Abstract
Background
Sputum eosinophilia has been shown to be a predictor of asthma patient response to therapies. However, quantitative cell counts and differentials of sputum are labor intensive. The objective of this study was to validate a novel ELISA-based assay of eosinophil peroxidase (EPX) in sputum as a rapid and reliable marker of airway eosinophils.
Methods
The utility of EPX-based ELISA as an eosinophil-specific assay was achieved through comparisons with sputum eosinophil differential counts in freshly prepared and archived patient samples from a variety of clinical settings.
Results
EPX levels in sputum correlated with eosinophil percentage (rs=0.84) in asthma patients with varying degrees of airway eosinophilia. Significantly, unlike assays of other eosinophil granule proteins (e.g., ECP and EDN), which often detect the presence of these proteins even in asthma patients with neutrophilic bronchitis, EPX-based ELISA levels are not increased in this subset of asthma patients or in COPD patients lacking evidence of an airway eosinophilia. Moreover, sputum EPX was a surrogate marker of airway eosinophilia in other patient studies (e.g., allergen inhalation and treatment trials the anti-(IL-5) therapeutic Mepolizumab™). Finally, EPX levels in cyto-centrifuged prepared sputum supernatants correlated with those from rapidly prepared non-centrifuged filtrates of sputum (rs 0.94).
Conclusions
EPX-based ELISA is a valid, reliable, repeatable, and specific surrogate marker of eosinophils and/or eosinophil degranulation in the sputum of respiratory patients.
Keywords: Sputum Eosinophils, EPX, ELISA, Asthma, COPD
INTRODUCTION
The presence of eosinophils in the airway is often an indicator of response to treatment with corticosteroids in patients with a variety of airway diseases such as asthma (1), COPD (2), and chronic cough (3). This airway eosinophilia is also often a predictor of response to therapies that indirectly target eosinophils such as anti-(IL-5) monoclonal antibodies (4–6). Indeed, assessments of airway eosinophils may provide a singularly valuable metric with which to manage the care of asthma patients (7). Airway eosinophils are reliably and non-invasively identified by quantitative cell counts and differentials of sputum recovered from patients (8). Therapeutic strategies that employed sputum eosinophil cell counts to guide treatment of asthma (9, 10) and COPD (11) patients led to significantly better clinical outcomes than current guideline based therapies. However, despite this enhanced efficacy, these assessments are not widely used in clinical practice because of the generally perceived view that they cannot be implemented in routine practice of most clinical settings (12).
We have recently developed (13) an ELISA-based strategy to quantify eosinophil peroxidase (EPX) and, in turn, detect the presence of eosinophils and/or evidence of eosinophil degranulation (14). We report here the potential clinical utility of this assay as a sensitive and reliable measure of eosinophil activation (i.e., degranulation) in sputum. In particular, we confirmed the observation we previously reported (13) that unlike assessments of ECP or EDN using commercially available ELISA kits, EPX-based ELISA of sputum is eosinophil-specific, representing a quantitatively accurate biomarker of sputum eosinophilia and/or eosinophil activities that can be performed rapidly. Significantly, EPX-based ELISA was also equally responsive in both laboratory-based (i.e., cyto-centrifuged) sputum supernatants and non-cytocentrifuged DTT-dispersed filtrates collected using a sputum filtration kit (Accufilter® (Cellometrics Inc, Hamilton, ON)). Collectively, these data showed that EPX-based ELISA of sputum is an easy to use assay that is sensitive, reproducible, and eosinophil-specific. These data also suggested that EPX-based ELISA will be a more efficacious alternative to sputum cell counts/differentials and applicable to routine clinical practice as a point-of-care diagnostic test to measure airway eosinophils/degranulation or as a means to monitor responses to available therapy(ies).
METHODS
Study subjects
Three groups of patients were recruited to obtain clinical samples for this study. Sputum was obtained from 43 subjects with asthma (with various intensities of eosinophilia or neutrophilia), 11 subjects with non-asthmatic COPD (with various intensities of eosinophilia), and 8 non-asthmatic healthy control subjects with normal sputum cell counts. The allergen responsiveness studies included 6 subjects who underwent an allergen inhalation in a cross-over study, and 13 subjects who participated in a randomized clinical trial of Mepolizumab™ (5 received Mepolizumab™ and 8 received matching placebo) (6). Plasma and serum samples were obtained from an additional group of 20 patients (10 with baseline eosinophil counts in sputum (normal controls) and 10 with high eosinophil counts in sputum (asthma patients)). In this report, asthma is defined based on a PC20 following methacholine challenge of <8 mg/ml or a >15% (and 200ml) improvement in FEV1 after inhaling a short-acting bronchodilator. Non-asthmatic COPD was defined as <12% improvement in FEV1 following administration of a short-acting bronchodilator and a post-bronchodilator FEV1/FVC of <70%. All subjects provided consent to use their sputum samples for biomarker discovery and the studies were approved by the Firestone Institute for Respiratory Health, St. Joseph’s Healthcare & McMaster University Hospital Research Ethics Board. A summary overview of the clinical characteristics of the study subjects is provided in Table 1.
Table 1.
Demographic Data on Control and Respiratory Disease Study Subjects†
| Variables | Assay Validation Studies | Comparison Studies | Responsiveness Studies | |||||
|---|---|---|---|---|---|---|---|---|
| Asthma (n = 30) | COPD (n = 11) | Healthy Controls (n = 8) | Assessments of Sputum Supernatants vs. Filtrates (n = 43) | Assessments of Plasma (n = 20) vs. Serum (n = 20) | Mepolizumab™ (n = 13) | Allergen (n = 6) | ||
| Plasma | Serum | |||||||
| Number of Males/Females | 9/21 | 2/9 | 4/4 | 17/28 | 9/11 | 5/15 | 8/5 | 3/3 |
| Age (years) | 46.0 ± 17.2 | 68.8 ± 4.7 | 54.0 ± 14 | 55.8 ± 16.8 | 46.7 ± 14.4 | 49.0 ± 15.4 | 58.5 ± 8.9 | 34.2 ± 12.6 |
| FEV1 (L) | 2.7 ± 1.1 | 1.6 ± 0.4 | 2.5 ± 1.1 | 1.9 ± 0.9 | 3.0 ± 0.9 | 2.2 ± 0.9 | 2.0 ± 0.8 | 3.4 ± 0.4 |
| FEV1 (%) | 84.9 ± 24.0 | 65.2 ± 21.1 | 86.9 ± 10.3 | 63.5 ± 29.3 | 87.1 ± 19.3 | 75.5 ± 23.1 | 65.1 ± 18.3 | 89.3 ± 8.2 |
| FEV1/FVC (%) | 75.0 ± 12.2 | 54.7 ± 17.0 | 81.4 ± 8.8 | 65.1 ± 18.8 | 85.5 ± 10.8 | 65.8 ± 28.5 | 66.3 ± 16.4 | 79.0 ± 7.9 |
| TCC (×10−6/g)¶ | 10.3 ± 16.2 | 6.80 ± 6.4 | 6.1 ± 2.5 | 10.9 ± 17.1 | 5.6 ± 4.5 | 3.8 ± 2.1 | 6.63 ± 7.2 | 5.2 ± 5.3 |
| Viability (%) | 63.6 ± 15.6 | 52.1 ± 20.6 | 78.0 ± 12.0 | 67.2 ± 18.1 | 63.4 ± 17.7 | 56.2 ± 18.7 | 57.0 ± 18.7 | 49.7 ± 20.0 |
| Neutrophil‡ (%) | 49.3 ± 31.3 | 53.3 ± 25.4 | 55.8 ± 19.9 | 59.7 ± 29.6 | 48.6 ± 24.6 | 44.4 ± 27.6 | 50.6 ± 26.3 | 30.3 ± 17.7 |
| Eosinophil‡ (%) | 3.6 [6.4] | 8.1 [5.1] | 0.2 [0.1] | 4.7 [10.5] | 3.8 [5.8] | 16.0 [24.2] | 11.6 [17.7] | 11.7 [12.6] |
Data are represented as mean ± SD, except for the % eosinophil which is expressed as median [IQR]
TCC - total cell count (×10−6/gram of sputum)
% of total sputum leukocytes
Study design
The validity of EPX-based ELISA assessments was established by comparing the levels of EPX (as determined by our ELISA) with the number of eosinophils (and/or eosinophil percentage) in the sputum of patients. Sputum samples with a range of eosinophils (>3%, 2–3%, 1–2%, 0–1%) and a total cell count of <10 × 106 cells/gram of sputum) were recovered from patients with asthma (n=30). Additional sputum samples were recovered from non-asthmatic COPD patients (n=11), and normal control subjects (n=8). Among the asthma patients examined we also included sputum samples in which free eosinophil granules where detectable in the absence of an available accurate differential cell count due either simply to their absence or recovery issues such as cell degeneration (n=13).
Specific metrics associated with the performance of the EPX-based ELISA were determined as a series of targeted experimental studies including assay sample and inter-observer repeatability, eosinophil-specificity of the assay, dynamic responsiveness of the assay, airway vs. systemic EPX levels as a function of disease, and ease of application to clinical samples.
Study methods
Sputum Recovery
As described in our previous studies (8), sputum from individual patients was induced by inhalation of hypertonic saline, selected from the expectorate, and processed for cell count, differential cell assessment, and recovery of cell-free supernatant. Briefly, the selection of sputum from the expectorate was performed using an inverted microscope. Recovered sputum was treated with ditheothreitol (DTT) to disperse the mucus for either processing by cyto-centrifugation (laboratory-based sputum supernatant) or filtration using an Accufilter® (Cellometrics Inc., Hamilton, ON Canada).
ELISA Assessments for ECP, EDN, and EPX
ECP and EDN sputum levels were determined using commercially available ELISA kits as per the manufacturer’s instructions (MBL International, Woburn, MA; www.mblintl.com). EPX-based ELISA assessments were performed with using antibodies and methodologies developed by our group (13).
Allergen Provocation
Allergen inhalations were performed as previously described (14).
Treatment of patients with anti-(IL-5) monoclonal antibody therapy (Mepolizumab™)
Patients participating in a randomized clinical trial (6), comparing the prednisone-sparing effect of Mepolizumab™ (n=9) relative to placebo (n=10), were used to assess the response of the EPX-based ELISA to this treatment. As a result of the present study being a post hoc analysis, patient sputum samples were available only for 5 subjects who received Mepolizumab™ and 8 subjects who received the placebo.
Statistical Analysis
Comparisons between paired data were made by paired t-test and between unpaired data by the Mann-Whitney U test. In addition, between groups comparisons were made using analysis of variance (ANOVA). In some cases, the significance of correlations between data sets was determined using Pearson or Spearman tests. The reproducibility of experimentally derived values was assessed using intraclass correlation coefficients (ICC). Statistical tests were done using the SPSS software, version 16.0 (Chicago, IL). p < 0.05 was considered significant.
RESULTS
EPX sputum levels correlate to the presence of sputum eosinophil numbers
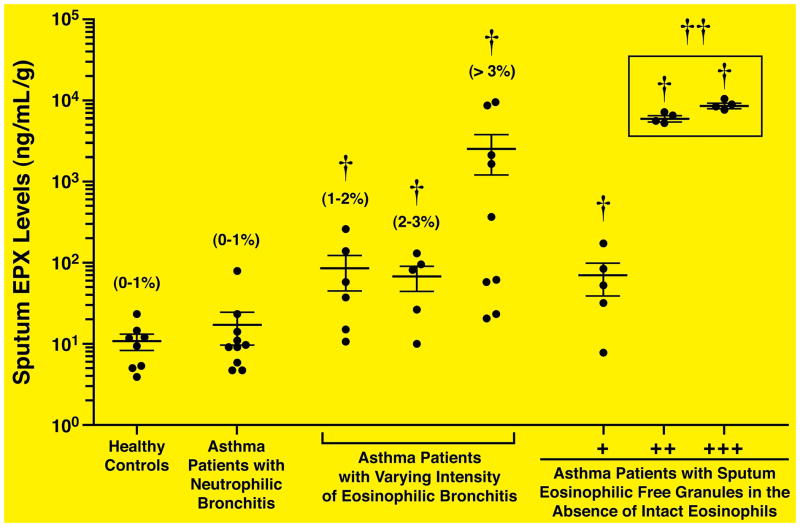
The utility of our novel EPX-based ELISA as a quantitative surrogate biomarker reflective of sputum eosinophila in clinical trial subjects was assessed in respiratory patients treated in the outpatient clinic of the Firestone Institute for Respiratory Health (St. Joseph’s Healthcare & McMaster University). Figure 1 showed that sputum EPX levels (ng of EPX/mL of sputum supernatant/gram of sputum (ng/mL-g)) is an accurate surrogate biomarker of sputum eosinophilia capable of distinguishing asthma patients with sputum eosinophilia from either normal healthy controls or patients identified as asthmatic with sputum neutrophilia (p<0.05). The mean, median, and standard deviation of sputum EPX levels in the healthy control subjects were 10.8ng/ml-g, 10.7ng/ml-g, and 6.5ng/ml-g, respectively. Thus, the upper limit of sputum EPX in healthy individuals is estimated to be ± 30.3ng/mL-g (i.e., three times the standard deviation above the measured mean value). Significantly, Figure 1 also includes assessments of diagnostically problematic asthma subjects (i.e., patients whose sputum cell differentials revealed evidence of eosinophil granules without the presence of identifiable intact eosinophils) also clearly showed that our EPX-based ELISA was capable of accurately linking this cohort of respiratory subjects with significant levels of occult eosinophils (p<0.05). Receiver Operating Characteristic (ROC) analysis demonstrated that sputum EPX levels ≥57ng/mL-g had 84% sensitivity and 84% specificity to identify a sputum eosinophil count of 3% (Supplemental Figure 1).
Figure 1. Sputum EPX levels increase in respiratory patients as a function of airway eosinophils and/or eosinophil-associated activities.
EPX-based ELISA was used to determine sputum EPX levels in cohorts of patients participating in clinical studies as part of their care in a pulmonary clinic. The clinical details of patient cohorts examined are described in the Materials and Methods section (Study Subjects) and Table 1. The data are presented as a scatter plot of the individual patients, presenting the mean with the error bars corresponding to the standard error of the mean for the data set in question. The airway eosinophilia of each cohort (percent (%) of total sputum leukocytes) is noted above the data derived from each patient cohort. The number of granules in asthma patients whose sputum cell evaluations failed to identify intact eosinophils were quantified as containing few (+), moderate (++), or many (+++) granules by a technologist who was blinded to the clinical details. †Significant difference (p < 0.05) relative to healthy controls. †† Significant difference (p < 0.05) relative to mild asthmatics with a 1–2% sputum eosinophilia.
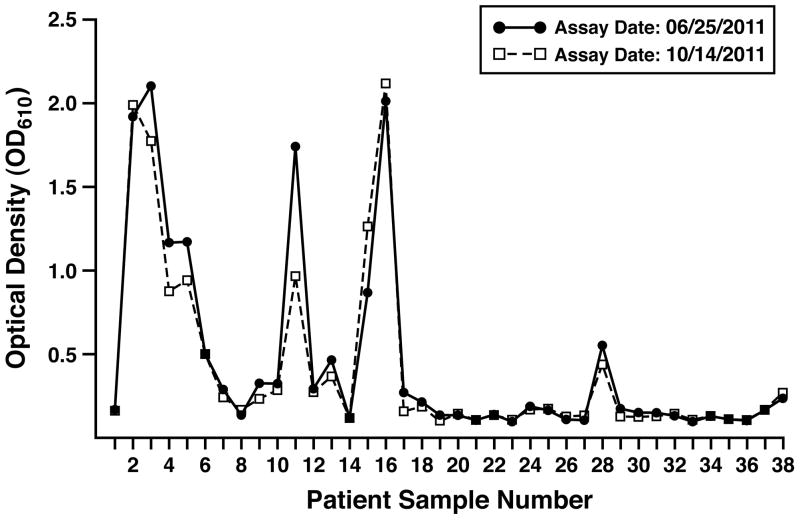
The reproducibility of the EPX-based ELISA measurements among these patients was determined as a function of two parameters: (i) the ability to obtain similar values following a freeze-thaw cycle of the sputum sample and (ii) the stability of the sputum samples stored for extended periods of time archived as a biospecimen stored at −70°C. Figure 2 plots the absolute values of the optical density response from the EPX-based ELISA derived from each patient following a successive freeze-thaw cycle separated by nearly four months. This study showed that the two data sets were remarkably similar (ICC = 0.9), and thus, EPX-based ELISA is a useful diagnostic tool for the evaluation of archived sputum samples even following multiple freeze-thaw cycles.
Figure 2. EPX-based ELISA represents a reproducibly robust assay capable of assessing EPX levels from archived sputum samples.
Sputum EPX levels derived from the patients of our study cohorts were assessed following consecutive freeze-thaw cycles (freeze-thaw 1 and 2) separated by a 4 month archive period at −70°C. The congruence of the repeated measurements (ICC = 0.9) also attested to the stability of the archived samples.
Assessments of sputum EPX levels in asthma patients (Supplemental Figure 2) demonstrated a significant correlation with the sputum eosinophilia in these patients (rs=0.76, p<0.001). Nonetheless, this was somewhat lower than the correlation displayed following the acute response obtained via segmental bronchial allergen challenge (rs=0.93 (13)), suggesting that the collective EPX-signature from sputum is likely derived from a dynamic mixture of accumulated intact eosinophils, EPX released via degranulation, and cells undergoing cytolysis; a conclusion also supported by evidence of sputum EPX in some patient samples devoid of intact eosinophils (see Figure 1).
Assessments of EPX levels in sputum, unlike assays of ECP and/or EDN levels, are eosinophil specific and diagnostic of respiratory patients
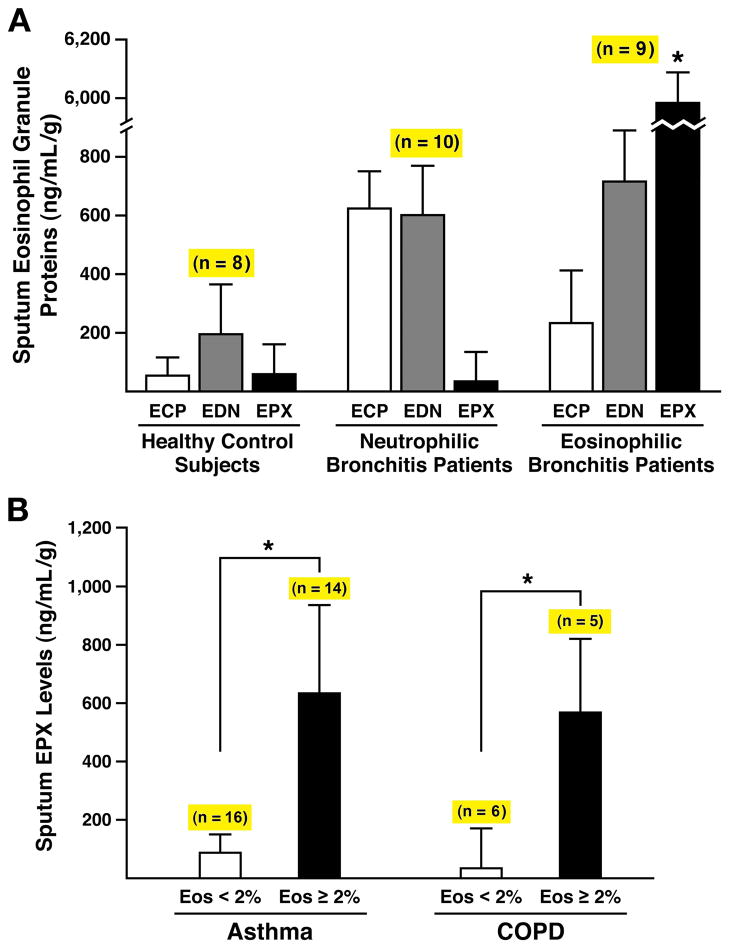
The usefulness of any assay reflective of eosinophils and/or eosinophil activation is absolutely dependent on the eosinophil-specific character of that biomarker. Our previous observations with EPX-based ELISA showed it to be an absolutely eosinophil-specific assay (13) and potential clinical value of this unique eosinophil-specificity was assessed relative to commercially available “eosinophil-specific” assays detecting either ECP or EDN (Note: neither of these assays are currently approved for clinical use). The data summarized in Figure 3(A) showed that EPX sputum levels were a robust surrogate marker reflective of sputum eosinophilia (rs=0.84, p<0.001). Specifically, high levels of EPX were detected in asthma patients with eosinophilic bronchitis ((<10 × 106 total cells/gram of sputum, ≥3% eosinophils, <3% neutrophils) and correspondingly low levels of EPX were detected in sputum from healthy control subjects as well as asthma patients with neutrophilic bronchitis ((>25 × 106 total cells/gram of sputum, ≥3% eosinophils, >80% neutrophils). Interestingly, these increased levels of EPX in asthma patients with eosinophilic bronchitis were restricted to sputum assessments. That is, serum and/or plasma levels of EPX in these subjects remained unchanged relative to healthy control subjects (Supplemental Figure 3) and we also failed to detect EPX in exhaled breath condensate from patients even with an intense sputum eosinophilia (data not shown). In contrast to the EPX assay, we have previously demonstrated (13) that while assessments of ECP and EDN clearly detected the presence of eosinophils these assays are definitively not eosinophil-specific. Assessments of sputum ECP and EDN levels using the commercially available ELISA kits confirmed these earlier data and were unable to discriminate respiratory patients with sputum eosinophilia from those with sputum neutrophilia (Figure 3(A)). This unique ability of EPX assessments to correlate with evidence of eosinophils in the sputum of asthma patients was also extended to a subset of subjects with COPD who concurrently displayed a sputum eosinophilia. These data showed that sputum EPX levels were elevated in subsets of both asthma and COPD patients as a function of the displayed sputum eosinophilia (Figure 3(B)).
Figure 3. ELISA assessments of the prominent eosinophil secondary granule protein levels in the sputum of respiratory patients demonstrated that EPX-based ELISA is the only “eosinophil-specific” assay capable of unambiguously identifying subsets of respiratory patients based on eosinophil involvement.
(A) Sputum levels (ng of eosinophil secondary granule protein/mL of supernatant/gram of sputum (ng/mL-g)) of ECP and EDN were determined using commercially available ELISA kits and compared to the data obtained with our EPX-based ELISA from three patient cohorts: Control subjects (i.e., healthy patients with no evidence of respiratory and/or allergic disease (<10 × 106 total cells/gram of sputum, <1% eosinophils, <3% neutrophils), neutrophilic bronchitis patients who displayed nominal improved airflow in response to a short acting beta-agonist (<12% increase in FEV1) and were unresponsive to steroid intervention (>25 × 106 total cells/gram of sputum, <3% eosinophils, >80% neutrophils), and eosinophilic bronchitis patients who displayed improved airflow in response to a short acting beta-agonist (>15% increase in FEV1) and also had symptomatic improvement following steroid intervention (<10 × 106 total cells/gram of sputum, ≥3% eosinophils, <3% neutrophils. The number of subjects (n) is shown above the histograms derived from each patient cohort. *Significant increase (p<0.0001) relative to levels observed in neutrophilic bronchitis patients. (B) Sputum EPX levels increased as a function of sputum (i.e., airway) eosinophilia independent of the diagnosed respiratory disease. Manual cell counts and differentials of sputum from asthma and COPD patients were used to stratify each cohort on the basis of a sputum eosinophilia (< or >2% of total of sputum leukocytes). The number of subjects (n) is shown above the data derived from the stratified patients within each cohort. *p<0.001.
The responsiveness of the EPX-based ELISA is sufficiently robust to be used as an outcome measure in clinical trials
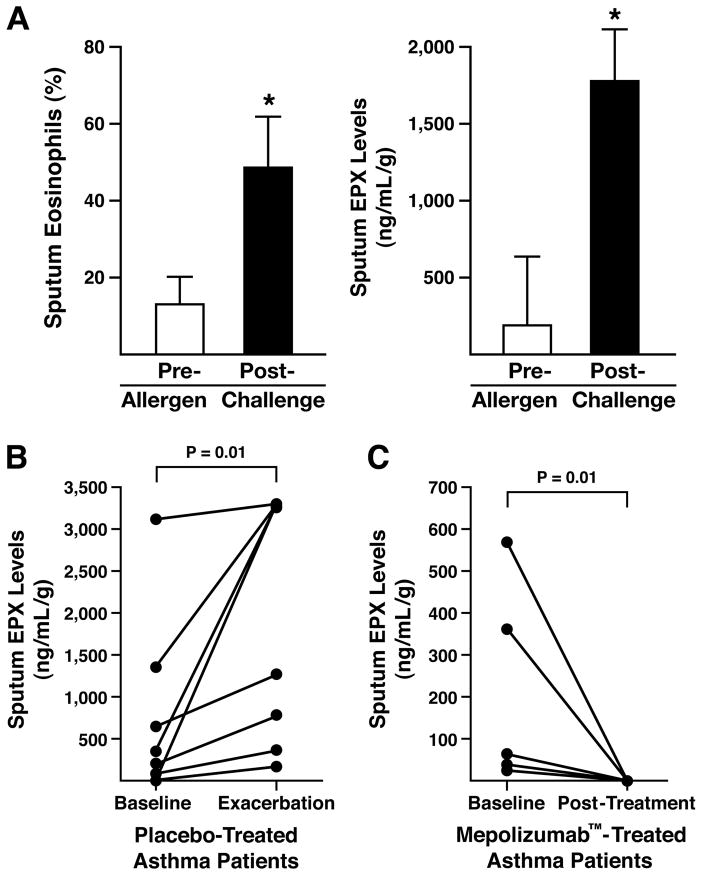
The responsiveness of the EPX-based ELISA in a clinical setting was determined in a series of post hoc assessments of sputum samples derived from two clinical studies. In the first of these post hoc assessments, we evaluated EPX sputum levels in allergic asthma patients before and after allergen-challenge. These data showed (Figure 4(A)) that as expected sputum EPX levels increased after allergen challenge in these patients as a function of the increasing number of sputum eosinophils accumulating in the airways. In a second post hoc assessment, data from a Mepolizumab™ clinical trial (6) was used to compare baseline sputum EPX levels in placebo-treated asthma patients at the start of an interventional study relative to the EPX levels determined at the first exacerbation event requiring these patients to seek medical treatment. The data showed that similar to the observed increase in sputum eosinophilia accompanying the exacerbation events among these patients (6), significant increases in sputum EPX levels were observed among the eight patients studied (Figure 4(B)). Sputum EPX levels in patients receiving Mepolizumab™ was decreased with 24 weeks of treatment to virtually zero (Figure 4(C)). The Spearman’s correlation coefficient between the change in sputum eosinophil % (pre-post intervention) and change in sputum EPX (pre-post intervention) was 0.7 (p<0.01).
Figure 4. Sputum EPX levels represented a reliable biomarker for the diagnostic evaluation of clinical study subjects.
(A) Post hoc assessments of asthma patients (n = 10) undergoing aeroallergen provocation as part of an investigatory study in a pulmonary clinic setting (6) demonstrated that relative to pre-challenge levels the post-challenge increase in sputum EPX accurately reflected the pre- vs. post-challenge increase in sputum eosinophil levels observed in these same patients. *p<0.01. (B) Sputum EPX levels (ng/mL-g) from post hoc assessments of asthma patients participating in a clinical study evaluating the anti-IL-5 therapeutic Mepolizumab™ (6). Patients receiving placebo (n = 8) were compared at the start of the study (baseline) and the first exacerbating event leading these patients to return to a clinical setting (exacerbation). *p<0.01. (C) Post hoc assessments of sputum EPX levels (ng/mL-g) from a cohort of asthma patients treated with Mepolizumab™ (n = 5) were also compared at the start of the study (baseline) and to the levels observed 24 weeks after the initiation of treatment. *p<0.01.
EPX-based ELISA represents an easy to use diagnostic assay of respiratory patients using sputum samples derived from rapid filtration-based fractionation
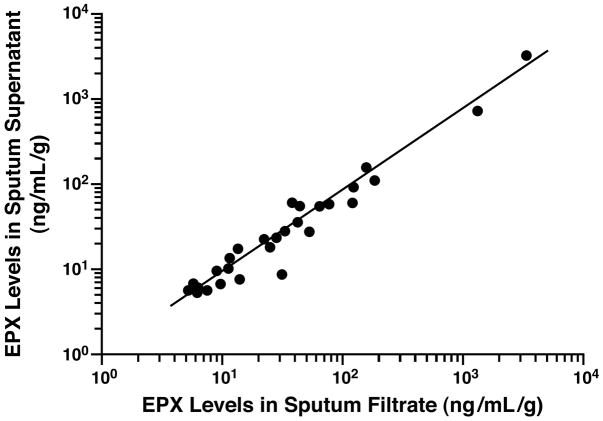
We evaluated the utility of the EPX-based ELISA to evaluate sputum samples in clinical settings with limited equipment and/or technology-based experience. In these studies, EPX levels were determined in sputum recovered from a syringe-based filtration unit (Accufilter®) relative to collecting sputum using established laboratory-based methods that include cyto-centrifugation. Figure 5 presents a graphical side-by-side comparison of the EPX-based ELISA assessments derived from these two sputum recovery methods. These data showed a significant correlation exists between the two assessments (rs = 0.94, p = 0.001), suggesting that accurate and reproducible EPX-based ELISA measurements are possible even with minimally processed sputum samples.
Figure 5. EPX levels in sputum filtrates recovered from a syringe-based filtration unit (Accufilter®) is not significantly different relative to the levels observed in sputum supernatants collected using established laboratory-based methods that include cyto-centrifugation.
Sputum was recovered by filtration vs. laboratory-based cyto-centrifugation from each patient prior to the determination of EPX levels (ng/mL-g). The resulting EPX levels were plotted relative to one another with (●) representing data from an individual patient and the line derived from a linear regression of the collective data set comparing these sputum processing strategies (rs = 0.94, p = 0.001).
DISCUSSION
The recent published reports of clinical trials utilizing antibodies specific for IL-5 demonstrated the potential importance/need to “phenotype” asthma based on the presence and/or absence of airway eosinophils ((4–6), reviewed in (15)). Nonetheless, these positive correlations have not led to the use of quantitative sputum eosinophil counts as a widely employed diagnostic strategy in patient care. As noted earlier, this failure likely stems from logistical issues including the loss of eosinophil integrity in some samples, the labor/equipment intensive character of sputum cell assessments, and the consensus that manual cell differentials cannot be implemented in routine practice (16). Surrogate biomarkers purportedly representative of pulmonary eosinophils have also been shown to be problematic in clinical settings. Fractional nitric oxide concentration in exhaled breath (FENO) is a widely regarded example of an easy to perform assay presumed reflective of disease severity (17). However, our recent studies have shown a significant disconnect between eosinophils and FENO levels in severe prednisone-dependent asthmatic patients (18), suggesting that FENO levels are not necessarily a valid surrogate biomarker of pulmonary eosinophil activities and, in turn, disease severity. Nonetheless, because treatment strategies based on the identification of sputum eosinophils have been demonstrated to be more effective than current guideline-based therapies for the management of asthma and COPD (19), it is likely that using a suitable surrogate biomarker of this airway eosinophilia would represent equally effective treatment strategies.
The use of our novel EPX-based ELISA is a reproducibly sensitive biomarker of sputum eosinophilia that represents an advancement over other potential diagnostic options:
Existing assays such as the commercially available ELISAs for ECP (20) and EDN (21) are not specific to eosinophils with both of these proteins detected in neutrophils (20, 21); as such, these assays are not approved for clinical use. Despite the development of an MBP-specific ELISA >20 years ago commercial versions of this assay are not available as a consequence of technical/biochemical issues (e.g., MBP solubility issues of post collection processing of samples (22)). This post-collection processing is a relatively complex procedure that is not easily performed outside a research lab environment. Finally, several studies have also demonstrated that while MBP is an abundant eosinophil granule protein, significant expression is also observed in other cells resident in the lungs of asthmatic such as mast cells and basophils (23, 24).
We extended the clinical utility of EPX-based ELISA over intact cell differentials by identifying eosinophil degranulation in degenerated samples of asthmatics with little to no intact cells available in their sputum.
The link between sputum eosinophils and observed EPX-levels was also not restricted to allergic airway diseases as even the subset of respiratory patients with COPD who displayed a concomitant airway eosinophilia were also identifiable using this EPX-based ELISA.
Sputum EPX levels in asthma patients were uniquely elevated in subjects with >3% sputum eosinophils relative to asthma patients with 1–2% or 2–3% eosinophils. In addition, EPX levels did not significantly vary among subjects with 1–3% sputum eosinophils confirming the widely held belief that 3% airway eosinophilia may be clinically relevant.
In summary, the studies presented provide evidence that this assay is a valuable biomarker easily adapted to a wide-variety of clinical practice settings. Indeed, our demonstration that DTT-dispersed sputum samples that are “quick-filtered” had levels of EPX comparable to those that are prepared by established laboratory protocols has significant consequences. That is, by bundling the EPX-based ELISA with the simple dispersion of sputum followed by filtration, we provide a rapid and easily performed high throughput assessment of patients that may be performed in research settings, clinical venues such as hospitals and doctor’s offices, and even potentially by patients themselves at home as part of self-management strategies of their disease.
Supplementary Material
Clinical Implication.
The novel EPX-assay is a valid and reproducible eosinophil-specific assay that can potentially be developed into a point-of-care assessment of eosinophil activity in airway secretions.
Acknowledgments
We wish to thank members of the Nair laboratory and integrated Lee Laboratories program for insightful discussions and critical comments. We also wish to acknowledge the invaluable assistance of the Mayo Clinic Arizona medical graphic artist (Marv Ruona), and the excellent administrative support provided by Linda Mardel and Shirley (“Charlie”) Kern. The Canada Research Chair Program, the Mayo Foundation, and grants from the United States National Institutes of Health [NAL (HL058723) and JJL (HL065228, RR0109709)] and the American Heart Association [NAL (05556392) and JJL (0855703)] were the sources of funding used in the performance of studies as well as data analysis.
Footnotes
Author Contributions
The corresponding authors (PN and JJL) had full access to all of the data reported in this study. PN, SIO, NAL, and JJL, designed the research study; PN, SIO, CP, KR, and AE performed the research; PN, SIO, NAL, and JJL analyzed the data; PN, NAL and JJL wrote the initial draft of the manuscript, and PN, SIO, CP, KR, AE, NAL, and JJL provided critical assessments during subsequent revisions leading to the final submitted manuscript. PN, SIO, CP, KR, AE, NAL, and JJL provide final approval of the final version of the manuscript submitted to Allergy.
Dedication
This manuscript is dedicated to the memory of Professor Freddy Hargreave who aspired to see a simple sputum eosinophil marker developed that could be used in routine clinical practice to enhance patient care. His encouragement and constantly optimistic view of life will be missed dearly by his family, friends, and colleagues.
Financial Disclosure: P. Nair is supported by a Canada Research Chair in Airway Inflammometry. P. Nair and A. Efthimiadis are listed on a patent for a sputum filtration device, Accufilter®. N.A. Lee and J.J. Lee are supported by the Mayo Foundation and grants from the United States National Institutes of Health [NAL (HL058723) and JJL (HL065228, RR0109709)] and the American Heart Association [NAL (05556392) and JJL (0855703)].
References
- 1.Kelly MM, Leigh R, Jayaram L, Goldsmith CH, Parameswaran K, Hargreave FE. Eosinophilic bronchitis in asthma: a model for establishing dose-response and relative potency of inhaled corticosteroids. J Allergy Clin Immunol. 2006;117(5):989–94. doi: 10.1016/j.jaci.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Leigh R, Pizzichini MMM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. European Respiratory Journal. 2006;27(5):964–971. doi: 10.1183/09031936.06.00072105. [DOI] [PubMed] [Google Scholar]
- 3.Pizzichini M, Pizzichini E, Parameswaran K, Clelland L, Efthimiadis A, Dolovich J, et al. Nonasthmatic chronic cough: No effect of treatment with an inhaled corticosteriod in patients without sputum eosinophilia. Canadian Respiratory Jouranal. 1999;6(4):323–330. doi: 10.1155/1999/434901. [DOI] [PubMed] [Google Scholar]
- 4.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro M, Mathur S, Hargreave F, Boulet L-P, Xie F, Young J, et al. Reslizumab for Poorly Controlled, Eosinophilic Asthma. American Journal of Respiratory and Critical Care Medicine. 2011;184(10):1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 6.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 7.Petsky HL, Cates CJ, Lasserson TJ, Li AM, Turner C, Kynaston JA, et al. A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils) Thorax. 2010 doi: 10.1136/thx.2010.135574. [DOI] [PubMed] [Google Scholar]
- 8.Pizzichini E, Pizzichini M, Efthimiadis A, Hargreave F, Dolovich J. Measurement of inflammatory indices in induced sputum: effects of selection of sputum to minimize salivary contamination. European Respiratory Journal. 1996;9(6):1174–1180. doi: 10.1183/09031936.96.09061174. [DOI] [PubMed] [Google Scholar]
- 9.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 10.Jayaram L, Pizzichini MM, Cook RJ, Boulet L-P, Lemière C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. European Respiratory Journal. 2006;27(3):483–494. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 11.Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. European Respiratory Journal. 2007;29(5):906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 12.D’Silva L, Hassan N, Wang HY, Kjarsgaard M, Efthimiadis A, Hargreave FE, et al. Heterogeneity of bronchitis in airway diseases in tertiary care clinical practice. Can Respir J. 2011;18(3):144–8. doi: 10.1155/2011/430317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Moqbel R, Lacy P, et al. The Development of a Sensitive and Specific ELISA for Mouse Eosinophil Peroxidase: Assessment of Eosinophil Degranulation Ex Vivo and in Models of Human Disease. Journal of Immunological Methods. 2012;375(1–2):138–47. doi: 10.1016/j.jim.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockcroft DW, Murdock KY, Kirby J, Hargreave F. Prediction of airway responsiveness to allergen from skin sensitivity to allergen and airway responsiveness to histamine. Am Rev Respir Dis. 1987;135(1):264–7. doi: 10.1164/arrd.1987.135.1.264. [DOI] [PubMed] [Google Scholar]
- 15.Hargreave FE, Nair P. Point: Is measuring sputum eosinophils useful in the management of severe asthma? Yes. Chest. 2011;139(6):1270–3. doi: 10.1378/chest.11-0618. [DOI] [PubMed] [Google Scholar]
- 16.D’Silva L, Neighbour H, Gafni A, Radford K, Hargreave F, Nair P. Quantitative sputum cell counts to monitor bronchitis: a qualitative study of physician and patiend perspectives. Canadian Respiratory Journal. 2012 doi: 10.1155/2013/248167. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, et al. Asthma outcomes: Biomarkers. J Allergy Clin Immunol. 2012;129(3 Suppl):S9–S23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair P, Kjarsgaard M, Armstrong S, Efthimiadis A, O’Byrne PM, Hargreave FE. Nitric oxide in exhaled breath is poorly correlated to sputum eosinophils in patients with prednisone-dependent asthma. J Allergy Clin Immunol. 2010;126(2):404–6. doi: 10.1016/j.jaci.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 19.Nair P. Eosinophil-targeted treatment of asthma. In: Lee JJ, Rosenberg HF, editors. Eosinophils in Health and Disease. Waltham, MA: Elsevier; 2012. pp. 462–466. [Google Scholar]
- 20.Sur S, Glitz DG, Kita H, Kujawa SM, Peterson EA, Weiler DA, et al. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukoc Biol. 1998;63(6):715–22. doi: 10.1002/jlb.63.6.715. [DOI] [PubMed] [Google Scholar]
- 21.Leigh R, Belda J, Kelly MM, Cox G, Squillace DL, Gleich GJ, et al. Eosinophil cationic protein relates to sputum neutrophil counts in healthy subjects. J Allergy Clin Immunol. 2000;106(3):593–4. doi: 10.1067/mai.2000.109057. [DOI] [PubMed] [Google Scholar]
- 22.Wassom DL, Loegering DA, Solley GO, Moore SB, Schooley RT, Fauci AS, et al. Elevated serum levels of the eosinophil granule major basic protein in patients with eosinophilia. J Clin Invest. 1981;67(3):651–61. doi: 10.1172/JCI110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252(3):418–25. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Matsumoto K, Suto H, Tanaka K, Ebisawa M, Tomita H, et al. Gene expression screening of human mast cells and eosinophils using high-density oligonucleotide probe arrays: abundant expression of major basic protein in mast cells. Blood. 2001;98(4):1127–34. doi: 10.1182/blood.v98.4.1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.