Abstract
Much of the literature on maternal behavior has focused on the role of infant experience and hormones in a canonical subcortical circuit for maternal motivation and maternal memory. Although early studies demonstrated that the cerebral cortex also plays a significant role in maternal behaviors, little has been done to explore what that role may be. Recent work though has provided evidence that the cortex, particularly sensory cortices, contains correlates of sensory memories of infant cues, consistent with classical studies of experience-dependent sensory cortical plasticity in non-maternal paradigms. By reviewing the literature from both the maternal behavior and sensory cortical plasticity fields, focusing on the auditory modality, we hypothesize that maternal hormones (predominantly estrogen) may act to prime auditory cortical neurons for a longer-lasting neural trace of infant vocal cues, thereby facilitating recognition and discrimination. This could then more efficiently activate the subcortical circuit to elicit and sustain maternal behavior.
Keywords: Estrogen, estradiol, maternal behavior, experience-dependent plasticity, auditory cortex, parenting, infant, natural stimulus, vocalization, memory
1. Introduction
Parental behaviors are an evolutionarily conserved social behavior observed across vertebrate species (Numan and Insel, 2003). These behaviors first arise after birth when parents respond to sensory cues from their offspring and are maintained until infants reach independence. In mothers, these behaviors are facilitated by a slew of hormonal changes that alter her overall physiological state during parturition. The major hormonal changes that accompany parturition are a surge of steroid hormones, namely estrogen and progesterone, which in turn modulate the release of other key neurochemicals like oxytocin and prolactin (Kendrick, 2000; Kinsley, 2008; Neumann, 2009). Changes in the levels of these steroid hormones and neurochemicals can then alter neural activity, gene expression, synaptic plasticity and neuronal structure within the circuitry of the maternal brain, ultimately initiating and retaining a mother’s behavioral responsiveness to infant cues.
Much of the work to understand the brain mechanisms of maternal behavioral has led to the delineation of a canonical hypothalamic-limbic circuit involved in the onset and maintenance of maternal responsiveness. This circuit – with key nodes in the medial preoptic area (MPOA), amygdala, ventral tegmental area (VTA), and nucleus accumbens (NAcc) – is critical for the motivational aspects of motherhood (Numan and Sheehan, 1997; Numan and Insel, 2003; Numan, 2007). However, even though some studies suggest a further role of this circuit in consolidating maternal memories (Lee et al., 1999b; Li and Fleming, 2003; Numan and Insel, 2003), we still do not completely understand where the long-term engram of offspring cues are stored. In particular, a usual assumption of the canonical model is that the neural input forward from sensory cortices representing these stimuli enters the subcortical circuit in the same way regardless of whether or not a female has been or is a mother. The main reason why mothers respond more positively to those cues than females that have never had or raised infants is then explained by intrinsic processing differences within the canonical circuit arising in part from hormonal modulation (Numan and Insel, 2003; Numan and Stolzenberg, 2009). However, this model does not fully incorporate the role of plasticity in the sensory input itself, which could arise as the cues from offspring become more salient. In fact, such sensory plasticity, possibly aided by hormonal priming in the maternal context, could be an important contributor to the formation and retention of maternal memories by providing a more efficient activation of the subcortical maternal circuit to more readily elicit the maternal responsiveness of experienced mothers.
The role of experience-dependent sensory cortical plasticity and its interplay with hormonal effects on neural circuits is a relatively new topic in the neurobiological research of both maternal behavior and sensory processing (Miranda and Liu, 2009; Pinaud and Tremere, 2012). Here, we make the argument for the view that sensory plasticity contributes to the establishment of maternal memories in order to facilitate the recognition of and differential response to infant cues. Since this topic lies at the intersection of otherwise separate bodies of research on sensory processing and maternal behavior, we try here to review the literature that may be unfamiliar to one or another side in the hopes that it helps explain the mutual interest in the topic, and spurs a deeper integration of the two. We begin by briefly reviewing work illustrating the importance of infant cues in triggering maternal behaviors, and the function of the canonical maternal motivation circuit in responding to those cues. We then discuss classic as well as recent studies implicating the cortex as a whole, and sensory cortex in particular, in maternal behavior and maternal memory. Although the processing of proximal sensory cues from offspring, such as olfactory and tactile stimuli, has been studied in more depth in the context of maternal behaviors, little is known about the processing of distal cues such as vocalizations that draw approach behavior. Hence, we focus here on the auditory cortex and its processing of vocal cues from offspring, using mice as a primary model, where more research has been conducted, and to which we have also contributed. We argue that experience-dependent plasticity, whose underlying neurobiology has been studied widely using (non-maternal) laboratory learning contexts, is likely a key mechanism in the maternal context through which a neural memory trace of offspring vocalizations is created in a mother’s auditory cortex. Finally, we discuss some recent data that leads us to hypothesize that this memory trace of offspring cues might be more robustly established in mothers through a combined role of rearing experience paired with a facilitatory action from steroid hormones like estrogen at parturition.
2. Importance of sensory processing for maternal behavior
Sensory stimulation by offspring is essential for the performance of maternal behaviors (Kinsley and Amory-Meyer, 2011; Lambert and Kinsley, 2012; Okabe et al., 2012). For example, olfactory cues from offspring act as powerful overall inhibitors or stimulators of maternal behavior in mammals. Female sheep are repelled by amniotic fluid throughout their estrous cycle and gestation, but for a few hours following parturition, they become attracted to it (Levy et al., 2004; Nowak et al., 2011). In fact, parturient ewes are more attracted to a fake lamb smeared with amniotic fluid than to one without amniotic fluid (Vince et al., 1985; Gonzalez-Mariscal, 2001), while anosmic female sheep are neither repelled by nor clearly attracted to amniotic fluid (Levy et al., 1983). Rabbits also display similar olfactory preferences in the maternal context: an absence of placentophagia in female rabbits is observed during estrus and pregnancy, whereas most females become placentophagic at parturition (Melo and Gonzalez-Mariscal, 2003). Even human mothers show olfactory preferences associated with motherhood. In one study, new mothers, 1-month postpartum mothers and nulliparous women were presented with a variety of infant and control odors, and were asked to provide hedonic ratings ranging from very pleasant to very unpleasant. In comparison to the other women, new mothers gave significantly more positive ratings to the infant body odors (Fleming et al., 1993). Finally, in some rodents like rats, offspring odors are initially aversive to virgins, but acquire a positive valence in mothers after parturition (Fleming et al., 1979; Kinsley and Bridges, 1990; Levy and Keller, 2009), when they become important cues for motivating maternal behaviors (Smotherman et al., 1974). In fact, changes in the interactions between olfactory and maternal systems are thought to underlie this new-found attractiveness by suppressing an intrinsic neural circuit for avoiding unfamiliar odors, which we describe further in Section 3.
As another example, vocal cues from offspring have also been shown to be key triggers for maternal behaviors, particularly for the distal recognition and localization of infants. For instance, when isolated from their mothers, lambs will emit bleats that vary in duration, mean frequency and spectral density of the calls. Ewes can use these acoustic features to individually recognize their own offspring from alien lambs (Searby and Jouventin, 2003). Similarly, sows can recognize and respond to their piglets by their calls (Maletinska et al., 2002). In humans, baby cries often reflect an infant’s different needs, and in response to these cries, both limbic and auditory processing areas of first-time mothers can show enhanced activation as measured by functional MRI (Lorberbaum et al., 2002; Swain et al., 2007; Swain et al., 2008; Del Vecchio et al., 2009; Landi et al., 2011; Montoya et al., 2012).
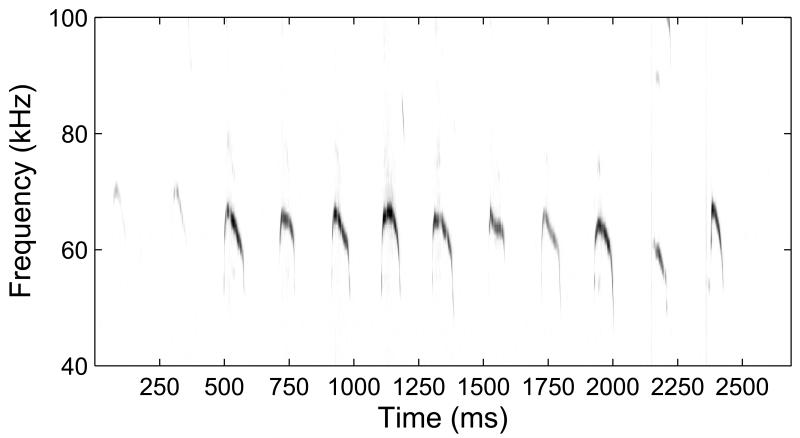
In rodents, which this review focuses on, pups from many species emit bouts of ultrasonic vocalizations (USVs) known as isolation calls when separated from the nest and fellow littermates (Sewell, 1968) (figure 1). Such vocalizations have been argued to arise as a result of dropping body temperature, since pups cannot fully thermoregulate until they reach approximately two weeks of age (Okon, 1970; Blumberg and Alberts, 1990; Blumberg et al., 1992). Others have made the case that they indicate anxiety or distress in the pup (Winslow and Insel, 1991; Hofer, 1996), or more general emotional arousal (Shair et al., 2003; Ehret, 2005). Regardless of their proximal cause, the calls are clearly communicative to those who have learned their meaning. Mothers and even pup-sensitized virgin female mice can recognize USVs and will respond by searching for pups and returning them to the nest (Sewell, 1970; Ehret et al., 1987). In the mouse maternal model, the playback of the calls alone is sufficient to elicit this approach behavior in motivated mothers (Haack et al., 1983; Lin et al., 2013), illustrating a key function for the auditory modality in the maternal context. On the other hand, virgin female mice do not naturally prefer the USVs and must learn their meaning through pup sensitization (Ehret, 1987), since even if they are instrumentally trained to respond to ultrasounds, they do not process them like communication sounds (Ehret, 1987). Furthermore, mouse pups are also known to emit other, lower frequency vocalizations known as wriggling calls when they are in the nest and trying to attach to the mother’s teats (Ehret and Riecke, 2002; Geissler and Ehret, 2002). These calls require different maternal responses (e.g. adjusting the nursing position, nest building and licking) from the search- and-retrieval elicited by USVs. This demonstrates that the auditory system needs to discriminate between distinct categories of offspring vocalizations to drive appropriate maternal responses.
Figure 1.
Example spectrogram of a USV bout from an individual postnatal day 7 mouse pup. Darker colors correspond to higher intensities in the sound signal at corresponding frequencies. These calls concentrate in the 60-80 kHz range, and feature moderate frequency modulation, as well as temporal structure in the form of a ~5 Hz repetition rate, which for comparison, is comparable to the typical syllable rate in human speech.
The role of the auditory system in maternal behaviors may therefore be more elaborate than to simply switch on/off overall maternal responsiveness. In fact, the different sensory modalities by which offspring cues facilitate maternal responses likely require different forms of information processing to elicit appropriate behaviors. This involves not only individual recognition processes that might require sensory memories, but also sensory discrimination between one stimulus requiring a particular response and another stimulus requiring a different response. From a sensory processing perspective, the maternal model sets up a great opportunity to use a natural behavior with robust stimulus-response links to elucidate neural coding along the various stages of information processing from signal input to behavioral output. This would help modernize paradigms for studying sensory coding, since it would require deeper consideration of how a system operates within the natural physiological states of an individual. Even though there will almost certainly be sensory modality-specific differences in the nature of the neural representation of offspring cues and how they are conveyed into the maternal circuit, commonalities are likely to emerge. We focus on one such developing theme, which is the interaction of sensory experience and hormones to alter the neural circuits involved in the maternal response to offspring cues.
3. The canonical subcortical circuit for maternal motivation to respond to infants
To better appreciate the interest in questions about sensory processing of infant cues, we first consider the output side of the behavior, and discuss the mechanisms underlying the “switch” that activates a mother’s behavioral responsiveness to those cues. One key mechanism for maternal motivation is a subcortical circuit that is thought to gate the expression of maternal behaviors. This maternal circuit has been studied in depth in rodents and mostly in rats (Numan and Insel, 2003), so most of our discussion on the neuroanatomy and hormonal regulation of the maternal circuit focuses on the rat brain, unless otherwise noted. Work from several labs has shown that specific subcortical areas are critical for maternal behaviors such as pup search (appetitive behavior), pup retrieval, anogenital licking and nursing (consummatory behavior). During gestation, parturition and the termination of pregnancy, a cascade of hormonal changes involving estrogen, progesterone and prolactin (Moltz et al., 1970) and their downstream effects help to modify neural activity within the maternal circuit, triggering the onset of maternal behaviors. At this stage, rat mothers find pups cues attractive and potentially rewarding, while the aversive nature of certain pup cues, like odors, is diminished to allow for the expression of maternal behaviors (Numan and Sheehan, 1997).
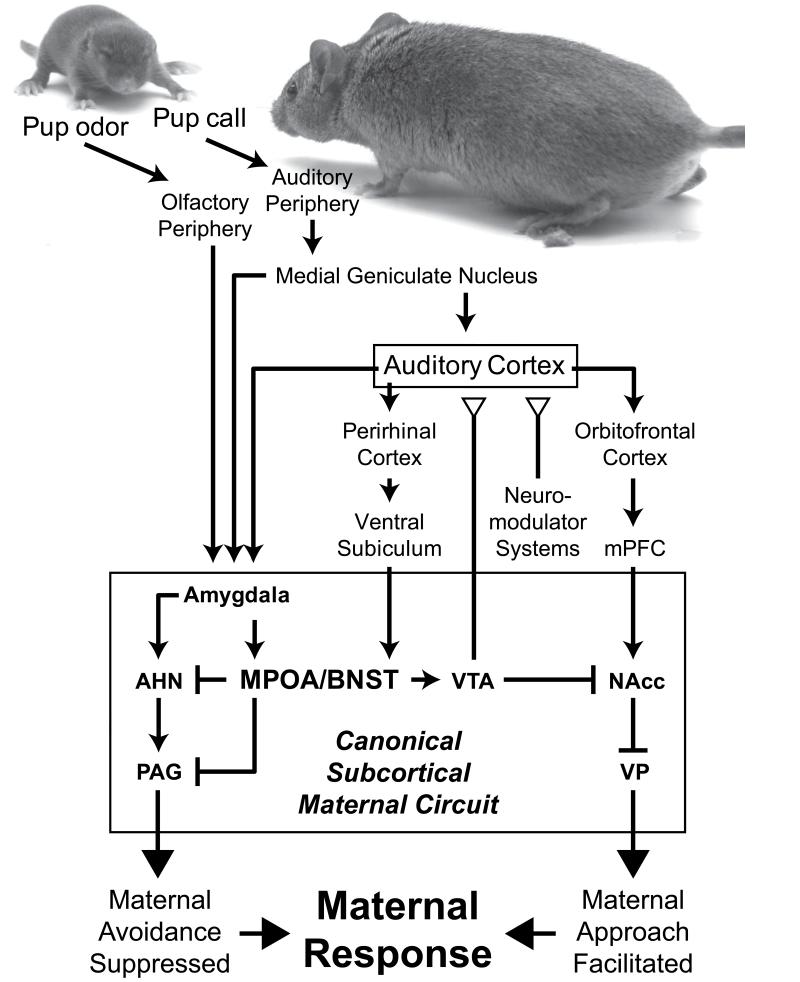
An essential brain area that mediates this maternal motivation is the MPOA (Numan and Stolzenberg, 2009), a developmentally telencephalic structure of hypothalamic function (Butler and Hodos, 2005), and the adjacent bed nucleus of stria terminalis (BNST). It is thought that this area serves as a site of integration for pup-related stimuli to drive subsequent behavioral output through its connections to motor nuclei (figure 2). For example, enhanced Fos (cFos and FosB) expression is observed in the MPOA and ventral BNST in mothers that display maternal behaviors when pups are presented to them (Kalinichev et al., 2000). Lesioning the MPOA or pharmacologically inhibiting it produces robust deficits in pup retrieval behaviors in pup-sensitized virgins, parturient and postpartum females (Numan and Insel, 2003; Pereira and Morrell, 2011). More than just playing a role in the motor aspects of responding to pups though, the MPOA may in fact be necessary for seeking the rewarding aspects of responding to pups as well. For instance, when lesioned, mothers will press a lever for food reward but will not press a lever for pup stimuli (Lee et al., 1999a). Moreover, when the MPOA is pharmacologically inhibited in mothers, they no longer prefer to stay in a chamber that had been paired with pup presentation in a conditioned place preference (CPP) test, a classic paradigm for testing the motivational effects of different sensory cues (Pereira and Morrell, 2010). Such data have therefore led to the suggestion that the MPOA/BNST complex controls the appetitive motivation to respond to offspring cues.
Figure 2.
Schematic of the neural pathways for olfactory and auditory cues from pups to reach the canonical subcortical maternal circuit based on the Numan model (Numan, 2007). Small arrows indicate predominantly excitatory connections; bars indicate presumed inhibitory connections; open triangles indicate neuromodulatory inputs. For clarity, not all connections are shown in order to concentrate on those that form the basis of our knowledge about the information flow, especially for auditory cues, which is the focus of this review. Auditory cortex is a key site for experience-dependent sensory plasticity, thought to be guided by neuromodulatory inputs from the dopaminergic, cholinergic, noradrenergic and other systems. Changes in the neural representation of pup vocalizations can be conveyed into the maternal circuit through limbic as well as prefrontal pathways, potentially modulating the drive for maternal responsiveness.
The mechanism for such regulation likely begins with the connection between the MPOA and the mesolimbic dopaminergic pathway (Numan, 2007), a pathway that is conserved across vertebrate species and that mediates reward-seeking in a variety of behavioral and addiction paradigms (O’Connell and Hofmann, 2011). The MPOA sends efferent input to the VTA (Simerly and Swanson, 1988; Geisler and Zahm, 2005), whose dopamine-synthesizing neurons project diffusely across the brain. These VTA projections are critical for the motivation required for appetitive maternal behaviors. In fact, if the VTA is inhibited in a mother prior to a CPP test of preference for either pups or cocaine, mothers that would otherwise choose pup stimuli in normal circumstances no longer display such a preference (Seip and Morrell, 2009; Pereira and Morrell, 2011). The key projection underlying the VTA’s role in motivating maternal behavior appears to be through the NAcc. The NAcc has a GABA-ergic projection to the ventral pallidum (VP), whose connection to motor areas regulates behavioral output. According to one influential model of maternal motivation (Numan and Sheehan, 1997), under the effect of hormonal priming and pup stimuli, the MPOA enhances motivation to respond to pups by disinhibiting the VP. It is argued that the MPOA’s excitatory input into the VTA drives increased dopamine release in NAcc, resulting in the activation of D1-like dopamine receptors that ultimately suppresses NAcc output. This inhibition of NAcc output releases the VP from its GABA-ergic inhibition, and thus increases the behavioral responsiveness to pups (figure 2).
Specific evidence in support of this model comes from several studies, which are more thoroughly reviewed recently in (Numan and Stolzenberg, 2009). First, a unilateral knife cut of lateral MPOA connections along with a contralateral electrolytic lesion of the VTA produces a drastic inhibition of nest building and pup retrieval, emphasizing the necessity of the bilateral connections between MPOA and VTA (Numan and Smith, 1984). Indeed, lesioning the VTA by applying the dopaminergic neurotoxin 6-hydroxydopamine also decreases the expression of maternal behaviors in postpartum rats (Hansen et al., 1991). The importance of the NAcc as a target of dopaminergic modulation comes from observations that dopamine release is in fact increased in the ventral striatum during maternal behaviors (Hansen et al., 1993). Significantly, depletion of NAcc dopamine produces a decrease in maternal behaviors (Hansen, 1994). Dopamine D1-like receptors in NAcc may be the primary target on which the neurotransmitter acts to modulate maternal motivation. Selective inhibition of D1-like receptors in NAcc decreases active components of maternal behaviors like pup retrieval (Numan et al., 2005b), whereas D1-like receptor stimulation enhances it (Stolzenberg et al., 2007 2010). The NAcc cell type expressing these D1-like receptors is still being investigated. Nevertheless, given that maternal interactions with pups normally increase the NAcc expression of the immediate early gene activity marker, cFos (Lonstein et al., 1998), it is thought that heightened activity of local inhibitory interneurons in NAcc (Numan, 2007) may suppress the NAcc’s GABA-ergic output to the VP. To test the idea that the VP may be normally disinhibited in mothers, injection of the GABA agonist muscimol into the VP of postpartum female rats decreases both retrieval and nursing behaviors, as predicted (Numan et al., 2005a). Finally, a unilateral neurochemical lesion to the MPOA with a contralateral VP lesion has been found to decrease pup retrieval in postpartum mothers, consistent with the view that an essential final pathway for MPOA’s effect on motivating maternal behavior flows through the VP (Numan et al., 2005a).
Besides this facilitatory gating circuit for maternal motivation, in female rats (at least) there is also an infant avoidance circuit that must be “switched” off in mothers. In virgins, this avoidance mechanism underlies the aversive nature of novel pup cues. It is mediated through the amygdala, a region known to regulate defensive behaviors and aggression. Olfactory cues from pups are conveyed from the primary and accessory olfactory systems to the medial amygdala, which then projects directly to the MPOA/BNST (Simerly and Swanson, 1986). When the medial amygdala is lesioned in virgins, they no longer avoid pups and develop full maternal behaviors upon pup exposure more quickly than sham-lesioned animals (Numan et al., 1993). Electrical stimulation of the medial amygdala predominantly inhibits MPOA neurons (Gardner and Phillips, 1977), and delays the onset of maternal behavior (Morgan et al., 1999). The facilitation of maternal behavior produced by amygdala lesions can be abolished by lesions of the MPOA, suggesting that the amygdala’s inhibitory role on maternal behavior must be mediated by interactions with the MPOA (Fleming et al., 1983). This interaction could be through their direct connections, or through neurons in the medial amygdala that project to the anterior hypothalamic nucleus (AHN), which in turn projects to the midbrain periaqueductal gray (PAG). These are both regions involved in fearfulness and avoidance behaviors, and it has been suggested that they may receive inhibitory input from the MPOA/BNST complex (Numan, 2006). Hence, in rat mothers, it is possible that under the influence of parturition related hormones, pup cues activate the MPOA, which then suppresses avoidance responses through its interaction with the AHN and PAG, thereby allowing maternal behaviors to be expressed. The same mechanism probably functions in wild mice (McCarthy and Vom Saal, 1985), but may not be essential in many strains of laboratory mice, since virgin females can often show spontaneous maternal responsiveness (Gandelman et al., 1970).
The amygdala is not the only source of sensory information into the maternal circuit. Early tracing studies found that the ventral subiculum likely relays sensory cortical information that converges in the perirhinal cortex, which provides input to the subiculum, to the lateral MPOA (Simerly and Swanson, 1986). There is also evidence that the MPFC, consisting of the anterior cingulate, prelimbic and infralimbic cortex, plays a sensorimotor integration role that contributes to the performance of maternal behaviors. The MPFC communicates with orbital prefrontal cortex, which is a well-known site for multisensory integration, and provides an important pathway for cortical output to limbic areas like the nucleus accumbens and amygdala (Öngür and Price, 2000; Morgane et al., 2005). Site specific inactivation within the MPFC has elucidated unique roles for each of its distinct components. For example, the anterior cingulate does not appear to play an essential role in maternal behavior, since lesions here only resulted in a slower rate of pup retrieval – perhaps because of retrograde damage in the anterior thalamus (Slotnick and Nigrosh, 1975). On the other hand, the infralimbic cortex, but not the prelimbic cortex, plays an important role in both pup retrieval and nursing during the early postpartum stage in mothers (Febo et al., 2010; Pereira and Morrell, 2011). As time progresses though, the necessary facilitatory role of the infralimbic cortex is diminished, and instead the prelimbic cortex begins contributing more to the expression of maternal behaviors (Febo et al., 2010; Pereira and Morrell, 2011). Therefore, MPFC components apparently act as an important pathway for integrated sensory cues emitted by pups to reach the maternal motivation circuit. Interestingly though, theories about the functioning of this maternal circuit leave out details about changes in the sensory input coming into it, and focus instead on intrinsic changes within it that may be hormonally modulated to switch on maternal behavior.
4. Maternal hormones facilitate the onset of maternal responsiveness and its consolidation
The hormonal environment during parturition plays a key role in the rapid onset of maternal behaviors. The critical changes in hormones include a rise in levels of estradiol, placental lactogens and pituitary prolactin superimposed on a steep fall in serum levels of progesterone which occurs at the end of a 22 day pregnancy in rats (Moltz et al., 1970; Zarrow et al., 1971) (reviewed in (Numan and Insel, 2003)). Early studies established that circulating hormones in a new mother act to quickly induce maternal responsiveness, even to the point that transfusing her blood into a virgin female can switch on the latter’s maternal behavior within a day (Terkel and Rosenblatt, 1972). Moreover, if ovariectomized and/or hysterectomized virgin rats are subjected to a maternal hormonal paradigm, the latency for most animals to display maternal behaviors is reduced to 1-2 days from 5+ days on exposure to pups (Moltz et al., 1970; Zarrow et al., 1971; Siegel and Rosenblatt, 1975), again suggesting that these hormonal changes facilitate the fast onset of maternal behaviors.
Given the subcortical maternal circuit outlined in the previous section, a natural question is where maternal hormones exert their effects on the onset of maternal behavior. Many key neuromodulatory brain areas, including those like VTA that are a part of the maternal circuit, express hormone receptors and could be potential targets (Miranda and Liu, 2009). The predominant theory though is that the MPOA is an essential actor. It is rich in estrogen receptors (Champagne et al., 2003), and when estrogen are directly implanted there versus just given subcutaneously, the latency to begin showing full maternal behavior is virtually eliminated in 16-day pregnant, hysterectomized, and ovariectomized female rats that would otherwise be slow to engage maternally with pups (Numan et al., 1977). It is therefore possible that hormonal priming of MPOA may lower the threshold to activate the downstream maternal gating circuit and suppress the avoidance circuit (Numan and Insel, 2003).
While maternal hormones act to immediately stimulate maternal behavior at birth, experience with infants is of course also needed. Interestingly, experience without a maternal hormonal profile can also produce animals that act maternally, even in ovariectomized virgin rats and mice (Rosenblatt, 1967; Ehret and Koch, 1989). However, at least in rats, this experience-based “sensitization” to infants generally takes longer to produce full maternal behavior than it does in parturient females (Rosenblatt, 1967), which is consistent with a facilitatory but not mandatory role for hormones during experience. In fact, once animals are maternally responsive, the maintenance of maternal behaviors only involves continued offspring experience, and does not require continued levels of maternal hormones. This is apparent from manipulations such as hypophysectomy (removal of the pituitary gland), ovariectomy, adrenalectomy, and progesterone administration that do not affect the expression of maternal behaviors (reviewed in (Numan and Insel, 2003)). Hence, the neurobiological changes that underlie an increased behavioral responsiveness to infants can be fostered with or without maternal hormones, but the latter acts to facilitate those changes.
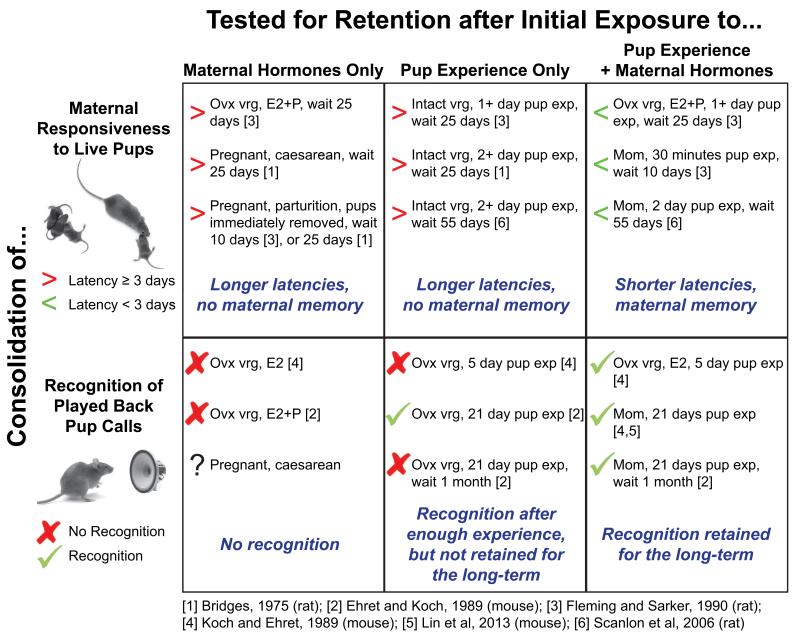
Direct infant experience also mediates a faster latency to reengage in consistent maternal behaviors after a prolonged separation from pups, an effect known as “maternal memory,” which has been extensively explored in rats. If experience is prevented by removing pups at birth or by caesarean section, these mothers tested from 1 to 3 weeks later are much slower to begin showing full maternal behavior than those who had some minimal pup care experience after parturition (Bridges, 1975; Fleming and Sarker, 1990). In another possible facilitatory role for maternal hormones, their presence during the initial experience caring for infants may help to strengthen maternal memory. Indeed, pairing maternal hormonal changes with pup experience facilitates future maternal responsiveness in the absence of additional hormonal triggers (figure 3). For example, if primiparous or pup-sensitized virgin females are allowed to provide full maternal care to pups for 2 days before separating from pups, and then tested 55 days later by re-exposing them to foster pups, primiparous mothers show shorter latencies to re-express full maternal behaviors (figure 2) (Scanlan et al., 2006). Such first-time mothers also have enhanced cFos expression in the NAcc and medial amygdala compared to the pup-sensitized virgins (Scanlan et al., 2006). Since their hormonal environments upon re-exposure are similar, the neural differences may well be due to more robust plasticity in the maternal hormonal environment during the initial pup exposure, which then helps establish a stronger maternal memory. It is therefore tempting to speculate on the possibility that parturition related hormonal events may be akin to developmental organizational events when pathways are laid out with the help of a series of profound endocrine events (Simerly, 2002; McCarthy, 2010; Adkins-Regan, 2012). Once mothers are exposed to pups at the end of parturition in tandem with her specific maternal physiological environment (high estrogen and decreasing progesterone levels), neural circuits are established such that no further hormonal stimulation is required for continued maternal responsiveness or its consolidation for long-lasting retention (Numan and Stolzenberg, 2009).
Figure 3.
Parallel effects for maternal hormones and pup experience on consolidation of maternal responsiveness and pup call recognition. Consolidation of maternal responsiveness is measured by the latency (in days) to express consistent maternal care for live pups weeks after an initial exposure to pups (or hormone treatment in the absence of pup experience). Without that exposure, or if that exposure is brief and occurs without maternal levels of hormones, the latency to express maternal behaviors on the retest is generally slow (i.e. longer than 3 days). However, in the presence of maternal hormones, an initial pup exposure as short as 30 minutes facilitates more rapid reinstatement of maternal care more than a week later. This pattern is mirrored for pup call recognition, which is measured in a two-alternative choice phonotaxis task between the playback of calls or a neutral sound. Artificially raising estradiol (E2) and progesterone (P) to maternal levels without exposure to pups is insufficient to acquire call recognition, as is brief exposure without hormones. Longer exposure without maternal hormones can be effective for establishing call recognition, but it is not maintained for the long-term, when tested a month later. The combination of maternal hormones during pup exposure appears sufficient to establish and maintain call recognition up to a month after weaning.
Where might maternal hormones act to help encode a stronger maternal memory? This seems to be still largely an open question. One distinct possibility is that synaptic plasticity occurs within the subcortical maternal circuit itself. For instance, during the initial experience with pups, connections within the MPOA may be strengthened so that in a future encounter with pups when maternal hormones are not at a level to facilitate MPOA activity, maternal responsiveness can still be readily engaged by the same sensory drive into the circuit. Alternatively, plasticity might be occurring within, or at least be facilitated through, other components of the maternal circuit. In particular, the shell region of the NAcc has been implicated in the consolidation of maternal memories, since lesioning it before but not 24 hours after pup experience disrupts the re-emergence of maternal behavior after 10 days of pup separation (Lee et al., 1999b; Li and Fleming, 2003). Importantly, these results indicate that the site of the long-term storage of the maternal memory lies outside the NAcc itself, either in other parts of the maternal circuit or beyond it. Where the memory is housed and whether maternal hormones might act to strengthen its encoding during infant experience are key questions that motivate this current review.
5. Sensory components of maternal memories
Is the basic nature of maternal memory just a matter of prior infant experience making the output “switch” for maternal responsiveness easier to turn “on”? That would presumably be a reasonable interpretation for plasticity within the maternal gating circuit itself. This could certainly be part of the answer, and there is some evidence for plasticity in the pup cue-elicited dopaminergic release in the NAcc of multiparous females compared to recently pup-sensitized virgins (Afonso et al., 2008). Even so though, it is also possible that such maternal circuit changes reflect alterations in the sensory input as a result of infant cues having acquired more salience through experience. In that sense, there are likely components of the maternal memory that are sensory in nature. In fact, individual recognition and discrimination between infant cues are problems that the sensory systems themselves are almost certainly solving using prior experience. Indeed, as we review in Section 6, experience-dependent plasticity in a sensory system, which is known to occur in many laboratory learning contexts, can also be found within the natural maternal context.
Where and how the memories of infant sensory cues are stored have therefore become relevant questions for understanding the consolidation of maternal responsiveness. One can begin to address the sensory-specific aspects of this maternal memory by stimulating individual modalities with infant cues and assaying behaviors that require correctly interpreting those cues, such as recognition and discrimination tasks. This goes beyond the traditional method of measuring the amount or quality of various maternal behaviors. This has, for example, been carried out in sheep, where olfactory recognition tests and auditory/visual two-choice paradigms have been used to assess how much experience with lambs ewes require before they are able to show a preference for their own lambs based only on the tested sensory modality (Keller et al., 2003). Using such paradigms, early studies of olfactory recognition of individual lambs by ewes suggest that key sites of neural plasticity lie within the sensory system itself (Kendrick et al., 1997). In particular, selective olfactory recognition of lambs is correlated with an increase in the responsiveness of mitral cells in the ewe’s olfactory bulb to her own lamb’s odors (Kendrick et al., 1992), and the neurochemical changes in the bulb that underlie this electrophysiological plasticity persist in multiparous ewes (Keverne et al., 1993). Furthermore, a seminal study by Kendrick and colleagues has demonstrated that nitric oxide released by mitral cells modulates the release of glutamate by both mitral and granule cells potentially contributing to olfactory memories in sheep (Da Costa et al., 1997; Kendrick et al., 1997). Plasticity mechanisms are even engaged in higher-order cortical areas processing sensory information, since initial lamb experience activates the plasticity-related immediate early gene, zif/268 in the ewe’s orbitofrontal cortex, potentially to encode the lamb’s olfactory memory (Da Costa et al., 1997).
Whether maternal hormones might facilitate experience-dependent sensory plasticity mechanisms for processing infant cues is not that well understood. Studies of auditory processing in the maternal context have begun to hint at this. First, an fMRI study in human mothers who delivered their infants either vaginally or through cesarean section (and thus did not have a full neurohormonal experience from vagino-cervical stimulation during birth) found that the former had greater activation in auditory cortex for baby cries in general and their own baby cries in particular, when scanned 2-4 weeks after birth (Swain et al., 2008). Second, studies in mice suggest a particular role for estrogen in the onset and retention of infant vocalization recognition. For example, the responsiveness of pup-sensitized virgin female “cocarers” to the playback of pup-like wriggling calls reportedly changes as a function of estrus, suggesting that ovarian hormones help regulate motivation to respond to pup vocalizations in virgin mice (Ehret and Schmid, 2009). However, estrogen acting alone does not by itself enable recognition of pup-like USVs, since ovariectomized virgin females given estrogen implants (OVX+E2) do not show a preference toward pups calls in the absence of pup experience (Koch and Ehret, 1989). After 5 days of pup experience though, OVX+E2 females as well as intact cocaring virgins prefer pup-like USVs (Ehret et al., 1987; Koch and Ehret, 1989). On the other hand, ovariectomized females that do not have estrogen implants do not prefer the USVs even after 5 days of pup experience (Koch and Ehret, 1989), and instead need 21 days of pup experience to develop a call preference (Ehret and Koch, 1989). These data suggest that in virgins, estrogen hastens and facilitates the learning of a salient auditory cue such as a pup call over a short period of interaction with pups. In addition, a longer period of interaction with pups allows for similar learning in the absence of normal physiological levels of estrogen. This pattern is reminiscent of the facilitatory but not mandatory role of maternal hormones on the onset of general maternal responsiveness, as discussed in Section 4. Interestingly, the retention of pup call recognition also follows a similar pattern. Intact mothers prefer pup-like USVs up to one month after the pups are weaned, while ovariectomized pup-sensitized cocarers no longer display such a preference by this time point (Ehret and Koch, 1989). Since hormonal cycles would have returned to normal in mothers by this late time point, those data suggest that the presence of estrogen may help strengthen the encoding or consolidation of auditory memories of infant vocalizations (figure 3).
Where and how might these auditory-specific infant memories be stored? Just as in the olfactory-specific case, a natural place to look for plasticity is within the auditory system itself, particularly at the cortical level. As we discuss in the next section, a large body of work based on non-maternal laboratory training paradigms suggests that the auditory cortex contains a physiological trace of behaviorally relevant sounds (Weinberger, 2004). In fact, some current models of long-term memory posit that experiences are consolidated during sleep through the coordinated replay of neural activity in the hippocampus and sensory cortex (Ji and Wilson, 2007). This is consistent with strong evidence from human patients and animal studies that the hippocampus and larger medial temporal lobe are involved in memory consolidation, but that “remote” memories of past experiences are actually distributed in synapses across a wide range of cortical areas that would be involved in processing the information contained in those memories (e.g. piriform cortex for olfactory memories) (Squire and Bayley, 2007; Takashima et al., 2009). Indeed, inhibiting in gustatory cortex the enzymatic activity of a specific protein kinase (PKMzeta) required for the maintenance of long-term potentiation in synapses, a presumed substrate for memory, has the practical effect of “erasing” the memory of a conditioned taste aversion that is mediated through that cortical area (Shema et al., 2007). Hence, plasticity within cerebral cortical areas in general, and sensory cortical regions in particular appear to be essential for the long-term storage of learned experiences. This thereby motivates a closer look at the role of the cortex in maternal behavior and memory.
In truth, there were early indications that the cerebral cortex in fact plays a role in maternal care, but this has been subsequently under-investigated in most animal research. Studies performed in the 1930s and 1940s suggested that the cortex as a whole, including sensory cortex, contributes to effective maternal behavior and maternal memory. Frank Beach found that when large portions of the cerebral cortex of adult primiparous female rats were destroyed 30+ days prior to parturition, maternal behaviors like nest-building, retrieval and care of new born pups were significantly impaired compared to control females. This was also true if animals were operated as juveniles and became mothers as adults, suggesting that animals are able to survive and mature without their cortex. Although the locus of the lesion did not seem to matter, the size of the cortical lesion was correlated to the extent of behavioral impairment observed (Beach, 1937; Beach Jr, 1938). A separate study showed that bilateral decortication greatly impairs the onset of maternal behaviors after parturition: such animals do not nurse, build nests, groom or retrieve their young (Davis, 1939). While none of the lesions in those studies were specific to sensory cortices, the damage certainly overlapped with them. Hence, the cerebral cortex overall, including sensory areas, plays a role in maternal behavior, although a caveat to these early studies is that whether maternal-specific or nonspecific disturbances in motor and sensory function were responsible could not be clearly dissociated. Nevertheless, since the subcortical maternal circuit in these animals was intact, the studies further suggest that this circuit’s actions in maternal behavior ultimately must be considered in the context of its connectivity to the whole brain, potentially including sensory input connections. Finally, we note that a few subjects in these lesion studies raised pups successfully prior to decortications, but were not successful mothers after decortications. Thus, prior experience cannot salvage the performance of decorticated mothers (Davis, 1939). This observation is consistent with the possibility that cortex not only processes infant cues, but could also aid in storing maternal memories of those cues, which would facilitate the reemergence of maternal behaviors in multiparous mothers.
6. Experience-dependent plasticity in the auditory cortex for learning behaviorally salient sounds
While the research on maternal behavior discussed above now motivates a deeper investigation into the role of the cerebral cortex in general and sensory cortex in particular, the converse is also true. Studies of sensory coding and plasticity are also beginning to find value in exploiting the maternal context as a natural paradigm for sensory learning and memory. A large body of research has already implicated primary sensory cortex as a potential site for long-term memory traces of behaviorally relevant stimuli, which underlies our hypothesis that it could play an essential role in the maternal context. Ongoing work in this field is elucidating the detailed form and time course of this trace, mostly at the electrophysiological level. In this section, we briefly review some key points from this research, which has generally employed traditional laboratory models of conditioning and learning, and explain how that has motivated more natural studies of sensory cortical plasticity in the maternal context.
A historically important concept in sensory neuroscience has been that of the neuronal receptive field or tuning curve. A sensory neuron at any stage of processing between the periphery and the cortex varies its action potential firing rate systematically in response to parameterized changes in a stimulus dimension. In the auditory modality, this dimension has traditionally been chosen to be the frequency of a purely tonal sound, or its intensity. The frequency tuning curves one derives can then be characterized by measures such as the “best frequency” (BF), which is the sound frequency that elicits the strongest response at any intensity. The value of such a characterization is that throughout the auditory pathway, BF is spatially organized so that neurons tuned to different frequencies are positioned along a gradient, referred to as a tonotopic gradient or map. Note though that despite each single neuron (or unit) or cluster of neurons (multiunit) having a defined BF, the tuning curve itself can have a broad tuning width, so that the unit may in fact respond to sounds even an octave frequency away from its BF. This is important because frequency tuning curves of auditory cortical neurons are plastic, so that responses to sounds within a unit’s tuning bandwidth can change. In particular, both the shape of a tuning curve as well as its BF can be altered.
In adult animals, the plasticity of the frequency tuning curve in primary auditory cortex was demonstrated early on in a fear conditioning paradigm (Bakin and Weinberger, 1990) by pairing a shock with the presentation of a specific tone frequency (conditioned stimulus, CS) while recording from cortical neurons in an anesthetized animal. This conditioning produced an increase in the neuron’s tuning curve near the paired frequency, often resulting in a shift of its BF towards the paired frequency. On a global scale, such receptive field plasticity would presumably occur in many neurons simultaneously during conditioning, thereby yielding an overall expansion in the proportion of primary auditory cortical neurons tuned near the paired frequency. This would therefore distort the tonotopic map, so that a larger cortical area would be responsive to the CS (Rutkowski and Weinberger, 2005).
This experience-dependent receptive field and corresponding map plasticity have been thought to be mediated primarily through the cholinergic neuromodulatory system during learning of the stimulus’ significance. In fact, direct pairing of a tone with electrical stimulation of the nucleus basalis, which provides the predominant source for cholinergic input into the forebrain, also produces robust receptive field shifts and auditory cortical tonotopic map expansion (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998). Pairing via stimulation of other neuromodulatory systems, like the dopaminergic VTA or the noradrenergic locus coeruleus, can similarly drive comparable plasticity in the tonotopic map (Bao et al., 2001) or receptive field (Edeline et al., 2010). Such results support the consensus that activation of neuromodulatory neurotransmitter systems, perhaps through arousal, attentional or motivational processes (Gu, 2002), facilitates sensory cortical plasticity. Importantly for the purposes of our discussion, most of these neuromodulatory systems are also activated by pup interactions in the maternal context, making it likely that sensory cortex is placed in a permissive state for plasticity to infant cues during maternal experiences.
The functional role of such retuning plasticity within the auditory cortex has been debated widely (Ohl and Scheich, 2005; Weinberger, 2007). There is evidence that it can correlate with altered perceptual discrimination (Recanzone et al., 1993; Han et al., 2007) and/or detection abilities for the learned sounds (McLin et al., 2002; Froemke et al., 2013). In fact, one study even suggests that the form of auditory cortical map plasticity observed directly relates to the perceptual relevance of behaviorally predictive acoustic features (Polley et al., 2006). Using the same behavioral apparatus, rats either learned that a tone frequency or intensity cue predicted reward. A double dissociation in primary auditory cortex was then revealed: rats that were trained on a frequency target exhibited only tonotopic map plasticity; those trained on an intensity target showed plasticity in their map of best sound intensity, but not BF. Moreover, these changes were correlated with the individual performance of animals in each task. Hence, retuning appears to provide a physiological memory trace for behaviorally relevant stimulus features within sensory cortex (Weinberger, 2004), which may then contribute to an improved functional processing of that sound. In fact, retuning has been reported in some cases to last for up to 8 weeks after training (Weinberger et al., 1993), presumably maintained through long-lasting changes in synaptic weights.
Despite this volume of work on auditory cortical plasticity using laboratory learning paradigms, a key question has persisted: is it relevant for learning in natural contexts? In studies like those mentioned above, single frequency pure tones that otherwise have no ethological meaning to an animal are used for intensive training or conditioning, thereby imbuing these synthetic sounds with behavioral significance. Using frequency receptive fields and tonotopic maps, which are based on the responses to such tones, makes sense in this case as a measure of plasticity. However, in real life, meaningful sounds that are encountered are more complex in their spectrotemporal characteristics. One study reported that low frequency tones that make up the spectrum of sounds from a water reward delivery system used in an instrumental training paradigm can also be overrepresented in the auditory cortex after extensive training with the device (Rutkowski and Weinberger, 2005). Even in this case though, would simply expanding one frequency range of representation constitute the only or final mechanism for storing the physiological traces of important sounds? What happens when sounds in different frequency ranges acquire different meaning over time? If the sensory cortical memory only involved expansion in the representation of the cortical map, there would be a limit to how many different natural stimuli could be stored.
This conundrum has led to the more recent hypothesis that large-scale retuning and map expansion contribute to learning, but that the long-term engram within auditory cortex lies within specific subsets of neural network connections that have been altered by the experience (Kilgard, 2012). This is supported by a recent study, which found that inducing cortical map expansion with nucleus basalis stimulation accelerates learning a tone discrimination task, but that map expansion can actually disappear over time even though behavioral performance is maintained (Reed et al., 2011). The absence of map plasticity after perceptual training was also observed in a few other studies (Brown et al., 2004; Takahashi et al., 2010). Which particular neurons and synapses are involved in storing an engram may well depend on the detailed specifics of the learning paradigm and acoustic features of the behaviorally relevant stimulus (Shepard et al., 2012). Moreover, the correlates of the engram may exist not just as changes in firing rates of those neurons, but potentially in the timing of the action potentials (Schnupp et al., 2006). These possibilities argue for looking at plasticity in the responses to the specific complex sounds themselves, rather than focusing simply on firing rate tuning curves for pure tones as the main measure of plasticity. Moreover, the potential that the exact form and time course that plasticity takes will be determined by the details of the learning context has highlighted the need to adopt more natural learning contexts to better understand how auditory cortical plasticity proceeds in real situations with natural stimuli and rewards. Thus, since a goal in auditory neuroscience has been to elucidate the auditory processing of communication sounds, studies are beginning to explore contexts where the meanings of species-specific vocalizations are learned through natural social interactions rather than instrumental training. This has therefore motivated interest in using the maternal mouse model to study the coding and plasticity underlying the acquisition of the behavioral significance of pup vocalizations.
7. Auditory cortical plasticity for infant calls in the maternal context
In the context of motherhood, experience-dependent plasticity studies such as those discussed above have led to the hypotheses that maternal experience will leave lasting changes on auditory cortical responses to infant vocalizations, which will then alter the nature of the sensory cortical representation that drives downstream input into the subcortical maternal circuit, thereby affecting maternal behavioral responses. As the debate on the function of map plasticity demonstrates, while these hypotheses are reasonable, they are by no means foregone conclusions. In fact, experience-dependent sensory cortical map plasticity in the maternal context was already observed transiently in the somatosensory system, but its correlates disappear soon after nursing experience ends. Specifically, 2-3 weeks of nursing in postpartum lactating rats enlarges the cortical representation of the ventral trunk region where the nipples are located (in comparison to postpartum rats separated from their pups at birth) (Xerri et al., 1994), but these changes are gone in the weeks after the end of nursing (Rosselet et al., 2006). Hence, in the context of infant vocalizations, a key question is whether there are changes in auditory cortical responses to pup calls that may then persist beyond the maternal experience (i.e. weaning), which might then help facilitate the reemergence of maternal behavior.
A series of studies from different laboratories have begun to find support for this in the mouse maternal model introduced in Section 2. The first hint that motherhood alters the auditory cortical response to pup calls in mice came from a cFos study of primiparous mothers with their 7-day old pups listening to the playback of USV models (Fichtel and Ehret, 1999). Significant differences in cFos expression relative to pup-naïve virgin females were found in both a core auditory cortical field (ultrasound field), as well as so-called higher order noncore fields (secondary auditory field and dorsoposterior field). The same lab using similar methods more recently reported that regions of the subcortical maternal circuit, including the MPOA, were differentially activated between mothers and recently pup-sensitized virgin cocarers by acoustic models of pup USVs (Geissler et al., 2012). Importantly, the level of MPOA cFos expression could not be simply explained by differences in pup retrieval performance during call playback, and thus was not just a read-out for maternal motivation. Instead, for mothers and virgins, the MPOA showed significantly different expression for different call models that were either more or less similar to the stereotyped natural pup USV, indicating that this key gateway into the maternal circuit is sensitive to acoustic features in potentially salient sounds. In fact, the authors interpreted the pattern of expression across groups as reflecting the perceived “news value” of the call models, which they argued as being different for mothers and recently pup-sensitized cocarers. Whether the neural activity driving MPOA already reflects this “news value” of the sounds is still unknown, and would ultimately need to be traced from its origin in the auditory system.
Electrophysiological measures of auditory cortical neural responses to pup USVs further demonstrate how the calls’ acquired behavioral relevance alters their representation, even in 1-2 week post-weaning mothers who are well beyond the period (by ~2+ weeks) when their pups would have reached their peak vocalization rates (Haack et al., 1983). Intriguingly, the plasticity is not apparent in as simple a manner as one might expect. Multiunit recordings in anesthetized post-weaning mothers and age-matched, pup-naïve virgins found that overall neural firing rates elicited within the core auditory cortex are not significantly different for various exemplars of pup USVs (Liu et al., 2006; Liu and Schreiner, 2007). Instead, the temporal pattern of multiunit spiking to individual natural calls (Liu and Schreiner, 2007) or bouts of calls (Liu et al., 2006) is significantly different in mothers, in agreement with other studies finding that temporal coding is important for the representation of vocalizations (Schnupp et al., 2006; Huetz et al., 2009). While altering the timing of responses may seem like a minor change, doing so can significantly improve the transmission of information for detecting and discriminating individual pup USVs in mothers (Liu and Schreiner, 2007). Moreover, it has the effect of enhancing the ability of the (anesthetized) auditory cortex to follow the individual vocalizations in a bout (Liu et al., 2006). Specifically, cortical multiunits in virgins can only be entrained well up to about a 3 Hz call repetition rate. However, natural call bouts are predominantly emitted by pups at a 5 Hz rate (Liu et al., 2003), a rate to which a mother’s auditory cortex can entrain well (Liu et al., 2006). This supports the notion that the auditory cortical plasticity produced when the behavioral relevance of a sound is gained naturally actually improves the sound’s functional processing for the long-term.
A final study in anesthetized mice provides some insight into possible neural mechanisms that may facilitate how the auditory system changes its response to pup vocalizations as their behavioral meaning is acquired. In vivo cell-attached recordings in the core auditory cortex of female mice found that exposure to pups’ body odor can modulate neural responses to pure tones and natural pup calls (Cohen et al., 2011). This multimodal interaction between pup olfactory and acoustic cues was most prominent in lactating mothers, but also significantly higher in pup-sensitized virgins and post-weaning mothers compared to pup-naïve virgins. Hence, the effects of pup olfactory cues (but not other smells) on auditory cortex were both experience-dependent and long-term. These results raise the interesting possibility that the normal presence of pup odors when pup vocalizations are heard modulates auditory cortical activity, which then helps to “teach” the auditory system about the calls’ relevance. In this context, the finding in sheep that auditory/visual cues from infant lambs require longer experience than olfactory cues to learn is quite suggestive (Keller et al., 2003). More generally, this study reveals a concrete interaction between sensory modalities excited by pup cues in the context of a highly multimodal natural behavior (Febo et al., 2008).
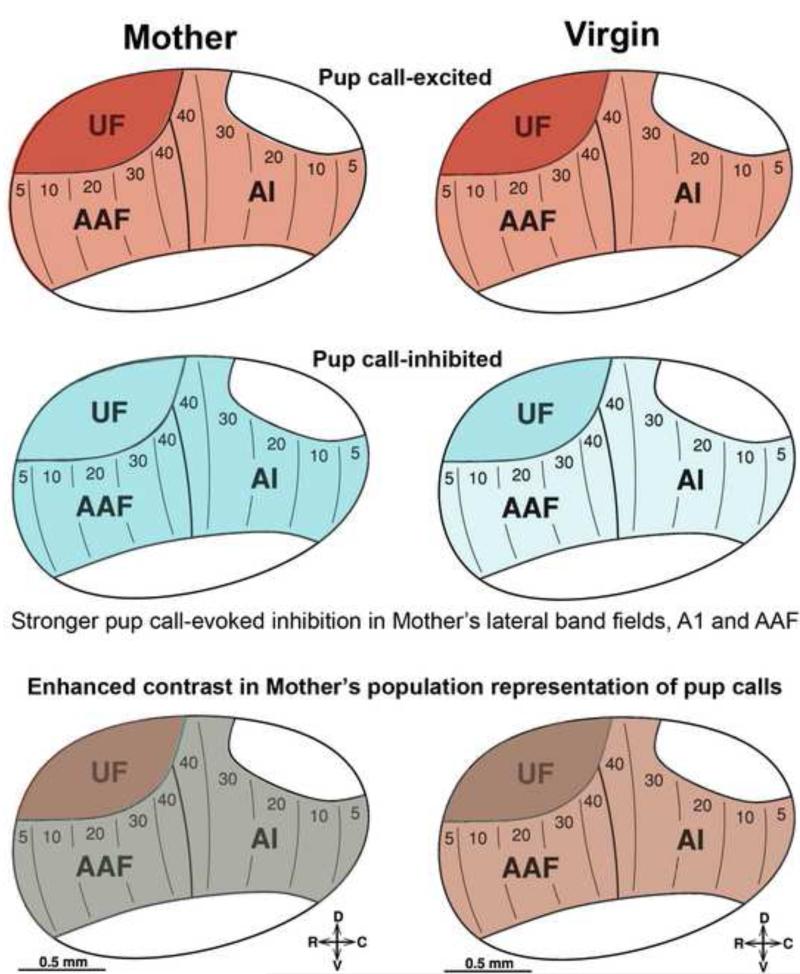
While studies in anesthetized animals are informative in illustrating differences between groups, they do not tell us how the auditory system performs during active communication in awake animals. As an intermediate step towards exploring auditory cortical activity in freely moving animals, we developed a head-restrained mouse preparation to deliver sounds precisely while recording high-quality single units and local field potentials from awake mice (Galindo-Leon et al., 2009). This then revealed a previously under-appreciated form of cortical response to vocalizations – call-evoked inhibitory responses, where inhibition here refers to the reduction of firing relative to baseline spontaneous activity. Single units in both post-weaning mothers and pup-naïve virgins show such evoked responses to a large library of pup vocalizations, but it is significantly stronger in mothers compared to virgins. In particular, this difference in call-evoked inhibition is most prominent at recording sites tuned to frequencies lower than the pup calls’ ultrasonic frequency range, which would fall into the non-ultrasonic core auditory cortical fields, primary and anterior auditory cortical fields (Lin et al, in press). This finding led us to hypothesize that the stronger so-called lateral band call-evoked inhibition observed in mothers may improve pup call detection by increasing the population neural activity contrast between call-evoked responses arising from high-ultrasonic and lateral frequency band neural populations (figure 4). This change in the signal-to-noise of the population representation of the calls could have a benefit if they are emitted in the presence of background noise, since call-evoked lateral band inhibition would help suppress that noise, thereby allowing neural activity in the high-ultrasonic band to stand out more clearly for downstream areas (Galindo-Leon et al., 2009).
Figure 4.
Schematic illustration of improved neural population contrast across auditory cortex in mothers for pup USVs. Call-evoked excitation is similar between mothers and pup-naïve virgins, with generally stronger (darker shade of red) excitatory responses in the ultrasound field, UF. Call-evoked inhibition is similar in UF between mothers and virgins, but stronger (darker shade of blue) in mothers for the lateral band core auditory cortical fields, A1 and AAF. Assuming downstream sites pool call-excitatory and inhibitory responses together, these patterns would produce a stronger contrast in activity between UF versus A1/AAF for mothers compared to virgins.
Plasticity in call-evoked inhibitory responses in post-weaning mothers raises the question of whether pup experience alone might be sufficient to produce these coding changes (Lin et al., 2013). Looking first in awake, pup-sensitized virgin cocarers after the pups they had cared for were weaned, we again observed call-evoked inhibition in a subpopulation of core auditory cortical single units. Importantly though, these experience-matched cocarers did not exhibit the same strength of inhibition as observed in post-weaning mothers, and instead more closely resembled pup-naïve virgins. However, recordings from cocarers with more immediate recent experience caring for pups (so-called early-cocarers removed from the litter when pups were 6 days old and recorded in the days afterwards) were more similar in their call-inhibited responses to post-weaning mothers. These results suggest that experience alone can alter this particular form of auditory cortical plasticity, but it is not sufficient by itself to maintain this plasticity for the long-term when the salience of the pup calls has receded into the past. This latter point was reinforced by behavioral results demonstrating that both post-weaning mothers and early-cocarers, which have stronger lateral band inhibition, show a significant behavioral preference for pup calls in a two-alternative choice phonotaxis task, but postweaning cocarers do not. Hence, the fact that mothers show a difference from naïve virgins and cocarers after weaning indicates that the physiology of parturition and/or lactation during her experience with pups likely plays a role in maintaining this inhibitory neural plasticity. In fact, the multimodal study described above found many of its effects to be stronger in the lactating mother, potentially hinting at a direct effect of maternal hormones on auditory cortical activity during pup experience (Cohen et al., 2011).
Taken together, these studies demonstrate that experience-dependent changes in auditory cortical coding of vocalizations do occur in a natural learning context, and that the mother’s physiological state during that experience appears to help these changes last for at least a few weeks beyond weaning. Since information from the core auditory cortex of rodents can be directly transmitted to the amygdala, perirhinal cortex and prefrontal cortex (Budinger and Scheich, 2009), a higher signal-to-noise representation pup calls in the auditory cortex of mothers may eventually impact behavioral responses to calls through this sensory area’s projections to the subcortical maternal circuit.
5. A potential role for estrogen in facilitating maternal memory in auditory cortex
The studies discussed above now raise new questions about whether priming by maternal hormones could help to more enduringly alter neural responses in the auditory cortex to salient cues from pup calls, and if so, what cellular and molecular mechanisms enable this. This possibility is particularly intriguing given that the hormonal environment plays an important role in the onset of maternal behaviors and in maternal behavioral memory, as reviewed in Sections 4 and 5. Cyclical modulation of reproductive hormones in females has also been linked to changes in sensory sensitivity (Parlee, 1983), learning (Maki et al., 2002) perception (Penton-Voak et al., 1999) and sensorineural activity (Walpurger et al., 2004), providing further impetus to consider their role in maternal sensory memories. Of special interest is the hormone estrogen, because of its role in initiating parturition and its necessity along with pup experience for driving a preference for pup calls in mice (Koch and Ehret, 1989), as mentioned in Section 5. Importantly, estrogen also has documented effects on learning and memory, as we describe in more detail below, leading us to formulate a tentative hypothesis that estrogen-dependent mechanisms may help facilitate the long-term storage of maternal memories in auditory cortex.
In rodents, the highest levels of estrogen are attained during the last part of gestation and return to baseline soon after parturition (Miranda and Liu, 2009). Therefore the phase of intense sensory stimulation from pups coincides with receding estrogen levels. High estrogen levels prior to and during parturition might therefore prime neural circuits for enhanced plasticity to process and store memories of sensory cues after the birth of offspring. Although virgin cocarers can also exhibit changes in auditory cortical responses to pup calls, a key difference from mothers lies in how long-lived those changes are. Here we consider how priming by estrogen might help to maintain plastic changes in the auditory cortex through myriad molecular and cellular pathways, which would then help enable a more efficient future response to salient pup cues.
Estrogen receptors are present in the auditory cortex of both male and female rodents. Immunohistochemistry performed in the rat cerebral cortex has demonstrated that both estrogen receptors alpha and beta are exclusively associated with neurons rather than glia in the cerebral cortex (Kritzer, 2002; Charitidi and Canlon, 2010). Cellular effects of estrogens are mediated through these receptors, which are present on either the cell surface or at intracellular locations. The biological effects of steroid hormones mainly arise through the classical mechanism of binding to specific intracellular receptors resulting in conformational change, dimerization and recruitment of coregulators for transcription-dependent genomic actions. Genomic actions are initiated when receptors in turn bind to classical estrogen response elements on DNA sequences resulting in synthesis of transcription factors that trigger the activation or inhibition of intracellular signaling cascades (McEwen and Alves, 1999; Luine and Frankfurt, 2012). A faster mechanism of estrogen action is mediated via an interaction with extranuclear estrogen receptors at the cell surface that then recruit G-protein coupled receptors and signaling kinases, enabling rapid effects of these hormones (Mani et al., 2012). Either or both these mechanisms of estrogen action could be relevant for hormonal priming of neurons within the auditory cortex during parturition, which would potentially allow neurons to be more receptive and malleable in response to pup calls.
A role for estrogen in behavioral memory has been tested in numerous paradigms in humans and rodents (Luine and Frankfurt, 2012; Pompili et al., 2012). When estrogen is administered over time, performance of rodents in memory tasks can be enhanced, whereas performance diminishes when subjects are ovariectomized, unless they are given supplemental estrogen (Luine and Frankfurt, 2012). A key aspect of memory is consolidation, the process by a short-term memories of experiences are converted into a long-term memories. Behavioral studies on the role of estrogen in memory consolidation have revealed that it can enhance consolidation in various learning tasks if delivered within 1 hour after the task is performed (Luine and Frankfurt, 2012). For example, in a test of object recognition memory, when estradiol or a synthetic estrogen diethylstilbestrol (DES) was administered in ovariectomized subjects immediately after behavioral training, and recognition retention was tested 4 hours later, DES-treated subjects discriminated between old and new objects, but ovariectomized subjects did not (Luine et al., 2003). Estrogen also enhanced spatial memory in rats performing a Morris water maze task, when administered immediately after the last learning trial. In a retention test 24 hours later, ovariectomized rats that received exogenous estradiol performed far better that ovariectomized controls (Packard, 1998). Even in the maternal context itself, multiparous mother rats display enhanced performance in hippocampal-based memory tasks as compared to inexperienced females (Kinsley et al., 2008; Macbeth et al., 2008).
Neurophysiologically, estrogen has been implicated in modulating several forms of plasticity, including neurogenesis, morphogenesis, and synaptic plasticity (Woolley et al., 1990; Woolley and McEwen, 1993; Brinton, 2009). In particular, estrogens have been shown to play an important role in such dendritic spine plasticity through both in vitro and in vivo studies (Srivastava et al., 2011; Srivastava, 2012). Changes in neuronal spine density and morphology can alter connectivity within neural circuits, and thus may provide a long-term substrate for enduring neuronal plasticity due to experience and learning (Ethell and Pasquale, 2005). Relevant for our hypothesis of estrogen effects on auditory cortical memories, treating cultures of cortical neurons with 17-beta estradiol increases dendritic spine protrusions within 30 minutes of application, and these changes can last for up to 60 minutes. Importantly, dendritic protrusions generated in this fashion can form functional connections with pre-synaptic terminals, suggesting that if estradiol levels are maintained, the new connections formed could contribute to circuit function. Since the estradiol-induced spines have a ‘thin’-like morphology, it is possible that the spines are associated with synapses that are dynamically active, so that they could be potentiated, stabilized or eliminated (Srivastava, 2012). That these processes actually occur in motherhood is already demonstrated in a study examining rat hippocampal dendritic spine densities. Both late-pregnant and lactating female rats, as well as ovariectomized virgins given an estrogen/progesterone regimen mimicking pregnancy, have significantly increased spine densities relative to controls with low hormone levels (Kinsley et al., 2006). These results help justify the focus on estrogen as a potential mediator for priming long-lasting plasticity in auditory cortical neurons.
In fact though, estrogen may not act only directly on cortical neurons to modulate synaptic plasticity and memory. Estrogen could have far-reaching effects on other neurochemical systems that may modulate plasticity in sensory areas (Miranda and Liu, 2009; Matragrano et al., 2011; Matragrano et al., 2012). For example, the forebrain cholinergic system that is frequently invoked in auditory cortical plasticity studies is maintained by estrogen (Gibbs and Aggarwal, 1998). Likewise, dopaminergic neurons in the VTA, which is directly activated by the MPOA in the canonical model for the maternal circuit, also express estrogen receptors (Shughrue et al., 1997; Creutz and Kritzer, 2002). Estrogen can also enhance the responses of glutamate AMPA and NMDA receptors, resulting in the activation of several signaling cascades (Cyr et al., 2001; McEwen, 2002).
Finally and importantly for motivating our hypothesis, recent research is accumulating evidence for an active role of estrogen in central auditory processing and memory, at least in avian species, where most work has focused on the zebra finch (Taeniopyggia guttata). Estrogen levels as measured by microdialysis are increased in the auditory area of the avian forebrain known as the caudomedial nidopallium (NCM) when a zebra finch hears conspecific song (Remage-Healey et al., 2008; Remage-Healey et al., 2010). The NCM is the songbird analog of the mammalian secondary auditory cortex, and it plays a key role in auditory discrimination and song memory. The enzyme aromatase and estrogen receptors are expressed at high levels in this region (Gahr et al., 1993; Peterson et al., 2005), and both locally synthesized and exogenously administered estradiol modulate auditory evoked activity in the NCM (Maney et al., 2006; Tremere et al., 2009; Caras et al., 2012). Estrogen has also been implicated in auditory memory storage in NCM. One study administered the aromatase inhibitor, fadrozole, systemically for days before exposing birds to songs (Yoder et al., 2012; Yoder and Vicario, 2012). Neural recordings in NCM several hours later then used an adaptation paradigm as a proxy for a neuronal memory of songs. Results from this experiment demonstrated that birds treated with fadrozole had significantly decreased memory for exposed songs compared to controls, but showed similar auditory processing of novel songs. Mechanistically, another study suggests that estradiol activates a MAPK cascade within NCM to facilitate long-lasting (several hour) changes in neural activity and coding (Tremere et al., 2012). Taken together, this research provides a proof-of-principle that high levels of estrogen can both immediately modulate central auditory neural activity evoked by sounds and mediate more persistent changes in this activity. It therefore lends support to our hypothesis that similar mechanisms may be active in the formation and maintenance of auditory memories in mammalian species.
Since the focus of this review has been on the role of the estrogen in establishing sensory maternal memories, in line with the focus of this Special Issue on steroid hormones, we have not elaborated much on the important role of other neurochemicals in the onset of maternal behaviors and the establishment of maternal sensory memories. However, two in particular bear some mention. First, the protein hormone, prolactin, has been demonstrated to help stimulate the onset of maternal behavior in virgin, steroidally primed female rats (Riddle et al., 1935; Zarrow et al., 1971; Bridges et al., 1985), where its primary site of action appears to be the MPOA (Bridges et al., 1990). Intriguingly, neurogenesis within the subventricular zone of pregnant mice is likely mediated by prolactin (Shingo et al., 2003), and gives rise to new olfactory interneurons that may help enable olfactory learning of infant odors and normal maternal responsiveness (Bridges and Grattan, 2003; Larsen and Grattan, 2010). It is possible this neurogenesis may also occur in sheep, and play a role in the immediate olfactory recognition of lambs by ewes discussed earlier (Keller et al., 2003). It is also worth noting that prolactin release is actually stimulated by estrogen (Chen and Meites, 1970), so that the latter could help orchestrate maternal behavior and memory through downstream effects on prolactin or other neurochemicals.
A second neurochemical of particular relevance is oxytocin, which contributes to social recognition and memory in mice (Ferguson et al., 2000; Ferguson et al., 2001; Choleris et al., 2009), and plays its own critical role in maternal behavior across species (Gimpl and Fahrenholz, 2001; Blanks and Thornton, 2003). In particular, if the major source of brain oxytocin, the hypothalamic paraventricular nucleus, is lesioned in pregnant rats, the induction of maternal behavior is significantly impaired (Insel and Harbaugh, 1989). This is likely due to the disruption of normal mechanisms that would mediate oxytocin’s facilitating effects on maternal behaviors, which have been demonstrated by infusing oxytocin directly into the paraventricular nucleus of sheep to presumably activate oxytocin autoreceptors there (Da Costa et al., 1996; Kendrick, 2000). A recent study also suggests that oxytocin may play an additional role in maternal memory. Infusing oxytocin antagonist into the postpartum rat’s NAcc, which is necessary for the consolidation of maternal memory as described in Section 4, impairs the reemergence of maternal behavior when tested 10 days after a 1 hour maternal experience (D’Cunha et al., 2011). While oxytocin expression can also be regulated by estrogen (Richard and Zingg, 1990), this may not occur strongly in the oxytocin-releasing neurons of the rat paraventricular nucleus (Peter et al., 1990; Gimpl and Fahrenholz, 2001), so that a more complete model of maternal sensory memories may require incorporation of distinct roles for both these hormones. Indeed, it should be emphasized that estrogens are only one of the important components of the molecular cascades that regulate maternal memory, but a complete review of all the potential players would be beyond the scope of the current review.
6. Conclusions
Overall, our synthesis of the literature on maternal behavior and memory with research on experience-dependent sensory cortical plasticity has led us to propose that the auditory cortex is a key site where an engram of pup vocalization cues is stored, and that estrogen-dependent mechanisms active in the period during pup care help prime auditory cortical neurons to create a longer-lasting memory of those sounds. Modified sensory circuits would presumably differentially activate the subcortical maternal circuit, which would then help facilitate the maintenance of maternal behaviors and mediate enhanced maternal responsiveness in multiparous females. Certainly a large number of experiments are needed to further test this hypothesis. For example, blocking estrogen receptors in the auditory cortex in mothers during parturition could shed light on the role of site-specific estrogen signaling for creating a long-term memory trace, both at the electrophysiological as well as behavioral levels. Other experiments could more deeply explore the molecular mechanisms of plasticity by assaying the expression of genes encoding key estrogen-modulated growth factors involved in synaptic plasticity, such as BDNF. In fact, there is evidence from sheep that BDNF and its receptor TrkB play roles in the consolidation of olfactory and visual memories of offspring. For example in one study (Broad et al., 2002), 2-3 hours following the initial formation of olfactory recognition memory for offspring, BDNF/TrkB-mediated plasticity changes are observed in brain areas involved in the consolidation of olfactory memory, such as the pyriform and entorhinal cortices. Similar changes also occur in areas of the brain involved in visual memory, face and object recognition (the temporal cortex, entorhinal cortex, hippocampal subfield CA1 and basolateral amygdala), and in regions with integrative and attentional functions (the medial frontal and anterior cingulate cortices and diagonal band). Finally, while our focus has been on sensory cortex’s role in maternal memory, there are obviously additional stages of processing before reaching the subcortical maternal circuit (figure 2); how these are modified by maternal experience and hormones remain to be explored. Nevertheless, we hope that the framework described here can provide a starting point to better understand the neurobiological mechanisms that transform behaviorally relevant sensory input into behavioral output in the maternal context.
Highlights.
New research is uncovering sensory cortex’s role in maternal behavior and memory
Infant cues are processed by sensory systems for recognition and discrimination
Experience-dependent cortical plasticity helps store an engram of infant cues
Maternal context provides natural paradigm for sensory cortical plasticity research
Estrogen-dependent mechanisms hypothesized to modulate sensory cortical plasticity
Acknowledgements
The authors thank NIH NIDCD (008343) and NSF CBN (IBN-9876754) for funding the original research in our lab on this topic. We also acknowledge Katy Shepard for providing feedback on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Regan E. Hormonal organization and activation: Evolutionary implications and questions. General and Comparative Endocrinology. 2012;176:279–285. doi: 10.1016/j.ygcen.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Afonso VM, Grella SL, Chatterjee D, Fleming AS. Previous maternal experience affects accumbal dopaminergic responses to pup-stimuli. Brain Research. 2008;1198:115–123. doi: 10.1016/j.brainres.2007.12.042. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces cs-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Research. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodeling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Beach FA., Jr. The neural basis of innate behavior. I. Effects of cortical lesions upon the maternal behavior pattern in the rat. Journal of Comparative Psychology. 1937;24:393–440. [Google Scholar]
- Beach FA., Jr The neural basis of innate behavior: II. Relative effects of partial decortication in adulthood and infancy upon the maternal behavior of the primiparous rat. The Pedagogical Seminary and Journal of Genetic Psychology. 1938;53:109–148. [Google Scholar]
- Blanks AM, Thornton S. The role of oxytocin in parturition. BJOG: An International Journal of Obstetrics & Gynaecology. 2003;110:46–51. doi: 10.1016/s1470-0328(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Alberts JR. Ultrasonic vocalizations by rat pups in the cold: An acoustic by-product of laryngeal braking? Behav Neurosci. 1990;104:808–817. doi: 10.1037//0735-7044.104.5.808. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Efimova IV, Alberts JR. Ultrasonic vocalizations by rat pups: The primary importance of ambient temperature and the thermal significance of contact comfort. Dev Psychobiol. 1992;25:229–250. doi: 10.1002/dev.420250402. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiology & Behavior. 1975;14:245–249. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Grattan DR. Prolactin-induced neurogenesis in the maternal brain. Trends in Endocrinology & Metabolism. 2003;14:199–201. doi: 10.1016/s1043-2760(03)00082-1. [DOI] [PubMed] [Google Scholar]
- Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science. 1985;227:782–784. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci U S A. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: Predictions for cognitive function. Trends in Pharmacological Sciences. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Mimmack ML, Keverne EB, Kendrick KM. Increased bdnf and trk-b mrna expression in cortical and limbic regions following formation of a social recognition memory. Eur J Neurosci. 2002;16:2166–2174. doi: 10.1046/j.1460-9568.2002.02311.x. [DOI] [PubMed] [Google Scholar]
- Brown M, Irvine DR, Park VN. Perceptual learning on an auditory frequency discrimination task by cats: Association with changes in primary auditory cortex. Cereb Cortex. 2004;14:952–965. doi: 10.1093/cercor/bhh056. [DOI] [PubMed] [Google Scholar]
- Budinger E, Scheich H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear Res. 2009 doi: 10.1016/j.heares.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative vertebrate neuroanatomy: Evolution and adaptation: Wiley-Liss. 2005 [Google Scholar]
- Caras ML, O’Brien M, Brenowitz EA, Rubel EW. Estradiol selectively enhances auditory function in avian forebrain neurons. J Neurosci. 2012;32:17597–17611. doi: 10.1523/JNEUROSCI.3938-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Canlon B. Estrogen receptors in the central auditory system of male and female mice. Neuroscience. 2010;165:923–933. doi: 10.1016/j.neuroscience.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Chen CL, Meites J. Effects of estrogen and progesterone on serum and pituitary prolactin levels in ovariectomized rats. Endocrinology. 1970;86:503–505. doi: 10.1210/endo-86-3-503. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009;30:442–459. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Cohen L, Rothschild G, Mizrahi A. Multisensory integration of natural odors and sounds in the auditory cortex. Neuron. 2011;72:357–369. doi: 10.1016/j.neuron.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: Regional, subregional, and cellular localization in the a10, a9, and a8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain nmda and ampa receptors. Brain Research Reviews. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- D’Cunha TM, King SJ, Fleming AS, Levy F. Oxytocin receptors in the nucleus accumbens shell are involved in the consolidation of maternal memory in postpartum rats. Hormones and Behavior. 2011;59:14–21. doi: 10.1016/j.yhbeh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Da Costa APC, Broad KD, Kendrick KM. Olfactory memory and maternal behaviour-induced changes in c-fos and zif/268 mrna expression in the sheep brain. Molecular Brain Research. 1997;46:63–76. doi: 10.1016/s0169-328x(96)00272-0. [DOI] [PubMed] [Google Scholar]
- Da Costa APC, Guevara-Guzman RG, Ohkura S, Goode JA, Kendrick KM. The role of oxytocin release in the paraventricular nucleus in the control of maternal behaviour in the sheep. Journal of Neuroendocrinology. 1996;8:163–177. doi: 10.1046/j.1365-2826.1996.04411.x. [DOI] [PubMed] [Google Scholar]
- Davis CD. The effect of ablations of neocortex on mating, maternal behavior and the production of pseudopregnancy in the female rat and on copulatory activity in the male. American Journal of Physiology. 1939;127:374–380. [Google Scholar]
- Del Vecchio T, Walter A, o,ÄôLeary SG. Affective and physiological factors predicting maternal response to infant crying. Infant Behavior and Development. 2009;32:117–122. doi: 10.1016/j.infbeh.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Hennevin E. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res. 2010;274:75–84. doi: 10.1016/j.heares.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Ehret G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature. 1987;325:249–251. doi: 10.1038/325249a0. [DOI] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds -- a gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Ehret G, Koch M. Ultrasound-induced parental behavior in house mice is controlled by female sex-hormones and parental experience. Ethology. 1989;80:81–93. [Google Scholar]
- Ehret G, Riecke S. Mice and humans perceive multiharmonic communication sounds in the same way. Proc Natl Acad Sci U S A. 2002;99:479–482. doi: 10.1073/pnas.012361999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Schmid C. Reproductive cycle-dependent plasticity of perception of acoustic meaning in mice. Physiol Behav. 2009;96:428–433. doi: 10.1016/j.physbeh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Ehret G, Koch M, Haack B, Markl H. Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften. 1987;74:47. doi: 10.1007/BF00367047. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Progress in Neurobiology. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Febo M, Felix-Ortiz AC, Johnson TR. Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats. Brain Research. 2010;1325:77–88. doi: 10.1016/j.brainres.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Stolberg TL, Numan M, Bridges RS, Kulkarni P, Ferris CF. Nursing stimulation is more than tactile sensation: It is a multisensory experience. Hormones and Behavior. 2008;54:330–339. doi: 10.1016/j.yhbeh.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Fichtel I, Ehret G. Perception and recognition discriminated in the mouse auditory cortex by c-{f}os labeling. Neuroreport. 1999;10:2341–2345. doi: 10.1097/00001756-199908020-00022. [DOI] [PubMed] [Google Scholar]
- Fleming A, Vaccarino F, Tambosso L, Chee P. Vomeronasal and olfactory system modulation of maternal behavior in the rat. Science. 1979;203:372–374. doi: 10.1126/science.760196. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Sarker J. Experience-hormone interactions and maternal behavior in rats. Physiology & Behavior. 1990;47:1165–1173. doi: 10.1016/0031-9384(90)90368-e. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Franks P, Surbey M, Schneider B, Steiner M. Postpartum factors related to mother’s attraction to newborn infant odors. Developmental psychobiology. 1993;26:115–132. doi: 10.1002/dev.420260204. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins AR, Zaika N, Bernstein H, Wachs M, Levis PA, Polley DB, Merzenich MM, Schreiner CE. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2013;16:79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Guttinger HA, Kroodsma DE. Estrogen receptors in the avian brain: Survey reveals general distribution and forebrain areas unique to songbirds. Journal of Comparative Neurology. 1993;327:112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- Galindo-Leon EE, Lin FG, Liu RC. Inhibitory plasticity in a lateral band improves cortical detection of natural vocalizations. Neuron. 2009;62:705–716. doi: 10.1016/j.neuron.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman R, Zarrow MX, Denenberg VH. Maternal behavior: Differences between mother and virgin mice as a function of the testing procedure. Developmental psychobiology. 1970;3:207–214. doi: 10.1002/dev.420030308. [DOI] [PubMed] [Google Scholar]
- Gardner CR, Phillips SW. The influence of the amygdala on the basal septum and preoptic area of the rat. Experimental Brain Research. 1977;29:249–263. doi: 10.1007/BF00237045. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. The Journal of Comparative Neurology. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geissler DB, Ehret G. Time-critical integration of formants for perception of communication calls in mice. Proc Natl Acad Sci U S A. 2002;99:9021–9025. doi: 10.1073/pnas.122606499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler DB, Sabine Schmidt H, Ehret G. Limbic brain activation for maternal acoustic perception and responding is different in mothers and virgin female mice. J Physiol Paris. 2012 doi: 10.1016/j.jphysparis.2012.05.006. in press. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Aggarwal P. Estrogen and basal forebrain cholinergic neurons: Implications for brain aging and alzheimer’s disease-related cognitive decline. Hormones and Behavior. 1998;34:98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G. Neuroendocrinology of maternal behavior in the rabbit. Hormones and Behavior. 2001;40:125–132. doi: 10.1006/hbeh.2001.1692. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Haack B, Markl H, Ehret G. Sound communication between parents and offspring. In: Willott JF, editor. The auditory psychobiology of the mouse. Charles C. Thomas; Springfield, IL: 1983. pp. 57–97. [Google Scholar]
- Han YK, Kover H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- Hansen S. Maternal-behavior of female rats with 6-ohda lesions in the ventral striatum - characterization of the pup retrieval deficit. Physiology & Behavior. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: A microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. Mesotelencephalic dopamine system and reproductive-behavior in the female rat - effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behavioral neuroscience. 1991;105:588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology. 1996;21:203–217. doi: 10.1016/0306-4530(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Huetz C, Philibert B, Edeline JM. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J Neurosci. 2009;29:334–350. doi: 10.1523/JNEUROSCI.3269-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Harbaugh CR. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol Behav. 1989;45:1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Nakabeppu Y, Morrell JI. Induction of c-fos like and fosb-like immunoreactivity reveals forebrain neuronal populations involved differentially in pup-mediated maternal behavior in juvenile and adult rats. Journal of Comparative Neurology. 2000;416:45–78. doi: 10.1002/(sici)1096-9861(20000103)416:1<45::aid-cne5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Keller M, Meurisse M, Poindron P, Nowak R, Ferreira G, Shayit M, Levy F. Maternal experience influences the establishment of visual/auditory, but not olfactory recognition of the newborn lamb by ewes at parturition. Developmental psychobiology. 2003;43:167–176. doi: 10.1002/dev.10130. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Oxytocin, motherhood and bonding. Experimental Physiology. 2000;85:111S–124S. doi: 10.1111/j.1469-445x.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Lévy F, Keverne EB. Changes in the sensory processing of olfactory signals induced by birth in sheep. Science. 1992;256:833–836. doi: 10.1126/science.1589766. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Guevara-Guzman R, Zorrilla J, Hinton MR, Broad KD, Mimmack M, Ohkura S. Formation of olfactory memories mediated by nitric oxide. Nature. 1997;388:670–674. doi: 10.1038/41765. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Levy F, Guevara-Guzman R, Kendrick KM. Influence of birth and maternal experience on olfactory bulb neurotransmitter release. Neuroscience. 1993;56:557–565. doi: 10.1016/0306-4522(93)90356-k. [DOI] [PubMed] [Google Scholar]
- Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 2012;35:715–722. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kinsley C, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Archives of Sexual Behavior. 2008;37:43–56. doi: 10.1007/s10508-007-9277-x. [DOI] [PubMed] [Google Scholar]
- Kinsley CH. The neuroplastic maternal brain. Hormones and Behavior. 2008;54:1–4. doi: 10.1016/j.yhbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Bridges RS. Morphine treatment and reproductive condition alter olfactory preferences for pup and adult male odors in female rats. Developmental psychobiology. 1990;23:331–347. doi: 10.1002/dev.420230405. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Amory-Meyer E. Why the maternal brain? Journal of Neuroendocrinology. 2011;23:974–983. doi: 10.1111/j.1365-2826.2011.02194.x. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EAA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Hormones and Behavior. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Koch M, Ehret G. Estradiol and parental experience, but not prolactin are necessary for ultrasound recognition and pup-retrieving in the mouse. Physiol Behav. 1989;45:771–776. doi: 10.1016/0031-9384(89)90293-x. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Regional, laminar, and cellular distribution of immunoreactivity for er alpha and er beta in the cerebral cortex of hormonally intact, adult male and female rats. Cerebral cortex. 2002;12:116–128. doi: 10.1093/cercor/12.2.116. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Kinsley CH. Brain and behavioral modifications that accompany the onset of motherhood. Parenting-Science and Practice. 2012;12:74–88. [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford H, Mencl E, Worhunsky P, Potenza MN, Mayes L. Maternal neural responses to infant cries and faces: Relationships with substance use. Frontiers in Psychiatry 2. 2011 doi: 10.3389/fpsyt.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: Effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behavioural brain research. 1999a;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Lee A, Li M, Watchus J, Fleming AS. Neuroanatomical basis of maternal memory in postpartum rats: Selective role for the nucleus accumbens. Behavioral neuroscience. 1999b;113:523–538. doi: 10.1037//0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- Levy F, Keller M. Olfactory mediation of maternal behavior in selected mammalian species. Behavioural Brain Research. 2009;200:336–345. doi: 10.1016/j.bbr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Levy F, Poindron P, Le Neindre P. Attraction and repulsion by amniotic fluids and their olfactory control in the ewe around parturition. Physiol Behav. 1983;31:687–692. doi: 10.1016/s0031-9384(83)80004-3. [DOI] [PubMed] [Google Scholar]
- Levy F, Keller A, Poindron P. Olfactory regulation of maternal behavior in mammals. Hormones and Behavior. 2004;46:284–302. doi: 10.1016/j.yhbeh.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behavioral neuroscience. 2003;117:426–445. doi: 10.1037/0735-7044.117.3.426. [DOI] [PubMed] [Google Scholar]
- Lin FG, Galindo-Leon EE, Ivanova TN, Mappus RC, Liu RC. A role for maternal physiological state in preserving auditory cortical plasticity for salient infant calls. Neuroscience. 2013;247C:102–116. doi: 10.1016/j.neuroscience.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. Plos Biology. 2007;5:1426–1439. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. European Journal of Neuroscience. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;114:3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Frontiers in Neuroendocrinology. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Gautreaux C, Luine VN. Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoaminergic neurotransmitters. Brain Res. 2008;1241:136–147. doi: 10.1016/j.brainres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Shayna Rosenbaum R. Implicit memory varies across the menstrual cycle: Estrogen effects in young women. Neuropsychologia. 2002;40:518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- Maletinska J, Spinka M, Vichova J, Stehulova I. Individual recognition of piglets by sows in the early post-partum period. Behaviour. 2002:975–991. [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Mani SK, Mermelstein PG, Tetel MJ, Anesetti G. Convergence of multiple mechanisms of steroid hormone action. Hormone and Metabolic Research. 2012;44:569–576. doi: 10.1055/s-0032-1306343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Sockman KW, Maney DL. Estradiol-dependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird. Eur J Neurosci. 2011;34:416–425. doi: 10.1111/j.1460-9568.2011.07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Beaulieu M, Sockman KW, Maney DL. Estradiol-dependent modulation of serotonergic markers in auditory areas of a seasonally breeding songbird. Behav Neurosci. 2012;126:110–122. doi: 10.1037/a0025586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. How it’s made: Organisational effects of hormones on the developing brain. Journal of Neuroendocrinology. 2010;22:736–742. doi: 10.1111/j.1365-2826.2010.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Vom Saal FS. The influence of reproductive state on infanticide by wild female house mice<i>(mus musculus)</i>. Physiology & behavior. 1985;35:843–849. doi: 10.1016/0031-9384(85)90248-3. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences. 2002;99:4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo AI, Gonzalez-Mariscal G. Placentophagia in rabbits: Incidence across the reproductive cycle. Developmental psychobiology. 2003;43:37–43. doi: 10.1002/dev.10118. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Liu RC. Dissecting natural sensory plasticity: Hormones and experience in a maternal context. Hearing Research. 2009;252:21–28. doi: 10.1016/j.heares.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltz H, Lubin M, Leon M, Numan M. Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiol Behav. 1970;5:1373–1377. doi: 10.1016/0031-9384(70)90122-8. [DOI] [PubMed] [Google Scholar]
- Montoya JL, Landi N, Kober H, Worhunsky PD, Rutherford HJV, Mencl WE, Mayes LC, Potenza MN. Regional brain responses in nulliparous women to emotional infant stimuli. PloS one. 2012;7:e36270. doi: 10.1371/journal.pone.0036270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HD, Watchus JA, Milgram NW, Fleming AS. The long lasting effects of electrical simulation of the medial preoptic area and medial amygdala on maternal behavior in female rats. Behavioural Brain Research. 1999;99:61–73. doi: 10.1016/s0166-4328(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Progress in Neurobiology. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Neumann ID. The advantage of social living: Brain neuropeptides mediate the beneficial consequences of sex and motherhood. Frontiers in Neuroendocrinology. 2009;30:483–496. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Nowak R, Keller M, Levy F. Mother-young relationships in sheep: A model for a multidisciplinary approach of the study of attachment in mammals. Journal of Neuroendocrinology. 2011;23:1042–1053. doi: 10.1111/j.1365-2826.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5:163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental psychobiology. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: Evidence for the involvement of preoptic projections to the ventral tegmental area. Behavioral neuroscience. 1984;98:712. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Annals of the New York Academy of Sciences. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. Springer; 2003. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. Journal of Comparative and Physiological Psychology. 1977;91:146. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, English JB. Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Horm Behav. 1993;27:56–81. doi: 10.1006/hbeh.1993.1005. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005a;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of d1 or d2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behavioral neuroscience. 2005b;119:1588. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. The Journal of Comparative Neurology. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H. Learning-induced plasticity in animal and human auditory cortex. Curr Opin Neurobiol. 2005;15:470–477. doi: 10.1016/j.conb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Okabe S, Nagasawa M, Mogi K, Kikusui T. The importance of mother-infant communication for social bond formation in mammals. Animal Science Journal. 2012;83:446–452. doi: 10.1111/j.1740-0929.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- Okon EE. The effect of environmental temperature on the production of ultrasound by isolated, non-handled, albino mouse pups. J Zool, London. 1970;162:71–83. [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Hormones and Behavior. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Parlee MB. Menstrual rhythms in sensory processes: A review of fluctuations in vision, olfaction, audition, taste, and touch. Psychol Bull. 1983;93:539–548. [PubMed] [Google Scholar]
- Penton-Voak IS, Perrett DI, Castles DL, Kobayashi T, Burt DM, Murray LK, Minamisawa R. Menstrual cycle alters face preference. Nature. 1999;399:741–742. doi: 10.1038/21557. [DOI] [PubMed] [Google Scholar]
- Pereira M, Morrell JI. The medial preoptic area is necessary for motivated choice of pup-over cocaine-associated environments by early postpartum rats. Neuroscience. 2010;167:216–231. doi: 10.1016/j.neuroscience.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Morrell JI. Functional mapping of the neural circuitry of rat maternal motivation: Effects of site-specific transient neural inactivation. Journal of Neuroendocrinology. 2011;23:1020–1035. doi: 10.1111/j.1365-2826.2011.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J, Burbach H, Adan RAH, van Tol HHM, Verbeeck MAE, Axelson JF, van Leeuwen FW, Beekman JM, Ab G. Regulation of the rat oxytocin gene by estradiol. Journal of Neuroendocrinology. 1990;2:633–639. doi: 10.1111/j.1365-2826.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Royal Society B: Biological Sciences. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Tremere LA. Control of central auditory processing by a brain-generated oestrogen. Nat Rev Neurosci. 2012;13:521–527. doi: 10.1038/nrn3291. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili A, Arnone B, Gasbarri A. Estrogens and memory in physiological and neuropathological conditions. Psychoneuroendocrinology. 2012;37:1379–1396. doi: 10.1016/j.psyneuen.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S, Zingg HH. The human oxytocin gene promoter is regulated by estrogens. Journal of Biological Chemistry. 1990;265:6098–6103. [PubMed] [Google Scholar]
- Riddle O, Lahr EL, Bates RW. Maternal behavior induced in virgin rats by prolactin. Proceedings of the Society for Experimental Biology and Medicine. 1935;32:730–734. [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Rosselet C, Zennou-Azogui Y, Xerri C. Nursing-induced somatosensory cortex plasticity: Temporally decoupled changes in neuronal receptive field properties are accompanied by modifications in activity-dependent protein expression. J Neurosci. 2006;26:10667–10676. doi: 10.1523/JNEUROSCI.3253-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan VF, Byrnes EM, Bridges RS. Reproductive experience and activation of maternal memory. Behavioral neuroscience. 2006;120:676. doi: 10.1037/0735-7044.120.3.676. [DOI] [PubMed] [Google Scholar]
- Schnupp JW, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci. 2006;26:4785–4795. doi: 10.1523/JNEUROSCI.4330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searby A, Jouventin P. Mother-lamb acoustic recognition in sheep: A frequency coding. Proc Biol Sci. 2003;270:1765–1771. doi: 10.1098/rspb.2003.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup-but not cocaine-paired contexts. Behavioral neuroscience. 2009;123:1325. doi: 10.1037/a0017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell GD. Ultrasound in rodents. Nature. 1968;217:682–683. [Google Scholar]
- Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- Shair HN, Brunelli SA, Masmela JR, Boone E, Hofer MA. Social, thermal, and temporal influences on isolation-induced and maternally potentiated ultrasonic vocalizations of rat pups. Dev Psychobiol. 2003;42:206–222. doi: 10.1002/dev.10087. [DOI] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of pkm zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Shepard KN, Kilgard MP, Liu RC. Experience-dependent plasticity and the auditory cortex. In: Cohen YE, Popper AN, Fay RR, editors. Neural correlates of auditory cognition. Springer; New York: 2012. pp. 293–327. [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mrna in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. Estrogen-induced maternal-behavior in hysterectomized ovariectomized virgin rats. Physiology & Behavior. 1975;14:465–471. doi: 10.1016/0031-9384(75)90012-8. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual Review of Neuroscience. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. The Journal of Comparative Neurology. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: A phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. The Journal of Comparative Neurology. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. Journal of Comparative and Physiological Psychology. 1975;88:118. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behavioral Biology. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Curr Opin Neurobiol. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP. Two-step wiring plasticity - a mechanism for estrogen-induced rewiring of cortical circuits. Journal of Steroid Biochemistry and Molecular Biology. 2012;131:17–23. doi: 10.1016/j.jsbmb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Waters EM, Mermelstein PG, Kramar E, Shors TJ, Liu F. Rapid estrogen signaling in the brain: Implications for the fine-tuning of neuronal circuitry. The Journal of Neuroscience. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine d1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behavioral neuroscience. 2007;121:907. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Funamizu A, Mitsumori Y, Kose H, Kanzaki R. Progressive plasticity of auditory cortex during appetitive operant conditioning. Biosystems. 2010;101:37–41. doi: 10.1016/j.biosystems.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis IL, Jensen O, Talamini LM, Rijpkema M, Fernandez G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–10093. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS. Humoral factors underlying maternal behavior at parturition: Cross transfusion between freely moving rats. Journal of Comparative and Physiological Psychology. 1972;80:365–371. doi: 10.1037/h0032965. [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. The Journal of Neuroscience. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Kovaleski RF, Burrows K, Jeong JK, Pinaud R. Mechanistic basis and functional roles of long-term plasticity in auditory neurons induced by a brain-generated estrogen. J Neurosci. 2012;32:16478–16495. doi: 10.1523/JNEUROSCI.3233-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vince MA, Billing AE, Baldwin BA, Toner JN, Weller C. Maternal vocalisations and other sounds in the fetal lamb’s sound environment. Early human development. 1985;11:179–190. doi: 10.1016/0378-3782(85)90105-7. [DOI] [PubMed] [Google Scholar]
- Walpurger V, Pietrowsky R, Kirschbaum C, Wolf OT. Effects of the menstrual cycle on auditory event-related potentials. Horm Behav. 2004;46:600–606. doi: 10.1016/j.yhbeh.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci U S A. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Infant rat separation is a sensitive test for novel anxiolytics. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:745–757. doi: 10.1016/0278-5846(91)90003-j. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. The Journal of Neuroscience. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Vicario DS. To modulate and be modulated: Estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. Behavioral neuroscience. 2012;126:17. doi: 10.1037/a0026673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Lu K, Vicario DS. Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport. 2012;23:922–926. doi: 10.1097/WNR.0b013e3283588b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrow MX, Gandelman R, Denenberg VH. Prolactin: Is it an essential hormone for maternal behavior in the mammal? Hormones and Behavior. 1971;2:343–354. [Google Scholar]