bar, CA) or, in heart failure subjects, 8 mm tip (Blazer, Boston Scientific, Natick, MA) catheter. In FIRM-guided subjects, FIRM was performed first via lesions to cover the AF source (rotational center of rotors, or focal source origin 8). The endpoint was AF termination or 10 minutes’ ablation (typically <5 minutes), whichever came first. If AF terminated, vigorous attempts were made to reinitiate AF. Patients in whom AF terminated but could be reinduced were labeled as ‘AF slowing’. FIRM was repeated for ≤ 3 sources (≤30 minutes permitted8), followed by conventional ablation.
Conventional ablation 12 , performed after FIRM in FIRM-guided patients and as sole therapy in FIRM-blinded patients, was standardized to comprise wide area isolation of left and right PV pairs verified with a circular mapping catheter (Lasso, Biosense-Webster). In persistent AF, a LA roof line was also performed. Atrial tachycardia or flutter were ablated appropriately. No other ablation was performed. If AF persisted after completion of the ablation protocol, cardioversion was performed.
Post-Procedure Clinical Management
Follow up for arrhythmia recurrence met or exceeded guidelines 12. Anti-arrhythmic medications (but not amiodarone) were continued for 3 months post-ablation, but repeat ablation was not permitted in this ‘blanking period’. Subjects were evaluated quarterly for recurrences using continuous implanted ECG monitors if possible, i.e. Reveal XT™ (Medtronic, Minneapolis, MN) once clinically available in 2009 (figure 1), or indicated pacemaker/ defibrillators. Remaining subjects received external event monitors quarterly and at times of symptoms.
Study Endpoints
The primary long-term efficacy endpoint was freedom from AF after one procedure, defined as <1% burden on continuous implanted ECG monitors (365 days/year monitoring; actual burden 0.1±0.2% in CONFIRM 8), or <30 seconds on intermittent monitors 12 (<28 days/year monitoring). Secondary efficacy measures included freedom from all atrial arrhythmias8.
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Normality was evaluated using the Kolmogorov-Smirnov test. Comparisons between 2 groups were made with Student’s t-tests and summarized with means and standard deviations for independent samples if normally distributed (e.g. age, LA diameter) or, if not normally distributed, with the Mann-Whitney U test and summarized with medians and quartiles. Nominal values are expressed as n (%) and compared with chi-square tests (e.g. numbers with obesity) or the Fisher exact test when expected cell frequency was<5 (e.g. numbers with kidney disease). Associations between continuous variables were evaluated with Pearson’s correlation. The comparison between two dependent correlations (e.g. rotors vs focal sources) was tested by equality of two dependent correlations 18. The specific tests used for each comparison are indicated in the Results section. Long-term outcome was assessed and reported after a single procedure, and raw event rates were compared with chi-square tests and event-free survival plots were made by the Kaplan-Meier method and compared with log-rank tests. A probability of<0.05 was considered statistically significant throughout.
Results
The population suffered from a wide range of comorbidities, including demographic factors of high risk for recurrent AF after ablation (Table 1).
Table 1.
Clinical Characteristics
| Characteristic | FIRM-blind (Conventional) |
FIRM-guided | p | |
|---|---|---|---|---|
| Type of AF | 52 | 21 | 0.919 | |
| Paroxysmal AF | 20 | 7 | ||
| Persistent AF | 25 | 11 | ||
| Long standing persistent AF | 7 | 3 | ||
| Age (years) | 61±8.8 | 62±9.1 | 0.787 | |
| Gender (Male/Female) | N=51/1 | N=19/2 | 0.197 | |
| LA diameter (mm) | 43.8±6.6 | 47.4±6.8 | 0.042 | |
| Left Ventricular Ejection Fraction (%) | 54.8±12 | 56.2±16 | 0.677 | |
| Comorbid Conditions | ||||
| Hypertension | 37/52 | 19/21 | 0.125 | |
| Diabetes | 17/52 | 9/21 | 0.412 | |
| BMI (mean) | 31.9±5.6 | 33.2±5.5 | 0.398 | |
| Normal weight (BMI <25) | 4/52 | 0/21 | 0.318 | |
| Overweight (BMI 25–29.9) | 15/52 | 5/21 | 0.662 | |
| Obese (BMI 30–39.9) | 28/52 | 12/21 | 0.798 | |
| Morbidly obese (BMI ≥40) | 4/52 | 2/21 | 1.000 | |
| Obstructive sleep apnea | 26/51 | 17/21 | 0.018 | |
| Congestive Heart Failure | 10/52 | 5/21 | 0.751 | |
| Systolic heart failure (EF <40%) | 6/52 | 5/21 | 0.277 | |
| Diastolic failure | 4/52 | 0/21 | 0.318 | |
| BNP | 206.5±186 | 171.5±147 | 0.551 | |
| Chronic Kidney Disease (CKD) | 6/50 | 5/21 | 0.282 | |
| GFR, ml/min (mean) | 75.8±16.3 | 72.6±17.6 | 0.486 | |
| Magnesium, mg/dl | 2.05±0.18 | 2.05±0.17 | 0.940 | |
Statistical comparisons were performed using the Chi-squared test (for incidences of persistent AF, diabetes mellitus, obstructive sleep apnea, overweight and obesity), the t-test (for age, LA diameter, LV ejection fraction, body mass index and laboratory data), and Fisher’s exact test (for gender, HTN, long standing persistent AF, chronic kidney disease, congestive heart failure, normal weight and morbid obesity).
Stable Localized Sources for Human AF
Intraprocedural AF was observed in 69/73 patients, in whom FIRM mapping revealed stable AF rotors or focal sources in 67 (97.1 %) for 2.1±1.1 concurrent sources each. The number of sources in prespecified demographic subgroups is shown in Table 2. Sources lay in diverse locations in left atrium (88%; 1.37±0.9 per patient) and right atrium (71.1%; 0.91±0.7 for each patient with biatrial mapping).
Table 2.
Number of localized sources in patient subgroups
| Variables | Number of Stable AF Sources |
Number of Rotors | Number of Focal Sources |
|---|---|---|---|
| All | 1.9±1.2 | 1.6±1.1 | 0.3±0.6 |
| Obstructive sleep apnea | 2.3±1.2 | 1.9±1.2 | 0.4±0.7 |
| LA enlargement | 2.1±1.3 | 1.8±1.2 | 0.3±0.6 |
| Systolic heart failure | 2.9±1.7 | 2.6±1.4 | 0.3±0.5 |
| Obesity | 2.1±1.4 | 1.8±1.2 | 0.3±0.6 |
| Persistent AF | 2.1±1.3 | 1.9±1.2 | 0.2±0.5 |
| Hypertension | 2.0±1.2 | 1.6±1.2 | 0.4±0.6 |
| Diabetes Mellitus | 2.1±1.5 | 1.6±1.4 | 0.5±0.8 |
| Hyperlipidemia | 2.1±1.3 | 1.7±1.2 | 0.4±0.6 |
Statistical analyses were done using paired samples t-test. The number of stable AF rotors was significantly higher than the number of focal sources for all subgroups (P<0.05 for each)
FIRM often ablated sources at locations that would not be targeted conventionally. For instance, figure 2A shows concurrent biatrial sources and figure 2B shows 2 RA AF rotors that were eliminated by FIRM but may have been missed by conventional ablation.
Demographic Associations with Numbers of Concurrent AF Sources, and Source Atrium
On univariate linear regression, several demographic factors were associated with the number of concurrent AF sources per patient (Table 3). On multivariate analysis, obstructive sleep apnea (p=0.004) and systolic dysfunction (p=0.001) associated with the number of concurrent AF sources.
Table 3.
Relationship Between Clinical Variables and the Total Numbers of Stable AF Sources on FIRM mapping
| Variables | p | r |
|---|---|---|
| Obstructive Sleep Apnea | 0.002 | 0.364 |
| Systolic heart failure | <0.001 | 0.424 |
| LA diameter (mm) | 0.021 | 0.283 |
| Magnesium (serum, mg/dL) | 0.017 | −0.307 |
| New York Heart Association (NYHA) class | 0.023 | 0.274 |
| LV ejection fraction (%) | 0.039 | −0.253 |
| Coronary artery disease | 0.050 | 0.236 |
| Persistent AF | 0.074 | 0.217 |
| Body Mass Index (BMI) | 0.103 | 0.202 |
| Chronic Kidney Disease (CKD) | 0.176 | 0.167 |
| DM | 0.185 | 0.162 |
| CKD stage | 0.194 | 0.161 |
| Hyperlipidemia | 0.216 | 0.151 |
| AF cycle length | 0.323 | −0.121 |
| Age | 0.338 | 0.117 |
| Hypertension | 0.349 | 0.114 |
Statistical analyses was performed using Pearson’s correlation.
Since conventional ablation is mostly LA while AF sources were biatrial, we studied demographic factors associated with right (versus left) atrial AF sources (e.g. figures 2A, B). As shown in table 4, the number of RA AF sources per patient rose with increasing BMI (p=0.015). Statistical significance was unchanged when analyzing the entire population in CONFIRM (i.e. including subjects with AF despite prior PVI; data not shown).
Table 4.
Relationship of Clinical Variables to Numbers of Left versus Right Atrial AF sources
| Variables | Relationship to Number of RA sources for AF |
Relationship to Number of LA sources for AF |
||
|---|---|---|---|---|
| P | r | P | r | |
| Systolic heart failure | 0.710 | 0.057 | <0.001 | 0.438 |
| Magnesium (serum, mg/dL) | 0.456 | 0.128 | 0.003 | −0.379 |
| NYHA class | 0.810 | 0.037 | 0.023 | 0.274 |
| LV ejection fraction (%) | 0.458 | −0.115 | 0.033 | −0.261 |
| OSA | 0.673 | 0.065 | 0.044 | 0.245 |
| Body mass index (BMI) | 0.015 | 0.367 | 0.805 | −0.031 |
| LA diameter (mm) | 0.060 | 0.290 | 0.166 | 0.173 |
| Persistent AF | 0.115 | 0.238 | 0.463 | 0.090 |
| DM | 0.567 | −0.088 | 0.144 | 0.178 |
| HL | 0.777 | −0.043 | 0.533 | 0.076 |
Statistical relationships Calculated Using Pearson’s correlation coefficient
Demographic Associations With Rotor Versus Focal AF Sources
On univariate analysis, the number of AF rotors was associated with LA diameter, systolic heart failure, low serum Magnesium, OSA and persistent AF (table 5). On multivariate analysis, systolic heart failure (p<0.001) and OSA (p=0.047) associated significantly with the number of rotors. When looking at the difference of univariate correlations between rotors and focal sources (table 5), there was a significant difference with presence of systolic heart failure (p=0.002) and serum Magnesium levels (p=0.028).
Table 5.
Relationship of Clinical Variables to Numbers of Rotors or Focal AF sources
| Variables | Relationship to AF Rotors | Relationship to AF Focal sources |
Significance of difference |
||
|---|---|---|---|---|---|
| p | r | P | r | p | |
| Systolic heart failure | <0.001 | 0.465 | 0.784 | −0.034 | 0.002 |
| LA diameter (mm) | 0.002 | 0.382 | 0.210 | −0.156 | 0.065 |
| Magnesium (serum, mg/dL) | 0.004 | −0.370 | 0.550 | 0.079 | 0.028 |
| Persistent AF | 0.004 | 0.339 | 0.096 | −0.202 | 0.180 |
| OSA | 0.020 | 0.282 | 0.141 | 0.180 | 0.251 |
| Body mass index (BMI) | 0.060 | 0.233 | 0.743 | −0.041 | 0.109 |
| AF cycle length | 0.223 | −0.149 | 0.748 | 0.039 | 0.242 |
Statistical relationships Calculated Using Pearson’s correlation coefficient and comparison of dependent correlation coefficients 18
Impact of Demographic Factors on Success of Ablation
Single procedure freedom from AF was 90% for patients receiving FIRM guided ablation versus 44% for conventional ablation. The higher success of FIRM was maintained across the prespecified high-risk groups in table 6 (for AF) and table 7 (for AF/all arrhythmias), in which row 1–7 analyses use the Chi-squared test, while rows 8–10 use Fisher’s exact for DM, systolic heart failure and paroxysmal AF.
Table 6.
Comparative Freedom from AF after FIRM-Blinded and FIRM-Guided Ablation For Prespecified Demographic Subgroups
| Characteristic | Single Procedure Freedom from AF, FIRM-Blinded (Conventional) |
Single Procedure Freedom from AF, FIRM-Guided |
p |
|---|---|---|---|
| All patients | 23/52 (44%) | 17/19 (90%) | 0.001 |
| Obesity (BMI≥30) | 14/32 (44%) | 11/12 (92%) | 0.004 |
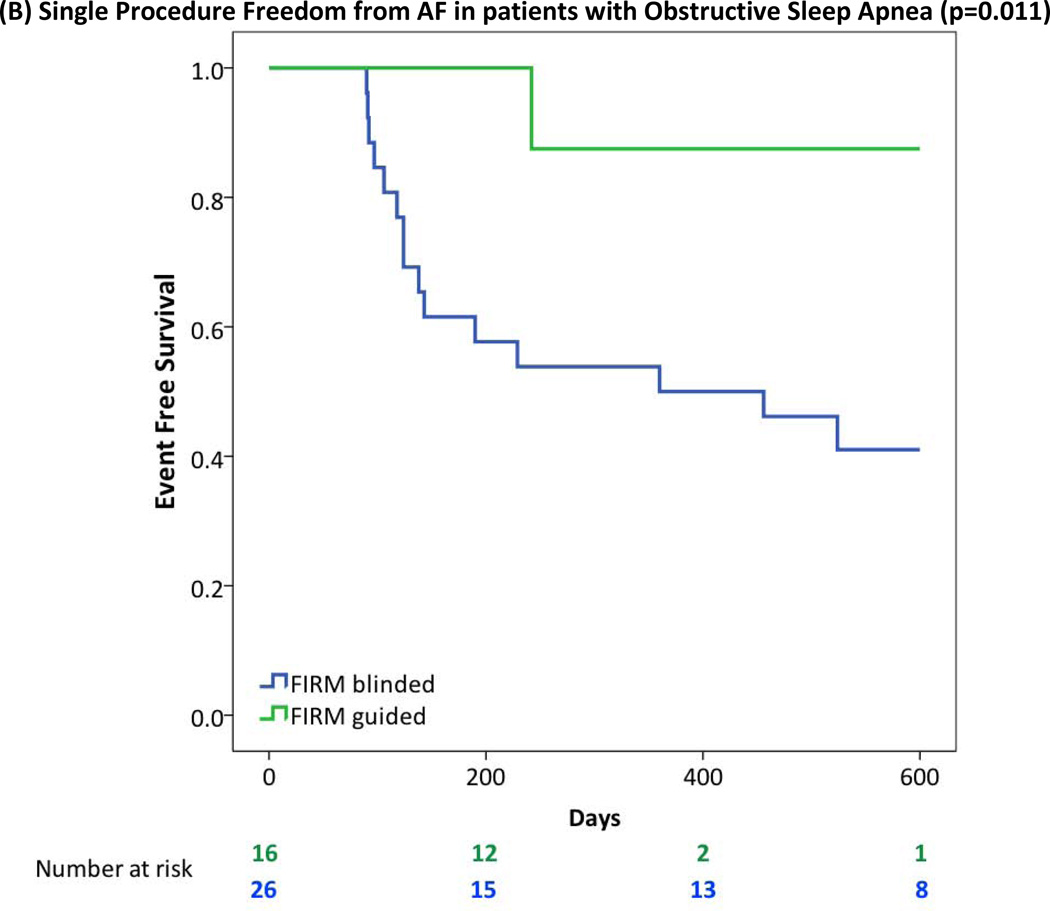
| Obstructive sleep apnea | 10/26 (39%) | 15/16 (94%) | <0.001 |
| Persistent AF | 12/32 (38%) | 12/13 (92%) | 0.001 |
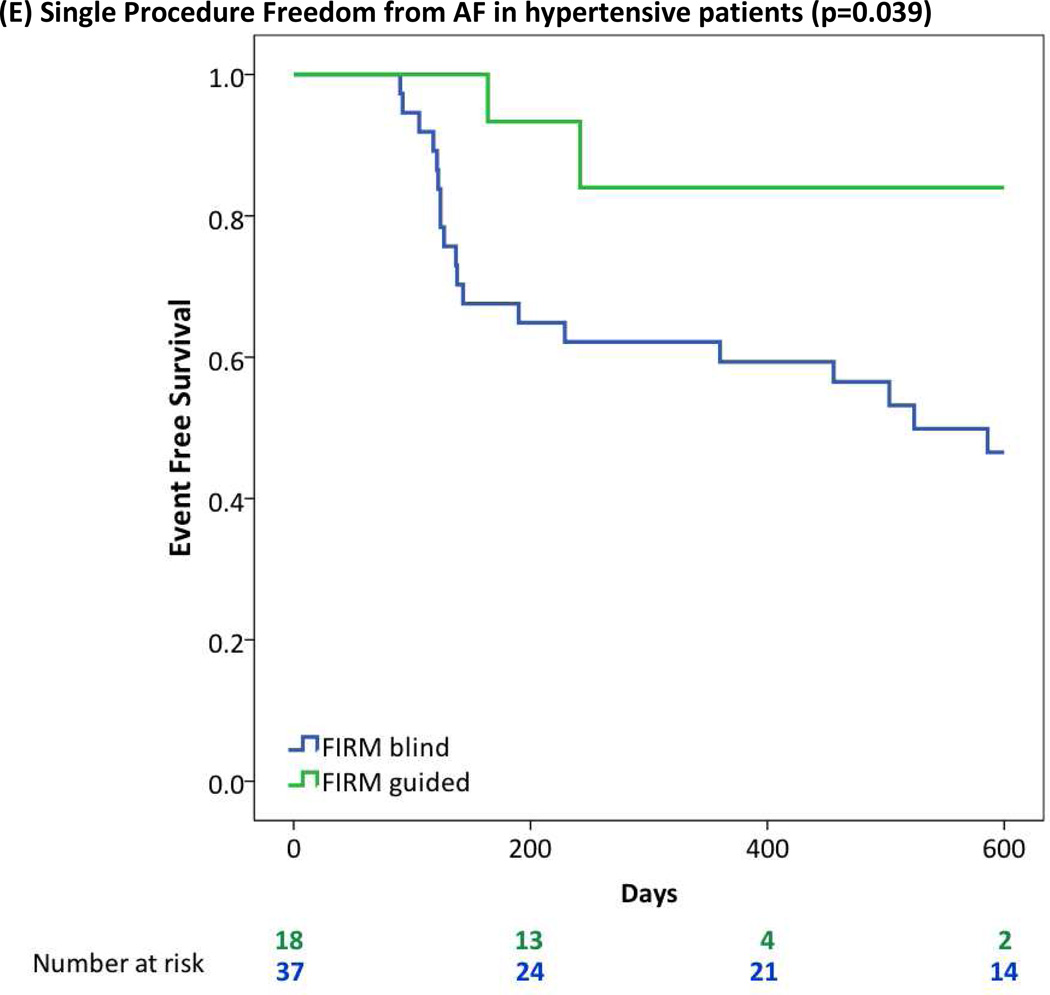
| HTN | 16/37 (43%) | 16/18 (89%) | 0.001 |
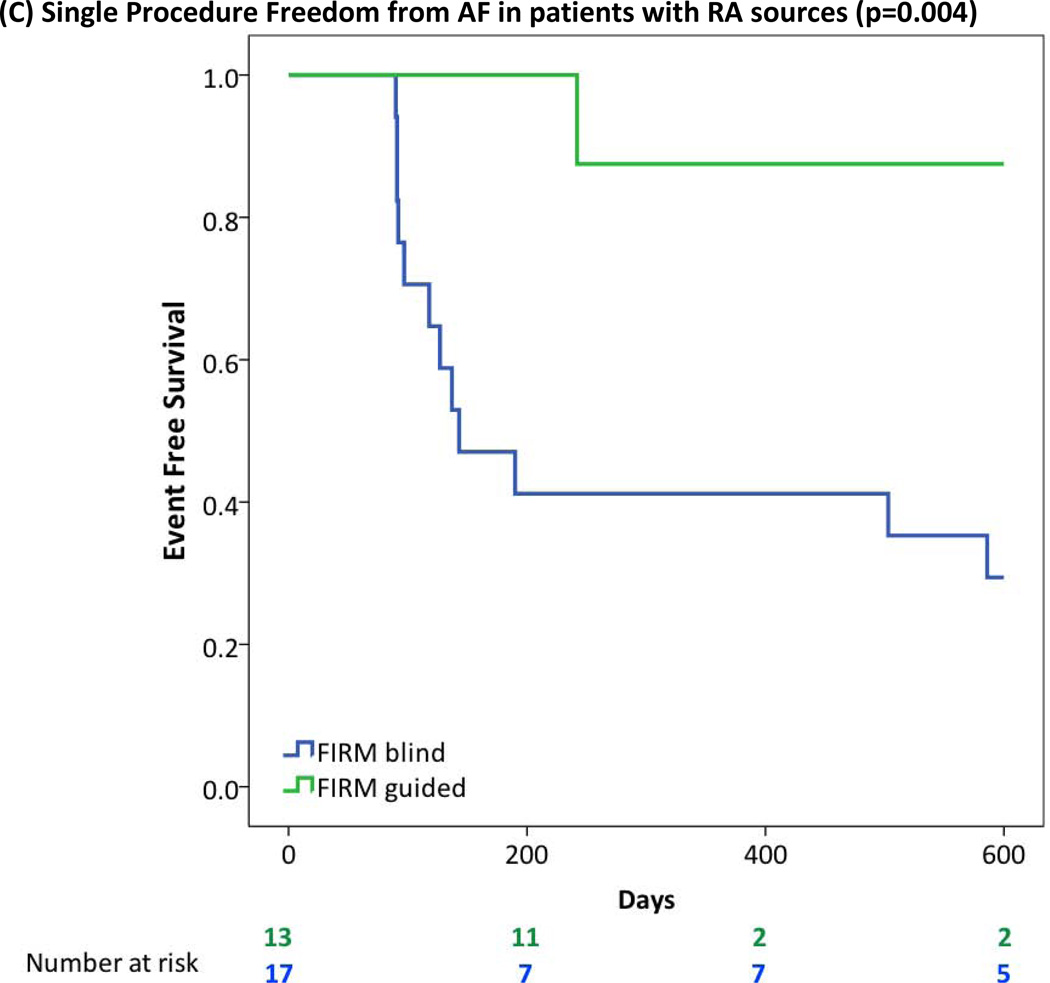
| Presence of RA sources | 5/17 (29%) | 12/13 (92%) | 0.001 |
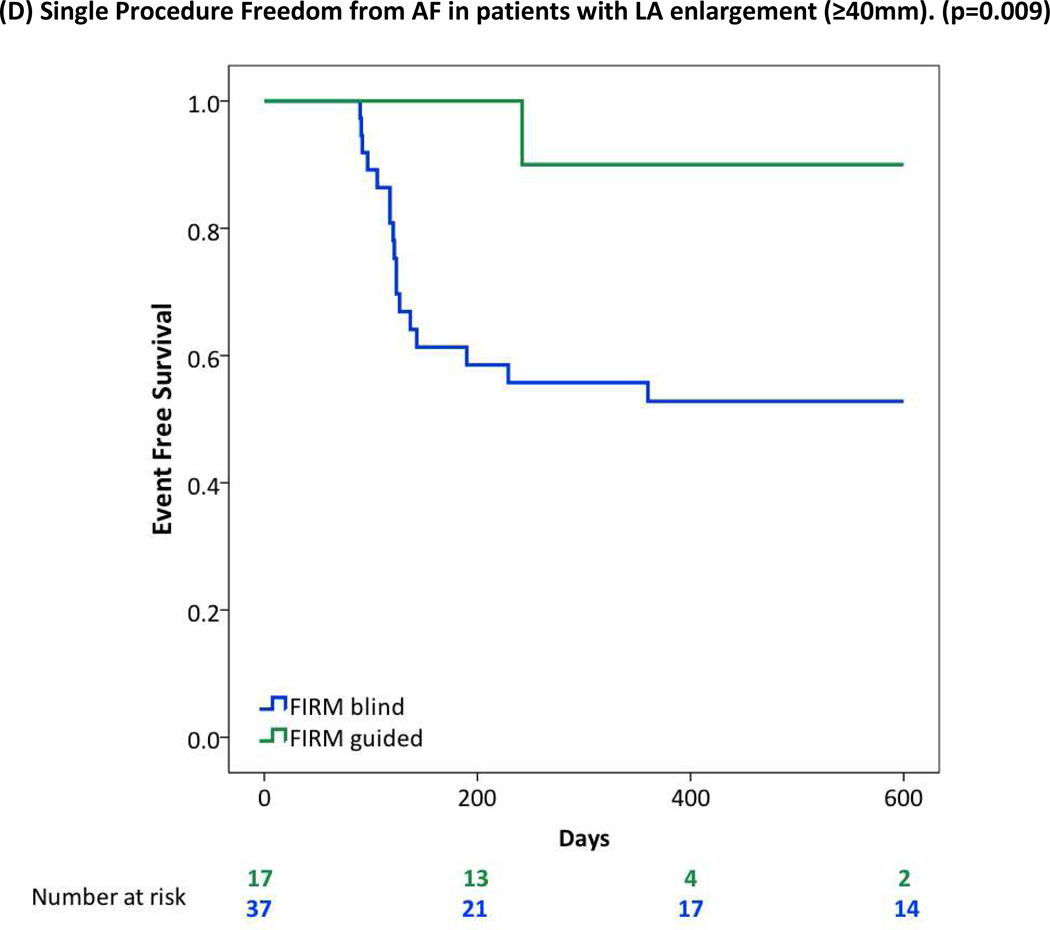
| LA enlargement | 19/37 (51%) | 16/17 (94%) | 0.002 |
| DM | 9/17 (53%) | 6/8 (75%) | 0.402 |
| Systolic heart failure | 4/6 (67%) | 4/4 (100%) | 0.467 |
| Paroxysmal AF | 11/20 (55%) | 5/6 (83%) | 0.352 |
Statistical differences calculated using Chi squared or Fisher’s exact tests.
Table 7.
Comparative Freedom from AT/AF after FIRM-Blinded and FIRM-Guided Ablation For Prespecified Demographic Subgroups
| Characteristic | Single Procedure Freedom from AF/AT, FIRM-Blind (Conventional) |
Single Procedure Freedom from AF/AT FIRM-Guided |
p |
|---|---|---|---|
| All patients | 20/52 (39%) | 14/19 (74%) | 0.009 |
| Persistent AF | 10/32 (31%) | 9/13 (69%) | 0.019 |
| Obesity (BMI≥30) | 12/32 (38%) | 9/12 (75%) | 0.027 |
| HTN | 15/37 (41%) | 13/18 (72%) | 0.027 |
| LA enlargement | 17/37 (46%) | 13/17 (77%) | 0.036 |
| Obstructive sleep apnea | 9/26 (35%) | 12/16 (75%) | 0.011 |
| Presence of RA sources | 5/17 (29%) | 9/13 (69%) | 0.030 |
| DM | 9/17 (53%) | 6/8 (75%) | 0.402 |
| Systolic heart failure | 3/6 (50%) | 4/4 (100%) | 0.200 |
| Paroxysmal AF | 10/20 (50%) | 5/6 (83%) | 0.197 |
Statistical differences calculated using chi square and Fisher’s exact tests
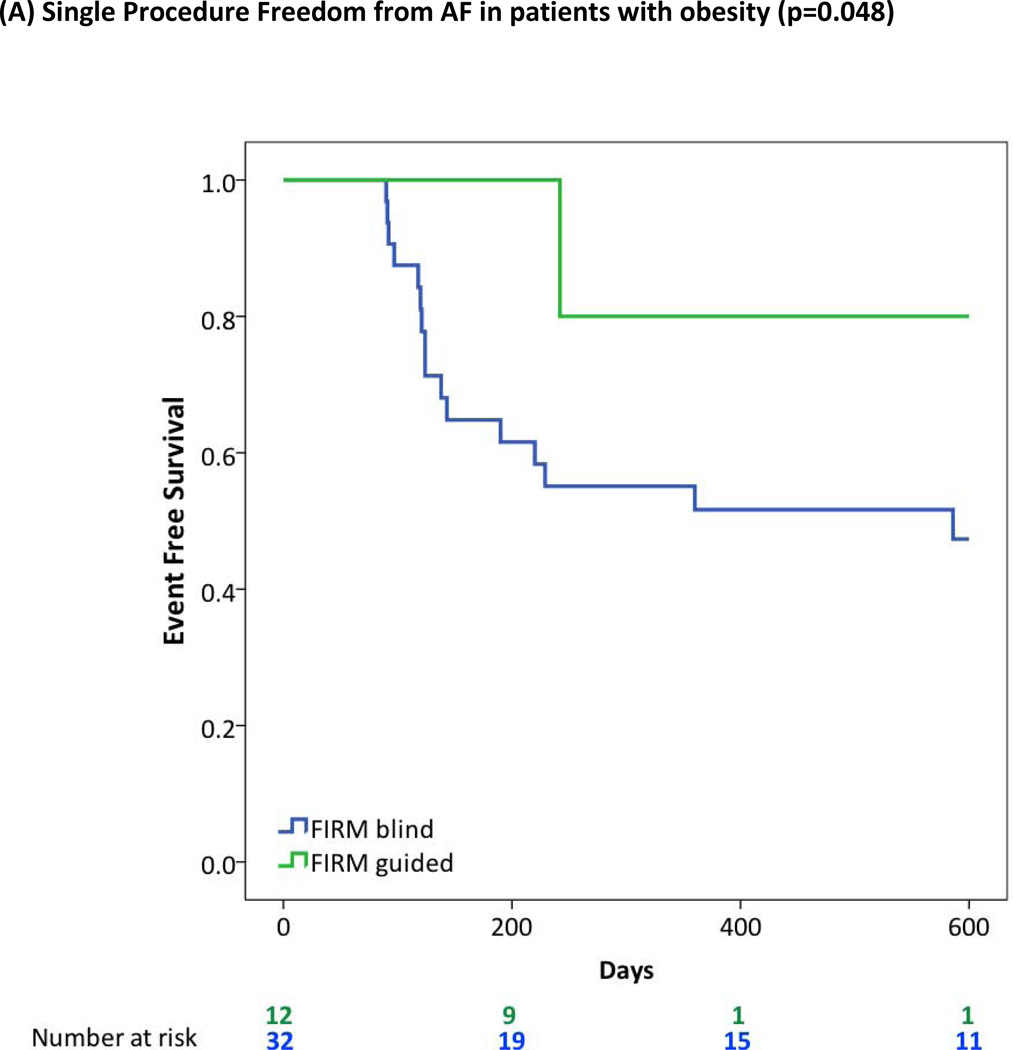
Figure 3 panels A-E illustrate Kaplan-Meier curves of cumulative single-procedure freedom from AF between FIRM and conventional ablation groups for these groups. Statistical significance was unchanged when analyzing the entire population in CONFIRM (i.e. including subjects with AF despite prior PVI; data not shown).
Figure 3. Cumulative Single Procedure Freedom from Atrial Fibrillation for FIRM-Guided versus Conventional Ablation for prespecified high risk subgroups.
FIRM-guided provided higher freedom from AF than FIRM-blinded ablation for patients with (A) Obesity or Morbid Obesity (BMI>30), (B) Obstructive Sleep Apnea Syndrome; (C) Right Atrial Sources; (D) LA Enlargement (diameter >40 mm); (E) Hypertension.
Discussion
In AF patients in whom demographic factors would predict failure of conventional ablation, targeted elimination of stable AF rotors and focal sources performed prior to conventional ablation substantially increased single procedure success. Mechanistically, AF in patients with obstructive sleep apnea, LA enlargement, obesity, hypomagnesemia and heart failure exhibited higher numbers of concurrent AF sources, while obese patients were more likely to show AF sources in the RA remote from conventional targets. These data provide a potential unifying explanation for the disappointing results of PVI in these patients, and may help realize the goal of patient-tailored ablation at AF mechanisms 19 in a wide AF population.
Demographic Markers of Risk for AF
Incident AF has long been associated with advanced age, male gender, hypertension and heart failure 20. More recently, incident AF has been linked with obesity and OSA 21 , 22 , 23, and low serum magnesium was recently shown to increase AF risk by 50% 24.
Freedom from AF after conventional ablation is also reduced by many of these same risk factors of obesity 4, 25, OSA 1 , 26, systolic heart failure2 and LA enlargement27. However, it has been mechanistically unexplained why these patients are ‘difficult-to-treat’, and thus unclear how to guide ablation procedures to circumvent these limitations.
Mechanistic Role of Stable AF Rotors and Focal Sources
This prespecified substudy of the CONFIRM trial shows that ‘difficult-to-treat’ AF patients show a higher number of concurrent stable AF rotors or focal sources than patients without these comorbidities. The ability of targeted FIRM ablation to maintain high long-term outcome in these patients strengthens the mechanistic role of stable AF sources across diverse populations, as demonstrated in the CONFIRM trial and now by other laboratories 9, 10.
At the cellular and tissue level, however, it remains unclear how AF sources functionally interact with specific patterns of myofibers 28, fibrosis or scar 29, electrical remodeling 30, autonomic innervation 31 or other factors. These issues may be resolved definitively only by translational studies in models that recapitulate human AF 6, 32. In the meanwhile, many of these results are pathophysiologically plausible in that the number of LA AF sources rose with the presence of heart failure and OSA, that increase LA pressure 33, and diabetes 34 that may cause atrial fibrosis, while RA sources rose with the presence of obesity, that can increase RA pressure 35. Intriguingly, low serum magnesium was also associated with numbers of concurrent LA rotors, independently confirming this recent epidemiological finding 24. Stable AF sources and their distributions may thus provide a mechanistic foundation explaining how structural and electrical remodeling interact to form AF “substrates”.
Clinical Implications
The apparent complexity of AF in ‘difficult-to-treat’ patients, evidenced by resistance to PV isolation, may simply reflect more AF sources in diverse non-PV locations. These locations may, in turn, reflect electrical or structural ‘remodeling’ mechanistically related to each comorbidity. By mapping AF sources regardless of right or left atrial location, FIRM-guided ablation maintained high single-procedure success rates even in these patients. Mechanistically, these data may help develop novel approaches to ameliorate AF such as reducing pulmonary arterial pressures in OSA patients 36 or therapies for heart failure.
Limitations
The CONFIRM study was non-randomized, although subjects were enrolled, mapped and treated prospectively. CONFIRM also had an active control group (conventional ablation) compared to prior studies that compared ablation to previously ineffective drugs. Although most AF ablation trials likely underestimated AF recurrence 37, 88% of FIRM-guided patients in CONFIRM were followed using implanted cardiac devices that is more rigorous than current guidelines 12. The present study is limited by excluding some patients from CONFIRM, but strengthened by documentation of comorbidities from each patient’s electronic medical record. Notably, associations of each comorbidity with source characteristics and the benefits of FIRM-guided versus conventional ablation were statistically unchanged if examining patients with AF after prior PVI. Finally, our patient population was mostly male, although studies from other centers 9, 10 show similar FIRM-guided mapping and ablation results in women.
Conclusions
Targeted ablation at stable AF rotors and focal sources (FIRM) combined with conventional PV isolation provided significantly higher single procedure efficacy than conventional ablation alone in traditionally ‘difficult-to-treat’ AF patients. Mechanistically, obesity, heart failure and obstructive sleep apnea are associated with greater numbers of AF sources often remote from the pulmonary veins and in the right atrium, that can be successfully identified and treated by FIRM guided ablation yet which explains lower the success of PV isolation in this population.
Brief Summary.
Atrial Fibrillation (AF) remains a major public health problem, for which ablation is increasingly performed. However, conventional ablation for AF is disappointing in patients with specific morbidities. We found that stable localized AF rotors, recently reported by several groups, are present in higher numbers and in atypical locations in these “difficult to treat” than other AF patients. Accordingly, ablation of AF rotors (FIRM) was more successful than conventional ablation in eliminating “difficult to treat” AF.
Acknowledgements
We thank Antonio Moyeda, RCVT, Kenneth Hopper, RCVT, Judy Hildreth, RN, Sherie Janes, RN, Stephanie Yoakum, RNP, Elizabeth Greer, RN, Donna Cooper, RN and Kathleen Mills, BA for helping to perform the clinical study and collecting follow-up data.
Funding Sources
This work was supported in part by grants from the American Heart Association to DEK, an ACC-Merck fellowship to AAS, and from the National Institutes of Health (HL70529, HL83359, HL103800) and Doris Duke Charitable Foundation to SMN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Baykaner: Reports no disclosures.
Mr Clopton: Reports no disclosures.
Dr. Lalani: Reports no disclosures.
Dr. Schricker: Reports no disclosures.
Dr. Krummen: His Institution has received fellowship support from Medtronic, Boston Scientific, St. Jude Medical and Biotronik.
Dr. Narayan: Dr. Narayan reports being co-inventor on intellectual property owned by the University of California and licensed to Topera, Inc. Dr. Narayan holds equity in Topera. Topera has not sponsored any research, including that presented here. Dr. Narayan also reports honoraria from Medtronic, St. Jude Medical and Biotronik Corporations. His Institution has received fellowship support from Medtronic, Boston Scientific, St. Jude Medical and Biotronik.
References
- 1.Jongnarangsin K, Chugh A, Good E, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:668–672. doi: 10.1111/j.1540-8167.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen MS, Marrouche NF, Khaykin Y, et al. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol. 2004;43:1004–1009. doi: 10.1016/j.jacc.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 3.Mohanty S, Mohanty P, Di Biase L, et al. Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. Journal of the American College of Cardiology. 2012;59:1295–1301. doi: 10.1016/j.jacc.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 4.Chilukuri K, Dalal D, Gadrey S, et al. A prospective study evaluating the role of obesity and obstructive sleep apnea for outcomes after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:521–525. doi: 10.1111/j.1540-8167.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- 5.Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. Iii. The "leading circle" concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 6.Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovascular research. 2011;89:766–775. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu K, Shroff SC, Sahadevan J, et al. Mapping of atrial activation during sustained atrial fibrillation in dogs with rapid ventricular pacing induced heart failure: Evidence for a role of driver regions. J Cardiovasc Electrophysiol. 2005;16:1348–1358. doi: 10.1111/j.1540-8167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 8.Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: Confirm (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivkumar K, Ellenbogen KA, Hummel JD, et al. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: First multicenter experience of focal impulse and rotor modulation (firm) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowal R, Daubert J, Day J, et al. Results of focal impulse and rotor modulation (firm) for atrial fibrillation are equivalent between patients treated in san diego compared with sites new to firm ablation: An extended multi-center experience. Heart Rhythm Society. 2013 [Google Scholar]
- 11.Narayan SM, Krummen DE, Clopton P, et al. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: On-treatment analysis of the confirm (conventional ablation for af with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calkins CH. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–696. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 13.Calvo D, Atienza F, Jalife J, et al. High-rate pacing-induced atrial fibrillation effectively reveals properties of spontaneously occurring paroxysmal atrial fibrillation in humans. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012 doi: 10.1093/europace/eus180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayan SM, Krummen DE, Rappel W-J. Clinical mapping approach to identify rotors and focal beats in human atrial fibrillation. J Cardiovascular Electrophysiology. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konings K, Kirchhof C, Smeets J, et al. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 16.Cuculich PS, Wang Y, Lindsay BD, et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Balk E, et al. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- 19.Nattel S. From guidelines to bench: Implications of unresolved clinical issues for basic investigations of atrial fibrillation mechanisms. The Canadian journal of cardiology. 2011;27:19–26. doi: 10.1016/j.cjca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the manitoba follow-up study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 22.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki Y-k, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm. 2012;9:1409–1416. doi: 10.1016/j.hrthm.2012.03.024. e1401. [DOI] [PubMed] [Google Scholar]
- 24.Khan AM, Lubitz SA, Sullivan LM, et al. Low serum magnesium and the development of atrial fibrillation in the community: The framingham heart study. Circulation. 2013;127:33–38. doi: 10.1161/CIRCULATIONAHA.111.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai L, Yin Y, Ling Z, et al. Predictors of late recurrence of atrial fibrillation after catheter ablation. International Journal of Cardiology. 2011 doi: 10.1016/j.ijcard.2011.06.094. [DOI] [PubMed] [Google Scholar]
- 26.Ng CY, Liu T, Shehata M, et al. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108:47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 27.Berruezo A, Tamborero D, Mont L, et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. European Heart Journal. 2007;28:836–841. doi: 10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 28.Klos M, Calvo D, Yamazaki M, et al. Atrial septopulmonary bundle of the posterior left atrium provides a substrate for atrial fibrillation initiation in a model of vagally mediated pulmonary vein tachycardia of the structurally normal heart. Circ Arrhythm Electrophysiol. 2008;1:175–183. doi: 10.1161/CIRCEP.107.760447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 31.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the ca2+ transient: An arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Comtois P, Nattel S. Impact of tissue geometry on simulated cholinergic atrial fibrillation: A modeling study. Chaos. 2011;21:013108. doi: 10.1063/1.3544470. [DOI] [PubMed] [Google Scholar]
- 33.Roux F, D'Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396–402. doi: 10.1016/s0002-9343(00)00302-8. [DOI] [PubMed] [Google Scholar]
- 34.Kato T, Yamashita T, Sekiguchi A, et al. What are arrhythmogenic substrates in diabetic rat atria? J Cardiovasc Electrophysiol. 2006;17:890–894. doi: 10.1111/j.1540-8167.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 35.de Divitiis O, Fazio S, Petitto M, et al. Obesity and cardiac function. Circulation. 1981;64:477–482. doi: 10.1161/01.cir.64.3.477. [DOI] [PubMed] [Google Scholar]
- 36.Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: Clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331–337. doi: 10.1016/j.hrthm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Charitos EI, Stierle U, Ziegler PD, et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: Insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012;126:806–814. doi: 10.1161/CIRCULATIONAHA.112.098079. [DOI] [PubMed] [Google Scholar]