Abstract
Background
A subset of HPV-associated oropharyngeal squamous cell carcinoma (HPV-OSCC) patients experience poor clinical outcomes. We explored prognostic risk factors on overall survival (OS) and recurrence free survival (RFS).
Methods
Patients with incident HPV-OSCC treated at the Johns Hopkins Hospital between 1997– 2008 with available tissue for HPV testing and demographic and clinicopathologic information (N=176) were included. Tissue was tested for HPV by in situ hybridization (ISH) and/or p16 immunohistochemistry (IHC). Demographic and clinicopathologic information was extracted from medical records.
Results
90% (157/176) of the OSCC cases were HPV-associated. In the HPV-OSCC patients, we observed a 3- and 5-year OS rate of 93% (95% CI: 88%–98%) and 89% (95% CI: 81%–97%), respectively. Lower survival was observed with older patient age (HR 2.33 per 10-year increase, CI: 1.05–5.16, p=0.038), advanced clinical T-stage (HR 5.78, 95% CI: 1.60–20.8, p=0.007), and current tobacco use (HR 4.38, 95% CI: 1.07–18.0, p=0.04). Disease recurrence was associated with advanced clinical T stage (HR 8.32, 95% CI: 3.06–23, p<0.0001), current/former alcohol use (HR 13, 95% CI: 1.33–120, p=0.03), and unmarried status (HR 3.28, 95% CI: 1.20–9.00, p=0.02).. Patients who remained recurrence-free for 5 years had an 8.6% chance of recurrence by 10 years (one-sided 95% CI upper bound is 19%, p=0.088).
Conclusions
Prognostic risk factors are identified for HPV-OSCC patients. Observed recurrence rates between 5 and 10 years following definitive therapy needs to be validated in additional studies to determine whether extended cancer surveillance is warranted in this cancer population.
Keywords: HPV, head and neck cancer, oropharyngeal cancer, gender, risk factors
INTRODUCTION
Despite decreases in per capita smoking prevalence and alcohol consumption in the U.S over the past several decades, the incidence of oropharyngeal squamous cell carcinomas (OSCC) has steadily increased1–3. The increasing incidence in OSCC is attributed to a growing number of cancers caused by the human papillomavirus (HPV)3, 4. Indeed, recent research has shown that HPV-associated OSCC (HPV-OSCC) is a distinct etiologic entity, and this subset of head and neck cancers is associated with an improved prognosis as compared to HPV-negative head and neck cancers5–13. Although the improved survival rates of HPV-OSCC patients have been well established, clinically there is a subset of patients who still experience poor outcomes.
Recent survival analysis demonstrated important prognostic differences between HPV-positive and HPV-negative OPSCC. However, prognostic factors for HPV-OSCC patients are less clear, but of significant interest given the consideration of de-escalation treatment strategies for this patient population. Therefore, we analyzed predictors of survival in a series of HPV-OSCC patients treated at a single academic institution.
METHODS
Study Subjects
Patients who were treated for OSCC (ICD0 codes C01.9, C02.4, C09.0–10.9) at the Johns Hopkins Hospital between 1997 to 2008 were screened for eligibility. Patients were included if they had a histologically confirmed diagnosis of incident OSCC, and available tissue for HPV testing. A modest number of individuals (n=46) who did not have tumor available for testing but had previously recorded HPV and/or p16 results were also included.
The medical records of all eligible patients were retrospectively reviewed to collect patient demographic (age, sex, race [as specified by patient during hospital registration], marital status) and clinicopathologic (site of tumor, date of cancer diagnosis, tumor stage, tobacco and/or alcohol use, primary treatment modality, treatment duration, recurrence, HPV status, and date of last follow-up or death) information. Follow-up was defined as time elapsed between the date when treatment was completed and last recorded date of clinic follow-up or date of death. If the demographic and/or clinicopathologic information could not be extracted from the medical records, patients were excluded from the study. Survival data were confirmed with the national death index database. IRB approval was obtained to perform this study and an exemption obtained to get informed consent from patients retrospectively since patients were de-identified after demographic extraction from the medical records.
Laboratory Analyses
Determination of HPV Status
Tumor HPV status was determined using either HPV-16 in situ hybridization (ISH), p16 immunohistochemistry (IHC), or both14. All slides were reviewed by a head and neck pathologist (WW) and HPV-16 ISH and p16 IHC status assessed. HPV tumor status was reported as positive if either ISH or IHC tests were positive. In cases in which there was a discrepancy between HPV-16 ISH and p16 IHC, a positive result by either test was considered HPV-associated.
HPV-16 in situ hybridization
Briefly, 5-µm thick paraffin-embedded tumor sections were deparaffinized. Heat-induced target retrieval was performed in citrate buffer. The samples then were digested with Proteinase K (20 µg/mL; Roche Diagnostics, Indianapolis, IN) and hybridized with a biotinylated HPV-16 DNA specific probe (Dako GenPoint, Carpinteria, CA). Streptavidin-horseradish peroxidase complex, biotinyl tyramide, and streptavidin-horseradish peroxidase complex were consecutively applied for signal amplification. Samples were incubated with chromogenic substrate diaminobenzidine. Punctate hybridization signals that localized to the tumor cell nuclei defined a HPV-positive tumor.
P16 immunohistochemistry
Briefly, 5-µm thick paraffin-embedded tumor sections were deparaffinized. Target retrieval was performed using heat-induced epitope retrieval with citrate buffer. Tissue sections were subsequently incubated with p16 monoclonal mouse antibody (1:500 dilution; MTM Laboratories, Heidelberg, Germany). Visualization of p16 antibody binding was achieved via the avidin-biotin-peroxidase technique (Dako LSAB kit, Dako Cytomation, Carpinteria, CA). P16 expression was scored as positive if strong and diffuse nuclear and cytoplasmic staining was present in ≥70% of the tumor.
Statistical Analysis
Descriptive statistics was used to characterize the study cohort. Differences in patient demographic and clinicopathologic characteristics between men and women diagnosed with HPV-OSCC were compared using Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables. Overall survival (OS) was computed as the interval from the date of end of treatment to the date of death from any cause, censored at the last follow-up time the subject was known to be alive. Recurrence free survival (RFS) was defined as the time from the date of end of treatment to the date of disease recurrence, censored at the earliest of the last follow-up or death prior to recurrence.
The associations of demographic and clinical factors with risk of death or disease recurrence in the HPV-positive cohort were first evaluated using the univariate Cox proportional hazards models and then the multivariable models. Unadjusted (univariate analysis) and adjusted (multivariate analysis) hazard ratios and corresponding 95% confidence intervals were estimated. In the multivariable analysis, race (white, black, and other), gender, age (continuous), marital status (married and unmarried (single, divorced or widowed)), alcohol use (never, former/current), tobacco use (never, former, and current), primary therapy (chemoradiation, radiation, and surgery), clinical T stage (Tx/T1/T2 and T3/T4) and N stage (Nx/N0/N1 and N2/N3) were considered in the initial models. All variables with p values <0.15 were retained in the final model with a backwards elimination procedure. As race and gender were of interest, these two variables were retained in the model regardless of statistical significance. OS and RFS were summarized using nonparametric Kaplan-Meier survival curves as well as direct adjusted survival curves that averaged the individual predicted survival curves based on unstratified Cox models15. The method of direct adjustment controls for possible confounders due to an imbalance of patient characteristics between groups. The above analyses were performed separately for OS and RFS. Proportional hazards assumption was verified by creating a time-dependent covariate for each predictor and testing time-dependent covariates all at once for its significance in the Cox model; no violations were noted. The 5-year conditional RFS of patients who were free of disease 5 years following the treatment was estimated as the 10-year RFS dividing by the 5-year RFS, and the corresponding standard error estimated with a variation of Greenwood’s formula. All statistical tests were two-sided, unless otherwise indicated, and considered statistically significant at p<0.05. The analyses were carried out using SAS (version 9.2, SAS Institute, Cary, NC) and R statistical software (version 2.12.0).
RESULTS
Four hundred forty-five patients with incident OSCC were treated at the Johns Hopkins Hospital between 1997 to 2008. Of these patients, 176 (40%) subjects had available tissue for HPV testing and complete demographic and clinicopathologic information and, thus, were included in this analysis.
HPV testing
We tested 176 OSCC for HPV status, including 130 tumors that were tested by both HPV16 ISH and p16 IHC, 43 tumors tested by HPV16 ISH only, and 3 tumors tested by p16 IHC only. Of the 130 samples tested by both HPV16 ISH and p16 IHC, 11 cases had discordant results (10 were HPV16 ISH-negative/p16-positive and one was HPV16 ISH-positive/p16-negative). A positive result by either ISH or IHC was considered HPV-associated (90%, 157/176 cases). HPV positivity was 90% (120/133 samples) by p16 IHC and lowered to 83% (144/173 samples) with HPV16 ISH testing. HPV16 ISH is considered to be a highly specific test but does not capture disease caused by other oncogenic HPV types, while p16 IHC is a highly sensitive method, which accounts for the higher HPV positivity rate using the latter testing method16.
Demographic and clinicopathologic characteristics
HPV-OSCC patients were primarily male (87%, 136/157) and white (78%, 122/157), with a median age of 54 years (range 28.7–80.3). HPV-positive patients were more likely to be married (79.6% vs 20.4%, p=0.042), never tobacco users (40.0% vs 15.2%, p=0.017) and diagnosed with an advanced N stage (77.1% vs 22.9%, p=0.007) than HPV-negative patients. We had a mixed population of patients treated with various treatment modalities. 51% (80/157) of the HPV-OSCC patients were treated with open surgery and 49% (77/157) treated with non-surgical therapy. Of the 80 surgical patients, 79 patients (98.8%) received adjuvant therapy: 40 patients (50%) received adjuvant concurrent chemoradiation therapy and 39 patients (48.8%) received adjuvant radiation therapy alone. Demographic and clinicopathologic characteristics of the HPV-positive and HPV-negative patients are presented in Table 1.
Table 1.
Patient demographic and clinicopathologic characteristics by HPV status
| Characteristic | Total (n=176) |
HPV | P value* | |
|---|---|---|---|---|
| Negative (n=19) |
Positive (n=157) |
|||
| Age at end of treatment (years) | 0.074 | |||
| Mean ± SD | 54.5 ± 9.0 | 57.7 ± 10.7 | 54.1 ± 8.7 | |
| Median (range) | 54 (28– 80) | 61 (30 – 73) | 54 (29– 80) | |
| Gender | 0.17 | |||
| Male | 150 (85.2) | 14 (73.7) | 136 (86.6) | |
| Female | 26 (14.8) | 5 (26.3) | 21 (13.4) | |
| Race | < 0.0001 | |||
| White | 153 (86.9) | 11 (57.9) | 142 (90.4) | |
| Black | 19 (10.8) | 8 (42.1) | 11 (7.0) | |
| Other | 4 (2.3) | 0 (0) | 4 (2.6) | |
| Marital status | 0.004 | |||
| Single/ Divorced/widowed | 42 (23.9) | 10 (52.6) | 32 (20.4) | |
| Married | 134 (76.1) | 9 (47.4) | 125 (79.6) | |
| Alcohol use | 0.008 | |||
| None | 38 (23.6) | 4 (22.2) | 34 (23.8) | |
| Former | 17 (10.6) | 6 (33.3) | 11 (7.7) | |
| Current | 106 (65.8) | 8 (44.4) | 98 (68.5) | |
| Tobacco use | 0.029 | |||
| None | 61 (37.2) | 3 (15.8) | 58 (40.0) | |
| Former | 74 (45.1) | 9 (47.4) | 65 (44.8) | |
| Current | 29 (17.7) | 7 (36.8) | 22 (15.2) | |
| Primary therapy | 0.22 | |||
| Chemoradiation | 73 (41.5) | 11 (57.9) | 62 (39.5) | |
| Radiation | 15 (8.5) | 0 (0) | 15 (9.5) | |
| Surgery | 88 (50) | 8 (42.1) | 80 (51.0) | |
| Clinical T stage | 0.155 | |||
| Tx/T1/T2 | 151 (85.8) | 14 (73.7) | 137 (87.3) | |
| T3/T4 | 25 (14.2) | 5 (26.3) | 20 (12.7) | |
| Clinical N stage | 0.775 | |||
| Nx/N0/N1 | 41 (23.3) | 5 (26.3) | 36 (22.9) | |
| N2/N3 | 135 (76.7) | 14 (73.7) | 121 (77.1) | |
| Clinical M stage | ||||
| M0 | 176 (100) | 19 (100) | 157 (100) | |
Nonparametric Wilcoxon rank sum test was used for continuous variables and Fisher’s exact test was used for categorical variables.
While the patient population was primarily white and male, there were 11 black patients and 4 patients of another race. In addition, there were 21 (13%) women diagnosed with HPV-OSCC. Demographic and clinicopathologic characteristics of the HPV-positive men and women are presented in Table 2. Analysis of RFS and OS in these HPV-positive patients was performed to determine risk factors which contributed to a poor prognosis.
Table 2.
Demographic and clinicopathologic characteristics among HPV+ patients
| Characteristics | Total (n=157) |
Gender | P* | |
|---|---|---|---|---|
| Women (n=21) |
Men (n=136) |
|||
| Age at end of treatment (years) | ||||
| Mean ± SD | 54.1 ± 8.7 | 54.4 (± 10.7) | 54.1 (± 8.4) | |
| Median (range) | 54 (28.7–80.3) | 53.6 (32.2–75.7) | 54.1 (28.7–80.3) | 0.648 |
| Race | 0.614 | |||
| White | 142 (90.4) | 20 (95.2) | 122 (89.7) | |
| Black | 11 (7.0) | 1 (4.8) | 10 (7.4) | |
| Other | 4 (2.6) | 0 (0) | 4 (2.9) | |
| Marital status | 0.042 | |||
| Single/ Divorced/widowed | 32 (20.4) | 8 (38.1) | 24 (17.7) | |
| Married | 125 (79.6) | 13 (61.9) | 112 (82.3) | |
| Alcohol use | 0.249 | |||
| Never | 34 (21.6) | 2 (11.8) | 32 (25.4) | |
| Former/Current | 109 (69.4) | 15 (88.2) | 94 (74.6) | |
| Tobacco use | 0.017 | |||
| Never | 58 (40.0) | 12 (70.6) | 46 (35.9) | |
| Former | 65 (44.8) | 3 (17.6) | 62 (48.4) | |
| Current | 22 (15.2) | 2 (11.8) | 20 (15.6) | |
| Primary therapy | 0.003 | |||
| Chemoradiation | 62 (39.5) | 12 (57.1) | 50 (36.8) | |
| Radiation | 15 (9.5) | 5 (23.8) | 10 (7.3) | |
| Surgery | 80 (51.0) | 4 (19.1) | 76 (55.9) | |
| Clinical T stage | 0.478 | |||
| Tx/T1/T2 | 137 (87.3) | 17 (80.9) | 120 (88.2) | |
| T3/T4 | 20 (12.7) | 4 (19.1) | 16 (11.8) | |
| Clinical N stage | 0.007 | |||
| Nx/N0/N1 | 36 (22.9) | 10 (47.6) | 26 (19.1) | |
| N2/N3 | 121 (77.1) | 11 (52.4) | 110 (80.9) | |
| Clinical M stage | ||||
| M0 | 157 (100) | |||
Nonparametric Wilcoxon rank sum test was used for continuous age and Fisher’s exact test was used for other categorical variables.
Overall survival analysis
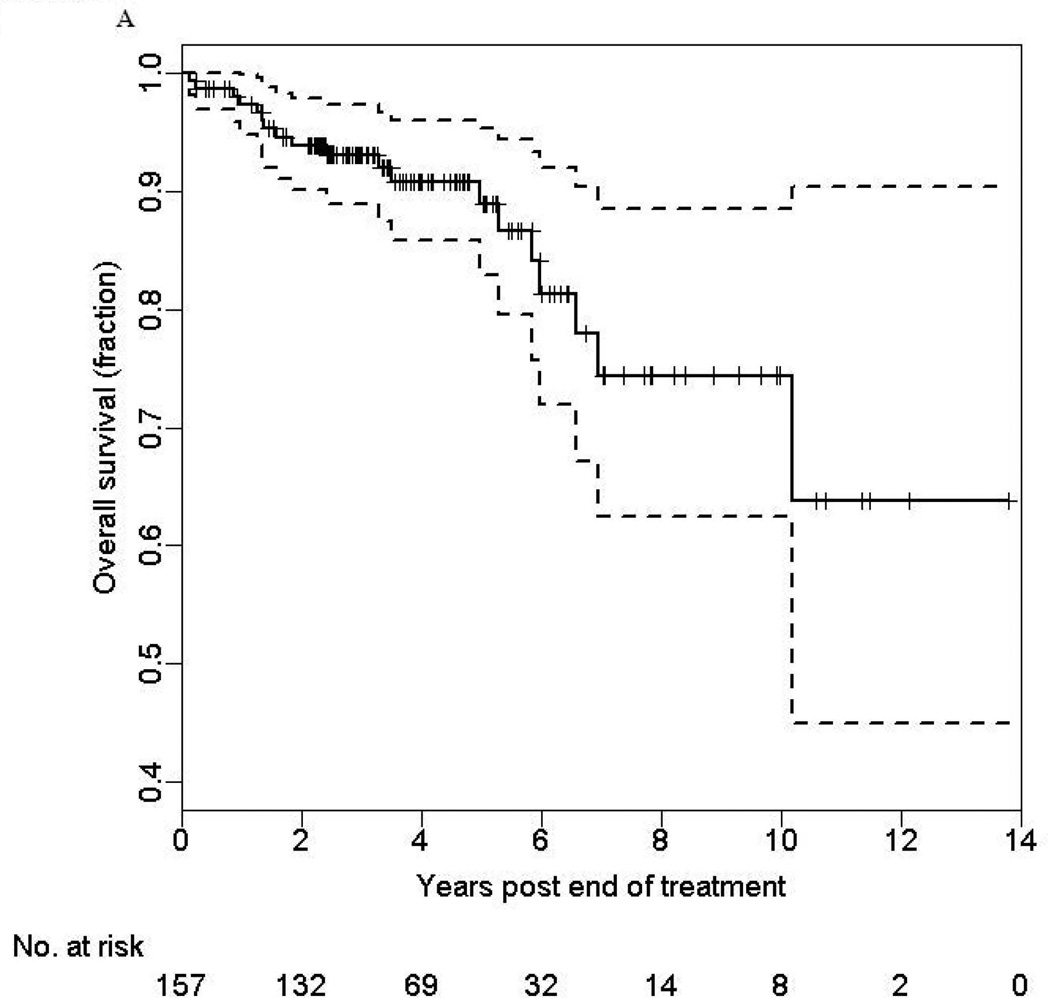
The OS rates at 3- and 5-years were 93% (95% CI: 88% –98%) and 89% (95% CI: 81% –97%), respectively (Figure 1a). The median follow-up was 42 months (range 1.8–167.0 months). Interestingly, we found that risk of disease specific death stabilized between years 2 through 5 and then increased between years 5 through 10. In univariate analysis, OS of HPV-positive patients was reduced among patients who were older (HR 2.56 per 10-year increase in age, 95% CI:1.41–4.67, p=0.002), had advanced clinical T-stage (HR 4.77, 95% CI:1.72–13.2, p=0.003), and were unmarried (HR 3.19, CI: 1.28–7.95, p=0.01) (Table 3).
FIGURE 1.
Table 3.
Association of risk factors with overall survival (OS) and recurrence free survival (RFS) among HPV-positive patients.
| Variable | Overall Survival | Recurrence-free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis2 | Univariate Analysis | Multivariate Analysis3 | |||||
| Hazard ratio (95% CI) |
P | Hazard ratio (95% CI) |
P | Hazard ratio (95% CI) |
P | Hazard ratio (95% CI) |
P | |
| Age at end of treatment (years) | 2.56 (1.41 – 4.67)1 | 0.002 | 2.33 (1.05 – 5.16)1 | 0.038 | 1.51 (0.86 – 2.64)1 | 0.15 | ||
| Gender | ||||||||
| Male | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Female | 1.98 (0.71, 5.5) | 0.19 | 2.11 (0.55, 8.0) | 0.274 | 1.51 (0.50, 4.54) | 0.46 | 0.87 (0.23, 3.31) | 0.84 |
| Race | 0.07 | 0.026 | 0.18 | 0.004 | ||||
| White | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Black | 0.65 (0.09, 4.9) | 0.67 | 2.14 (0.23, 20) | 0.505 | 2.40 (0.69, 8.33) | 0.17 | 4.77 (0.95, 24) | 0.06 |
| Other | 5.38 (1.22, 24) | 0.03 | 16.5 (2.15, 126) | 0.007 | 4.10 (0.54, 31) | 0.17 | 47 (4.20, 528) | 0.002 |
| Marital status | ||||||||
| Married | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Single/Divorced/Widowed | 3.19 (1.28, 8) | 0.01 | 2.63 (0.89, 7.84) | 0.082 | 2.63 (1.05, 6.62) | 0.04 | 3.28 (1.20, 9.00) | 0.02 |
| Alcohol use | ||||||||
| Never | 1.00 | - | - | 1.00 | - | 1.00 | - | |
| Former/Current | 0.85 (0.30, 2.42) | 0.76 | 6.02 (0.80, 45) | 0.08 | 13 (1.33, 120) | 0.03 | ||
| Tobacco use | 0.38 | 0.117 | 0.95 | |||||
| Never | 1.00 | - | 1.00 | - | 1.00 | - | ||
| Former | 1.16 (0.40, 3.36) | 0.79 | 1.69 (0.50, 5.71) | 0.40 | 0.91 (0.34, 2.43) | 0.85 | ||
| Current | 2.20 (0.70, 7.0) | 0.18 | 4.38 (1.07, 18) | 0.04 | 0.79 (0.17, 3.74) | 0.77 | ||
| Primary therapy | 0.66 | 0.81 | ||||||
| Radiation | 1.00 | - | - | 1.00 | - | |||
| Chemo-radiation | 1.21 (0.22 – 6.6) | 0.83 | 1.28 (0.27, 6.07) | 0.76 | ||||
| Surgery | 1.76 (0.38 – 8.1) | 0.47 | 0.94 (0.20, 4.42) | 0.94 | ||||
| Clinical T stage | ||||||||
| Tx/T1/T2 | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| 3/T4 | 4.77 (1.72 – 13) | 0.003 | 5.78 (1.60, 21) | 0.007 | 7.30 (2.95, 18) | <0.0001 | 8.32 (3.06, 23) | <0.0001 |
| Clinical N stage | ||||||||
| N2/N3 | 1.00 | - | 1.00 | - | 1.00 | - | ||
| Nx/N0/N1 | 2.45 (0.98, 6.1) | 0.06 | 4.94 (1.53, 16) | 0.007 | 1.79 (0.71, 4.48) | 0.22 | ||
Hazard ratio per 10-year increase in age.
Final model for OS included age at end of treatment, gender, race, marital status, tobacco use, clinical T stage and N stage.
Final model for RFS included gender, race, marital status, alcohol use, and clinical T stage.
In multivariate analysis of the HPV-positive patients, OS was worse among patients with an older age (HR 2.33 per 10-year increase, 95% CI: 1.05–5.16, p=0.038), advanced clinical T-stage (HR 5.78, 95% CI: 1.60–20.8, p=0.007), less advanced clinical N-stage (HR 4.94, 95% CI: 1.53–16.0, p=0.007), and current tobacco use as compared to never tobacco use (HR 4.38, 95% CI: 1.07–18.0, p=0.04) (Table 3). Alcohol use and primary therapy modality were not associated with differences in OS. Gender and race did not have statistically significant differences in survival, however the hazard ratios for women and non-white individuals were all above 2.0 (Table 3).
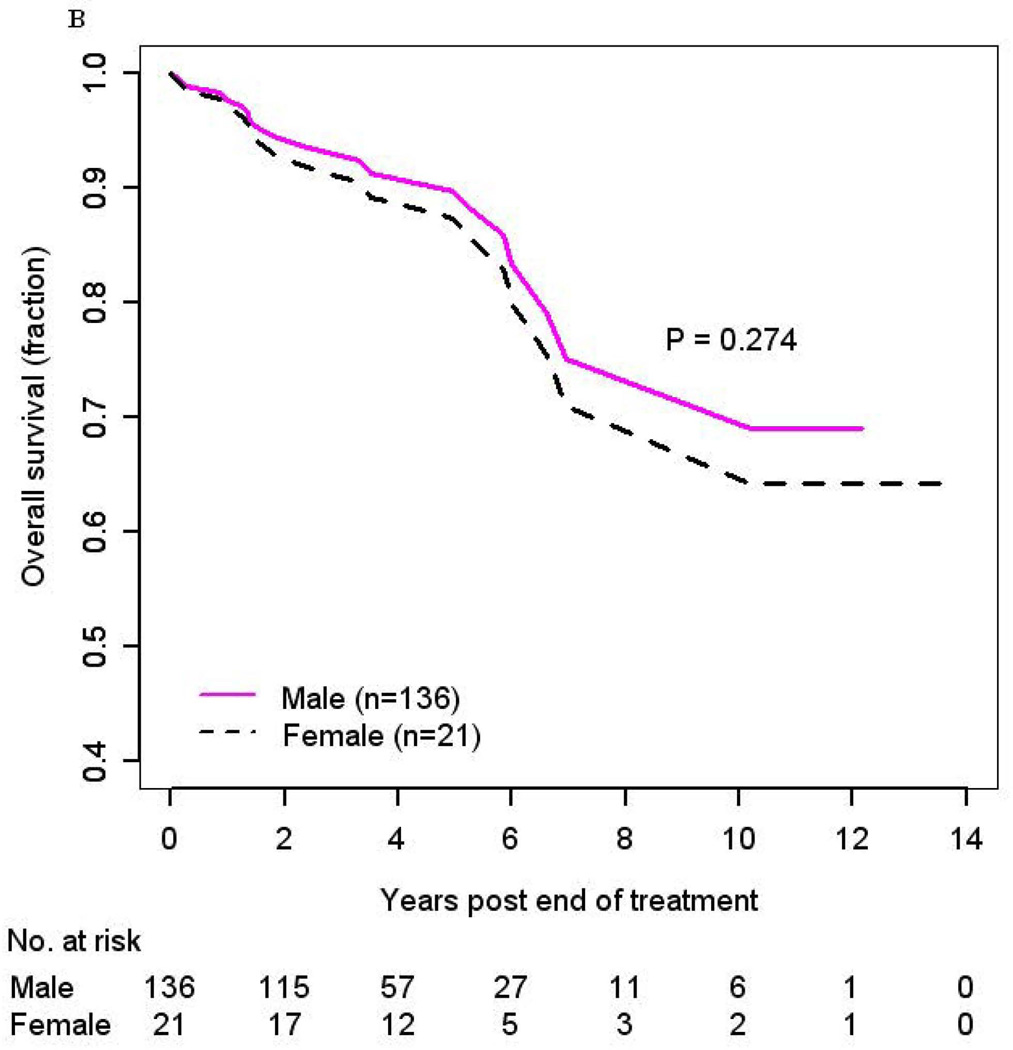
The impact of gender on OS was further evaluated with Kaplan-Meier curves. There was a trend that women (N=21) might have a higher risk of death as compared to men (N=136) in both adjusted (HR 2.11, 95% CI: 0.55–8.0, p=0.274) (Figure 1b) and unadjusted (Supplemental Figure 1) (HR 1.98, 95% CI: 0.71–5.5, p=0.19) analysis, however this difference did not reach statistical significance.
The effect of race on OS in HPV-positive patients was also explored. A slightly higher but not statistically different risk of death was observed in adjusted (HR 2.14, 95% CI: 0.23–20, p=0.51) analysis among blacks (N=11) with HPV-OSCC compared to whites (N=142) with HPV-OSCC (Supplemental Figure 2). The small number of death events among blacks (N=1) as compared to whites (N=12) during a similar follow-up period (median 48 months for blacks and 42 months for whites) limited the power for this analysis. A significant increased risk of death was observed among cases of other race (HR 16.5, 95% CI: 2.15–126, p=0.007), although this was based upon only 4 cases of other race (Table 3). A sensitivity analysis was performed to determine whether this small group affected our results; and the results remained unchanged with the exclusion of these 4 patients of other race.
Recurrence free survival (RFS) analysis
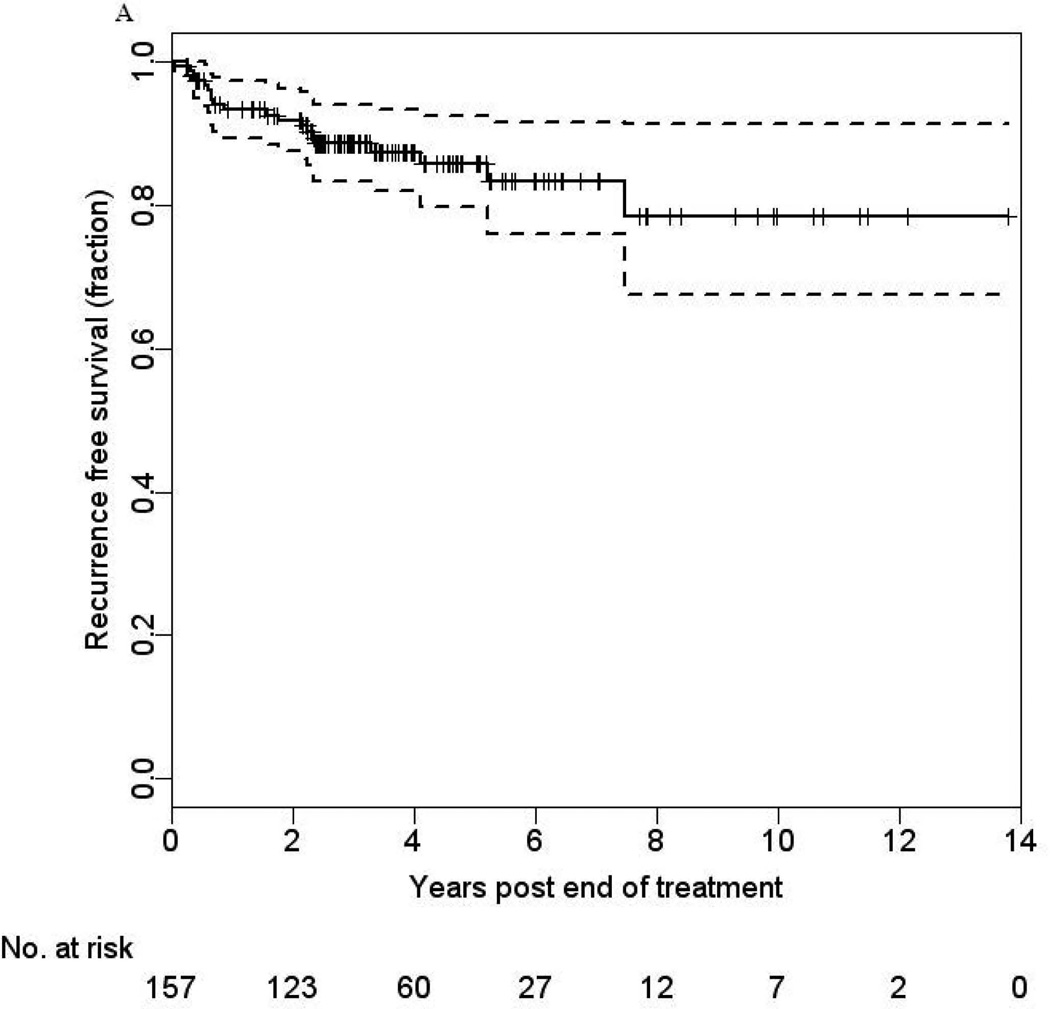
The RFS rates at 3- and 5-years were 89% (95% CI: 82% –95%) and 86% (95% CI: 76% –96%), respectively (Figure 2a). To examine whether patients who had been free of disease 5 years after completion of treatment were still at risk for recurrence, we estimated their 5-year conditional RFS. We found that having remained recurrence-free for 5 years (N=40), patients had a cumulative risk of recurrence of 8.6% by 10 years (one-sided 95% CI upper bound is 19%, p=0.088), suggesting an appreciable level of risk of recurrence for the subsequent 5 years. All recurrences were confirmed to be related to their primary head and neck cancer through histopathologic evaluation and HPV-16 ISH testing of the diagnostic biopsies at the recurrent sites, all of which had integrated HPV-16 DNA. Recurrences occurred in the following locoregional sites: oropharynx, parotid, neck, hard palate, tongue, tonsil, and retromolar trigone. Distant site involvement included the lung (n=9), brain (n=1), and temporal bone (n=1).
FIGURE 2.
In univariate analysis, variables associated with RFS included advanced clinical T-stage (HR 7.30, 95% CI: 2.95–18.0, p<0.0001) and unmarried status (HR 2.63, 95% CI: 1.05–6.62, p=0.04) (Table 3).
In multivariate analysis, advanced clinical T-stage (HR 8.32, 95% CI: 3.06–23, p<0.0001), unmarried status (HR 3.28, 95% CI: 1.20–9.00, p=0.02), and current/former alcohol use (HR 13, 95% CI: 1.33–120, p=0.03) as compared to never alcohol use remained significantly associated with increased risk of disease recurrence (Table 3). In contrast to risk factors for OS, older age, lower N stage, and current tobacco use were not associated with RFS.
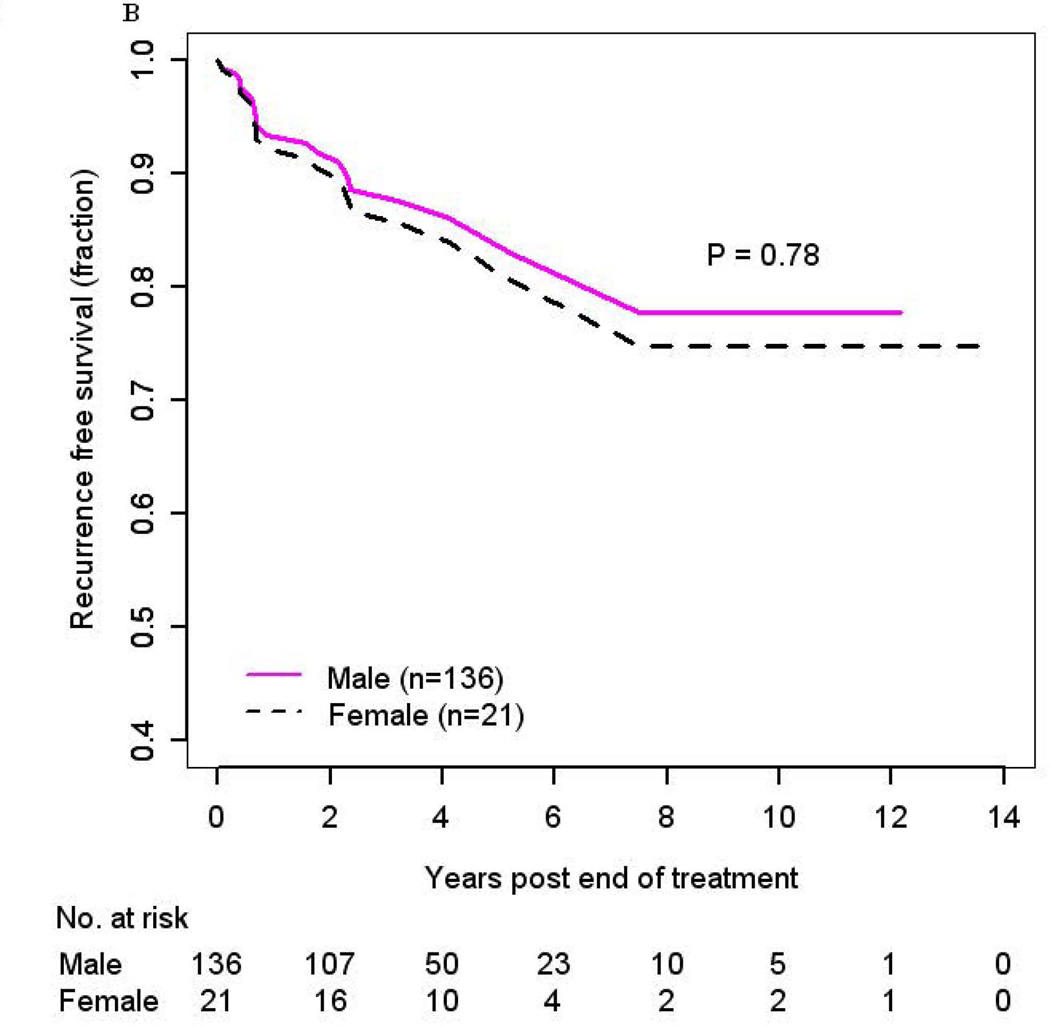
No difference in RFS was observed in men as compared to women (HR 0.87, 95% CI: 0.23–3.31, p=0.84, adjusted (Figure 2b); HR 1.51, 95% CI: 0.50–4.54, p=0.46, unadjusted) (Supplemental Figure 3). When evaluating race, risk of recurrence was higher in blacks (HR 4.77, 95% CI: 0.95–24, p=0.06) as compared to whites in analysis adjusted for gender, marital status, alcohol use, and clinical T-stage but did not reach statistical significance (Supplemental Figure 4). A significant increased risk of recurrence was also observed among cases of other race (HR 47, 95% CI: 4.20–528, p=0.002), although again this was based upon a limited number of cases (Table 3). Similar to the analysis of OS, a sensitivity analysis excluding these 4 patients of other race was performed to determine whether this small group affected our results; and the results remained unchanged.
DISCUSSION
Although patients with HPV-OSCC have improved prognosis as compared to patients with HPV-negative OSCC, there exists a subset of patients with HPV-OSCC who experience a poor clinical outcome. Identification of the risk factors for poor outcome among HPV-OSCC patients is of particular interest since it would facilitate the stratification of those patients who may benefit from more aggressive intervention or conversely those who may benefit from de-escalation treatment approaches.
In order to identify risk factors for poor outcome in HPV-OSCC patients, we retrospectively analyzed the OS and RFS rates of 157 patients with HPV- OSCC treated at the Johns Hopkins Hospital. To date, this is one of the largest studies evaluating predictors of survival among HPV-positive patients, some of whom have been followed for more than 10 years after definitive treatment. We demonstrated a 5-year OS of 89%, which was reduced by older patient age, advanced clinical T-stage, and current tobacco use, and RFS of 86%, which was reduced by advanced clinical T-stage, current/former alcohol use, and unmarried status.
Maxwell J et al reported that tobacco use in HPV-OSCC patients is associated with a higher risk of disease recurrence as compared to never-tobacco users17. Our study provides further evidence that current tobacco use increases risk of poor OS for HPV-OSCC. It is unclear whether there is a true interaction between tobacco use and HPV. It has been hypothesized that the direct effect of tobacco on the pathogenesis of cancer is secondary to the carcinogenic properties of tobacco smoke, which can increase one’s risk of developing genetic alterations18. Tobacco is also considered immunosuppressive and, in the setting of a virus-related cancer, tobacco may inhibit the body’s natural ability to mount effective immunologic responses to eradicate the virus and any virus-related cancer cells19.
Advanced clinical T stage has been reported to be a significant risk factor for recurrence and death in patients with OSCC. Studies of OSCC patients treated with concurrent chemoradiation20 or with transoral laser microsurgery21 have shown poorer survival in OSCC patients with T3–4 than T1–2 disease, similar to that observed in our study. We also found that a lower clinical N-stage was a poor prognostic factor which may in part be explained by the clinical characteristics of the HPV-OSCC patient population. HPV-OSCC patients often have cystic cervical lymph nodes which can result in a higher clinical N stage22. In our study, the HPV-positive patients were diagnosed with an advanced N stage as compared to the HPV-negative patients (77.1% vs 22.9%, p=0.007) and, among the patients with a higher N stage, 89% had a lower T stage (Tx, T1–2). Given the association of higher N stage with lower T stage in our study group, the prognostic effect of T and N stage may have been confounded. Additional studies need to be performed to evaluate the prognostic effect of N stage in HPV-OSCC patients.
We had a mixed population of patients treated with various modalities. 51% of the patients were treated with open surgeries and 49% treated with non-surgical approaches. Post-operative adjuvant concurrent chemoradiation therapy is often administered based on aggressive pathological features, including extracapsular extension (ECE). Although ECE was not evaluated specifically in our study, of the 80 surgical patients included, 50% received adjuvant concurrent chemoradiation therapy and 48.8% adjuvant radiation therapy alone. Based on previously published studies, ECE in head and neck SCC usually predicts worse outcomes. However, in the era of HPV-OSCC, this is an area of controversy. A recent study evaluated 101 OSCC cases in which 90% of the cancers were p16 positive. ECE as defined as extension into soft tissue was not significantly associated with poorer overall (p=0.14), disease free (p=0.2), or disease-specific survival (p=0.09) in multivariate analysis. The study concluded that the impact of ECE in nodal metastases is limited in OSCC23. Thus, ECE may not be an applicable prognostic factor for HPV-OSCC. These results are intriguing and ECE, as well as other traditionally aggressive pathologic features, in HPV-OSCC needs to be evaluated in future studies.
HNSCC is traditionally associated with recurrence rates of 50% with approximately 80–90% occurring within the first 2–4 years following definitive therapy24–31. These rates of recurrence have changed in the era of HPV-OSCC. In the HPV-OSCC patient population, recurrences have been documented to occur at approximately 13.6% at local sites and 8.7% at distant sites within 3-years after completion of definitive treatment13. One study reported on the risk factors for distant metastasis in HPV-OSCC and identified patients with N2c disease have reduced distant control when treated with radiotherapy alone suggesting that these patients may not be candidates for de-intensification strategies that do not administer chemotherapy32. In our study, we report a cumulative incidence of recurrence in HPV-OSCC of 14% after 5 years and, in those patients who were recurrence free for 5 years, the cumulative risk of recurrence at 10 years was 8.6%. This finding may provide us with further observational insight into the pathophysiology and natural course of this disease process. The indolent growth of these tumors may explain the increased number of recurrences 10 years post-treatment as compared to HPV-negative HNSCC. Nonetheless, these observations need to be further confirmed in additional studies to determine whether extended cancer surveillance is warranted in this cancer population.
Prior studies suggest marital status is of prognostic significance in head and neck cancer patients, with partnered patients experiencing lower rates of recurrence and better overall survival compared to unpartnered patients33–35. We observed a similar trend towards poorer OS and significant recurrence rates in unmarried as compared to married patients diagnosed with HPV-OSCC. These findings highlight that social support continues to be an important consideration in caring for head and neck cancers patients, independent of HPV status.
Gender differences in survival have been difficult to study since men have a higher incidence of HPV-OSCC as compared to women4, 13. Our study is one of the largest series which compares survival in women as compared to men with HPV-OSCC and survival of women was not statistically different to that of men suggesting that HPV-OSCC is a distinct clinical and pathologic entity that is driven predominantly by tumor biology rather than gender differences.
Our institution is a large referral base that treats patients from a diverse geographical and demographic background. Having all patients treated at a single institution has the inherent advantage that all patients are treated and clinically monitored for recurrence in a uniform manner. An additional strength of the study is that all of these tumors were tested for HPV status. Overall, our findings suggest that demographic and clinical features, such as advanced patient age, tobacco and alcohol use, advanced clinical T-stage, and unmarried status, which have been reported to be poor prognostic factors in head and neck cancer patients continue to be applicable prognostic markers in HPV-associated OSCC patients, independent of treatment modality. Interestingly, we found a trend towards increased risk of recurrence after the conventional 5-year cancer surveillance period following definitive therapy, which needs to be further investigated to determine whether extended cancer surveillance is warranted in this cancer population.
Supplementary Material
Acknowledgment
Funding source: NIH/NIDCR Head and Neck Spore Program P50DE01932 and P30 CA006973
Funding
This work was supported by the National Institute of Craniofacial and Dental Research at the National Institutes of Health (P50 DE019032 to WW, SIP).
Footnotes
Conflict of interest: None
Financial disclosures: None.
REFERENCES
- 1.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the US population ages 20–44 years. Cancer. 2005;103(9):1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 3.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90(21):1626–1636. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 7.Andl T, Kahn T, Pfuhl A, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58(1):5–13. [PubMed] [Google Scholar]
- 8.Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21(10):1510–1517. doi: 10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 9.van Houten VM, Snijders PJ, van den Brekel MW, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93(2):232–235. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 10.Hafkamp HC, Speel EJ, Haesevoets A, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5–8. Int J Cancer. 2003;107(3):394–400. doi: 10.1002/ijc.11389. [DOI] [PubMed] [Google Scholar]
- 11.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 12.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerstens HM, Poddighe PJ, Hanselaar AG. A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem. 1995;43(4):347–352. doi: 10.1177/43.4.7897179. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan JA, Boyle JO, Koch WM, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332(11):712–717. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 19.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Sedaghat AR, Zhang Z, Begum S, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119(8):1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 21.Rich JT, Milov S, Lewis JS, Jr, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119(9):1709–1719. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JS, Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24(11):1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brockstein B, Haraf DJ, Rademaker AW, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15(8):1179–1186. doi: 10.1093/annonc/mdh308. [DOI] [PubMed] [Google Scholar]
- 25.Bourhis J, Le Maitre A, Baujat B, Audry H, Pignon JP. Individual patients' data meta-analyses in head and neck cancer. Curr Opin Oncol. 2007;19(3):188–194. doi: 10.1097/CCO.0b013e3280f01010. [DOI] [PubMed] [Google Scholar]
- 26.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 27.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 28.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 29.Hall SF, Groome PA, Irish J, O'Sullivan B. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope. 2008;118(8):1362–1371. doi: 10.1097/MLG.0b013e318173dc4a. [DOI] [PubMed] [Google Scholar]
- 30.Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239–247. doi: 10.1002/cncr.22401. [DOI] [PubMed] [Google Scholar]
- 31.Boysen M, Lovdal O, Tausjo J, Winther F. The value of follow-up in patients treated for squamous cell carcinoma of the head and neck. Eur J Cancer. 1992;28(2–3):426–430. doi: 10.1016/s0959-8049(05)80068-1. [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 33.Dilling TJ, Bae K, Paulus R, et al. Impact of gender, partner status, and race on locoregional failure and overall survival in head and neck cancer patients in three radiation therapy oncology group trials. Int J Radiat Oncol Biol Phys. 2011;81(3):e101–e109. doi: 10.1016/j.ijrobp.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konski AA, Pajak TF, Movsas B, et al. Disadvantage of men living alone participating in Radiation Therapy Oncology Group head and neck trials. J Clin Oncol. 2006;24(25):4177–4183. doi: 10.1200/JCO.2006.06.2901. [DOI] [PubMed] [Google Scholar]
- 35.Wong YK, Tsai WC, Lin JC, et al. Socio-demographic factors in the prognosis of oral cancer patients. Oral Oncol. 2006;42(9):893–906. doi: 10.1016/j.oraloncology.2005.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.