Abstract
Background
Atopy varies by ethnicity even within Latino groups. This variation may be due to environmental, socio-cultural or genetic factors.
Objective
To examine risk factors for atopy within a nationwide study of U.S. Latino children with and without asthma.
Methods
Aeroallergen skin test repsonse was analyzed in 1830 US latino subjects. Key determinants of atopy included: country / region of origin, generation in the U.S., acculturation, genetic ancestry and site to which individuals migrated. Serial multivariate zero inflated negative binomial regressions, stratified by asthma status, examined the association of each key determinant variable with the number of positive skin tests. In addition, the independent effect of each key variable was determined by including all key variables in the final models.
Results
In baseline analyses, African ancestry was associated with 3 times as many positive skin tests in participants with asthma (95% CI:1.62–5.57) and 3.26 times as many positive skin tests in control participants (95% CI: 1.02–10.39). Generation and recruitment site were also associated with atopy in crude models. In final models adjusted for key variables, Puerto Rican [exp(β) (95%CI): 1.31(1.02–1.69)] and mixed ethnicity [exp(β) (95%CI):1.27(1.03–1.56)] asthmatics had a greater probability of positive skin tests compared to Mexican asthmatics. Ancestry associations were abrogated by recruitment site, but not region of origin.
Conclusions
Puerto Rican ethnicity and mixed origin were associated with degree of atopy within U.S. Latino children with asthma. African ancestry was not associated with degree of atopy after adjusting for recruitment site. Local environment variation, represented by site, was associated with degree of sensitization.
Keywords: Latino, atopy, region of origin, genetic ancestry, immigration, skin test, aeroallergen
Introduction
Atopy and atopic disorders such as asthma are thought to be caused by the interaction of genetic, social and environmental factors.1 Although atopy is understudied among U.S. minority populations, there are well described ethnic differences in atopic diseases such as asthma. Asthma prevalence and severity differ between Latino groups, where Puerto Ricans have the highest prevalence and severity of asthma while Mexicans have among the lowest burden.2, 3 There are many potential explanations for this observation, including place of birth and generation in the U.S.,4–7 acculturation,8 early life exposures9–12 and genetic predisposition.1, 13, 14 Prior research has demonstrated that African ancestry is associated with asthma in African-origin populations,15, 16 and IgE levels in Afro-Caribbean populations.15
Many studies evaluating ethnicity as a risk factor for atopy do not disaggregate Latinos by country or region of origin.14, 17–20 Studies of atopy in Latino populations have either been small in total numbers,21, 22 inadequately representative of different national origins,23, 24 or both.21 Furthermore, few studies have evaluated factors that are associated with atopy in U.S. Latino populations.22, 25 Given the size of the U.S. Latino population26 and the diversity of this group regarding country of origin, it is important to describe patterns of atopy and determine factors contributing to variable childhood atopy susceptibility in this population.
Latinos are admixed and share varying proportions of African, Native American and European ancestry.27, 28 The complexity of genetic ancestry among Latinos may complicate biomedical research studies in this population. On the other hand, precisely because of this complexity, Latinos also present a unique opportunity to disentangle the clinical, social, environmental, and genetic underpinnings of population differences in health outcomes. Specifically, the mixed ancestry of Latinos provides the intrinsic variability needed to untangle complex gene-environment interactions, which may help to explain the striking differences in atopic disorders seen among Latinos and other populations.
We sought to determine whether country of origin, genetic ancestry, generation and acculturation were associated with atopy in U.S. Latino children. To study this question, we leveraged a large multi-center study of U.S. Latino children with and without asthma to examine the independent effects of local environment, socio-cultural factors relevant to immigration, and genetic ancestry on atopy.
Methods
Recruitment
Latino children were enrolled as a part of the ongoing Genes-environments & Admixture in Latino Americans (GALA II) case-control study. From July 2008 through November 2011, 4,157 children (2,022 participants with asthma and 2,135 healthy controls) were recruited from five centers (Chicago, Illinois; Bronx, New York; Houston, Texas; San Francisco Bay Area, California; and Puerto Rico) using a combination of community- and clinic-based recruitment. Participants were eligible if they were 8–21 years of age and all four grandparents self-identified as Latino. Asthma cases were defined as participants with a history of physician diagnosed asthma and the presence of two or more symptoms of coughing, wheezing, or shortness of breath in the 2 years preceding enrollment. Healthy controls were recruited from the community and clinics with the same catchment area as cases. Controls were defined as participants with no reported history of asthma, lung disease, or chronic illness over their lifetime, and no reported symptoms of coughing, wheezing or shortness of breath in the last two years. Controls were frequency matched on age (within 1 year), sex, and study center. Participants were excluded if they reported any of the following: (1) 10 or more pack-years of smoking; (2) any smoking within 1 year of recruitment date; (3) history of lung diseases other than asthma (cases) or chronic illness (cases and controls); or (4) pregnancy in the third trimester. All local institutional review boards approved the study, and all participants/parents provided appropriate written assent/consent.
Trained interviewers, proficient in both English and Spanish, administered questionnaires to gather baseline demographic data, as well as information on general health, asthma status, acculturation, social, and environmental exposures. For this analysis, only mainland U.S. participants who had skin prick test data available were studied. Puerto Rican subjects from Puerto Rico were not included as the analysis is focused on patterns of atopy in the mainland U.S. and evaluates factors including acculturation and migrant generation which are not relevant to this population. This resulted in 1,129 participants with asthma and 1,167 healthy controls. Participants missing data on age (n=8), mother's education (n=31), first-born child status (n=36), breastfeeding (n=83), mother smoking during pregnancy (n=12),, postnatal smoking exposure (n=249), cat or dog ownership in the first year of life (n=11), and daycare attendance in the first 3 years of life (n=24) were excluded from the analyses, yielding an analytical sample size of 1830 (914 patients with asthma and 916 controls).
Outcome measurements
Skin testing was performed on the volar aspect of the forearm, using the Multi-Test II Device (Lincoln Diagnostics, Decatur IL) for a panel of 14 aeroallergens including dust mite (Dermatophygoides pteronyssinus, Dermatophygoides farinae), dog, cat, cockroach (mix of German and American cockroach), mouse epithelium, rat epithelium, tree (oak and olive/elm), grass (Perennial ryegrass and Timothy), short ragweed, Alternaria tenius, Hormodendrum cladosporioides, and Aspergillus mix. Any allergen with a mean wheal diameter of at least 3 mm greater than the saline control was considered positive. The number of positive skin tests was summed for each individual to provide the count variable used in these analyses. For secondary analyses, we classified any subject with a positive test as atopic and those with all negative tests as non-atopic.
Key independent variables
Origin
The participant’s country of birth, parents’ country of birth, and self-reported country of birth of all four grandparents were used to determine country or region of origin. Firstly, for participants born outside the U.S., their origin was determined by their country of birth. For U.S.-born participants, origin was determined by their parents' country of birth (or grandparent’s country of origin if their parents were born in the U.S.). For those with missing information for one parent, origin defaulted to the origin of the known parent. Participants were then classified as Puerto Rican, Mexican, South American, Central American, non-Puerto Rican Caribbean, or mixed Latino (for individuals of multiple origins who did not fit in the prior mentioned categories).
Generation in the U.S
The same variables used to assign the participant's origin were also used to determine their generation of immigration. Foreign-born participants were classified as first generation immigrants, U.S. born participants with at least one foreign-born parent as second generation, and U.S.-born participants with both U.S.-born parents as third generation or greater. For participants with missing information for one parent, immigrant status was determined from information of the known parent.
Maternal Acculturation
Acculturation status was determined using the mother’s characteristics as a proxy for the child’s home environment, since early life factors 9–12 have been shown to be important atopy risk factors and it is not known whether these factors would differ depending on degree of U.S. acculturation. Consistent with previous studies,29–32 acculturation status was determined by aggregating the reported country of birth for the mother, language preference of the mother, and length of time the mother had been living in the U.S. A five-level acculturation variable was constructed: U.S.-born mother who prefers English, foreign-born mother living in the U.S. for 10 or more years and prefers English, foreign-born mother living in the U.S. for 15 or more years and prefers Spanish, foreign-born mother living in the U.S. for 10–15 years and prefers Spanish, and foreign-born mother living in the U.S. for <10 years and prefers Spanish.
Ancestry proportions
To estimate the ancestral admixture of GALA II participants, we used available genome-wide genotyping data from the Affymetrix Axiom Latino Array.33 We removed single nucleotide polymorphisms (SNPs) with >5% missing values and failing platform specific SNP quality criteria (n=63,328), along with those out of Hardy-Weinberg equilibrium (n=1845; p<10−6) within their respective populations. After combining HapMap European(CEU) and African (YRI) ancestral data with Native American samples (kindly provided by Cheryl Winkler, Andres Moreno, Karla Sandoval and Carlos Bustamante) a final sample of 568,037 autosomal SNPs with relevant ancestral data were used to estimate the three-way ancestry using the program ADMIXTURE.34 Native American and African Ancestry were chosen for modeling because African and European ancestries are along the same axis of assortment in Latino populations.
Recruitment site/site to which migration occurred
We included recruitment site as a proxy for unmeasured local environmental factors which may vary by site.
Covariates
Covariates for all models were selected based on the literature and included: gender,35 age,36, 37 mother's highest education level, first-born child status,38 breastfeeding,39 mother smoking during pregnancy,40 postnatal smoking exposure,41 cat or dog ownership in the first year of life,42–44 and daycare attendance in the first 3 years of life.45, 46 Aside from a priori covariates, a number of regional air pollution exposure variables (PM10, PM2.5, ozone, and distance from major traffic arteries) were evaluated. Estimates for each subject were based on geocoded addresses at 1–3 years of age and at time of recruitment mapped to the Environmental Protection Agency’s Air Quality System Data and averaged for each year. These were subsequently removed from the analyses due to lack of significance.
Statistical methods
Descriptive statistics for selected variables were presented for the overall study population and according to asthma status. All analyses were stratified by asthma status to avoid inflating the effects of asthma.47 To determine significant differences between children with and without asthma, we used Mann-Whitney test, t-tests, and chi-square tests. Zero inflated negative binomial (ZINB) regression models were used to estimate associations of each key variable (origin, recruitment site, ancestry proportions, generation, and acculturation) with the number of positive skin tests. ZINB regression models count data with an excess of zeros, and assumes the zeros are distributed from a different process than non-zero observations (the “count portion”), and are thus modeled independently. The count portion models the negative binomial distribution (log link), and the mass at zero models a binary distribution. Since we are interested in the contribution of the relevant exposures to the number of positive skin tests to aeroallergens, we extract the coefficients from the “count” portion of the zeroinf() function in the R package (pscl), and exponentiate these to get the expected impact of our predictors on the number of positive skin prick tests. In addition, the independent effect of each key variable was determined by adjusting key variables in the final models, stratified by children with and without asthma. Because acculturation and migrant generation were defined using mother’s place of birth, they were not included in the same model to avoid multicollinearity. Similarly, we created two separate final models for country/region of origin and recruitment site due to collinearity of these two variables. As a secondary analysis, we evaluated the association of these factors with presence of any sensitization with logistic models, using the same baseline covariates as with the models for number of positive skin tests. These models comprised all key variables, including generation, region of origin and ancestry proportion. All analyses were performed using the statistical software R version 2.13.1.
Results
Cohort Characteristics
The characteristics of the cohort are displayed in Table 1. The average age of participants was 13.1±3.4years. The two largest groups of participants were from Mexico (57.8%) and Puerto Rico (12.2%) with smaller numbers from South America, Central America, and the Caribbean. Over two-thirds of GALA II children were second generation migrants (70.2%) with low levels of acculturation, and 71.3% of mothers preferred Spanish. GALA II is a primarily low socioeconomic status (SES) cohort where 95.1% of participants had a maternal education level at high school or less. We also present the distribution of skin test positivity to each of the evaluated allergens by region of origin in supplementary Table 1. Notably, mouse and cockroach were more common sensitizers in New York.
Table 1.
Characteristics of GALA II Case-Control Study participants: 2006–2011
| Total N (%) | Case N (%) | Control N (%)> | p* | |
|---|---|---|---|---|
| N | 1830 (100) | 914 (100) | 916 (100) | |
| Total number of positive skin prick tests† | 2 (0 – 4) | 3 (1 – 5) | 1 (0 – 3) | <0.001 |
| Demographics | ||||
| Average age‡ | 13.1 (3.4) | 12.5 (3.2) | 13.6 (3.5) | <0.001 |
| Male | 894 (48.9) | 504 (55.1) | 390 (42.6) | <0.001 |
| Region of Origin | <0.001 | |||
| Mexico | 1057 (57.8) | 491 (53.7) | 566 (61.8) | |
| Puerto Rico | 223 (12.2) | 152 (16.6) | 71 (7.8) | |
| Central America | 190 (10.4) | 85 (9.3) | 105 (11.5) | |
| Caribbean | 95 (5.2) | 36 (3.9) | 59 (6.4) | |
| South America | 33 (1.8) | 11 (1.2) | 22 (2.4) | |
| Mixed | 226 (12.3) | 134 (14.7) | 92 (10) | |
| Missing data | 6 (0.3) | 5 (0.5) | 1 (0.1) | |
| Global Ancestry | ||||
| Average % of African ancestry‡ | 10.3 (12.3) | 11.2 (12.7) | 9.3 (11.7) | <0.001 |
| Average % of European ancestry‡ | 43.4 (18.4) | 46.2 (17.6) | 40.5 (18.9) | <0.001 |
| Average % of Native American ancestry‡ | 46.3 (25.2) | 42.5 (24.1) | 50.2 (25.9) | <0.001 |
| Missing data | 183 (10) | 71 (7.8) | 112 (12.2) | |
| Generation in the U.S. | <0.001 | |||
| 1st generation | 338 (18.5) | 92 (10.1) | 246 (26.9) | |
| 2nd generation | 1285 (70.2) | 687 (75.2) | 598 (65.3) | |
| 3rd+ generation | 178 (9.7) | 121 (13.2) | 57 (6.2) | |
| Missing data/not applicable | 29 (1.6) | 14 (1.5) | 15 (1.6) | |
| Mother’s acculturation level | <0.001 | |||
| Foreign born, living in U.S.<10 yrs, prefers Spanish | 241 (13.2) | 73 (8) | 168 (18.3) | |
| Foreign born, living in U.S. 10–15 yrs, prefers Spanish | 357 (19.5) | 177 (19.4) | 180 (19.7) | |
| Foreign born, living in U.S. 15+ yrs, prefers Spanish | 706 (38.6) | 341 (37.3) | 365 (39.8) | |
| Foreign born, living in U.S. 10+ yrs, prefers English | 116 (6.3) | 70 (7.7) | 46 (5) | |
| U.S. born, prefers English | 314 (17.2) | 205 (22.4) | 109 (11.9) | |
| Missing data/not applicable | 96 (5.2) | 48 (5.3) | 48 (5.2) | |
| Mother’s education level | 0.561 | |||
| Bachelor’s degree and above | 89 (4.9) | 47 (5.1) | 42 (4.6) | |
| High school degree | 782 (42.7) | 399 (43.7) | 383 (41.8) | |
| No High school degree | 959 (52.4) | 468 (51.2) | 491 (53.6) | |
| Variables associated with sensitization | ||||
| First-born child | 875 (47.8) | 398 (43.5) | 477 (52.1) | <0.001 |
| Breastfed | 1316 (71.9) | 605 (66.2) | 711 (77.6) | <0.001 |
| Mother smoking during pregnancy | 89 (4.9) | 54 (5.9) | 35 (3.8) | 0.049 |
| Adults smoking during pregnancy | 588 (32.1) | 329 (36.0) | 259 (28.3) | <0.001 |
| Owned dog/cat in 1st year of life | 511 (27.9) | 235 (25.7) | 276 (30.1) | 0.040 |
| Attended daycare | 427 (23.3) | 242 (26.5) | 185 (20.2) | 0.002 |
P values calculated from Mann-Whitney test (all ordinal variables), t-tests (all continuous variables), and chi-square tests (all categorical non-ranked variables).
Reported as median (IQR)
Reported as mean (SD)
Finally, there were differences in total number of positive skin tests between asthmatic cases [median: inter-quartile range (IQR): 3 (1–5)] and controls [median (IQR):1 (0–3)], respectively (p<0.001), and any positive skin test (77% vs 54.4% in cases and controls, respectively) and the sensitivity to dust mites (52.5% and 29.7% in cases and controls, respectively). Along with these sensitivity differences, participants with asthma were also different from controls in gender, region of origin, estimates of global ancestry, generation of migrant, acculturation, as well as variables associated with sensitization including first born child and breast feeding (p-values <0.01).
Origin, Migrant Generation, Ancestry, Acculturation, Recruitment Site, and Atopy
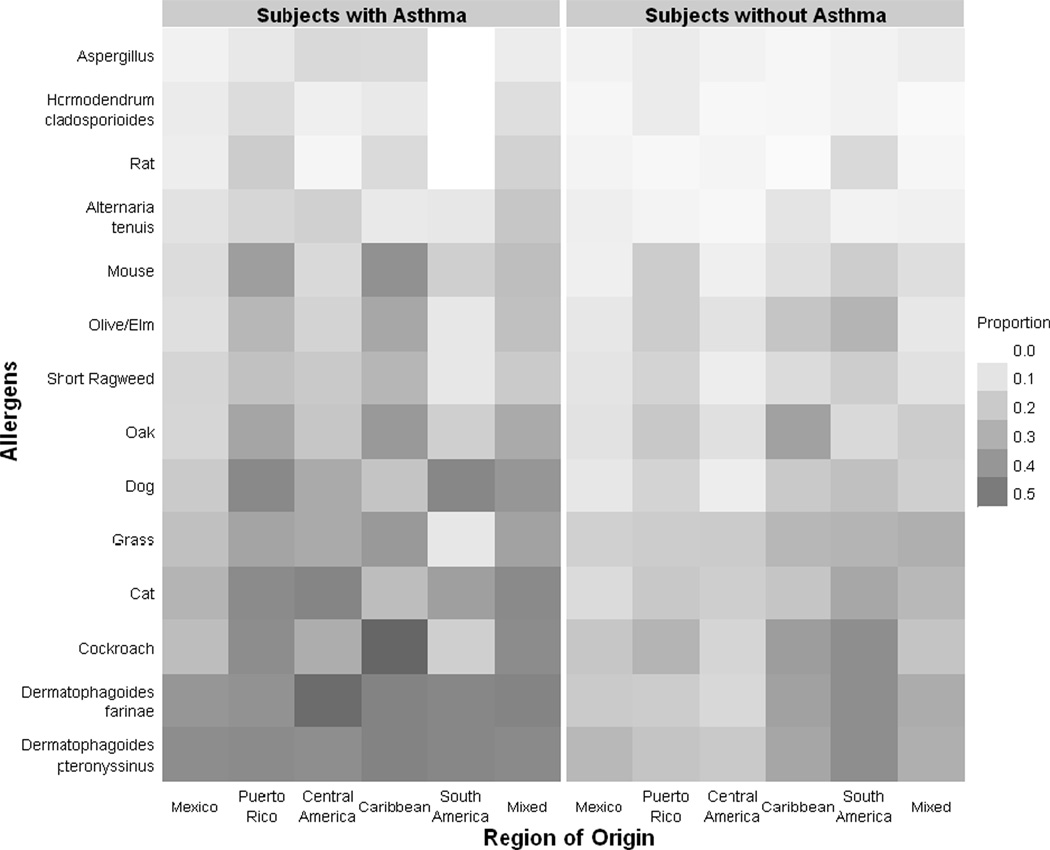
The distribution of positive skin tests by region of origin is presented in Figure 1 for participants with asthma and healthy controls, where darker squares represent a greater proportion of sensitized individuals. There were differences in the proportion of individuals sensitized to these aeroallergens by asthma status, and by region of origin within both asthmatic and non-asthmatic participants.
Figure 1. Positive skin prick tests by region of origin.
This figure represents the proportion of participants with a positive skin test to allergens tested by region of origin. The proportions are represented on a scale in increments of 0.1 ranging from 0 (white) to 1.0 (black).
We display the ZINB regression-based associations between our key determinants and atopy for children with and without asthma (Table 2). The associations were adjusted for the covariates as noted, but each predictor variable (region of origin, ancestry, generational status, recruitment site, and acculturation) was tested without accounting for the other predictors in this first analytical step.
Table 2.
Zero-inflated negative bionomial regression analysis evaluating potential primary determinants of number of positive skin prick tests among Latino children with asthma
| Asthmatic Participants | Non-Asthmatic Participants | |||
|---|---|---|---|---|
| N | Relative number of positive skin prick tests (95%CI)* |
N | Relative number of positive skin prick tests (95%CI)* |
|
| Model of country of origin | 909 | 915 | ||
| Mexico | 491 | 1.0 (Referent) | 566 | 1.0 (Referent) |
| Puerto Rico | 152 | 1.48(1.25–1.75) | 71 | 1.27(0.91–1.77) |
| Central | 85 | 1.12(0.91–1.36) | 105 | 1.01(0.75–1.36) |
| Caribbean | 36 | 1.39(1.06–1.83) | 59 | 1.34(0.98–1.83) |
| South | 11 | 1.17(0.64–2.15) | 22 | 1.49(0.95–2.35) |
| Mixed | 134 | 1.4(1.19–1.65) | 92 | 0.97(0.73–1.3) |
| Model of ancestry | 843 | 804 | ||
| African Ancestry proportion | 3.00(1.62–5.57) | 3.26(1.02–10.39) | ||
| Native American Ancestry proportion | 0.92(0.64–1.31) | 1.28(0.76–2.15) | ||
| Model of generation | 900 | 901 | ||
| 1st generation migrant | 92 | 1.0 (Referent) | 246 | 1.0 (Referent) |
| 2nd generation migrant | 687 | 1.01(0.81–1.26) | 598 | 1.18(0.94–1.5) |
| 3rd+ generation migrant | 121 | 1.31(1.00–1.72) | 57 | 1.52(1.04–2.23) |
| Model of mother’s acculturation | 866 | 868 | ||
| Foreign born, in U.S. <10 yrs, prefers Spanish | 73 | 1.0 (Referent) | 168 | 1.0 (Referent) |
| Foreign born, in U.S. 10–15 yrs, prefers Spanish | 177 | 1.1 (0.84 – 1.45) | 180 | 0.97 (0.73 – 1.3) |
| Foreign born, in U.S. 15+ yrs, prefers Spanish | 341 | 1.07 (0.83 – 1.38) | 365 | 1.18 (0.9 – 1.54) |
| Foreign born, in U.S. 10+ yrs, prefers English | 70 | 1.31 (0.95 – 1.8) | 46 | 1.04 (0.66 – 1.64) |
| U.S. born, prefers English | 205 | 1.29 (0.99 – 1.69) | 109 | 1.16 (0.83 – 1.63) |
| Model of recruitment site | 914 | 916 | ||
| New York, NY | 273 | 1.0 (Referent) | 246 | 1.0 (Referent) |
| Chicago, IL | 211 | 0.65(0.56–0.76) | 232 | 0.59(0.47–0.74) |
| San Francisco Bay Area, CA | 300 | 0.51(0.44–0.59) | 341 | 0.72(0.58–0.88) |
| Houston, TX | 130 | 0.69(0.58–0.82) | 97 | 0.51(0.36–0.71) |
All models of key determinants are adjusted for gender, age, mother’s highest education level, first-born child status, breastfeeding, prenatal smoke exposure, pet ownership in the first year of life, and daycare attendance.
Bold indicates significant antilog-β at α=0.05.
In participants with asthma, children from Puerto Rico (1.48, 95%CI: 1.25–1.75), the Caribbean (1.39, 95%CI: 1.06–1.83), and of mixed national origin (1.40, 95%CI: 1.19–1.65) were 40%–50% more likely to have positive skin prick tests (Table 2) than children from Mexico. Increasing African Ancestry was also associated with 3 times as many positive skin tests in participants with asthma [exp(β)(95%CI): 3.00(1.62— 5.57)]. The association of the number of positive skin tests with third generation status alone [exp(β)(95%CI): 1.31(1.00 — 1.72)] and highest level of acculturation [exp(β)(95%CI): 1.29(0.99— 1.69)] were marginally significant, but additional associations with acculturation lacked precision.
In participants without asthma, African ancestry was associated with 3.26 times as many positive skin tests [exp(β)(95%CI): 3.26 (1.02 — 10.39)]. Native American ancestry was not associated with number of positive skin tests. Compared to first generation subjects, 3rd generation status was associated with 1.52 times as many positive skin tests [exp(β)(95%CI): 1.52 (1.04 — 2.23)] (Table 2). The association with Caribbean origin [exp(β)(95%CI): 1.34(0.98–1.83)] was marginally significant, but no other obvious national origin differences were present in controls.
Compared to New York City, being recruited in Chicago, the San Francisco Bay Area, or Houston was associated with lower risk of sensitization for both participants with and without asthma. These associations remained nearly identical after controlling for ancestry and generation in the US (Table 3).
Table 3.
Evaluation of recruitment site, ancestry and migrant generation as determinants of positive skin prick test differences among Latino children with and without asthma
| Asthmatic Participants | Non-Asthmatic Participants | |||
|---|---|---|---|---|
| N | Relative number of positive skin prick tests (95%CI)* |
N | Relative number of positive skin prick tests (95%CI)* |
|
| Model for number of positive skin prick tests | 829 | 790 | ||
| New York, NY | 246 | 1.0 (Referent) | 223 | 1.0 (Referent) |
| Chicago, IL | 190 | 0.68(0.56–0.81) | 207 | 0.56(0.43–0.72) |
| San Francisco Bay Area, CA | 267 | 0.53(0.44–0.64) | 266 | 0.74(0.58–0.95) |
| Houston, TX | 126 | 0.74(0.6–0.9) | 94 | 0.49(0.35–0.7) |
| African Ancestry proportion | 1.53(0.82–2.86) | 0.8(0.22–2.93) | ||
| Native American Ancestry proportion | 1.18(0.83–1.69) | 1(0.58–1.72) | ||
| 1st generation migrant | 1.0 (Referent) | 1.0 (Referent) | ||
| 2nd generation migrant | 0.97(0.78–1.2) | 1.19(0.94–1.51) | ||
| 3rd+ generation migrant | 1.13(0.85–1.49) | 1.41(0.96–2.08) | ||
Adjusted for gender, age, mother’s highest education level, first-born child status, breastfeeding, prenatal smoke exposure, pet ownership in the first year of life, and daycare attendance.
Bold indicates significant antilog-β at α=0.05.
Ancestry, Origin, and Generation of Immigration
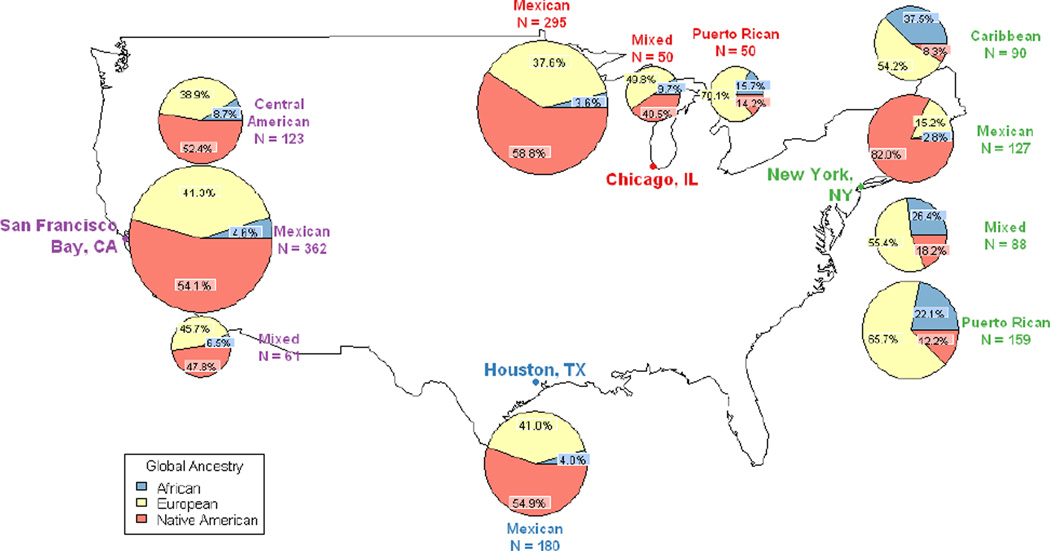
In Figure 2, we display the mean proportions of African, European, and Native American ancestry for each region of origin at each recruitment site. Participants with Caribbean or Puerto Rican origin had higher levels of African ancestry (p <0.001) than participants with other regions of origin, consistent with previous studies.48
Figure 2. Global ancestry proportions of GALA II Latinos by origin and recruitment site.
Diameter of pie charts represents size of group recruited at each site. Blue, yellow, and orange wedges represent African, European, and Native American ancestry respectively. Global ancestry proportions for population sizes less than 20 are not displayed on this map.
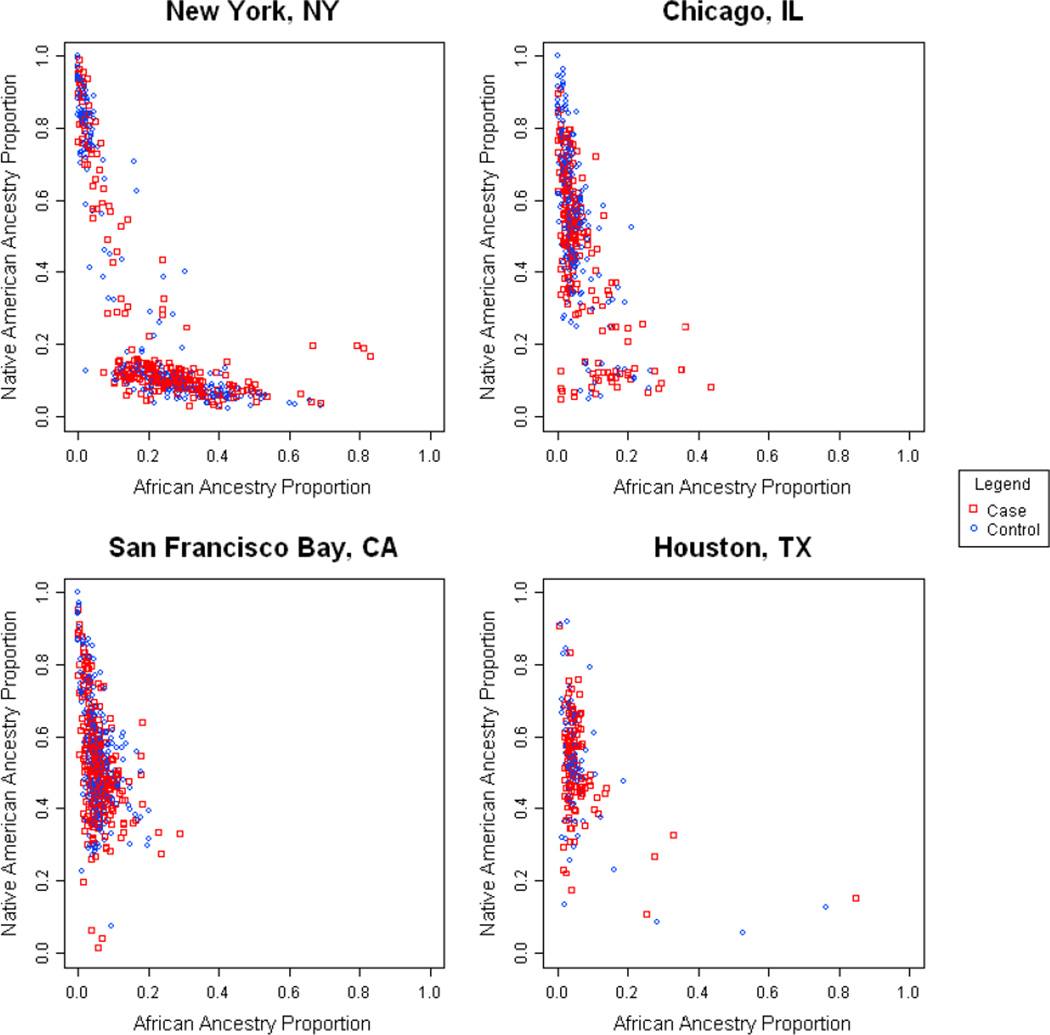
Table 3 evaluated whether addition of local effects (recruitment site/site to which migration occurred) and generation of immigration status attenuated the effects of genetic ancestry. Indeed, only recruitment site remained significantly associated with atopy, abrogating the effects of African ancestry and generation of immigrant. To further examine the effect of recruitment site on ancestry associations, we evaluated whether there were differences in ancestry distribution across sites (Figure 3) and found that there were clear differences in African ancestry between New York and other sites.
Figure 3. Ancestral proportions and recruitment site and case/control status.
Each plot on these graphs represents a recruitment site. The Y – axis represents global estimates of individual Native American ancestral proportion and the X axis represents global estimates of individual African ancestral proportion. Asthmatic subjects are represented by square symbols and non-asthmatics are represented by circular symbols
Finally, we sought to investigate whether the effects of recruitment site on ancestry associations were due to differential migration patterns of Latino national origin groups. We tested whether addition of country of origin and generation of immigration status attenuated the effects of genetic ancestry (i.e., whether recruitment site/local effects were proxies for migration patterns). Among participants with asthma (Table 4), the associations for African ancestry [exp(β)(95% CI): 3.13 (1.63 — 6.01)] were slightly higher than the effects seen in Table 2. In participants without asthma (Table 4), the magnitude of the association between African ancestry and positive skin tests was even greater after controlling for other key variables [exp(β)(95% CI):4.99 (1.19 — 20.91)]. There were no significant associations between Native American ancestry and positive skin tests in participants without asthma. However, there was a linear trend between generation of immigrant with the number of positive skin tests, in which third generation Latinos had a 65% greater likelihood of positive number of skin tests compared to first generation Latinos [exp(β)(95% CI):1.65 (1.05 — 2.59)]. These results suggest that the local effects of recruitment site are not simply proxies for differential migration patterns of different ethnic groups since region or country of origin did not abrogate the associations in the same manner as recruitment site.
Table 4.
Evaluation of origin, ancestry and migrant generation as determinants of positive skin prick test differences among Latino children with and without asthma
| Asthmatic Participants | Non-asthmatic Participants | |||
|---|---|---|---|---|
| N | Relative number of positive skin prick tests(95%CI)* |
N | Relative number of positive skin prick tests(95%CI)* |
|
| Model for number of positive skin prick tests | 825 | 790 | ||
| Region of origin | ||||
| Mexico | 446 | 1.0 (Referent) | 486 | 1.0 (Referent) |
| Puerto Rico | 127 | 1.31(1.02–1.69) | 59 | 0.96(0.61–1.52) |
| Central | 77 | 1(0.81–1.23) | 91 | 0.96(0.69–1.32) |
| Caribbean | 36 | 1.17(0.83–1.66) | 55 | 0.94(0.58–1.54) |
| South | 11 | 1.03(0.49–2.15) | 19 | 1.55(0.98–2.47) |
| Mixed | 128 | 1.27(1.03–1.56) | 80 | 0.81(0.59–1.11) |
| Global ancestry proportion | ||||
| African | 3.13(1.63–6.01) | 4.99(1.19–20.91) | ||
| Native American | 1.47(0.95–2.27) | 1.51(0.84–2.71) | ||
| Generation in the U.S. | ||||
| First generation | 1.0 (Referent) | 1.0 (Referent) | ||
| Second generation | 0.97(0.77–1.22) | 1.21(0.94–1.55) | ||
| Third generation | 1.14(0.84–1.55) | 1.65(1.05–2.59) | ||
Adjusted for gender, age, mother’s highest education level, first-born child status, breastfeeding, prenatal smoke, pet ownership in the first year of life, and daycare attendance.
Bold indicates significant antilog-β at α=0.05.
As a secondary analysis, we evaluated the contribution of the key variables to the presence of any sensitization in a model that accounted for origin, ancestry, and migrant status. In asthmatic subjects, we found that of our key variables, only region of origin was associated with atopic status [Central America OR =3.99 (1.71 — 9.3), Caribbean OR = 3.57 (1.03 — 12.42), and mixed OR=2.14 (1.15 — 3.97), reference = Mexican]. Similarly, in control subjects, of our key variables, only region of origin was associated with atopic status [Puerto Rican subjects OR =2.23 (1.01 — 4.92), Caribbean OR = 3.77 (1.41 — 10.06), South American OR = 3.37 (1.06 — 10.69), and mixed OR=2.54 (1.38 — 4.66), reference = Mexican].
Discussion
GALA II is the largest nationwide genetic study of Latino children with and without asthma. This study has carefully collected data that allow estimation of the effects of acculturation, migration patterns, and genetic ancestry, and other asthma-related factors on lung function and atopy. By leveraging these strengths of GALA II, we were able to evaluate the independent associations of social, environmental, and genetic factors with degree of atopy. Our findings suggest that country/region of origin and local exposures may have the greatest influence on the degree of atopy in Latino children. In multivariable analysis, genetic ancestry was not independently associated with atopy in this population. Since Latinos of different countries migrate preferentially to various sites, the associations of genetic ancestry in the U.S. may be proxies for local exposures.
Our findings on ancestry are in keeping with another study that examined the association of sensitization with African ancestry and did not find an ancestry specific association with atopy in white and African American populations.49 In that study by Williams et al., the effects of ancestry on sensitization were explained by local neighborhood effects. However, our results are in contrast with the association between African ancestry and atopy reported by other investigators in Afro-Caribbean15 and multi-ethnic U.S. populations.13 While we did observe an association between African ancestry and degree of atopy, these results were not independent of local environmental factors. New York had the highest rates of sensitization to cockroach and mouse epithelium and also had the highest proportions of Afro-Caribbean Latino subjects. The initially observed ancestry effect may be confounded by patterns of migration to this local environment. Our results move beyond these prior investigations in that we had a large number of children from multiple Latin American countries that migrated to a number of different regions in the U.S. As such, we are uniquely positioned to differentiate genetic from socioeconomic, sociocultural, and unmeasured local factors. Environmental factors such as SES may co-vary with African ancestry1 and atopic diseases.50 We accounted for SES, generation status, degree of acculturation, and unmeasured local site effects that could vary by differential patterns of immigration among Latinos from different countries to different areas of the U.S. Our study was also rigorous in adjusting for traditional factors affecting atopy including gender,35 age,36, 37 mother's highest education level, first-born child status,38 breastfeeding,39 maternal smoking during pregnancy,40 postnatal smoking exposure,41 cat or dog ownership in the first year of life,42–44 and daycare attendance in the first 3 years of life.
Interestingly, differences in degree of atopy by country of origin were only seen in participants with asthma. It is possible that some of the differential associations by asthma status are modified by other variables that co-vary with asthma. While we have included a number of covariates that influence the development of atopy, it is possible that our estimates may be residually confounded by unmeasured factors that are associated with asthma and influence atopy (e.g., dietary influences in early life9–12). Alternately, given lower levels of atopy in participants without asthma, we may not have had sufficient power to see differences in numbers of positive skin tests by region of origin. This last possibility is supported by the fact that we did see differences in the presence of atopy by region of origin in both asthmatic and control subjects.
We also found that generation status had modest and variable effects in increasing risk of positive skin tests to aeroallergens, which were abrogated after adjusting for recruitment site. Other studies have suggested that migration as a child (first generation) or birth in a high-income country (second generation) increases the risk of atopic disease.6, 7, 51, 52 First generation immigrants who arrive in a country as adults may have less risk of atopy than their second generation offspring,6, 51 suggesting that early life exposures are important in this phenomena. However, the majority of our first generation participants, the reference group, were children and not first generation adult participants. These subjects would have had similar early life environments in the U.S. compared to higher generation immigrants. Finally, we did not observe associations by acculturation status despite prior studies suggesting that U.S. lifestyle was associated with atopy.53, 54 One implication of this finding may be that biological factors associated with growing up in the U.S. modulate early life immune development11, 55, 56 and may be more relevant than other environmental factors associated with acculturation.
Our study has a number of limitations. First, the sample size of participants from different Latin American regions varies and is smaller for South America and Caribbean origin participants. This limits our ability to distinguish differences between groups and limits the number of Latinos with a higher proportion of African ancestry. However, the distribution by region of origin in our sample is representative of national immigration patterns and similar to U.S. Census proportions. Additionally, GALA II was limited to the ages 8–21, restricting our ability to evaluate age of immigration on the development of atopic disease. Finally, there may be some residual confounding from unmeasured factors, such as discrimination and stress in pregnancy,57, 53 that vary by skin pigmentation59 and are also associated with atopic disease. The local effects may be due to other unmeasured environmental factors including levels of allergen in settled dust,60–63 levels of endotoxin exposure,64–67 or the microbiome.56 We examined annual regional air pollution exposures such as PM10, PM 2.5, ozone, and distance from major traffic arteries as potential covariates, but these variables were not significantly associated with atopy in this population. Finally, GALA II is a case-control design, which may affect the representativeness of our results in terms of prevalence; however separate case/control analyses allow for interpretations independent of asthma prevalence.
By using GALA II, the largest nationwide genetic study of Latino children with and without asthma, we were able to describe patterns of atopy and also evaluate the independent contributions of factors potentially important in the U.S. Latino population (acculturation, migration pattern, and genetic ancestry). Without this type of data, key determinants of atopy would be missed in this growing and understudied U.S. minority group. In summary, there are differences in atopy by country of origin among Latino participants with asthma in this nationwide sample. Furthermore, in contrast to prior studies, genetic estimates of African ancestry were not associated with increased risk of atopy in multivariate analyses and may be proxies for local environmental factors since there are differences in patterns of immigration from Latino countries to sites within the U.S. Finally, our associations vary by national origin and highlight that Latinos from different countries of origin should be evaluated independently in studies of atopy.
Clinical Implications.
Latino children in the U.S. have variable degrees of atopy based on both country of origin and local environment. In particular, Puerto Rican children are at higher risk.
Acknowledgments
The authors acknowledge the families and patients for their participation and thank the numerous health care providers and community clinics for their support and participation in GALA II and SAGE II. In particular, the authors thank study coordinator Sandra Salazar; and the recruiters who obtained the data: Duanny Alva MD, Gaby Ayala-Rodriguez, Ulysses Burley, Lisa Caine, Elizabeth Castellanos, Jaime Colon, Denise DeJesus, Iliana Flexas, Blanca Lopez, Brenda Lopez MD, Louis Martos, Vivian Medina, Juana Olivo, Mario Peralta, Esther Pomares MD, Jihan Quraishi, Johanna Rodriguez, Shahdad Saeedi, Dean Soto, Ana Taveras.
Funding: This research was supported in part by National Institutes of Health (ES015794, AI077439, HL088133, HL078885, CA113710, AI079139, AI061774, HL079055, DK064695, and M01-RR00188; HHSN26120080001E; MD006902); Flight Attendant Medical Research Institute (FAMRI); RWJF Amos Medical Faculty Development Award (to EGB); the Sandler Foundation; the American Asthma Foundation (to EGB); Ernest S. Bazley Grant (to PCA); NHLBI K23 to RK (K23HL093023). RK and EGB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. None of the authors have a conflict of interest pertaining to this work.
Abbreviations used
- CI
confidence interval
- GALA II
Genes-environments & Admixture in Latino Asthmatics study
- IgE
immunoglobulin E
- IQR
interquartile range
- tIgE
total IgE
- U.S.
United States
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choudhry S, Seibold MA, Borrell LN, Tang H, Serebrisky D, Chapela R, et al. Dissecting complex diseases in complex populations: asthma in latino americans. Proc Am Thorac Soc. 2007;4:226–233. doi: 10.1513/pats.200701-029AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117:43–53. doi: 10.1542/peds.2004-1714. [DOI] [PubMed] [Google Scholar]
- 3.Homa DM, Mannino DM, Lara M. Asthma mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban heritage, 1990–1995. Am J Respir Crit Care Med. 2000;161:504–509. doi: 10.1164/ajrccm.161.2.9906025. [DOI] [PubMed] [Google Scholar]
- 4.Brugge D, Lee AC, Woodin M, Rioux C. Native and foreign born as predictors of pediatric asthma in an Asian immigrant population: a cross sectional survey. Environ Health. 2007;6:13. doi: 10.1186/1476-069X-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brugge D, Woodin M, Schuch TJ, Salas FL, Bennett A, Osgood ND. Community-level data suggest that asthma 462 prevalence varies between U.S. and foreign-born black subpopulations. J Asthma. 2008;45:785–789. doi: 10.1080/02770900802179957. [DOI] [PubMed] [Google Scholar]
- 6.Leung RC, Carlin JB, Burdon JG, Czarny D. Asthma, allergy and atopy in Asian immigrants in Melbourne. Med J Aust. 1994;161:418–425. doi: 10.5694/j.1326-5377.1994.tb127522.x. [DOI] [PubMed] [Google Scholar]
- 7.Hjern A, Rasmussen F, Hedlin G. Age at adoption, ethnicity and atopic disorder: a study of internationally adopted young men in Sweden. Pediatric Allergy & Immunology. 1999;10:101–106. doi: 10.1034/j.1399-3038.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 8.Eldeirawi KM, Persky VW. Associations of physician-diagnosed asthma with country of residence in the first year of life and other immigration-related factors: Chicago asthma school study. Annals of Allergy, Asthma, & Immunology. 2007;99:236–243. doi: 10.1016/S1081-1206(10)60659-X. [DOI] [PubMed] [Google Scholar]
- 9.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32:43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]
- 10.Hijazi N, Abalkhail B, Seaton A. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax. 2000;55:775–779. doi: 10.1136/thorax.55.9.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20:682–687. doi: 10.1097/MOP.0b013e3283154f26. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Murray CS, Woodcock A, Simpson A, Custovic A. Dietary antioxidant intake, allergic sensitization and allergic diseases in young children. Allergy. 2009;64:1766–1772. doi: 10.1111/j.1398-9995.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 13.Celedon JC, Sredl D, Weiss ST, Pisarski M, Wakefield D, Cloutier M. Ethnicity and skin test reactivity to aeroallergens among asthmatic children in Connecticut. Chest. 2004;125:85–92. doi: 10.1378/chest.125.1.85. [DOI] [PubMed] [Google Scholar]
- 14.Litonjua AA, Celedon JC, Hausmann J, Nikolov M, Sredl D, Ryan L, et al. Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol. 2005;115:751–757. doi: 10.1016/j.jaci.2004.12.1138. [DOI] [PubMed] [Google Scholar]
- 15.Vergara C, Caraballo L, Mercado D, Jimenez S, Rojas W, Rafaels N, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet. 2009;125:565–579. doi: 10.1007/s00439-009-0649-2. [DOI] [PubMed] [Google Scholar]
- 16.Flores C, Ma SF, Pino-Yanes M, Wade MS, Perez-Mendez L, Kittles RA, et al. African ancestry is associated with asthma risk in African Americans. PLoS One. 2012;7:e26807. doi: 10.1371/journal.pone.0026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scirica CV, Gold DR, Ryan L, Abulkerim H, Celedon JC, Platts-Mills TA, et al. Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol. 2007;119:81–88. doi: 10.1016/j.jaci.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Platts-Mills TA, Satinover SM, Naccara L, Litonjua AA, Phipatanakul W, Carter MC, et al. Prevalence and titer of IgE antibodies to mouse allergens. J Allergy Clin Immunol. 2007;120:1058–1064. doi: 10.1016/j.jaci.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Basehore MJ, Howard TD, Lange LA, Moore WC, Hawkins GA, Marshik PL, et al. A comprehensive evaluation of IL4 variants in ethnically diverse populations: association of total serum IgE levels and asthma in white subjects. J Allergy Clin Immunol. 2004;114:80–87. doi: 10.1016/j.jaci.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Lester LA, Rich SS, Blumenthal MN, Togias A, Murphy S, Malveaux F, et al. Ethnic differences in asthma and associated phenotypes: collaborative study on the genetics of asthma. J Allergy Clin Immunol. 2001;108:357–362. doi: 10.1067/mai.2001.117796. [DOI] [PubMed] [Google Scholar]
- 21.Chew GL, Reardon AM, Correa JC, Young M, Acosta L, Mellins R, et al. Mite sensitization among Latina women in New York, where dust-mite allergen levels are typically low. Indoor Air. 2009;19:193–197. doi: 10.1111/j.1600-0668.2008.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons TW, Wakefield DB, Cloutier MM. Mold and Alternaria skin test reactivity and asthma in children in Connecticut. Annals of Allergy, Asthma, & Immunology. 2011;106:301–307. doi: 10.1016/j.anai.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ly NP, Soto-Quiros ME, Avila L, Hunninghake GM, Raby BA, Laskey D, et al. Paternal asthma, mold exposure, and increased airway responsiveness among children with asthma in Costa Rica. Chest. 2008;133:107–114. doi: 10.1378/chest.07-2130. [DOI] [PubMed] [Google Scholar]
- 24.Hunninghake GM, Lasky-Su J, Soto-Quiros ME, Avila L, Liang C, Lake SL, et al. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177:830–836. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galanter JM, Torgerson D, Gignoux CR, Sen S, Roth LA, Via M, et al. Cosmopolitan and ethnic-specific replication of genetic risk factors for asthma in 2 Latino populations. J Allergy Clin Immunol. 2011;128:37–43. doi: 10.1016/j.jaci.2011.03.050. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. http://www.census.gov/.] Available from http://www.census.gov/popest/national/asrh/NC-EST2008/NC-EST2008-03.xls.
- 27.Salari K, Choudhry S, Tang H, Naqvi M, Lind D, Avila PC, et al. Genetic admixture and asthma-related phenotypes in Mexican American and Puerto Rican asthmatics. Genet Epidemiol. 2005;29:76–86. doi: 10.1002/gepi.20079. [DOI] [PubMed] [Google Scholar]
- 28.Salari K, Burchard EG. Latino populations: a unique opportunity for epidemiological research of asthma. Paediatr Perinat Epidemiol. 2007;21(Suppl 3):15–22. doi: 10.1111/j.1365-3016.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 29.Martin MA, Shalowitz MU, Mijanovich T, Clark-Kauffman E, Perez E, Berry CA. The effects of acculturation on asthma burden in a community sample of Mexican American schoolchildren. Am J Public Health. 2007;97:1290–1296. doi: 10.2105/AJPH.2006.092239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan LK, Sobal J, Martorell R. Acculturation, socioeconomic status, and obesity in Mexican Americans, Cuban Americans, and Puerto Ricans. Int J Obes Relat Metab Disord. 1997;21:91–96. doi: 10.1038/sj.ijo.0800367. [DOI] [PubMed] [Google Scholar]
- 31.Foster BA, Read D, Bethell C. An analysis of the association between parental acculturation and children's medication use. Pediatrics. 2009;124:1152–1161. doi: 10.1542/peds.2008-2746. [DOI] [PubMed] [Google Scholar]
- 32.Deyo RA, Diehl AK, Hazuda H, Stern MP. A simple language-based acculturation scale for Mexican Americans: validation and application to health care research. Am J Public Health. 1985;75:51–55. doi: 10.2105/ajph.75.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann TJ, Zhan Y, Kvale MN, Hesselson SE, Gollub J, Iribarren C, et al. Design and coverage of high throughput genotyping arrays optimized for individuals of East Asian, African American, and Latino race/ethnicity using imputation and a novel hybrid SNP selection algorithm. Genomics. 2011;98:422–430. doi: 10.1016/j.ygeno.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson CC, Peterson EL, Ownby DR. Gender differences in total and allergen-specific immunoglobulin E (IgE) concentrations in a population-based cohort from birth to age four years. Am J Epidemiol. 1998;147:1145–1152. doi: 10.1093/oxfordjournals.aje.a009413. [DOI] [PubMed] [Google Scholar]
- 36.Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy. 2000;55:240–245. doi: 10.1034/j.1398-9995.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 37.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 38.McKeever TM, Lewis SA, Smith C, Collins J, Heatlie H, Frischer M, et al. Siblings, multiple births, and the incidence of allergic disease: a birth cohort study using the West Midlands general practice research database. Thorax. 2001;56:758–762. doi: 10.1136/thorax.56.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sears MR, Greene JM, Willan AR, Taylor DR, Flannery EM, Cowan JO, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360:901–907. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 40.Kerkhof M, Wijga A, Smit HA, de Jongste JC, Aalberse RC, Brunekreef B, et al. The effect of prenatal exposure on total IgE at birth and sensitization at twelve months and four years of age: The PIAMA study. Pediatr Allergy Immunol. 2005;16:10–18. doi: 10.1111/j.1399-3038.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- 41.Hagendorens MM, Bridts CH, Lauwers K, van Nuijs S, Ebo DG, Vellinga A, et al. Perinatal risk factors for sensitization, atopic dermatitis and wheezing during the first year of life (PIPO study) Clin Exp Allergy. 2005;35:733–740. doi: 10.1111/j.1365-2222.2005.02254.x. [DOI] [PubMed] [Google Scholar]
- 42.Campo P, Kalra HK, Levin L, Reponen T, Olds R, Lummus ZL, et al. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy Clin Immunol. 2006;118:1271–1278. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gern JE, Reardon CL, Hoffjan S, Nicolae D, Li Z, Roberg KA, et al. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol. 2004;113:307–314. doi: 10.1016/j.jaci.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. Jama. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 45.Celedon JC, Litonjua AA, Weiss ST, Gold DR. Day care attendance in the first year of life and illnesses of the upper and lower respiratory tract in children with a familial history of atopy. Pediatrics. 1999;104:495–500. doi: 10.1542/peds.104.3.495. [DOI] [PubMed] [Google Scholar]
- 46.Nicolaou NC, Simpson A, Lowe LA, Murray CS, Woodcock A, Custovic A. Day-care attendance, position in sibship, and early childhood wheezing: a population-based birth cohort study. J Allergy Clin Immunol. 2008;122:500–506. doi: 10.1016/j.jaci.2008.06.033. e5. [DOI] [PubMed] [Google Scholar]
- 47.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–420. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryc K, Velez C, Karafet T, Moreno-Estrada A, Reynolds A, Auton A, et al. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8954–861. doi: 10.1073/pnas.0914618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James J Yang EGB, Shweta Choudhry, Christine C Johnson, Dennis ROwnby, David Favro, Justin Chen, Matthew Akana, Connie Ha, Pui-Yan Kwok, Richard Krajenta, Suzanne L Havstad, Christine L Joseph, Max A Seibold, Mark D Shriver, L Keoki Williams. Differences in allergic sensitization by self-reported race and genetic ancestry. The Journal of Allergy & Clinical Immunology. 2008;122:820–827. doi: 10.1016/j.jaci.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternthal MJ, Coull BA, Chiu YH, Cohen S, Wright RJ. Associations among maternal childhood socioeconomic status, cord blood IgE levels, and repeated wheeze in urban children. J Allergy Clin Immunol. 2011;128:337–345. doi: 10.1016/j.jaci.2011.05.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braback L, Vogt H, Hjern A. Migration and asthma medication in international adoptees and immigrant families in Sweden. Clinical & Experimental Allergy. 2011;41:1108–1115. doi: 10.1111/j.1365-2222.2011.03744.x. [DOI] [PubMed] [Google Scholar]
- 52.Leung R, Ho P. Asthma, allergy, and atopy in three south-east Asian populations. Thorax. 1994;49:1205–1210. doi: 10.1136/thx.49.12.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Mutius E, Martinez FD, Fritzsch C, Nicolai T, Roell G, Thiemann HH. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med. 1994;149:358–364. doi: 10.1164/ajrccm.149.2.8306030. [DOI] [PubMed] [Google Scholar]
- 54.Matricardi PM. Prevalence of atopy and asthma in eastern versus western Europe: why the difference? Ann Allergy Asthma Immunol. 2001;87:24–27. doi: 10.1016/s1081-1206(10)62336-8. [DOI] [PubMed] [Google Scholar]
- 55.Thornton CA, Macfarlane TV, Holt PG. The hygiene hypothesis revisited: role of materno-fetal interactions. Curr Allergy Asthma Rep. 2010;10:444–452. doi: 10.1007/s11882-010-0148-5. [DOI] [PubMed] [Google Scholar]
- 56.Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646–652. doi: 10.1016/j.jaci.2011.04.060. e1–5. [DOI] [PubMed] [Google Scholar]
- 57.Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatr Perinat Epidemiol. 2007;21(Suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 58.Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113:1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 59.Borrell LN, Kiefe CI, Williams DR, Diez-Roux AV, Gordon-Larsen P. Self-reported health, perceived racial discrimination, and skin color in African Americans in the CARDIA study. Soc Sci Med. 2006;63:1415–1427. doi: 10.1016/j.socscimed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 61.Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J Allergy Clin Immunol. 2001;107:41–47. doi: 10.1067/mai.2001.111143. [DOI] [PubMed] [Google Scholar]
- 63.Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Curtin-Brosnan J, et al. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. J Allergy Clin Immunol. 2003;112:87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- 64.Bolte G, Bischof W, Borte M, Lehmann I, Wichmann HE, Heinrich J. Early endotoxin exposure and atopy development in infants: results of a birth cohort study. Clin Exp Allergy. 2003;33:770–776. doi: 10.1046/j.1365-2222.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- 65.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 66.Celedon JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TA, et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007;120:144–149. doi: 10.1016/j.jaci.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gehring U, Bischof W, Fahlbusch B, Wichmann HE, Heinrich J. House dust endotoxin and allergic sensitization in children. Am J Respir Crit Care Med. 2002;166:939–944. doi: 10.1164/rccm.200203-256OC. [DOI] [PubMed] [Google Scholar]