Abstract
Proteoglycans are a component of the extracellular matrix and are critical for cellular and tissue function. Mutations in proteoglycan components and enzymes involved in proteoglycan synthesis have been implicated in several growth disorders, with common features including short stature and skeletal dysplasia. For example, mutations in B4GALT7, a gene whose protein product catalyzes proteoglycan synthesis, have been associated with the rare progeroid variant of Ehlers-Danlos syndrome. Here, we conducted exome sequencing in a patient with a previously undiagnosed growth disorder and identified compound heterozygous mutations in B4GALT7. This patient is just the fourth individual with genetically confirmed progeroid variant of Ehlers-Danlos syndrome. The mutations include a previously characterized c.808C>T p.Arg270Cys substitution, and a novel c.122T>C p.Leu41Pro substitution. We demonstrate that the novel mutation caused decreased levels of the enzyme, supporting the pathogenicity of the mutation. Our report identifies a novel mutation in B4GALT7 causing the progeroid variant of Ehlers-Danlos syndrome and contributes an extensive phenotypic characterization of a patient with the syndrome. We also reviewed the previous literature in addition to the present patient, and conclude that the key features associated with B4GALT7 deficiency are short stature, developmental anomalies of the forearm bones and elbow, and bowing of the extremities, in addition to the classic features of Ehlers-Danlos syndrome. This report helps define the phenotype of the progeroid variant of Ehlers-Danlos syndrome and furthers our understanding of the effect of proteoglycan defects in growth disorders.
Keywords: proteoglycans, growth disorder, Ehlers-Danlos syndrome, whole exome sequencing
INTRODUCTION
Proteoglycans are a component of the extracellular matrix and are important for connective tissue structure and function. Proteoglycans help mediate processes such as cell proliferation, differentiation, and migration, and participate in signal transduction [Bishop et al., 2007; Hacker et al., 2005]. Proteoglycans are comprised of a core protein and one or more attached glycosylaminoglycans. Glycosylaminoglycans (GAGs) are long polysaccharides consisting of repeating disaccharide units and are highly hydrophilic to help impart volume and elasticity to tissues [Prydz and Dalen, 2000]. All GAGs, with the exception of keratan sulfate and hyaluronan, are connected to the serine residue of a core protein through a tetrasaccharide linkage region. This tetrasaccharide linkage, an O-linked glycosylation, is formed by the serial addition of one xylose, two galactoses, and one glucuronic acid. The various steps involved in synthesizing proteoglycans are catalyzed by a host of enzymes and occurs primarily within the secretory pathway [Wopereis et al., 2006; Prydz and Dalen, 2000]. A variety of congenital disorders have been associated with mutations in genes encoding these enzymes [Freeze, 2006].
Mutations in B4GALT7, a gene that encodes an enzyme utilized in one step of the formation of the tetrasaccharide linkage in proteoglycan synthesis, have been reported to cause the progeroid variant of Ehlers-Danlos syndrome (EDS) [Almeida et al., 1999; Okajima et al., 1999; Seidler et al., 2006]. As a result of these mutations, patients have defective synthesis of proteoglycans that contain heparan, dermatan, and chondroitin sulfate [Seidler et al., 2006; Gotte et al., 2008]. There are just eight reported patients with the progeroid variant of EDS in the medical literature, only three of which have supporting genetic diagnoses [Hernandez et al., 1979; Hernandez et al., 1981; Hernandez et al., 1986; Faiyaz-Ul-Haque et al., 2004; Kresse et al., 1987]. Similar to other more common forms of EDS, patients with the progeroid variant present with features including joint laxity, skin hyperextensibility, and poor wound healing. In contrast, the patients with the progeroid variant have additional features including congenital joint dislocations and short stature.
We conducted exome sequencing in a child with severe short stature and a skeletal dysplasia who despite an extensive evaluation had no clinical diagnosis. In this patient, exome sequencing allowed us to identify compound heterozygous mutations in the B4GALT7 gene, providing a genetic diagnosis of the progeroid variant of EDS. We also conducted functional experiments to demonstrate the pathogenicity of a novel mutation in the gene. This study highlights the utility of whole exome sequencing to identify rare genetic disorders and further delineates the clinical features of the extremely rare progeroid variant of EDS.
METHODS
Approval
This study was approved by the Institutional Review Board of Boston Children’s Hospital. All participants or their legal guardians provided written informed consent.
Sequencing
Exome sequencing was conducted on the patient, his unaffected brother, and both parents as previously described [Dauber et al., 2013]. Causal variants in B4GALT7 were confirmed by Sanger sequencing. Additional details are provided in the Supplementary Methods.
Plasmid Generation and Cell Culture
We generated C-terminal FLAG-tagged plasmids with wild type B4GALT7 and with the patient’s mutations. The plasmids were transiently transfected into HEK-293 cells and whole cell lysates were collected (see Supplementary Methods in supporting information online).
Western Blotting
Twenty µg of protein were loaded into a 4–15% gradient precast polyacrylamide gel (Bio-Rad, Hercules, CA). Protein was then transferred to a PVDF membrane and blocked for 2 hours with 5% milk suspended in PBS-T buffer (Phosphate Buffered Saline with 0.1% Tween 20). The membrane was then blotted overnight at 4 °C with primary mouse antibodies against FLAG (1:10,000 dilution; Sigma-Aldrich) or β-actin (1:5,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). It was then washed three times with PBS-T and incubated with HRP-linked anti-mouse IgG (1:1,000 dilution; Cell Signaling Technology, Danvers, MA) for 1 hour. The membrane was again washed three times with PBS-T and signal detected with SuperSignal Luminol/Enhancer Solution (Thermo Fisher Scientific, Waltham, MA).
RT-PCR
RNA was collected from transiently transfected cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany). cDNA was then synthesized using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) using random hexamers according to manufacturer’s instructions. PCR was then performed from cDNA using the HotStarTaq PCR kit (Qiagen) according to manufacturer’s instructions. A forward primer (5’-AACAGCACGGACTACATTGCC-3’) annealing to the coding sequence of B4GALT7 and a reverse primer (5’-TCGTCGTCATCCTTGTAATCGCTG-3’) annealing to the FLAG tag were used for amplification. The PCR product was then electrophoresed on a 1% agarose gel.
RESULTS
Clinical Report
The patient was the 2977g., 45.7 cm (AGA) product of a 40.5 week gestation to a 37-year-old mother and 38-year-old father. At 4 months, the infant was seen in a genetics clinic due to concern about bowing of the forearms and marked joint flexibility. Throughout childhood, the patient exhibited slow growth in height and weight, remaining below the 5th centile in both. At age 4 years, a random growth hormone (GH) level was 2.85 ng/mL, and a GH stimulation test at age 5 years showed a peak response of 8.96 ng/ml. Based on this moderate response to GH stimulation, the patient was initiated on recombinant GH therapy from age 5.5 years to age 10 years. There was no convincing evidence of a significant change in growth velocity after initiation of GH. Thyroid hormone, IGF-1, and IGFBP-3 levels were all normal.
The remainder of the medical history was significant for one hospitalization at 3 months for bronchiolitis and surgical resection of the levator palpabrae for correction of congenital unilateral ptosis. He also had severe hyperopia with astigmatism and intermittent exotropia. The patient did not report ease of bruising or scarring, but did have a wound that showed difficulty healing. He also reported skin hyperextensibility. He had no history of fractures or sprains. He reported pain upon contact to the elbows and pain upon knee hyperextension. The patient reached all developmental milestones and was socially well adjusted. He did have a mild learning disability, with delayed reading ability. The father was 184 cm in height (+1.07 SD), and the mother was 163 cm (−0.12 SD). There was no significant family history.
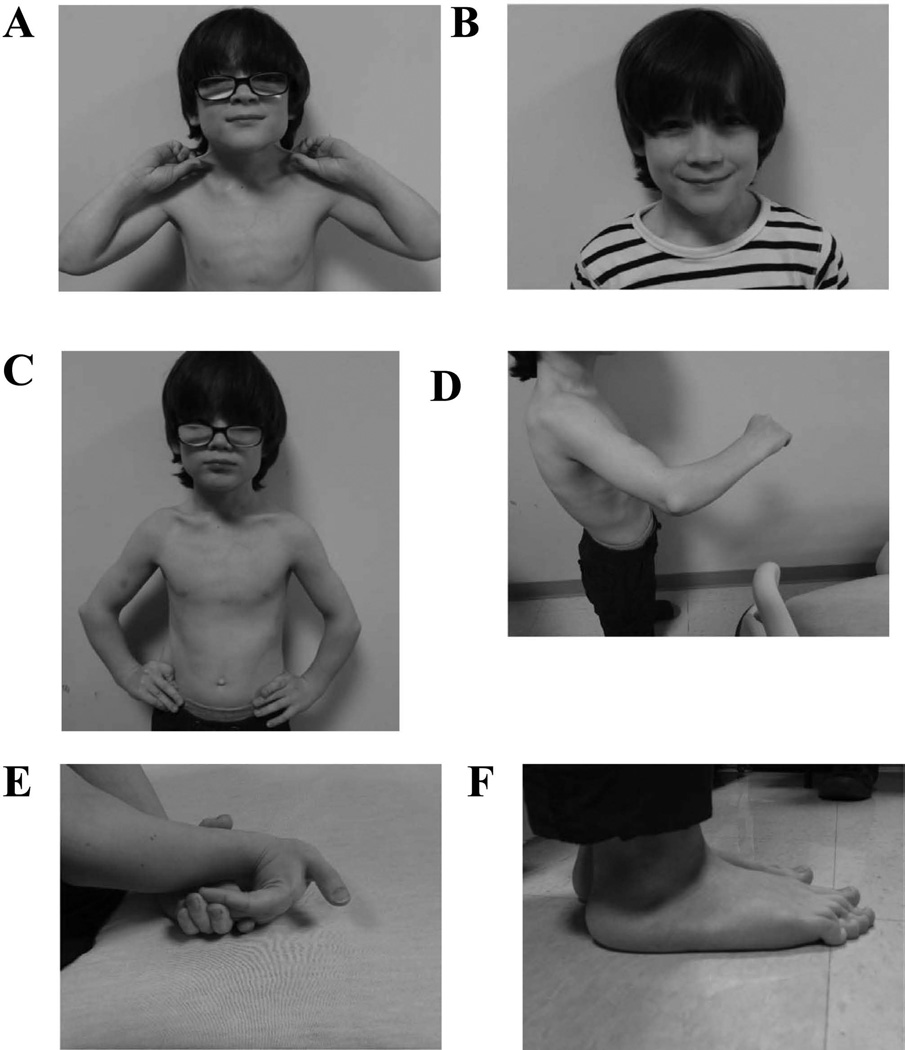
Physical examination at 10 years of age showed height of 116.4 cm (−3.5 SD), weight of 25.4 kg (~10th centile), and a head circumference of 55 cm (94th centile). The short stature was proportionate. His skin was soft, velvety, hyperextensible, and without wrinkles (Fig. 1A). There was a wide scar on his left forehead only. There was a prominent venous pattern on his chest. His hair had normal texture and distribution. He had a prominent forehead and his face was triangular with a pointed chin (Fig. 1B). He had short ears (measuring about −2 SD), a smooth philtrum and a normal palate. There was a mild pectus carinatum (Fig. 1C). His genitalia were consistent with a normal pre-pubertal male with descended testes. There was no scoliosis, kyphosis, or lordosis. There was slight ulnar bowing of his forearm (Fig. 1D). There were bilateral elbow contractures with decreased supination. There was hyperextension of the fingers, knees, shoulders, and wrists (Fig. 1E). There was varus bowing of the lower legs, marked pes planus, and long toes (Fig. 1F). Neurologic examination was notable for mild hypotonia. The remainder of the physical exam was unremarkable.
Figure 1.
Photographs taken at 10 years of age demonstrating various clinical features of the patient. A) Marked skin hyperextensibility. B) Normal facial features. C) Mild pectus carinatum. D) Ulnar bowing of forearms. E) Hyperflexible joints. F) Pes planus. Color figures can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-4833.
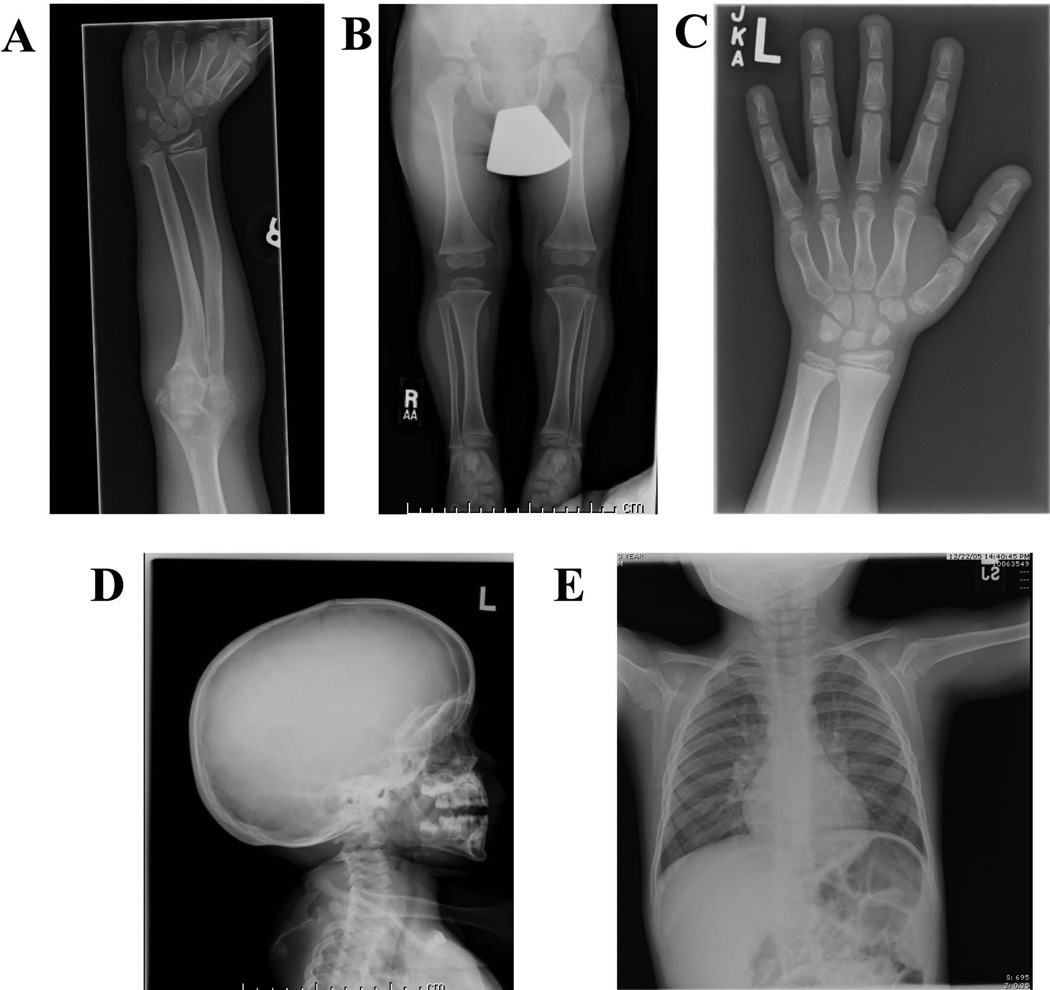
Review of the imaging showed bilateral posterior subluxation of the proximal radial head with shortening of the ulna and modest bowing of the radial shaft (Fig. 2A). The lower leg was slightly shortened with prominent flaring of the tibial metaphyses (Fig. 2B), as well as less prominent bowing of the lower leg. Hand radiographs demonstrated a bulbous appearance to the distal phalangeal tufts (Fig. 2C). A radiograph of the head highlighted a modest degree of dolichocephaly (Fig. 2D). The patient did not have prominently shortened or dysmorphic clavicles (Fig. 2E). No other marked skeletal abnormalities were noted on radiograph, and there was no remarkable osteopenia evident on the images.
Figure 2.
Radiographs highlighting various skeletal features in the patient. Radiographs were taken from various skeletal surveys conducted at different ages. A) Ulnar bowing of radial shaft and radioulnar synostoses (9 years old). B) Flaring of proximal tibial metaphyses (3 years old). C) Bulbing of phalangeal tufts (8 years old). D) Dolichocephaly (3 years old). D) Chest x-ray showing normal clavicles (3 years old).
Based on these clinical features, the patient was suspected to have a syndromic form of dyschondrosteosis, but clinical SHOX gene mutation testing was negative. Notably, EDS was also considered, and clinical collagen testing was conducted (University of Washington, Seattle, WA) on the patient’s fibroblasts for EDS Type VII. Testing showed that the patient had slightly decreased procollagen type 1, but within normal limits. Testing for mutations in the RMRP gene for cartilage-hair hypoplasia was also negative. Thus, despite numerous laboratory tests and extensive clinical evaluations, no unifying genetic diagnosis was made.
Sequencing Results
The patient, his parents, and unaffected sibling underwent exome sequencing with the goal of uncovering a unifying genetic diagnosis. Following sequencing, we applied a variety of filters as described in Supplementary Methods to generate a list of candidate variants (Supplementary Table I in supporting information online) and searched in the OMIM database and PubMed for known disease associations with our candidate genes. Considering the patient’s clinical features and this list of candidate genes, we made a presumptive genetic diagnosis of progeroid variant of EDS due to deficiency of the B4GALT7 gene (encoding β1–4-galactosyltransferase 7).
Mutation Analysis
The patient was compound heterozygous for mutations in B4GALT7. The patient had a c.122T>C transversion, which predicts p.Leu41Pro). The patient also had a c.808C>T (rs28937869) variant, which predicts(p.Arg270Cys), which has been reported as a pathogenic variant [Rahuel-Clermont et al., 2010; Gotte et al., 2008]. The father carried the p.Leu41Pro variant, while the mother carried the p.Arg270Cys variant. The unaffected sibling had neither mutation. These results were confirmed by Sanger sequencing (Supplementary Fig. 2 in supporting information online).
Protein Expression of β1–4-galactosyltransferase 7
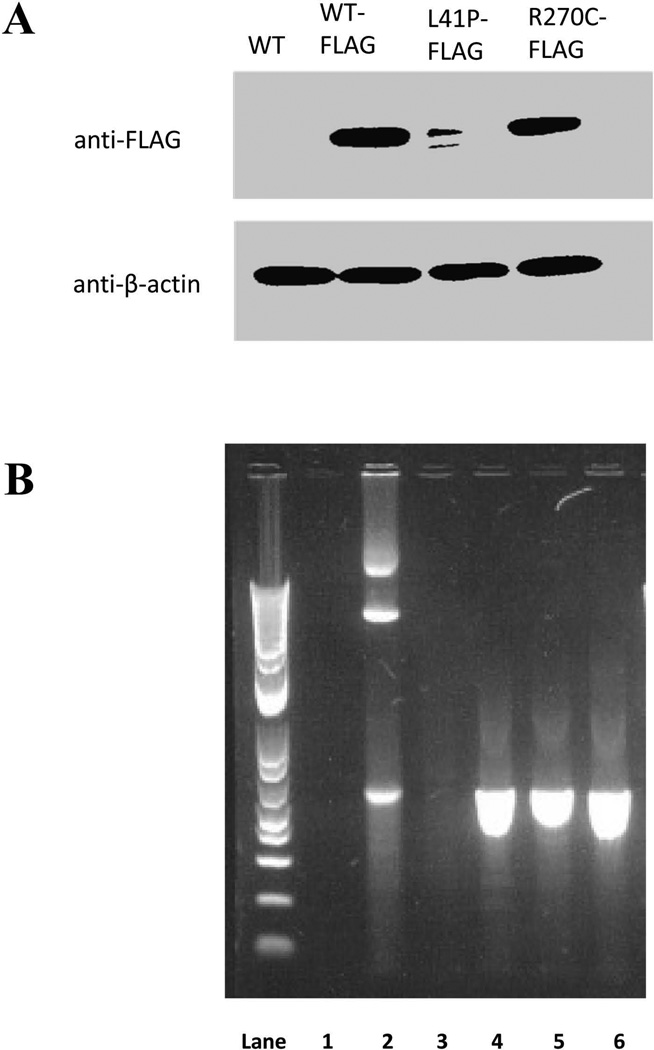
FLAG-tagged wild type, L41P, and R270C B4GALT7 constructs were cloned into a eukaryotic expression vector and transiently transfected into HEK-293 cells. Western blotting of whole cell lysates demonstrated decreased protein levels of B4GALT7-L41P-FLAG as compared to B4GALT7-WT-FLAG or B4GALT7-R270C-FLAG (Fig. 3A). This suggested degradation of the p.Leu41Pro mutant. It was not clear why there is an additional band in the L41P-FLAG lane, but it likely represented a partial degradation product. RT-PCR of RNA isolated from transfected cells was used to confirm transcription of the plasmid-encoded gene (Fig. 3B).
Figure 3.
Functional analysis of patient’s mutations. Wild type and mutant FLAG-tagged constructs were transiently transfected into HEK-293 cells. A) Protein levels as assessed by western blotting against the FLAG tag. The approximate size of the bands is 37 kDa. A wild type construct with no FLAG tag was used as a negative control, and β-actin levels were used as a loading control. B) As a control, RT-PCR was used to confirm transcription of plasmid-encoded B4GALT7 sequence in the transfected cell lines. Lane 1 is a RT-PCR off of RNA isolated from untransfected HEK-293 cells. Lane 2 is a PCR done directly from a WT-FLAG plasmid, performed as a positive control. Lanes 3, 4, 5, and 6 are RT-PCRs from RNA isolated from cells transfected with WT, WT-FLAG, L41P-FLAG, and R270C-FLAG plasmids, respectively.
Expression of B4galt7 at Rat Growth Plate
Unlike other forms of EDS, the progeroid variant was associated with short stature. We examined the expression of B4galt7 mRNA in the growth plate of rodents, experiments that were performed as part of a recently published paper [Lui et al., 2012]. Microarray analysis showed that B4galt7 was highly expressed in the mouse growth plate as compared to the mouse heart (4.5 fold increase, p-value 1.3×10−11), kidney (5.0 fold increase, p-value 5.0×10−12), or lungs (3.8 fold increase, p-value 8.5×10−11). Furthermore, B4galt7 was found to have a significant decrease in expression between the proliferative and hypertrophic zones of the rat growth plate (−1.8 fold decrease, p-value 0.0011). The differential expression of the gene in rodent growth plates suggested a possible connection between B4galt7 and endochondral ossification.
DISCUSSION
We report on the fourth patient with genetically confirmed progeroid variant of EDS in a boy with compound heterozygous mutations in B4GALT7. B4GALT7 encodes a 327 amino acid protein that catalyzes the addition of a galactose moiety onto a xylosyl group in the tetrasaccharide linker of proteoglycans. β1–4-galactosyltransferase 7 is normally localized to the Golgi membrane, where it performs its catalytic function [Wopereis et al., 2006; Prydz and Dalen, 2000]. The p.Arg270Cys mutation has been reported in two related patients [Faiyaz-Ul-Haque et al., 2004]. Numerous biochemical studies have shown that the mutation results in loss of catalytic activity [Gotte et al., 2008; Rahuel-Clermont et al., 2010]. The p.Leu41Pro mutation has not been reported in the literature.
PolyPhen2 [Adzhubei et al., 2010] analysis of the p.Leu41Pro mutation was predicted to be probably damaging. In agreement with previous studies suggesting loss of catalytic activity [Gotte et al., 2008; Rahuel-Clermont et al., 2010], the p.Arg270Cys mutation was also predicted to be probably damaging. Both residues were conserved across many species (Supplementary Fig. 3A in supporting information online), which further suggests that the patient’s mutations would result in loss of protein function.
Sequence annotation from the UniProt database (www.uniprot.org) predicted that β1–4-galactosyltransferase 7 (UniProt ID: Q9UBV7) is a Type II transmembrane protein, with residues 1–30 in the cytoplasm, 31–51 as a helical transmembrane domain, and 52–327 as a Golgi lumenal domain (Supplementary Fig. 3B in supporting information online). The location of the arginine 270 in the Golgi lumen is consistent with previous evidence that p.Arg270Cys substitution ablates catalytic function [Gotte et al., 2008; Rahuel-Clermont et al., 2010]. The predicted p.Ala186Asp and p.Leu206Pro mutations, both demonstrated to result in loss of catalytic function, were similarly predicted to reside in the lumenal domain [Okajima et al., 1999; Rahuel-Clermont et al., 2010; Gotte and Kresse, 2005]. Based on the transmembrane location of leucine 41, we hypothesized that that the novel p.Leu41Pro mutation would disrupt alpha helical structure and diminish the ability for the protein to localize to the Golgi membrane. Accordingly, we provided functional evidence that a novel p.Leu41Pro substitution in B4GALT7 results in decreased levels of the protein. We also found that the gene is differentially expressed in rodent growth plates, which suggests a role for B4GALT7 in endochondral ossification and a possible connection with the short stature seen in the patients. However, while expression of the gene in the growth plate is consistent with a role in bone development and height, it does not definitively prove causality as there are many genes expressed in the growth plate that do not regulate height. Additionally, B4GALT7 is highly expressed in many other tissues suggesting additional functions.
Comparison with Previous Reports of Progeroid Variant of EDS
There have been eight patients with the progeroid variant of EDS reported in the medical literature. The first five patients were described in three separate reports [Hernandez et al., 1979; Hernandez et al., 1981; Hernandez et al., 1986]. The authors labeled this novel disorder as a progeroid variant of EDS based on the aged facial features of these patients in conjunction with their skin hyperextensibility and joint laxity. Notably, these five patients did not undergo sequencing, so it is unknown if they had mutations in B4GALT7. A separate team later described a Danish boy with compound heterozygous mutations in B4GALT7 predicting p.Ala186Asp and p.Leu206Pro substitutions [Kresse et al., 1987; Almeida et al., 1999]. The authors assigned a diagnosis of progeroid variant of EDS based on similarities in the subject’s clinical features with the previous five patients and connected the syndrome with mutations in B4GALT7. Later, two patients from a large family from Qatar were also found to have the syndrome, and both had homozygous mutations resulting in p.Arg270Cys substitutions [Faiyaz-Ul-Haque et al., 2004].
Since there is no genetic evidence in the first five patients described by Hernandez et al. [1979; 1981; 1986], we cannot be certain that they have the same disorder as the Danish boy [Kresse et al., 1987] and the two Qatari patients [Faiyaz-Ul-Haque et al., 2004], who all had a genetic diagnosis of B4GALT7 mutations. Thus, we compared clinical features observed in these eight previously reported patients, along with the current patient (Table I). Short stature, radioulnar synostoses, and bowing of extremities appear to be consistent features in patients with demonstrated B4GALT7 mutations. The radial head subluxation falls along a clinical spectrum that ranges from radial head subluxation to dislocation to more severe radioulnar synostoses that are seen in a variety of congenital conditions [Elliott et al., 2010]. We note that some features such as cardiovascular abnormalities and nevi are present in the five patients reported by Hernandez et al. [1979; 1981; 1986] but are absent in the patients with demonstrated B4GALT7 mutations. Importantly, cognitive disability, developmental delay, and progeroid facial features were much more prominent features and more consistently observed in the five patients of Hernandez et al. [1979; 1981; 1986] than in the four patients with proven mutations in B4GALT7. The progeroid facies were quite mild in the Danish boy [Kresse et al., 1987] and not present in the two patients from Qatar [Faiyaz-Ul-Haque et al., 2004]. One of the authors of the present paper (DWB) had the opportunity to personally examine the two patients from Qatar (currently ages 15 and 43 years) and it is clear that these patients were not have progeroid features, confirming that they did not develop a progeroid facies over time. Thus, progeroid facial features do not appear to be strongly associated with mutations in B4GALT7.
Table I.
Comparison of clinical features with all eight previously reported patients. Five patients were originally reported by Hernandez et al. [1986; 1981; 1979], but these patients did not have a genetic diagnosis. A Danish boy reported by Kresse et al. [1987] and two individuals from a Qatari family reported by Faiyaz-Ul-Haque et al. [2004] all had genetic diagnoses of B4GALT7 mutations.
| Five patients reported by Hernandez et al. [1979; 1981; 1986] |
Patient from Kresse et al. [1987] |
Patient 1 from Faiyaz-Ul-Haque et al. [2004] |
Patient 2 from Faiyaz-Ul-Haque et al. [2004] |
Present patient | |
|---|---|---|---|---|---|
| Short Stature | + (4/5) | + | + | + | + |
| Radioulnar Synostoses | −* | + | + | + | + |
| Bowed extremities | −* | + | + | + | + |
| Joint Hypermobility | + (5/5) | + | + | + | + |
| Skin Hyperextensibility | + (5/5) | + | + | + | + |
| Delayed Wound Healing | + (5/5) | + | N/R | + | + |
| Pes Planus | + (5/5) | + | N/R | equinovarus | + |
| Nevi | + (5/5) | N/R | N/R | N/R | − |
| Cardiovascular abnormalities | + (1/5) | N/R | N/R | N/R | − |
| Other Findings | |||||
| Developmental Delay | + (5/5) | + | + | + | Mild learning disability |
| Decreased Bone Density | N/R | + | + | N/R | − |
| Facial Features | Progeroid (5/5) | Mildly progeroid; narrow and short face, proptosis, narrow mouth | Narrow and short face, midface hypoplasia, narrow mouth | Narrow and short face, midface hypoplasia, narrow mouth | Normal facial features |
| Dental Abnormalities | + (5/5) | + | + | N/R | − |
| Ophthalmologic Abnormalities | N/R | − | mild esotropia and mild hyperopia | N/R | severe hyperopia, congenital ptosis, intermittent exotropia |
| Hypotonia | N/R | + | + | + | + |
| Broad fingertips | N/R | N/R | + | N/R | + |
A “+” indicates that the feature was reported in that patient, or the presence could be inferred from the patient description. A “−” indicates the absence of that feature, or the absence could be inferred from the patient description. For the five patients from Hernandez et al. the numbers inside the parentheses indicate how many out of the five patients exhibited a given feature. “NR” indicates that the presence or absence of a feature was not reported and could not be confidently inferred from the patient description.
Although the five patients described by Hernandez et al. [1979; 1981; 1986] did not specifically report on the presence or absence of radioulnar synostoses or bowed extremities, these patients did have skeletal surveys and these abnormalities were not reported. We therefore assumed that these features were not present.
Based on these differences, we suggest that the five patients reported by Hernandez et al. [1979; 1981; 1986] might not actually be syndromes resulting from B4GALT7 deficiency, but might represent a different genetic disorder. Nonetheless, it is possible that the original patients reported by Hernandez et al. [1979; 1981; 1986] also have causative mutations in B4GALT7 and represent part of a wider phenotypic spectrum resulting from mutations in the gene. Since we do not have a genetic diagnosis for these patients, we only draw definitive conclusions about the phenotypic spectrum of the disorder based on the four patients with genetic evidence. Notably, the phenotype of the patient reported here appears generally milder than that of the patients reported by Kresse et al. [1987] and Faiyaz-Ul-Haque et al. [2004] as evidenced by the lack of dysmorphic facial features and more subtle developmental delay in the present patient. This reflects a spectrum of phenotypic severity associated with B4GALT7 deficiency, and suggests that patients with milder phenotypes may lack some of the features we have associated with the syndrome and be more difficult to diagnose in the clinic. It is possible that the present patient’s milder features are due to a less deleterious effect of his novel transmembrane variant as compared to the previously reported variants which all reside in the catalytic domain.
In summary, based on the patients with confirmed B4GALT7 deficiency, we suggest that key features of the progeroid variant of EDS are short stature, developmental anomalies of the forearm bones and elbow, and bowing of extremities, in addition to the classic stigmata of EDS. Significant developmental delay is not a consistent feature in these patients. As progeroid facial appearance is not strongly associated with B4GALT7 deficiency, it should not be regarded as a key feature of the syndrome, and we advocate for the removal of the term “progeroid variant” from this syndrome. This misnomer may lead to under-diagnosis of the syndrome.
Comparison with Other Proteoglycan Disorders
A common tetrasaccharide linkage connects GAGs to a core protein in the synthesis of proteoglycans. The formation of this tetrasaccharide linkage involves several stepwise additions of sugar moieties, one of which is catalyzed by β1–4-galactosyltransferase-7. Interestingly, a recent report connected mutations in B3GAT3 (which encodes the protein needed to catalyze the addition of the final sugar moiety in the tetrasaccharide linker) to a Larsen-like syndrome [Baasanjav et al., 2011]. Clinical features of patients with mutations in B3GAT3 included cardiovascular abnormalities, short stature, multiple joint dislocations, and various dysmorphic craniofacial features. These patients all had congenital dislocations of the elbow and broad fingertips, in addition to joint laxity. These features are similar to the present patient and other patients described with the B4GALT7 deficiency, although the patients with B3GAT3 deficiency did not have any reported skin abnormalities and had prominent cardiac anomalies. The other genes involved in the synthesis of the tetrasaccharide linker, XYLT1/XYLT2 and B3GALT6, have not been linked to any syndromes. However, we expect that they might eventually be associated with syndromes with features similar to those found in patients with B4GALT7 and B3GAT3 deficiency.
Other syndromes caused by deficiency in various steps of GAG synthesis have also been described. For example, mutations in carbohydrate sulfotransferase 3 (CHST3) are known to cause spondyloepiphyseal dysplasia with congenital joint dislocations [Thiele et al., 2004], with features that include dysplasia and subluxation of the elbow and short stature. Other genes encoding enzymes involved in GAG synthesis, such as CHST14 [Sonoda and Kouno, 2000], CHSY1 [Tian et al., 2010], EXT1 [Francannet et al., 2001] have also been variably associated with short stature. It is interesting to note that these disorders, which share common molecular mechanisms of defects in proteoglycan synthesis, have overlapping phenotypic features.
Our report highlights how exome sequencing technology can help make rapid diagnoses of extremely rare disorders, reducing the time-consuming and costly diagnostic odyssey of individually testing suspected genes. As exome sequencing becomes more widely utilized in routine clinical practice and research settings, we believe that other patients with this syndrome and other syndromes resulting from defects in proteoglycan synthesis will become more widely reported. As demonstrated by our report, having a cohort with genetic diagnoses can help develop insights on clinical phenotype, variability, and disease classification. Importantly, this will allow for a better understanding of the clinical sequelae of these syndromes and improve our management of the affected individuals.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patient and family for their participation. We also thank Jennifer Moon for her technical assistance and Dr. Martin Götte for B4GALT7 plasmids.
Funding: This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health. This work was also supported by NIH grant 1K23HD073351-01 (AD), a fellowship grant from the Genentech Center for Clinical Research in Endocrinology (AD), March of Dimes grant 6-FY09-507 (JNH), and support of the Translational Research Program at Boston Children’s Hospital. ON was supported by an ESPE Research Fellowship Grant and grants from the Swedish Research Council (K2007-52X-20316-01-4, K2012-99X-21998-01-3), the Stockholm County Council, the Swedish Society of Medicine, Her Royal Highness Crown Princess Lovisa’s Foundation for Pediatric Care, Wera Ekstrom’s Foundation for Pediatric Research, Märta och Gunnar V Philipson’s Foundation, Sällskapet Barnavård, and Karolinska Institutet.
Footnotes
Competing Interests: The authors report no competing interests.
REFERENCES
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida R, Levery SB, Mandel U, Kresse H, Schwientek T, Bennett EP, Clausen H. Cloning and expression of a proteoglycan UDP-galactose:Beta-xylose beta1,4-galactosyltransferase I. A seventh member of the human beta4-galactosyltransferase gene family. J Biol Chem. 1999;274:26165–26171. doi: 10.1074/jbc.274.37.26165. [DOI] [PubMed] [Google Scholar]
- Baasanjav S, Al-Gazali L, Hashiguchi T, Mizumoto S, Fischer B, Horn D, Seelow D, Ali BR, Aziz SA, Langer R, Saleh AA, Becker C, Nurnberg G, Cantagrel V, Gleeson JG, Gomez D, Michel JB, Stricker S, Lindner TH, Nurnberg P, Sugahara K, Mundlos S, Hoffmann K. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am J Hum Genet. 2011;89:15–27. doi: 10.1016/j.ajhg.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Dauber A, Stoler J, Hechter E, Safer J, Hirschhorn JN. Whole exome sequencing reveals a novel mutation in CUL7 in a patient with an undiagnosed growth disorder. J Pediatr. 2013;162:202.e1–204.e1. doi: 10.1016/j.jpeds.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AM, Kibria L, Reed MH. The developmental spectrum of proximal radioulnar synostosis. Skeletal Radiol. 2010;39:49–54. doi: 10.1007/s00256-009-0762-2. [DOI] [PubMed] [Google Scholar]
- Faiyaz-Ul-Haque M, Zaidi SH, Al-Ali M, Al-Mureikhi MS, Kennedy S, Al-Thani G, Tsui LC, Teebi AS. A novel missense mutation in the galactosyltransferase-I (B4GALT7) gene in a family exhibiting facioskeletal anomalies and Ehlers-Danlos syndrome resembling the progeroid type. Am J Med Genet A. 2004;128A:39–45. doi: 10.1002/ajmg.a.30005. [DOI] [PubMed] [Google Scholar]
- Francannet C, Cohen-Tanugi A, Le Merrer M, Munnich A, Bonaventure J, Legeai-Mallet L. Genotype-phenotype correlation in hereditary multiple exostoses. J Med Genet. 2001;38:430–434. doi: 10.1136/jmg.38.7.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- Gotte M, Kresse H. Defective glycosaminoglycan substitution of decorin in a patient with progeroid syndrome is a direct consequence of two point mutations in the galactosyltransferase I (beta4GalT-7) gene. Biochem Genet. 2005;43:65–77. doi: 10.1007/s10528-005-1068-2. [DOI] [PubMed] [Google Scholar]
- Gotte M, Spillmann D, Yip GW, Versteeg E, Echtermeyer FG, van Kuppevelt TH, Kiesel L. Changes in heparan sulfate are associated with delayed wound repair, altered cell migration, adhesion and contractility in the galactosyltransferase I (beta4GalT-7) deficient form of Ehlers-Danlos syndrome. Hum Mol Genet. 2008;17:996–1009. doi: 10.1093/hmg/ddm372. [DOI] [PubMed] [Google Scholar]
- Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: The sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Aguirre-Negrete MG, Gonzalez-Flores S, Reynoso-Luna MC, Fragoso R, Nazara Z, Tapia-Arizmendi G, Cantu JM. Ehlers-Danlos features with progeroid facies and mild mental retardation. further delineation of the syndrome. Clin Genet. 1986;30:456–461. doi: 10.1111/j.1399-0004.1986.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Aguirre-Negrete MG, Liparoli JC, Cantu JM. Third case of a distinct variant of the Ehlers-Danlos syndrome (EDS) Clin Genet. 1981;20:222–224. doi: 10.1111/j.1399-0004.1981.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Aguirre-Negrete MG, Ramirez-Soltero S, Gonzalez-Mendoza A, Martinez y Martinez R, Velazquez-Cabrera A, Cantu JM. A distinct variant of the Ehlers-Danlos syndrome. Clin Genet. 1979;16:335–339. doi: 10.1111/j.1399-0004.1979.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Kresse H, Rosthoj S, Quentin E, Hollmann J, Glossl J, Okada S, Tonnesen T. Glycosaminoglycan-free small proteoglycan core protein is secreted by fibroblasts from a patient with a syndrome resembling progeroid. Am J Hum Genet. 1987;41:436–453. [PMC free article] [PubMed] [Google Scholar]
- Lui JC, Nilsson O, Chan Y, Palmer CD, Andrade AC, Hirschhorn JN, Baron J. Synthesizing genome-wide association studies and expression microarray reveals novel genes that act in the human growth plate to modulate height. Hum Mol Genet. 2012;21:5193–5201. doi: 10.1093/hmg/dds347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima T, Fukumoto S, Furukawa K, Urano T. Molecular basis for the progeroid variant of Ehlers-Danlos syndrome. identification and characterization of two mutations in galactosyltransferase I gene. J Biol Chem. 1999;274:28841–28844. doi: 10.1074/jbc.274.41.28841. [DOI] [PubMed] [Google Scholar]
- Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J Cell Sci. 2000;113(Pt 2):193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- Quentin E, Gladen A, Roden L, Kresse H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: Galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc Natl Acad Sci U S A. 1990;87:1342–1346. doi: 10.1073/pnas.87.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahuel-Clermont S, Daligault F, Piet MH, Gulberti S, Netter P, Branlant G, Magdalou J, Lattard V. Biochemical and thermodynamic characterization of mutated beta1,4-galactosyltransferase 7 involved in the progeroid form of the Ehlers-Danlos syndrome. Biochem J. 2010;432:303–311. doi: 10.1042/BJ20100921. [DOI] [PubMed] [Google Scholar]
- Seidler DG, Faiyaz-Ul-Haque M, Hansen U, Yip GW, Zaidi SH, Teebi AS, Kiesel L, Gotte M. Defective glycosylation of decorin and biglycan, altered collagen structure, and abnormal phenotype of the skin fibroblasts of an Ehlers-Danlos syndrome patient carrying the novel Arg270Cys substitution in galactosyltransferase I (beta4GalT-7) J Mol Med (Berl) 2006;84:583–594. doi: 10.1007/s00109-006-0046-4. [DOI] [PubMed] [Google Scholar]
- Sonoda T, Kouno K. Two brothers with distal arthrogryposis, peculiar facial appearance, cleft palate, short stature, hydronephrosis, retentio testis, and normal intelligence: A new type of distal arthrogryposis? Am J Med Genet. 2000;91:280–285. [PubMed] [Google Scholar]
- Thiele H, Sakano M, Kitagawa H, Sugahara K, Rajab A, Hohne W, Ritter H, Leschik G, Nurnberg P, Mundlos S. Loss of chondroitin 6-O-sulfotransferase-1 function results in severe human chondrodysplasia with progressive spinal involvement. Proc Natl Acad Sci U S A. 2004;101:10155–10160. doi: 10.1073/pnas.0400334101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Ling L, Shboul M, Lee H, O'Connor B, Merriman B, Nelson SF, Cool S, Ababneh OH, Al-Hadidy A, Masri A, Hamamy H, Reversade B. Loss of CHSY1, a secreted FRINGE enzyme, causes syndromic brachydactyly in humans via increased NOTCH signaling. Am J Hum Genet. 2010;87:768–778. doi: 10.1016/j.ajhg.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wopereis S, Lefeber DJ, Morava E, Wevers RA. Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: A review. Clin Chem. 2006;52:574–600. doi: 10.1373/clinchem.2005.063040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.