Abstract
Background
Previous studies reported altered autonomic nervous system (ANS) responses in IBS at baseline and to colonic balloon distension. This study examined heart rate variability (HRV) and plasma catecholamines as an index of ANS responsiveness in IBS during flexible sigmoidoscopy (FS) and explored associations of HRV with clinical measures.
Methods
Rome III positive IBS patients and healthy controls completed questionnaires measuring GI and psychological symptoms. HRV measures were calculated using electrocardiogram (ECG) data at rest and during FS. Plasma catecholamines were measured before and after the FS. Linear mixed effects models were used to compare HRV with IBS status and IBS duration across six time points. Significance was assessed at the 0.05 level.
Results
36 IBS patients (53% F, mean age 37.89) and 31 controls (58% F, mean age 37.26) participated. After adjusting for age, sex, BMI, and HAD anxiety, IBS patients had a non-significant lower cardiovagal tone (p=0.436) and higher cardiosympathetic balance (p=0.316) at rest. During FS, controls showed a transient increase in cardiosympathetic balance and decrease in cardiovagal tone. However, IBS patients had significantly less cardiosympathetic and cardiovagal responsiveness both leading up to (p=0.003, p=0.005) and following (p=0.001, p=0.001) this stimulus. Those with longer duration of disease had less cardiosympathetic (p=0.014) and cardiovagal (p=0.009) responsiveness than those with shorter duration. No differences in catecholamines between IBS and controls were found.
Conclusion
IBS demonstrated dysregulated ANS responses to a visceral stressor which could be related to disease duration. Therefore, autonomic dysregulation is an objective physiologic correlate of IBS.
Keywords: autonomic dysregulation, autonomic nervous system, blunting, duration of disease, heart rate variability, irritable bowel syndrome, physiologic correlate, plasma catecholamines, somatic stressor, visceral stressor
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder affecting 7–20% of the U.S. population, occurring twice as frequently in women than men (1, 2). It is characterized by chronic or recurrent abdominal pain with bowel habit changes, such as diarrhea and/or constipation (1, 3). Although its pathophysiology is not completely understood, IBS is considered a stress-sensitive disorder. Stress activates the hypothalamic-pituitary-adrenal (HPA) axis and the locus coeruleus-noradrenergic (LC-NE) systems (4). Dysregulations in basal and stress-induced activity of both the HPA axis and autonomic nervous system (ANS) have been demonstrated in IBS (5). The two branches of the ANS, the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS), communicate with the enteric nervous system to modulate and coordinate functions such as GI motility, secretion, and immune functions (5–7), and thus ANS dysregulation may play an important role in IBS pathophysiology.
Various components of heart rate variability (HRV) can be used to index function of the SNS and PNS (8–11). While HRV is a measure of cardiac autonomic function it has been used as an indirect marker of central autonomic control and to some extent GI ANS function (12, 13). Relative power in high frequency (HF) represents cardiovagal, or cardiac parasympathetic activity. The ratio of low frequency power to HF power (LF/HF) represents cardiosympathetic balance (14–19).
Given the higher incidence of IBS in women compared to men, sex may have an effect on ANS function in IBS. However studies examining the effect of sex on ANS function in IBS and controls are scarce. Most studies include women only and those that include both sexes show inconsistent findings (13). We previously found that during rectosigmoid balloon placement and phasic sigmoid distensions, IBS men demonstrated greater ANS dysregulation than IBS women, who were not significantly different from healthy controls. IBS men had a higher cardiosympathetic and lower cardiovagal tone both before and during the visceral stressor than both IBS women and controls (16).
In addition to HRV, catecholamine levels including norepinephrine (NE) and epinephrine (EPI) can be used to assess SNS activity. Studies have shown higher levels of NE and EPI in IBS men and IBS women at baseline (20, 21) compared to controls however limited data is available with respect to visceral stimulation. In a previous study, we found significantly higher plasma NE levels in IBS vs. controls before and after rectal balloon distension associated with auditory stress but they did not change in response to the stress and distension conditions (22).
We have previously shown that the HPA axis is activated in response to a visceral stressor in IBS patients and healthy controls (23). The aims of the current study were to: 1) measure ANS function (i.e., cardioautonomic tone, plasma catecholamines) in IBS patients and healthy controls in response to a novel visceral stressor (flexible sigmoidoscopy [FS]), 2) perform exploratory analyses to determine if ANS responses are associated with IBS characteristics (i.e., bowel habit, disease duration, IBS symptom severity), 3) determine how sex affects ANS response in IBS and controls.
Materials and Methods
Study Subjects and Recruitment
IBS patients 18–55 years of age who fulfilled Rome III diagnostic criteria (1, 24) were recruited from community advertisements. A gastroenterologist with expertise in IBS (LC) confirmed the diagnosis. Additionally, bowel habit subclassification was determined using Rome III criteria (24). IBS patients were excluded if they had a current history of a psychiatric disorder. Healthy controls were recruited by newspaper or internet advertisement from the community. Controls only had no history of IBS, other chronic pain conditions, or psychiatric illness, and were not taking beta-adrenergic blockers or centrally-acting drugs or those that could affect ANS function (anxiolytics, narcotics, antidepressants). (25) Subjects were excluded if they smoked more than 0.5 packages of cigarettes daily, had 400 mg caffeine daily (equivalent to a 16oz cup of standard-brew coffee), or exercised 1 hour or more per day. For premenopausal women not taking oral contraceptive agents or provera, menstrual cycle phase was determined by the count forward/backward method (menses: first three days of menses; follicular: days 4–14; luteal: day 14 - onset of menses). Women who took oral contraceptive agents were studied during the 4–14 days of their menstrual cycle, similar to the follicular phase. Plasma progesterone was collected at baseline (blood draw #1, see Table 1) to help confirm cycle phase. None of the post-menopausal women were taking hormone replacement therapy. Subjects were compensated for participating in the study. Informed consent was obtained from all subjects.
Table 1.
Periods for Data Acquisition
| Period* | Description |
|---|---|
| Rest | Baseline, patient lying still supine for 15 minutes before data collection |
| BD #1 | Blood draw #1, data collected at start of venipuncture |
| Pre-FS | Period prior to starting sigmoidoscopy, patient in procedure room lying still supine for 15 minutes before data collection |
| FS | Insertion of flexible sigmoidoscope and position at 40 cm from anal verge, data collected when sigmoidoscope first reaches appropriate location (total duration of FS ~5–10 minutes) |
| Post-FS | Immediately after sigmoidoscope removal from anus |
| BD #2 | Blood draw #2, data collected at start of venipuncture |
Abbreviations: FS= flexible sigmoidoscopy, BD= blood draw
5 minutes of ECG data collected for each period
The study was approved by the University of California Los Angeles (UCLA) Institutional Review Board and was conducted in accordance with the institutional guidelines regulating human subjects research.
Symptom Measures
At the screening visit, a bowel symptom questionnaire was used to assess the presence and severity of IBS symptoms and duration of disease (26). Duration of disease is defined as current age minus the age of first onset of IBS symptoms. Current depression and anxiety symptoms were measured using the Hospital Anxiety and Depression (HAD) scale, a self-assessment scale utilized to detect current symptoms of depression and anxiety in subjects at baseline (27). Each subject also underwent a structured psychiatric interview (MINI) to measure past or current psychiatric disease (28).
At the study visit, a verbal descriptor anchored visual analog scale (VDVAS) (29) which assesses sensory intensity and unpleasantness of GI symptoms was administered prior to the procedure to measure the severity of IBS symptoms over past 24 hours and also immediately after the sigmoidoscopy to measure abdominal symptoms during the procedure.
Protocol
At the study visit, sigmoidoscopy to at least 40 cm from the anal verge was performed in the Medical Procedures Unit in the early afternoon (between 12 pm and 2 pm). Subjects used two tap-water enemas for bowel preparation. The 2-lead electrocardiogram (ECG) data was collected using the BioPac recording system (MP100A-CE, BioPac Inc., Santa Barbara, CA, USA) for 5-minute periods at 6 different time-points (see Table 1) before, during, and after the FS visceral stressor. All ECG measurements were taken in supine position with minimal movement. Two blood draws for catecholamine levels were performed, one at baseline (blood draw #1) and one immediately following the sigmoidoscopy (blood draw #2). Plasma catecholamines (NE and EPI) were assayed at ARUP Laboratories (Salt Lake City, UT, USA) using high performance liquid chromatography. Additionally, sigmoid colonic mucosal biopsies were obtained after positioning of the sigmoidoscope for use in an associated study (30). If any signs of organic gastrointestinal disease were found on sigmoidoscopy, the subject was excluded from the study and referred to a clinician for further diagnostic evaluation.
Heart Rate Variability (HRV) Analysis
ECG data was manually screened for artifacts and the AcqKnowledge peak detection algorithm (V3.8.2, BioPac Inc., Santa Barbara, CA, USA) was used to determine the intervals between adjacent QRS complexes. The inter-beat (R-R) interval data was used for HRV analysis (Kubios HRV 2.0, Biosignal Analysis and Medical Imaging Group, Dept. of Physics, University of Kuopio, Finland). Autoregression (AR) frequency analysis and Fast Fourier transform (FFT) analysis was performed on one continuous 2-minute segment (epoch) of data in each 5-minute period in order to avoid inclusion of artifacts in the majority of samples, as any single artifact can distort HRV analysis (10). Epochs were chosen from the center of the period and were shifted, first downstream then upstream, to avoid artifacts when possible. The AR and FFT analysis yielded measures of high frequency (HF) power in normalized power based on a frequency band of 0.15–0.4 Hz and the LF/HF ratio with low frequency (LF) power defined as the interval between 0.04–0.15 Hz. A model order of 16 was used in the AR analysis with no spectral factorization. FFT analysis utilized Welch's periodogram method with a window width of 256 seconds and window overlap of 50%.
Our primary measures of HRV are based on the AR approach, which takes the data as a composite of deterministic and stochastic components and utilizes a best-fit model to compute the spectral values. AR analysis excludes “noise” by concentrating on more significant data points, allowing for improved resolution compared to FFT analysis, which treats all data as deterministic components (19). However, much of the HRV literature utilizes FFT analysis as it is considered more descriptive (19) so in a secondary analysis in this study, we performed FFT spectral analysis on the interbeat interval data and compared the results to the AR analysis to allow for comparison with previous HRV studies as well as demonstrate the robustness of our findings.
Statistical analysis
Descriptive statistics of baseline measures were summarized for categorical (frequency [%]) and continuous (mean±SE) variables. The Kruskal-Wallis and Fisher's exact test were utilized to test group differences among continuous variables and categorical variables, respectively. Spearman's correlation was used to assess the association between continuous variables. Linear mixed effects models were used to compare HRV values measured across six time points (Table 1) to the interaction of IBS status with time while controlling for clinical traits (gender, age, BMI and HAD anxiety). Time plots indicated that there was a change in slope after the FS and, therefore time was modeled such that the rate of change (slope 1) from the rest period to the FS was allowed to differ from the FS to blood draw #2 (slope 2) for the IBS and control comparisons. The interaction of sex and disease status (IBS, controls) on ANS measures was also evaluated using linear mixed models. To examine the effect of IBS duration on ANS responses, a linear time model was assumed. We also evaluated the association of change in ANS measures over time with IBS symptoms (current intensity and unpleasantness over past 24 hours, intensity and unpleasantness of the FS procedure, and duration of disease). IBS duration was coded as a continuous variable while controlling for the same clinical traits. For the linear mixed effects models, non-Gaussian variables were log transformed to achieve approximate normality. Changes in ANS responses were depicted in figures using a LOESS function which smoothes out the individual fluctuations at each time period for better visualization of patterns over time. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or R version 2.15.1 and significance was assessed at the 0.05 level.
Results
Baseline Clinical Characteristics
All baseline clinical characteristics of the subjects are summarized in Table 2 and are evaluated using a Kruskal-Wallis or Fisher's exact test as appropriate. The 67 subjects included 36 IBS patients (19 women and 17 men) and 31 healthy controls (18 women and 13 men). The three main bowel habit subtypes were similarly represented within the IBS group. There were no significant differences in menstrual cycle phase distribution between IBS and control women. IBS patients did not differ significantly from controls in mean age, however age did significantly affect our HRV in our models, a finding previously reported in literature (31, 32), and was controlled for in our analysis. IBS patients and controls did not significantly differ in mean body mass index (BMI).
Table 2.
Subject Characteristics
| IBS Patients (n=36) | Controls (n=31) | p-value | |
|---|---|---|---|
| Age (Years±SE) | 37.89±1.79 | 37.26±1.83 | 0.744 |
| BMI (kg/m2±SE) * | 26.83±1.00 | 25.18±0.80 | 0.299 |
| Women (%) | 19 (53%) | 18 (58%) | 0.810 |
| Menstrual Cycle Phase Distribution for Women | 0.498 | ||
| Menses | 1 | 0 | |
| Luteal Phase | 3 | 2 | |
| Follicular Phase | 10 | 14 | |
| Peri-menopausal | 1 | 1 | |
| Menopausal | 4 | 1 | |
| HAD Scores (0–20±SE) | |||
| Anxiety | 6.42±0.90 | 2.55±0.4 | 0.002 |
| Depression | 3.25±0.62 | 0.90±0.27 | 0.005 |
| VDVAS Current Symptom Severity Scores (0–20±SE) ** | |||
| Abdominal Symptoms Unpleasantness Rating Past 24 hours (IBS only) | 7.64±0.52 | NA | |
| Abdominal Symptoms Intensity Rating Past 24 hours (IBS only) | 9.68±0.7 | NA | |
| Unpleasantness Rating of Abdominal Symptoms During the Procedure | 9.5±0.58 | 5.67±0.56 | <0.001 |
| Intensity Rating of Abdominal Symptoms During the Procedure | 11.46±0.74 | 6.15±0.87 | <0.001 |
| Bowel Habits (%) | |||
| Constipation | 11 (31%) | NA | |
| Diarrhea | 14 (39%) | NA | |
| Mixed/Alternating | 10 (28%) | NA | |
| Unspecified | 1 (2%) | NA | |
| Duration of IBS (Years±SE) | 10.88 ± 1.48 | NA | |
| Duration of IBS - Constipation Subtype | 9.7±2.62 | NA | |
| Duration of IBS - Diarrhea Subtype | 10 ± 2.16 | NA | |
| Duration of IBS - Mixed/Alternating Subtype | 12.9±3.35 | NA | |
| Duration of IBS - Unspecified Subtype | 13 | NA |
Compared to healthy controls, IBS patients had significantly higher mean HAD anxiety and depression symptom scores, but the majority of the anxiety (75%) and depression (92%) scores for IBS patients were within either the non-case (0–7) or doubtful case (8–10) range (27) and all of the anxiety and depression scores for controls were within the non-case or doubtful case range. Within the IBS group, only 9 subjects had anxiety scores in the definite case range of 11 or greater and only 3 had depression scores in this range. Neither HAD anxiety nor depression scores significantly affected the ANS responses. Compared to controls, IBS patients rated their abdominal symptoms during the FS procedure as significantly more unpleasant (9.5 vs. 5.67, p<0.001) and intense (11.46 vs. 6.15, p<0.001), but these ratings did not correlate with ANS responses.
None of the IBS patients were taking beta-adrenergic blockers and the majority (86%) of IBS patients were not currently taking centrally acting drugs or those that could affect ANS function. Only one IBS subject was taking an antidepressant medication (i.e., SSRI) at the time of the study.
HRV in IBS vs. Controls
One healthy control man was excluded due to irregular cardiac rhythm which interfered with the HRV analysis. More than 90% of subjects had usable HRV data for all periods except blood draw #2, where only 64% of subjects had usable HRV data (Table 3). Missing period data are accounted for by ECG lead detachment or malfunction (21 out of 402 data points, 5.2%), computer malfunction (4 data points, 1.0%), movement artifact (3 data points, 0.7%), and procedural error (3 data points, 0.7%).
Table 3.
Number of Patients Who Had ANS Data for Each Period
| Period | IBS Patients (n=36) | Healthy Controls (n=31) | Total |
|---|---|---|---|
| Rest | 34 | 26 | 60 |
| BD #1 | 35 | 30 | 65 |
| Pre-FS | 34 | 28 | 62 |
| FS | 34 | 30 | 64 |
| Post-FS | 31 | 29 | 60 |
| BD #2 | 22 | 21 | 43* |
Abbreviations: FS= flexible sigmoidoscopy, BD= blood draw
See Table 1 for period descriptions.
Twenty-four subjects missing data for blood draw #2: blood draw #2 was not attempted in 16 of 67 subjects (24%) when blood samples from the subject could not be obtained during blood draw #1, ECG leads detached or malfunctioned in 3 subjects (4.5%), there was a procedure error for 3 subjects (4.5%), 1 subject became hypovolemic in blood draw, and the computer system malfunctioned during the procedure for 1 subject.
Baseline HRV Measures
After adjusting for age, gender, HAD anxiety, and BMI, HF power (cardiovagal tone, p = 0.436, Fig. 1) and LF/HF (cardiosympathetic balance, p = 0.316, Fig. 2) was similar in IBS patients and controls.
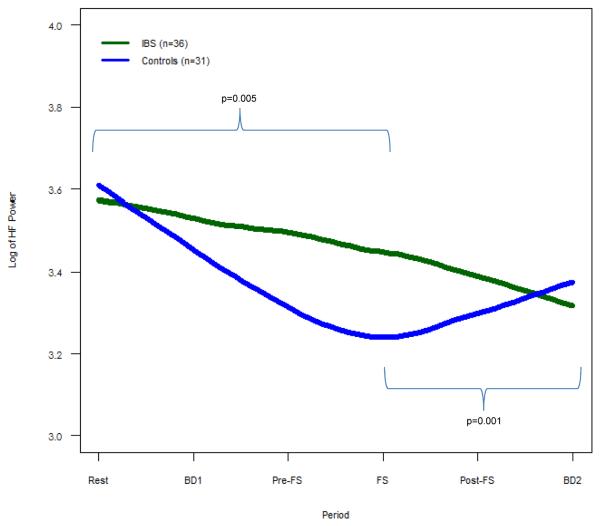
Figure 1. HF Power for IBS vs. Controls.
Mean HF power, representing cardiovagal tone, is shown. IBS subjects demonstrate less change in HF power both leading up to FS (p = 0.005) and following FS (p=0.001) compared to controls.
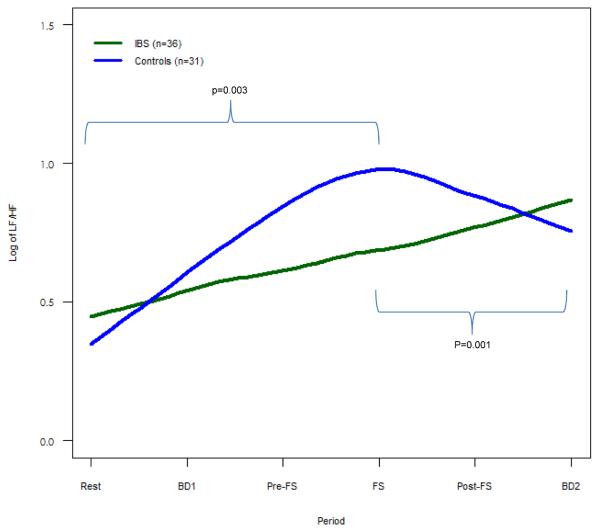
Figure 2. LF/HF for IBS vs. Controls.
Mean LF/HF, representing cardiosympathetic balance, is shown. IBS subjects demonstrate less change in LF/HF both leading up to FS (p = 0.003) and following FS (p=0.001) compared to controls.
ANS Response to the FS procedure
During the baseline period and blood draw #1 prior to the FS procedure controls showed a decrease in HF power (i.e., cardiovagal tone) and increase in LF/HF (i.e., cardiosympathetic balance), while IBS patients had significantly less change in both HF power (p=0.005) and LF/HF (p=0.003) (Fig. 1–2). This pattern was seen again following the FS. As controls showed an increase in HF power and decrease in LF/HF after FS, IBS showed significantly less change in both HF power (p=0.001) and LF/HF (p=0.001) (Fig. 1–2). Of note, differences in the mean values of these measures were statistically significant during the FS procedure (Supplemental Table 1).
Correlation between HRV and clinical characteristics
Sex differences
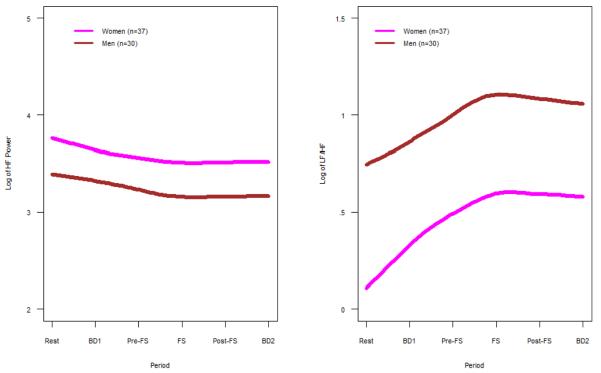
Women had significantly higher HF power (p = 0.008) and lower LF/HF (p=0.002) compared to men throughout all periods (Fig. 3A–B, Supplemental Table 2). Both men and women demonstrated the same trend in ANS measures across periods with a decrease in HF power (cardiovagal, p=0.001) and increase in LF/HF (cardiosympathetic balance, p<0.001) leading up to the FS procedure. Despite overall sex differences, there was no significant effect of sex on the ANS findings when comparing IBS patients and controls both prior to and after the FS.
Figure 3.
A. HF Power for Women vs. Men: Mean HF power, which represents cardiovagal tone, is shown for women vs. men for all subjects. Women had significantly higher HF power across all periods compared to men (p = 0.008).
B. LF/HF Power for Women vs. Men: Mean LF/HF, which represents cardiosympathetic balance, is shown for women vs. men for all subjects. Women had significantly lower LF/HF across all periods compared to men (p = 0.002).
Duration of Disease
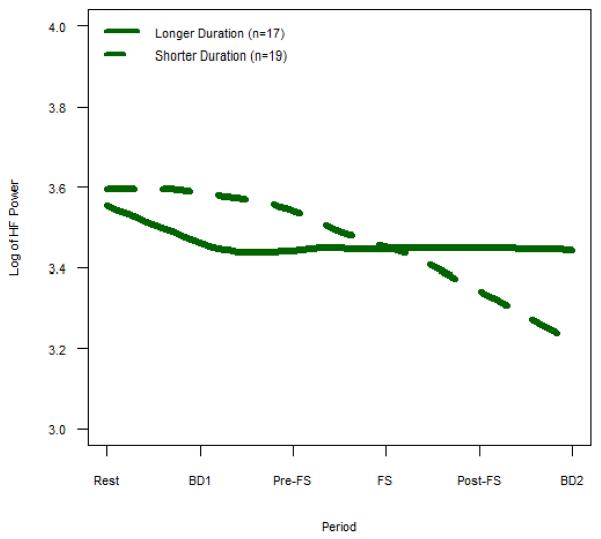
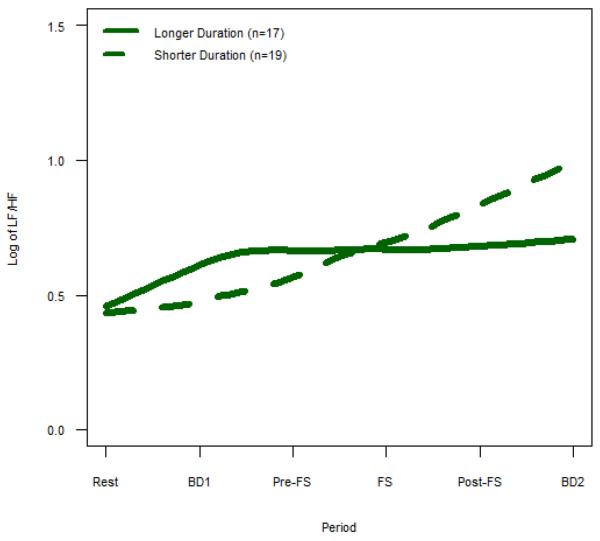
While IBS patients with a shorter duration of disease demonstrated a significant decrease in HF power across all periods, those with a longer duration of disease had significantly less change in HF power throughout the study (p=0.009, Supplemental Table 3). Similarly, while IBS patients with a shorter duration of disease showed a significant increase in LF/HF across all periods, patients with IBS for a longer duration of time had significantly less change in LF/HF (p=0.014, Supplemental Table 3). In Fig. 4–5, we presented the duration of disease as a categorical variable of longer and shorter duration using the median split with longer duration ≥ 9 years (mean age 40.0 ±2.3yrs) and shorter duration < 9 years (mean age 36.6±1.5).
Figure 4. HF Power for Longer Duration vs. Shorter Duration IBS.
Mean HF power, which represents cardiovagal tone, is shown for IBS subjects with longer vs. shorter duration of disease. Longer duration IBS subjects demonstrate less change in HF power across all periods compared to shorter duration IBS (p = 0.009).
Figure 5. LF/HF Power for Longer Duration vs. Shorter Duration IBS.
Mean LF/HF, which represents cardiosympathetic balance, is shown for IBS subjects with longer vs. shorter duration of disease. Longer duration IBS subjects demonstrate less change in LF/HF across all periods compared to shorter duration IBS (p = 0.014).
Current IBS Symptom Severity
Current IBS symptom unpleasantness and intensity scores in the past 24 hours of the study did not significantly affect the ANS responses in IBS vs. controls.
IBS Bowel Habit Subtype
ANS response did not significantly differ between the IBS bowel habit subgroups.
HRV using FFT Analysis instead of AR Analysis
When comparing the AR models to FFT models, all of our findings were in the same direction, but some of the interaction terms only reached borderline significance. IBS demonstrated less change in both HF power and LF/HF compared to controls, both prior to the FS (p=0.087 and p=0.096, respectively) and after the FS (p=0.027 and p=0.022, respectively). The sex effect was upheld with women having higher HF power and lower LF/HF (p=0.002 and p=0.003, respectively) compared to men across all periods with no significant difference in the rate of change, or trend across periods, between men and women for either HF power (p=0.133) or LF power (p=0.384). Lastly IBS subjects with longer duration of disease had significantly less change in HF power and LF/HF throughout compared to controls (p = 0.029 and p=0.015 respectively).
Catecholamine levels in IBS vs. Controls
Of the total number of subjects, 22 (61%; 13 are women) of the IBS patients and 21 (70%; 12 are women) of the healthy controls had catecholamine data both at baseline (blood draw #1) and following the FS (blood draw #2). Two IBS patients had catecholamine data at baseline only. All missing catecholamine data was due to the inability to obtain sufficient plasma samples for catecholamine analysis from the subject during the blood draws. IBS patients and healthy controls did not show significant differences at baseline for NE (mean ± SE: 275±22 vs. 247±20 pg/mL, p= 0.475) or EPI (24±4 vs. 28±5 pg/mL, p= 0.569). Similarly there were no differences in catecholamines for IBS vs. controls following the FS in terms of NE (303±27 vs. 294±28 pg/mL, p=0.915) or EPI (26 ±5 vs. 29±4 pg/mL, p= 0.349). There were no significant changes in catecholamines from blood draw #1 to blood draw #2 for either IBS or controls and there were no sex differences.
Discussion
The main study results are: 1) IBS patients showed a significant blunting of ANS response to FS which was not seen in controls, 2) this blunting became more prominent with longer disease duration, and 3) Overall, women had higher cardiovagal tone and lower cardiosympathetic balance compared to men across all time points. The lack of difference in ANS measures between IBS and controls at baseline differs from most previous HRV analysis findings which show IBS patients having higher cardiosympathetic tone and/or lower cardiovagal tone compared to healthy controls (6, 33). Our main finding of ANS blunting in IBS in response to visceral stressor differs from the literature which generally shows greater cardiosympathetic activity in IBS patients during and after both rectal/colonic distensions (16) and meals (12, 34) compared to controls.
Our study is novel in several ways: 1) a standard GI procedure in clinical practice, FS, served as the visceral stressor, 2) the specific finding of ANS blunting across multiple distinct periods of time in IBS patients has not been previously reported, 3) an association of ANS and duration of disease has not been reported in the past in IBS, and 4) the sex difference in ANS may not be specific to IBS.
There are two previous studies which examined cardioautonomic tone across multiple time points including during rectosigmoid balloon distension. Both demonstrate significant dysregulation in ANS response to visceral stressor however the specific dysregulation patterns differ from the ones reported in this study. In one study, we conducted HRV analysis in 40 IBS patients and 16 controls to measure response to a rectosigmoid balloon distension and found that IBS had higher cardiosympathetic balance and lower cardiovagal tone than controls at all 3 time points: baseline, after balloon was positioned in colon, and during phasic sigmoid balloon distension (16). Additionally we discovered that IBS men had greater cardiosympathetic balance and lower cardiovagal tone compared to IBS women across all time points. In the second study, Ng et al. (2007) measured HRV in 8 IBS patients (mean age 39 yrs, 87.5% women; bowel habit 75% diarrhea, 25% constipation) and 8 healthy controls (mean age 38 yrs; 87.5% women) in response to colonic distension in both fasting and then fed states (1000 kcal liquid meal given 60 minutes after distension protocol) (34). They found no difference in cardiovagal tone between IBS and controls at any of the four time points: pre-distension fasting, during distension fasting, pre-distension fed, and during distension fed. IBS had significantly greater cardiosympathetic balance than controls in the pre-distension fed state only. In response to feeding, IBS patients demonstrated a significant decrease in cardiovagal tone and an increase in cardiosympathetic balance while controls showed no significant change in either measure. The only significant response to a visceral stressor was in IBS patients who demonstrated a decrease in cardiosympathetic tone only in the post-prandial state (34). Of note, while other studies may have performed sigmoidoscopies to place colonic balloons for distension, our current study differs in that we measured ANS measures immediately before, during and after the sigmoidoscopy, which also included collection of colonic mucosal biopsies. However, the biopsies were collected within a few minutes and did not cause additional abdominal discomfort or pain. Nonetheless, it is possible that the addition of colonic biopsies could have contributed to the ANS differences between our study's findings and that of previous ones.
The phenomenon of ANS blunting, though novel to functional gastrointestinal disorders, has been reported in chronic somatic pain syndromes. A previous study conducted in patients with chronic pelvic pain syndrome (CPPS) showed that men with CPPS had no significant change in HF power or cardiovagal tone upon standing compared to controls (35). Another study conducted several years later showed that individuals with somatoform disorders have less cardiovagal reactivity during emotional stimuli compared to controls (36). In addition, a pediatric study showed that children with chronic fatigue syndrome had relatively unchanged cardiovagal and cardiosympathetic tone in response to head-up-tilt compared to both a healthy control group and patients evaluated for syncope (37). The occurrence of ANS blunting in both IBS and chronic pain syndromes suggests a shared pathophysiology involving dysregulation of the ANS. This idea is further supported by a 2010 population study in Minnesota, which found that of 129 women with pelvic pain, 7% met diagnostic criteria for IBS, demonstrating a significant overlap between the two diseases (38). The blunted ANS response to a visceral stressor in IBS suggests that autonomic dysregulation may extend to the interactions between the enteric nervous system and central nervous system within the brain-gut axis (39, 40).
Our study's finding that ANS blunting in IBS patients became more pronounced with duration of disease further supports the hypothesis that ANS blunting is a physiologic correlate of the “wear and tear” of the ANS over time in IBS. One theoretical confounder is the effect of age on ANS dysregulation, however our findings were still significant after controlling for age. An additional possible confounder is that IBS patients may have had greater past exposures to the flexible sigmoidoscopy and thus may demonstrate a blunted ANS response to the visceral stimulus as they are “familiar” with the procedure compared to healthy controls. However, in our study, the number of subjects with previous experiences of FS was comparable between the IBS and control groups (4 vs. 2 subjects). Additionally, subjects in the IBS group demonstrated significantly higher unpleasantness and intensity ratings of the procedure compared to controls, and thus this hypothesis of familiarity with the visceral stimulus is less likely.
The progressive degradation of ANS responsiveness over time has never been reported in GI functional disorders or other chronic pain disorders according to our knowledge, and hypotheses to explain the mechanism are scarce. Interestingly, the degree of ANS dysregulation showed no correlation with bowel habit type or the reported symptom severity, which differs from a recent study of 103 IBS and 49 healthy control women which showed significant ANS dysregulation only among IBS subjects with symptoms rated as severe and differing patterns of ANS derangement based on specific bowel habit subtype (17). Of note our study measured only current symptom severity and not severity over a prolonged period of time. Nonetheless, our study suggests progressive ANS dysregulation may be particularly affected by duration of disease and less so by IBS severity or symptom subtype.
Another possible confounder to our study is the ANS response to the nociceptive pain from the needle stick in blood draw #1 and blood draw #2. Somatic pain as a stressor in healthy subjects is known to activate the sympathetic nervous system which in turn can facilitate ANS descending inhibition of the nociceptive transmission (41). In IBS patients however, the response to somatic pain can differ significantly, particularly if they do not have a coexistent chronic somatic pain disorder. A study by Tousignant-Laflamme et al. (2006) which evaluated the ANS response to pain with a cold water immersion test in 14 IBS women and 13 healthy control women showed that the IBS patients had an ANS response to pain which was opposite of expected from healthy controls. IBS patients showed an increase in cardiovagal tone and decrease in cardiosympathetic balance in response to pain from cold water immersion (42). This ANS response to a somatic stimulus is opposite to that of a visceral stimulus and may be due to the fact that IBS patients without a known coexistent somatic pain disorder have a greater somatic pain tolerance to this particular stimulus compared to healthy controls, suggestive of somatic hypoalgesia (43, 44). Nevertheless, our analysis did not find any significant change in either HF power or LF/HF attributable to blood draw #1 or blood draw #2 alone. The nociceptive pain from the blood draws may be contributing to the overall effect of the visceral stressor on the ANS response, however it is difficult to decipher based on the study's experimental protocol.
Another key finding of this study is that women had lower cardiosympathetic balance and higher cardiovagal tone compared to men throughout most time points. This sex difference in ANS activity is consistent with the literature which shows women having lower cardiosympathetic tone and/or higher cardiovagal tone at baseline than men in both healthy populations (45, 46) and IBS populations (16). No clear mechanism has been suggested to explain this overall ANS sex difference and studies looking at the relationship between the ANS disparities and hormonal differences frequently report conflicting and ultimately inconclusive results (46). In this study, we found that there was no sex effect on ANS differences between IBS and controls. This lack of sex effect on cardioautonomic activity in IBS suggests that autonomic dysregulation alone is not a predominant mechanism to explain the higher prevalence of IBS in women compared to men and reinforces the multifactorial etiology of IBS.
The possible weakness of this study is that blood levels for catecholamines could only be collected in about two-thirds of subjects. Therefore, failure to determine group and condition differences in catecholamines, such as the utility of plasma catecholamines as a gauge of SNS function, could be due to low statistical power.
In summary, we found that IBS patients had ANS dysregulation prior to, during and after a visceral stressor; and this autonomic dysregulation was more pronounced in patients with greater duration of disease suggestive of progressive autonomic dysfunction over time. The ANS dysregulation was not attributed to current symptom severity, bowel habit or sex. Further research is needed to elucidate the mechanism of this ANS dysregulation and integrate it into the understanding of the pathophysiology of IBS.
Supplementary Material
Acknowledgement
We would like to thank Cathy Liu for her assistance with database management and Aditi Joshi, PhD. for her guidance in the use of the BioPac Inc. equipment as well as the Kubios HRV 2.0 analysis software.
Funding: The project described was supported by NIH grants P50 DK64539 (EAM, LC), R01 AR46122 (LC), R24 AT002681 (EAM), and the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ANS
autonomic nervous system
- AR
autoregression
- BD
blood draw
- EPI
epinephrine
- FFT
fast Fourier transform
- FS
flexible sigmoidoscopy
- GI
gastrointestinal
- HADS
hospital anxiety and depression scale
- HF
high frequency
- HRV
heart rate variability
- IBS
irritable bowel syndrome
- LF
low frequency
- NE
norepinephrine
- n.u.
normalized units
- PNS
parasympathetic nervous system
- SNS
sympathetic nervous system
- VDVAS
Verbal Descriptor Visual Analog Scales
Footnotes
Author Contributions:
PC: Data analysis & interpretation, writing of manuscript
WS: Data analysis & interpretation, mixed models statistical analysis, assistance with manuscript preparation and review
MA: Study Coordinator, data acquisition
AP: Overview of statistical analysis, manuscript review
AL: Study Coordinator, data acquisition
EM: Funding, concept and design, critical review of manuscript
BN: Concept and design, critical review of manuscript
LC: Principal Investigator, funding, concept and design, performed medical history and physical examinations, performed sigmoidoscopy with biopsies, analysis and interpretation of data, writing of manuscript
Disclosure: There are no conflicts of interest to disclose for all authors.
References
- 1.Khan S, Chang L. Diagnosis and management of IBS. Nature reviews Gastroenterology & hepatology. 2010;7(10):565–81. doi: 10.1038/nrgastro.2010.137. Epub 2010/10/05. [DOI] [PubMed] [Google Scholar]
- 2.Videlock EJ, Chang L. Irritable bowel syndrome: current approach to symptoms, evaluation, and treatment. Gastroenterology clinics of North America. 2007;36(3):665–85. x. doi: 10.1016/j.gtc.2007.07.002. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. doi: 10.1053/j.gastro.2005.11.061. Epub 2006/05/09. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues in clinical neuroscience. 2006;8(4):367–81. doi: 10.31887/DCNS.2006.8.4/bmcewen. Epub 2007/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140(3):761–5. doi: 10.1053/j.gastro.2011.01.032. Epub 2011/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manabe N, Tanaka T, Hata J, Kusunoki H, Haruma K. Pathophysiology underlying irritable bowel syndrome--from the viewpoint of dysfunction of autonomic nervous system activity. Journal of smooth muscle research = Nihon Heikatsukin Gakkai kikanshi. 2009;45(1):15–23. doi: 10.1540/jsmr.45.15. Epub 2009/04/21. [DOI] [PubMed] [Google Scholar]
- 7.Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain, behavior, and immunity. 2011;25(3):386–94. doi: 10.1016/j.bbi.2010.11.010. Epub 2010/11/26. [DOI] [PubMed] [Google Scholar]
- 8.Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Casali KR, et al. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neuroscience and biobehavioral reviews. 2009;33(2):71–80. doi: 10.1016/j.neubiorev.2008.07.006. Epub 2008/08/19. [DOI] [PubMed] [Google Scholar]
- 9.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84(2):482–92. doi: 10.1161/01.cir.84.2.482. Epub 1991/08/01. [DOI] [PubMed] [Google Scholar]
- 10.Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R. Heart rate variability today. Progress in cardiovascular diseases. 2012;55(3):321–31. doi: 10.1016/j.pcad.2012.09.001. Epub 2012/12/12. [DOI] [PubMed] [Google Scholar]
- 11.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. The American journal of physiology. 1985;248(1 Pt 2):H151–3. doi: 10.1152/ajpheart.1985.248.1.H151. Epub 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 12.van Orshoven NP, Andriesse GI, van Schelven LJ, Smout AJ, Akkermans LM, Oey PL. Subtle involvement of the parasympathetic nervous system in patients with irritable bowel syndrome. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2006;16(1):33–9. doi: 10.1007/s10286-006-0307-x. Epub 2006/02/16. [DOI] [PubMed] [Google Scholar]
- 13.Mazurak N, Seredyuk N, Sauer H, Teufel M, Enck P. Heart rate variability in the irritable bowel syndrome: a review of the literature. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24(3):206–16. doi: 10.1111/j.1365-2982.2011.01866.x. Epub 2012/01/20. [DOI] [PubMed] [Google Scholar]
- 14.Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. The American journal of gastroenterology. 2001;96(2):460–6. doi: 10.1111/j.1572-0241.2001.03526.x. Epub 2001/03/10. [DOI] [PubMed] [Google Scholar]
- 15.Elsenbruch S, Lovallo WR, Orr WC. Psychological and physiological responses to postprandial mental stress in women with the irritable bowel syndrome. Psychosomatic medicine. 2001;63(5):805–13. doi: 10.1097/00006842-200109000-00014. Epub 2001/09/27. [DOI] [PubMed] [Google Scholar]
- 16.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54(10):1396–401. doi: 10.1136/gut.2004.058685. Epub 2005/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heitkemper M, Jarrett M, Cain KC, Burr R, Levy RL, Feld A, et al. Autonomic nervous system function in women with irritable bowel syndrome. Digestive diseases and sciences. 2001;46(6):1276–84. doi: 10.1023/a:1010671514618. Epub 2001/06/21. [DOI] [PubMed] [Google Scholar]
- 18.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European heart journal. 1996;17(3):354–81. Epub 1996/03/01. [PubMed] [Google Scholar]
- 19.Berntson GG, Bigger JT, Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. Epub 1997/12/24. [DOI] [PubMed] [Google Scholar]
- 20.Mazur M, Furgala A, Jablonski K, Mach T, Thor P. Autonomic nervous system activity in constipation-predominant irritable bowel syndrome patients. Medical science monitor : international medical journal of experimental and clinical research. 2012;18(8):CR493–9. doi: 10.12659/MSM.883269. Epub 2012/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heitkemper M, Jarrett M, Cain K, Shaver J, Bond E, Woods NF, et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. The American journal of gastroenterology. 1996;91(5):906–13. Epub 1996/05/01. [PubMed] [Google Scholar]
- 22.Dickhaus B, Mayer EA, Firooz N, Stains J, Conde F, Olivas TI, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. The American journal of gastroenterology. 2003;98(1):135–43. doi: 10.1111/j.1572-0241.2003.07156.x. Epub 2003/01/16. [DOI] [PubMed] [Google Scholar]
- 23.Videlock EJ, Adeyemo M, Licudine A, Hirano M, Ohning G, Mayer M, et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137(6):1954–62. doi: 10.1053/j.gastro.2009.08.058. Epub 2009/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130(5):1377–90. doi: 10.1053/j.gastro.2006.03.008. Epub 2006/05/09. [DOI] [PubMed] [Google Scholar]
- 25.Jarrett ME, Burr RL, Cain KC, Rothermel JD, Landis CA, Heitkemper MM. Autonomic nervous system function during sleep among women with irritable bowel syndrome. Digestive diseases and sciences. 2008;53(3):694–703. doi: 10.1007/s10620-007-9943-9. Epub 2007/10/16. [DOI] [PubMed] [Google Scholar]
- 26.Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, et al. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112(1):55–63. doi: 10.1016/s0016-5085(97)70219-1. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. Epub 1983/06/01. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 4-57. Epub 1999/01/09. [PubMed] [Google Scholar]
- 29.Gracely RH, McGrath F, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5(1):5–18. doi: 10.1016/0304-3959(78)90020-9. Epub 1978/06/01. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. The American journal of gastroenterology. 2012;107(2):262–72. doi: 10.1038/ajg.2011.423. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucy SD, Kowalchuk JM, Hughson RL, Paterson DH, Cunningham DA. Blunted cardiac autonomic responsiveness to hypoxemic stress in healthy older adults. Canadian journal of applied physiology = Revue canadienne de physiologie appliquee. 2003;28(4):518–35. doi: 10.1139/h03-040. Epub 2003/09/10. [DOI] [PubMed] [Google Scholar]
- 32.Cowan MJ, Pike K, Burr RL. Effects of gender and age on heart rate variability in healthy individuals and in persons after sudden cardiac arrest. Journal of electrocardiology. 1994;27(Suppl):1–9. doi: 10.1016/s0022-0736(94)80037-5. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 33.Mazur M, Furgala A, Jablonski K, Madroszkiewicz D, Ciecko-Michalska I, Bugajski A, et al. Dysfunction of the autonomic nervous system activity is responsible for gastric myoelectric disturbances in the irritable bowel syndrome patients. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2007;58(Suppl 3):131–9. Epub 2007/12/06. [PubMed] [Google Scholar]
- 34.Ng C, Malcolm A, Hansen R, Kellow J. Feeding and colonic distension provoke altered autonomic responses in irritable bowel syndrome. Scandinavian journal of gastroenterology. 2007;42(4):441–6. doi: 10.1080/00365520600965749. Epub 2007/04/25. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz U, Liu YW, Berger RE, Yang CC. Autonomic nervous system changes in men with chronic pelvic pain syndrome. The Journal of urology. 2007;177(6):2170–4. doi: 10.1016/j.juro.2007.01.144. discussion 4. Epub 2007/05/19. [DOI] [PubMed] [Google Scholar]
- 36.Pollatos O, Herbert BM, Wankner S, Dietel A, Wachsmuth C, Henningsen P, et al. Autonomic imbalance is associated with reduced facial recognition in somatoform disorders. Journal of psychosomatic research. 2011;71(4):232–9. doi: 10.1016/j.jpsychores.2011.03.012. Epub 2011/09/14. [DOI] [PubMed] [Google Scholar]
- 37.Stewart J, Weldon A, Arlievsky N, Li K, Munoz J. Neurally mediated hypotension and autonomic dysfunction measured by heart rate variability during head-up tilt testing in children with chronic fatigue syndrome. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 1998;8(4):221–30. doi: 10.1007/BF02267785. Epub 1998/10/29. [DOI] [PubMed] [Google Scholar]
- 38.Choung RS, Herrick LM, Locke GR, 3rd, Zinsmeister AR, Talley NJ. Irritable bowel syndrome and chronic pelvic pain: a population-based study. Journal of clinical gastroenterology. 2010;44(10):696–701. doi: 10.1097/MCG.0b013e3181d7a368. Epub 2010/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annual review of medicine. 2011;62:381–96. doi: 10.1146/annurev-med-012309-103958. Epub 2010/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SE, Chang L. Overlap between functional GI disorders and other functional syndromes: what are the underlying mechanisms? Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24(10):895–913. doi: 10.1111/j.1365-2982.2012.01993.x. Epub 2012/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlereth T, Birklein F. The sympathetic nervous system and pain. Neuromolecular medicine. 2008;10(3):141–7. doi: 10.1007/s12017-007-8018-6. Epub 2007/11/09. [DOI] [PubMed] [Google Scholar]
- 42.Tousignant-Laflamme Y, Goffaux P, Bourgault P, Marchand S. Different autonomic responses to experimental pain in IBS patients and healthy controls. Journal of clinical gastroenterology. 2006;40(9):814–20. doi: 10.1097/01.mcg.0000225607.56352.ce. Epub 2006/10/04. [DOI] [PubMed] [Google Scholar]
- 43.Iovino P, Tremolaterra F, Consalvo D, Sabbatini F, Mazzacca G, Ciacci C. Perception of electrocutaneous stimuli in irritable bowel syndrome. The American journal of gastroenterology. 2006;101(3):596–603. doi: 10.1111/j.1572-0241.2006.00414.x. Epub 2006/02/09. [DOI] [PubMed] [Google Scholar]
- 44.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84(2–3):297–307. doi: 10.1016/s0304-3959(99)00215-8. Epub 2000/02/10. [DOI] [PubMed] [Google Scholar]
- 45.Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and clinical electrophysiology : PACE. 2010;33(11):1407–17. doi: 10.1111/j.1540-8159.2010.02841.x. Epub 2010/07/29. [DOI] [PubMed] [Google Scholar]
- 46.Smetana P, Malik M. Sex differences in cardiac autonomic regulation and in repolarisation electrocardiography. Pflugers Archiv : European journal of physiology. 2013 doi: 10.1007/s00424-013-1228-x. Epub 2013/02/14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.