Abstract
Obesity is a common disease affecting adults and children. The incidence of obesity in Canada is increasing. Laparoscopic sleeve gastrectomy (LSG) is a relatively new and effective procedure for weight loss. Owing to an increase in the number of bariatric surgical procedures, general surgeons should have an understanding of the complications associated with LSG and an approach for dealing with them. Early postoperative complications following LSG that need to be identified urgently include bleeding, staple line leak and development of an abscess. Delayed complications include strictures, nutritional deficiencies and gastresophageal reflux disease. We discuss the principles involved in the management of each complication.
Abstract
L’obésité est une maladie fort répandue, tant chez les adultes que chez les enfants, et son incidence est en hausse au Canada. La gastrectomie longitudinale laparoscopique (GLL) est une intervention relativement nouvelle et efficace pour la perte de poids. Compte tenu de l’augmentation du nombre d’interventions chirurgicales bariatriques, les chirurgiens généralistes doivent se familiariser avec les complications associées à la GLL et avec leur prise en charge. Les complications postopératoires immédiates de la GLL qu’il faut savoir reconnaître sans retard sont l’hémorragie, les fuites le long de la ligne d’agrafes et la formation d’abcès. Parmi les complications plus tardives, mention-nons les sténoses, les carences alimentaires et le reflux gastro-œsophagien. Nous présentons les principes qui sous-tendent la prise en charge de chaque complication.
Obesity is a common disease affecting more than 300 million adults worldwide.1 It is defined as a body mass index greater than 30. In Canada, the prevalence of obesity has increased almost 3-fold in the past 2 decades.2 Approximately 25% of the Canadian population is now classified as obese.3
Current options for bariatric surgery are categorized by several principles. Purely restrictive procedures include laparoscopic adjustable gastric banding and sleeve gastrectomy. Roux-en-Y gastric bypass is a restrictive surgery with a minor malabsorption approach. Largely malabsorptive procedures with a restrictive component include duodenal switch and biliopancreatic diversion. Almost purely malabsorptive procedures include jejuno-ileal bypass.
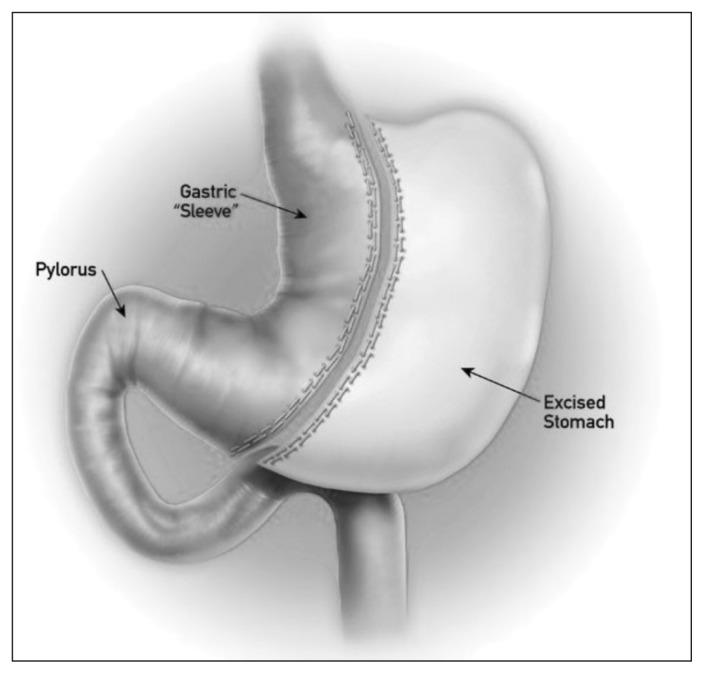
Laparoscopic sleeve gastrectomy (LSG), also known as longitudinal or vertical gastrectomy, is a relatively new and effective surgical option for the management of morbid obesity (Fig. 1). It was initially introduced in 1990 as an alternative to distal gastrectomy with the duodenal switch procedure to reduce the rate of complications.4,5 Sleeve gastrectomy was first performed laparoscopically by Ren and colleagues in 1999.6 At the time, LSG was considered a first-stage operation in high-risk patients before biliopancreatic diversion or Roux-en-Y gastric bypass.7 Laparoscopic sleeve gastrectomy was subsequently found to be effective as a single procedure for the treatment of morbid obesity.8 Although LSG functions as a restrictive procedure, it may also cause early satiety by removing the ghrelin-producing portion of the stomach.9
Fig. 1.
Sleeve gastrectomy.
The incidence of obesity in Canada is on the rise, and more patients are undergoing bariatric surgical procedures.10 This growth is compounded with the escalating incidence of medical tourism wherein patients are travelling abroad for surgical care, particularly bariatric surgery.11 This inevitably translates to an increased incidence of complications associated with such procedures. It is therefore essential for all general surgeons, including those practising in smaller communities, to be aware of these potential complications and to have a basic understanding of how to manage them and when to ask for guidance from a bariatric surgeon. The purpose of this article is to shed some light on basic principles in the management of complications after LSG. We present our operative approach to LSG and review the major acute (within 2 wk of surgery) and late complications that can arise in patients following LSG (Table 1).
Table 1.
Complications associated with laparoscopic sleeve gastrectomy
| Complication | Chronicity | Diagnosis | Management |
|---|---|---|---|
| Hemorrhage | Acute | Physical findings, serial CBC | Transfusion with or without laparoscopy/laparotomy |
| Leak | Acute/chronic | Physical findings, UGI series | Drainage (infrared laparoscopy), antibiotics with or without stenting and/or repair |
| Abscess | Chronic | CT scan, ultrasound | Drainage, antibiotics |
| Stricture | Chronic | Endoscopy, UGI series | Endoscopy (dilatation), surgery (seroyotomy) |
| Nutrient deficiency | Chronic | Physical findings, blood work | Nutritional supplements |
| GERD | Chronic | History, endoscopy | Treatment with proton pump inhibitor |
CBC = complete blood count; CT = computed tomography; GERD = gastroesophageal reflux disease; UGI = upper gastrointestinal.
Operative technique
The patient is placed in a supine position with the arms spread apart. Pneumoperitoneum is achieved using a closed technique with a Veress needle placed in the left subcostal area of the abdomen. Two 10 mm ports are placed in the supraumbilical and left midabdominal areas. An additional 15 mm port is placed in the right mid-abdomen to pass the stapler. Finally, 2 additional 5 mm ports are placed in the left and right upper quadrants of the abdomen. The left lobe of the liver is retracted medially using a Nathanson retractor placed in the subxiphoid area.
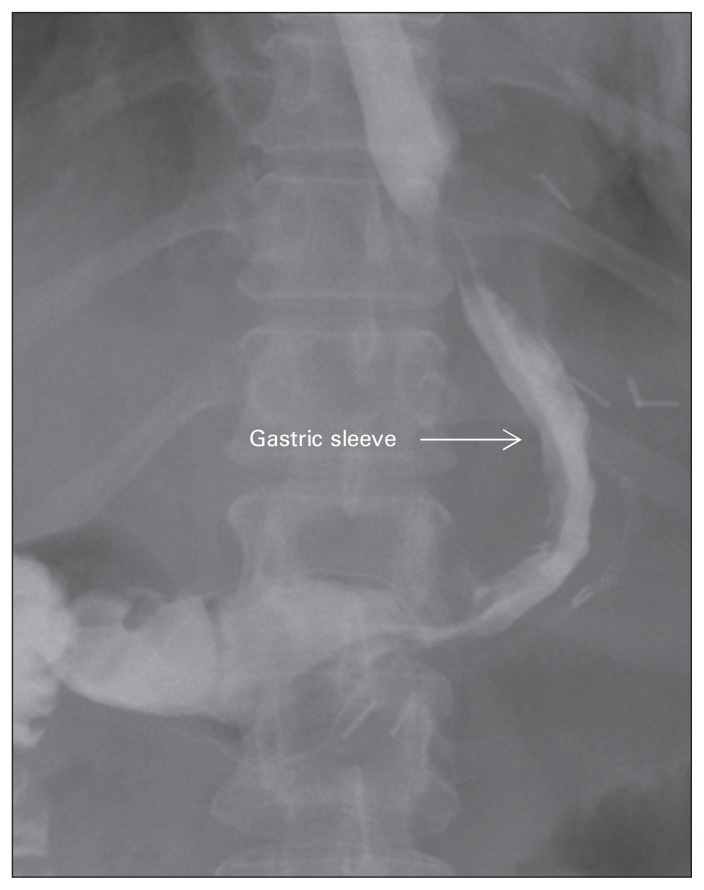
The stomach is decompressed at the beginning of the operation by placing an orogastric tube. The surgeon stands to the patient’s right with the first assistant standing to the patient’s left. The angle of His is taken down bluntly using the Goldfinger dissector (Ethicon Endo-Surgery), exposing the left crus of the diaphragm. Dissection is started about 6 cm proximal to the pylorus by taking down the gastrocolic ligament using the Harmonic Scalpel (Ethicon Endo-Surgery). Dissection is carried out proximally toward the short gastric vessels. This releases attachments to the greater curvature of the stomach and gastric fundus. The orogastric tube is then removed and replaced by a 50-French bougie placed in the stomach by the anesthesiologist and guided laparoscopically to sit in the lesser curvature of the stomach just distal to the pylorus. A 60 mm Endo GIA tri-stapler is then used to divide the stomach. We use 2 black cartridges initially to divide the distal stomach, starting 6 cm proximal to the pylorus. Next, 4–6 60 mm purple cartridges are used to complete the division of the remainder of the stomach. The specimen is then taken out of the abdominal cavity through the 15 mm port. The bougie is then removed, and intraoperative gastroscopy is performed with air insufflation and methylene blue to rule out any leaks. We routinely close our 15 mm port fascia under direct vision before exsufflation of the abdomen. Patients receive nothing by mouth after surgery. On postoperative day 1, an upper gastrointestinal study is done using gastrografin to rule out staple line leakage (Fig. 2). A clear fluids diet is subsequently initiated.
Fig. 2.
Radiograph showing a normal image of the stomach after laparoscopic sleeve gastrectomy.
Acute complications
Hemorrhage
The risk of postoperative bleeding has been reported to be between 1% and 6% after LSG.4,12 The source of bleeding can be intra- or extraluminal. Intraluminal bleeding from the staple line usually presents with an upper gastrointestinal bleed. Common symptoms include hematemesis or melena stools. Diagnosis and management of intraluminal bleeding follows the common algorithm taken for an upper gastrointestinal bleed. This includes establishment of large bore intravenous lines for fluid resuscitation, administration of packed red blood cells if necessary, accurate measurement of urine output with insertion of a Foley catheter and an urgent gastroscopy to diagnose and control the source of bleeding.
Extraluminal bleeding usually presents with a serial drop in serum hemoglobin levels or signs of tachycardia or hypotension. Common sources for extraluminal bleeding include the gastric staple line, spleen, liver or abdominal wall at the sites of trocar entry. We suggest a second-look laparoscopy in any patient who presents with extraluminal bleeding with a sustained heart rate greater than 120 beats per minute and a drop in hemoglobin of more than 10 g/L postoperatively. Urgent laparoscopy facilitates a diagnosis and allows evacuation of the clot as well as surgical control of the source of bleeding. Many times the actual source cannot be identified, but we believe that evacuation of the hematoma and placement of a closed suction drain often serves as a helpful adjunct to patient resuscitation.
A number of buttressing materials are commercially available to attempt to reduce the rate of bleeding from the staple line. These include glycolide trimethylene carbonate copolymer (Gore Seamguard; W.L. Gore and Associates), bovine pericardium strips (Synovis Surgical Innovations) or porcine small intestinal submucosa (Surgisis Biodesign, Cook Medical). Whether the use of buttressing material reduces the rate of bleeding remains controversial. In a recent prospective randomized trial, Dapri and colleagues13 compared the rate of staple line bleeding after LSG using 3 different techniques: stapling the stomach with no reinforcement, or reinforcement with either suturing or buttressing with Gore Seamguard. These investigators observed a significantly lower rate of bleeding with the use of buttressing material. There was no difference in the incidence of a leak. Albanopoulos and colleagues,14 however, did not observe a significant difference in their rate of postoperative bleeding between patients with staple line suturing or buttressing with Gore Seamgard after LSG. These investigators had a 6% and 0% complication rate (bleeding and leak) in their suturing and Seamgard arms, respectively, but this did not reach significance. Therefore at our institution we do not routinely use any reinforcement materials (sutures or buttresses) for LSG.
Staple line leak
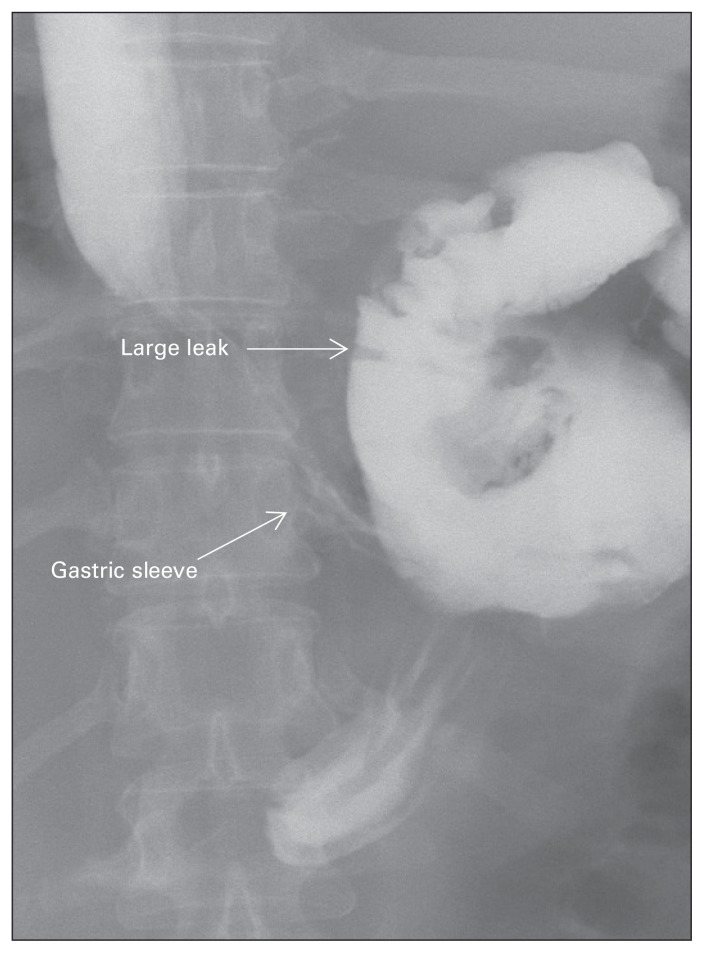
Gastric leak is one of the most serious and dreaded complications of LSG (Fig. 3). It occurs in up to 5% of patients following LSG.8,12 Several classifications exist based on the radiologic findings and time of diagnosis.15 Based on upper gastrointestinal contrast study, gastric leak can be classified into 2 types. A type I or subclinical leak is controlled either through a surgical drain or through a fistulous tract into the abdominal or chest cavity. A type II or clinical leak is a disseminated leak with diffusion of the contrast into the abdominal or chest cavities.16 Based on the time of diagnosis, gastric leaks are classified as early or late. An early leak is generally diagnosed within the first 3 days after surgery, whereas a delayed leak is usually diagnosed more than 8 days after surgery.17
Fig. 3.
Radiograph showing a leak following laparoscopic sleeve gastrectomy.
Gastric leaks can be diagnosed either incidentally on a routine upper gastrointestinal series performed postoperatively without any clinical signs or during exploratory laparoscopy/laparotomy performed owing to unexplained tachycardia. In a study by Kolakowski and colleagues,18 a combination of clinical signs of fever, tachycardia and tachypnea was found to be 58.33% sensitive and 99.75% specific for detection of anastomotic leaks. Diabetes mellitus and sleep apnea were associated with a greater incidence of anastomotic leak. Therefore, we suggest an exploratory laparoscopy for diagnosis in patients who show these signs in the early postoperative period. In the presence of a leak, an abdominal washout with surgical repair of the leak (if technically feasible) and establishment of an enteral feeding route should be performed. Because the stomach is limited in size, the preferred choice for enteral feeding is typically a feeding jejunostomy.
In contrast, treatment of a delayed gastric leak is more challenging surgically owing to the presence of an inflammatory reaction. In this setting, attempts to repair the leak are usually futile. Treatment options include conservative or surgical management. This depends on the patient’s hemodynamic condition and on physical and radiologic findings. In the absence of hemodynamic instability and physical findings suggestive of peritonitis, conservative management may be initiated. This entails fluid resuscitation, initiation of intravenous antibiotics, nothing by mouth, percutaneous drainage of intra-abdominal collections (if drainable) and intraluminal stenting.19 In a septic patient with radiological evidence of a leak with diffuse intra-abdominal fluid collections, surgical drainage of the fluid collection is warranted.
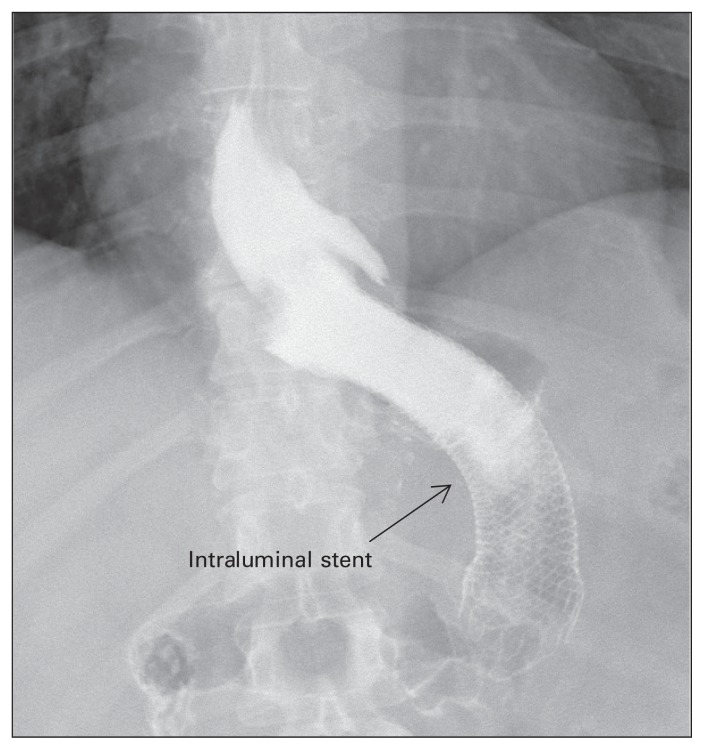
At our institution, we have successfully managed delayed gastric leaks with drainage (either surgical or percutaneous), establishment of a feeding route (enteral or parenteral) and placement of gastric stents for approximately 2–4 weeks (Fig. 4). Other investigators have also used intraluminal stents for the management of gastric leaks.20,21 Himpens and colleagues20 reported their experience in the management of 29 patients with gastric leak after sleeve gastrectomy with stenting. These investigators left the stents in situ on average for 7 weeks. Immediate success was observed in 19 patients after placement of the first stent, whereas 5 patients required placement of a second stent. Two patients had persistent leaks requiring a surgical intervention.
Fig. 4.
Radiograph showing an intraluminal stent for the treatment of a leak following laparoscopic sleeve gastrectomy.
Abscess
Intra-abdominal abscess is another possible complication after LSG. It usually presents with symptoms of abdominal pain, fever/chills or nausea and vomiting. If there are clinical suspicions, one should obtain a computed tomography scan of the abdomen to rule out the presence of intra-abdominal abscess. In a series of 164 patients undergoing LSG, Lalor and colleagues22 reported 1 patient with an abscess (0.7%). Treatment includes percutaneous drainage and antibiotics.
Chronic complications
Stricture
Formation of stricture is another potential complication occurring after LSG. It could present either acutely after surgery due to tissue edema or more commonly in a delayed fashion. Presenting symptoms include food intolerance, dysphagia or nausea and vomiting. Although kinking of the stomach following LSG has been reported,23 the most common site of stenosis is at the incisura angularis.24 An upper gastrointestinal study or endoscopy is usually diagnostic.
Treatment options depend on the time of presentation. A stricture diagnosed acutely after surgery can sometimes be treated conservatively with bowel rest (nothing by mouth), rehydration with intravenous fluids and close observation. In the absence of other pathologies (e.g., abscess, leak), these strictures will spontaneously resolve with no need for further intervention. Failure of conservative management warrants endoscopic dilation.
In contrast, chronic strictures usually require further intervention. These include either endoscopic or surgical treatments. Treatment options depend on the length of stenosis. Endoscopic dilatation is an invaluable tool used in this setting of a short segment stenosis.25 Successive treatments in 4- to 6-week intervals are adequate to treat stricture and ameliorate patient symptoms. In contrast, long segment stenosis and failure of endoscopic management demands a surgical intervention. Options include laparoscopic or open seromyotomy or conversion to Roux-en-Y gastric bypass. Dapri and colleagues26 reported their experience with laparoscopic seromyotomy in patients who had LSG. These investigators reported successful results with this treatment. Parikh and colleagues25 reported an incidence of 3.5% of symptomatic stenosis following LSG in their series of 230 patients; 2 patients required conversion to a Roux-en-Y gastric bypass owing to failure of endoscopic management.
Nutritional deficiencies
Nutritional deficiencies are common after bariatric surgery. The etiology is multifactorial owing to impaired absorption and decreased oral intake. In a recent study by Gehrer and colleagues,27 the prevalence of vitamin B12, vitamin D, folate, iron and zinc deficiency were reported to be 3%, 23%, 3%, 3% and 14%, respectively, after LSG. In general, these investigators found micronutrient deficiencies to be less prevalent after LSG than Roux-en-Y gastric bypass; however, folate deficiency was slightly more common after LSG than Roux-en-Y gastric bypass (22% v. 12%).27 Routine blood work is therefore warranted after LSG to diagnose vitamin and mineral deficiencies. At our institution, we routinely monitor patients’ serum vitamin B12, vitamin D, folate, iron and calcium levels at 3, 6 and 12 months after surgery and treat them accordingly, if necessary.
Gastroesophageal reflux disease
Gastroesophageal reflux disease (GERD) is a condition seen commonly in the bariatric surgery population. Although some operations, such as Roux-en-Y gastric bypass, are known to be associated with a reduced incidence of reflux postoperatively, this is controversial for LSG. In a recent systematic review by Chiu and colleagues,28 the authors found the data to be inconclusive with respect to the effect of LSG on GERD. Of the included studies, 4 showed an increased incidence of GERD postoperatively, whereas 7 showed a decrease in the incidence of GERD. Carter and colleagues29 performed a retrospective study on patients who underwent LSG and found 47% of their patients to have persistent (> 30 d) GERD symptoms. The most common reported symptoms included heartburn (46%) and regurgitation (29%). Management of patients with persistent GERD involves treatment with proton pump inhibitors. These patients require close clinical follow-up. If their symptoms persist despite the use of proton pump inhibitors, we usually perform a gastroscopy for diagnosis.
Conclusion
Laparoscopic sleeve gastrectomy is a new and effective procedure for the surgical management of morbid obesity. Therefore, the number of patients undergoing this procedure will continue to rise. Basic understanding of common complications and available treatment options is essential for all practising general surgeons. This paper offers basic management guidelines for the treatment of complications after LSG. By early diagnosis and treatment of these complications, patient morbidity and mortality might be reduced.
Footnotes
Competing interests: None declared for K. Sarkhosh and A. Sharma. D.W. Birch declares having received consultant and speaker fees from Johnson & Johnson, Ethicon Endo-Surgery, Covidien, Olympus, Bard and Baxter. S. Karmali declares having received speaker fees from Ethicon and Covidien.
Contributors: All authors designed the study. K. Sarkhosh acquired the data, which K. Sarkhosh and A. Sharma analyzed. K. Sarkhosh and S. Karmali wrote the article, which all authors reviewed and approved for publication.
References
- 1.Deitel M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg. 2003;13:329–30. doi: 10.1381/096089203765887598. [DOI] [PubMed] [Google Scholar]
- 2.Katzmarzyk PT, Mason C. Prevelance of class I, II and III obesity in Canada. CMAJ. 2006;174:156–7. doi: 10.1503/cmaj.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields M, Carroll MD, Ogden CL. Adult obesity prevalence in Canada and the United States. NCHS Data Brief. 2011;56:1–8. [PubMed] [Google Scholar]
- 4.Frezza EE. Laparoscopic vertical sleeve gastrectomy for morbid obesity. The future procedure of choice? Surg Today. 2007;37:275–81. doi: 10.1007/s00595-006-3407-2. [DOI] [PubMed] [Google Scholar]
- 5.Marceau P, Hould FS, Simard S, et al. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947–54. doi: 10.1007/s002689900498. [DOI] [PubMed] [Google Scholar]
- 6.Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg. 2000;10:514–23. doi: 10.1381/096089200321593715. [DOI] [PubMed] [Google Scholar]
- 7.Regan JP, Inabnet WB, Gagner M, et al. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg. 2003;13:861–4. doi: 10.1381/096089203322618669. [DOI] [PubMed] [Google Scholar]
- 8.Moon Han S, Kim WW, Oh JH. Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients. Obes Surg. 2005;15:1469–75. doi: 10.1381/096089205774859227. [DOI] [PubMed] [Google Scholar]
- 9.Gumbs AA, Gagner M, Dakin D, et al. Sleeve gastrectomy for morbid obesity. Obes Surg. 2007;17:962–9. doi: 10.1007/s11695-007-9151-x. [DOI] [PubMed] [Google Scholar]
- 10.Padwal RS, Lewanczuk RZ. Trends in bariatric surgery in Canada 1993–2003. CMAJ. 2005;172:735. doi: 10.1503/cmaj.045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birch DW, Vu L, Karmali S, et al. Medical tourism in bariatric surgery. Am J Surg. 2010;199:604–8. doi: 10.1016/j.amjsurg.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Melissas J, Koukouraki S, Askoxylakis J, et al. Sleeve gastrectomy: A restrictive procedure? Obes Surg. 2007;17:57–62. doi: 10.1007/s11695-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 13.Dapri G, Cadiere GB, Himpens J. Reinforcing the staple line during laparoscopic sleeve gastrectomy: prospective randomized clinical study comparing three different techniques. Obes Surg. 2010;20:462–7. doi: 10.1007/s11695-009-0047-9. [DOI] [PubMed] [Google Scholar]
- 14.Albanopoulos K, Alevizos L, Flessas J, et al. Reinforcing the staple line during laparoscopic sleeve gastrectomy: prospective randomized clinical study comparing two different techniques. Preliminary results. Obes Surg. 2012;22:42–6. doi: 10.1007/s11695-011-0421-2. [DOI] [PubMed] [Google Scholar]
- 15.Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic–sleeve gastrectomy for obesity. Obes Surg. 2009;19:1672–7. doi: 10.1007/s11695-009-9884-9. [DOI] [PubMed] [Google Scholar]
- 16.Csendes A, Diaz JC, Burdiles P, et al. Classification and treatment of anastomotic leakage after extended total gastrectomy in gastric carcinoma. Hepatogastroenterology. 1990;37:174–7. [PubMed] [Google Scholar]
- 17.Csendes A, Burdiles P, Burgos AM, et al. Conservative management of anastomotic leaks after 557 open gastric bypasses. Obes Surg. 2005;15:1252–6. doi: 10.1381/096089205774512410. [DOI] [PubMed] [Google Scholar]
- 18.Kolakowski S, Kirkland ML, Scuricht AL. Routine postoperative upper gastrointestinal series after Roux-en-Y gastric bypass. Arch Surg. 2007;142:930–4. doi: 10.1001/archsurg.142.10.930. [DOI] [PubMed] [Google Scholar]
- 19.Márquez MF, Ayza MF, Lozano RB, et al. Gastric leak after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20:1306–11. doi: 10.1007/s11695-010-0219-7. [DOI] [PubMed] [Google Scholar]
- 20.Himpens J, Dapri G, Cadiere GB. Treatment of leaks after sleeve gastrectomy. Bariatric Times. Sep, 2009. [(accessed 2012 Oct. 29)]. Available: http://bariatrictimes.com/treatment-of-leaks-after-sleeve-gastrectomy/
- 21.Oshiro T, Kasama K, Umezawa A, et al. Successful management of refractory staple line leakage at the esophagogastric junction after a sleeve gastrectomy using the HANAROSTENT. Obes Surg. 2010;20:530–4. doi: 10.1007/s11695-009-9976-6. [DOI] [PubMed] [Google Scholar]
- 22.Lalor PF, Tucker ON, Szomstein S, et al. Complications after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2008;4:33–8. doi: 10.1016/j.soard.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Uglioni B, Wolnerhanssen B, Peters T, et al. Midterm results of primary versus secondary laparoscopic sleeve gastrectomy (LSG) as an isolated operation. Obes Surg. 2009;19:401–6. doi: 10.1007/s11695-009-9804-z. [DOI] [PubMed] [Google Scholar]
- 24.Cottam D, Qureshi F, Mattar S, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20:859–63. doi: 10.1007/s00464-005-0134-5. [DOI] [PubMed] [Google Scholar]
- 25.Parikh A, Alley JB, Peterson RM, et al. Management options for symptomatic stenosis after laparoscopic vertical sleeve gastrectomy in the morbidly obese. Surg Endosc. 2012;26:738–46. doi: 10.1007/s00464-011-1945-1. [DOI] [PubMed] [Google Scholar]
- 26.Dapri G, Cadiere GB, Himpens J. Laparoscopic seromyotomy for long stenosis after sleeve gastrectomy with or without duodenal switch. Obes Surg. 2009;19:495–9. doi: 10.1007/s11695-009-9803-0. [DOI] [PubMed] [Google Scholar]
- 27.Chiu S, Birch DW, Shi X, et al. Effect of sleeve gastrectomy on gastro-esophageal reflux disease: a systematic review. Surg Obes Relat Dis. 2011;7:510–5. doi: 10.1016/j.soard.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Gehrer S, Kern B, Peters T, et al. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-en-Y-gastric bypass (LRYGB) — a prospective study. Obes Surg. 2010;20:447–53. doi: 10.1007/s11695-009-0068-4. [DOI] [PubMed] [Google Scholar]
- 29.Carter PR, LeBlanc KA, Hausmann MG, et al. Association between gastroesophageal reflux disease and laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:569–72. doi: 10.1016/j.soard.2011.01.040. [DOI] [PubMed] [Google Scholar]