Abstract
Noonan syndrome is a heterogenous rasopathy typically presenting with short stature, characteristic facial features, cardiac abnormalities including pulmonic valve stenosis, ASD and hypertrophic cardiomyopathy (HCM), cryptorchidism, ectodermal abnormalities and learning differences. The phenotype is variable, and limited genotype phenotype correlation exists with SOS1 mutations often associated with normal cognition and stature, RAF1 mutations entailing a high HCM risk, and certain PTPN11 mutations predisposing to juvenile myelomonocytic leukemia. The recently identified SHOC2 mutation (p.Ser2Gly) causes Noonan syndrome with loose anagen hair. We report five patients with this mutation. All had skin hyperpigmentation, sparse light colored hair, increased fine wrinkles, ligamentous laxity, developmental delay and 4/4 had a structural cardiac anomaly. Hypotonia and macrocephaly occurred in 4/5 (80%); 3/5 (60%) had polyhydramnios, increased birth weight or required use of a feeding tube. Distinctive brain abnormalities included relative megalencephaly and enlarged subarachnoid spaces suggestive of benign external hydrocephalus, and a relatively small posterior fossa as indicated by a vertical tentorium. The combination of a large brain with a small posterior fossa likely resulted in the high rate of cerebellar tonsillar ectopia (3/4) (75%). Periventricular nodular heterotopia was seen in one patient with a thick and dysplastic corpus callosum. We report on the first hematologic neoplasm, myelofibrosis, in a 2-year-old patient with SHOC2 mutation. Myelofibrosis is exceedingly rare in children and young adults. The absence of a somatic JAK2 mutation, seen in the majority of patients with myelofibrosis, is noteworthy as it suggests that germline or somatic SHOC2 mutations are causally involved in myelofibrosis.
Keywords: Chiari 1 malformation, Heterotopia, Rasopathy, Noonan syndrome with loose anagen hair, SHOC2, malignancy, myelofibrosis
INTRODUCTION
Noonan syndrome was delineated based on its clinical findings of short stature, characteristic facial features, wide or webbed neck, cryptorchidism and congenital heart disease including pulmonic stenosis [for review see Roberts et al., 2013]. Following the identification of PTPN11 accounting for about half of all patients with Noonan syndrome, mutations were found in other genes, with SOS1 and RAF1 more commonly affected than KRAS, NRAS or CBL [for review see Allanson and Roberts, 2011]. These genes encode proteins interacting within the Ras mitogen activated protein kinase (MAPK) pathway, and germline mutations result in dysregulation of this signaling cascade. Syndromic conditions resulting from germline mutations in additional Ras-MAPK protein encoding genes, for example cardio-facio-cutaneous and Costello syndrome, share overlapping physical and developmental findings with Noonan syndrome. Despite the phenotypic similarities associated with germline mutations in these genes, distinctive characteristics are associated with certain genes or mutations, such as the increased risk for hypertrophic cardiomyopathy with RAF1 mutations, the often normal height with SOS1 mutation, or the increased risk for juvenile myelomonocytic leukemia with specific PTPN11 mutations. Similarly, the recently identified SHOC2 mutation, predicted to result in a p.Ser2Gly amino acid substitution, causes Noonan syndrome with loose anagen hair. Loose anagen hair presents as easily pluckable, sparse, thin and slow growing hair due to an abnormal hair bulb lacking inner and outer root sheaths on microscopy. Only a few patients with the recurrent de novo SHOC2 mutation have been reported, and the associated phenotype has not been comprehensively assessed [Cordeddu et al., 2009; Komatsuzaki et al., 2010; Ekvall et al., 2011; Hoban et al., 2012; Capalbo et al., 2012a].
MATERIALS AND METHODS
In our ongoing clinical and molecular study on Costello syndrome and related disorders, we enrolled patients with clinical and/or molecular diagnoses of Costello syndrome and related conditions. Laboratory testing for pathogenic mutations is performed, and newly identified disease causing genes may be analyzed in samples from clinically diagnosed individuals without a molecular diagnosis. Identification of a pathogenic mutation in a proband is followed by testing of both biological parents for the respective change. The study is approved by our institutional review board (A. I. duPont Hospital for Children #2005-051).
CLINICAL REPORTS
Patient 1 (CS# 229; LR13–109)
This male was born to his 37-year-old mother and 37-year-old father, he had two older and one younger sibling and a maternal half-sister. The pregnancy was complicated by polyhydramnios and a cystic hygroma noted during the third trimester (Table I). Amniocentesis had a normal 46, XY karyotype and a fetal MRI had reportedly normal results. He was born by cesarean at 38-weeks-gestation for macrosomia. His length was 53.3 cm (>90th centile; 50th centile for 1 month); weight 4.16 kg (>90th centile; 50th centile for 1 month) and OFC was not recorded. Apgar values were 8 and 9, at one and five minutes. Projectile vomiting beginning at age 10 days led to surgical pyloric stenosis repair. Persistent feeding difficulties and severe failure-to-thrive resulted in gastrostomy tube placement at age 5 months. Echocardiography at age 3 months revealed mild pulmonic valve stenosis. The pulmonic valve was mildly thickened and doming. The gradient across the valve had a mean of 7 mm with a peak of 15 mm at rest, and increased to 34 mm upon agitation and crying. Low muscle tone and unusual eye movement described as “eyes rolling back into his head” resulted in neurologic evaluation at age 10 weeks, and low axial tone was diagnosed. Ophthalmologic evaluation did not reveal structural anomalies or nystagmus, and brain MRI showed small optic nerves (Table II). Motor development was delayed with pulling to stand at 18 months and cruising at 2 4/12 years. He used words at age 1 year and had about 15 words at 2 4/12 years.
Table I.
Patient Characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Cordeddu et al. [2009] | Capalbo et al. [2012b] | |
|---|---|---|---|---|---|---|---|
| Polyhydramnios | + | + | + | − | − | N/A | 1 /2 |
| Increased birth weight |
+ | + | + | − | − | N/A | 0/2 |
| Short stature | − | 3rd-5th centile |
10th centile | + | + | 24/24 | 2/2 |
| Macrocephaly | + | + | + | − | + | 23/25 | 2/2 |
| Feeding tube use | + | − | + | − | + | N/A | N/A |
| GH deficiency | N/A | N/A | N/A | N/A | N/A | 12/15 | 2/2 |
| Delayed development |
+ | +, intellectual disability |
+ | + mild- moderate intellectual disability |
+ | 20/24 Intellectual disability |
1 /2 Intellectual disability |
| Hypotonia | + | + | − | + | + | N/A | N/A |
| ADHD | N/A | N/A | N/A | N/A | + | 13/21 | N/A |
| Pulmonic valve stenosis |
+ | N/A | + | − | − | 9/25 | N/A |
| Mitral/tricuspid valve anomaly |
− | N/A | Aortic insufficiency |
− | Mitral valve abnormality |
8/24 | N/A |
| ASD/VSD | − | N/A | − | ASD | − | 8/25 ASD 2/25 VSD |
1 / 2 ASD |
| Hypertrophic cardiomyopathy |
− | N/A | + | − | − | 5/25 | N/A |
| Hyperpigmentation | + | + | + | + | + | 16/24 | 2/2 |
| Loose anagen hair | Fine, sparse | Sparse | Slightly sparse |
Sparse, slow growing |
Sparse, slow growing |
12/12 | 2/2 |
| Long eye lashes |
+ | N/A | + | + | + | N/A | N/A |
| Inceased fine wrinkles |
+ | + | + | + | + | N/A | N/A |
| Eczema | N/A | + | + | − | − | 8/25 | 0/2 |
| Hemangioma | + | − | − | − | + | N/A | N/A |
| Webbed/short neck |
+ | − | − | + | − | 17/25 | 2/2 |
| Pectus anomaly | − | − | − | + | + | 20/25 | N/A |
| Ligamentous laxity | + | + | + | + | + | N/A | N/A |
| Other GU anomaly | N/A | Inguinal hernias |
− | − | Duplicated renal collecting system |
N/A | N/A |
| Malignancy | Myelofibrosis | − | − | − | − | None | None |
| Other | Pyloric stenosis; late closing anterior fontanel |
Ptosis repair; |
Right bundle branch block; pervasive developmental disorder; Osteoporosis; strabismus |
Rectal prolapse; Strabismus; astigmatism |
Patent ductus arteriosus |
N/A | Seizures 1 /2 |
| SHOC2 p.Ser2Gly | +, de novo | +, de novo | + | + | + | + | + |
| Normal sequence results |
PTPN11, SOS1, RAF1, KRAS, HRAS |
HRAS |
PTPN11, SOS1; RAF1; BRAF; MEK1; MEK2; KRAS; HRAS, NRAS |
FLNA, PTPN11, SOS1, RAF1, KRAS, MEK1, MEK2, BRAF, HRAS |
BRAF, HRAS, KRAS, MEK1, MEK2, NRAS,PTPN11, SOS1, RAF1, CBL, SPRED1 |
N/A: not available or not assessed.
Table II.
Central Nervous System Findings
| Patients (this series) | Literature reports | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Mazzanti et al. [2003] | Ekvall et al. [2011] | Capalbo et al. [2012a] | Capalbo et al. [2012b] | TOTAL | |
| Macrocephaly | + | + | + | − | + | + | + | + | + | 8/9 |
| Prominent forehead or dolichocephaly | + | + | + | + | + | + | + | … | … | 7/7 |
| Ventriculomegaly (++, hydrocephalus) | − | + | − | + | − | ++ | … | … | … | 3/6 |
| Arachnoid spaces enlarged (++, moderate) | ++ | + | + | ++ | + | … | + | … | … | 6/6 |
| Periventricular nodular heterotopia | − | − | … | + | − | … | … | … | … | 1/4 |
| Corpus callosum thin (++, hypogenesis) | + | ++ | … | − | + | … | … | + | … | 4/5 |
| White matter volume decreased | − | + | + | + | − | … | … | + | … | 4/6 |
| Tentorium and straight sinus abnormally vertical (++, more severe) |
+ | ++ | … | ++ | + | … | … | … | … | 4/4 |
| Cerebellar hypoplasia, mild | − | − | … | − | + | … | … | … | … | 1/4 |
| Cerebellar tonsillar ectopia (++, with Chiari) | + | + | … | ++ | − | ++ (4y) | … | … | ++ (9y) | 5/6 |
| Neurosurgical management | − | NUS1 | − | − | − | NUS2 | − | − | − | 2/9 |
| Optic nerves small | + | − | … | − | − | … | … | … | … | 1/4 |
Abbreviations: …, Not available or not assessed. When information was based on a report, rather than review of the original images, and a feature was not specifically comment upon, … is used as well; NUS1, evacuation of subdural effusion; NUS2, ventriculo-peritoneal shunt at 2 years; y, years.
At age 2 3/12 years, he was diagnosed with possible myeloid leukemia and juvenile myelomonocytic leukemia was suspected. A clinical diagnosis of Costello syndrome was proposed. At age 2 4/12 years, his height was 86.3 cm (10–25th centile), weight 12.8 kg (10–25th centile), and OFC 54.3 cm (+3 SD). His anterior fontanel measured 5 × 3 cm and his head was dolichocephalic. His sparse and thin hair was described as “very fine”. Eyes showed bilateral mild ptosis and downslanting palpebral fissures (Fig. 1). He had a short neck and narrow shoulders. The abdomen appeared full due to hepatosplenomegaly. Testicles were descended. His skin showed scattered hemangiomata over his right shoulder, a single hyperpigmented nevus measuring 2 ×1 cm on his right knee and numerous fine wrinkles in his hands. His knees appeared hypermobile, but there was no apparent ligamentous laxity affecting his small joint. Muscle tone was decreased. A detailed neurological and developmental assessment was not possible due to his overall poor health. The patient developed increasing hepatosplenomegaly and blasts in his peripheral blood, with decreasing red cell counts, but repeated bone marrow studies were not diagnostic of acute leukemia. He died at age 2 ½ years after splenectomy in preparation for a bone marrow transplant.
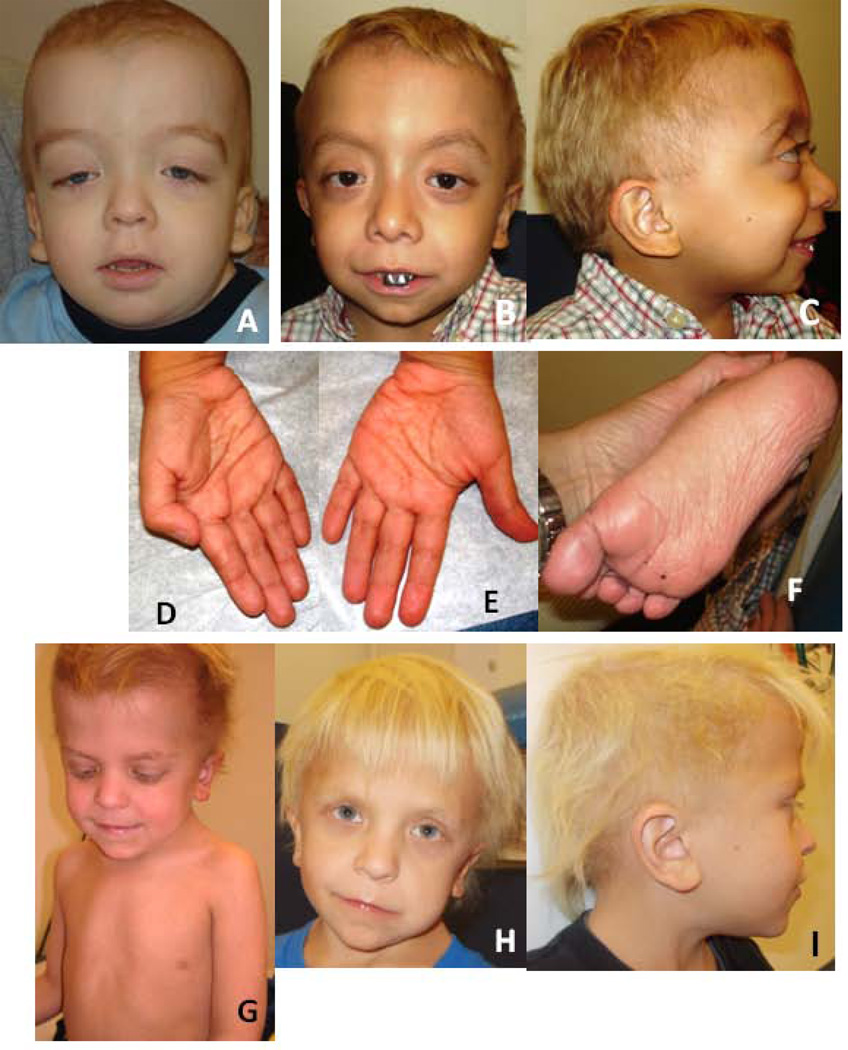
Figure 1.
Facial photograph of Patient 1 (A) at age 2 3/12 years, note sparse hair and very long eyelashes (dolichocilia); tall forehead with mild bitemporal narrowing; downslanting palpebral fissures, long and deeply grooved philtrum and low-set ears with prominent lobes. Facial photograph of Patient 4 (B) at age 5 5/12 years, note sparse hair and dolichocilia, tall forehead, long and deeply grooved philtrum, capped teeth, and low-set ears on lateral view (C). His palms (D, E) and soles (F) showed an increased number of fine wrinkles and a hyperpigmented lesion. Photographs of Patient 5 at ages 5 (G) and 8 years (H, I), showing pectus excavatum (G), tall forehead and sparse hair (H), dolichocephaly, dolichocilia and low-set ears (I). Note hair texture and light color, unusual for the family background, in Patients 4 and 5.
Flow cytometry immunophenotyping was not diagnostic of acute leukemia. Bone marrow biopsies revealed markedly hypercellular marrow with crush artifacts suggestive of fibrosis and marked generalized increase in reticulin staining consistent with myelofibrosis. Karyotype on bone marrow was 46, XY, at a 400 band level, in 30 cells. FISH studies for monosomy 7 had a normal result. Allele specific PCR assay for the JAK2 p.Val617Phe mutation associated with non-CML myeloproliferative disorders on bone marrow derived DNA was negative. All bone marrow study results were most consistent with primary myelofibrosis.
Postmortem testing of stored buccal cell derived DNA performed on a research basis revealed the recurrent SHOC2 c.4A>G mutation. This change was not present in either parent’s buccal cell derived DNA.
Patient 2 (CS#113; LR13–110)
This male was the second child born to his 21-year-old mother and a 40-year-old father after a pregnancy complicated by polyhydramnios. Delivery was vaginally at term, birth weight was 4.14 kg (95th centile). Early severe feeding difficulties and failure-to-thrive prompted a hospitalization at age 3 months, but no permanent feeding tube was placed. At this time, weight was 5.05 kg (10–25thth centile), length 57 cm (10th centile) and OFC 44.5 cm (+2SD). Macrocephaly, unilateral ptosis, posteriorly angulated ears, increased wrinkling of the palms, loose skin, bilateral inguinal hernias and hypotonia were noted, and Costello syndrome was considered. Neurosurgical drainage of presumed bilateral chronic subdural hematomata revealed clear fluid. Echocardiography reportedly had normal results. Bone age was delayed with 4.5 years at chronological age 6 years. The patient had notable intellectual disability with walking independently at age 5 years and reportedly using few single words at 9 years.
At 9 5/12 years, his height was 125 cm (3rd-5th centile; 50th centile for 7.5 years), weight 30.6 kg (50th centile), and OFC 56.4 cm (+2SD). His hair was sparse bitemporally and cropped very short. He had mild frontal bossing and his forehead appeared broad and square. Palpebral fissures slanted downward, and there was mild residual left ptosis. Ears were posteriorly angulated, slightly prominent and had anteverted lobes. His neck, chest and back were non dysmorphic. Hands showed deep palmar creases and mild redundancy of the soft tissue, skin appeared thickened with a thick callus on the knuckles of the left hand. Fingers appeared short and had increased joint laxity. He had pes planus with deep plantar creases and hyperextensible toes, with the 4th overriding the 3rd toe bilaterally. His genitals appeared normal, Tanner stage I. Skin was dry, with a hyperpigmented lesion below his left scapula measuring about 3.5 cm × 1cm; and apparent acanthosis nigricans around his neck. With exception of generalized hypotonia, his neurologic examination was normal. The patient was friendly, but non verbal during the examination and wearing a diaper.
Laboratory results included a 46,XY karyotype, for further studies see Table I.
Patient 3 (CS#134)
This male was born at 36 weeks gestation after a pregnancy complicated by third trimester polyhydramnios, late diagnosis of gestational diabetes and fetal macrosomia to a 30-year-old mother and a 28-year-old father. Birth weight was 4.36 kg (>90th centile) and length 46 cm (25th centile). He was hospitalized for 48 days due to tracheostenosis, chronic lung disease and seizure on day-of-life 4. He had right bundle branch block, and mild to moderate biventricular hypertrophic cardiomyopathy (HCM) at age 6 months. A gastrostomy tube was placed at age 16 months for failure-to-thrive. At age 3 years his height was 90 cm (3–10th centile), weight 15.5 kg (50–75th centile) and OFC 57 cm (+4 SD). The combination of HCM, macrocephaly, loose appearing skin, deep palmar creases, developmental delay and short stature suggested a clinical diagnosis of Costello syndrome. Subsequently, aortic insufficiency and mild pulmonic stenosis occurred, and his HCM remained stable. Eczema and dry skin were controlled with lotions.
Patient 3 walked independently at age 24 months and used four words consistently at 30 months. Aggressive behavior began at age 3 ½ years, necessitating referral to a behavioral specialist at 5 years, to a psychiatrist at 6 years, and hospitalization at 7 years. At age 8 years his developmental level was typical for age 3–4 years. He was diagnosed with pervasive developmental disorder and Tourette syndrome at 9 years and continued to use psychotropic medications.
His height was 125 cm (10th centile), weight 29.4 kg (50th centile) and OFC 60 cm (+5 SD) at age 9 years. Hair was coarse and straight. Mild ptosis and mildly coarse facial features were noted. A single café-au-lait lesion and increased wrinkling of the palms were present. Sudden outbursts of aggression limited the examination.
Patient 4 (LR#12–389)
This male was born to a 32-year-old G2P1-2 mother and a 41-year-old father of Mexican ancestry at 37 weeks gestation with a weight of 2.9 kg (50–75th centile). At age 4 months he was hospitalized for poor growth (weight was 4.6 kg, <3rd centile; with height at 3rd and OFC at 5th centile) and bronchiolitis. He had nonsymptomatic elevations of PT/PTT. Physical findings were suggestive of Noonan syndrome, but molecular analysis was initially non-diagnostic (Table I). Rectal prolapse required surgical repair at age 3 years. Strabismus, astigmatism and nystagmus were treated with glasses. Chronic ear infections resulted in tube placement. Use of caloric supplements caused dental decay. A persistent foramen ovale and an ASD with trivial mitral valve regurgitation did not require treatment. He had severe eczema and his blond hair grew poorly, was easily pluggable and significantly lighter in color than his parents’ and sister’s. His very long eye lashes were never cut.
Development was globally delayed with walking independently at 2 3/12 years and using words at 1 6/12 years. A formal developmental evaluation at age 4 years resulted in a diagnosis of mild to moderate intellectual disability, and he had an individualized education plan in school. A brain MRI revealed mild ventriculomegaly and subependymal heterotopia (Fig. 2). Subsequent brain and spinal MRIs performed at 6 years due to headaches revealed development of a Chiari 1 malformation (Table II) in the context of platybasia and basilar invagination, and segmental fusion of the C3 and C4 vertebrae. Accompanying head MRAs showed mild tortuosity and ectasia of intracranial vertebral and basilary arteries, and unusual venous anatomy with absent transverse sinuses bilaterally, large cortical veins emptying directly into the bilateral sigmoid sinuses, a superior sagittal sinus draining to the right marginal sinus, and the straight sinus flowing into the occipital sinus. Perfusion images demonstrated normal symmetric cerebral blood flow.
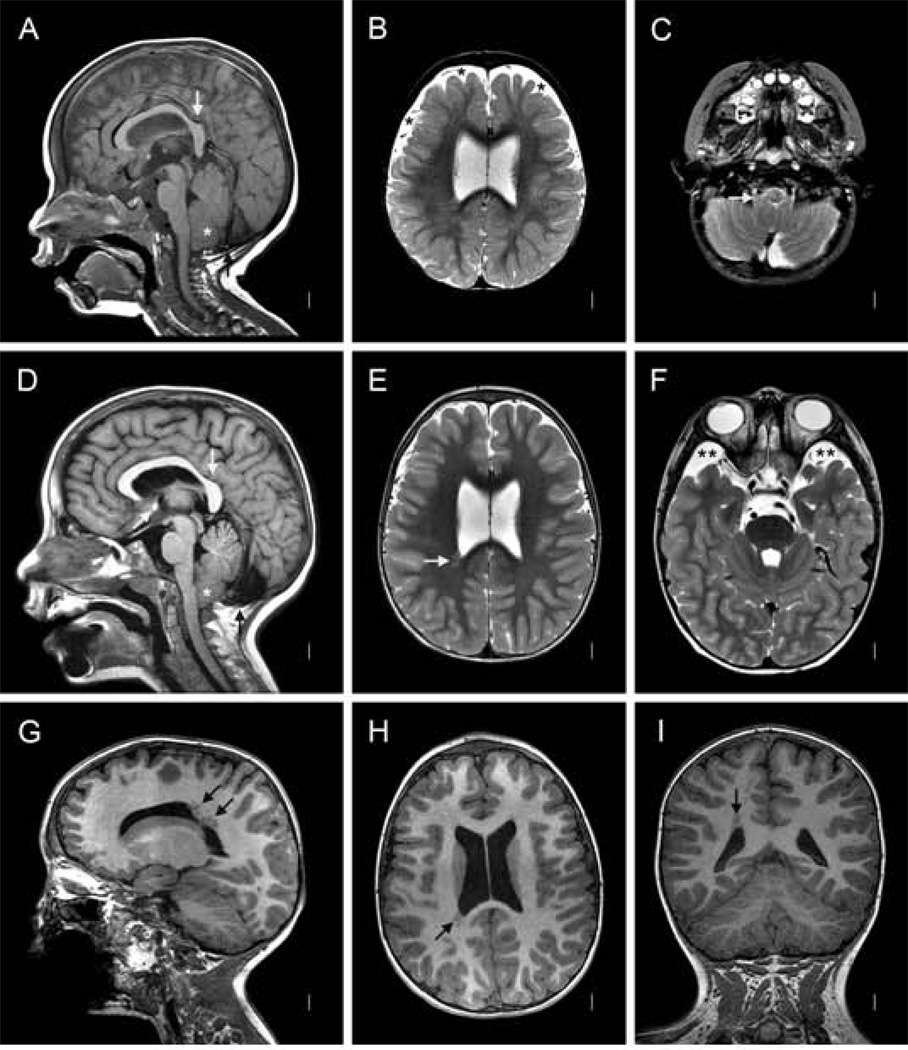
Figure 2.
Magnetic resonance images of Patient 4 (LR12-389) at age 15 months (A–C) and 6 years (D–I). Midline sagittal images (A, D) show an unusual posterior cranial and brain configuration characterized by a small posterior fossa, a sharply angled splenium of the corpus callosum (vertical white arrows in A and D), vertical tentorium (black arrows in A and D), and large occiput that extends down well below the cerebellum. The first scan shows moderate cerebellar tonsillar ectopia (asterisk in A). The second shows a more horizontal basi-occiput and more severe tonsillar ectopia sufficient for Chiari malformation (asterisk in D). The corpus callosum appears mildly thick and the anterior commisure very thick (angled white arrow in D). Axial T2-weighted images at the level of the lateral ventricles (B and E) show enlarged extra-axial space over the frontal lobes that disappeared on the second scan. Lower T2 weighted images show the tonsils wrapping around the medulla (white arrow in C), and prominent arachnoid cysts anterior to the temporal poles (double asterisks in F). Numerous images show an unusual cluster of periventricular nodular heterotopia along the posterior right lateral ventricle that extend up into the deep subcortical white matter, not touching the ventricular wall (arrows in E, G, H and I). Comparable normal images are shown in Figure 3.
At age 5 5/12 years height was 101 cm; (<3rd centile; 50th centile 3 9/12 years), weight 13.9 kg (<3rd centile) and OFC 52.7 cm (about 75th centile). Hair was blonde, thin and sparse, and his eye lashes were long. Supraorbital ridges were flat, resulting in a proptotic appearance. Palpebral fissures showed mild downslant (Fig. 1). Both ears were slightly low-set and posteriorly angulated. The neck was mildly webbed and thoracic kyphosis was mildly increased. Pectus excavatum with hypoplastic and widely spaced appearing nipples (inter nipple distance 14 cm; chest circumference 54 cm) were noted. Increased ligamentous laxity affected the small joints, and feet were flat. He had increased fine wrinkles in the palms and three café-au-lait macules. Neurologic findings included mild hypotonia and brisk deep tendon reflexes.
Patient 5 (LR#12–454)
This male was conceived with donor oocytes and his father was 43-year-old at delivery. A cesarian was performed at 38-weeks-gestation due to maternal hypertension. His birth weight was 3345 g (75th centile), length 48.3 cm (50th centile) and OFC 35.5 cm (90th centile). His neonatal course was complicated by hypotonia and feeding difficulties, resulting in naso-gastric feeding tube use until about age 1 year. A patent ductus arteriosus closed after medication treatment. A brain MRI at age 3 weeks (Fig. 3) showed a relatively small cerebellar vermis and hemispheres with prominent fluid spaces above and below the cerebellum, and increased extra-axial fluid spaces over the frontal lobes and sylvian fissures. The patient received early intervention therapy, but walked independently at age 14 months. No therapies were provided after age 2 years. At age 8 3/12 years, his height was 113 cm (<3rd centile; 50th centile for 5 1/2 years), weight 19.4 kg (<3rd centile) and OFC 56.1 cm (+2 SD). Facial features are shown in Figure 1, H-I. He had a wide chest with mild pectus excavatum. He had several small hemangiomata, and dry and hyperpigmented skin. His hair grew slowly and had never been cut, and he never had hair loss or easily pluckable hair. He attended school in a typical class with an individualized education plan and occupational therapy for fine motor delay. Attention deficit disorder was treated with medication. Echocardiography showed an abnormal mitral valve with three papillary muscles and redundant mitral valve chordae. There was no obstruction, and criteria for prolapse were not met.
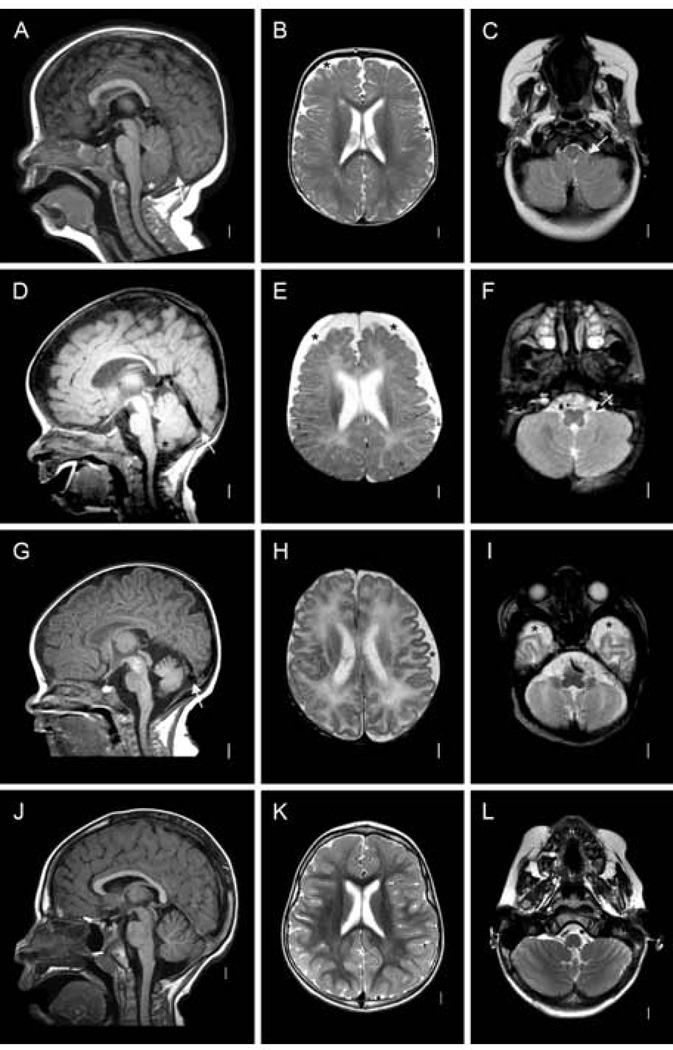
Figure 3.
Magnetic resonance images of Patient 1 (LR13-109) at 12 months (A–C), Patient 2 (LR13-110) at 5 months (D–F), and Patient 5 (LR12-454) at 3 weeks (G–I), and images from a normal child at 3 years (J–L). Midline sagittal images show small posterior fossa volume with abnormal vertical tentorium (white arrows in A, D and G), borderline small cerebellar vermis hypoplasia, and mild cerebellar tonsillar ectopia without Chiari malformation (A and D). Axial images show mildly (asterisks in B and H) or moderately (asterisk in E) enlarged subarachnoid spaces over the frontal lobes, and anterior temporal lobes (asterisks in I), and tonsils wrapped partly around the low medulla (arrows in C and F). Comparable images from a 3-year-old girl are shown in the bottom row (J–L).
RESULTS
Patients 1–3 were originally clinically diagnosed with Costello syndrome or a related rasopathy, resulting in enrollment into our research study. Patient 4 was referred after molecular diagnosis of a SHOC2 mutation, and Patient 5 was followed clinically for suspected Costello syndrome. Physical, facial and developmental findings in all patients were consistent with Noonan syndrome with loose anagen hair (Table I); however, myelofibrosis, periventricular nodular heterotopia, vertical tentorium, and postnatal development of a Chiari 1 malformation have not previously been reported in this condition.
DISCUSSION
Following the identification of the recurrent SHOC2 p.Ser2Gly mutation underlying Noonan syndrome with loose anagen hair, Cordeddu et al. [2009] found this mutation in 4 of 96 individuals with Noonan syndrome and without a molecular diagnosis. Komatsuzaki et al. [2010] identified 8 SHOC2 mutation positive individuals amongst 92 with Noonan syndrome or a Noonan-like condition and without identified mutation. Assuming no overlap between these case series, the incidence of a SHOC2 mutation is 12/188, or about 6%. However, at least 70% of all Noonan syndrome patients have a mutation in a previously reported gene; thus 6% of the remaining 30% would reflect 1.8% of all individuals with Noonan syndrome. It is noteworthy that Patients 1, 2, 3 and 5 in our series were suspected of having Costello syndrome, a rasopathy sharing phenotypic findings with Noonan syndrome. Costello syndrome is characterized by severe feeding difficulties in infancy, and feeding tube use was necessary in 3/5 patients reported here. Further, developmental delay was universally present. Skin findings in the patients reported here were more significant than typical for Noonan syndrome: In addition to the previously reported hyperpigmentation, the increased fine wrinkles on the palms (Fig. 1) were striking. No patient reported here was formally diagnosed with loose anagen hair, which requires microscopic analysis of the root sheaths, but all showed the sparse, very slow growing hair of unruly texture and unusually light coloring (see blonde hair in Patient 4 of Hispanic ancestry; Fig. 1 B,C). In contrast to the slow growing scalp hair, all had unusually long eye lashes, but none requiring trimming of the lashes. This combination of light colored, slow growing and unruly textured scalp hair with dolichocilia (extremely long eye lashes) is typical for Costello syndrome resulting from an uncommon HRAS change, p.Gly13Cys [Gripp et al., 2011]. Interestingly, individuals with HRAS p. Gly13Cys have less short stature and appear less cognitively impaired than those with the most common Costello syndrome mutation resulting in p.Gly12Ser. Subjectively, individuals with HRAS p.Gly13Cys and SHOC2 p.Gly2Ser closely resemble each other in their ectodermal, growth and developmental findings. One might thus anticipate that, like Patients 1, 2, 3 and 5, additional individuals with a suspected diagnosis of Costello syndrome will have the SHOC2 mutation, supporting the use of a panel approach to molecular testing in patients with a rasopathy phenotype.
Malignancy in Noonan syndrome
An increased incidence of juvenile myelomonocytic leukemia (JMML) in Noonan syndrome is related to the shared mechanism of PTPN11 missense mutations, of germline origin in Noonan syndrome and somatic in sporadic JMML [Schubbert et al., 2005; Schubbert et al., 2007]. In addition, reports of neuroblastoma, acute lymphoblastic leukemia, low grade glioma and rhabdomyosarcoma result in a cumulative cancer incidence of approximately 4% in individuals with Noonan syndrome by age 20 years [Kratz et al., 2011]. Patients with a PTPN11 mutation have a cumulative cancer risk of 23% by age 55 years, a 3.5-fold increase over the general population (95% confidence interval, 2.0–5.9), based on a study of 297 Dutch Noonan syndrome individuals [Jongmans et al., 2011].
The Ras/MAPK pathway’s important primary role in cell differentiation and proliferation permits an equally significant role in carcinogenesis and accounts for the cancer predisposition associated with germline mutations. In this context it is not surprising that we report the first hematologic malignancy in a patient with a SHOC2 mutation. Primary myelofibrosis is classified under the myeloproliferative myeloid neoplasms by the World Health Organization Classification [Tefferi and Vardiman, 2008]. This rare condition affects 1.46/100,000 individuals, with a mean age at diagnosis of 67 years [Mesa et al., 1999]. A somatic gain-of-function mutation in JAK2 (p.Val617Phe), with secondary loss-of-heterozygosity of the wildtype allele, can result in myelofibrosis and is present in the majority of patients with myelodysplastic conditions [Kralovics et al., 2005; Levine et al., 2007]. Janus kinase 2 is a tyrosine phosphatase involved in early signal transduction after membrane bound receptor activation. The p.Val617Phe substitution results in constitutive activation of the abnormal protein product. While the activation of signal transducers and activators of transcription (STATs) is the best recognized target of JAKs, they also recruit and activate other molecules involved in signal transduction, including the Ras/MAPK and the PI3K pathway [for review see Rane and Reddy, 2000]. This interrelatedness of the JAK and Ras-MAPK pathway may be the biological mechanism behind the overlapping myelodysplastic conditions associated with mutations resulting in constitutive activation of either pathway. The absence of the common JAK2 mutation in Patient 1 is noteworthy and suggests that the SHOC2 mutation may have been the predisposing change for myelofibrosis. Similarly, germline mutations in CBL result in a Noonan syndrome phenotype [Martinelli et al., 2010] with predisposition to JMML [Niemeyer et al., 2010], whereas the more common somatic CBL mutations are seen in myelodysplastic syndromes [Schwaab et al., 2012].
Extramedullary hematopoiesis resulted in a thoracic mass in a 1-month-old with a de novo SHOC2 and a familial PTPN11 p.Gly409Ala mutation [Ekvall et al., 2011]. This patient also had a surgically removed spinal neuroblastoma. No further information on the neuroblastoma or the extramedullary hematopoiesis was provided at the patient’s age of 11 years [Ekvall et al., 2011]. Leukocytosis was noted in a premature infant [Hoban et al., 2012] and a 5-year-old without an obvious infection [Komatsuzaki et al., 2010], but no somatic SHOC2 mutation has been reported in hematologic malignancies. Komatsuzaki et al. [2010] did not identify a SHOC2 mutation in leukemic cells from 82 patients. We suggest to study JAK2 mutation negative myelofibrosis cases for SHOC2 p.Ser2Gly.
Structural brain anomalies
Few reports of structural brain findings in SHOC2 mutation patients are available, and the information provided is limited. Brain imaging studies in children with SHOC2 mutations (Table II) demonstrate two distinct abnormalities: Relative megalencephaly and small posterior fossa. First, most patients had short stature and macrocephaly after birth, which supports a relative megalencephaly as observed in other rasopathies such as Costello syndrome [Gripp et al., 2010]. All patients had mildly enlarged subarachnoid spaces, especially over the frontal region, which appears most consistent with "benign external hydrocephalus". This poorly understood disorder is seen in children with macrocephaly between 6 and 18 months of age, and largely resolves thereafter [Zahl et al., 2011]. The two patients with the most striking subarachnoid space enlargement, Patients 2 and 4, were scanned at age 1 year, and subsequent scans in Patient 4 demonstrated partial resolution. Benign external hydrocephalus is commonly associated with ventriculomegaly and mildly reduced white matter volume, also seen in the patients reported here. Benign arachnoid cysts were present in Patient 4 (Fig. 2) and specifically commented upon in the patient with SHOC2 and PTPN11 mutation reported by Ekvall et al. [2011].
Second, all four patients had relatively small posterior fossa size as indicated by the vertical tentorium, and Patient 4 had a vertical splenium of the corpus callosum as well. The combination of relative megalencephaly and small posterior fossa likely resulted in Patient 4’s postnatal development of a Chiari I malformation. Mazzanti et al. [2003] reported a patient in whom an MRI performed at age 2 5/12 years showed hydrocephalus requiring shunt placement, but a Chiari 1 malformation was first seen at age 4 1/12 years, also suggesting a postnatally developed anomaly. Adding the patient in whom a MRI obtained at age 9 years revealed a Chiari 1 malformation [Capalbo et al. 2012b], 3/8 patients in whom MRIs were obtained had a Chiari 1. The high rate of cerebellar tonsillar ectopia, defined as downward displacement of the tonsils into the low posterior fossa, and Chiari 1 malformation is not surprising given the combination of a large brain (relative megalencephaly) and small posterior fossa.
Due to limited data on the previously reported patients, Chiari 1 may be underascertained in SHOC2 mutation positive individuals. In addition to several single case reports of Chiari 1 in Noonan syndrome [Ball and Peiris, 1982; Holder-Espinasse and Winter, 2003; Galarza et al., 2010; Massimi et al., 2011; Keh et al., 2013] and the allelic Noonan syndrome with multiple lentigines [Beier et al., 2009], Smpokou et al. [2012] found self-reported Chiari 1 malformation in 7/35 (20%) of adolescents or adults with Noonan syndrome, supporting an increased incidence compared to the general population. While Chiari 1 malformation has rarely been reported in patients with CFC syndrome [Gripp et al., 2010], other structural abnormalities, delayed myelination and brain atrophy as well as seizures have been reported more commonly [Yoon et al., 2007; Papadopoulou et al., 2011; Adachi et al., 2012]. Periventricular nodular heterotopia had not been reported in patients with SHOC2 mutation, and does not appear to be common in rasopathies.
Physical and behavioral findings in this cohort
In addition to the CNS findings and the malignancy discussed above, physical findings not typically seen in Noonan syndrome include the pyloric stenosis, chronic lung disease and rectal prolapse (Table I). Since each finding occurred in a single patient, they may be coincidental. However, pyloric stenosis is more common in Costello syndrome than in the general population [Gripp et al., 2008], as reviewed following the identification of a patient with an unusual HRAS mutation, pyloric stenosis and severe behavior problems requiring medication [Gripp et al., 2008; Gripp et al. 2012]. Severe behavior problems requiring psychotropic medication are uncommon in Costello and Noonan syndrome, but were present in one patient reported here as well.
In conclusion, we report structural brain abnormalities including benign external hydrocephalus, relative megalencephaly and small posterior fossa, postnatally developed Chiari 1 malformation and heterotopia with SHOC2 mutation. We report the first myeloproliferative malignancy, myelofibrosis, in a SHOC2 mutation positive individual. In the absence of the common somatic JAK2 mutation this suggests activation of the Ras/MAPK pathway as an alternative initiating step towards myeloproliferative disorders. We subjectively noted facial and ectodermal features resembling those of individuals with Costello syndrome, in particular due to HRAS p.Gly13Cys. The well recognized overlap of phenotypic findings amongst the rasopathies supports the use of gene panel based molecular diagnostic testing.
ACKNOWLEDGMENTS
We thank the patients and their families for sharing this information. Dr. James Barkovich reviewed the MR images shown in Figure 2. We thank the Center for Pediatric Research COBRE-funded Molecular Core for sequencing support. Dr. Sol-Church is partially funded by grant 8P20GM103464-08 from the National Institute of General Medical Sciences.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- Adachi M, Abe Y, Aoki Y, Matsubara Y. Epilepsy in RAS/MAPK syndrome: two cases of cardio-facio-cutaneous syndrome with epileptic encephalopathy and a literature review. Seizure. 2012;21:55–60. doi: 10.1016/j.seizure.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Allanson JE, Roberts AE. Noonan syndrome. [Accessed 12-4-2012];GeneReviews at GeneTests: Medical Genetics Information Resource [database online] 2011 Available at http://www.genetests.org.
- Ball MJ, Peiris A. Chiari (type 1) malformation and syringomyelia in a patient with Noonan’s syndrome. J Neurol Psychiatry. 1982;45:753–754. doi: 10.1136/jnnp.45.8.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier AD, Barrett RJ, Burke K, Kole B, Soo TM. Leopard syndrome and Chiari type I malformation: a case report and review of the literature. Neurologist. 2009;15:37–39. doi: 10.1097/NRL.0b013e31817833c4. [DOI] [PubMed] [Google Scholar]
- Capalbo D, Melis D, De Martino L, Palamaro L, Riccomagno S, Bona G, Cordeddu V, Pignata C, Salerno M. Noonan-like syndrome with loose anagen hair associated with growth hormone insensitivity and atypical neurological manifestations. Am J Med Genet A. 2012a;158A:856–860. doi: 10.1002/ajmg.a.35234. [DOI] [PubMed] [Google Scholar]
- Capalbo D, Scala MG, Melis D, Minopoli G, Improda N, Palamaro L, Pignata C, Salerno M. Clinical Heterogeneity in two patients with Noonanlike Syndrome associated with the same SHOC2 mutation. Ital J Pediatr. 2012b;38:48. doi: 10.1186/1824-7288-38-48. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeddu V, Di Schiavi E, Pennacchio LA, Ma’ayan A, Sarkozy A, Fodale V, Cecchetti S, Cardinale A, Martin J, Schackwitz W, Lipzen A, Zampino G, Mazzanti L, Diglio MC, Martinelli S, Flex E, Lepri F, Bartholdi D, Kutsche K, Ferrero GB, Anichini C, Selicorni A, Rossi C, Tenconi R, Zenker M, Merlo D, Dallapiccola B, Iyengar R, Bazzicalupo P, Gelb BD, Tartaglia M. Mutation of SHOC2 promotes aberrant protein N-myristolation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022–1026. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekvall S, Hagenäs L, Allanson J, Annerén G, Bondeson M-L. Co-occurring SHOC2 and PTPN11 mutations in a patient with severe/complex Noonan syndrome-like phenotype. Am J Med Genet Part A. 2011;155:1217–1224. doi: 10.1002/ajmg.a.33987. [DOI] [PubMed] [Google Scholar]
- Galarza M, Martínez-Lage JF, Ham S, Sood S. Cerebral anomalies and Chiari type 1 malformation. Pediatr Neurosurg. 2010;46:442–449. doi: 10.1159/000327220. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Doyle D, Dobyns WB. High incidence of progressive postnatal cerebellar enlargement in Costello syndrome: Brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. Am J Med Genet Part A. 2010;152A:1161–1168. doi: 10.1002/ajmg.a.33391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Serrano A, Leonard NJ, Stabley DL, Sol-Church K. Transmission of the rare HRAS mutation (c. 173C>T; p.T58I) further illustrates its attenuated phenotype. Am J Med Genet Part A. 2012;158A:1095–1101. doi: 10.1002/ajmg.a.35294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Sol-Church K, Stabley DL, Axelrad ME, Doyle D, Dobyns WB, Hudson C, Johnson J, Tenconi R, Graham GE, Sousa AB, Heller R, Piccione M, Corsello G, Herman GE, Tartaglia M, Lin AE. Phenotypic analysis of individuals with Costello syndrome due to HRAS p.G13C. Am J Med Genet Part A. 2011;155A:706–716. doi: 10.1002/ajmg.a.33884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Innes AM, Axelrad ME, Gillan TL, Parboosingh JS, Davies C, Leonard NJ, Lapointe M, Doyle D, Catalano S, Nicholson L, Stabley DL, Sol-Church K. Costello syndrome associated with novel germline HRAS mutations: An attenuated phenotype? Am J Med Genet Part A. 2008;146A:683–690. doi: 10.1002/ajmg.a.32227. [DOI] [PubMed] [Google Scholar]
- Hoban R, Roberts AE, Demmer L, Jethva R, Shephard B. Noonan syndrome due to a SHOC2 mutation presenting with fetal distress and fatal hypertrophic cardiomyopathy in a premature infant. Am J Med Genet Part A. 2012;158A:1411–1413. doi: 10.1002/ajmg.a.35318. [DOI] [PubMed] [Google Scholar]
- Holder-Espinasse M, Winter RM. Type 1 Arnold-Chiari malformation and Noonan syndrome: a new diagnostic feature? Clin Dysmorph. 2003;12:275. doi: 10.1097/00019605-200310000-00013. [DOI] [PubMed] [Google Scholar]
- Jongmans MCJ, van der Burgt I, Hoogerbrugge PM, Noordam K, Yntema HG, Nillesen WM, Kuiper RP, Ligtenberg MJL, van Kessel AG, van Krieken JHJM, Kiemeney ALM, Hoogerbrugge N. Cancer risk in patients with Noonan syndrome carrying a PTPN11 mutation. Europ J Hum Genet. 2011;19:870–874. doi: 10.1038/ejhg.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keh YS, Abernethy L, Pettorini B. Association between Noonan syndrome and Chiari I malformation: a case-based update. Childs Nerv Syst. 2013 doi: 10.1007/s00381-012-2000-9. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Komatsuzaki S, Aoki Y, Niihori T, Okamoto N, Hennekam RC, Hopman S, Ohashi H, Mizuno S, Watanabe Y, Kamasaki H, Kondo I, Moriyama N, Kurosawa K, Kawame H, Okuyama R, Imaizumi M, Rikiishi T, Tsuchiya S, Kure S, Matsubara Y. Mutation analysis of the SHOC2 gene in Noonan-like syndrome and in hematologic malignancies. J Hum Genet. 2010;55:801–809. doi: 10.1038/jhg.2010.116. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo S-S, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. New Eng J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Kratz CP, Rapisuwon S, Reed H, Hasle H, Rosenberg PS. Cancer in Noonan, Costello, cardiofaciocutaneous and LEOPARD syndromes. Am J Med Genet Part C Semin Med Genet. 2011;157:83–89. doi: 10.1002/ajmg.c.30300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- Martinelli S, De Luca A, Stellacci E, Rossi C, Checquolo S, Lepri F, Caputo V, Silvano M, Buscherini F, Consoli F, Ferrara G, Digilio MC, Cavaliere ML, van Hagen JM, Zampino G, van der Burgt I, Ferrero GB, Mazzanti L, Screpanti I, Yntema HG, Nillesen WM, Savarirayan R, Zenker M, Dallapiccola B, Gelb BD, Tartaglia M. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am J Hum Genet. 2010;87:250–257. doi: 10.1016/j.ajhg.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimi L, Della Pepa GM, Tamburrini G, Di Rocco C. Sudden onset of Chiari malformation Type I in previously asymptomatic patients. J Neurosurg Pediatr. 2011;8:438–442. doi: 10.3171/2011.8.PEDS11160. [DOI] [PubMed] [Google Scholar]
- Mazzanti L, Cacciari E, Cicognani A, Bergamaschi R, Scarano E, Forabosco A. Noonan-like Syndrome with Loose anagen Hair: A New Syndrome? Am J Med Genet. 2003;118A:279–286. doi: 10.1002/ajmg.a.10923. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. Am J Hematol. 1999;61:10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Niemeyer CM, Kang MW, Shin DH, Furlan I, Erlacher M, Bunin NJ, Bunda S, Finklestein JZ, Sakamoto KM, Gorr TA, Mehta P, Schmid I, Kropshofer G, Corbacioglu S, Lang PJ, Klein C, Schlegel PG, Heinzmann A, Schneider M, Starý J, van den Heuvel-Eibrink MM, Hasle H, Locatelli F, Sakai D, Archambeault S, Chen L, Russell RC, Sybingco SS, Ohh M, Braun BS, Flotho C, Loh ML. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat Genet. 2010;42:794–800. doi: 10.1038/ng.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou E, Sifakis S, Sol-Church K, Klein-Zighelboim E, Stabley DL, Raissaki M, Gripp KW, Kalmanti M. CNS Imaging is a key diagnostic tool in the evaluation of patients with CFC syndrome: Two cases and literature review. Am J Med Genet Part A. 2011;155:605–611. doi: 10.1002/ajmg.a.33787. [DOI] [PubMed] [Google Scholar]
- Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–5679. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381:333–342. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert S, Lieuw K, Rowe SL, Lee CM, Li X, Loh ML, Clapp DW, Shannon KM. Functional analysis of leukemia-associated PTPN11 mutations in primary hematopoietic cells. Blood. 2005;106:311–317. doi: 10.1182/blood-2004-11-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Schwaab J, Ernst T, Erben P, Rinke J, Schnittger S, Ströbel P, Metzgeroth G, Mossner M, Haferlach T, Cross NC, Hochhaus A, Hofmann WK, Reiter A. Activating CBL mutations are associated with a distinct MDS/MPN phenotype. Ann Hematol. 2010;91:1713–1720. doi: 10.1007/s00277-012-1521-3. [DOI] [PubMed] [Google Scholar]
- Smpokou P, Tworog-Dube E, Kucherlapati RS, Roberts AE. Medical complications, clinical findings, and educational outcomes in adults with Noonan syndrome. Am J Med Genet Part A. 2012;158A:3106–3111. doi: 10.1002/ajmg.a.35639. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- Yoon G, Rosenberg J, Blaser S, Rauen KA. Neurological complications of cardio-facio-cutaneous syndrome. Dev Med Child Neurol. 2007;49:894–899. doi: 10.1111/j.1469-8749.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- Zahl SM, Egge A, Helseth E, Wester K. Benign external hydrocephalus: a review, with emphasis on management. Neurosurg Rev. 2011;34:417–432. doi: 10.1007/s10143-011-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]