Abstract
We conducted a preliminary RCT among 40 HIV-positive Latinos of Mexican descent on the U.S.-Mexico border who indicated imperfect adherence and depressive symptomatology. Participants were randomly assigned to culturally adapted cognitive-behavioral therapy for adherence and depression (CBT-AD) with an alarmed pillbox or usual care. Outcomes were depressive symptoms (self-report and blind clinician ratings), adherence (self-report and electronic pillbox), and biological markers. The intervention, delivered in English and Spanish, proved feasible and acceptable. Generalized estimating equations in intent-to-treat analyses showed some effects of “moderate” to “large” size, with maintenance over time. For example, intervention (vs. control) participants demonstrated at post-intervention a greater drop in BDI scores (OR = - 3.64, p = .05) and greater adherence according to the electronic pillbox (OR = 3.78, p = .03). Biological markers indicated some relative improvement for CD4 count but not VL. The promising results suggest a larger trial to determine efficacy is warranted.
Keywords: HIV/AIDS, ART adherence, depression, CBT, Latino/Hispanic
HIV disproportionately impacts Latinos in the U.S., who are more than twice as likely as non-Hispanic Whites to be living with HIV [1]. Latinos, especially in the impoverished U.S.-Mexico border region, have limited access to prevention and health care services, delay entry into care, report sub-optimal adherence to HIV medications, and experience significant untreated mental health problems [2-4]. Specifically, in a study of 81 monolingual Spanish speakers in Los Angeles, area, only 32% of participants were consistently adherent, and the most frequently cited barriers to adherence were feeling depressed or overwhelmed (21%) or simply forgetting to take medication doses (19%)[5].
Despite a large and growing field of research aimed at developing and evaluating behavioral interventions to promote ART adherence [6-8], we could locate only one published report of such an intervention that worked with Latinos [9], which described a culturally adapted program addressing health literacy and patient-provider communication with low-income, low-literacy, Spanish-speaking Latinos. Thus, the present study focused on Latinos along the U.S.-Mexico border, with a dual approach to promoting adherence by treating depression with a culturally adapted intervention and addressing forgetfulness with an electronic pillbox reminder system.
Depression is an important potential target for any adherence intervention because of its high prevalence among people living with HIV/AIDS (PLWHA) [10, 11], including those on the U.S.-Mexico border [12], and its association with poor HIV adherence [13-16]. The link between depression and non-adherence may be due to the symptoms of depression themselves (e.g., sadness, poor concentration, psychomotor retardation, suicidal ideation) or to the impairments in problem solving and coping that can be secondary to depression [17]. Depression can be successfully treated among HIV-positive individuals, particularly with cognitive-behavioral therapy (CBT) [18-20], and its alleviation can lead to improved health outcomes [14, 21-24].
We selected as a suitable intervention for our target population evidence-based Cognitive-Behavioral Therapy for Adherence and Depression (CBT-AD) [25, 26], a CBT-based approach to treating depression and non-adherence among persons with chronic illness. It was originally based on traditional CBT approaches for the treatment of depression, clinical experience with HIV-positive individuals, and intervention techniques most applicable to persons with chronic illness in general [19, 27, 28]. It integrates adherence counseling following the Life Steps program [29], a brief, evidenced-based [11] approach to improving adherence to medical treatment and medications. In a two-arm preliminary RCT with 45 HIV-positive individuals diagnosed with mood disorders, Safren and colleagues [30] found strong and sustained effects of the intervention on mood symptoms and adherence, compared to an enhanced treatment-as-usual condition. As CBT-AD was originally developed with a largely non-Latino, English-speaking sample on the East Coast of the U.S., we adapted it culturally and linguistically for a Latino population of Mexican descent.
Simultaneously learning adherence reminder strategies (see Life-Steps adherence counseling below) while receiving treatment for depression may be particularly useful in combatting non-adherence, as “simply forgetting” is the most frequently cited reason for missing doses, reported by 34-52% of patients [31-33]. There is evidence that prospective memory, in particular, may play a role in non-adherence [34]. One approach to treating non-adherence in the face of depression is the use of electronic reminders to counteract forgetfulness [35, 36]. Specifically, in a study of 224 individuals attending an HIV primary care clinic in Seattle, a reminder system of text messages sent to pagers was found to improve biological outcomes of CD4 count and VL at post-intervention, an improvement that persisted through follow-up, with greater participant engagement in the pager system associated with better outcomes [37].
The present study examined the feasibility, acceptability, and potential efficacy of the adapted CBT-AD intervention among Latinos of Mexican descent. Primary outcomes were depressive symptoms and medication non-adherence, both assessed with self-report as well as more objective measures. Secondary outcomes were biological markers of HIV disease state -- viral load (VL) and CD4 cell counts. This preliminary RCT was undertaken to ascertain approximate effect sizes rather than powered for statistical tests to determine efficacy.
Method
Procedure
Data were collected in a two-stage process from 10/21/2009 – 8/31/2011 at a publicly-funded community health clinic in El Paso, TX, on the U.S.-Mexico border. In the first stage, flyers advertising a survey study were posted in the clinic, and referrals were encouraged from clinic providers, staff, and the patients themselves. Study staff provided information about the scope of the survey and, if interested, patients who were on antiretroviral medications for at least 30 days and over 18 years of age were interviewed immediately or given appointments to return. The 1-hour interviewer-administered survey assessed adherence as well as other psychosocial and attitudinal variables (e.g., adherence self-efficacy, substance use, sexual risk behaviors, social support, coping, psychological adaptation, and quality of life).
In the second stage, eligibility for the trial was determined based on survey responses and clinic staff input. Eligible participants were: (a) currently receiving HIV care at the clinic; (b) 18 years of age or older; (c) self-identified as being of Mexican heritage; (d) English- or Spanish-speaking; (e) capable of giving informed consent; (f) currently on a prescribed antiretroviral regimen; (g) sub-optimally adherent (demonstrated either by a VL load taken in the last 12 months that was above the undetectable threshold of 48 mL copies or self-report of less than perfect adherence—i.e., missing at least one dose in the past 7 days, less than 100% of doses taken in the past 30 days, or direct mention to study staff of any adherence problems); and (h) exhibiting at least mild depressive symptomatology (Beck Depression Inventory-IA [BDI-IA] scores of 10 or above). Patients were excluded if, (a) according to provider report, they showed signs of active psychosis or dementia, or (b) they reported the use of crack, cocaine, heroin, or methamphetamines to any extent in the past 30 days. Those who (c) were planning on being away from the area for any extended period during the study (as in the case of seasonal workers) or (d) had household members already enrolled in the study also were excluded. Those who qualified for the study were then given a detailed description of what participation would involve and were invited back for a baseline appointment at which they provided written informed consent.
We obtained human subjects participation approval from the clinic, as well as the participating universities. Additionally, we secured a Federal Certificate of Confidentiality. Primary care providers of all trial participants were sent a letter informing them that the patients had exhibited evidence of depressive symptoms and HIV medication non-adherence, and had been enrolled in the RCT. Providers were not informed of to which study arm patients were assigned but were asked to provide patients with the care they deemed appropriate.
Eligible participants were randomly assigned to the intervention or treatment as usual (TAU) control condition, both enhanced with the notification letter to provider. Allocation concealment involved the use of sequentially numbered, opaque, sealed envelopes containing the study arm assignment, which study staff opened at the moment of randomization after baseline assessment. An external statistician had used a computerized random number generator to select random permuted blocks of four. Due to the nature of the intervention, participants and interventionists could not be blinded to study arm assignment, but to the extent possible, study staff performing subsequent assessments were.
All participants were subsequently assessed at post-intervention (approximately 6 months), and follow-up (9 months.) They were reimbursed $20 for each assessment, with a $20 bonus if they completed both outcome assessments and returned the electronic pillbox.
Control group participants were asked to return for brief monthly visits during the intervention phase, at which they were asked if their medication regimen had changed, electronic pillbox data were uploaded, and staff inquired about any problems that patients had experienced with the pillbox or participation in the study. They were reimbursed $20 for each of these visits.
In the treatment condition, participants returned approximately weekly for therapy sessions and were reimbursed $20 for each therapy session they attended. Retention efforts included unscripted reminder phone calls 1 week and 1 day before each scheduled interview appointment and mailed correspondence in the case of non-response to phone calls.
Experimental Intervention
The cultural and linguistic adaptation of the intervention, which was guided by the principles of community-based participatory research methods and the CDC Prevention Map of Adaptation Process [38] and is to be fully described in a separate report, included adjustments in both content and process. For example, an initial Cultural Exploration module was added to assess ways in which certain culturally based issues (e.g., family relationships, stigma, spirituality) might inform patients’ experiences with depression and medication adherence and to provide an opportunity for therapists to build confianza (a culturally valued sense of mutual trust) with clients. Efforts were made to incorporate information gleaned from this module into the rest of the intervention, and later therapy goals often included issues related to these topics.
The first treatment session after the Cultural Exploration module focused solely on adherence counseling following the Life Steps approach. This session covered 11 informational, problem-solving, and cognitive-behavioral steps [29]. In each step, the clinician helped the participant articulate a specific adherence goal, identify barriers to achieving the goal, and make a plan and back-up plan for each goal. Participants also received medication adherence tools such as assistance in formulating a schedule.
Subsequent sessions then integrated continued Life Steps adherence counseling with traditional CBT techniques for the treatment of depression. Module 1 (≈1 session) provided psychoeducation about HIV and depression, and a motivational interviewing exercise designed to set the stage for behavioral change. Module 2 (≈1 session) focused on behavioral activation and activity scheduling, designed to increase pleasure and mastery. Module 3 (≈3 sessions), cognitive restructuring, involved training in adaptive thinking, such as identifying and restructuring negative automatic thoughts. Module 4 (≈3 sessions), problem-solving, involved training in selecting an action plan for problems and breaking this plan into manageable steps [39]. Module 5 (≈1 session), relaxation, involved training in progressive muscle relaxation and diaphragmatic breathing.
Sessions were approximately 50 minutes long and occurred every 7-14 days, with the goal of completing treatment in approximately four months, with 2 booster sessions at months 5 and 6. Flexibility in the number of sessions devoted to any module was allowed to address the complexity and variability of issues facing participants with HIV and depression, as well as the challenges that this generally low-income sample faced in attending weekly sessions. All sessions were audio-recorded for quality assurance monitoring and therapist supervision.
Study interventionists were five bilingual, bicultural, Latino graduate students in psychology. Three had masters’ degrees and two were enrolled in an M.A. program in clinical psychology. Only one of the therapists had substantial psychotherapy practicum experience with adults before participating in the study. All therapists were trained by study personnel to conduct the CBT-AD intervention and adapt the cognitive behavioral techniques for Latinos [40] with co-morbid medical and psychiatric conditions [41, 42]. Additionally, they participated in weekly clinical supervision with a licensed psychologist. Supervision focused both on maintaining fidelity and ensuring flexibility to address the cultural issues relevant to providing the intervention to this Latino population. A formal intervention fidelity procedure involved having therapists listen to others’ sessions and rate the extent to which they adhered to the manualized intervention. All graduate students delivering the intervention either received credit for an (unpaid) clinical externship or were paid through research assistantships.
Electronic Pillbox Intervention
An electronic drug monitor (EDM) in the form of an electronic pillbox developed by Medsignals® was provided to all intervention participants. The pillbox is portable, provides storage for up to four medications, prompts correct dosing times and warnings, and records data on bin openings (i.e., presumptive dosing) that can be uploaded through a telephone line or directly to a computer during a clinic visit. Management of the pillbox, including programming of times and downloading of data, was web-based.
After randomization, the study coordinator distributed to each participant a pillbox that was already programmed with that participant’s daily medication regimen and dosing times (confirmed by the clinic pharmacist), and given an overview about its functioning and assessment capability. Those participants who were randomized to the treatment group were given pillboxes with an activated alert function. At the time of the scheduled dose of each of their medications, the pillbox would alert the participant in three different ways (i.e., beeping, flashing bin light, and LED screen instructions). The opening of the appropriate bin or pressing a button (recording no bin opening) would silence the audible alerts. Bin compartments operated independently of one another. If beeps were silenced but the compartment bin went unopened during the designated time, the LED continued to flash until the bin was opened. Otherwise, if the beeping was not silenced, both the audio and visual signaling would continue intermittently until the bin was opened. Once the bin was opened, the alerts involved displaying the number of pills to be taken on the LCD screen of the device, a commensurate number of beeps and flashes and a voice announcement specifying how many pills the participant needed to take and any additional instructions required for that particular medication (e.g., “Take with food”). These announcements were programmed in either English or Spanish, depending on the preferred language of the participant. The participants who were randomized to the control group received an identical pillbox with the alert system deactivated. These control participants were advised simply to use the pillbox as a storage device, but to take their medications normally. After completion of the study, all participants had the option of keeping the device for free or returning it for the $20 incentive.
Usual Care
All study participants continued to receive medical care at the participating community clinic as usual. At the clinic, medically stable patients are scheduled for appointments with a primary care provider (a physician or consulting pharmacist) every three months, at which time they regularly have laboratory tests conducted, including VL tests and CD4 cell counts. Patients who report psychiatric symptoms are typically first evaluated by the primary care provider. For relatively uncomplicated psychiatric concerns, providers may prescribe psychoactive medications. At the discretion of the providers, patients may be referred to counseling services provided by masters-level therapists at a local mental health clinic. However, those services may be provided by therapists generally unfamiliar with the HIV/AIDS disease and treatment context. Patients who are judged to pose a risk to themselves or to others due to psychiatric symptomatology are immediately referred to emergency care. Patients judged by providers to be non-adherent to medication are initially screened and counseled by the provider and may be referred for adherence education provided by the consulting pharmacist.
Evaluation of Intervention Fidelity, Feasibility, Acceptability
Fidelity to the manualized therapy intervention was independently rated from audio recordings of two sessions per treatment group participant, using a multidimensional rating scale with behavioral anchors. All sessions were rated for review of medication adherence, depressive symptoms, and previous week’s symptomatology and homework; as well as therapist’s adherence to the CBT model; attentiveness to cognitions, behavioral skills, and emotions; assignment of homework; and active engagement of the participant. Each module was then rated separately for content coverage. The feasibility of implementing the intervention in this context with this sample was assessed by tracking initial interest and willingness of patients to be involved and consent to randomization, extent of attendance at intervention sessions and loss to follow-up, as well as barriers to full participation. Exit interview items on participant acceptability of the intervention reviewed each module individually, along with the use of the electronic pillbox.
Outcome Measures
Adherence and depressive symptoms were measured at baseline, and 6-month and 9-month follow-up appointments. The primary adherence outcomes were assessed with self-report and the EDM device. The primary depressive symptoms outcomes were assessed with self-report and a semi-structured interview by an independent rater blind to treatment condition. All self-report and clinician-administered scales were translated from English into Mexican Spanish by a certified translator. They were then independently back-translated by a second certified translator. The translations were reviewed and discrepancies were resolved by consensus of a committee consisting of both translators and bilingual content experts [43]. Secondary outcomes of VL and CD4 cell count were extracted by study staff from participants’ medical charts.
HIV Medication Adherence
Past 7-day self-reported percent adherence was assessed with a visual analog scale (VAS) [44]. The VAS involved asking participants to indicate, with a mark along a 10cm line, the percentage of doses of all their HIV medications taken in the past 7 days, with intervals of 10 percentage points indicated from 0% to 100%.
Past 2-week EDM percent adherence was calculated as the number of valid bin openings divided by the total prescribed doses. A bin opening was counted as valid if it occurred within 12 or 6 hours of an alarm for once or twice a day dosing, respectively. Duplicate openings within each alarm window were discarded to restrict adherence to a maximum of 100%. Baseline adherence was calculated for the two-week period after monitoring was initiated, with 6 and 9-month adherence calculated for the two weeks prior to each of the follow-up assessments.
Depressive Symptoms
Self-reported depressive symptoms were assessed at each time point using the revised Beck Depression Inventory-IA (BDI-IA) [45], which consists of 21 items, each with a 4-point response scale anchored with descriptive statements. While the psychometric properties of the BDI-IA are well documented, the Spanish translation of the BDI has also demonstrated internal consistency reliability, test-retest reliability, and convergent validity [46, 47], and has been shown to have both positive and negative predictive value when compared to clinical interviews (80% and 95% correct classification, respectively) [48]. To avoid confounding symptoms of depression and HIV [49], the somatic items were omitted, leaving the first 13 items constituting the cognitive/affective factor [45].
Clinician-administered ratings of depressive symptoms were conducted using the Montgomery-Åsberg Depression Rating Scale (MADRS) [50], a semi-structured interview designed to capture the severity of ten symptoms over the past seven days. Each of the ten symptoms is rated from zero to six using a scoring index anchored with descriptive statements. The MADRS has good internal consistency (α = .88) [51] and inter-rater reliability (r = .86) [52] and correlates well with independent ratings done with the Hamilton Depression Rating Scale (r = .82). All interviewers were trained to administer the MADRS and completed mock MADRS administrations during training. Weekly group supervision using audio recording of MADRS administrations was conducted to prevent rater drift, and recordings of 20% of all interviews were reviewed in this fashion.
Biological markers
All VL and CD4 cell counts were extracted from patient medical records by study staff after obtaining consent. We used lab results from samples drawn prior to, or no later than 14 days after, study enrollment as our baseline measure. We selected 6 and 9-month lab results in closest temporal proximity to the 6 and 9-month follow-up appointment dates. For consistency across testing methods of differing precision, all results indicating fewer than 48 copies per mL were considered undetectable and set to zero. A log transformation was performed to account for the non-normal distribution of VL.
Statistical Methods
All randomized participants were included in the analyses (i.e., an intent-to-treat approach). To evaluate efficacy, repeated-measures analyses were conducted using generalized estimating equations (GEE) [53]. An exchangeable working correlation structure was specified to account for correlated outcome data across assessments, making these analyses comparable to repeated measures analysis of variance (ANOVA). In contrast to traditional repeated measures ANOVA, the GEE approach permits analysis using all available outcome data, allowing for participants with at least one assessment to be retained. Depression and biomarker outcomes were modeled as normally distributed continuous variables with a Gaussian link function and percent adherence outcomes were modeled as binomially distributed continuous variables (i.e., a proportion between 0 and 1) with a logit link function. Robust standard error corrections were applied to all GEE models of adherence outcomes as recommended when analyzing percentage data [54].
In the GEE analyses, each outcome was regressed on CBT-AD intervention, time (assessment point), and the CBT-AD × time interaction. The effect of time was partitioned into planned contrasts of baseline assessment against each follow-up (i.e., BL vs. 6 months, BL vs. 9 months). The statistical tests of the intervention effects were the CBT-AD × time interactions. The interaction effects were reported as beta coefficients for the continuous outcomes and as odds ratio for the percent adherence outcomes. The odds ratio reflected the relative probability of adherence over non-adherence (i.e., 100% vs. 0% of doses taken) in the CBT-AD group compared with treatment as usual.
This preliminary RCT was undertaken to ascertain approximate effect sizes and was not powered for statistical tests of efficacy. Interpretation focuses, therefore, on the magnitude and valence of the intervention effects, with p values provided for reference purposes.
Results
Flow of Participants
As seen in Figure 1, of the 295 referrals for the screening survey, 42 were ineligible, declined to be screened, or could not be scheduled. Among the resulting 253 who completed the screening survey, most were excluded because they were not of Mexican descent (48; 23%) or failed to meet the criterion for depressive symptomatology (135; 55%). Only one otherwise eligible individual declined to participate.
Figure 1.
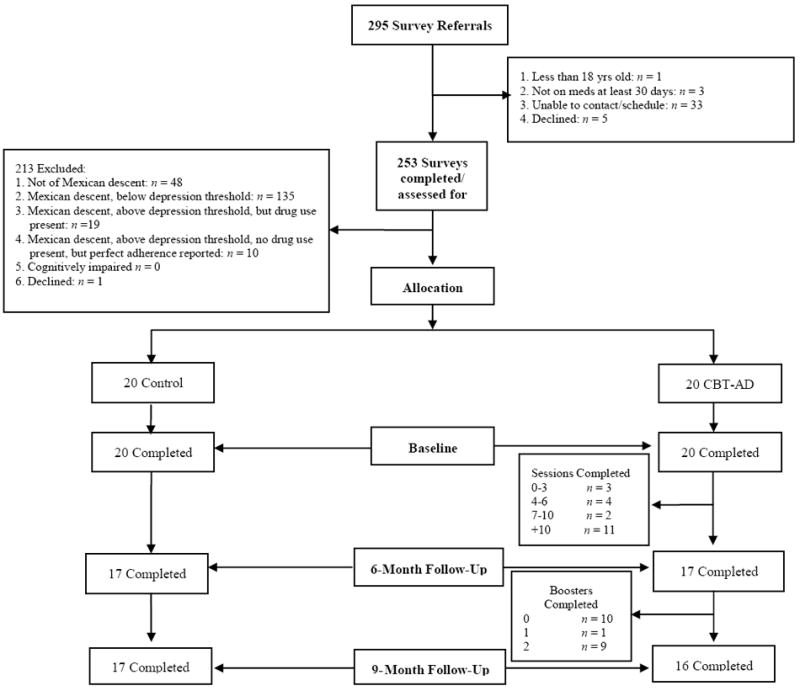
Flow chart of participants.
Baseline assessment data are available for the 40 participants allocated to the intervention (n=20) and TAU (n= 20) arms; 83% of participants had complete data, 3% missed a single follow-up assessment, and 14% missed both follow-up assessments. Intervention participants completed an average of 10.8 sessions (SD = 7.5). There were no significant differences in retention by study arm.
Participants
See Table 1 for a description of the 40 participants enrolled in the trial. All were of Mexican descent, ranging in age from 24 to 63 years; 73% were male and 53% completed the intervention in Spanish. With respect to baseline medication, 47% of participants were on a once-daily regimen and 53% were on a twice-daily regimen. Participants were prescribed between 1 and 4 antiretroviral products, either individual or combination tablets (M/SD = 2.6/1.2). Of the 40 participants, 3 admitted missing one dose of medication over the reporting period but had undetectable VLs, 14 said they had missed more than one dose but had undetectable VLs, 7 with undetectable VLs indicated to study staff of having problems with adherence during initial survey (thus making them eligible to enroll). One said they had only missed one dose and had a detectable VL, and 6 admitted missing multiple doses and had detectable VLs. The remaining 9 had missing VL data during the survey, with 2 reporting missing one dose, 4 reporting missing more than one dose, and 3 admitting to staff of having problems with adherence.
Table I.
Description of Sample of HIV-Positive Latinos by Treatment Condition at Baseline
| TAU (n = 20) |
CBT-AD (n=20) |
Total (N=40) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Frequency (%) | Frequency (%) | Frequency (%) | χ2 | df | p | |
| Gender | 0.13 | 1 | .72 | |||
| Female | 6 (30) | 5 (25) | 11 (27.5) | |||
| Male | 14 (70) | 15 (75) | 29 (72.5) | |||
| Sexual orientation | 0.75 | 2 | .69 | |||
| Heterosexual | 8 (40) | 8 (40) | 16 (40) | |||
| Bisexual | 3 (15) | 5 (25) | 8 (20) | |||
| Gay male | 9 (45) | 7 (35) | 16 (40) | |||
| Relationship status | 3.96 | 1 | .05 | |||
| Partnered | 4 (20) | 10 (50) | 14 (35) | |||
| Not partnered | 16 (80) | 10 (50) | 26 (65) | |||
| Preferred language | 0.10 | 1 | .75 | |||
| English | 10 (50) | 9 (45) | 19 (47.5) | |||
| Spanish | 10 (50) | 11 (55) | 21 (52.5) | |||
| Employment | 1.23 | 2 | .54 | |||
| Full-time | 1 (5) | 0 (0) | 1 (2.5) | |||
| Part-time | 8 (40) | 7 (35) | 15 (37.5) | |||
| None | 11 (55) | 13 (65) | 24 (60) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | t | df | p | |
| Age in years | 44.8 (10.7) | 47.3 (10.7) | 46.0 (10.6) | 0.73 | 38 | .47 |
| Years since diagnosis | 9.7 (6.7) | 10.5 (6.4) | 10.1 (6.5) | 0.41 | 38 | .68 |
| Median (SIQ) | Median (SIQ) | Median (SIQ) | z | p | ||
| Household income† | 9,480 (4,995) | 11,134 (4,002) | 10,360 (4,524) | 0.38 | .70 | |
Figures are medians (SIQ; semi-interquartile range) in US$ due to a highly positively skewed distribution.
p < .05
Baseline comparison of participants in the two arms demonstrated that randomization was successful. No important differences (i.e., p < .20) were found on any socio-demographic, regimen, or outcome variable except that more participants in the CBT-AD arm (50%, n=10) were in a partnered relationship at baseline than those receiving treatment as usual (20%, n=4), χ2(1) = 3.96, p = .05. Additionally, CBT-AD participants had higher BDI scores at baseline (M/SD = 22.0/2.06) than those receiving standard care (M/SD = 16.8/1.41), t(38) = -2.08, p = .04.
Missing Data
To assess for differences between participants with complete self-report data (83%) and those who missed one or more assessments (17%), Fisher’s exact χ2 tests and Satterthwaite t tests for unequal variances were conducted, respectively, on categorical and continuous socio-demographic characteristics and baseline levels of the outcomes. Participants with missing self-report data, compared to those with complete data, were more likely to be English-speaking (86% vs. 39%), Fisher’s exact χ2(1) = 4.97, p = .04; were younger (M/SD = 34.2/6.1 vs. 48.5/9.7), t(13.4) = 5.00, p < .001; and were more recently diagnosed, in years, with HIV (M/SD = 4.8/3.1 vs. 11.2/6.5), t(19.3) = 3.99, p = .001. With respect to baseline outcomes, there was a trend towards lower baseline CD4 count among participants with missing self-report data, compared to those with complete data (M/SD = 319.4/194.2 vs. 489.8/283.1), t(7.5) = 1.67, p = .14.
Missing EDM adherence data were tabulated separately as these data could be available even if a self-report assessment was missing. For EDM adherence, 73% of participants had complete data and 28% were missing one or more assessments. Participants with missing EDM data, compared to those with complete data, were marginally more likely to be English-speaking (73% vs. 38%), Fisher’s exact χ2(1) = 3.87, p = .08; younger (M/SD = 37.9/8.5 vs. 49.1/9.8), t(20.9) = 3.55, p = .002; and more recently diagnosed with HIV (M/SD = 6.9/5.4 vs. 11.3/6.5), t(21.5) = 2.19, p = .04. With respect to baseline outcomes, there was a trend towards lower baseline CD4 count among participants with missing EDM data, compared to those with complete data (M/SD = 315.3/246.2 vs. 519.7/270.2), t(15.7) = 2.07, p = .06.
Intervention Fidelity, Feasibility, Acceptability
Therapy fidelity was independently rated from audio recordings for two sessions per treatment group participant, using a multidimensional rating scale with behavioral anchors. Therapists achieved an average of 93% coverage of prescribed content, suggesting close adherence to the manualized intervention protocol. The intervention proved to be highly feasible, with much initial interest and only 20% of participants lost to follow; 70% of participants completed at least half the sessions and 60% completed all sessions. Only one eligible patient declined outright to participate.
However, there were challenges in retaining patients in care, including barriers related to international border crossing, frequent moves due to financial instability, and incarceration. Indeed, only 50% of participants attended booster sessions, partly for reasons noted above. Challenges in the intervention delivery included both literacy and language issues, with cognitive restructuring and thought records homework requiring adaptation.
Exit interview items on acceptability indicated all patients in the intervention arm found that overall the intervention was a good experience, with psycho-education and activity scheduling universally deemed helpful and all but two participants commenting positively on the problem-solving and relaxation components. However, four participants reported that cognitive restructuring was difficult and/or intrusive and required more time than allotted.
Descriptive Data on Outcomes
Descriptive data (means/SD) are presented in Table II for all outcomes at baseline and 6 and 9 months. For the entire sample, we report general patterns of outcomes below.
Table II.
Outcome Data by Treatment Condition at all Assessment Points with Generalized Estimating Equations Estimates of Treatment Effect
| All participants | CBT-AD participants | TAU participants | Treatment effect (CBT-AD × time) | |||
|---|---|---|---|---|---|---|
|
M(SD) N = 40 |
M(SD) n = 20 |
M(SD) n = 20 |
OR | 95% CI | p | |
|
| ||||||
| Adherence | ||||||
| VAS% (7-day) | Omnibus: χ2(2) = 10.48 | .01 | ||||
| Baseline | 87.2 (19.5) | 84.4 (24.4) | 90.6 (11.1) | – | – | – |
| 6 Months | 89.3 (12.7) | 92.6 (9.4) | 86.0 (14.7) | 3.43 | 1.62 – 7.26 | .001 |
| 9 Months | 92.7 (8.2) | 93.5 (9.0) | 91.9 (7.7) | 2.11 | 0.91 – 4.85 | .08 |
| EDM % (2-week) | Omnibus: χ2(2) = 5.32 | .07 | ||||
| Baseline (2 weeks) | 49.4 (40.6) | 43.2 (43.5) | 55.6 (37.6) | – | – | – |
| 6 Months | 37.3 (40.6) | 45.8 (47.8) | 28.8 (31.0) | 3.78 | 1.17 – 12.18 | .03 |
| 9 Months | 25.4 (38.3) | 31.1 (44.9) | 20.0 (31.5) | 3.44 | 0.64 – 18.56 | .15 |
| Depressive symptomatology | ||||||
| BDI | Omnibus: χ2(2) = 7.33 | .03 | ||||
| Baseline | 19.4 (8.2) | 22.0 (9.2) | 16.8 (6.3) | – | – | – |
| 6 Months | 13.4 (7.3) | 15.0 (7.4) | 11.8 (7.0) | -3.64 | -7.26 – -0.01 | .05 |
| 9 Months | 13.9 (8.0) | 14.2 (7.0) | 13.5 (9.0) | -4.80 | -8.46 – -1.14 | .01 |
| MADRS | Omnibus: χ2(2) = 2.34 | .31 | ||||
| Baseline | 23.6 (9.7) | 25.1 (9.7) | 22.1 (9.8) | – | – | – |
| 6 Months | 18.5 (11.6) | 17.8 (12.2) | 19.2 (11.2) | -5.14 | -11.90 – 1.62 | .14 |
| 9 Months | 18.7 (12.7) | 19.7 (12.7) | 17.7 (12.9) | -1.42 | -8.25 – 5.41 | .68 |
| Biological markers | ||||||
| HIV-1 RNA viral load (Log10 copies/mL) | Omnibus: χ2(2) = 0.10 | .95 | ||||
| Baseline | 1.5 (1.1) | 1.3 (1.0) | 1.7 (1.2) | – | – | – |
| 6 Months | 1.4 (1.0) | 1.2 (0.6) | 1.5 (1.2) | 0.14 | -0.75 – 1.03 | .75 |
| 9 Months | 1.2 (0.8) | 1.1 (0.2) | 1.3 (1.0) | 0.07 | -0.91 – 1.05 | .89 |
| CD4 count (cells/mm3) | Omnibus: χ2(2) = 3.28 | .19 | ||||
| Baseline | 464.0 (276.0) | 525.2 (289.9) | 412.9 (261.0) | – | – | – |
| 6 Months | 468.8 (311.5) | 541.0 (300.2) | 414.7 (318.2) | 69.45 | -6.16 – 145.05 | .07 |
| 9 Months | 568.9 (391.0) | 618.0 (467.5) | 532.0 (340.0) | 25.71 | -58.91 – 110.32 | .55 |
Note. VAS = Visual analogue scale; EDM = Electronic drug monitor; BDI = Beck Depression Inventory; MADRS = Montgomery-Åsberg Depression Rating Scale; CBT-AD = Cognitive behavioral therapy for depression and adherence; TAU = treatment as usual; OR = odds ratio; CI = confidence interval; mL = milliliter; mm3 = cubic millimeter
The overall average self-reported adherence, as assessed by the VAS, was high at initiation and rose slowly over time from 87% at baseline, to 89% at 6 months, and 93% at 9 months. In contrast, the EDM data for the entire sample indicated that average adherence was low at initiation and declined over time from 49% at baseline (i.e., 2 weeks), to 37% at 6 months, and 25% at 9 months.
With respect to depressive symptoms in all participants, the average self-reported symptoms as assessed by the BDI were initially moderate in severity (i.e., 19.4 at baseline), decreasing to 13.4 at 6 months, and remaining stable at 13.9 at 9 months. Similarly, average clinician-assessed depressive symptoms using the MADRS were moderate in severity at baseline (23.6), decreasing to 18.5 at 6 and remaining stable at 18.7 at 9 months.
With respect to biological markers for all participants, log10 VL decreased across assessments from 1.5 at baseline, to 1.4 at 6 months, and 1.2 at 9 months. Notably, over 80% of our data points for VL were undetectable, creating ceiling effects that likely limited our power to note any differences over time or by arm. CD4 count remained high from baseline to 6 months (464-469 cells/mm3) and increased to 569 cells/mm3 by 9 months.
Evaluation of Intervention Effects
Adherence
As seen in Table II and Figure 2, the CBT-AD intervention apparently led to improved adherence. An examination of the self-report 7-day adherence means indicated that even though participants in the treatment arm reported lower adherence at baseline, they reported gains by 6 months that amounted to higher levels of adherence than those in the TAU arm, which were then maintained at 9 months. Participants in the TAU arm, conversely, reported a drop in adherence over 6 months with a return to approximately the baseline level by the 9-month follow-up. The longitudinal GEE analysis of the self-report percent adherence outcome revealed a significant omnibus CBT-AD × time effect, χ2(2) = 10.48, p = 0.01, with a significant CBT-AD × time (baseline vs. 6 months) contrast, indicating a more than three-fold increase in the odds of 100% adherence for the CBT-AD arm participants at post-intervention (OR = 3.43, SE = 1.31, 95% CI = 1.62 – 7.26, p = .001) and a marginally significant CBT-AD × time (baseline vs. 9 months) interaction (OR = 2.11, SE = 0.90, 95% CI = 0.91 – 4.85, p = .08) in the same direction.
Figure 2.
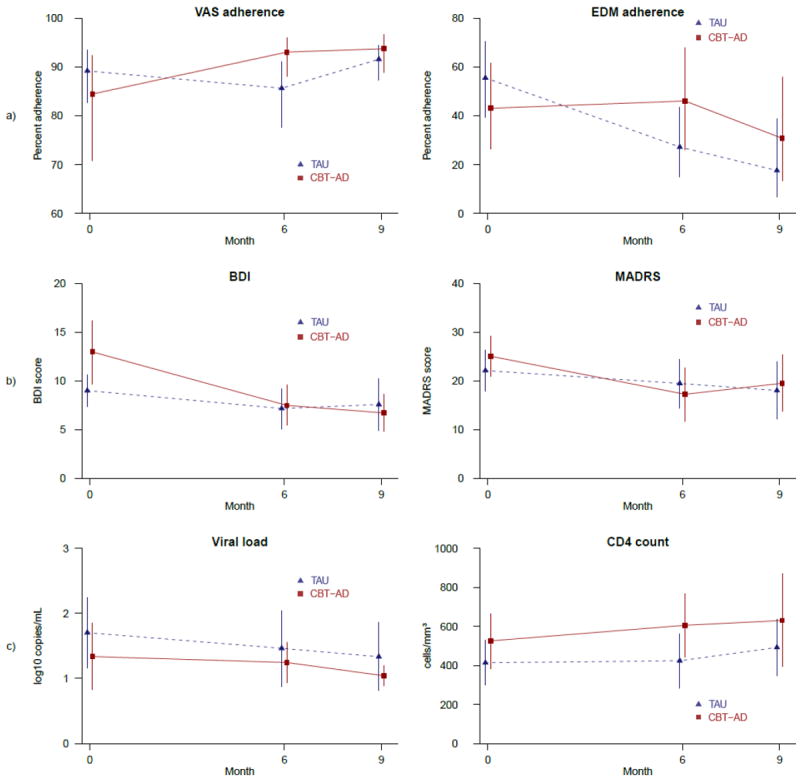
Primary and secondary outcomes at 0, 6, and 9 months by study arm according to the longitudinal GEE models: a) Self-report and EDM adherence, b) BDI and MADRS depression, c) Viral load and CD4 count.
Findings with respect to EDM 2-week adherence, compared to self-report, indicated lower mean levels of adherence and a deteriorating trajectory, yet the same superiority of CBT-AD over the TAU arm. Specifically, the means indicated treatment participants began with lower adherence that rose slightly at 6 months but then decreased to below baseline levels by the 9-month follow-up. TAU participants, on the other hand, reported adherence that dropped precipitously by 6 months and then to even lower levels by 9 months. The GEE analysis of the 2-week dose percent adherence outcome revealed a marginally significant omnibus CBT-AD × time effect, χ2(2) = 5.32, p = .07, with a significant CBT-AD × time (baseline vs. 6 months) contrast, indicating a nearly four-fold greater odds of 100% adherence for treated participants at post-intervention (OR = 3.78, SE = 2.26, 95% CI = 1.17 – 12.18, p = .03). In the CBT-AD × time (baseline vs. 9 months) contrast (OR = 3.44, SE = 2.96, 95% CI = 0.64 – 18.56, p = .15), the direction and size of the effect were comparable.
Depressive symptomatology
Intervention effects were noted as well with respect to depressive symptoms assessed by the BDI and, to a lesser extent, the MADRS (see Table II and Figure 2). Across study arms, BDI scores dropped from baseline to 6 months, with improvements maintained at 9 months. For treatment arm participants, however, depression scores were higher at baseline and dropped farther over time than for TAU participants. The GEE analysis of the BDI outcome revealed a statistically significant omnibus CBT-AD × time effect, χ2(2) = 7.33, p = .03, with a significant CBT-AD × time (baseline vs. 6 months) contrast, indicating a nearly four-point greater drop in depressive symptoms for those receiving the intervention at post-intervention (OR = -3.64, SE = 1.85, 95% CI = -7.26 – -0.01, p = .05) and a significant CBT-AD × time (baseline vs. 9 months) contrast indicating a nearly five-point drop in depressive symptoms associated with intervention at 9 months (OR = -4.80, SE = 1.87, 95% CI = -8.46 – -1.14, p = .01).
MADRS mean scores indicated that clinician-rated depression in both arms also dropped at 6 months, with improvement maintained at the 9-month follow-up. There was a larger drop in the treatment versus TAU arm (7 versus 3 points). However, the omnibus test of the CBT-AD × time interaction for clinician-rated depression was not statistically significant, χ2(2) = 2.34, p = .31. Within the omnibus test, planned contrasts suggested a trend towards a greater reduction in clinician-rated depression for those receiving the intervention from baseline to 6 months (Beta = -5.14, SE = 3.45, 95% CI = -11.90 – -1.62, p = .14), but not at 9 months.
Biological markers
With respect to biological markers, CD4 but not VL outcomes were associated with treatment arm participation. Specifically, means for VL, though lower initially for the treatment arm, dropped comparably at each assessment point for both arms. The omnibus test of the CBT-AD × time interaction for the log10 VL outcome suggested almost no association between the intervention and change in HIV VL over time, χ2(2) = 0.10, p = 0.95. Within the omnibus test, planned contrasts also indicated no association between intervention and change in HIV VL from baseline to 6 or 9 months. CD4 findings indicated immunologic gains occurred in both arms, but, especially for the 6-month assessment point, they were greater in the intervention versus TAU arm. Within the omnibus test of the CBT-AD × time interaction, χ2(2) = 3.28, p = 0.19, there was a marginally significant CBT-AD × time (baseline vs. 6 months) contrast, indicating a 69 cell/mm3 greater improvement at post-intervention for those receiving the intervention (Beta = 69.45, SE = 38.57, 95% CI = -6.16 – 145.05, p = .07), yet little difference in the CBT-AD × time (baseline vs. 9 months) contrast, (Beta = 25.71, SE = 43.17, 95% CI = -58.91 – 110.32, p = .55), suggesting that the marginal post-intervention improvement in immunological functioning was not maintained at follow-up.
Discussion
We culturally adapted and then evaluated a CBT program (CBT-AD) to treat depression and medication non-adherence in a preliminary RCT involving 40 HIV-positive Latino men and women on ART at a community-based HIV clinic on the U.S.-Mexico border. Specially trained graduate student clinicians were able to faithfully implement the manualized intervention in both Spanish and English, which indicated good feasibility and participant acceptability. Although this preliminary trial was not powered for tests of statistical significance, findings were promising, indicating multiple moderate and at least one large-sized effect in the hypothesized direction. Specifically, self-reported adherence was moderate and rose more steeply in the treatment versus the TAU arm. EDM data, as is typical in adherence studies, suggested lower adherence and descending trajectories across arms, but overall better outcomes for participants receiving the CBT-AD intervention. Self-reported levels of depressive symptomatology according to the BDI were supportive of a treatment effect as were, to a lesser degree, the clinician-administered MADRS interviews. The differences in results pertaining to depressive symptomatology may be due to different measurement modalities (self-report versus blind clinician-rated interviews). There were ceiling effects for VL (which may have led to not finding a difference between arms), but immunological outcomes according to CD4 counts were better for the treatment participants at the 6-month assessment point.
The size and endurance of the effect sizes with respect to adherence are promising, given reports from meta-analyses that behavioral interventions to improve HAART adherence have minor effects on adherence that often diminish over time [8, 55]. This may be attributable to the dual approach of addressing mental health needs and using technology to address “simply forgetting” of dosing times. Indeed, this study is responsive to recent calls to integrate mental health treatment directly into HIV primary care [56]. Specifically, Sikkema and colleagues [56] have posited a model of the purported effects of mental health treatment, namely improved health outcomes and reduced transmission risks.
Overall, the results suggest a larger trial is warranted to investigate whether the intervention might prove efficacious and generalize to other resource-poor areas in the U.S.-Mexico Border region, most of which has been designated as a Health Professions Shortage Area in the domain of mental health by the Secretary of the Department of Health and Human Services [57], and perhaps for resource-limited regions in other countries. Implementation of such an intervention does require some resources such as space and equipment, but staff may be selected from mental health training programs such as interns training in psychology, social work, nursing and counseling. Doctoral-level providers in short supply in the border region, and many doctoral-level providers there and elsewhere in the U.S. are ill-equipped to serve the needs of monolingual Spanish-speaking clients and have little experience in the unique aspects of mental health issues presenting in the HIV treatment context. The present study suggests one method of responding to calls to increase access to mental health services and decrease the burden of mental illness and associated conditions in underserved areas [58].
The findings must be interpreted in the light of several limitations of the study. It was clearly a preliminary evaluation, with a relatively small sample size, an abbreviated follow-up period, and the absence of a true time or attention control comparison study arm (although TAU participants were reimbursed $20 for monthly check-in visits and had letters sent to their provider indicating they were enrolled in the study and presenting with depressive symptoms). Additionally, the combined delivery of the therapy intervention with the electronic pillbox reminder precludes our ability to distinguish the most efficacious component of the intervention. Another limitation was the between-arm differences at baseline (intervention arm participants were more likely to be partnered and reported higher levels of depression), suggesting randomization was not completely successful. A review of our randomization procedures revealed no systematic bias. With small, convenience samples, randomization is often not optimal [59] due to the increased likelihood of chance imbalances, further supporting the need for a full trial with a larger sample size. Our broad inclusion criteria are another potential limitation with respect to capturing persons with less than perfect adherence. Participants reporting only 1 missed dose (i.e., near perfect adherence) or verbally expressing problems with adherence to study staff including were eligible to participate. Thus, the effect sizes obtained in this study are likely conservative estimates, since showing statistically significant treatment effects with a sample of participants reporting near perfect adherence is difficult. In this preliminary trial we could not control for differences in baseline factors such as participants in the treatment arm being more likely to have a partner. This and other factors such as acculturation levels may influence the outcome of an intervention and might be explored in future research [60].
Recent years have seen an increase in the use of community-based participatory research (CBPR) with Latinos [61], and there has been substantial work done on mental health service utilization [62-64], and inclusion and retention in clinical trials among Latinos [65, 66]. However, the current study is the first to adapt an evidence-based intervention for depression and non-adherence in Latinos living with HIV [67]. In conclusion, the HIV epidemic in low-resource settings such as the U.S.-Mexico border continues to require innovative and cost-effective solutions. Interventions such as the one described here that capitalize on existing personnel resources (i.e., students with limited professional experience), integrate easily with clinic care, and address key cultural values merit further attention.
Acknowledgments
We are grateful to the patients and staff of Centro de Salud Familiar la Fe CARE Center, Inc. for their participation and facilitation of this study. We thank members of our community and administrative advisory boards as well as research consultants Jaime Anaya, Kurt C. Organista, Steffanie A. Strathdee, Karina Walters, and Randolph Whitworth. We would also like to thank those who aided with project implementation, including Jessica Armendariz, Teresa Frias, Carolina Lara, Chrisie Lemon, Virginia Longoria, Elsa Martin, Antonio Martinez, Miriam Pando, Tatiana Rodriguez, and Cesar Villareal Ramos from the University of Texas at El Paso, and Kimberly Nelson, Cynthia Pearson, and Samantha Yard from the University of Washington. This research project was supported by the National Institute of Mental Health (grant # 1R34MH08674 and minority research supplement award # R34MH084674-S1) and in part by the Center for AIDS Research (grant # P30 AI027757).
References
- 1.CDC. HIV Surveillance Report, 2009. [29 September 2011]; Available from http://www.cdc.gov/hiv/topics/surveillance/resource/reports/
- 2.del Rio C. Latinos and HIV care in the southeastern United States: new challenges complicating longstanding problems. Clin Infect Dis. 2011;53(5):488–89. doi: 10.1093/cid/cir440. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez MA, Lemp GF, Magis-Rodríguez C, Bravo-García E, Carter S, Ruiz JD. The epidemiology of HIV among Mexican migrants and recent immigrants in California and Mexico. J Acquir Immune Defic Syndr. 2004;37(Suppl 4):S204–S214. doi: 10.1097/01.qai.0000141253.54217.24. [DOI] [PubMed] [Google Scholar]
- 4.Santos G, Puga AM, Medina C. HAART, adherence, and cultural issues in the US Latino community. AIDS Read. 2004;14(Suppl 10):S26–S29. [PubMed] [Google Scholar]
- 5.Murphy DA, Roberts KJ, Hoffman D, Molina A, Lu MC. Barriers and successful strategies to antiretroviral adherence among HIV-infected monolingual Spanish-speaking patients. AIDS Care. 2003;15(2):217–30. doi: 10.1080/0954012031000068362. [DOI] [PubMed] [Google Scholar]
- 6.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Infect Dis Rep. 2008;10(6):515–21. doi: 10.1007/s11908-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Servellen G, Nyamathi A, Carpio F, et al. Effects of a treatment adherence enhancement program on health literacy, patient-provider relationships, and adherence to HAART among low-income HIV-positive Spanish-speaking Latinos. AIDS Patient Care STDS. 2005;19(11):745–59. doi: 10.1089/apc.2005.19.745. [DOI] [PubMed] [Google Scholar]
- 10.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–30. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 11.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 12.Longoria V, Wiebe JS, Jones A. A nurse-based disease management model of HIV/AIDS care on the U.S./Mexico Border. In: Curiel H, editor. Innovative strategies for HIV/AIDS prevention & care along the U S /Mexico Border. Washington, D.C.: HRSA; 2007. [Google Scholar]
- 13.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acqui Immune Defic Syndr. 2011;58(2):181–87. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherr L, Clucas C, Harding R, Sibley E, Catalan J. HIV and depression-A systematic review of interventions. Psychol Health Med. 2011;16(5):493–527. doi: 10.1080/13548506.2011.579990. [DOI] [PubMed] [Google Scholar]
- 15.Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. [June 28, 2012];AIDS Behav. doi: 10.1007/s10461-012-0212-3. [published online ahead of print May 30 2012], http://www.springerlink.com/content/q00283l02312tjw7/ [DOI] [PMC free article] [PubMed]
- 16.Wagner GJ, Goggin K, Remien RH, et al. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behave Med. 2011;42(3):352–60. doi: 10.1007/s12160-011-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safren SA, Radomsky AS, Otto MW, Salomon E. Predictors of psychological well-being in a diverse sample of HIV-positive patients receiving highly active antiretroviral therapy. Psychosomatics. 2002;43(6):478–85. doi: 10.1176/appi.psy.43.6.478. [DOI] [PubMed] [Google Scholar]
- 18.Kelly JA, Murphy DA, Bahr GR, et al. Outcome of cognitive-behavioral and support group brief therapies for depressed, HIV-infected persons. Am J Psychiatry. 1993;150(11):1679–1686. doi: 10.1176/ajp.150.11.1679. [DOI] [PubMed] [Google Scholar]
- 19.Mulder CL, Emmelkamp PM, Antoni MH, Mulder JW, Sandfort TG, de Vries MJ. Cognitive-behavioral and experiential group psychotherapy for HIV-infected homosexual men: a comparative study. Psychosom Med. 1994;56(5):423–31. doi: 10.1097/00006842-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Thomason BT, Bachanas PJ, Campos PE. Cognitive behavioral interventions with persons affected by HIV/AIDS. Cogn Behav Pract. 1996;3:417–42. [Google Scholar]
- 21.Balfour L, Kowal J, Silverman A, et al. A randomized controlled psycho-education intervention trial: improving psychological readiness for successful HIV medication adherence and reducing depression before initiating HAART. AIDS Care. 2006;18(7):830–38. doi: 10.1080/09540120500466820. [DOI] [PubMed] [Google Scholar]
- 22.Laperriere A, Ironson GH, Antoni MH, et al. Decreased depression up to one year following CBSM+ intervention in depressed women with AIDS: the smart/EST women’s project. J Health Psychol. 2005;10(2):223–31. doi: 10.1177/1359105305049772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olatunji BO, Mimiaga MJ, O’Cleirigh C, Safren SA. Review of treatment studies of depression in HIV. Top HIV Med. 2006;14(3):112–24. [PubMed] [Google Scholar]
- 24.Yun LWH, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005;38(4):432–38. doi: 10.1097/01.qai.0000147524.19122.fd. [DOI] [PubMed] [Google Scholar]
- 25.Safren SA, Gonzalez JS, Soroudi N. Coping with chronic illness: cognitive behavioral therapy for adherence and depression in individuals with chronic illness, client workbook. New York, NY: Oxford University Press; 2007a. [Google Scholar]
- 26.Safren SA, Gonzalez JS, Soroudi N. Coping with chronic illness: cognitive behavioral therapy for adherence and depression in individuals with chronic illness, therapist guide. New York, NY: Oxford University Press; 2007b. [Google Scholar]
- 27.Nezu AM, Nezu CM, Friedman SH, Faddis S, Houts PS. Helping cancer patients cope: a problem-solving approach. Washington, D.C.: American Psychological Association; 1998. [Google Scholar]
- 28.Nezu A, Nezu C, Friedman SH, Houts P, Faddis S. Project Genesis: application of problem-solving therapy to individuals with cancer. Behave Ther. 1997;9:155–58. [Google Scholar]
- 29.Safren S, Otto M, Worth J. Life-steps: applying cognitive behavioral therapy to HIV medication adherence. Cogn Behav Pract. 1999;6(4):332–41. [Google Scholar]
- 30.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brigido LF, Rodrigues R, Casseb J, et al. Impact of adherence to antiretroviral therapy in HIV-1-infected patients at a university public service in Brazil. AIDS Patient Care STDS. 2001;15(11):587–93. doi: 10.1089/108729101753287685. [DOI] [PubMed] [Google Scholar]
- 32.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiser S, Wolfe W, Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003;34(3):281–88. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Zogg JB, Woods SP, Sauceda JA, Wiebe JS, Simoni JM. The role of prospective memory in medication adherence: a review of an emerging literature. J Behav Med. 2011;35(1):47–62. doi: 10.1007/s10865-011-9341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrade ASA, McGruder HF, Wu AW, et al. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infec Dis. 2005;41(6):875–82. doi: 10.1086/432877. [DOI] [PubMed] [Google Scholar]
- 36.Safren SA, Hendriksen ES, Desousa N, Boswell SL, Mayer KH. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care. 2003;15(6):787–93. doi: 10.1080/09540120310001618630. [DOI] [PubMed] [Google Scholar]
- 37.Simoni JM, Huh D, Frick PA, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52(4):465–73. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKleroy VS, Galbraith JS, Cummings B, et al. Adapting evidence-based behavioral interventions for new settings and target populations. AIDS Educ Prev. 2006;18(4 Suppl A):59–73. doi: 10.1521/aeap.2006.18.supp.59. [DOI] [PubMed] [Google Scholar]
- 39.Nezu AM, Nezu CM, Felgoise SH, McClure KS, Houts PS. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. J Consult Clin Psychol. 2003;71(6):1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- 40.Organista K. Cognitive-behavioral therapy with Latinos and Latinas. In: Hays PA, Iwamasa GY, editors. Culturally responsive cognitive-behavior therapy: assessment, practice, and supervision. Washington, DC: American Psychological Association; 2006. pp. 73–96. [Google Scholar]
- 41.Bedoya CA, Safren SA. Capturing (and communicating) complexity: adapting CBT for clients with multiple diversity [commentary on the case of Felix: an example of gay-affirmative cognitive-behavioral therapy] Pragmatic Case Studies in Psychotherapy. 2009;5(2):22–27. [Google Scholar]
- 42.Trinh NT, Bedoya CA, Chang TE, Flaherty K, Fava M, Yeung A. A study of a culturally focused psychiatric consultation service for Asian American and Latino American primary care patients with depression. BMC Psychiatry. 2011;11(1):166. doi: 10.1186/1471-244X-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brislin RW. Translation and content analysis of oral and written material. In: Triandis HC, Berry JW, editors. Handbook of cross-cultural psychology. Boston, MA: Allyn & Bacon; 1980. pp. 389–444. [Google Scholar]
- 44.Amico KR, Fisher WA, Cornman DH, et al. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr. 2006;42(4):455–59. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- 45.Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 46.Bonicatto S, Dew AM, Soria JJ. Analysis of the psychometric properties of the Spanish version of the Beck Depression Inventory in Argentina. Psychiatry Res. 1998;79(3):277–85. doi: 10.1016/s0165-1781(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 47.Sanz J, Vázquez C. Fiabilidad, validez, y datos normativos del inventario para la depresión de Beck [Reliability, validity, and normative data from the Beck Depression Inventory] Psicothema. 1998;10:303–18. [Google Scholar]
- 48.Suárez-Mendoza AA, Cardiel MH, Caballero-Uribe CV, Ortega-Soto HA, Márquez-Marín M. Measurement of depression in Mexican patients with rheumatoid arthritis: validity of the Beck Depression Inventory. Arthritis Care Res. 1997;10(3):194–99. doi: 10.1002/art.1790100307. [DOI] [PubMed] [Google Scholar]
- 49.Kalichman SC, Rompa D, Cage M. Distinguishing between overlapping somatic symptoms of depression and HIV disease in people living with HIV-AIDS. J Nerv Ment Dis. 2000;188(10):662–70. doi: 10.1097/00005053-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–89. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 51.Lobo A, Chamorro L, Luque A, et al. Validation of the Spanish versions of the Montgomery-Asberg depression and Hamilton anxiety rating scales. Med Clin (Barc) 2002;118(13):493–99. doi: 10.1016/s0025-7753(02)72429-9. [DOI] [PubMed] [Google Scholar]
- 52.Kørner A, Nielsen BM, Eschen F, et al. Quantifying depressive symptomatology: inter-rater reliability and inter-item correlations. J Affect Disord. 1990;20(2):143–49. doi: 10.1016/0165-0327(90)90128-u. [DOI] [PubMed] [Google Scholar]
- 53.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 54.Huh D, Flaherty BP, Simoni JM. Optimizing the analysis of adherence interventions using logistic generalized estimating equations. AIDS Behave. 2011;16(2):422–31. doi: 10.1007/s10461-011-9955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–97. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 56.Sikkema KJ, Watt MH, Drabkin AS, Meade CS, Hansen NB, Pence BW. Mental health treatment to reduce HIV transmission risk behavior: a positive prevention model. AIDS Behav. 2010;14(2):252–62. doi: 10.1007/s10461-009-9650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Health Professional Shortage Areas (HRSA) Health Professional Shortage Area NHSC Fulfillment of Mental Health Care HPSA Needs Summary, 2005. [1 September 2007]; Available from http://datawarehouse.hrsa.gov/hpsadetail.aspx.
- 58.Kazdin AE, Blase SL. Rebooting psychotherapy research and practice to reduce the burden of mental illness. Perspect Psychol Sci. 2011;6(1):21–37. doi: 10.1177/1745691610393527. [DOI] [PubMed] [Google Scholar]
- 59.Altman DG, Bland JM. How to randomize. Brit Med J. 1999;319(7211):703–704. doi: 10.1136/bmj.319.7211.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robbins RN, D’Aquila E, Morgello S, Byrd D, Remien RH, Mindt MR. Cultural influences on antiretroviral therapy adherence among HIV-infected Puerto Ricans. J Assoc Nurses AIDS Care. 2012;23(6):531–538. doi: 10.1016/j.jana.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Las Nueces D, Hacker K, DiGirolamo A, Hicks LS. A systematic review of community-based participatory research to enhance clinical trials in racial and ethnic minority groups. Health Serv Res. 2012;47(2):1363–1386. doi: 10.1111/j.1475-6773.2012.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becker MA, Martinez-Tyson D, DiGennaro J, Ochshorn E. Do Latino and Non-Latino White Medicaid-enrolled adults differ in utilization of evidence-based treatment for major depressive disorder? J Immigr Minor Health. 2011;13(6):1048–1054. doi: 10.1007/s10903-011-9508-z. [DOI] [PubMed] [Google Scholar]
- 63.Keyes KM, Martins SS, Hatzenbuehler ML, Blanco C, Bates LM, Hasin DS. Mental health service utilization for psychiatric disorders among Latinos living in the United States: the role of ethnic subgroup, ethnic identity, and language/social preferences. Soc Psychiatry Psychiatr Epidemiol. 2012;47(3):383–94. doi: 10.1007/s00127-010-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soller M, Kharrazi N, Prentiss D, et al. Utilization of psychiatric services among low-income HIV-infected patients with psychiatric comorbidity. AIDS Care. 2011;23(11):1351–1359. doi: 10.1080/09540121.2011.565024. [DOI] [PubMed] [Google Scholar]
- 65.Brown SD, Lee K, Schoffman DE, King AC, Crawley LM, Kiernand M. Minority recruitment into clinical trials: experimental findings and practical implications. Contemp Clin Trials. 2012;33(4):620–23. doi: 10.1016/j.cct.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rivera-Goba MV, Dominguez DC, Stoll P, Grady C, Ramos C, Mican JM. Exploring decision-making of HIV-infected Hispanics and African Americans participating in clinical trials. J Assoc Nurses AIDS Care. 2011;22(4):295–306. doi: 10.1016/j.jana.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez JS, Hendriksen ES, Collins EM, Durán RE, Safren SA. Latinos and HIV/AIDS: examining factors related to disparity and identifying opportunities for psychosocial intervention research. AIDS Behav. 2008;13(3):582–602. doi: 10.1007/s10461-008-9402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


