Abstract
Sex-steroid hormones are well-known regulators of vocal motor behavior in several organisms. A large body of evidence now indicates that these same hormones modulate processing at multiple levels of the ascending auditory pathway. The goal of this review is to provide a comparative analysis of the role of estrogens in vertebrate auditory function. Four major conclusions can be drawn from the literature: First, estrogens may influence the development of the mammalian auditory system. Second, estrogenic signaling protects the mammalian auditory system from noise- and age-related damage. Third, estrogens optimize auditory processing during periods of reproductive readiness in multiple vertebrate lineages. Finally, brain-derived estrogens can act locally to enhance auditory response properties in at least one avian species. This comparative examination may lead to a better appreciation of the role of estrogens in the processing of natural vocalizations and may provide useful insights toward alleviating auditory dysfunctions emanating from hormonal imbalances.
Keywords: Steroid, Hormone, Estrogen, Estradiol, Comparative, Auditory, Hearing, Sensory, Seasonal
1. Introduction: Sex-steroid hormones modulate sensory processing
A fundamental area of neurobiological research is the hormonal modulation of neural circuits and behavior. Hormones regulate both internal states, such as mood, stress, fluid balance, and appetite, and optimize interactions with the outside environment, modulating aggressive and reproductive encounters in a wide range of vertebrate taxa. In humans, hormone synthesis and hormone receptors are the targets of many therapeutic drugs aimed at alleviating disease and improving the quality of life during development, adulthood, and aging. Therefore, research on this topic has far-reaching implications for advancing knowledge in basic biomedical sciences and improving human health.
Sex-steroids are one class of hormones that have received particular attention for their involvement in sexual differentiation, mating, gestation, parturition, parental care, and aggression. Derived from cholesterol, they are synthesized in the gonads, adrenal gland and brain (Schlinger and Remage-Healey, 2012). Importantly, the central nervous system may be modulated by both peripherally synthesized steroids (“neuroactive steroids”) and brain-derived steroids (“neurosteroids”).
In addition to their known involvement in the behaviors mentioned above, sex-steroids play an important role in the regulation of vocal communication systems in various non-human species. Vocal communication necessitates both the production of a sound signal by a sender, and the reception of a sound signal by a receiver. Extensive research in birds, fish, and anuran amphibians (frogs and toads) has demonstrated robust, hormonally-mediated plasticity of vocal motor behaviors and their underlying neural substrates (for reviews see Bass, 2008; Brenowitz, 2004; Zornik, 2011). A growing body of literature is consistent with the notion that sex-steroid hormones also regulate auditory physiology and perceptual processes across a broad range of animal taxa. The primary goal of this review is to summarize these latter findings from a comparative standpoint.
Specifically, this review focuses on the influence of one of the most potent estrogens, 17β-estradiol (E2), on central auditory structure and function. First, I discuss the importance of estrogenic signaling in the human auditory system under both normal and pathological conditions. Next, I present findings from research in rodents, anurans, fish and birds, highlighting both species-specific and species-wide estrogenic effects. I then review several cellular and molecular mechanisms that may underlie these findings. It is hoped that the insight gained from such a comparative approach will improve our understanding of hormonally-mediated plasticity in sensory systems, and indicate potential sensory benefits and consequences of clinical hormone treatments.
2. Estrogens modulate human auditory function
For several decades, research has suggested that estrogenic signaling both modulates normal human auditory function, and underlies various auditory pathologies. Circumstantial and direct evidence supporting these claims is outlined below.
2.1 Men and women differ in their auditory capabilities
A number of reports have documented sex differences in human auditory perception. For example, women reliably demonstrate lower thresholds (or better perceptual sensitivity) for high-frequency sounds than males (Chung et al., 1983; Jönsson et al., 1998; Snihur and Hampson, 2011). In addition, while both men and women underestimate the arrival time and the terminal distance of an approaching auditory object, women consistently perceive such objects to be closer than men do (Neuhoff et al., 2009). Some have hypothesized that this systematic underestimation is not due to a neural computational “error,” but instead is part of an early warning system, allowing an organism to react more quickly to an oncoming predator (Guski, 1992). If during evolutionary history women were at greater risk of predation, these findings might suggest that better auditory sensitivity and enhanced detection of approaching auditory objects in females represents an adaptive mechanism to increase their chances of survival.
Women do not, however, perform better than men in all areas of auditory perception. For instance, Zundorf and colleagues (2011) presented men and women with naturally occurring, non-speech sounds and asked subjects to judge the location of the sounds in the horizontal plane. When sounds were presented in isolation, men and women performed equally well at localizing the auditory object. When sounds were presented simultaneously, however, and subjects were asked to localize one target sound while ignoring the distractors, men were more accurate in their location estimates than women. In a related study, Lewald (2004) found that when subjects were only allowed to use their right ear to localize a sound in the vertical plane, men outperformed women. Thus, men demonstrate an advantage over women in spatial auditory tasks; similar results from experiments testing visuospatial abilities suggest that this sex difference may be hormonally based (for review see Clint et al., 2012).
Physiological measurements also have identified consistent sex differences in human auditory processing. Two commonly used diagnostic assessments of auditory function, otoacoustic emissions (OAEs) and auditory brainstem responses (ABRs), have been used extensively in such investigations. These measures are described briefly here, as they will be mentioned frequently throughout this review.
OAEs are low intensity sounds that are emitted by the ear, and result from cochlear amplification. These sounds can be recorded easily using small microphones placed at the opening of the ear canal (Kemp, 1978). OAEs can occur spontaneously (SOAEs) or can be evoked by auditory stimuli, including clicks (CEOAEs), and simultaneously presented tones that result in distortion products (DPOAEs). OAEs are typically quantified by counting the number of emissions present and/or measuring their amplitude (in units of decibels sound pressure level, or dB SPL). The presence of strong, numerous OAEs indicates a healthy cochlea (Kemp, 2002).
The ABR is a pooled, multi-wave neural response that occurs within 10-15 msec after the presentation of a sound stimulus and is recorded with electrodes placed on the scalp (Hall, 2007; Jewett and Williston, 1971; Jewett et al., 1970). Standard quantification of ABRs includes measuring peak latencies (the time between stimulus onset and the peaks of successive ABR waves), inter-peak intervals (the time between the peaks of particular ABR waves), and in some cases, wave amplitude (the voltage difference between the peak and trough of a single ABR wave). ABR latencies and inter-peak intervals provide rough estimates of the transmission time along the ascending auditory pathway, though it is important to acknowledge that each wave has multiple neural generators. In practice, prolonged ABR latencies and reduced peak amplitudes are associated with decreased auditory function (Hall, 2007).
Both OAEs and ABRs demonstrate reliable sexual differences (for review see McFadden, 1998). Consistently, women generate more numerous SOAEs (Burns et al., 1992; McFadden and Loehlin, 1995; McFadden and Pasanen, 1999; Snihur and Hampson, 2011; Talmadge et al., 1993) and stronger CEOAEs (McFadden et al., 1996; McFadden and Pasanen, 1998; McFadden et al., 2009a; Snihur and Hampson, 2011) than males. Women also have shorter wave-V ABR latencies, shorter wave-I-V inter-peak intervals, and larger wave-V amplitudes than males (Dehan and Jerger, 1990; Jerger and Hall, 1980; Jerger and Johnson, 1988; McFadden and Champlin, 2000; McFadden et al., 2010). Thus, as expected from the perceptual studies described previously, women appear to have more sensitive auditory systems than men, and this advantage is manifest at the peripheral processing level.
Notably, these sex differences are present in infants (OAEs: Burns et al., 1992; ABRs: Eldredge and Salamy, 1996; Maurizi et al., 1988), raising the possibility that they are the result of “organizational” effects of sex-steroid hormones during prenatal development. In support of this notion, McFadden (1993) reported that females with male co-twins have significantly fewer SOAEs than females without male co-twins. This finding was interpreted to mean that the androgens produced by the developing male embryo “masculinized” the cochlea of the female twin (for reviews on this topic see McFadden 2002, 2008, 2009, 2011; for more information about the role of intrauterine position in the exposure to and effects of sex-steroid hormones on prenatal development, see vom Saal, 1989). Similarly, it has been proposed that prenatal hormone exposure may contribute both to the development of a non-heterosexual orientation, and masculinized CEOAEs (McFadden and Pasanen, 1998), SOAEs (McFadden and Pasanen, 1999), and wave-V ABR latencies (McFadden and Champlin, 2000) in homosexual and bisexual women. In a direct test of this hypothesis, McFadden and colleagues measured OAEs in rhesus monkeys (Macaca mulatta; McFadden et al., 2006), and Suffolk sheep (Ovis aries, McFadden et al., 2009b) that had been administered testosterone during prenatal development. In both cases, they found that testosterone treatment masculinized the CEOAEs of female offspring. Intriguingly, there was a trend for prenatal E2 treatment to similarly masculinize sheep CEOAEs (McFadden et al., 2009b), but the sample size of only 2 animals was too small to allow for any firm conclusions. Nevertheless, these findings collectively raise the interesting possibility that prenatal masculinization of the auditory periphery and brainstem may be mediated by testosterone and/or its estrogenic metabolites, leading to fundamental differences in auditory detection and localization.
2.2 Female auditory function fluctuates during the menstrual cycle
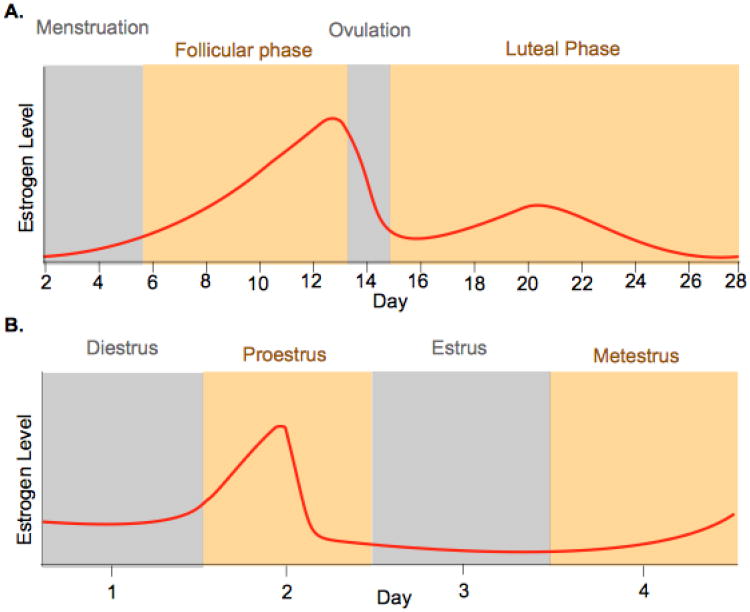
In addition to the possible hormonal influence on prenatal auditory development described above, estrogenic signaling has important “activational” effects on the mature auditory system, examples of which are detailed here and in the following sections. Case reports linking the female reproductive cycle and auditory function date back over 40 years. In general, patients experience fluctuating hearing loss in the luteal phase of their cycle, when E2 levels are moderately elevated (Andreyko and Jaffe 1989; Miller and Gould, 1967; Fig. 1A). One study, however, reported a patient with hearing loss, tinnitus and right ear blockage during menstruation, when E2 levels are low (Souaid and Rappaport, 2001; Fig. 1A).
Figure 1. Estrogen levels during the menstrual and estrus cycles.
A. The human menstrual cycle Estrogen levels gradually rise during the follicular phase, peaking just prior to ovulation. A smaller estrogen surge occurs after ovulation, during the mid-luteal phase. Estrogen levels drop at the end of the luteal phase and remain low during menstruation. B. The rat estrus cycle is similar to the human menstrual cycle. Estrogen levels peak once, during proestrus. Estrogen levels remain low throughout estrus, and gradually begin to rise at the end of metestrus, and into diestrus. Estrus cycle length is species-dependent. In rats (depicted here), the entire cycle lasts 4 days.
These anecdotal findings led some researchers to study this phenomenon in more detail. Two studies that compared pure-tone audiometric thresholds in normal women across the ovulatory cycle reported poorer sensitivity during menstruation (Cox, 1980; Swanson and Dengerink, 1988). More recent studies have demonstrated cyclical ABR and OAE fluctuations. During the late follicular and ovulatory phases, when E2 levels peak (Fig. 1A), OAE frequencies are highest (Al-Mana et al., 2010; Bell, 1992; Penner, 1995), ABR wave-V latencies are delayed (Al-Mana et al., 2010; Dehan and Jerger, 1990; Elkind-Hirsch et al., 1992b), and the interval between wave-I and V is prolonged (Elkind-Hirsch et al., 1992b; Souaid and Rappaport, 2001; but see Caruso et al., 2003a for a contradictory finding). Other indicators of auditory function also may change across the reproductive cycle, including medial olivocochlear suppression (a reduction of cochlear gain mediated by an efferent input to the outer hair cells; Al-Mana et al., 2010), binaural beat detection (Tobias, 1965), sound localization capabilities (Haggard and Gaston, 1978), temporary threshold shifts (Swanson and Dengerink, 1988), and acoustic reflex thresholds (Laws and Moon, 1986). These cyclical changes are abolished when hormonal fluctuations are disrupted by oral contraception (Caruso et al., 2003a; Elkind-Hirsch et al., 1992b; Snihur and Hampson, 2012; Swanson and Dengerink, 1988).
The functional indicators described above have various underlying neural mechanisms, suggesting that E2 likely exerts its effects at multiple relays in the ascending auditory pathway. Little is known, however, about estrogen receptor (ER) expression in the human auditory system. Only a single study has explored the expression pattern of ERs in the human inner-ear. Stenberg et al. (2001) reported that in adult women, ER immunostaining was restricted to the stria vascularis (the structure responsible for maintaining the fluid and ion balance of the cochlea) and the type I spiral ganglion neurons (thick myelinated afferents that primarily innervate inner hair cells). As the authors noted, caution is warranted in interpreting these results because of the difficulty in obtaining well-preserved human tissue (Stenberg et al., 2001). Additional research is needed to elucidate the distribution pattern of ERs throughout the human auditory system, particularly during different stages of development and different stages of the menstrual cycle, so that one can more accurately predict the specific sites of estrogenic action.
2.3 Auditory dysfunction is prevalent during pregnancy
During pregnancy, when E2 levels rise continuously until parturition, correlated changes in auditory function also occur. For example, throughout pregnancy, low-frequency audiometric thresholds significantly worsen, and during the third trimester, women perceive lower levels of sound as uncomfortably loud (Sennaroglu and Belgin, 2001). Self-reports and case studies indicate that other auditory symptoms, such as fullness in the ear, tinnitus, and autophonia, are more prevalent during pregnancy and symptoms resolve after giving birth (Gurr et al., 1993; Mukhophadhyay et al., 2007; Schmidt et al., 2010; Tsunoda et al., 1999). Physiological findings support these claims: Tandon et al. (1990) found that ABR inter-peak intervals are prolonged during pregnancy. Additionally, in rare circumstances pregnancy may cause sudden deafness (Goh and Hussain, 2012; Kenny et al., 2011). The exact etiology of these symptoms is still unknown, though many hypotheses have been proposed, including hormonally-mediated osmotic shifts that alter the ionic balance of the cochlea, and cochlear hypoxia resulting from compromised circulation (for review see Goh and Hussain, 2012). These findings indicate that in certain circumstances, alterations in sensory function may result from indirect hormonal actions on other physiological processes.
2.4 Auditory function is diminished after menopause, but may be restored by estrogen replacement
Deficits in auditory function also accompany menopause. For instance, Wharton and Church (1990) found that ABR peak latencies increase and ABR peak amplitudes decrease as a function of age, but these changes are greater in females compared to males, possibly as a result of hormonal changes that underlie menopause. This notion is supported by Kim et al. (2002), who found that the incidence of hearing loss in a large sample of postmenopausal women was inversely correlated with serum E2 levels.
Further evidence comes from studies that examined the effects of hormone replacement therapy. In general, hormone replacement improves pure-tone audiometric thresholds (Hederstierna et al., 2007; Kilicdag et al., 2004), shortens ABR peak latencies (Caruso et al., 2003b; Khaliq et al., 2005, 2003; Sator et al., 1999), and increases ABR peak amplitudes (Khaliq et al., 2005, 2003) in postmenopausal women. Conversely, one study found that estrogen replacement therapy actually increases ABR latencies and inter-peak intervals (Elkind-Hirsch et al., 1992a). One possible explanation for this apparent contradiction lies in the fact that the subjects in the study by Elkind-Hirsch and colleagues were relatively young women (29-42 years old) being treated for premature ovarian failure. In contrast, the subjects in the other studies ranged from 45-70 years, suggesting that estrogen sensitivity may be age-dependent. Regardless, these findings indicate that proper E2 levels and/or estrogenic sensitivity are important for the maintenance of auditory health in adult and aging women.
2.5 Auditory pathology is common in Turner's syndrome patients
Clinical disorders that cause disruptions in normal hormonal homeostasis provide further insight into the role of estrogens in auditory function. Turner's syndrome is one such abnormality that results in the complete or partial loss of one × chromosome. Women with this syndrome do not develop ovaries, and therefore, are estrogen deficient. Recurring episodes of middle-ear inflammation (otitis media) is frequent among this patient population (Hultcrantz, 2003; Stenberg et al., 1998). In addition, sensorineural hearing loss is common, and the rate of hearing decline over time is higher than in the general population (Güngör et al., 2000; Hederstierna et al., 2009b; Hultcrantz and Sylven, 1997; Hultcrantz et al., 1994; Hultcrantz, 2003). Furthermore, ABRs also may exhibit abnormalities. For example, Güngör and colleagues (2000) found that wave-I and III latencies were lengthened in Turner's syndrome patients relative to controls; however, Hederstierna et al. (2009a) found no such difference. This disparity partially may be explained by differences in the criteria used for subject selection: Güngör et al. included patients with conductive hearing loss; Hederstierna et al. did not. Additionally, (and somewhat paradoxically), Hederstierna et al. (2009a) reported that on average, Turner's syndrome patients demonstrated shorter wave-V latencies compared to controls. The authors posited that Turner's syndrome patients may have shorter auditory nerves, and this length difference could give rise to an earlier wave-V peak latency; to date, however, the true cause of this finding remains unknown. Finally, other central deficits are manifest in these women, including difficulty with sound source localization (Hederstierna et al., 2009a). Thus, proper estrogenic tone may be necessary for normal auditory development and the maintenance of auditory health, not only in aging women, as outlined in the previous section but young, premenopausal women as well.
3. Estrogens and auditory function in animal models
The human studies summarized above strongly suggest that estrogens influence auditory physiology and perception. For obvious ethical reasons, however, we must rely heavily on animal models to test the causality of these relationships and elucidate underlying mechanisms. Thus, the following sections will discuss key findings from research performed on a wide range of vertebrate taxa. It is hoped that the wealth of information that has been and continues to be generated from such studies will improve our understanding of hormone-mediated plasticity, and create new avenues for both basic and translational research.
3.1 Estrogens and the rodent auditory system
3.1.1 Behavioral discrimination changes during the rodent estrus cycle
Similar to humans, adult rodent auditory processing appears to be sensitive to changes in hormonal state that are associated with the reproductive cycle. For example, Ehret and Schmid (2009) compared the behavioral responses of virgin mice (Mus musculus) to synthetic models of pup “wriggling” calls at different stages of the estrus cycle. Mouse pups generate these “wriggling” calls when an adult female is in a nursing or warming position; such females, in turn, respond to these calls by licking pups, building nests, and adjusting their body position. The authors reported that pup call discrimination fluctuated with the estrus cycle. Discrimination was worst during the estrus phase, when circulating levels of E2 are low, and best during the diestrus phase, when E2 levels are beginning to rise (see figure 1 for a comparison between the human menstrual and rodent estrus cycle). While it remains unclear whether this effect is specific to the auditory system, it should be noted that ERα gene expression within the mouse cochlea fluctuates over the course of the estrus cycle as well, with downregulated expression during periods of high E2 (Charitidi et al., 2012). Similar findings were observed in the rat (Rattus norvegicus) inner-ear, with weaker immunohistochemical staining for ERs during late pregnancy (when E2 levels are elevated) compared to early pregnancy (Simonoska et al., 2009). Thus, natural oscillations of estrogenic signaling in the rodent cochlea may contribute to altered neural discrimination and behavioral responsiveness.
3.1.2 Changes in estrogenic signaling may contribute to age-related auditory decline in the rodent
Rodent auditory function also has been explored in the context of menopause and hormone replacement therapy. To start with, Guimaraes and colleagues (2004) examined ABRs and DPOAEs in male and female mice at three different ages. Young adult mice (2.1-2.9 months old) showed no sex differences in DPOAE levels or ABR magnitudes. Male mice began to show decreased DPOAE levels in middle age (14-16.4 months old) and demonstrated decreased ABR magnitudes in old age (24.3-29.0 months old). Females, on the other hand, only began to show declines in DPOAE levels in old age (after menopause), and did not show deficits in ABR function at any age tested. These findings suggest that estrogenic signaling may help to maintain and/or protect the health of the cochlea, leading to a reduced rate of functional decline in females during adulthood and maturity.
Coleman et al. (1994) examined the effect of estrogen replacement on ABR latencies in young adult (90-day-old) ovariectomized rats. They found that moderate doses of estrogen replacement shortened ABR latencies and inter-peak intervals relative to vehicle-treated females, but larger estrogen doses actually had the opposite effect, prolonging some peak latencies. Conversely, the same moderate dose of estrogen that was effective in young adult rats did not affect ABR latencies in aged (20-month-old) ovariectomized rats (Cooper et al., 1999). Finally, a study by Price et al. (2009) revealed that prolonged administration of systemic E2 to middle-aged, ovary-intact mice (15-17 months old) worsened ABR thresholds. Taken together, these data suggest a clear sensitivity of the rodent auditory system to E2, but specific effects appear to result from a complex interplay of age and estrogenic signaling.
Several immunohistochemical studies support the idea that the impact of E2 on the rodent auditory system is age-dependent. For example, the intensity of ER immunostaining is reduced in the cochleae of aged male and female mice, compared to younger adults (Motohashi et al., 2010). Similarly, Charitidi and colleagues found that the distribution pattern of ERα and ERβ in the mouse ascending auditory pathway changed as a function of age for both males and females (Charitdi and Canlon, 2010; Charitidi et al., 2010). In prepubertal (4-week-old) mice, ERα and ERβ were spatially segregated, such that ERβ+ neurons were primarily found in the auditory brainstem and periphery, and ERα+ neurons were concentrated in the inferior colliculus and (to a smaller degree) the auditory cortex. Aged mice (26-28 months old), on the other hand, expressed both ER subtypes throughout the auditory pathway. These results suggest that age-related changes in the estrogenic sensitivity of auditory structures may contribute to the efficacy of hormone replacement after natural or surgically-induced menopause, as previously suggested by human menopausal studies. Further research on this topic is warranted.
3.1.3 ERβ protects the mouse auditory system from damage
The findings presented above have led some researchers to hypothesize that ERα and ERβ serve different functions in the mammalian auditory system (Charitidi et al., 2012). Two studies investigated this issue directly in ERα and ERβ knockout mice. Meltser and colleagues (2008) measured ABR thresholds in 12-22 week old male and female mice before and after prolonged exposure to high-intensity broadband noise. This type of acoustic trauma induces a temporary threshold shift that recovers within 48 hours. Noise exposure induced significantly greater threshold shifts in mice lacking ERβ than in wild-type (WT) or ERα knockouts. Furthermore, WT mice that were pre-treated with a selective ERβ agonist (2,3-bis (4-hydroxylphenyl)-triyl-trisphenol, DPN) before noise exposure demonstrated reduced threshold shifts compared to vehicle-treated mice. Thus, ERβ-dependent signaling appears to protect the mouse auditory system from acoustic trauma.
Similarly, Simonoska et al. (2009b) examined auditory physiology and morphology in female ERβ -/-mice and WT littermates at two different ages. While no functional or morphological differences were observed between the groups at 3 months, ERβ knockouts showed significant deficits by 1 year of age. Specifically, ERβ-/- mice lacked measurable ABRs, showed a marked loss of hair cells and spiral ganglion cells, and displayed gross cochlear degeneration. Thus, in the mouse, ERβ expression is important for the maintenance of normal auditory morphology and function. Collectively, these two studies suggest that ERβ-mediated signaling cascades protect the mammalian auditory periphery from environmental and age-related damage; additional evidence indicates that brain-derived neurotrophic factor (BDNF) may be involved in such protection (for details see section 4.1 below).
3.1.4 E2 regulates cortical and behavioral responses to pup vocalizations in female mice
Apart from its role in auditory development and health, E2 appears to be a key regulator in experience-dependent auditory plasticity. An excellent example of such regulation comes from studies of pup retrieval by female mice (for review see Miranda and Liu, 2009). When a mouse pup is isolated from the nest, it emits an ultrasonic vocalization, which prompts search and retrieval behavior by the mother. Data suggest that hormonal state and prior experience with pup care interact to regulate vocalization recognition and/or salience. For example, mothers, but not virgins or ovariectomized females, demonstrate a behavioral preference for playback of pure-tones that are spectrally matched to pup ultrasonic vocalizations (Ehret and Koch, 1989; Ehret et al., 1987). Five days of pup experience, however, are sufficient to induce vocalization preference in virgins and in E2-treated ovariectomized females; longer exposure periods are required to generate the same behavioral effect in untreated ovariectomized animals (Ehret and Koch, 1989). Furthermore, while vocalization preference is retained in gonadally-intact mothers one month after being separated from their pups, it is lost in mothers that were ovariectomized after the separation event (Ehret and Koch, 1989). Thus, E2 appears to enhance the acquisition and retention of behavioral preferences for pup isolation calls, though it could do so by targeting any of a number of neural circuits, including those that are non-auditory.
Fichtel and Ehret (1999) provided the first supporting evidence that differences in auditory function may underlie these effects. They exposed mothers and pup-naïve virgin mice to synthetic calls for a 45-minute period. Immediately after exposure, animals were sacrificed and the brains were labeled for c-Fos, an immediate-early gene marker for recent neural activity. Mothers and virgins demonstrated different spatial patterns of c-Fos expression across the auditory cortex subfields.
A separate set of experiments explored this finding in more detail by comparing activity from the auditory cortex of mothers and virgins that lacked previous pup experience. Liu and colleagues (2006) presented anesthetized mice with sets of pup isolation calls that varied in repetition rate. When calls were presented at the naturally occurring rate of 5 Hz, cortical multi-unit responses from mothers demonstrated robust responses to each call within a bout, thus demonstrating temporal entrainment. Responses from virgins, on the other hand, were only able to entrain temporal modulations when calls were presented at a rate of 3 Hz or less. Furthermore, an information-based analysis revealed that when single- and multi-unit auditory cortex responses are analyzed with a fine temporal window, responses from recent mothers convey more bits of information about pup call detection and discrimination compared to responses from pup-naïve virgin females (Liu and Schreiner, 2007). Finally, Galindo-Leon et al. (2009) examined single-unit and local-field-potential recordings from the auditory cortex of awake, head-restrained mice. They reported that mothers and pup-naïve virgins display differences in inhibition for neural sites tuned to frequencies lower than 50 kHz. Specifically, they found that at these sites, mothers demonstrate a greater magnitude and duration of call-evoked inhibition, shorter latencies to inhibition onset, and greater trial-to-trial reliability of the inhibitory response. These neural sites were tuned to just below (i.e. laterally to) the frequency range of pup vocalizations (60-80 kHz), suggesting that enhanced lateral band inhibition serves to improve cortical contrast and detection of pup calls in maternal caregiving females. While carefully controlled hormone manipulations and measurements are required to draw any further conclusions, it is important to note that the mouse auditory cortex is likely sensitive to endogenous E2, as evidenced by the expression of aromatase (the enzyme responsible for converting testosterone into E2; Tremere et al., 2011), ER mRNA (Tremere et al., 2011) and ER protein (Charitidi and Canlon, 2010) in males and females of reproductive age. Taken together, these findings suggest that differences in estrogenic state and pup care experience regulate the processing of vocal stimuli in the mouse auditory cortex.
3.2 Estrogens and the anuran auditory system
For over three decades, anurans species, which use vocal communication extensively in courtship behaviors, have been popular model organisms to investigate auditory plasticity, particularly in a seasonal context (for review see Arch and Narins, 2009). Seasonal plasticity consists of adaptive changes in the brain and behavior of an organism in response to environmental variations in day length (photoperiodicity), ambient temperature, rainfall, and food availability (Brenowitz, 2004). Such environmental cues stimulate the production of sex-steroid hormones, including estrogens, which can dramatically reshape the neural substrates underlying reproductive and communication behaviors (Bass, 2008; Meitzen and Thompson, 2008; Sisneros, 2009c).
The majority of studies that have investigated seasonal auditory plasticity in anurans have focused on the torus semicircularis (TS), a midbrain homolog to the mammalian inferior colliculus. As early as 1980, Walkowiak demonstrated the existence of seasonal plasticity in the TS of the fire-bellied toad (Bombina bombina). By obtaining extracellular single-unit recordings, he revealed an elevated level of spontaneous activity during the breeding season, when compared with the non-breeding season. A similar finding was described a short time later by Hillery (1984), who showed that sound-evoked activity also exhibits a seasonal pattern of plasticity. During the breeding period, TS auditory-evoked potentials are larger, and multi-unit pure-tone thresholds are lower (better) in the treefrog (Hyla chrysoscelis; Hillery, 1984). Seasonal differences in tuning and temporal properties of auditory midbrain neurons also have been described. For example, during the breeding period, single-units in the TS of male Northern leopard frogs (Rana pipiens pipiens) show stronger phase-locking to amplitude-modulated tones designed to mimic the envelope of a natural advertisement call (Goense and Feng, 2005). The authors postulated that such enhanced time-locking may be beneficial for call discrimination during periods of intense background noise, such as during the conspecific mating chorus. Furthermore, the authors observed seasonal shifts in the distribution of single-unit characteristic frequencies, such that the number of neurons tuned to low frequencies (100-500 Hz) increased gradually throughout the summer and fall, the number of neurons tuned to intermediate frequencies (700-1200 Hz) gradually declined, and the number of neurons tuned to higher frequencies remained constant (Goense and Feng, 2005). Neurons sensitive to 100-1200 Hz likely receive their input from the amphibian papilla, one of two peripheral auditory structures found in anurans. Because high-frequency neurons receive input from the other end organ, the basilar papilla, the authors hypothesized that the observed seasonal differences in frequency tuning may originate in the periphery (Goense and Feng, 2005). Finally, a related study reported stronger auditory-evoked multi-unit response strengths in the TS of unmated female green treefrogs (Hyla cinerea) compared to their recently mated counterparts (Miranda and Wilczynski 2009). These findings indicate that during periods of reproduce receptivity, neuronal discharge patterns and properties of the anuran auditory midbrain are enhanced in a manner that could aid in the processing of acoustic mating and/or territorial calls.
While these studies provide only indirect evidence of estrogenic effects on the auditory circuits that process communication calls, a separate report demonstrated that intraventricular administration of E2 increases the amplitude of tone-evoked potentials in the TS of female Northern leopard frogs (Yovanof and Feng, 1983). In addition, it is interesting to note that mRNA for both nuclear ER subtypes, ERα and ERβ, is expressed in the auditory midbrain of male and female túngara frogs during the reproductive period (Physalaemus pustulosus; Chakraborty and Burmeister, 2010). Thus, auditory coding in the anuran auditory midbrain may be modulated by the direct action of gonadal and/or brain-derived E2.
It is increasingly clear that E2 plays an active role in the regulation of auditory processing, but the reciprocal also is true: acoustic cues can modulate E2 production, and these modulations can be influenced by social context. For example, in female túngara frogs, circulating levels of E2 increase after acoustic exposure to a mate chorus, but remain steady after exposure to spectrally-matched synthetic stimuli (Lynch and Wilczynski, 2006). Based on neuroanatomical and neurophysiological evidence, it is thought that this endocrine response is mediated by auditory-evoked activity carried by projections from the TS and the thalamus to the preoptic area and hypothalamus (for review see Wilcynzski et al., 1993). Thus, estrogenic and auditory function can interact in a bidirectional manner to optimize behavioral responses to salient stimuli.
Collectively, these findings suggest that in anuran species, E2 mediates seasonal- and experience-dependent changes in auditory midbrain physiology that may lead to enhanced processing of conspecific vocalizations during periods of reproductive readiness. Additional research is needed to determine whether E2 similarly affects other regions in the ascending auditory pathway.
3.3 Estrogens and the fish auditory system
Additional insight into estrogenic modulation of auditory function can be gleaned from a growing number of studies on teleost species, members of the ray-finned fish (Actinopterygii) taxonomic class. One compelling series of experiments that focused on the midshipman fish (Porichthys notatus) again highlights the utility of assessing such effects in the context of seasonal plasticity (for reviews see Sisneros 2009a,c). In this species, nest-building males generate a “hum”-like advertisement call to attract gravid females to their nest. Extracellular recordings of single auditory nerve afferents in such females revealed that during the summer, maximum tone-evoked firing rates increase, phase-locking strengthens and frequency-tuning shifts upwards, enabling better encoding of the high-frequency harmonics that dominate the male advertisement call (Sisneros and Bass, 2003). Notably, these effects can be induced in non-reproductive females in the laboratory by systemic treatment with E2 (Sisneros et al., 2004). These changes are paralleled by enhanced auditory sensitivity of the inner-ear end organ, the saccule (Rohmann and Bass, 2011; Sisneros, 2009b). In a recent study, Coffin et al. (2012) identified seasonally-mediated saccular hair cell addition as the potential anatomical substrate underlying these functional modulations. Additionally, during the breeding period, ERα mRNA is expressed in the female midshipman saccular epithelia (Sisneros et al., 2004; Forlano et al., 2005), and the enzyme aromatase localizes to auditory nerve ganglion cell bodies (Forlano et al., 2005). Together, these findings suggest that in the reproductive female midshipman fish, gonadally and locally synthesized E2 initiates signaling cascades in the auditory nerve that may influence hair cell survival, peripheral auditory physiology, and ultimately, the perception of and behavioral response to male advertisement calls. It will thus be interesting to further explore whether E2 directly modulates auditory responses in more central subregions of the midshipman, such as the TS, which also expresses ERα mRNA (Forlano et al., 2005).
In a separate set of experiments, Maruska and colleagues investigated the role of estrogens in the auditory system of the Tanganyikan cichlid fish (Astatotilapia burtoni). The authors recorded auditory evoked potentials in females that were in one of two reproductive states: gravid (i.e. ready to spawn), or mouthbrooding (a period that occurs post-spawning when developing young are reared in the mouth; Maruska et al., 2012). It was found that gravid females display better sensitivity to low-frequency sounds that match the spectral properties of male courtship signals compared with mouthbrooding females. Moreover, systemic hormone measurements revealed a correlation between circulating levels of E2 and auditory performance, such that higher E2 levels were associated with better auditory thresholds (Maruska et al., 2012). In a related work, ER and aromatase mRNA expression levels varied in the cichlid inner-ear as a function of reproductive condition (Maruska and Fernald 2010). Circulating levels of E2 in gravid females were elevated, while concomitantly, the expression of ERα, ERβa (one of two ERβ subtypes found in teleost fish) and aromatase were diminished compared to females that had recently spawned Thus, estrogenic signaling in the cichlid auditory periphery may regulate the encoding of auditory stimuli in an experience-dependent manner, such that mate-ready females demonstrate enhanced sensitivity to courtship signals.
Together, these studies indicate that in some teleost fish, E2 action optimizes behavioral responses to acoustic mating displays by synchronizing periods of reproductive readiness and maximum auditory receptivity. Additional research on diverse fish species may provide a better understanding as to whether these findings represent an evolutionary-conserved mechanism in teleosts, as might be suggested by presence of aromatase in the peripheral and central auditory system of the goldfish (Carassius auratus; Gelinas and Callard, 1997), or whether they represent independent evolutionary events, as might be suggested by the absence of seasonal/hormonal auditory plasticity in the Lusitanian toadfish (Halobatrachus didactylus; Vasconcelos et al., 2011) and the association of high E2 levels with poorer auditory thresholds in the round goby (Neogobius melanostomus; Zeyl et al., 2013).
3.4 Estrogens and the songbird auditory system
Songbirds, which rely heavily on learned vocalizations for mate attraction and territorial defense, have contributed greatly to our understanding of hormonal regulation of vocal motor systems over the past 3 decades (Brenowitz, 2008). In recent years, however, they also have become increasingly popular experimental models to investigate sex-steroid-mediated auditory plasticity. This section of the review will focus on two different, but complementary lines of research, each supporting a role for estrogenic modulation of songbird auditory physiology.
3.4.1 Brain-derived E2 modulates auditory processing in the zebra finch caudomedial nidopallium
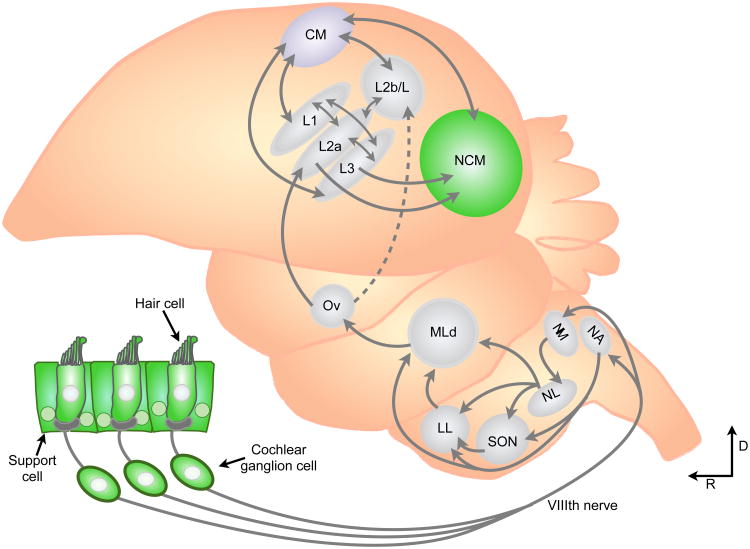
The first line of songbird research evaluated here focuses on the caudomedial nidopallium (NCM) of the zebra finch (Taeniopygia guttata). NCM is a second-order auditory forebrain region specialized for processing conspecific songs, and is thought to be analogous to mammalian auditory association cortex (Fig 2.) Several independent lines of evidence have demonstrated that E2 is an important regulator of auditory processing in NCM neurons. For starters, aromatase is expressed in adult zebra finch NCM cell bodies and presynaptic terminals in both sexes (Peterson et al., 2005). Playback of conspecific song (but not white-noise) increases E2 levels in NCM of males and females within 30 minutes, without affecting circulating levels of E2, or its precursor, testosterone (Remage-Healey et al., 2008, 2012). This fluctuation in E2 concentration depends on excitatory glutamatergic signaling and presynaptic voltage-gated calcium influx (Remage-Healey et al., 2008, 2011). Thus, exposure to a conspecific acoustic stimulus rapidly regulates locally synthesized E2.
Figure 2. Distribution of ERs in the songbird ascending auditory system.
The dashed line connecting Ov to L2b indicates that this projection is a smaller input than the one to L2a, and its origin is restricted to the medial portion of Ov. Areas in green indicate sites of known ER expression in songbirds. Note that portions of this schematic have been simplified for clarity. L1, L2a, L2b/L and L3 refer to individual subregions in the field L complex. NA nucleus angularis; NM, nucleus magnocellularis; SON, superior olivary nucleus; NL, nucleus laminaris; LL, lateral lemniscus; MLd, mesencephalicus pars dorsalis; Ov, ovidalis; CM, caudal mesopallium; NCM, caudomedial nidopallium; D, dorsal; R, rostral.
Because NCM of both sexes not only produces E2, but also expresses ERs, (Jeong et al., 2011; Metzdorf et al., 1999), NCM neurons are logical targets of estrogenic action. For example, infusion of exogenous E2 into NCM increases multi- and single-unit sound-evoked activity, and auditory response strengths in both males and females (Remage-Healey et al., 2010; 2012; Remage-Healey and Joshi, 2012; Tremere et al., 2009; Tremere and Pinaud, 2011). Similarly, local inhibition of aromatase decreases firing rates and response strengths (Remage-Healey et al., 2012; Tremere et al., 2009; Tremere and Pinaud, 2011).
In addition, the impact of estrogenic signaling in NCM extends to at least one downstream sensorimotor nucleus: HVC (used as a proper name). Direct administration of E2 to NCM of adult males enhances neural selectivity in HVC for the bird's own song (BOS) compared to the song of a conspecific. This finding is consistent with the fact that application of fadrozole (an aromatase inhibitor) to NCM decreases this selectivity (Remage-Healey and Joshi, 2012). Notably, direct pharmacological manipulation of HVC itself, or another nearby auditory region, the caudomedial mesopallium (CMM), has no effect on HVC electrophysiological response properties, indicating that the site of estrogenic action is specific to NCM. These effects are thought to depend on a membrane-bound receptor, as infusion of a membrane-impermeable E2-biotin conjugate into NCM recapitulates several of these findings, including increased auditory response strengths in NCM (Remage-Healey et al., 2012) and increased neural BOS selectivity in HVC (Remage-Healey and Joshi, 2012). Furthermore, male zebra finches display an innate behavioral preference for BOS, or for the song of their male tutor, than for the songs of other conspecific males. Playback experiments demonstrate that disruption of E2 synthesis in NCM abolishes this natural preference (Remage-Healey et al., 2010; Tremere and Pinaud, 2011). Collectively, these findings show that the reception of ethologically-relevant acoustic stimuli rapidly upregulates E2 synthesis in NCM, which in turn, enhances auditory response properties in NCM and HVC, ultimately shaping behavioral song preferences and/or discrimination capabilities.
3.4.2 Systemic E2 mediates seasonal auditory plasticity in several songbird species
The studies summarized above indicate that brain-derived E2 plays an important role in regulating auditory function in the zebra finch NCM and HVC. A separate line of research, however, focusing on hormonal modulation of the songbird auditory system in a seasonal and reproductive context, reveals that E2 actually affects neuronal response properties throughout the ascending auditory pathway and in multiple songbird species. Key issues emerging from this growing body of literature are discussed below.
Lucas and colleagues performed a series of experiments to explore seasonal effects on peripheral and brainstem auditory function in males and females of several avian species. They found that both Carolina chickadees (Poecile carolinensis) and house sparrows (Passer domesticus) exhibited increased ABR peak amplitudes during the spring, compared with other times of the year (Henry and Lucas, 2009; Lucas et al., 2002, 2007). Cochlear microphonic and frequency-following-response measurements in chickadees indicate that some of these seasonal differences include both sensory and neural components (Lucas et al., 2007). Conversely, spring ABR peak amplitudes are lower, and ABR peak latencies are prolonged compared to winter responses in both white-breasted nuthatches (Sitta carolinensis) and downy woodpeckers (Picoides pubescens; Lucas et al., 2002, 2007). Together, these findings suggest that seasonal plasticity in peripheral and brainstem auditory processing may be a widespread phenomenon among avian species, including non-songbirds (the woodpecker), though the nature of the effects may depend on species-specific differences in food-gathering strategies, predation risk, or reproductive state.
The results of the Lucas et al. studies provoke additional thought as to whether seasonal plasticity in the avian auditory periphery and brainstem is vulnerable to E2-dependent mechanisms. Immunohistochemical analysis has revealed ERα expression in the hair cells, support cells and cochlear ganglion cell bodies in both sexes of two songbird species: the zebra finch (Noirot et al., 2009) and the white-crowned sparrow (Zonotrichia leucophrys gambelii; similar patterns observed under both breeding and non-breeding conditions; Yuan Wang, Eliot Brenowitz and Edwin Rubel, unpublished observations). Similarly, zebra finch hair cells also express aromatase (Noirot et al., 2009). These findings suggest that estrogenic signaling may indeed mediate seasonal plasticity in the avian inner-ear and auditory brainstem relays. Two additional studies shed further light on this issue.
In one set of experiments, Gall et al. (2013) presented house sparrows with two sets of acoustic stimuli and measured ABRs. The first stimulus set, tone-bursts embedded in spectrally-notched white-noise, served to assess auditory filter bandwidth, or frequency selectivity. The second stimulus set, pairs of tone bursts separated by various time intervals, was used to determine temporal resolution. Their results revealed that during the spring, female house sparrows had sharper auditory filters and poorer temporal resolution than in the autumn. While the precise adaptive significance of this finding is unclear, one possibility is that during the breeding season, spectral cues in the advertisement song honestly indicate male quality, and thus females sacrifice precise temporal processing in order to enhance frequency discrimination and improve their chances of identifying a high-quality mate. In addition, systemic E2 levels were greater in the spring than in the autumn, implying that this synergistic change in the auditory system is triggered by elevated E2. These results strongly suggest that estrogenic signaling shapes seasonal changes in auditory response properties of female house sparrows.
In related research, Caras et al. (2010) explored peripheral and auditory brainstem function in a closely related species, and pronounced seasonal breeder, Gambel's white-crowned sparrow. Wild-caught female birds were brought into breeding and non-breeding conditions in the laboratory using previously validated photoperiod and hormone manipulations. Specifically, to induce breeding condition, birds were exposed to long days, typical of their Alaskan summer breeding grounds (20 hours of light; 4 hours of darkness) and implanted with a subcutaneous pellet that released supplementary E2 systemically. To induce non-breeding condition, birds were exposed to short day lengths and did not receive any supplementary E2. ABR measurements from females housed under breeding condition (when plasma E2 levels are high) revealed poorer thresholds and prolonged peak latencies compared with ABRs recorded from non-breeding females (Caras et al., 2010). These findings are unlikely to be the result of impaired non-linear inner-ear amplification because DPOAEs were unaffected by hormonal state. Thus, direct manipulation of E2 levels in a wild-caught songbird led to corresponding changes in peripheral and/or brainstem neural activity.
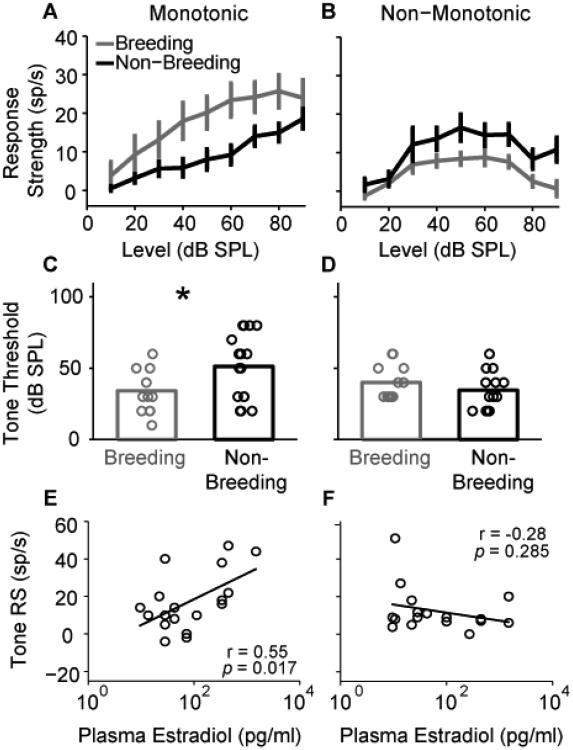
In order to better understand the implications of the above findings, a separate group of female white-crowned sparrows were brought into breeding and non-breeding condition in the laboratory. Extracellular single-unit responses to pure-tone and conspecific song stimuli from field L (Fig. 2), the avian analogue of the mammalian primary auditory cortex, were subsequently recorded (Caras et al., 2012). The authors examined the effect of E2 on two functionally distinct cell types that are common in the auditory system: Monotonic cells steadily increase their firing rates when presented with a pure-tone stimulus of increasing intensity, eventually reaching a saturation point at high sound levels. Non-monotonic cells, on the other hand, increase their firing rates only up to a given sound level; at higher intensities, their firing rates are suppressed. The authors found that for monotonic cells, E2 treatment increased spontaneous firing rates, maximum evoked firing rates and auditory response strengths across a wide range of stimulus intensities (Fig. 3A). This increased activation led to enhanced pure-tone sensitivity (Fig. 3C) and an expanded dynamic range for conspecific vocalizations. Furthermore, the response properties of individual monotonic neurons strongly correlated with the concentration of circulating E2, revealing a robust dose dependence of central sensory physiology on systemic E2 (Fig. 3E). Notably, these effects were absent or were in the opposite direction in non-monotonic cells (Fig. 3B,D and F). Based on the level-tolerance model (Sadagopan and Wang, 2008), which suggests that nonmonotonic neurons allow the spectral content of a time-varying stimulus to be encoded by neuronal firing rates without the confounding effect of stimulus intensity, the authors hypothesized that E2 might act selectively on monotonic neurons to enhance signal detection in the reproductive season, while the stability of the non-monotonic neurons may act to preserve signal recognition throughout the year. Thus, in the white-crowned sparrow, high levels of circulating E2, typical of the breeding season, simultaneously diminishes auditory brainstem function (Caras et al., 2010), and enhances the function of a select cell population in the primary auditory forebrain (Caras et al., 2012).
Figure 3. Estradiol enhances auditory forebrain tone responses in a cell-specific and dose-dependent manner.
Single-unit responses to pure-tones at characteristic frequencies were recorded in the primary auditory forebrain, field L, of female white-crowned sparrows brought into breeding (high E2) or non-breeding condition (low E2) in the laboratory. A-B. Mean +/- S.E.M pure-tone response strengths are plotted as a function of stimulus intensity. In cells with monotonic rate-level functions (A), breeding condition significantly elevates firing rate response strengths compared to non-breeding condition (p < 0.05). B. Breeding condition decreases response strengths of neurons with non-monotonic rate level functions (p < 0.05). C-D. Individual single unit pure-tone thresholds (circles) are plotted for each experimental group, along with their means (bars). C. Breeding condition significantly decreases pure-tone thresholds of monotonic neurons (p < 0.05), but has no effect on non-monotonic thresholds (D). E-F. Individual single unit pure-tone response strengths (circles) are plotted as a function of the circulating E2 concentration. Data shown were collected at 50 dB SPL, but similar results were found for all stimulus intensities tested. E. Monotonic Response strengths are significantly and positively correlated with circulating levels of plasma E2. F. Response strengths and E2 levels are uncorrelated in non-monotonic neurons. Adapted with permission from Caras et al., 2012.
In order to speculate about the adaptive significance of these findings, one must make note of the fact that during the breeding season, songbirds are capable of singing at extremely high intensities, ranging from 74-105 dB SPL at a distance of 1 meter (Brackenbury, 1979). Thus, one possibility worth considering is that estrogenic signaling reduces responsiveness of the auditory periphery and brainstem to protect the system from noise-induced damage. Conversely, because accurate song perception is important for female mate choice, it would be disadvantageous to have diminished auditory function throughout the auditory pathway. Thus, E2 may simultaneously enhance the responses of monotonic field L neurons in part to offset the effects at the periphery.
Other researchers also have reported enhanced songbird auditory forebrain function during the reproductive season. For example, extracellular single- and multi-unit recordings have shown that in the sensorimotor nucleus HVC of the canary (Serinus canaria), long-day photoperiods significantly increase spontaneous activity, increase the proportion of putative interneurons, and enhance neural selectivity (in males) for BOS (Del Negro and Edeline, 2002; Del Negro et al., 2005, 2000). On some level, these results are similar to those reported by Remage-Healey and Joshi (2012) for HVC neurons after manipulation of local E2 levels in NCM of the non-seasonally breeding zebra finch (see section 3.4.1 above). Thus, these findings raise the interesting possibility that in seasonal breeders, such as the canary, changes in day length may modulate the synthesis of local E2 in NCM.
Several additional songbird studies have investigated seasonal patterns of neural activation by examining expression of the immediate-early gene zenk (also known as zif-268, egr-1, krox-24 and NGFI-A), and its protein product ZENK, both of which are hallmarks of recent neural activity (for reviews see Maney and Pinaud, 2011; Mello et al., 2004). In the seasonally-breeding white-throated sparrow (Zonotrichia albicollis), systemic administration of E2 modulates ZENK expression in two auditory forebrain regions (NCM and CMM), as well as the midbrain nucleus mesencephalicus lateralis pars dorsalis (the avian homologue of the mammalian inferior colliculus; see figure 2). Specifically, in E2-treated females, song exposure resulted in a higher density of ZENK+ cells than pure-tone exposure. This stimulus specificity was absent in untreated birds (Maney et al., 2006; Sanford et al., 2010). A similar result was reported for freely-living male black-capped chickadees (Poecile atricapillus): in the breeding season, there was a higher density of ZENK+ cells in NCM of birds exposed to chickadee songs and calls, compared to those that heard songs of a heterospecific (Phillmore et al., 2011). These findings may underlie behavioral responsiveness to acoustic cues, as E2 treatment increases the frequency of song-evoked copulation solicitation displays (a stereotyped behavior evoked by mate-ready females) in white-throated sparrows (Maney et al., 2006, 2008; Sanford et al., 2010). All together, the above findings suggest that in seasonally-breeding songbird species, natural fluctuations in systemic E2 shape stimulus-evoked response properties in the songbird auditory periphery, brainstem, and forebrain, ultimately enhancing neural and behavioral selectivity for conspecific vocalizations.
4. What are the molecular mechanisms?
As the previous sections have made clear, E2 appears to be an important regulator of auditory function across a wide range of animal taxa, including humans, and acts on multiple auditory structures. The classical view of estrogenic signaling is that E2 crosses the plasma membrane and binds to an intracellular receptor, which dimerizes and directly regulates gene transcription by binding to estrogen response elements (EREs) in promoter regions of DNA. Various non-classical mechanisms also exist, including, but not limited to, ERE-independent co-regulation of DNA transcription, membrane receptor-initiated changes in cellular excitability, and ligand-independent activation of ERs by other second messengers (Charitidi et al., 2009; Chow et al., 2010; Kelly and Rønnekleiv, 2009; Zakon, 1998). Thus, in order to more fully understand the influence of E2 on sensory circuits, and determine its potential role in the treatment of auditory dysfunction, one must identify the downstream signaling cascades that underlie its effects. Therefore, the following sections discuss some of the molecules that are known to be modulated by estrogens, and highlight their role in auditory processing.
4.1 BDNF mediates estrogenic protection of the mammalian auditory system
Among the several growth-promoting factors, brain-derived neurotrophic factor, or BDNF, is a widely-expressed protein important for the development and maintenance of cells in the peripheral and central nervous system. Like other neurotrophins, BDNF is secreted by target cells and acts on innervating neurons by binding to the p75 neurotrophin receptor or the tropomyosin-related kinase receptor, TrkB (Ramekers et al., 2012).
Several lines of evidence support the hypothesis that estrogenic regulation of BDNF signaling impacts auditory function. BDNF is expressed in both the developing and mature auditory system (Green et al., 2012) and supports the survival of spiral-ganglion neurons after an ototoxic challenge (Staecker et al., 1996). Estrogens modulate BDNF gene expression; however, whether such modulation leads to an increase or decrease in BDNF levels depends on the specific brain region, and the presence or absence of other sex-steroid hormones, such as progesterone (for review see Sohrabji and Lewis, 2006).
Meltser and colleagues (2008) explored the relationship between estrogenic signaling and BDNF expression in detail by making use of male and female transgenic knockout mice. Initially, the authors found significantly lower levels of BDNF protein in the cochleae of ERβ and aromatase knockout mice, compared with wild-type litter-mates. Administration of a selective ERβ agonist (DPN) significantly increased BDNF concentration in aromatase knockouts, supporting the idea that ERβ-dependent signaling cascades modulate BDNF expression. The authors then exposed the mice to high-intensity broadband noise, designed to induce a temporary threshold shift. ERβ knockouts were more susceptible to this acoustic trauma than wild-type mice, as evidenced by significantly greater ABR threshold shifts. Moreover, while wild-type mice demonstrated a robust reduction in BDNF levels after noise exposure, ERβ knockouts only exhibited mild decreases in BDNF expression. These findings suggest that under normal conditions, E2 regulates and maintains relatively high levels of BDNF expression in the cochlea by binding specifically to ERβ; after acoustic trauma, this BDNF is released, protecting auditory sensitivity.
In addition to its protective effects, BDNF also regulates auditory processing under non-pathological conditions. A recent study by Zuccotti et al. (2012) conditionally eliminated BDNF from hair cells, spiral ganglion cells, the dorsal cochlear nucleus, and the inferior colliculus of male and female mice. Such BDNF transgenics demonstrated poorer ABR thresholds, reduced exocytosis from basally located inner hair cells, fewer ribbon synapses in mid-basal regions of the cochlea, and fewer afferent fibers. Notably, ribbon synapse number was normal at the age of hearing onset, indicating that BDNF is involved in synaptic maintenance, rather than development. Collectively, these studies strongly support a role for BDNF in estrogenic modulation of auditory function, and suggest that direct manipulation of BDNF levels may be of therapeutic value in the treatment of auditory symptoms in estrogen-deficient clinical populations.
4.2 GABA may mediate estrogenic enhancement of songbird auditory activity
Gamma-Aminobutyric acid, or GABA, is the primary inhibitory neurotransmitter in the adult central nervous system. Its synthesis is regulated by the rate-limiting enzyme, glutamic acid decarboxylase (GAD), which has two known isoforms that differ in molecular weight: GAD65 (encoded by the gad2 gene) and GAD67 (encoded by the gad1 gene). GABA affects the electrochemical gradient of cells by binding to one of its three known receptor subtypes. In adults, ligand binding to the GABAA and GABAC receptors, which gate ionotropic chloride channels, result in chloride influx and dampen neural activity via hyperpolarization. By contrast, the GABAB receptor is metabotropic and is found at both pre- and postsynaptic loci. GABA binding to postsynaptic GABAB receptors dampens excitability by inhibiting calcium influx (for review see Chalifoux and Carter, 2011). GABA binding of presynaptic GABAB receptors, on the other hand, reduces calcium influx and subsequent vesicle release (for reviews see Watanabe et al., 2002; Grothe and Koch, 2011).
GABAergic transmission helps maintain balanced excitation and inhibition in central nervous system networks, and serves several important functions in auditory processing. In the auditory brainstem, GABA-receptor activation modulates binaural processing, which has important implications for sound localization and sound segregation (for review see Grothe and Koch, 2011). Numerous in vivo studies utilizing bicuculline, a GABAA receptor antagonist, have highlighted the importance of GABAergic inhibition in shaping frequency tuning at multiple levels of the ascending auditory pathway (Fukui et al., 2010; LeBeau et al., 2001; Suga et al., 1997; Yang et al., 1992). Additional reports indicate that inhibition modulates the selectivity of single inferior colliculus neurons for conspecific vocalizations, in part by changing receptive field properties (Klug et al., 2002; Mayko et al., 2012; Xie et al., 2005). These findings are similar to estrogenic effects on auditory processing in a number of organisms, raising the possibility that in some circumstances, E2 shapes auditory function by regulating GABA expression or release.
In fact, several lines of evidence indicate that E2 can influence both of these processes. Much of this evidence comes from studies of non-auditory brain regions, particularly the hippocampus. As it is beyond the scope of this review to summarize this rich body of literature, only a few key studies are highlighted here. For example, E2 regulates GAD65 and GAD67 mRNA levels (McCarthy et al., 1995) and acts via the classical, receptor-dependent pathway to directly modulate transcriptional activation of the gad2 promoter (Hudgens et al., 2009). At an anatomical level, E2 increases dendritic spine formation in cultured hippocampal neurons by temporarily downregulating GABAergic inhibitory transmission (Murphy et al., 1998) and similar regulation of GABA signaling has been shown in vivo (Rudick and Woolley 2001). Functionally, E2 suppresss hippocampal inhibitory synaptic transmission by acting through an ERα-dependent mechanism that decreases the probability of GABA release (Huang and Woolley, 2012). Thus, E2 is capable of altering synaptic plasticity by regulating GABAergic inhibition.
Surprisingly, few studies have examined the relationship between E2 and GABA in the auditory system. Jeong et al. (2011) demonstrated that GAD65 mRNA is co-expressed with aromatase and ER mRNA in male and female zebra finch NCM. Additionally, Tremere and colleagues (2009) made whole-cell patch-clamp recordings from zebra finch NCM neurons in an acute slice preparation. Bath application of E2 decreased the frequency of miniature inhibitory postsynaptic currents (mIPSCs), whereas tamoxifen, a putative ER antagonist, increased the mIPSC frequency. Miniature IPSCs were largely abolished by application of the GABAA receptor antagonist, bicuculline, suggesting that in NCM, E2 increases neural activity by suppressing GABAA-mediated inhibitory transmission. Future studies should determine whether similar mechanisms shape auditory function in the mammalian auditory cortex, in which cells similarly co-express GAD65, ER and aromatase mRNA (Tremere et al., 2011).
4.3 Norepinephrine may mediate estrogenic regulation of song selectivity
Based on the available evidence, one of the most likely candidates for mediating estrogenic effects on auditory processing is the catecholaminergic neuromodulator norepinephrine (NE). In the biosynthesis of NE, also known as noradrenaline, two important enzymes stand out: dopamine beta-hydroxylase (DBH), which converts dopamine into NE, and tyrosine hydroxylase (TH), the rate-limiting enzyme responsible for converting the amino acid tyrosine into the dopamine preursor L-Dopa. The primary source of NE in the central nervous system is the brainstem nucleus, locus coeruleus (LoC), which provides extensive innervation to the forebrain (Berridge and Waterhouse, 2003). As several noradrenergic receptor subtypes exist, NE has a wide range of effects on both intrinsic membrane physiology and synaptic transmission (Berridge and Waterhouse, 2003). In sensory networks, the implications of noradrenergic signaling are well known; NE enhances signal-to-noise ratios, shapes receptive fields, and fine-tunes temporal coding (Hurley et al., 2004).
The majority of evidence suggesting that estrogenic effects on auditory function may be mediated through the noradrenergic system comes from studies on songbird species. For example, catecholaminergic cells in the zebra finch LoC concentrate E2 (Heritage et al. 1980) and TH+ LoC cells in the male canary express ER mRNA (Maney et al., 2001). Additionally, Barclay and Harding (1990) found that administration of E2 to castrated zebra finches restored normal NE levels and turnover rates in multiple brain regions. Together, these findings indicate that in at least two songbird species, the noradrenergic system is sensitive to E2.
Physiological, genomic and behavioral studies have revealed a bidirectional interaction between NE and the songbird auditory system. For instance, infusion of NE directly into sound responsive regions of male zebra finches affects single- and multi-unit spontaneous and stimulus-evoked firing rates (Cardin and Schmidt, 2004; Dave et al., 1998), though whether activity is enhanced or suppressed depends on a non-monotonic dose response curve (Cardin and Schmidt, 2004). Additionally, Velho and colleagues (2012) demonstrated that expression of the activity-dependent marker zenk, and auditory memory formation requires normal noradrenergic signaling in NCM of female zebra finches. At the behavioral level, several studies have shown that NE depletion or noradrenergic antagonism affects the detection, preference, and/or discrimination of conspecific vocalizations (Canaries: Appeltants et al., 2002; European starlings: Pawlisch et al., 2011; Riters and Pawlisch, 2007; Zebra finches: Vyas et al., 2008). In contrast, sound exposure increases the number of ZENK+ catecholaminergic cells in the LoC of female zebra finches (Lynch et al., 2012), and rapidly modulates TH activation in the white-throated sparrow auditory forebrain (Matragrano et al., 2012b). Thus, in the songbird, auditory stimulation activates the noradrenaline system, and NE in turn shapes neural and behavioral responses to acoustic cues.
Over the past decade, the Maney laboratory conducted a series of detailed studies investigating NE-mediated estrogenic effects on the auditory system of female white-throated sparrows. Their work demonstrates that the E2-mediated increase in ZENK song selectivity in NCM (Maney et al., 2006 and summarized earlier) is accompanied by elevated TH immunoreactivity in the auditory forebrain and LoC (LeBlanc et al., 2007), increased noradrenergic fiber density in midbrain and forebrain auditory structures, and elevated levels of NE in the auditory forebrain (Matragrano et al., 2011). Collectively, these findings strongly implicate NE in the estrogenic modulation of songbird auditory function. Future studies are needed to determine the precise functional role other monoamines play in this process, as a separate study similarly implicated serotonin in mediating the effects of E2 (Matragrano et al., 2012a). Additionally, it is worth noting that the rodent LoC is also sensitive to estrogens (Helena et al., 2006; Serova et al., 2004), which suggests that these findings may have important implications for non-avian species.
5. Summary and conclusions
This review has evaluated our current state of knowledge regarding the impacts of estrogenic signaling on information processing in vertebrate auditory circuits. As research on this topic continues to expand, it is becoming increasingly clear that E2 serves several important functions. First, it appears that E2 may influence the development of the mammalian auditory system. This deduction is supported by: 1) the existence of sex differences in human infant auditory function, 2) the presence of auditory pathology in Turner's syndrome children, and 3) the masculinization of OAEs in rhesus monkeys and sheep after prenatal administration of testosterone, the biosynthetic precursor to E2. It must be cautioned, however, that androgenic signaling may directly mediate these effects. Future studies employing non-aromatizable androgens, aromatase inhibitors, and/or androgen receptor antagonists are needed to conclusively identify the hormones and receptors involved in these organizational processes, and to determine the precise developmental time-window during which the auditory system is most sensitive to hormonal signaling.
Another principal that emerges from this review is the notion that estrogenic action protects the mammalian auditory system from noise-induced and age-related damage, possibly via ERβ-dependent biochemical signaling cascades. This idea is supported by: 1) an increased rate of hearing decline in women with Turner's syndrome compared to the normal population, 2) delayed onset of age-related hearing loss in female mice compared to male mice, 3) increased susceptibility to acoustic trauma in ERβ knockout mice, and 4) reduced susceptibility to acoustic trauma in mice pre-treated with an ERβ agonist. Together, these findings raise the possibility of estrogen receptor-targeted approaches for treatments aimed at combating or preventing hearing loss.
An additional key role of E2 appears to be the augmentation of auditory processing during periods of reproductive readiness. Evidence for this idea is revealed by: 1) enhanced response properties of the anuran torus semicircularis during the breeding season and reduced responses after mating, 2) an E2-dependent shift in peripheral frequency tuning towards the dominant harmonic of the male advertisement call in female midshipman fish during the summer breeding season, 3) better auditory sensitivity of gravid cichlid fish compared to mouthbrooding females, 4) elevated neural activity and selectivity in HVC of canaries during long-day photoperiods, 5) increased response strengths of select cell populations in E2-treated white-crowned sparrows, 6) heightened ZENK and behavioral selectivity for conspecific songs in E2-treated white-throated sparrows and reproductively active black-capped chickadees, and 7) enhanced auditory sensitivity of women outside of menstruation. Collectively, these findings strongly imply that estrogen-mediated auditory plasticity is widespread among the vertebrate lineage, and plays a crucial role in optimizing behavioral responses to courtship-related acoustic cues.
Finally, this review has highlighted the importance of brain-derived E2 in regulating auditory responses in the zebra finch forebrain. Here, exposure to conspecific vocalizations upregulates the synthesis of E2 in NCM, which acts rapidly via a membrane-bound receptor to enhance sound-evoked cellular properties Additional research is needed to determine whether this finding is unique to zebra finches, or is a common regulatory mechanism that may have clinical implications.
While it may be far-fetched to imply that estrogenic mechanisms in the myriad of species discussed here have direct correlates in humans, it is hoped that this review will nonetheless provoke thought on potential comprehensive strategies towards the restoration of auditory function in patients with hormonal imbalances.
Highlights.
Estrogens influence mammalian auditory development
Estrogens protect the mammalian auditory system from damage
Estrogens enhance auditory function during breeding periods
Brain-derived estrogens can enhance local response properties in the avian forebrain
Acknowledgments
I am deeply indebted to Dr. Vibhu Kotak, Dr. Dennis McFadden, one anonymous reviewer, and Dr. Jacques Balthazart for their insightful and constructive comments on this manuscript. This work was supported by NIH/NIDCD F31DC010938, the Achievement Rewards for College Scientists (ARCS) Foundation, and the Washington Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM. Alteration in auditory function during the ovarian cycle. Hear Res. 2010;268:114–122. doi: 10.1016/j.heares.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Andreyko JL, Jaffe RB. Use of a gonadotropin-releasing hormone agonist analogue for treatment of cyclic auditory dysfunction. Obstet Gynecol. 1989;74:506–509. [PubMed] [Google Scholar]
- Appeltants D, Del Negro C, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res. 2002;133:221–235. doi: 10.1016/s0166-4328(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Arch VS, Narins PM. Sexual hearing: the influence of sex hormones on acoustic communication in frogs. Hear Res. 2009;252:15–20. doi: 10.1016/j.heares.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- Bass AH. Steroid-dependent plasticity of vocal motor systems: novel insights from teleost fish. Brain Res Rev. 2008;57:299–308. doi: 10.1016/j.brainresrev.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Bell A. Circadian and menstrual rhythms in frequency variations of spontaneous otoacoustic emissions from human ears. Hear Res. 1992;58:91–100. doi: 10.1016/0378-5955(92)90012-c. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Brackenbury JH. Power capabilities of the avian sound-producing system. J Exp Biol. 1979;78:163–166. [Google Scholar]
- Brenowitz EA. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the song control system in adult birds. In: Zeigler P, Marler P, editors. Neuroscience of birdsong. New York: Cambridge University Press; 2008. p. 332. [Google Scholar]
- Burns EM, Arehart KH, Campbell SL. Prevalence of spontaneous otoacoustic emissions in neonates. J Acoust Soc Am. 1992;91:1571. doi: 10.1121/1.402438. [DOI] [PubMed] [Google Scholar]
- Caras ML, Brenowitz E, Rubel EW. Peripheral auditory processing changes seasonally in Gambel's white-crowned sparrow. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:581–599. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras ML, O'Brien M, Brenowitz EB, Rubel EW. Estradiol selectively enhances auditory function in avian forebrain neurons. J Neurosci. 2012;32:17597–17611. doi: 10.1523/JNEUROSCI.3938-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Rugolo S, Intelisano G, Farina M, Cocuzza S, Serra A. Auditory brainstem response in premenopausal women taking oral contraceptives. Human Reproduction. 2003a;18:85–89. doi: 10.1093/humrep/deg003. [DOI] [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Agnello C, Garozzo A, Di Mari L, Serra A. Effects of patch or gel estrogen therapies on auditory brainstem response in surgically postmenopausal women: a prospective, randomized study. Fertil Steril. 2003b;79:556–561. doi: 10.1016/s0015-0282(02)04763-5. [DOI] [PubMed] [Google Scholar]
- Chakraborty M, Burmeister SS. Sexually dimorphic androgen and estrogen receptor mRNA expression in the brain of túngara frogs. Horm Behav. 2010;58:619–627. doi: 10.1016/j.yhbeh.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptor modulation of synaptic function. Curr Opin Neurobiol. 2011;21:339–344. doi: 10.1016/j.conb.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Tahera Y, Canlon B. Functional responses of estrogen receptors in the male and female auditory system. Hear Res. 2009;252:71–78. doi: 10.1016/j.heares.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Canlon B. Estrogen receptors in the central auditory system of male and female mice. Neuroscience. 2010;165:923–933. doi: 10.1016/j.neuroscience.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Frisina RD, Vasilyeva ON, Zhu X, Canlon B. Expression patterns of estrogen receptors in the central auditory system change in prepubertal and aged mice. Neuroscience. 2010;170:1270–1281. doi: 10.1016/j.neuroscience.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Canlon B. Estradiol treatment and hormonal fluctuations during the estrous cycle modulate the expression of estrogen receptors in the auditory system and the prepulse inhibition of acoustic startle response. Endocrinology. 2012 doi: 10.1210/en.2012-1416. [DOI] [PubMed] [Google Scholar]
- Chow RW, Handelsman DJ, Ng MK. Minireview: rapid actions of sex steroids in the endothelium. Endocrinology. 2010;151:2411–2422. doi: 10.1210/en.2009-1456. [DOI] [PubMed] [Google Scholar]
- Chung DY, Mason K, Gannon RP, Willson GN. The ear effect as a function of age and hearing loss. J Acoust Soc Am. 1983;73:1277. doi: 10.1121/1.389276. [DOI] [PubMed] [Google Scholar]
- Clint EK, Sober E, Garland T, Jr, Rhodes JS. Male superiority in spatial navigation: adaptation or side effect? The Quarterly review of biology. 2012;87:289–313. doi: 10.1086/668168. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Mohr RA, Sisneros JA. Saccular-specific hair cell addition correlates with reproductive state-dependent changes in the auditory saccular sensitivity of a vocal fish. J Neurosci. 2012;32:1366–1376. doi: 10.1523/JNEUROSCI.4928-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80:209–215. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Cooper WA, Ross KC, Coleman JR. Estrogen treatment and age effects on auditory brainstem responses in the post-breeding Long-Evans rat. Audiology. 1999;38:7–12. doi: 10.3109/00206099909072996. [DOI] [PubMed] [Google Scholar]
- Cox JR. Hormonal influence on auditory function. Ear Hear. 1980;1:219–222. doi: 10.1097/00003446-198007000-00008. [DOI] [PubMed] [Google Scholar]
- Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- Dehan CP, Jerger J. Analysis of gender differences in the auditory brainstem response. Laryngoscope. 1990;100:18–24. doi: 10.1288/00005537-199001000-00005. [DOI] [PubMed] [Google Scholar]
- Del Negro C, Kreutzer M, Gahr M. Sexually stimulating signals of canary (Serinus canaria) songs: evidence for a female-specific auditory representation in the HVc nucleus during the breeding season. Behav Neurosci. 2000;114:526–542. doi: 10.1037//0735-7044.114.3.526. [DOI] [PubMed] [Google Scholar]
- Del Negro C, Edeline JM. Sex and season influence the proportion of thin spike cells in the canary HVc. Neuroreport. 2002;13:2005–2009. doi: 10.1097/00001756-200211150-00003. [DOI] [PubMed] [Google Scholar]
- Del Negro C, Lehongre K, Edeline JM. Selectivity of canary HVC neurons for the bird's own song: modulation by photoperiodic conditions. J Neurosci. 2005;25:4952–4963. doi: 10.1523/JNEUROSCI.4847-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Koch M, Haack B, Markl H. Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften. 1987;74:47. doi: 10.1007/BF00367047. [DOI] [PubMed] [Google Scholar]
- Ehret G, Koch M. Ultrasound-induced parental behaviour in house mice is controlled by female sex hormones and parental experience. Ethology. 1989;80:81–93. [Google Scholar]
- Ehret G, Schmid C. Reproductive cycle-dependent plasticity of perception of acoustic meaning in mice. Physiol Behav. 2009;96:428–433. doi: 10.1016/j.physbeh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Eldredge L, Salamy A. Functional auditory development in preterm and full term infants. Early Hum Dev. 1996;45:215–228. doi: 10.1016/0378-3782(96)01732-x. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch KE, Wallace E, Stach BA, Jerger JF. Cyclic steroid replacement alters auditory brainstem responses in young women with premature ovarian failure. Hear Res. 1992a;64:93–98. doi: 10.1016/0378-5955(92)90171-i. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch KE, Stoner WR, Stach BA, Jerger JF. Estrogen influences auditory brainstem responses during the normal menstrual cycle. Hear Res. 1992b;60:143–148. doi: 10.1016/0378-5955(92)90016-g. [DOI] [PubMed] [Google Scholar]
- Fichtel I, Ehret G. Perception and recognition discriminated in the mouse auditory cortex by c-Fos labeling. Neuroreport. 1999;10:2341–2345. doi: 10.1097/00001756-199908020-00022. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner-ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Fukui I, Burger RM, Ohmori H, Rubel EW. GABAergic inhibition sharpens the frequency tuning and enhances phase locking in chicken nucleus magnocellularis neurons. J Neurosci. 2010;30:12075–12083. doi: 10.1523/JNEUROSCI.1484-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Leon EE, Lin FG, Liu RC. Inhibitory plasticity in a lateral band improves cortical detection of natural vocalizations. Neuron. 2009;62:705–716. doi: 10.1016/j.neuron.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall MD, Salameh TS, Lucas JR. Songbird frequency selectivity and temporal resolution vary with sex and season. Proc Biol Sci. 2013;280:20122296. doi: 10.1098/rspb.2012.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas D, Callard GV. Immunolocalization of aromatase- and androgen receptor-positive neurons in the goldfish brain. Gen Comp Endocrinol. 1997;106:155–168. doi: 10.1006/gcen.1997.6891. [DOI] [PubMed] [Google Scholar]
- Goense JB, Feng AS. Seasonal changes in frequency tuning and temporal processing in single neurons in the frog auditory midbrain. J Neurobiol. 2005;65:22–36. doi: 10.1002/neu.20172. [DOI] [PubMed] [Google Scholar]
- Goh AY, Hussain SS. Sudden hearing loss and pregnancy: a review. J Laryngol Otol. 2012;126:337–339. doi: 10.1017/S0022215112000114. [DOI] [PubMed] [Google Scholar]
- Green SH, Bailey E, Wang Q, Davis RL. The Trk A, B, C's of neurotrophins in the cochlea. Anat Rec (Hoboken) 2012;295:1877–1895. doi: 10.1002/ar.22587. [DOI] [PubMed] [Google Scholar]
- Grothe B, Koch U. Dynamics of binaural processing in the mammalian sound localization pathway--the role of GABA(B) receptors. Hear Res. 2011;279:43–50. doi: 10.1016/j.heares.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Gurr P, Owen G, Reid A, Canter R. Tinnitus in pregnancy. Clinical Otolaryngology & Allied Sciences. 1993;18:294–297. doi: 10.1111/j.1365-2273.1993.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Guski R. Acoustic tau: An easy analogue to visual tau? Ecological Psychology. 1992;4:189–197. [Google Scholar]
- Güngör N, Böke B, Belgin E, Tunçbilek E. High frequency hearing loss in Ullrich-Turner syndrome. Eur J Pediatr. 2000;159:740–744. doi: 10.1007/pl00008338. [DOI] [PubMed] [Google Scholar]
- Haggard M, Gaston JB. Changes in auditory perception in the menstrual cycle. Br J Audiol. 1978;12:105–118. doi: 10.3109/03005367809078862. [DOI] [PubMed] [Google Scholar]
- Hall JW. New Handbook of Auditory Evoked Responses. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- Hederstierna C, Hultcrantz M, Collins A, Rosenhall U. Hearing in women at menopause. Prevalence of hearing loss, audiometric configuration and relation to hormone replacement therapy. Acta Otolaryngol. 2007;127:149–155. doi: 10.1080/00016480600794446. [DOI] [PubMed] [Google Scholar]
- Hederstierna C, Hultcrantz M, Rosenhall U. Estrogen and hearing from a clinical point of view; characteristics of auditory function in women with Turner syndrome. Hear Res. 2009a;252:3–8. doi: 10.1016/j.heares.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Hederstierna C, Hultcrantz M, Rosenhall U. A longitudinal study of hearing decline in women with Turner syndrome. Acta Otolaryngol. 2009b;129:1434–1441. doi: 10.3109/00016480902741962. [DOI] [PubMed] [Google Scholar]
- Helena CV, de Oliveira Poletini M, Sanvitto GL, Hayashi S, Franci CR, Anselmo-Franci JA. Changes in alpha-estradiol receptor and progesterone receptor expression in the locus coeruleus and preoptic area throughout the rat estrous cycle. J Endocrinol. 2006;188:155–165. doi: 10.1677/joe.1.06268. [DOI] [PubMed] [Google Scholar]
- Henry KS, Lucas JR. Vocally correlated seasonal auditory variation in the house sparrow (Passer domesticus) J Exp Biol. 2009;212:3817–3822. doi: 10.1242/jeb.033035. [DOI] [PubMed] [Google Scholar]
- Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–1379. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- Hillery M. Seasonality of Two Midbrain Auditory Responses in the Treefrog, Hyla chrysoscelis. Copeia. 1984;4:844–852. [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgens ED, Ji L, Carpenter CD, Petersen SL. The gad2 promoter is a transcriptional target of estrogen receptor (ER)alpha and ER beta: a unifying hypothesis to explain diverse effects of estradiol. J Neurosci. 2009;29:8790–8797. doi: 10.1523/JNEUROSCI.1289-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultcrantz M, Sylvén L, Borg E. Ear and hearing problems in 44 middle-aged women with Turner's syndrome. Hear Res. 1994;76:127–132. doi: 10.1016/0378-5955(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Sylven L. Turner's syndrome and hearing disorders in women aged 16-34. Hear Res. 1997;103:69–74. doi: 10.1016/s0378-5955(96)00165-7. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M. Ear and hearing problems in Turner's syndrome. Acta Otolaryngol. 2003;123:253–257. doi: 10.1080/00016480310001097. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Burrows K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. Eur J Neurosci. 2011;34:283–291. doi: 10.1111/j.1460-9568.2011.07743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Archives of Otolaryngology—Head & Neck Surgery. 1980;106:387. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Jerger J, Johnson K. Interactions of age, gender, and sensorineural hearing loss on ABR latency. Ear Hear. 1988;9:168. doi: 10.1097/00003446-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Jewett DL, Romano MN, Williston JS. Human auditory evoked potentials: possible brain stem components detected on the scalp. Science. 1970;167:1517–1518. doi: 10.1126/science.167.3924.1517. [DOI] [PubMed] [Google Scholar]
- Jewett DL, Williston JS. Auditory-evoked far fields averaged from the scalp of humans. Brain. 1971;94:681–696. doi: 10.1093/brain/94.4.681. [DOI] [PubMed] [Google Scholar]
- Jönsson R, Rosenhall U, Gause-Nilsson I, Steen B. Auditory function in 70- and 75-year-olds of four age cohorts. A cross-sectional and time-lag study of presbyacusis. Scand Audiol. 1998;27:81–93. doi: 10.1080/010503998420324. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Br Med Bull. 2002;63:223–241. doi: 10.1093/bmb/63.1.223. [DOI] [PubMed] [Google Scholar]
- Kenny R, Patil N, Considine N. Sudden (reversible) sensorineural hearing loss in pregnancy. Ir J Med Sci. 2011;180:79–84. doi: 10.1007/s11845-010-0525-z. [DOI] [PubMed] [Google Scholar]
- Khaliq F, Tandon OP, Goel N. Auditory evoked responses in postmenopausal women on hormone replacement therapy. Indian J Physiol Pharmacol. 2003;47:393–399. [PubMed] [Google Scholar]
- Khaliq F, Tandon OP, Goel N. Differential effects of exogenous estrogen versus a estrogen-progesterone combination on auditory evoked potentials in menopausal women. Indian J Physiol Pharmacol. 2005;49:345–352. [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Bagis T, Tarim E, Erkan AN, Kazanci F. Effects of estrogen therapy on hearing in postmenopausal women. Am J Obstet Gynecol. 2004;190:77–82. doi: 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang BM, Chae HD, Kim CH. The association between serum estradiol level and hearing sensitivity in postmenopausal women. Obstet Gynecol. 2002;99:726–730. doi: 10.1016/s0029-7844(02)01963-4. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol. 2002;88:1941–1954. doi: 10.1152/jn.2002.88.4.1941. [DOI] [PubMed] [Google Scholar]
- Laws DW, Moon CE. Effects of the menstrual cycle on the human acoustic reflex threshold. J Aud Res. 1986;26:197–206. [PubMed] [Google Scholar]
- LeBeau FE, Malmierca MS, Rees A. Iontophoresis in vivo demonstrates a key role for GABA(A) and glycinergic inhibition in shaping frequency response areas in the inferior colliculus of guinea pig. J Neurosci. 2001;21:7303–7312. doi: 10.1523/JNEUROSCI.21-18-07303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- Lewald J. Gender-specific hemispheric asymmetry in auditory space perception. Brain Res Cogn Brain Res. 2004;19:92–99. doi: 10.1016/j.cogbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Krishnan A, Long GR. A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:981–992. doi: 10.1007/s00359-002-0359-x. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Long GR, Krishnan A. Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:201–215. doi: 10.1007/s00359-006-0180-z. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Social regulation of plasma estradiol concentration in a female anuran. Horm Behav. 2006;50:101–106. doi: 10.1016/j.yhbeh.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KS, Diekamp B, Ball GF. Colocalization of immediate early genes in catecholamine cells after song exposure in female zebra finches(Taeniopygia guttata) Brain Behav Evol. 2012;79:252–260. doi: 10.1159/000337533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney D, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol. 2011;32:287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311:189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Steroid receptor expression in the fish inner-ear varies with sex, social status, and reproductive state. BMC Neurosci. 2010;11:58. doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Ung US, Fernald RD. The African cichlid fish Astatotilapia burtoni uses acoustic communication for reproduction: sound production, hearing, and behavioral significance. PLoS One. 2012;7:e37612. doi: 10.1371/journal.pone.0037612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Sockman KW, Maney DL. Estradiol-dependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird. Eur J Neurosci. 2011;34:416–425. doi: 10.1111/j.1460-9568.2011.07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Beaulieu M, Sockman KW, Maney DL. Estradiol-dependent modulation of serotonergic markers in auditory areas of a seasonally breeding songbird. Behav Neurosci. 2012a;126:110–122. doi: 10.1037/a0025586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Beaulieu M, Phillip JO, Rae AI, Sanford SE, Sockman KW, Maney DL. Rapid effects of hearing song on catecholaminergic activity in the songbird auditory pathway. PLoS One. 2012b;7:e39388. doi: 10.1371/journal.pone.0039388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M, Ottaviani F, Paludetti G, Almadori G, Pierri F, Rosignoli M. Effects of sex on auditory brainstem responses in infancy and early childhood. Scand Audiol. 1988;17:143–146. doi: 10.3109/01050398809042184. [DOI] [PubMed] [Google Scholar]
- Mayko ZM, Roberts PD, Portfors CV. Inhibition shapes selectivity to vocalizations in the inferior colliculus of awake mice. Front Neural Circuits. 2012;6:73. doi: 10.3389/fncir.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Kaufman LC, Brooks PJ, Pfaff DW, Schwartz-Giblin S. Estrogen modulation of mRNA levels for the two forms of glutamic acid decarboxylase (GAD) in female rat brain. J Comp Neurol. 1995;360:685–697. doi: 10.1002/cne.903600412. [DOI] [PubMed] [Google Scholar]
- McFadden D. A masculinizing effect on the auditory systems of human females having male co-twins. Proc Natl Acad Sci U S A. 1993;90:11900–11904. doi: 10.1073/pnas.90.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Loehlin JC. On the heritability of spontaneous otoacoustic emissions: A twins study. Hear Res. 1995;85:181–198. doi: 10.1016/0378-5955(95)00045-6. [DOI] [PubMed] [Google Scholar]
- McFadden D, Loehlin JC, Pasanen EG. Additional findings on heritability and prenatal masculinization of cochlear mechanisms: click-evoked otoacoustic emissions. Hear Res. 1996;97:102–119. [PubMed] [Google Scholar]
- McFadden D. Sex differences in the auditory system. Developmental Neuropsychology. 1998;14:261–298. [Google Scholar]
- McFadden D, Pasanen EG. Comparison of the auditory systems of heterosexuals and homosexuals: Click-evoked otoacoustic emissions. Proceedings of the National Academy of Sciences. 1998;95:2709–2713. doi: 10.1073/pnas.95.5.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Spontaneous otoacoustic emissions in heterosexuals, homosexuals, and bisexuals. J Acoust Soc Am. 1999;105:2403–2413. doi: 10.1121/1.426845. [DOI] [PubMed] [Google Scholar]
- McFadden D, Champlin CA. Comparison of auditory evoked potentials in heterosexual, homosexual, and bisexual males and females. J Assoc Res Otolaryngol. 2000;1:89–99. doi: 10.1007/s101620010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D. Masculinization effects in the auditory system. Arch Sex Behav. 2002;31:99–111. doi: 10.1023/a:1014087319682. [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, Raper J, Lange HS, Wallen K. Sex differences in otoacoustic emissions measured in rhesus monkeys (Macaca mulatta) Horm Behav. 2006;50:274–284. doi: 10.1016/j.yhbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- McFadden D. What do sex, twins, spotted hyenas, ADHD, and sexual orientation have in common? Perspectives on Psychological Science. 2008;3:309–323. doi: 10.1111/j.1745-6924.2008.00082.x. [DOI] [PubMed] [Google Scholar]
- McFadden D. Masculinization of the mammalian cochlea. Hear Res. 2009;252:37–48. doi: 10.1016/j.heares.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Martin GK, Stagner BB, Maloney MM. Sex differences in distortion-product and transient-evoked otoacoustic emissions compared. J Acoust Soc Am. 2009a;125:239–246. doi: 10.1121/1.3037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, Valero MD, Roberts EK, Lee TM. Effect of prenatal androgens on click-evoked otoacoustic emissions in male and female sheep (Ovis aries) Horm Behav. 2009b;55:98–105. doi: 10.1016/j.yhbeh.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Hsieh MD, Garcia-Sierra A, Champlin CA. Differences by sex, ear, and sexual orientation in the time intervals between successive peaks in auditory evoked potentials. Hear Res. 2010;270:56–64. doi: 10.1016/j.heares.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D. Sexual orientation and the auditory system. Front Neuroendocrinol. 2011;32:201–213. doi: 10.1016/j.yfrne.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Thompson CK. Seasonal-like growth and regression of the avian song control system: neural and behavioral plasticity in adult male Gambel's white-crowned sparrows. Gen Comp Endocrinol. 2008;157:259–265. doi: 10.1016/j.ygcen.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Velho TA, Pinaud R. Song-induced gene expression: a window on song auditory processing and perception. Ann N Y Acad Sci. 2004;1016:263–281. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- Miller MH, Gould WJ. Fluctuating sensorineural hearing impairment associated with the menstrual cycle. Journal of Auditory Research. 1967;7:373–385. [Google Scholar]
- Miranda JA, Liu RC. Dissecting natural sensory plasticity: hormones and experience in a maternal context. Hear Res. 2009;252:21–28. doi: 10.1016/j.heares.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Female reproductive state influences the auditory midbrain response. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:341–349. doi: 10.1007/s00359-008-0410-7. [DOI] [PubMed] [Google Scholar]
- Motohashi R, Takumida M, Shimizu A, Konomi U, Fujita K, Hirakawa K, Suzuki M, Anniko M. Effects of age and sex on the expression of estrogen receptor alpha and beta in the mouse inner-ear. Acta Otolaryngol. 2010;130:204–214. doi: 10.3109/00016480903016570. [DOI] [PubMed] [Google Scholar]
- Mukhophadhyay S, Biswas S, Vindla S. Severe tinnitus in pregnancy, necessitating caesarean delivery. J Obstet Gynaecol. 2007;27:81–82. doi: 10.1080/01443610601062689. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff JG, Planisek R, Seifritz E. Adaptive sex differences in auditory motion perception: looming sounds are special. J Exp Psychol Hum Percept Perform. 2009;35:225–234. doi: 10.1037/a0013159. [DOI] [PubMed] [Google Scholar]
- Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, Balthazart J, Ball GF. Presence of aromatase and estrogen receptor alpha in the inner-ear of zebra finches. Hear Res. 2009;252:49–55. doi: 10.1016/j.heares.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Pawlisch BA, Stevenson SA, Riters LV. α1 -Noradrenegic receptor antagonism disrupts female songbird responses to male song. Neurosci Lett. 2011;496:20–24. doi: 10.1016/j.neulet.2011.03.078. [DOI] [PubMed] [Google Scholar]
- Penner MJ. Frequency variation of spontaneous otoacoustic emissions during a naturally occurring menstrual cycle, amenorrhea, and oral contraception: a brief report. Ear Hear. 1995;16:428–432. doi: 10.1097/00003446-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillmore LS, Veysey AS, Roach SP. Zenk expression in auditory regions changes with breeding condition in male Black-capped chickadees (Poecile atricapillus) Behav Brain Res. 2011;225:464–472. doi: 10.1016/j.bbr.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Price K, Zhu X, Guimaraes PF, Vasilyeva ON, Frisina RD. Hormone replacement therapy diminishes earing in peri-mesal mice. Hear Res. 2009;252:29–36. doi: 10.1016/j.heares.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Grolman W, Klis SF. Neurotrophins and their role in the cochlea. Hear Res. 2012;288:19–33. doi: 10.1016/j.heares.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Pawlisch BA. Evidence that norepine ences responses to male courtship song and activity within song control regions and the ventromedial nucleus of the hypothalamus in female European starlings. Brain Res. 2007;1149:127–140. doi: 10.1016/j.brainres.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Rohmann KN, Bass AH. Seasonal plasticity of auditory hair cell frequency sensitivity correlates with plasma steroid levels in vocal fish. J Exp Biol. 2011;214:1931–1942. doi: 10.1242/jeb.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopan S, Wang X. Level invariant representation of sounds by populations of neurons in primary auditory cortex. J Neurosci. 2008;28:3415–3426. doi: 10.1523/JNEUROSCI.2743-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford SE, Lange HS, Maney DL. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Dev Neurobiol. 2010;70:73–86. doi: 10.1002/dneu.20757. [DOI] [PubMed] [Google Scholar]
- Sator MO, Franz P, Egarter C, Gruber DM, Wölfl G, Nagele F. Effects of tibolone on auditory brainstem responses in postmenopausal women--a randomized, double-blind, placebo-controlled trial. Fertil Steril. 1999;72:885–888. doi: 10.1016/s0015-0282(99)00373-8. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Remage-Healey L. Neurosteroidogenesis: insights from studies of songbirds. J Neuroendocrinol. 2012;24:16–21. doi: 10.1111/j.1365-2826.2011.02150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PM, Flores Fda T, Rossi AG, Silveira AF. Hearing and vestibular complaints during pregnancy. Braz J Otorhinolaryngol. 2010;76:29–33. doi: 10.1590/S1808-86942010000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennaroglu G, Belgin E. Main articles audiological findings in pregnancy. The Journal of Laryngology & Otology. 2001;115:617–621. doi: 10.1258/0022215011908603. [DOI] [PubMed] [Google Scholar]
- Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004;1015:1–8. doi: 10.1016/j.brainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Simonoska R, Stenberg A, Masironi B, Sahlin L, Hultcrantz M. Estrogen receptors in the inner ear during different stages of pregnancy and development in the rat. Acta Otolaryngol. 2009;129:1175–1181. doi: 10.3109/00016480802691150. [DOI] [PubMed] [Google Scholar]
- Simonoska R, Stenberg AE, Duan M, Yakimchuk K, Fridberger A, Sahlin L, Gustafsson JA, Hultcrantz M. Inner ear pathology and loss of hearing in estrogen receptor-beta deficient mice. J Endocrinol. 2009b;201:397–406. doi: 10.1677/JOE-09-0060. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. J Neurosci. 2003;23:1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Sisneros JA. Adaptive hearing in the vocal plainfin midshipman fish: getting in tune for the breeding season and implications for acoustic communication. Integr Zool. 2009a;4:33–42. doi: 10.1111/j.1749-4877.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- Sisneros JA. Seasonal plasticity of auditory saccular sensitivity in the vocal plainfin midshipman fish, Porichthys notatus. J Neurophysiol. 2009b;102:1121–1131. doi: 10.1152/jn.00236.2009. [DOI] [PubMed] [Google Scholar]
- Sisneros JA. Steroid-dependent auditory plasticity for the enhancement of acoustic communication: recent insights from a vocal teleost fish. Hear Res. 2009c;252:9–14. doi: 10.1016/j.heares.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snihur AW, Hampson E. Sex and ear differences in spontaneous and click-evoked otoacoustic emissions in young adults. Brain Cogn. 2011;77:40–47. doi: 10.1016/j.bandc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Snihur AW, Hampson E. Oral contraceptive use in women is associated with defeminization of otoacoustic emission patterns. Neuroscience. 2012;210:258–265. doi: 10.1016/j.neuroscience.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souaid JP, Rappaport JM. Fluctuating sensorineural hearing loss associated with the menstrual cycle. The Journal of otolaryngology. 2001;30:246. doi: 10.2310/7070.2001.20181. [DOI] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van De Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Nylén O, Windh M, Hultcrantz M. Otological problems in children with Turner's syndrome. Hear Res. 1998;124:85–90. doi: 10.1016/s0378-5955(98)00113-0. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Wang H, Fish J, 3, Schrott-Fischer A, Sahlin L, Hultcrantz M. Estrogen receptors in the normal adult and developing human inner-ear and in Turner's syndrome. Hear Res. 2001;157:87–92. doi: 10.1016/s0378-5955(01)00280-5. [DOI] [PubMed] [Google Scholar]
- Suga N, Zhang Y, Yan J. Sharpening of frequency tuning by inhibition in the thalamic auditory nucleus of the mustached bat. J Neurophysiol. 1997;77:2098–2114. doi: 10.1152/jn.1997.77.4.2098. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Dengerink HA. Changes in pure-tone thresholds and temporary threshold shifts as a function of menstrual cycle and oral contraceptives. J Speech Hear Res. 1988;31:569–574. [PubMed] [Google Scholar]
- Talmadge CL, Long GR, Murphy WJ, Tubis A. New off-line method for detecting spontaneous otoacoustic emissions in human subjects. Hear Res. 1993;71:170–182. doi: 10.1016/0378-5955(93)90032-v. [DOI] [PubMed] [Google Scholar]
- Tandon OP, Misra R, Tandon I. Brainstem auditory evoked potentials (BAEPs) in pregnant women. Indian J Physiol Pharmacol. 1990;34:42–44. [PubMed] [Google Scholar]
- Tobias JV. Consistency of sex differences in binaural-beat perception. International Journal of Audiology. 1965;4:179–182. [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Burrows K, Jeong JK, Pinaud R. Organization of estrogen-associated circuits in the mouse primary auditory cortex. J Exp Neurosci. 2011;2011:45–60. doi: 10.4137/JEN.S7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda K, Takahashi S, Takanosawa M, Shimoji Y. The influence of pregnancy on sensation of ear problems--ear problems associated with healthy pregnancy. J Laryngol Otol. 1999;113:318–320. doi: 10.1017/s0022215100143877. [DOI] [PubMed] [Google Scholar]
- Vasconcelos RO, Sisneros JA, Amorim MC, Fonseca PJ. Auditory saccular sensitivity of the vocal Lusitanian toadfish: low frequency tuning allows acoustic communication throughout the year. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011;197:903–913. doi: 10.1007/s00359-011-0651-8. [DOI] [PubMed] [Google Scholar]
- Velho TA, Lu K, Ribeiro S, Pinaud R, Vicario D, Mello CV. Noradrenergic control of gene expression and long-term neuronal adaptation evoked by learned vocalizations in songbirds. PLoS One. 2012;7:e36276. doi: 10.1371/journal.pone.0036276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS. Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J Anim Sci. 1989;67:1824–1840. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Harding C, McGowan J, Snare R, Bogdan D. Noradrenergic neurotoxin, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4), treatment eliminates estrogenic effects on song responsiveness in female zebra finches (Taeniopygia guttata) Behav Neurosci. 2008;122:1148–1157. doi: 10.1037/0735-7044.122.5.1148. [DOI] [PubMed] [Google Scholar]
- Walkowiak W. The coding of auditory signals in the torus semicircularis of the fire-bellied toad and the grass frog: responses to simple stimuli and to conspecific calls. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1980;138:131–148. [Google Scholar]
- Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- Wharton JA, Church GT. Influence of menopause on the auditory brainstem response. Audiology. 1990;29:196–201. doi: 10.3109/00206099009072850. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Allison JD, Marler CA. Sensory pathways linking social and environmental cues to endocrine control regions of amphibian forebrains. Brain Behav Evol. 1993;42:252–264. doi: 10.1159/000114159. [DOI] [PubMed] [Google Scholar]
- Xie R, Meitzen J, Pollak GD. Differing roles of inhibition in hierarchical processing of species-specific calls in auditory brainstem nuclei. J Neurophysiol. 2005;94:4019–4037. doi: 10.1152/jn.00688.2005. [DOI] [PubMed] [Google Scholar]
- Yang L, Pollak GD, Resler C. GABAergic circuits sharpen tuning curves and modify response properties in the mustache bat inferior colliculus. J Neurophysiol. 1992;68:1760–1774. doi: 10.1152/jn.1992.68.5.1760. [DOI] [PubMed] [Google Scholar]
- Yovanof S, Feng AS. Effects of estradiol on auditory evoked responses from the frog's auditory midbrain. Neurosci Lett. 1983;36:291–297. doi: 10.1016/0304-3940(83)90015-0. [DOI] [PubMed] [Google Scholar]
- Zakon HH. The effects of steroid hormones on electrical activity of excitable cells. Trends Neurosci. 1998;21:202–207. doi: 10.1016/s0166-2236(97)01209-5. [DOI] [PubMed] [Google Scholar]
- Zeyl JN, Love OP, Higgs DM. Condition-dependent auditory processing in the round goby (Neogobius melanostomus): links to sex, reproductive condition, and female estrogen levels. J Exp Biol. 2012;216:1075–1084. doi: 10.1242/jeb.076935. [DOI] [PubMed] [Google Scholar]
- Zornik E, Kelley DB. A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Front Neuroendocrinol. 2011;32:353–366. doi: 10.1016/j.yfrne.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotti A, Kuhn S, Johnson SL, Franz C, Singer W, Hecker D, Geisler HS, Köpschall I, Rohbock K, Gutsche K, Dlugaiczyk J, Schick B, Marcotti W, Rüttiger L, Schimmang T, Knipper M. Lack of brain-derived neurotrophic factor hampers inner hair cell synapse physiology, but protects against noise-induced hearing loss. J Neurosci. 2012;32:8545–8553. doi: 10.1523/JNEUROSCI.1247-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zündorf IC, Karnath HO, Lewald J. Male advantage in sound localization at cocktail parties. Cortex. 2011;47:741–749. doi: 10.1016/j.cortex.2010.08.002. [DOI] [PubMed] [Google Scholar]