Abstract
Introduction
All humans have natural, protective antibodies directed against phosphorylcholine (PC) epitopes, a common inflammatory danger signal appearing at sites of cell injury, oxidative stress, and on bacterial capsules. In large human cohorts, low levels of anti-PC IgM were associated with a significantly increased risk of stroke or myocardial infarction. However it is not known if these antibodies protect against the premature closure of arterial reconstructions.
Methods
A prospective, observational study of patients undergoing elective, infrainguinal, autogenous vein bypasses for atherosclerotic occlusive disease of the legs was conducted. Clinical data were recorded prospectively, and preoperative levels of anti-PC IgM measured with the CVDefine kit from Athera Biotechnologies. The principal clinical endpoint was the loss of primary patency (loss of graft flow, or any intervention for stenosis). Patients were followed regularly by duplex ultrasound at 1, 3, 6, 12, 18 months, and yearly thereafter.
Results
Fifty-six patients were studied, for an average of 1.3 years. Indications for surgery were claudication (33.9%), ischemic rest pain (17.9%), and ischemia with ulceration or gangrene (48.2%). Seventeen (30.4%) patients experienced loss of primary patency (10 graft occlusions, 7 surgical or endovascular revisions of graft stenoses). Kaplan-Meier survival analysis showed that the quartile of patients with the lowest anti-PC IgM levels had significantly worse primary graft patency (p=.0085, log rank test). Uni- and multivariate Cox proportional hazards analysis revealed that the preoperative anti-PC IgM level was an important predictor of graft failure. Patients with IgM values in the lowest quartile had a 3.6-fold increased risk of graft failure (95% confidence interval: 1.1-12.1), even after accounting for other significant clinical or technical factors such as indication for surgery, site of distal anastomosis, or vein graft diameter.
Conclusions
A naturally occurring IgM antibody directed against the pro-inflammatory epitope phosphorylcholine may be protective against vein graft stenosis and failure, through anti-inflammatory mechanisms. Measurement of this antibody may be a useful prognostic indicator, although larger studies of more diverse populations will be needed to confirm these results. The biological actions of anti-PC IgM suggest it may be useful in developing immunotherapies to improve bypass longevity.
Introduction
Atherosclerosis is an inflammatory, immune-mediated disease1-3. The appearance of oxidized lipids and related pathologic neo-antigens in the vessel wall are commonly the inciting events that attract inflammatory cells to the site of injury, beginning a cascade of inflammatory events that lead to intimal thickening, smooth muscle cell proliferation, matrix deposition and ultimately complex plaque formation3, 4. Our increasing knowledge of arterial injury and repair has led to an understanding that there are common mechanisms of cellular and humorally-mediated inflammation that govern primary atherosclerosis, neointimal hyperplasia, and adaptive vascular remodeling. While much research has emphasized the pro-inflammatory mediators, some natural anti-inflammatory mechanisms have also been known for some time5.
Among these are natural IgM antibodies that belong to the innate immune system, and are present at birth6-8. These natural antibodies are programmed to recognize a restricted pattern of “danger signals”, and hence protect against bacterial invasion, control oxidative stress, and neutralize pro-inflammatory oxidized lipid moieties9, 10. As outlined by Lutz and others8, during oxidative stress at sites of vascular injury, the abundant lipid phosphatidylcholine undergoes oxidation to expose normally hidden phosphorylcholine (PC) headgroups. Dying cells and cellular debris also expose and release this neo-antigen of phosphorylcholine, which is recognized by the immune system as “altered self”, and instigates a complex inflammatory response5. These normally cryptic PC epitopes are also a prominent part of the capsular polysaccharide of many bacteria. Thus, abnormally exposed PC is one of the major antigens recognized by our innate immune system11. Research and diagnostic tools have now been developed to study natural IgM that recognizes exposed phosphorylcholine (PC) in humans and animals12
The putative vascular protective mechanisms of this antibody include the inhibition of macrophage uptake of oxidized LDL, blockade of macrophage IL-6 production13, and suppression of endothelial activation by inflammatory mediators10, 14. In vivo, anti-PC IgM is thought to attenuate the inflammatory response to PC that is exposed at sites of vascular injury. Low levels of anti-PC IgM may thus lead to excessive activation of inflammatory responses. In large human cohorts, low levels of anti-PC IgM were associated with a significantly increased risk of stroke or myocardial infarction14-16, 16, 17, and mortality in dialysis patients18. Furthermore, in animal models, enhancement of anti-PC antibodies through immunization or supplementation reduced atherosclerosis and intimal hyperplasia in a mouse vein bypass model19, 20. Twin studies suggest that forty percent of the variability in human anti-PC IgM levels is attributable to genetic variance21.
Autogenous vein grafts used to bypass atherosclerotic occlusive disease of the lower extremities are prone to early failure due to stenosis and thrombosis. Atherosclerosis, per se, is not the cause of these early failures, which typically occur within the first one to two years after surgery22. However both early graft failure and atherosclerosis have in common the derangement of the injury/repair process by activation of thrombo-inflammatory pathways, leading to intimal hyperplasia, smooth muscle cell proliferation, and induction of a prothrombotic local vascular environment. Thus, it is possible that anti-PC IgM antibodies might modulate and normalize the vascular healing process in vein grafts, just as they modulate the progression of cardiovascular arterial disease. Previous human studies illustrated the importance of low levels of anti-PC IgM, as they were associated with loss of atheroprotection, and adverse events. We conducted this pilot study to determine if there was a relationship between low levels of anti-PC IgM antibodies, and the loss of primary patency of infrainguinal autogenous vein grafts.
Patients and Methods
We designed a prospective, longitudinal observational study of all eligible patients undergoing infrainguinal bypass at a single institution. After informed consent, patients scheduled for elective, infrainguinal bypass surgery using autologous vein were prospectively recruited at the VA Puget Sound Health Care System, a large regional referral center for vascular surgery. The research was approved by the Institutional Review Board of the Human Research Protection Program of the VA Puget Sound. All elective infrainguinal bypasses using autogenous vein were eligible for study. Exclusion criteria for the study were: operations for non-atherosclerotic or aneurysmal disease, concurrent aorto-bifemoral or femoral-femoral bypass, use of a prosthetic bypass conduit, active treatment for malignancy (i.e. chemotherapy), chronic hemodialysis, perioperative thrombosis of the graft, or a diagnosed systemic inflammatory disease (e.g. lupus or other treatment with immunosuppressive drugs) or thrombophilia. At surgery, completion intraoperative imaging was performed in all cases (angiography or duplex ultrasound) to confirm patency and freedom from stenosis or technical defects. Patients were classified as diabetic if the diagnosis was in their problem list and they were being chronically treated with an oral hypoglycemic agent or insulin. Active smoking was self-reported by the patient. We included as “smokers” those who had quit in the past week prior to surgery. The non-smoker classification included those former smokers who had quit months to years previously.
The clinical severity of their occlusive disease at the time of surgery (Leg Status) was classified as claudication (Fontaine stage II, Rutherford grade I), rest pain (Fontaine stage III, Rutherford grade II), or critical ischemia with ulceration (Fontaine stage IV, Rutherford grade III), according to the guidelines of the Society for Vascular Surgery23. Several other surgical factors known to influence the primary patency of lower extremity bypass grafts were also recorded prospectively: a history of a previously failed bypass graft, the location of the distal anastomosis, and the minimum diameter of the pressurized vein conduit. Finally, technical aspects of the surgical procedures that have been associated with a higher risk of failure were noted, which we denoted as Higher Risk Vein Graft. This factor was considered positive if veins other than the greater saphenous vein were used in part or whole or if there were any technical problems during surgery requiring intraoperative revisions.
The long term patency of the grafts was monitored by arterial color duplex ultrasound and ankle/brachial indices at 4-6 weeks, then at 3, 6, 12, 18 months, and yearly thereafter. Standard duplex graft surveillance was performed on Acuson Antares equipment, using protocols established by Bandyk et al.24, 25. The primary clinical endpoint was the loss of primary patency (graft failure), defined as any graft revision to maintain patency, or graft thrombosis. The first of these events to occur triggered the primary endpoint. Graft revision was defined as any open surgical or endovascular procedure performed on a patent graft to correct stenosis or narrowing. The treating surgeon made the independent clinical decision regarding the need for revision, based on the accepted definitions for a hemodynamically significant vein graft stenosis (i.e. diameter reduction of >75%, peak systolic velocity > 300 cm/sec, velocity ratio > 3.5)25. Graft occlusion was defined (per published guidelines23) as clinical loss of flow, which was confirmed by complementary imaging when necessary.
Peripheral blood samples were obtained to measure anti-PC IgM preoperatively, using evacuated tubes containing potassium-EDTA (1.8mg/ml final concentration, Becton Dickinson). Within one hour of collection, the tubes were centrifuged at 200×g for 10 minutes at room temperature, and the platelet-rich plasma transferred to polypropylene microcentrifuge tubes. These tubes were centrifuged at 13,000×g for 10 minutes, and the platelet-free plasma was removed, aliquoted, and stored at -70°C until assay. The manufacturer of the kits states that samples tolerate at least 3-4 cycles of freeze thawing, although this was avoided by making aliquots. IgM anti-PC is also stable for years at -70 °C. Anti-PC IgM levels were determined by ELISA using the CVDefine kit (Athera Biotechnologies)12, an enzyme-linked immunosorbent assay which employs an immobilized, conjugated phosphorylcholine antigen, and human anti-PC IgM for standards. Briefly, thawed plasma was centrifuged 5 minutes at 13,000×g to remove aggregates, then diluted as directed in the provided assay diluent. Samples, standards, and controls provided in the kit were added in duplicate to microwells coated with PC. After washing, the bound IgM was reacted with anti-IgM conjugated to horseradish peroxidase, measured with TMB as substrate. The standard curve was generated using the cubic spline algorithm (GraphPad Prism), and sample concentrations were expressed as arbitrary units/ml as defined by the manufacturer.
Statistical Analyses
Analyses were conducted in the statistical software package R (version 2.15.2). Survival analyses were made with the Kaplan–Meier survival curve and the Cox proportional hazard model, stratified by dichotomization of anti-PC IgM values into the lowest 25% and the remaining 75%. This was justified by the biological behavior of this IgM antibody, as observed in clinical studies. The univariate and multivariate Cox regression analyses are presented as hazard ratios [HR; 95% confidence intervals (CI)]. Cox adjustments were done with correction for the known predictors of graft failure outlined below. Values of IgM were log-transformed where noted. Log rank tests were also completed to determine whether the survival distributions between groups were equal. To determine if the value of pre-operative IgM was associated with clinical characteristics of the patients, uni- and multivariate linear regression models were completed for each clinical parameter.
Results
Patient Characteristics
A total of 68 patients were initially recruited to the study. Eight of these patients were excluded from further study because they did not have a qualifying operation (e.g. prosthetic graft, or never had surgery), 3 had perioperative occlusions before discharge, and 1 was excluded for lack of intraoperative completion imaging. All of the subsequent primary endpoints in the remaining 56 patients occurred more than 30 days after their surgery. Thus, 56 consecutive, eligible patients were enrolled in the study between 2007 and 2012, and all had their levels of plasma anti-PC IgM antibodies measured preoperatively. Fifty-four subjects were Caucasian, two African-American, and three were female. Table I describes the main characteristics of these subject's medical conditions, their mean and median follow up, and the relevant details of their surgeries. Thirteen patients received the designation of Higher Risk Vein Graft, based on technical aspects of the surgery (veins other than the greater saphenous, 3 patients) or any technical problems requiring intraoperative revisions (10 patients). Technical revisions included autogenous patch angioplasties of narrow anastomoses, and intraoperative repairs or resections of venous conduit abnormalities.
Table I. Clinical and Surgical Characteristics of Patient Population.
| Characteristic | Mean | Median | Range or Percentage |
|---|---|---|---|
| Age | 64.8 | 63.0 | 50 – 81 |
| Follow Up - All Patients (days) | 464 | 377 | 31 – 1580 |
| Time to Loss of Primary Patency (days) | 305 | 184 | 38 – 1261 |
| Clinical Factors | |||
| Smoking | 55.4% | ||
| Diabetes | 37.5% | ||
| Hypertension | 87.5% | ||
| Hyperlipidemia | 87.5% | ||
| Statin use | 80.4% | ||
| Aspirin | 92.9% | ||
| Clopidogrel | 8.9% | ||
| Warfarin | 7.1% | ||
| Leg Status | |||
| Claudication | 33.9% | ||
| Rest Pain | 17.9% | ||
| Ulceration | 48.2% | ||
| Ankle-Brachial Index | 0.46 | 0.47 | 0-0.96 |
| White Blood Cell Count (K/uL) | 8.2 | 7.7 | 5-13.6 |
| Prior Failed Graft | 12.5% | ||
| Operative Factors | |||
| Above Knee distal anastomosis | 33.9% | ||
| Below Knee distal anastomosis | 28.6% | ||
| Tibial distal anastomosis | 37.5% | ||
| High risk vein graft | 23.2% | ||
| Diam. Of Vein (mm) | 4.06 | 4.00 | 3-7 |
Relationship between Anti-PC IgM Levels and Outcomes
The average preoperative IgM value was 74.4 units/ml (± 11.9 sem) and the median was 38.0 units/ml. The values were not normally distributed, so a log transformation was completed and the log values were normally distributed. Seventeen of the fifty-six patients experienced a primary endpoint (30.4%), of which ten were graft occlusion, and seven graft revisions for a hemodynamically significant stenosis. The clinical characteristics of these patients are described in the on-line, Supplemental Table I.
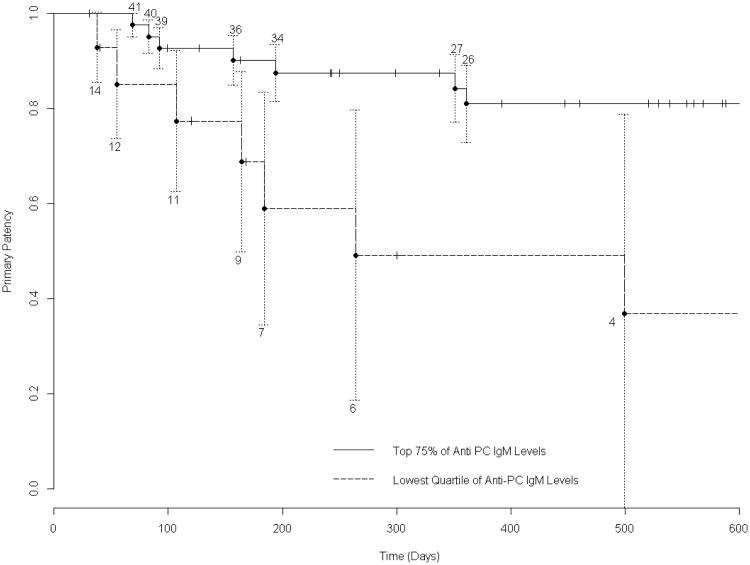
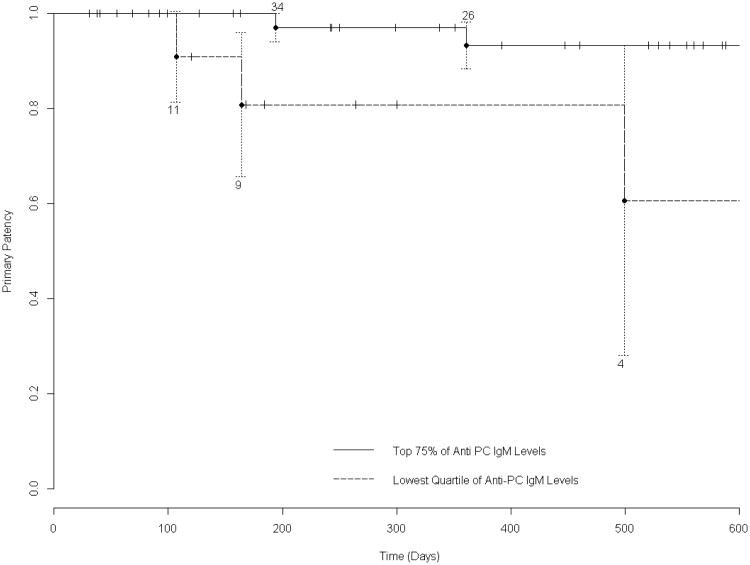
The primary patency of the overall group is presented as a Kaplan-Meier survival curve in Figure 1. For survival analysis of the effect of anti-PC IgM, the preoperative valueswere dichotomized, comparing those in the lowest quartile (n=14) to the other 75% (n=42). This analytic approach was taken because prior research suggests that low levels of IgM are specifically associated with the loss of protective effects, while a much wider range of higher levels are associated with freedom from adverse outcomes. Those patients in the lowest quartile experienced a significantly inferior primary graft survival, compared to the higher 75% (p=.0085, log rank test, Figure 2). We tested alternative dichotomization schemes, stratifying patients by the lowest quintile versus the highest 80%, or those below the median value versus those above. Each of these analyses (log rank test 0.019 and 0.035 respectively) were statistically significant, but stratification by the lowest quartile gave the best discrimination of clinical outcome. Graft survival was also analyzed from the perspective of freedom from intervention for stenosis, excluding those primary endpoints that arose due to graft thrombosis. Figure 3 illustrates the Kaplan-Meier curves, and shows that those patients in the lowest quartile of anti-PC IgM values had a higher incidence of graft stenosis (log rank test 0.023) .
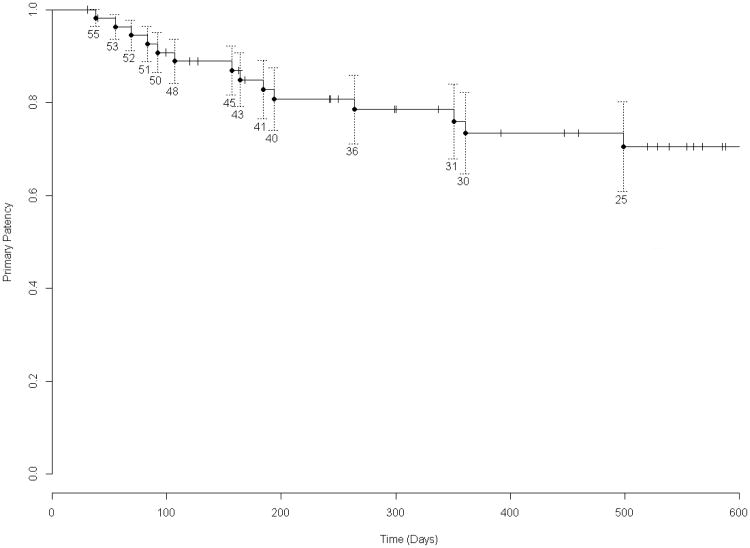
Figure 1. Kaplan-Meier Survival Analysis of Overall Primary Graft Patency.
Freedom from loss of primary patency is illustrated for all 56 patients studied. The numbers above and below the graph lines indicate the number of subjects at risk at each interval. SEM's are shown.
Figure 2. Kaplan-Meier Survival Analysis of Overall Primary Graft Patency According to IgM Level.
The difference in primary graft patency (stenosis requiring intervention, and graft occlusion) between those in the bottom quartile of anti-PC IgM values, and those in the top 75% was significant (p=.0085 log-rank test). The numbers above and below the graph lines indicate the number of subjects at risk at each interval. SEM's are shown, with truncation of the graph to 600 days, where the SEM of the primary group exceeds 10%.
Figure 3. Kaplan-Meier Survival Analysis of Freedom from Stenosis According to IgM Level.
Considering the endpoint of stenosis (requiring intervention) alone, the differences in graft survival were significant (p=.023 log-rank test). Technical details of the graph are the same as figure 2.
The preoperative IgM values were significantly lower in those subjects who suffered loss of primary patency (median 27.9 units/ml, 20.45-55.08 interquartile range [IQR]) compared with those whose grafts remained patent (median 46.1, 34.78-134.02 IQR). Because the majority of graft failures occur within the first year of follow up, we included only the 42 subjects with at least one full year of surveillance of their patent grafts. Figure 4 illustrates these differences, which were significant (P=.015, Mann-Whitney rank sum test), and also illustrates a scatter plot of all preoperative values.
Figure 4. Anti-PC IgM Levels According to Primary Graft Patency.
42 subjects with at least one full year of follow-up were included. The box plots show the mean (dotted line), median (solid line), 25-75 percentile (box), and 10-90 percentile (error bars). P= .015, Mann-Whitney rank sum. The central scatter plot illustrates the range of preoperative IgM values among all 56 patients.
To identify the specific factors that might be associated with the duration of primary patency, we performed Cox proportional hazards analyses of the entire cohort of 56 patients, first testing individually the variables that might affect the loss of primary patency (Table II). The variables that were individually significant at alpha level .05 were distal anastomosis (P=.02, tibial vs. above knee), minimum vein diameter (P=.001), and pre-operative IgM. The variables of Leg Status (P=.06, ulceration vs. claudication) and high risk vein graft (P=.07) were of borderline significance, so they were included in the multivariate Cox proportional hazards model. Subsequent multivariate analysis yielded a parsimonious model for graft failure in which the minimum vein graft diameter, and the preoperative anti-PC IgM value were the two most significant factors associated with graft failure. Table III shows that patients in the lowest quartile of anti-PC IgM values (below ∼27 units/ml) had a 3.62 fold greater risk of graft failure than the other 75%, even accounting for the diameter of vein conduit, leg status, site of distal anastomosis, and high risk vein graft characteristics. Also, for each 1 mm reduction in vein graft diameter from the median (4 mm), the risk of graft failure increases by 5.56 fold, after adjustment for distal anastomosis, high risk vein graft characteristics and preoperative IgM quartile.
Table II. Univariate Cox Proportional Hazards Model for Primary Endpoint – Loss of Primary Patency.
These clinical characteristics were tested individually for their relationship to the occurrence of the primary endpoint over time. The preoperative IgM value, whether considered as the lowest quintile, quartile, or below the median, was significantly associated with loss of primary patency. The following factors were chosen for inclusion in the subsequent multivariate hazards model because they were significant, or approached significance: Leg status, Distal anastomosis, High risk vein graft, Minimum diameter, and lowest quartile of IgM value.
| Variable | Level | Hazard Ratio | Lower 95% | Upper 95% | P-value for Z statistic |
|---|---|---|---|---|---|
| Age | - | 1.04 | 0.98 | 1.11 | 0.22 |
| Leg Status (reference level = claudication) | Rest Pain | 1.81 | 0.40 | 8.13 | 0.44 |
| Ulceration | 3.20 | 0.96 | 10.67 | 0.06 | |
| Diabetes | Yes | 1.54 | 0.59 | 3.99 | 0.38 |
| Smoker | Yes | 1.03 | 0.39 | 2.71 | 0.96 |
| Distal Anastomosis (reference level = above knee) | Below knee | 1.94 | 0.43 | 8.73 | 0.39 |
| Tibial | 5.05 | 1.29 | 19.77 | 0.02 | |
| Prior Failed Graft | Yes | 2.22 | 0.70 | 7.00 | 0.18 |
| High Risk Vein Graft | Yes | 2.53 | 0.91 | 7.02 | 0.07 |
| Index Leg Ankle Brachial Index (for each .10 decrease) | - | 1.19 | 0.96 | 1.48 | 0.11 |
| Minimum Diameter | - | 0.23 | 0.10 | 0.56 | 0.001 |
| Pre-operative IgM value – Quintile | Lowest 20% | 3.37 | 1.15 | 9.87 | 0.03 |
| Pre-operative IgM value – Quartile | Lowest 25% | 3.53 | 1.30 | 9.58 | 0.01 |
| Pre-operative IgM value – Median | Below Median | 2.94 | 1.03 | 8.40 | 0.04 |
Table III. Multivariate Cox Proportional Hazards Model for Primary Endpoint – Loss of Primary Patency.
In this multivariate hazards model, Minimum diameter of the vein graft, and the lowest quartile of preoperative IgM values were significantly associated with loss of primary patency.
| Variable | Level | Hazard Ratio | Lower 95% | Upper 95% | P-value for Z statistic |
|---|---|---|---|---|---|
| Leg Status (reference level = claudication) | Rest Pain | 1.07 | 0.21 | 5.40 | 0.93 |
| Ulceration | 1.40 | 0.36 | 5.48 | 0.63 | |
| Distal Anastomosis (reference level = above knee) | Below knee | 2.68 | 0.56 | 12.74 | 0.22 |
| Tibial | 2.86 | 0.59 | 13.87 | 0.19 | |
| High Risk Vein Graft | Yes | 1.51 | 0.45 | 5.09 | 0.50 |
| Minimum Diameter (for each decrement of 1mm from median) | - | 5.56 | 1.94 | 15.95 | 0.001 |
| Pre-operative IgM value – quartile | Lowest 25% | 3.62 | 1.08 | 12.07 | 0.037 |
We conducted a similar analysis, considering exclusively the endpoint of graft stenosis greater than 75% - all of which underwent intervention and lost primary patency. Tables IV and V illustrate the univariate and multivariate analyses. By univariate analysis, the preoperative IgM quartile was significant, and the characteristics of Prior Failed bypass and High Risk Vein Graft were of borderline significance. Including all these in a multivariate analysis shows that those patients in the lowest quartile of IgM values had a 7.8 fold increased risk of graft stenosis requiring revision.
Table IV. Univariate Cox Proportional Hazards Model for Incidence of Graft Stenosis.
These clinical characteristics were tested individually for their relationship to the occurrence of the endpoint of graft stenosis. The following factors were chosen for inclusion in the subsequent multivariate hazards model because they were significant, or approached significance: Prior failed graft, High risk vein graft, and lowest quartile of IgM value.
| Variable | Level | Hazard Ratio | Lower 95% | Upper 95% | P-value for Z statistic |
|---|---|---|---|---|---|
| Age | - | 1.04 | 0.94 | 1.15 | 0.49 |
| Leg Status (reference level = claudication) | Rest Pain | 0.82 | 0.085 | 7.96 | 0.87 |
| Ulceration | 1.74 | 0.32 | 9.61 | 0.53 | |
| Diabetes | Yes | 0.69 | 0.13 | 3.57 | 0.66 |
| Smoker | Yes | 1.63 | 0.31 | 8.44 | 0.56 |
| Distal Anastomosis(reference level = above knee) | Below knee | 1.01 | 0.17 | 6.06 | 0.99 |
| Tibial | 1.33 | 0.2 | 8.98 | 0.77 | |
| Prior Failed | Yes | 3.87 | 0.65 | 23.23 | 0.14 |
| High Risk Vein Graft | Yes | 4.92 | 0.96 | 25.09 | 0.06 |
| Minimum Diameter (for each decrement of 1mm from median) | - | 2.07 | 0.56 | 7.62 | 0.27 |
| Index Leg Ankle Brachial Index (for each .10 decrease) | - | 1.07 | 0.74 | 1.54 | 0.73 |
| Pre-operative IgM value – Quartile | Low 25% | 5.47 | 1.07 | 27.87 | 0.04 |
Table V. Multivariate Cox Proportional Hazards Model for Incidence of Graft Stenosis.
In this multivariate hazards model, the lowest quartile of preoperative IgM values was significantly associated with graft stenosis requiring intervention.
| Variable | Level | Hazard Ratio | Lower 95% | Upper 95% | P-value for Z statistic |
|---|---|---|---|---|---|
| Prior Failed | Yes | 3.45 | 0.39 | 30.83 | 0.27 |
| High Risk Vein Graft | Yes | 3.5 | 0.47 | 26.03 | 0.22 |
| Pre-operative IgM value – quartile | Low 25% | 7.79 | 1.27 | 47.82 | 0.027 |
| Log rank test, P = 0.013 for this model | |||||
Relationship Between Clinical Factors and IgM Levels
Patients with different clinical characteristics (e.g. diabetes, or indication for surgery) might disproportionately have higher or lower IgM values. To investigate these relationships, we performed univariate and multivariate linear regressions. Neither analysis revealed any significant statistical relationship between IgM values and the clinical characteristics (age, Leg Status, Diabetes, Smoking, Distal Anastomosis, Prior Failed Graft, High Risk Vein Graft, and Minimum Diameter). These results are summarized in the on-line Supplemental Table II. The values of preoperative anti-PC IgM were not significantly different between diabetics/non-diabetics, or according to indication for surgery (on-line Supplemental Table III). Twenty-eight percent of the lowest quartile of IgM values had high risk bypasses, and 21% of the highest three quartiles had high risk bypasses. This difference was not significant (Fisher's exact test, p=1.0).
Discussion
This most durable of infrainguinal revascularization procedures, lower extremity bypass with vein, is still prone to stenosis and graft failure that can affect 20-35% of infrainguinal grafts within the first year of surgery22, 26-28. In this prospective, longitudinal study, we have shown that the preoperative level of a naturally occurring IgM antibody directed against the inflammatory antigen, phosphorylcholine, is closely associated with the primary patency of autogenous infrainguinal vein grafts. Even accounting for other well-known factors that influence the longevity of bypass grafts, such as the severity of occlusive disease, the quality of the vein conduit, and technical aspects of the surgery, the preoperative anti-PC IgM value was a significant predictor of loss of primary patency. When the analysis was restricted to loss of primary patency due to hemodynamically significant vein graft stenosis, the results were similar.
We confirmed that Fontaine class, the site of the distal anastomosis, vein diameter and intraoperative technical factors, when considered individually, may be prognostic indicators of future vein graft stenosis and occlusion29-32. They are useful in postoperative decision making regarding antithrombotic therapy and the intensity of graft surveillance. Until now, biological measurements that directly reflect a patient's predisposition to graft stenosis or occlusion have been lacking. Our data suggest that the preoperative level of anti-PC IgM may be another potential tool for prognostication and postoperative management. The mechanisms of action of this natural IgM may also lend new insights into the mechanisms of graft failure, and the development of preventative strategies.
Previous studies of anti-PC IgM and cardiovascular disease have shown that low levels are most predictive of adverse outcomes. Many studies suggest that a wide range of IgM levels might be healthy, while levels below a certain threshold lead to the loss of anti-inflammatory, atheroprotective effects, which is why we analyzed the IgM values as a discrete variable. Fiskesund and colleagues, in a study of 227 subjects with 455 matched controls found the strongest association between IgM and first stroke below the 30th percentile of IgM values. The association with stroke was more pronounced at lower percentiles and primarily in women but not in men15. Population-based studies involving almost 5,000 subjects, from the Malmo Diet and Cancer Study cohort and a Stockholm screening project, found that low levels of anti-PC IgM were most strongly associated with cardiovascular events in men14, 33. The interactions between gender and anti-PC IgM effects are still unclear. Women in general have higher levels of this IgM, which may be one factor contributing to the lower incidence and later onset of vascular disease in females33, 34. Our current study cannot resolve this issue, as our subjects were predominantly male.
The vascular-protective mechanisms of anti-PC IgM center on its ability to block cellular inflammatory responses to phosphorylcholine. This epitope is normally hidden, but becomes exposed when low density lipoprotein becomes oxidized, when there is cellular damage and death, or when certain bacteria are present. Su et al. found that the expression of adhesion molecules by human endothelial cells in response to platelet activating factor (itself a PC derivative) was inhibited by anti-PC IgM35. de Faire and colleagues demonstrated that anti-PC IgM blocked the uptake of oxidized LDL by macrophages14. Research in animal models has shown that inducing higher levels of anti-PC IgM retards the progression of atherosclerosis, and prevents vein graft thickening, providing evidence of a cause and effect relationship20, 36. These studies suggest that the innate anti-PC IgM antibody may play an important role in the modulation of vascular inflammation.
As in atherosclerosis, vascular inflammation is a key factor in graft failure. Although the etiologies of autogenous graft failure are complex and diverse, loss of primary patency in the first two years is primarily the result of neointimal hyperplasia and constrictive remodeling37, 38. The activation of inflammatory and thrombotic pathways (which are tightly intertwined) is thought to be a prime driver of the derangements of vascular healing that result in graft stenosis and failure22, 39. Patients with peripheral arterial occlusive disease (PAD) are known to have pathological activation of these thrombo-inflammatory pathways40, 41.
Synthesizing our current knowledge – that anti-PC IgM plays an anti-inflammatory role against the appearance of a common inflammatory molecule; that vascular inflammation contributes to graft stenosis and failure; and that PAD patients have exaggerated inflammatory profiles – the results of our current study are consistent and confirmatory. Patients with the lowest levels of anti-PC IgM were significantly more prone to graft failure than those with higher levels.
Biological strategies to abrogate the deranged response to injury have uniformly failed in humans, and the human variability in the response to vascular injury is poorly understood. The biological actions of anti-PC IgM raise the possibility of new strategies to normalize vascular healing, by suppressing of one of the earliest events in vascular healing – the exposure of inflammatory phosphorylcholine. Such strategies could include conferring passive immunity by the administration of an engineered anti-PC antibody to those patients with low levels, or the induction of enhanced immunity by vaccination. Both strategies have been successful in animal models19,20.
This study has limitations. This was an observational study, so we did not dictate patient management or antithrombotic treatments. Some variables, like the use of aspirin (92.9%) and statins (80.4%) were so highly prevalent that analysis of their influence on primary patency was not possible. The study population does not shed light on possible gender or racial differences in anti-PC IgM levels, nor the prognostic significance of such factors. We are currently conducting larger and broader studies to address these questions. Despite these limitations, and in spite of a relatively small cohort, the relationship between anti-PC IgM levels and loss of primary patency was strong and consistent, even after accounting for other important variables.
Supplementary Material
Supplemental Table I. Clinical Characteristics of Losses of Primary Patency.
Supplemental Table II. The relationships between the clinical characteristics of the patient population and the preoperative anti-PC IgM level were tested. IgM values were log transformed. The univariate analysis revealed no statistical relationship between IgM values and the clinical characteristics listed. A multivariate regression model including all of these clinical characteristics did not reveal any significant relationships either. Correlation coefficients analysis showed no relationship between vein graft diameter and IgM quartile, nor did logistic regression reveal any unequal distribution of vein graft diameters between the lowest quartile and highest 75% of IgM values (not shown).
Supplemental Table III. The median values of preoperative IgM were not significantly different according to diabetic status or preoperative leg status (i.e. indication for surgery).
Acknowledgments
Funding: This work was supported by grants from the Department of Veterans Affairs, Veterans Health Administration, Merit Review Agency (MS) and Research Career Scientist Award (RCS 05-196 - XHZ); National Institutes of Health T32 HL007828 and R01HL030946 (AC, MS). We are grateful to the Athera Biotechnology Corporation, for their generous donation of CVDefine assay kits. We wish to thank the Clinical Research Unit at the VA Puget Sound Health Care System, and especially Susan Bigda, R.N., and Teresita Cornell, BSN for their invaluable assistance.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002 Mar 5;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Keaney JF., Jr Immune modulation of atherosclerosis. Circulation. 2011 Nov 29;124(22):e559–e560. doi: 10.1161/CIRCULATIONAHA.111.074096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011 May;105(1):S34–S42. doi: 10.1160/THS10-11-0717. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012 Aug 31;111(6):778–99. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002 Nov;8(11):1218–26. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 6.Gronwall C, Vas J, Silverman GJ. Protective Roles of Natural IgM Antibodies. Front Immunol. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010 Nov;10(11):778–86. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 8.Lutz HU, Binder CJ, Kaveri S. Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol. 2009 Jan;30(1):43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Binder CJ. Naturally occurring IgM antibodies to oxidation-specific epitopes. Adv Exp Med Biol. 2012;750:2–13. doi: 10.1007/978-1-4614-3461-0_1. [DOI] [PubMed] [Google Scholar]
- 10.de Faire U, Frostegard J. Natural antibodies against phosphorylcholine in cardiovascular disease. Ann N Y Acad Sci. 2009 Sep;1173:292–300. doi: 10.1111/j.1749-6632.2009.04748.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011 Jan 21;108(2):235–48. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Athera Biotechnologies - CVDefine Product Sheet and References. 2013 [Google Scholar]
- 13.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van LG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008 Apr 18;133(2):235–49. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Faire U, Su J, Hua X, Frostegard A, Halldin M, Hellenius ML, et al. Low levels of IgM antibodies to phosphorylcholine predict cardiovascular disease in 60-year old men: effects on uptake of oxidized LDL in macrophages as a potential mechanism. J Autoimmun. 2010 Mar;34(2):73–9. doi: 10.1016/j.jaut.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Fiskesund R, Stegmayr B, Hallmans G, Vikstrom M, Weinehall L, de Faire U, et al. Low levels of antibodies against phosphorylcholine predict development of stroke in a population-based study from northern Sweden. Stroke. 2010 Apr;41(4):607–12. doi: 10.1161/STROKEAHA.109.558742. [DOI] [PubMed] [Google Scholar]
- 16.Gronlund H, Hallmans G, Jansson JH, Boman K, Wikstrom M, de Faire U, et al. Low levels of IgM antibodies against phosphorylcholine predict development of acute myocardial infarction in a population-based cohort from northern Sweden. Eur J Cardiovasc Prev Rehabil. 2009 Jun;16(3):382–6. doi: 10.1097/HJR.0b013e32832a05df. [DOI] [PubMed] [Google Scholar]
- 17.Caidahl K, Hartford M, Karlsson T, Herlitz J, Pettersson K, de Faire U, et al. IgM-phosphorylcholine autoantibodies and outcome in acute coronary syndromes. Int J Cardiol. 2012 Feb 3; doi: 10.1016/j.ijcard.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Carrero JJ, Hua X, Stenvinkel P, Qureshi AR, Heimburger O, Barany P, et al. Low levels of IgM antibodies against phosphorylcholine-A increase mortality risk in patients undergoing haemodialysis. Nephrol Dial Transplant. 2009 Nov;24(11):3454–60. doi: 10.1093/ndt/gfp309. [DOI] [PubMed] [Google Scholar]
- 19.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, et al. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol. 2007 Aug 7;50(6):540–6. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 20.Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, et al. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006 Nov;189(1):83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Rahman I, Atout R, Pedersen NL, de Faire U, Frostegard J, Ninio E, et al. Genetic and environmental regulation of inflammatory CVD biomarkers Lp-PLA2 and IgM anti-PC. Atherosclerosis. 2011 Sep;218(1):117–22. doi: 10.1016/j.atherosclerosis.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006 Apr;43(4):742–51. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 23.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997 Sep;26(3):517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 24.Bandyk DF. Infrainguinal vein bypass graft surveillance: how to do it, when to intervene, and is it cost-effective? J Am Coll Surg. 2002 Jan;194(1 Suppl):S40–S52. doi: 10.1016/s1072-7515(01)01117-6. [DOI] [PubMed] [Google Scholar]
- 25.Tinder CN, Chavanpun JP, Bandyk DF, Armstrong PA, Back MR, Johnson BL, et al. Efficacy of duplex ultrasound surveillance after infrainguinal vein bypass may be enhanced by identification of characteristics predictive of graft stenosis development. J Vasc Surg. 2008 Sep;48(3):613–8. doi: 10.1016/j.jvs.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 26.Mills JL, Wixon CL, James DCM, Devine J, Westerband A, Hughes JD. The natural history of intermediate and critical vein graft stenosis: Recommendations for continued surveillance or repair. J Vasc Surg. 2001;33:273–80. doi: 10.1067/mva.2001.112701. [DOI] [PubMed] [Google Scholar]
- 27.Gupta AK, Bandyk DF, Cheanvechai D, Johnson BL. Natural history of infrainguinal vein graft stenosis relative to bypass grafting technique. J Vasc Surg. 1997 Feb;25(2):211–20. doi: 10.1016/s0741-5214(97)70344-6. [DOI] [PubMed] [Google Scholar]
- 28.Visser K, Idu MM, Buth J, Engel GL, Hunink MG. Duplex scan surveillance during the first year after infrainguinal autologous vein bypass grafting surgery: costs and clinical outcomes compared with other surveillance programs. J Vasc Surg. 2001 Jan;33(1):123–30. doi: 10.1067/mva.2001.109745. [DOI] [PubMed] [Google Scholar]
- 29.Towne JB, Schmitt DD, Seabrook GR, Bandyk DF. The effect of vein diameter on patency of in situ grafts. J Cardiovasc Surg (Torino) 1991 Mar;32(2):192–6. [PubMed] [Google Scholar]
- 30.Idu MM, Buth J, Hop WC, Cuypers P, van de Pavoordt ED, Tordoir JM. Factors influencing the development of vein-graft stenosis and their significance for clinical management. Eur J Vasc Endovasc Surg. 1999 Jan;17(1):15–21. doi: 10.1053/ejvs.1998.0676. [DOI] [PubMed] [Google Scholar]
- 31.Wengerter KR, Veith FJ, Gupta SK, Ascer E, Rivers SP. Influence of vein size (diameter) on infrapopliteal reversed vein graft patency. J Vasc Surg. 1990 Apr;11(4):525–31. doi: 10.1067/mva.1990.18327. [DOI] [PubMed] [Google Scholar]
- 32.Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007 Dec;46(6):1180–90. doi: 10.1016/j.jvs.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Sjoberg BG, Su J, Dahlbom I, Gronlund H, Wikstrom M, Hedblad B, et al. Low levels of IgM antibodies against phosphorylcholine-A potential risk marker for ischemic stroke in men. Atherosclerosis. 2009 Apr;203(2):528–32. doi: 10.1016/j.atherosclerosis.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Su J, Georgiades A, Wu R, Thulin T, de Faire U, Frostegard J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis. 2006 Sep;188(1):160–6. doi: 10.1016/j.atherosclerosis.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008 Aug;47(8):1144–50. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 36.Cesena FH, Dimayuga PC, Yano J, Zhao X, Kirzner J, Zhou J, et al. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE-/- mice. Atherosclerosis. 2012 Jan;220(1):59–65. doi: 10.1016/j.atherosclerosis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Owens CD, Ho KJ, Conte MS. Lower extremity vein graft failure: a translational approach. Vasc Med. 2008 Feb;13(1):63–74. doi: 10.1177/1358863X07083432. [DOI] [PubMed] [Google Scholar]
- 38.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: clinical implications. J Vasc Surg. 2010 Mar;51(3):736–46. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens CD, Rybicki FJ, Wake N, Schanzer A, Mitsouras D, Gerhard-Herman MD, et al. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg. 2008 Jun;47(6):1235–42. doi: 10.1016/j.jvs.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaparala RP, Orsi NM, Lindsey NJ, Girn RS, Homer-Vanniasinkam S. Inflammatory profiling of peripheral arterial disease. Ann Vasc Surg. 2009 Mar;23(2):172–8. doi: 10.1016/j.avsg.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Moreno K, Murray-Wijelath J, Yagi M, Kohler T, Hatsukami T, Clowes A, et al. Circulating inflammatory cells are associated with vein graft stenosis. J Vasc Surg. 2011 Oct;54(4):1124–30. doi: 10.1016/j.jvs.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table I. Clinical Characteristics of Losses of Primary Patency.
Supplemental Table II. The relationships between the clinical characteristics of the patient population and the preoperative anti-PC IgM level were tested. IgM values were log transformed. The univariate analysis revealed no statistical relationship between IgM values and the clinical characteristics listed. A multivariate regression model including all of these clinical characteristics did not reveal any significant relationships either. Correlation coefficients analysis showed no relationship between vein graft diameter and IgM quartile, nor did logistic regression reveal any unequal distribution of vein graft diameters between the lowest quartile and highest 75% of IgM values (not shown).
Supplemental Table III. The median values of preoperative IgM were not significantly different according to diabetic status or preoperative leg status (i.e. indication for surgery).