Abstract
Background
As a first step toward developing effective strategies to control symptoms associated with head and neck cancer (HNC) and its treatment, we sought to describe the pattern of symptoms experienced before radiation therapy.
Methods
Subjects completed the MD Anderson Symptom Inventory—Head and Neck Module before beginning radiation therapy.
Results
270 patients participated. Symptom severity and interference varied between treatment-naïve patients and those with prior treatment. Cluster analyses revealed that 33% of patients had high symptom burden. Symptoms most often rated moderate-to-severe were fatigue, sleep disturbance, distress, pain, and problems chewing and swallowing. Poorer performance status, higher T classification, and receipt of previous treatment correlated with higher symptom burden.
Conclusions
A substantial proportion of patients were experiencing high symptom burden. Because few interventions currently exist for several of the most problematic symptoms, research in symptom reduction that targets the pattern of symptoms described here is greatly needed.
Keywords: head and neck cancer, patient-reported outcomes, symptom burden, MDASI-HN, symptom research
Introduction
Control of symptoms associated with head and neck cancer (HNC) and its treatment is a high priority for patients (1), and the need to control such symptoms extends beyond the time of diagnosis and treatment to afterward, during the survivorship phase (2). Radiation therapy is used in the treatment of more than half of HNC patients and is commonly associated with substantial patient symptoms. Even before radiation-based treatment is begun, patients with HNC may be experiencing high levels of distress associated with their recent cancer diagnosis. They may also have significant symptoms related to their tumors and/or any prior therapy, such as recent chemotherapy or surgery. To our knowledge, HNC patient–reported symptoms occurring during this pre-radiation therapy interval have not been documented in a prospective study.
Although the benefit of optimal symptom control to avoid unnecessary patient suffering is intuitive, adequate symptom assessment and management during the pretreatment period are important for several other reasons. First, unrecognized or uncontrolled potentially treatable symptoms may lead to delays in starting curative therapy, poorer tolerance of radiation or chemoradiation, or exacerbation of expected symptoms during the course of therapy. A high symptom burden and poor tolerance of therapy can also lead to unplanned radiation treatment breaks or other noncompliance with the planned treatment regimen that can negatively affect tumor control (3). Preventing the development of symptoms, or recognizing symptoms and intervening early while they are still perceived as being “mild,” can prevent subsequent high-grade symptoms or severe adverse events (4). In addition, the degree of symptom burden (a concept describing both the severity of the symptoms and the patient’s perception of their impact on the patient’s activities (5)) may have implications for prognosis (6, 7), and many symptoms persist well after completion of cancer therapy (8), underscoring the need for early and effective symptom prevention and intervention. Furthermore, patients with high symptom burden might be subject to potential physician bias and selected for less aggressive therapies which otherwise might be appropriate based upon other patient and tumor factors.
The MD Anderson Symptom Inventory is a brief, psychometrically validated multisymptom assessment tool that assesses symptoms commonly associated with cancer or its treatment (9). Because patients with HNC have unique disease site and treatment-related symptoms, the MD Anderson Symptom Inventory–Head and Neck Module (MDASI-HN) was designed and validated in order to capture and quantify such symptoms and the degree of symptom interference. The MDASI-HN provides clinicians with pertinent, easily attainable information to help guide patient-specific evaluations and interventions (10). As a first step toward our ultimate goal of developing effective programmatic symptom prevention and intervention strategies and designing future symptom-reduction clinical trials that focus on patient-reported outcome (PRO) endpoints using the MDASI-HN, the goals of the present study were to 1) describe the pattern of symptoms experienced by HNC patients before planned radiation or concurrent chemoradiation, 2) explore potential differences in patterns of symptoms experienced by various subgroups, and 3) identify potential patient-, tumor- and previous treatment-related factors that correlate with higher overall symptom burden.
Methods and Materials
Patients
This single-institution, prospective questionnaire-based study was conducted after approval of the institutional review board. Consecutive adult patients who were able to read and understand English and who were scheduled to undergo radiation or chemoradiation with curative intent for HNC were recruited at the time of their evaluation in our Head and Neck Planning and Development Clinic (11). All participating patients provided study-specific written informed consent and were enrolled before starting radiation-based treatment. Information on demographics, tumor characteristics, and any previous therapies (e.g., chemotherapy or surgery) was collected at the time of enrollment. Prior surgery was defined as a standard cancer operation in which the tumor was completely excised, but diagnostic surgical procedures such as diagnostic tonsillectomy and excisional lymph node biopsy were not defined as surgery for this analysis. Patient performance status was rated by the treating radiation oncologist according to the Eastern Cooperative Oncology Group (ECOG) scale (12). Tumor and lymph node status was recorded according to the American Joint Committee on Cancer (AJCC) 2002 (6th edition) staging system. Tumor site was assigned based on the anatomic location of the primary tumor, previous surgical volume, and planned radiation therapy target volume. For example, a minor salivary gland tumor in the oral cavity was coded as “oral cavity.” Unknown primary carcinomas for which elective mucosal irradiation was to be delivered were coded as “unknown primary,” but unknown primary carcinomas of suspected skin origin for which the radiation volume was to include the skin with or without draining nodal basins or parotid bed but no planned mucosal target were coded as “skin.” Exclusion criteria for this study were being treated with palliative intent, distant metastatic disease, previous radiation therapy to the head and neck, prior treatment outside our institution without clinical information available on those treatments, and failure to complete at least 50% of the MDASI-HN items.
The MDASI–HN multisymptom assessment tool
The MDASI-HN is a 28-item questionnaire containing 13 core items representing common symptoms across all cancer types, 9 HNC items, and 6 items on the extent to which symptoms interfere with major activities of daily life (10). All MDASI-HN symptom items are rated on numeric 0-to-10 scales from “not present” to “as bad as you can imagine,” and the MDASI-HN interference items are rated on numeric 0-to-10 scales from “did not interfere” to “interfered completely." Participating patients completed the self-administered MDASI-HN via pencil and paper before starting radiation therapy. Although patients also completed the MDASI-HN at various times during and after radiation therapy, this analysis involved only the “baseline” (pre-radiotherapy) data.
Statistical analysis
Descriptive statistics were used to summarize patient demographic, clinical characteristics, and MDASI-HN ratings. There were 22 patients who failed to respond to a mean 1.4 (range: 1–3) MDASI-HN items. The missing data were imputed by replacing the value of a missing symptom item or items with the mean symptom value (average of non-missing symptoms) for that particular patient. Analysis of variance and t tests were used to test for differences among subgroups. Hierarchical cluster analysis of patients by using Ward’s method on squared Euclidian distance among the 22 symptoms was used to classify patients who reported higher versus lower levels of symptom burden. Since clinicians typically find it simpler and clinically useful to compare and contrast two groups instead of 3 or more groups, for this analysis we specified a priori that we would fit (identify) two groups: a high symptom group and a low symptom group. Hierarchical cluster analysis of symptoms was also used to provide a pictorial representation of how symptoms clustered together. We further considered and describe the proportions of patients reporting moderate-to-severe (ratings of ≥5 on the 0–10 scale) and severe (ratings of ≥7) symptoms both by symptom burden group and by receipt of prior chemotherapy or surgery (13, 14).
Correlates of having a high or low symptom burden were then determined by using a two-step method. First, in a univariate approach, we tested the relationship of a specified list of covariates selected a priori with low or high symptom burden, one at a time. These covariates were sex, age, T classification, N classification, disease site, receipt of previous treatment, time since surgery, time since chemotherapy, and ECOG performance status. Only those associated variables with p≤0.1 on univariate testing were considered further. Second, we identified the final set of correlates with symptom burden by using multivariate logistic regression. We compared the results of forward and backward variable selection procedures. P values of <0.05 were deemed statistically significant. All tests were two-tailed. Analyses were performed using PASW Statistics 17.0 (SPSS).
Results
Patients, tumors, and previous treatment
From March 2005 through July 2007, 294 patients were enrolled and 270 met the criteria for analysis. The main reasons for exclusion were previous radiation to the head and neck (9/24) and treatment with palliative intent (4/24). Patient, tumor, and previous treatment characteristics are presented in Table 1. Median patient age was 58.5 years (standard deviation [SD] 11.9). Most tumors (208) were of squamous histology; 9 were papillary thyroid carcinoma, 8 nasopharyngeal carcinoma, 7 adenocarcinoma, and 7 adenoid cystic carcinoma. Among the 72 patients who had received prior chemotherapy, the most common regimens were a platin and taxane doublet (n=24), cetuximab, paclitaxel, and carboplatin (n=19), docetaxel, cisplatin, and fluorouracil (n=16), and paclitaxel, ifosfamide, and carboplatin (n=8). Seventy-nine patients had received surgery, 74 alone and 5 with chemotherapy. The median number of days from the final day of chemotherapy or the date of surgery to completion of the MDASI-HN were 21.6 (SD 25.3) or 39.1 (SD 25.7), respectively.
Table 1.
Patient, disease, and previous treatment characteristics
| Previous treatment group | ||||
|---|---|---|---|---|
| Entire cohort | No previous treatment | Previous chemotherapy only |
Previous surgery only or chemotherapy and surgery |
|
| No. of patients = 270 | No. of patients = 124 | No. of patients = 67 | No. of patients = 79 | |
| Characteristic | No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) |
| Age | ||||
| < 60 years | 143 (53.0) | 62 (50.0) | 45 (67.2) | 36 (45.6) |
| ≥ 60 years | 127 (47.0) | 62 (50.0) | 22 (32.8) | 43 (54.4) |
| Sex | ||||
| Female | 66 (24.4) | 23 (18.5) | 14 (20.9) | 29 (36.7) |
| Male | 204 (75.6) | 101 (81.5) | 53 (79.1) | 50 (63.3) |
| Educational level | ||||
| ≤ 12th grade | 100 (37.0) | 45 (36.3) | 22 (32.8) | 33 (41.8) |
| > 12th grade | 169 (62.6) | 78 (62.9) | 45 (67.2) | 46 (58.2) |
| Ethnicity | ||||
| White non-Hispanic | 220 (81.5) | 109 (87.9) | 56 (83.6) | 55 (69.6) |
| Black non-Hispanic | 18 (6.7) | 6 (4.8) | 5 (7.5) | 7 (8.9) |
| Hispanic | 21 (7.8) | 7 (5.6) | 3 (4.5) | 11 (13.9) |
| Other | 11 (4.1) | 2 (1.6) | 3 (4.5) | 6 (7.6) |
| Employment status | ||||
| Employed outside the home | 127 (47.0) | 72 (50.8) | 34 (50.7) | 30 (38.0) |
| Homemaker | 10 (3.7) | 2 (1.6) | 3 (4.5) | 5 (6.3) |
| Retired | 91 (33.7) | 39 (31.5) | 15 (22.4) | 37 (46.8) |
| Medical leave of absence | 16 (5.9) | 4 (3.2) | 9 (13.4) | 3 (3.8) |
| Disabled due to illness | 16 (5.9) | 11 (8.9) | 3 (4.5) | 2 (2.5) |
| Unemployed | 7 (2.6) | 3 (2.4) | 2 (3.0) | 2 (2.5) |
| Other | 3 (1.1) | 2 (1.6) | 1 (1.5) | 0 (0.0) |
| Disease site | ||||
| Oropharynx | 119 (44.1) | 72 (58.1) | 47 (70.1) | 0 (0.0) |
| Oral Cavity | 28 (10.4) | 4 (3.2) | 0 (0.0) | 24 (30.4) |
| Nasopharynx | 4.1 (11) | 5 (4.0) | 5 (7.5) | 1 (1.3) |
| Larynx | 27 (10.0) | 19 (15.3) | 5 (7.5) | 3 (3.8) |
| Hypopharynx | 9 (3.3) | 7 (5.6) | 2 (3.0) | 0 (0.0) |
| Thyroid/Trachea | 13 (4.8) | 1 (0.8) | 0 (0.0) | 12 (15.2) |
| Major salivary gland | 17 (6.3) | 1 (0.8) | 1 (1.5) | 15 (19.0) |
| Nasal cavity/Paranasal sinus | 20 (7.4) | 4 (3.2) | 3 (4.5) | 13 (16.5) |
| Skin | 11 (4.1) | 0 (0.0) | 1 (1.5) | 10 (12.7) |
| Unknown primary | 15 (5.6) | 11 (8.9) | 3 (4.5) | 1 (1.3) |
| ECOG performance grade | ||||
| Grade 0 | 157 (58.1) | 99 (79.8) | 25 (37.3) | 33 (41.8) |
| Grade 1 | 89 (33.0) | 19 (15.3) | 36 (53.7) | 34 (43.0) |
| Grade 2 | 21 (7.8) | 5 (4.0) | 5 (7.5) | 11 (13.9) |
| Grade 3 | 1 (0.4) | 1 (0.8) | 0 (0.0) | 0 (0.0) |
| T classification | ||||
| Tx | 10 (3.7) | 5 (4.0) | 0 (0.0) | 5 (6.3) |
| T0 | 18 (6.7) | 11 (8.9) | 4 (6.0) | 3 (3.8) |
| T1 | 52 (19.3) | 31 (25.0) | 11 (16.4) | 10 (12.7) |
| T2 | 77 (28.5) | 31 (29.8) | 22 (32.8) | 18 (22.8) |
| T3 | 45 (16.7) | 22 (17.7) | 16 (23.9) | 7 (8.9) |
| T4 | 48 (17.8) | 16 (12.9) | 14 (20.9) | 18 (22.8) |
| Recurrent | 18 (6.7) | 2 (1.6) | 0 (0.0) | 16 (20.3) |
| NA | 2 (0.7) | 0 (0.0) | 0 (0.0) | 2 (2.5) |
| N classification | ||||
| Nx | 9 (3.3) | 6 (4.8) | 0 (0.0) | 3 (3.8) |
| N0 | 86 (31.9) | 44 (35.5) | 3 (4.5) | 39 (49.4) |
| N1 | 27 (10.0) | 15 (12.1) | 2 (3.0) | 10 (12.7) |
| N2 | 124 (45.9) | 57 (46.0) | 48 (71.6) | 19 (24.1) |
| N3 | 17 (6.3) | 2 (1.6) | 14 (20.9) | 1 (1.3) |
| Recurrent | 7 (2.6) | 0 (0.0) | 0 (0.0) | 7 (8.9) |
| Previous treatment | ||||
| No previous treatment | 124 (45.9) | |||
| Induction chemotherapy only | 67 (24.8) | |||
| Induction chemotherapy and surgery | 5 (1.9) | |||
| Surgery only | 74 (27.4) | |||
Pattern of symptom burden
Mean MDASI-HN symptom ratings, with SDs, are reported in Table 2. Analysis of variance showed differences in mean symptom severity (p=0.01) and mean symptom interference (p=0.006) depending on receipt of previous treatment (none, chemotherapy only, or surgery with or without chemotherapy). Follow-up analysis showed that mean symptom severity for the group that had not had prior treatment (“treatment naïve”) was different from both the previous chemotherapy group (p=0.005) and the previous surgery group (p=0.04). Mean interference for the treatment naïve group was different from the previous chemotherapy (p=0.005) but not from the previous surgery group (p=0.057). However, neither the mean symptom severity nor mean interference was different in the groups that had previous chemotherapy or previous surgery.
Table 2.
Mean scores (with standard deviations) on the MD Anderson Symptom Inventory—Head & Neck Module items according to receipt of prior treatment
| Previous treatment group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entire cohort | No previous treatment | Previous chemotherapy only |
Previous surgery only or chemotherapy and surgery |
|||||
| No. of patients = 270 | No. of patients = 124 | No. of patients = 67 | No. of patients = 79 | |||||
| MDASI-HN items | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 13 Core items | ||||||||
| Fatigue | 2.2 | 2.5 | 1.6 | 2.4 | 3.1 | 2.5 | 2.5 | 2.6 |
| Disturbed sleep | 1.9 | 2.6 | 1.9 | 2.7 | 2.1 | 2.6 | 1.9 | 2.5 |
| Distress | 1.8 | 2.3 | 1.7 | 2.2 | 1.9 | 2.4 | 2.1 | 2.5 |
| Pain | 1.6 | 2.5 | 1.8 | 2.8 | 1.7 | 2.5 | 1.3 | 2.1 |
| Drowsiness | 1.6 | 2.3 | 1.3 | 2.2 | 2.0 | 2.5 | 1.6 | 2.0 |
| Sadness | 1.5 | 2.2 | 1.1 | 1.9 | 1.5 | 2.2 | 2.1 | 2.7 |
| Difficulty remembering | 1.1 | 1.7 | 0.8 | 1.5 | 1.5 | 1.9 | 1.3 | 1.8 |
| Numbness/Tingling | 1.1 | 2.0 | 0.5 | 1.3 | 2.0 | 2.6 | 1.3 | 2.0 |
| Dry mouth | 1.1 | 2.2 | 0.8 | 1.8 | 1.2 | 2.1 | 1.4 | 2.7 |
| Lack of appetite | 1.0 | 2.0 | 0.9 | 1.9 | 1.6 | 2.5 | 0.8 | 1.6 |
| Shortness of breath | 0.7 | 1.6 | 0.5 | 1.4 | 1.2 | 2.1 | 0.6 | 1.3 |
| Nausea | 0.6 | 1.5 | 0.4 | 1.4 | 1.0 | 1.9 | 0.5 | 1.2 |
| Vomiting | 0.1 | 0.8 | 0.1 | 1.0 | 0.1 | 1.0 | 0.1 | 0.3 |
| 9 HNC-specific items | ||||||||
| Swallowing/Chewing | 1.6 | 2.5 | 1.5 | 2.4 | 1.2 | 2.3 | 2.1 | 2.7 |
| Voice/Speech | 1.3 | 2.2 | 0.9 | 1.5 | 0.9 | 1.9 | 2.4 | 2.9 |
| Mucus in mouth and throat | 1.2 | 2.2 | 1.0 | 2.1 | 1.0 | 2.0 | 1.8 | 2.6 |
| Tasting food | 1.0 | 1.9 | 0.5 | 1.3 | 2.0 | 2.5 | 0.8 | 1.9 |
| Constipation | 1.0 | 2.0 | 0.7 | 1.6 | 1.5 | 2.5 | 0.9 | 1.8 |
| Teeth/Gums | 0.9 | 2.1 | 0.5 | 1.5 | 1.4 | 2.6 | 1.1 | 2.2 |
| Mouth/Throat sores | 0.7 | 1.8 | 0.7 | 1.8 | 0.9 | 2.0 | 0.7 | 1.8 |
| Choking/Coughing | 0.7 | 1.8 | 0.6 | 1.6 | 0.7 | 1.8 | 0.9 | 2.2 |
| Skin pain/burning/rash | 0.5 | 1.6 | 0.2 | 0.8 | 1.4 | 2.7 | 0.2 | 0.8 |
| 6 Interference items | ||||||||
| Work | 2.1 | 2.9 | 1.4 | 2.6 | 3.1 | 3.2 | 2.3 | 2.8 |
| Enjoyment of life | 2.0 | 2.7 | 1.6 | 2.7 | 2.3 | 2.6 | 2.2 | 2.7 |
| General activity | 2.0 | 2.7 | 1.4 | 2.4 | 2.9 | 3.1 | 2.1 | 2.5 |
| Mood | 1.8 | 2.4 | 1.5 | 2.3 | 2.0 | 2.3 | 2.1 | 2.5 |
| Walking ability | 1.4 | 2.5 | 1.0 | 2.3 | 2.2 | 3.0 | 1.3 | 2.3 |
| Relations with others | 1.1 | 2.0 | 1.0 | 1.9 | 1.3 | 2.0 | 1.3 | 2.1 |
The proportions of patients with moderate-to-severe and severe levels of each MDASI-HN symptom item are presented in Supplementary Table 1. Ten percent or more of the patients in the entire cohort, the treatment-naïve group, the previous chemotherapy group, and the previous surgery group reported moderate-to-severe levels for 10, 7, 15, and 8 of the MDASI-HN symptom items, respectively. Figures 1 and 2 show the proportions of patients experiencing moderate-to-severe and severe levels of the top symptoms (i.e., those symptoms experienced at moderate-to-severe levels by at least 10% of patients in any previous treatment group).
Figure. 1.
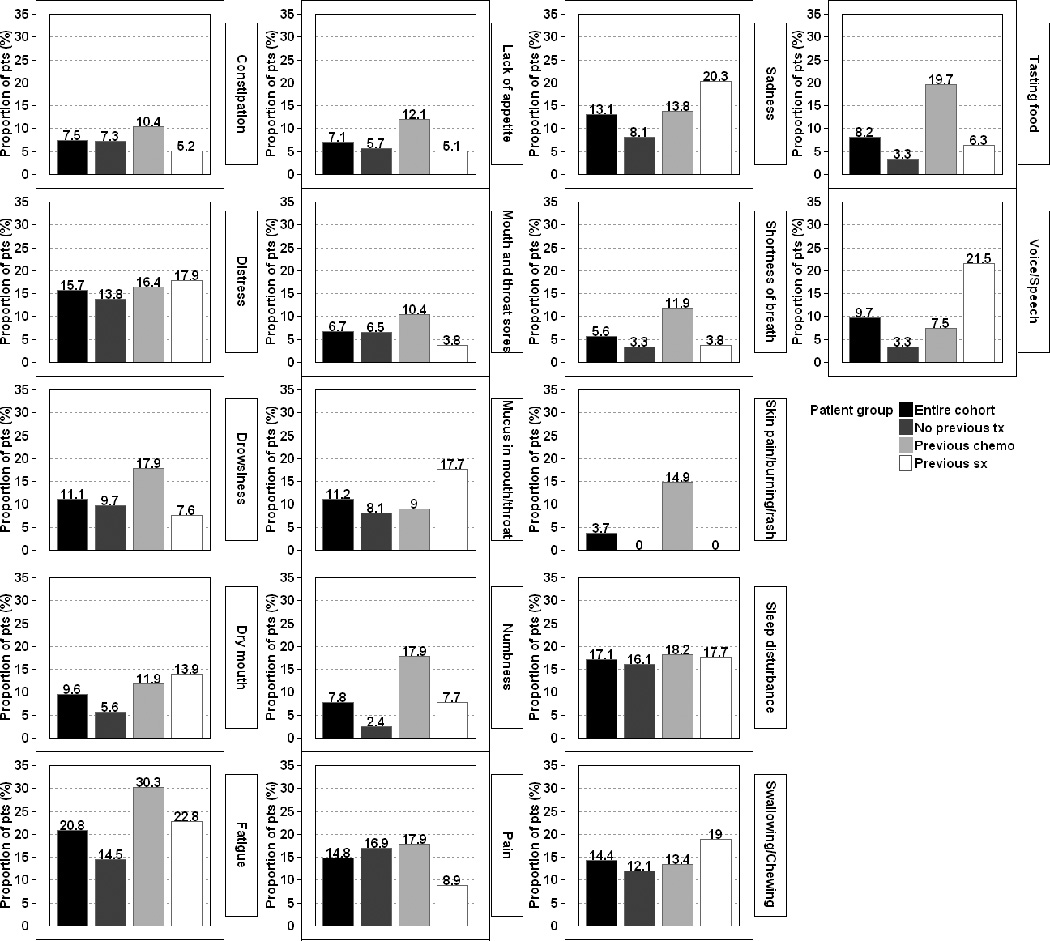
Proportions of patients experiencing moderate-to-severe levels (ratings of ≥5) of the 17 most commonly experienced symptoms of the MD Anderson Symptom Inventory—Head and Neck Module, according to receipt of prior therapy.
Figure. 2.
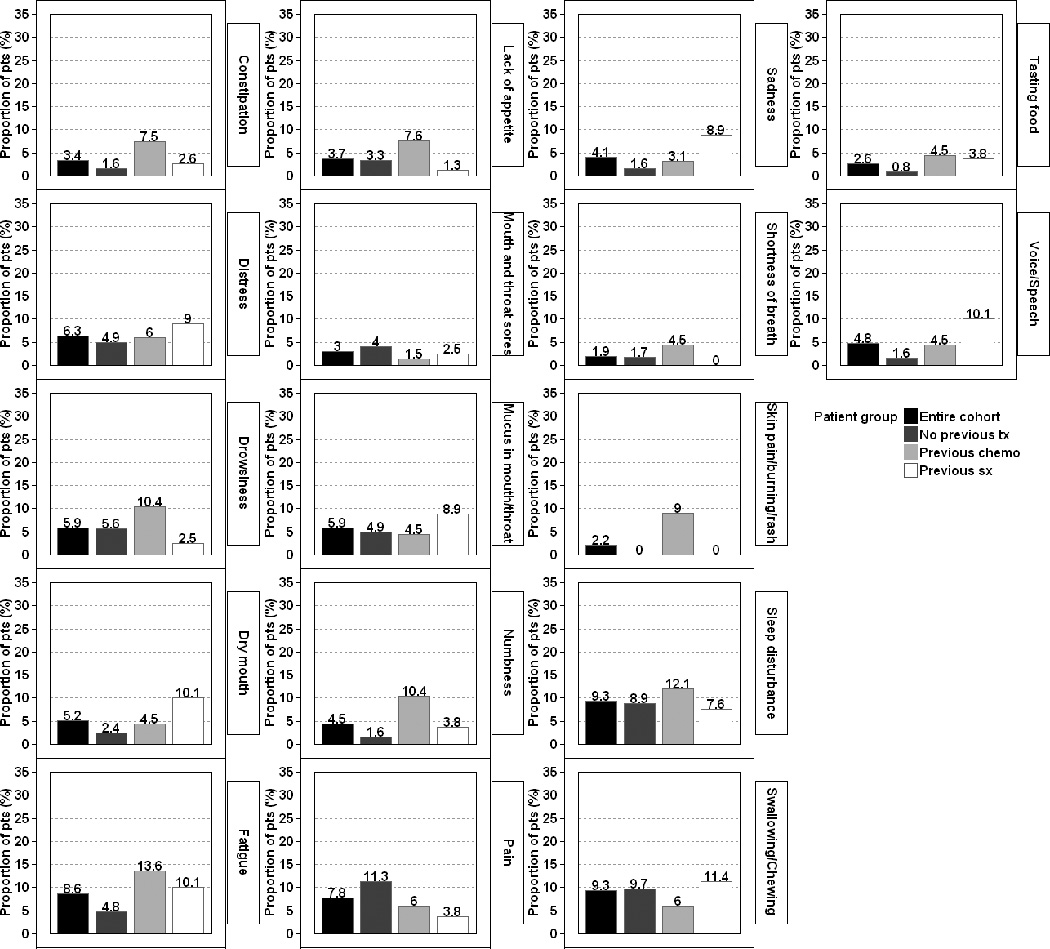
Proportions of patients experiencing severe levels (ratings of ≥7) of the 17 most commonly experienced symptoms of the MD Anderson Symptom Inventory—Head and Neck Module, according to receipt of prior therapy.
Cluster analysis of mean differences across MDASI-HN symptom items revealed that 32.6% of patients were experiencing high symptom burden and 67.4% were experiencing low symptom burden. The proportion of patients in the high symptom burden group experiencing moderate-to-severe and severe levels of individual symptoms are reported in Figure 3. Overall, the average proportions of patients experiencing moderate-to-severe symptom levels across all MDASI-HN symptom items were 26.3% for the high symptom burden group and 13.6% for the low symptom burden group; corresponding percentages of patients with severe symptoms were 1.2% and 0.2%. One hundred twenty-two of the 270 patients (45.2%) reported moderate-to-severe levels of at least one MDASI-HN symptom item. Patients in the higher symptom burden group experienced moderate-to-severe levels of a mean number of 5.78 (±SD 4.06) individual symptoms versus 0.27 (±SD 0.62) in the lower symptom burden group (p<0.001). Results of symptom cluster analysis are illustrated in Figure 4.
Figure. 3.
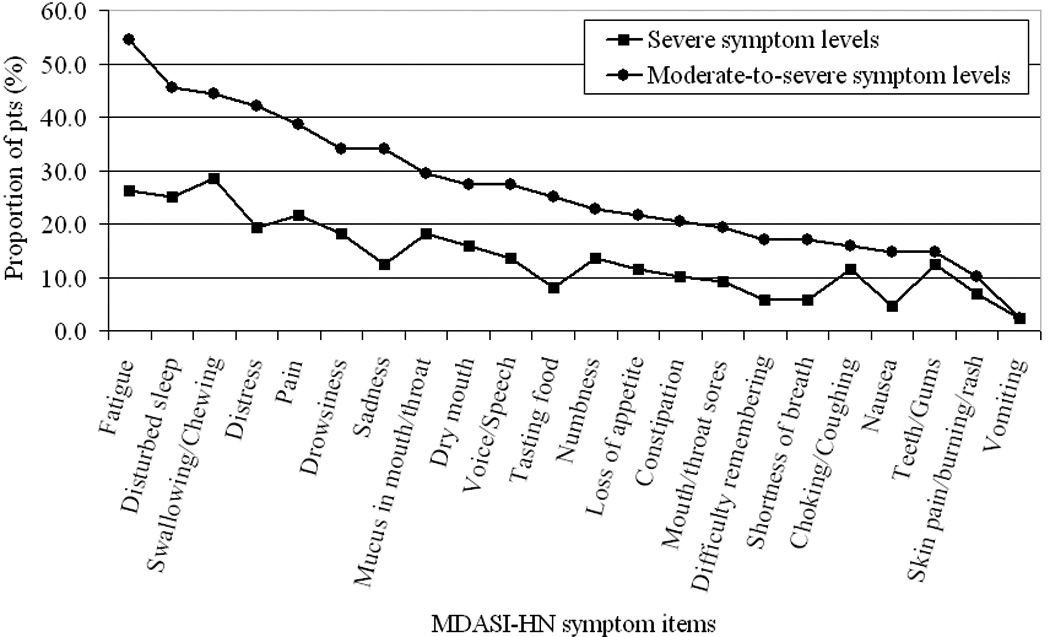
Proportions of patients with high symptom burden who have moderate-to-severe (rated ≥5) and severe levels (rated ≥7) of symptoms listed on the MD Anderson Symptom Inventory—Head and Neck Module.
Figure. 4.
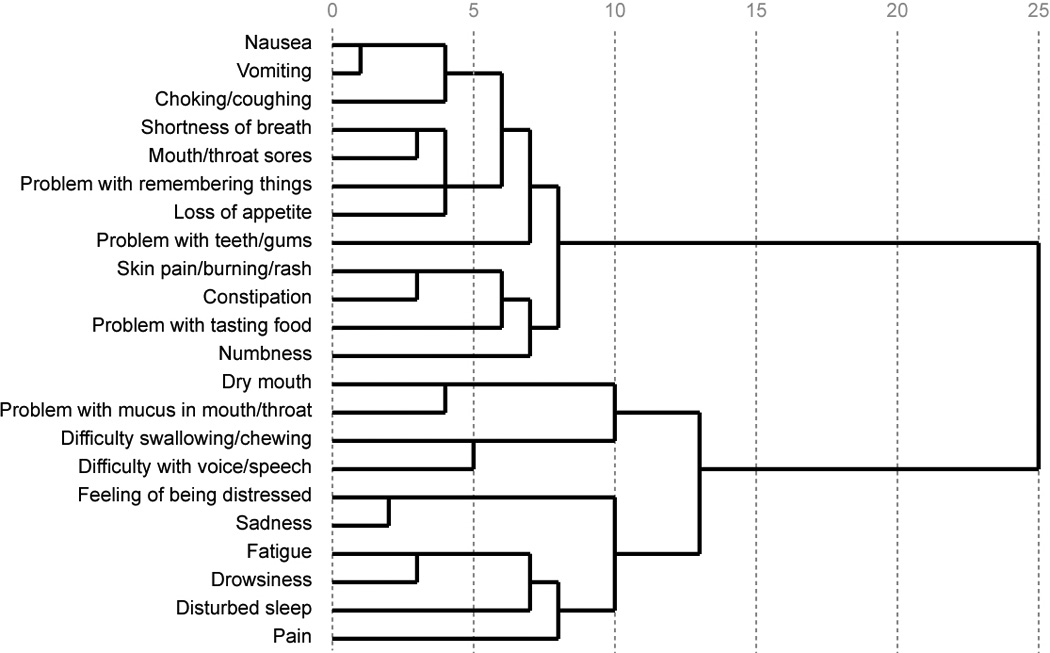
Dendrogram of symptom cluster analysis. Looking at the figure from left to right, the items will join together. The items that join with others sooner along the relative distance scale of 0–25 (more to the left in the figure) were rated by patients more similarly. For example, it can be seen that the items ‘nausea’ and ’vomiting’ join together very quickly suggesting that patients perceived and rated these items similarly.
Correlates of symptom burden
Univariate analysis showed that ECOG performance status (p<0.001), T classification (p<0.01), N classification (p=0.03), receipt of previous treatment (p<0.001), and disease site (p=0.035) were associated with symptom burden group membership. Tested demographic variables and time since previous treatment were not associated with symptom burden. On multivariate logistic regression analysis, having poorer ECOG performance status (ECOG 2–3) (odds ratio [OR]=3.71, 95% confidence interval [CI] 1.96–7.03, p<0.01), advanced (T3 or T4) tumors (OR=2.0, 95% CI 1.09–3.65, p=0.025), previous chemotherapy (OR=2.31, 95% CI 1.10–4.87, p=0.027), or previous surgery (OR=2.47, 95% CI 1.15–5.3, p=0.021) were associated with having a higher symptom burden.
Discussion
We conducted this prospective study using the MDASI-HN to describe the nature and pattern of symptoms before radiation-based treatment. Our results showed that by using the MDASI-HN we were able to identify and describe a subgroup of patients who were experiencing more, and more severe, symptoms and to identify the top (most common) such symptoms. Although the mean ratings of individual MDASI-HN items for the entire cohort were low (range 0.1–2.2), we found that a substantial proportion of patients reported moderate-to-severe levels of many symptoms (ratings of ≥5 on a 0-to-10 scale). Although we found differences in mean symptom severity reports according to receipt and type of previous treatment, we believe that considering the proportions of patients with higher symptom reports for individual symptoms of interest or particular symptom clusters would be more useful both for treatment and for future clinical trial design.
Working groups such as the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) (15) and Assessing the Symptoms of Cancer using Patient-Reported Outcomes (ASCPRO) (16) have emphasized the importance of using validated PRO tools in lieu of physician ratings of toxicity in clinical effectiveness research, and the U.S. Food and Drug Association has provided guidelines for the design, use, and implementation of PRO instruments in drug- and device-based studies (17). The National Institutes of Health consensus statement on symptom management in cancer also supports the routine use of brief symptom assessment tools to help optimize symptom control (18). Furthermore, use of patient-reported symptoms rather than patient-reported health-related quality of life measures may be preferable in clinical decision-making and outcomes research because symptoms are thought to more directly reflect the disease process and treatment course (16). To our knowledge, this is the first description of the symptom burden reported by patients with HNC before beginning radiation-based treatment.
We found that certain symptoms clustered in patients with HNC similar to findings from a study by Murphy et al. (19). However, unlike the Murphy study population, which consisted mostly of patients who were receiving or had received chemoradiation, our symptom reports were collected before radiation-based treatment had begun; as a result, the symptom ranking and magnitude would be expected to be quite different. One potential approach to symptom management could be to intervene for a particular cluster of symptoms, with the rationale that improving one symptom in a certain cluster might improve the commonly associated symptoms and that improving one or more clusters of symptoms could greatly improve the overall symptom burden.
Many patients in our study reported moderate-to-severe levels of several types of symptoms, suggesting that these patients are experiencing a substantial symptom burden (5). This finding provides further justification for using the MDASI-HN before treatment is begun to identify such patients for appropriate interventions or referral for additional supportive care measures, particularly because many of these symptoms are expected to increase during the course of radiation or chemoradiation. Although we found some differences in the pattern of symptom burden according to the receipt or type of previous treatment, overall the top five symptoms reported as being moderate-to-severe by the largest proportions of patients were, in decreasing proportions of patients, fatigue, sleep disturbance, distress, pain, and problems chewing and swallowing. These symptoms should be prioritized for screening by the patient care team and they should be part of the focus of symptom intervention strategies. Fatigue and pain in particular have been shown to correlate most strongly with poor health-related quality of life among cancer survivors (20).
This study did have some limitations. The study cohort represents a fairly homogeneous subset of patients with HNC who were treated in a single tertiary care specialty hospital with a relatively consistent treatment philosophy. Most of the patients in this study were of white non-Hispanic origin and were working at the time of MDASI-HN collection, thus precluding the ability to reach any conclusions regarding the influence of differences in ethnicity or employment status or other economic factors on patient-reported symptoms. However, our study population was reflective of current epidemiologic trends in HNC, in that our patients were mostly younger than 60 years of age, most were male, and cancer of the oropharynx was most common (21).
Our multivariate analysis showed that performance status, higher T classification, and receipt of previous treatment (chemotherapy or surgery) correlated with having a higher symptom burden. It should be noted that many of the variables we tested would be expected to be highly correlated. For example, reflective of our treatment philosophy at the time of this study, most of the patients with N3 disease had received chemotherapy, most of the patients with disease of the oral cavity had received surgery, and most of the patients with disease of the oropharynx had not received prior treatment (Table 1). Our analysis is limited by the absence of reliable data for other potentially important variables that may be associated with symptom burden, such as smoking status, alcohol use, nutritional status and weight loss, the presence of a feeding tube or tracheotomy, and numbers and types of comorbid conditions. Therefore, our findings on patient-, tumor- and treatment-related correlates of symptom burden should be considered exploratory. Also, because we excluded patients who were being treated with palliative intent and because the patients in this study had already been deemed fit by their treating physicians to receive radiation-based treatment, most of these patients had good to excellent performance status. Thus, our results may not be applicable to patients with advanced distant disease or incurable local disease, or those who are not fit for aggressive therapy. Most of the patients in this study had not received prior treatment; nevertheless, a larger cross-sectional study of patients with untreated HNC is needed to better quantify symptoms in previously untreated patients with representation across a wider variety of types of HNC patients.
Notably, these symptom reports were collected at a specific predetermined time point (before planned radiation or chemoradiation), and we evaluated only basic details on previous chemotherapy or surgery. For these reasons, our results are not meant to reflect the pattern of symptoms associated with chemotherapy or surgery for HNC, but rather to illustrate the pattern of symptoms typical of patients presenting for radiation-based treatment. Nevertheless, contrary to what might be expected, few patients who had received previous chemotherapy reported having problems with nausea/vomiting, and pain was not a substantial problem for patients who had previous surgery. These findings are probably attributable to the use of established and effective interventions for chemotherapy associated nausea and vomiting and postoperative pain, the opportunity to have recovered from these symptoms prior to presenting for radiation planning, or perhaps the tendency of clinicians to prioritize control of these symptoms.
Our results show that fatigue was the most commonly reported symptom, a finding consistent with other studies (9, 22). Other than treating any underlying reversible medical cause, there is currently little to offer in the way of effective interventions for fatigue, and thus our findings support the need for future studies of fatigue interventions for patients with HNC. As is true for fatigue, few interventions are available for other common symptoms noted here (e.g., distress and problems chewing and swallowing), again highlighting the need for symptom intervention research and for early functional swallowing rehabilitation in patients presenting for radiation-based treatment (23).
On the basis of these results, we are currently conducting prospective, randomized clinical trials to evaluate different medical interventions that target symptom development and symptom expression pathways for the top symptoms identified here. We are also performing longitudinal analyses to profile the symptom burden during and after completion of radiation and chemoradiation that will evaluate the influence of these presenting symptoms on subsequent symptom trajectory and patient outcomes.
In conclusion, most patients with HNC presenting for radiation-based treatment in this study had a low symptom burden. However, the MDASI-HN allowed for identification of a substantial subgroup of patients with a high symptom burden, their top most commonly reported and most severe symptoms, and symptom clusters. Clinicians who treat patients with HNC should be prepared to screen patients for these common symptoms, to intervene when effective interventions are available, and to identify patients with substantial symptom burden who are in need of additional symptom support. Because few interventions exist at this time for several of the most problematic symptoms, research in symptom reduction that targets the pattern of symptoms described here is greatly needed.
Supplementary Material
Acknowledgements
The authors extend a special thanks to Christine F. Wogan for her contribution in manuscript preparation and editing.
Grant or financial support: Supported in part by Award Number CA026582 from the National Cancer Institute to Charles Cleeland, PhD and by Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Prior presentation [at meeting(s)]: In part at American Society for Radiation Oncology, 52nd Annual Meeting, November 2010, San Diego, California.
Conflicts of interest: E.Y.H.: consultancy Medtronic, Inc.
References
- 1.List MA, Stracks J, Colangelo L, et al. How do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J Clin Oncol. 2000;18:877–884. doi: 10.1200/JCO.2000.18.4.877. [DOI] [PubMed] [Google Scholar]
- 2.Levy MH, Back A, Benedetti C, et al. NCCN clinical practice guidelines in oncology: palliative care. J Natl Compr Canc Netw. 2009;7:436–473. doi: 10.6004/jnccn.2009.0031. [DOI] [PubMed] [Google Scholar]
- 3.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radia Oncol Biol Phys. 2007;68:654–661. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441–1449. doi: 10.1093/annonc/mdl137. [DOI] [PubMed] [Google Scholar]
- 5.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monographs. 2007:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 6.Fang F-M, Liu Y-T, Tang Y, et al. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer. 2004;100:425–432. doi: 10.1002/cncr.20010. [DOI] [PubMed] [Google Scholar]
- 7.Pugliano FA, Piccirillo JF, Zequeira MR, et al. Symptoms as an index of biologic behavior in head and neck cancer. Otolaryngol Head Neck Surg. 1999;120:380–386. doi: 10.1016/S0194-5998(99)70279-2. [DOI] [PubMed] [Google Scholar]
- 8.Harrington CB, Hansen JA, Moskowitz M, et al. It’s not over when it’s over: long-term symptoms in cancer survivors--a systematic review. Int J Psychiatry Med. 2010;40:163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 9.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M.D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29:923–931. doi: 10.1002/hed.20602. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal DI, Asper JA, Barker JL, Jr, et al. Importance of patient examination to clinical quality assurance in head and neck radiation oncology. Head Neck. 2006;28:967–973. doi: 10.1002/hed.20446. [DOI] [PubMed] [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 13.Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 14.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Sloan JA. Assessing the Symptoms of Cancer Using Patient-Reported Outcomes (ASCPRO): searching for standards. J Pain Symptom Manage. 2010;39:1077–1085. doi: 10.1016/j.jpainsymman.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2009 ( http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf.)
- 18.NIH State-of-the-Science Statement on symptom management in cancer: pain, depression, and fatigue. NIH Consens State Sci Statements. 2002;19:1–29. [PubMed] [Google Scholar]
- 19.Murphy BA, Dietrich MS, Wells N, et al. Reliability and validity of the Vanderbilt Head and Neck Symptom Survey: a tool to assess symptom burden in patients treated with chemoradiation. Head Neck. 2010;32:26–37. doi: 10.1002/hed.21143. [DOI] [PubMed] [Google Scholar]
- 20.Shi Q, Smith TG, Michonski JD, et al. Symptom burden in cancer survivors 1 year after diagnosis: A Report From the American Cancer Society’s Studies of Cancer Survivors. Cancer. 2011;117:2779–2790. doi: 10.1002/cncr.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 22.Barbera L, Seow H, Howell D, et al. Symptom burden and performance status in a population - based cohort of ambulatory cancer patients. Cancer. 2010;116:5767–5776. doi: 10.1002/cncr.25681. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


