Abstract
Inhibition of polyol pathway enzyme aldose reductase (AR) has been shown to prevent colon cancer cells growth in culture and in nude mice xenografts. However, the role of AR in the mediation of growth factor-induced colon cancer cells growth is not well understood. In this study, we have investigated how AR inhibition prevents tumor growth via regulation of microRNA (miR)-21-mediated PDCD4 expression in colon cancer cells in in vitro and in vivo. Treatment of colon cancer cells (HT29, SW480 and Caco-2) with EGF caused increased expression of miR-21 and inhibition of AR prevented it. Further, AR inhibition also increased PDCD4, a putative target of miR-21 in human colon cancer cells. Inhibition of AR also prevented EGF-induced phosphorylation of PDCD4. Treatment of HT29 cells with AR inhibitor, fidarestat, prevented the EGF-induced phosphorylation of mTOR, Raptor, eIF4E, S6K and 4E-BP1 and increased the phosphorylation of AMPK. Similarly, in nude mice xenograft tissues, PDCD4 and 4E-BP1 levels were significantly higher in AR inhibitor-treated mice compared to controls. Collectively, these results indicate that AR inhibition prevents growth factors-induced colon cancer growth by down-regulating miR-21 expression and increasing PDCD4 levels through the ROS/AMPK/mTOR/AP1/4E-BP1 pathway.
1. Introduction
While we are beginning to get to grips with many of the contributing factors and cellular mechanisms such as increased inflammatory cytokines and oxidative stress that underlie the pathophysiology of colorectal cancer (CRC), and despite the development of numerous effective new therapies over the past 2 to 3 decades, mortality associated with CRC remains a major problem worldwide.1,2 Therefore, novel therapeutic approaches are required to prevent/treat colon cancer. Recently, we have investigated the role of the polyol pathway enzyme aldose reductase (AR) in the pathophysiology of CRC. Our studies have demonstrated that AR inhibitors such as fidarestat (which has already undergone Phase III clinical trials in diabetic neuropathy for 52 weeks and was found to be safe in humans with no major irreversible side effects) is very effective in preventing CRC growth and metastasis in cellular and mouse models.3-7 Specifically, we have shown that AR inhibitors or siRNA ablation of AR prevent the growth factors–induced proliferation of human CRC cells and the expression of inflammatory cytokines and other inflammatory factors such as COX-2 and PGE2.3,5 Further, our studies have also shown that AR inhibition prevents tumor growth in nude mice xenografts, aberrant crypt foci formation in azoxymethane-treated mice and CRC metastasis in mice.7,8 Although, based upon these results, we can assume that AR inhibitors could be used as chemo-preventive/-therapeutic drugs against CRC, the molecular mechanism(s) by which AR inhibition prevents CRC growth is not clearly understood.
In this study, we examined how AR regulates growth factor-induced reactive oxygen species (ROS) activate miR-21 via AP-1 transcription factor and mTOR pathway, which in turn promotes colon cancer cell proliferation. AP-1 is known to transcribe miR-21 in cancer cells that targets various tumor suppressor genes/proteins which in turn promote cancer cell proliferation.9 However, mTOR promotes cell survival via activating its downstream targets eIF4E, S6K and 4E-BP1.10,11,12 S6K phosphorylates PDCD4, a known tumor suppressor and valid target of miR-21, causes its inactivation and degradation.13,14 It has been shown that PDCD4 inactivates AP-1, thereby prevents tumor growth.15,16 However, loss of PDCD4 expression has been associated with various cancers including CRC.17,18 Recently, we have shown that the inhibition of AR prevents human colon cancer growth by down-regulating miR-21 and increasing PTEN.19 However, the role of AR in regulation of PDCD4, 4E-BP1 and mTOR pathway in colon cancer cells is not known. We demonstrate that the AR inhibition suppresses miR-21, increases PDCD4 and 4E-BP1, which in turn prevents the proliferation of human CRC cells via the ROS/AMPK/mTOR/AP-1/miR-21/PDCD4/4E-BP1 signaling pathway. These unique findings provided further understanding of the molecular mechanisms by which AR inhibition prevents human CRC.
2. Materials and Methods
2.1. Materials
McCoy's medium, RPMI 1640, penicillin/streptomycin solution, and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA). Antibodies against PDCD4, Phospho-PDCD4 (S67), Phospho-PDCD4 (S457), c-Jun, Phospho-c-Jun (S73), eIF4E, Phospho-eIF4E, 4E-BP1, Phospho-4E-BP1, FLIP and GAPDH were purchased from Cell Signal, Inc. Fidarestat was obtained as a gift chemical from Livwel Therapeutics Inc, CA, USA. EGF, basic-FGF (bFGF), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT), and other reagents used in Western blot analysis were obtained from Sigma (St. Louis, MO). On-Target plus SMARTpool siRNA for PDCD4 and c-Jun were purchased from Dharmacon RNAi Technologies, USA.
2.2. Cell culture
Human colon cancer cells (SW480, Caco-2, and HT29) were obtained from the American Type Culture Collection. SW480, and Caco-2 cells were grown in RPMI 1640, and DMEM supplemented with 10% FBS and 1% penicillin/streptomycin, respectively. HT29 cells were maintained and grown in McCoy's 5A medium supplemented with 10% FBS and 1% penicillin/streptomycin.
2.3. Quantitative real-time PCR (Q-PCR) analysis of miR-21 expression
HT29 cells were serum-starved in McCoy's 5A medium containing 0.1% FBS in the presence or absence of fidarestat (2μM) for 24 h and then stimulated with bFGF for another 8 h. miR-enriched total RNA was extracted from HT29 cells using the mirVana miRNA isolation kit (Ambion). Quantification of miR-21 was performed using TaqMan MicroRNA Assays (Applied Biosystems). U6 RNA was used for normalization of miR expression. Analysis and fold changes were determined using the comparative threshold cycle (Ct) method.
2.4. Measurement of Cytotoxicity
HT29 cells were cultured in McCoy's medium in a 96-well plate. Subconfluent cells were growth-arrested in 0.1% FBS with or without the AR inhibitor, fidarestat (2 μM) or transfected with control siRNA and PDCD4 siRNA using Hiperfect transfection reagent (Qiagen). After 24 h, cells were treated with EGF (10 ng/ml) and incubated for 24 h. Cell viability was determined by MTT assay as described previously.4
2.5. Transient transfection with miRNA Mimic and miRNA Inhibitor
MiR-21 mimic and inhibitor were transfected into HT29 cells according to the manufacturer's protocol. Briefly, HT-29 cells were serum-starved in McCoy's 5A medium containing 0.1% FBS and transfected with 500 nM of inhibitor and 50nM of mimic of miR-21 (Qiagen, Hilden, Germany) for 24 h, and followed by stimulation with EGF (10 ng/ml) for another 24 h.
2.6. Western blots
The expression of AMPK, Phospho-AMPK, PDCD4, Phospho- PDCD4 (S67), Phospho-PDCD4 (S457), c-FOS, Phospho-c-Fos (S32), c-Jun, Phospho-c-Jun (S73), eIF4E, Phospho-eIF4E, 4E-BP1, Phospho-4E-BP1, FLIP and GAPDH proteins were determined by Western blot analysis. The antigen-antibody complex was detected by enhanced chemiluminescence (Pierce).
2.7. Determinations of ROS and immunofluroscence
Determination of ROS using ROS-sensitive indicator, dihydroethidium (DHE) from Invitrogen, Carlsbad, CA and immunofluroscence for PDCD4 protein were performed in Caco-2 cells treated with or without fidarestat. Briefly, Caco-2 cells were cultured in DMEM medium in culture slides. Subconfluent cells were growth-arrested in 0.1% FBS with or without the fidarestat (2 μM). After 24 h, cells were treated with EGF (10ng/ml) and bFGF (15ng/ml) and incubated for 24 h. Subsequently, cells were washed and incubated with 5μM DHE in PBS at 37°C for 30 min. Cells were washed in PBS twice and fixed in 90% ethanol. Expression of PDCD4 was visualized with a monoclonal antibodies against PDCD4 followed by the addition of FITC-labeled anti-rabbit antisera. DAPI (blue) staining was also performed to visualize the nuclei.
2.8. Western blot and immunohistochemistry of tumor samples obtained from human colon cancer cells (HT29) in nude mice xenografts
PDCD4 and 4E-BP1 in archived tumor samples (human colon cancer cells (HT29) nude mice xenografts) from control and fidarestat-treated nude mice were analyzed by Western blot and immunohistochemistry. Effect of AR inhibition on tumor growth in HT29 human colon cancer nude mice model was studied as described earlier (19).
2.9. Statistical analysis
Data presented as mean ± SE and P-values were determined by unpaired Student's t test using Microsoft Office Excel 2010 software. P < 0.05 was considered as statistically significant.
3. Results
3.1. AR regulates GF-induced PDCD4 expression in colon cancer cells
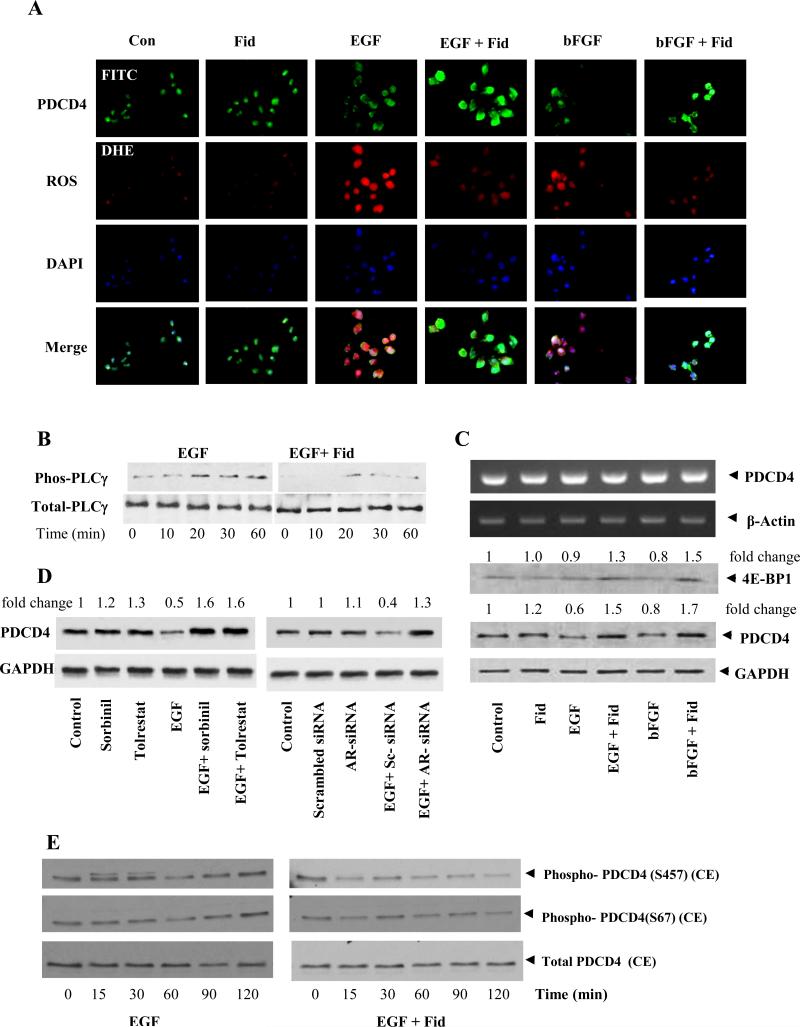
Recent studies indicate that PDCD4 is a suppressor of tumorigenesis and tumor progression invasion and growth.15-18 To examine how AR inhibition prevents colon cancer cells growth via regulating the expression of PDCD4, we first treated colon cancer cells with growth factors in the absence and presence of AR inhibitor, fidarestat. Our results indicate that EGF and bFGF caused decrease in the expression of PDCD4 protein and inhibition of AR increased PDCD4 protein expression (Fig 1A and 1C). AR inhibitor also decreased the growth factors-induced ROS formation (Fig 1A). To examine how AR inhibition prevents EGF-induced ROS, we have measured the activation of PLC which is well known to generate ROS via NADPH oxidase. Our results suggest that AR inhibition prevents EGF-induced phosphorylation by PLC Fig 1B. Further, mRNA levels of PDCD4 were not affected when cells were treated with growth factors alone or in the presence of fidarestat (Fig 1C). To examine specificity of fidarestat, HT-29 cells were treated with two other AR inhibiors, sorbinil and tolrestat or transfected with scrambled and AR-siRNA and measured the expression of PDCD4. Results shown in the Fig 1D indicate that AR inhibition by pharmacological inhibitors or AR ablations by AR siRNA prevent EGF-induced PDCD4 in colon cancer cells. We next examined the effect of AR inhibition on EGF-induced phosphorylation of the PDCD4. As shown in Fig 1E, stimulation of HT29 cells with EGF in a time-dependent manner increased the phosphorylation of PDCD4 and AR inhibitor significantly decreased the phosphorylation of PDCD4.
Fig 1. Effect of AR inhibition on ROS, 4E-BP1 and PDCD4 in growth factor-induced in human colon cancer cells.
(A) Growth-arrested Caco-2 cells in 0.1% FBS without or with fidarestat (2 μM, 24 h) were treated with EGF (10ng/ml) and bFGF (15ng/ml) and incubated for another 24 h. Subsequently, the cells were washed, incubated with 5μM DHE for 30 min for ROS and fixed in 90% ethanol. Expression of PDCD4 was visualized with a specific rabbit monoclonal antibody against PDCD4 followed by the addition of FITC (green)-labeled anti-rabbit antisera. DAPI (blue) staining was also performed to visualize the nuclei. (B) HT −29 cells were incubated with EGF for indicated time points in the absence and presence of fidarestat and examined the activation of PLC by Western blot analysis. (C) Growth-arrested HT29 cells were preincubated with fidarestat for 24 h followed by stimulation with EGF and bFGF for another 8h for mRNA (RT-PCR) and 24h for protein Western blot analysis. 4E-BP1 expression was analyzed by Western blot. The same blots were stripped and reprobed with GAPDH antibody to verify equal protein loading. Fold changes were calculated by densitometric analysis after normalizing with the respective loading controls. (D) To examine specificity of fidarestat, HT-29 cells were treated with two other AR inhibiors, sorbinil and tolrestat and transfected with scrambled and AR-siRNA and measured the expression of PDCD4. (E) HT29 cells were growth-arrested in McCoy's medium containing 0.1% serum with or without fidarestat and stimulated with EGF for the indicated time periods. The pooled cytoplasmic extracts from three independent experiments were subjected to SDS-PAGE and Western blots using antibodies against specific proteins. The antibody binding was detected by enhanced picochemiluminescence.
3.2. AR regulates GF-induced PDCD4 expression by modulating miR21 in colon cancer cells
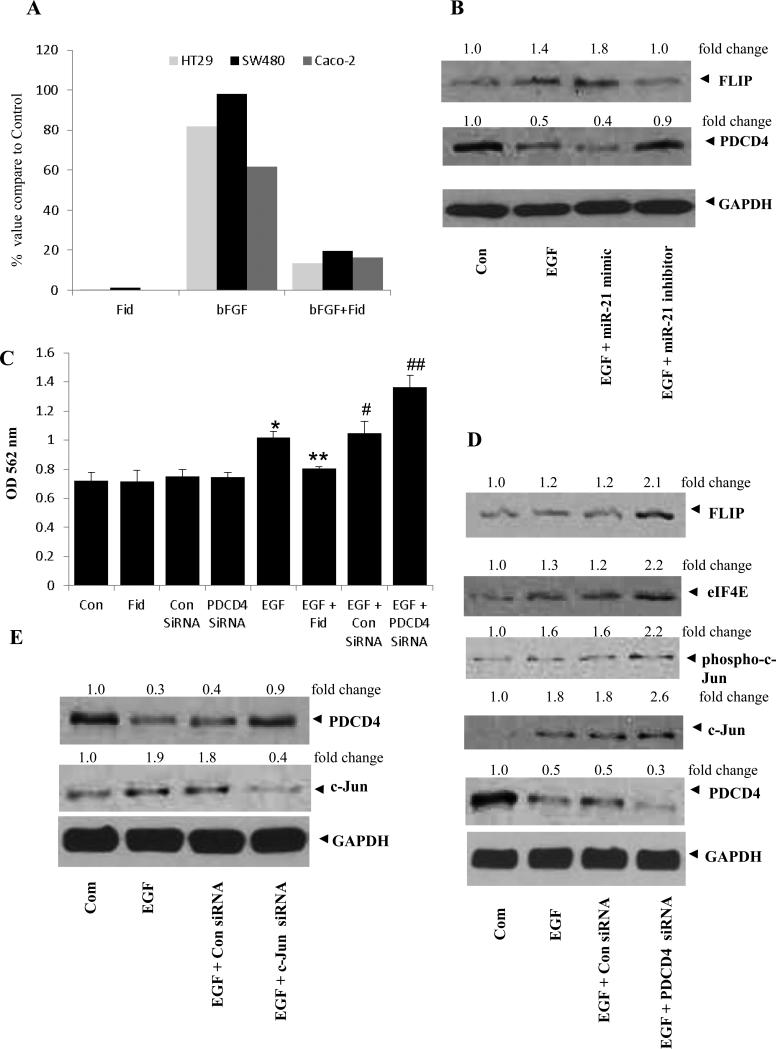
Since PDCD4 expression is downregulated by miR-21 in cancer cells, we next examined the role of AR in mediation of miR-21-regulated PDCD4 expression and cancer cell proliferation. We first examined the growth factor-induced expression of miR-21 in human colon cancer cells (HT29, SW480 and Caco-2) in the absence and presence of AR inhibitor. Our results indicate that bFGF caused increased expression of miR-21 and pre-incubation of the cells with fidarestat significantly inhibited it (Fig 2A). Further, treatment of HT29 cells with EGF alone or EGF + miR-21-mimic down-regulated the PDCD4 expression, and treatment with EGF + miR-21 inhibitor significantly restored PDCD4 expression (Fig 2B). Thus, our results indicate that inhibition of AR prevents colon cancer growth by upregulating the expression of PDCD4 via miR-21. Further, treatment of HT29 cells with EGF alone or EGF + miR-21-mimic increased the expression of anti-apoptotic protein FLIP, and treatment with EGF + miR-21 inhibitor significantly inhibited FLIP expression (Fig 2B) To further confirm the role of PDCD4 in cancer cell proliferation, we ablated PDCD4 in HT29 colon cancer cells with siRNA and incubated the cells with EGF ± AR inhibitor. Our results shown in the Fig 2C, indicate that cell proliferation was higher in PDCD4 ablated cells when compared with EGF alone. Since, it is well known that AP-1 transcriptionally regulates miR-21, we next investigated the expression of PDCD4 in HT29 cells treated with AP-1 (c-Jun)-siRNA. Our results show that c-Jun-ablated cells showed increased PDCD4 expression compared to EGF alone (Fig 2E). Our results also show that ablation of PDCD4 increased the total and phospho-c-Jun levels in colon cancer cells (Fig 2D).
Fig 2. Effect of AR inhibition, miR-21 mimics and inhibitors, and PDCD4 and c-Jun ablation on growth factor-induced human colon cancer cells.
(A) Growth-arrested SW480, Caco-2 and HT-29 cells were preincubated with fidarestat for 24 h followed by stimulation with bFGF for 8 h. The miR-21 expression was detected by real-time PCR using U6 RNA for normalization. (B) Growth-arrested HT-29 cells were treated with or without AR inhibitor, miR-21 mimic and miR-21 inhibitor or Hiperfect transfection reagent. PDCD4 and FLIP expression was analyzed by Western blots. (C) and (D) HT-29 cells were growth-arrested in 0.1% FBS and transfected with or without AR inhibitor, Control siRNA, PDCD4 siRNA, Hiperfect transfection reagent (Qiagen). After 24 h, cells were treated with EGF (10 ng/ml) and incubated for 24 h. Cell viability was determined by MTT assay (C) and protein expression was analyzed by Western blot (D). Bars represent mean ± S.E. (n = 6); * p < 0.05 vs. control; ** P<0.01 vs. EGF; # p<0.05 vs. control siRNA and ## p<0.01 vs. PDCD4 siRNA (E) HT-29 cells transfected with Control siRNA, and c-Jun siRNA were treated with EGF (10 ng/ml) and incubated for another 24 h. Protein expression was analyzed by Western blot. The same blots were stripped and reprobed with GAPDH antibody to verify equal protein loading. Fold changes were calculated by densitometric analysis.
3.3. AR regulates GF-induced PDCD4 phosphorylation by mTOR pathway
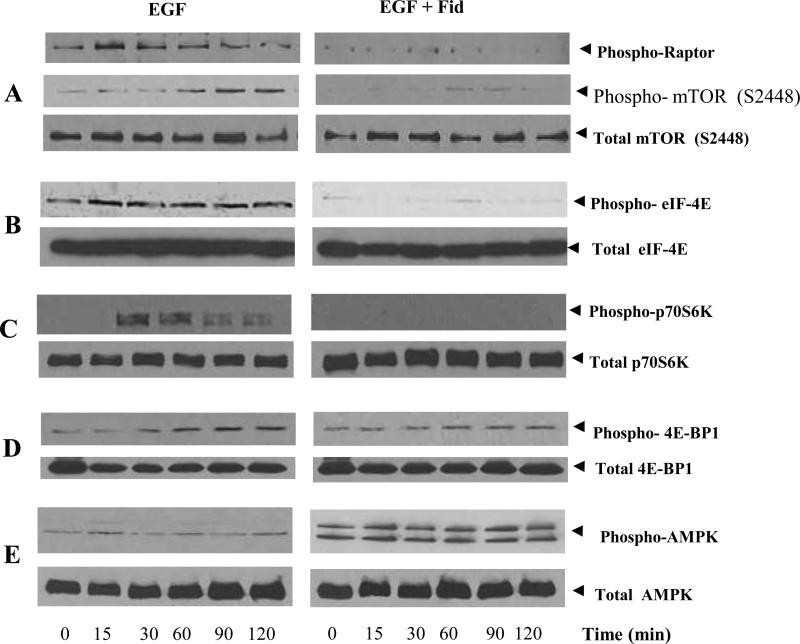
To further understand the mechanism by which AR regulates PDCD4 phosphorylation, we examined the activation of mTOR pathway in colon cancer cells. As shown in Fig 3A, stimulation of HT29 cells with EGF caused phosphorylation of mTOR and Raptor and inhibition of AR prevented it. Since mTOR causes the phosphorylation of downstream targets such as eIF4E, S6K and 4E-BP1, we next examined the affect of AR inhibition on EGF-induced phosphorylation of eIF4E, S6K and 4E-BP1 in HT-29 cells. Our results showed that AR inhibition prevented the phosphorylation of eIF4E, S6K and 4E-BP1 in a time-dependent manner (Fig 3B, 3C and 3D). Since S6K causes the phosphorylation of PDCD4, we next examined the effect of AR inhibition on EGF-induced phosphorylation of PDCD4. As shown in Fig 1C, that AR inhibition prevented EGF-induced phosphorylation of PDCD4. Most importantly, AR inhibition increased the EGF-induced AMPK activity suggesting that by activating AMPK, AR inhibition could prevent mTOR activation (Fig 3E). Collectively, these results suggest that AR inhibition prevents EGF-induced PDCD4 and 4E-BP1 phosphorylation via regulation of mTOR/Raptor/S6K pathway.
Fig 3. Effect of AR inhibition on EGF-induced activation of mTOR, Raptor, S6K, eIF4E and 4E-BP1 and inactivation of AMPK in HT29 colon cancer cells.
HT29 cells were treated with or without fidarestat and stimulated with EGF for the indicated time periods. The pooled cytoplasmic extract from three independent experiments were subjected Western blot analysis using antibodies against Phospho-Raptor, phospho- and total mTOR (A); phospho- and total eIF-4E (B); phospho- and total p70S6K (C); phospho- and total 4E-BP1 (D) and phospho- and total AMPK (E). The antibody binding was detected by enhanced picochemiluminescence.
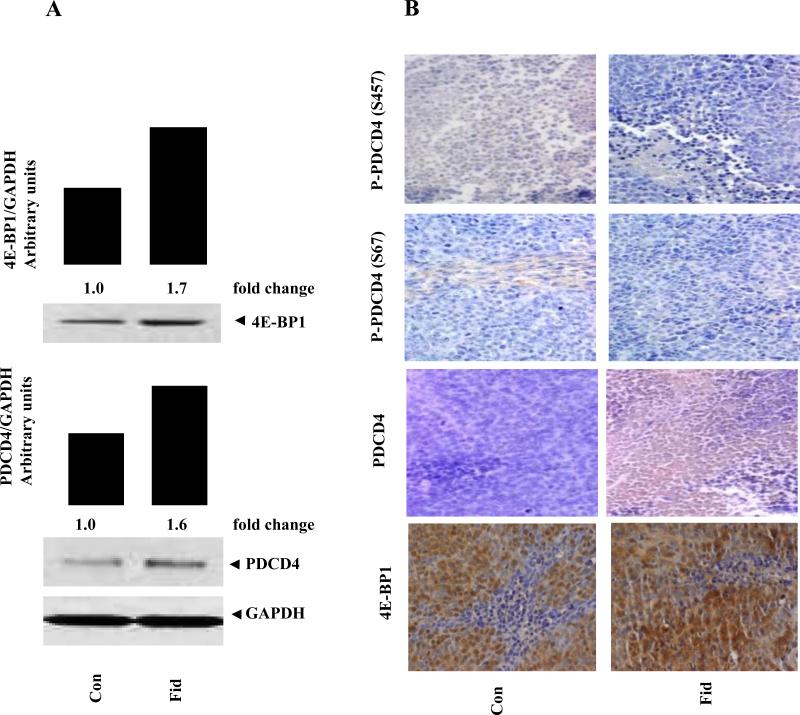
3.4 Inhibition of AR increases PDCD4 and 4E-BP1 expression in nude mice xenografts
We have recently shown that inhibition of AR prevented tumor growth in HT-29 human colon cancer cells nude mice xenografts (19). To confirm the increase of PDCD4 and 4E-BP1 by AR inhibition in in vitro, we determined the expression of PDCD4 and 4E-BP1 in HT29 colon cancer xenografts obtained from the nude mice treated with or without AR inhibitor, fidarestat. As shown in Fig 4A, AR inhibition significantly increased the expression of PDCD4 and 4E-BP1 in xenograft obtained from mice treated with AR inhibitor compared to that obtained from control mice. Immunostaining of tumor sections against antibodies to PDCD4 also showed increased expression and decreased phosphorylation of PDCD4 in fidarestat-treated mice compared to control mice, suggesting that AR inhibition increases the expression and activation of PDCD4 and 4E-BP1 in nude mice xenografts (Fig.4B). Collectively, our results indicate that AR inhibition could prevent colon cancer growth by downregulating the expression of miR-21 and upregulating the expression of PDCD4 and 4E-BP1 via mTOR pathway.
Fig 4. Effect of AR inhibition on PDCD4 and 4E-BP1 expression in HT29 xenografts.
HT-29 xenografts samples obtained from the nude mice treated with or without AR inhibitor, fidarestat were analyzed by Western blot (A) and immunohistochemistry (B). The same blots were stripped and reprobed with GAPDH antibody to verify equal protein loading. Fold changes were calculated by densitometric analysis after normalizing with the respective loading controls.
Discussion
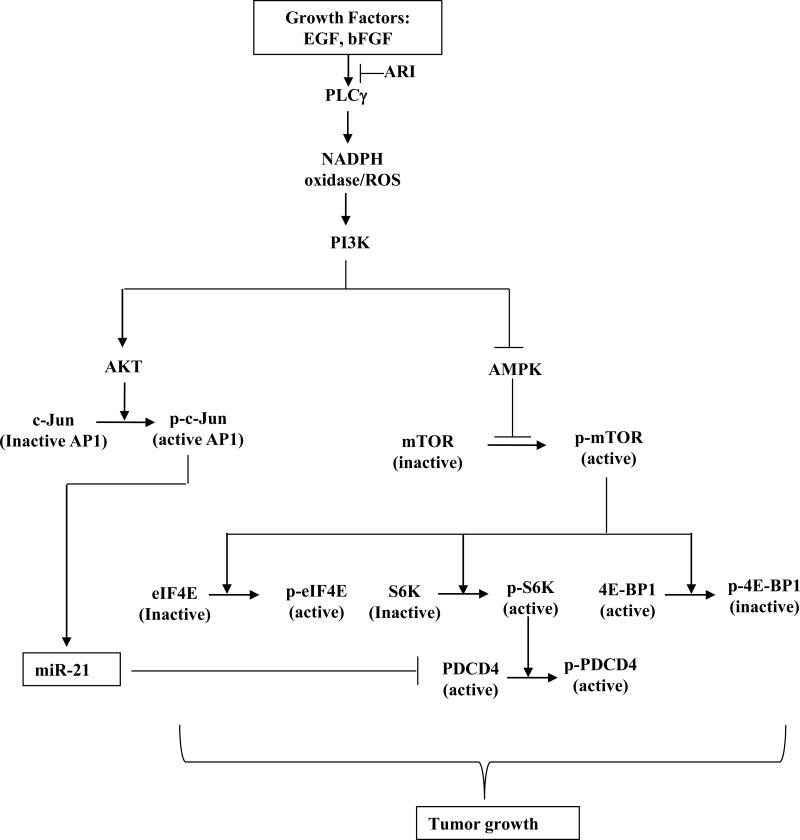
To further understand the underlying molecular mechanism (s) how AR inhibition prevents human CRC in cellular and animal models, we investigated the effect of AR inhibition on miR-21, PDCD4, 4E-BP1, c-Jun, AMPK and mTOR pathway in human colon cancer cells. Our results demonstrate that AR inhibition prevents growth factor-induced colon cancer growth by activating AMPK which in turn downregulates mTOR pathway leading to activation of PDCD4. Further, AR inhibition also prevents growth factor-induced miR-21 expression and thereby increases PDCD4 expression via downregulating AP-1 transcription factor activation in CRC cells (Fig.5).
Fig. 5.
Schematic representation of the role of AR in mediation of growth factor-induced colon cancer via PDCD4 and miR-21.
Oncogenic miR-21 has been shown to promote growth in several forms of cancers by down-regulating its target proteins such as PTEN and PDCD4. 20-22 Recently, we have shown that miR-21 is overexpressed in CRC cells that promoted the CRC cell proliferation, and AR inhibition prevented the expression of miR-21 dependent CRC growth by upregulation of PTEN.19 In the present study, we investigated the effect of AR inhibition in PDCD4, another target of miR-21 in CRC cells. It has been shown that PDCD4 is inactivated by S6K and downregulated by miR-21 in various cancers. 13,14,18,22 Further, EGF and FGF were shown to decrease the expression of PDCD4 expression23. Our results showed that AR inhibition downregulates miR-21 expression and increases PDCD4 expression, thereby prevents the CRC growth. Our results also showed that AR inhibition prevented the phosphorylation of PDCD4 by S6K, thereby making it active in CRC cells. To further confirm that miR-21 regulates PDCD4, we transfected HT29 cells with miR-21 mimics and inhibitors. HT29 cells transfected with the miR-21 mimics and stimulated with EGF showed decreased PDCD4 expression compared to EGF alone. These results inversely correlated with miR-21 inhibitors and confirmed that miR-21 downregulates of PDCD4. To confirm the role of PDCD4 in proliferation and regulation of various proteins, we ablated the HT29 cells with PDCD4-siRNA, and stimulated with EGF. PDCD4-ablated cells showed increased proliferation compared to EGF alone. This confirms that PDCD4 arrests the CRC growth. Further, PDCD4-ablated cells showed increased total and phospho-cJun, eIF4E, and FLIP expression, suggesting that PDCD4 may prevent the transcription of miR-21 by inhibition of c-Jun, inhibit the translation of various oncogenic proteins by decreasing the expression and binding affinity of eIF4E to respective mRNA, and inhibit the expression of FLIP. Inconsistent with our data, Yang et al. have also reported that PDCD4 could inhibit c-Jun activation in human colon RKO cells.15
Synthesis of aberrant oncogenic proteins through deregulated cap-dependent translation and various cell signaling pathways are the main contributor to tumorigenesis. An important step of cap-dependent mRNA translation is the binding of eIF4E to mRNA molecules with a 5′-terminal 7-methyl-GTP cap. Binding of eIF4E to mRNA is regulated by mTOR and 4E-BP1.11,12,24,25,26 4E-BP1 is a negative regulator of eIF4E. However, 4E-BP1 is phosphorylated by mTOR which makes it inactive. Phosphorylation of 4E-BP1 reduces it affinity to bind eIF4E, thereby promoting the translation of various cell survival proteins.12,24,25 In addition to this, phosphorylation of eIF4E by various kinases such as mTOR reduces its affinity to bind 4E-BP1.12,24,25 Collectively, overexpression of phospho-eIF4E and phospho-4E-BP1, which increases the binding affinity of eIF4E to mRNA that in turn translate various oncogenic proteins through deregulated cap-dependent translation, has been observed in various cancers.24,25,26 Therefore, we investigated the effect of AR inhibition on the activation of mTOR, eIF4E, S6K and 4E-BP1 in CRC cells. Our results show that AR inhibition prevents the EGF-induced phosphorylation/activation of mTOR, Raptor, eIF4E, and S6K, thereby making these oncogenic proteins inactive in colon cancer cells. We also showed that AR inhibition prevents the phosphorylation of 4E-BP1 in a time dependent manner and increases its expression in HT29 cells, which prevents the proliferation of cancer cells.
In summary, we have demonstrated a novel role of AR in the modulation of miR-21, PDCD4 and 4E-BP1. Our findings suggest that the inhibition of AR suppresses miR-21, increases PDCD4 and 4E-BP1, which in turn prevent the proliferation of human CRC cells via the ROS/AMPK/mTOR/AP-1/miR-21 signaling pathway. These unique findings provide further understanding of the molecular mechanisms by which AR promotes growth factor-induced colon cancer cell proliferation and AR inhibitor prevents it.
Acknowledgments
Supported by NIH grant CA129383 to SKS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: None
References
- 1.American Cancer Society . Cancer Facts and Figures 2011. American Cancer Society; Atlanta, Ga: 2011. [Google Scholar]
- 2.Shike M, Winawer SJ, Greenwald PH, Bloch A, Hill MJ, Swaroop SV. Primary prevention of colorectal cancer. The WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bull World Health Organ. 1990;68:377–85. [PMC free article] [PubMed] [Google Scholar]
- 3.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–13. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 4.Tammali R, Saxena A, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents hypoxia-induced increase in hypoxia-inducible factor-1alpha (HIF-1alpha) and vascular endothelial growth factor (VEGF) by regulating 26 S proteasome-mediated protein degradation in human colon cancer cells. J Biol Chem. 2011;286:24089–100. doi: 10.1074/jbc.M111.219733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–92. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 6.Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol Cancer Ther. 2010;9:813–24. doi: 10.1158/1535-7163.MCT-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tammali R, Reddy AB, Saxena A, Rychahou PG, Evers BM, Qiu S, Awasthi S, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents colon cancer metastasis. Carcinogenesis. 2011;32:1259–67. doi: 10.1093/carcin/bgr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talotta F, Cimmino A, Matarazzo MR, Casalino L, De Vita G, D'Esposito M, Di Lauro R, Verde P. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28:73–84. doi: 10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
- 10.Dobashi Y, Suzuki S, Sato E, Hamada Y, Yanagawa T, Ooi A. EGFR-dependent and independent activation of Akt/mTOR cascade in bone and soft tissue tumors. Mod Pathol. 2009;22:1328–40. doi: 10.1038/modpathol.2009.104. [DOI] [PubMed] [Google Scholar]
- 11.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo SH, Hsu CH, Chen LT, Lu YS, Lin CH, Yeh PY, Jeng HJ, Gao M, Yeh KH, Cheng AL. Lack of compensatory pAKT activation and eIF4E phosphorylation of lymphoma cells towards mTOR inhibitor, RAD001. Eur J Cancer. 2011;47:1244–57. doi: 10.1016/j.ejca.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–71. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 14.Carayol N, Katsoulidis E, Sassano A, Altman JK, Druker BJ, Platanias LC. Suppression of programmed cell death 4 (PDCD4) protein expression by BCR-ABL-regulated engagement of the mTOR/p70 S6 kinase pathway. J Biol Chem. 2008;283:8601–10. doi: 10.1074/jbc.M707934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, Tan TH, Colburn NH. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene. 2003;22:3712–20. doi: 10.1038/sj.onc.1206433. [DOI] [PubMed] [Google Scholar]
- 17.Schmid T, Bajer MM, Blees JS, Eifler LK, Milke L, Rübsamen D, Schulz K, Weigert A, Baker AR, Colburn NH, Brüne B. Inflammation-induced loss of Pdcd4 is mediated by phosphorylation-dependent degradation. Carcinogenesis. 2011;32:1427–33. doi: 10.1093/carcin/bgr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allgayer H. Pdcd4, a colon cancer prognostic that is regulated by a microRNA. Crit Rev Oncol Hematol. 2010;73:185–91. doi: 10.1016/j.critrevonc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Saxena A, Tammali R, Ramana KV, Srivastava SK. Aldose Reductase Inhibition Prevents Colon Cancer Growth by Restoring Phosphatase and Tensin Homolog Through Modulation of miR-21 and FOXO3a. Antioxid Redox Signal. 2012 Oct 25; doi: 10.1089/ars.2012.4643. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 21.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–9. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liwak U, Thakor N, Jordan LE, Roy R, Lewis SM, Pardo OE, Seckl M, Holcik M. Tumor suppressor PDCD4 represses internal ribosome entry site-mediated translation of antiapoptotic proteins and is regulated by S6 kinase 2. Mol Cell Biol. 2012;32:1818–29. doi: 10.1128/MCB.06317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, Straub J, Xiao D, Singh SV, Yang HS, Sonenberg N, Vatsyayan J. Phenethyl isothiocyanate, a cancer chemopreventive constituent of cruciferous vegetables, inhibits cap-dependent translation by regulating the level and phosphorylation of 4E-BP1. Cancer Res. 2007;67:3569–73. doi: 10.1158/0008-5472.CAN-07-0392. [DOI] [PubMed] [Google Scholar]
- 25.Grosso S, Pesce E, Brina D, Beugnet A, Loreni F, Biffo S. Sensitivity of global translation to mTOR inhibition in REN cells depends on the equilibrium between eIF4E and 4E-BP1. PLoS One. 2011;6(12):e29136. doi: 10.1371/journal.pone.0029136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pons B, Peg V, Vázquez-Sánchez MA, López-Vicente L, Argelaguet E, Coch L, Martínez A, Hernández-Losa J, Armengol G, Ramon Y, Cajal S. The effect of p-4E-BP1 and p-eIF4E on cell proliferation in a breast cancer model. Int J Oncol. 2011;39:1337–45. doi: 10.3892/ijo.2011.1118. [DOI] [PubMed] [Google Scholar]