Abstract
Objective
To examine the impact of a weight loss intervention that focused on daily self-weighing for self-monitoring as compared to a delayed control group among 91 overweight adults.
Design and Methods
The 6-month intervention included a cellular-connected “smart” scale for daily weighing, web-based weight loss graph, and weekly emails with tailored feedback and lessons. An objective measure of self-weighing frequency was obtained. Weight was measured in clinic at 3 and 6 months. Caloric intake and expenditure, and perceptions of daily self-weighing were also measured.
Results
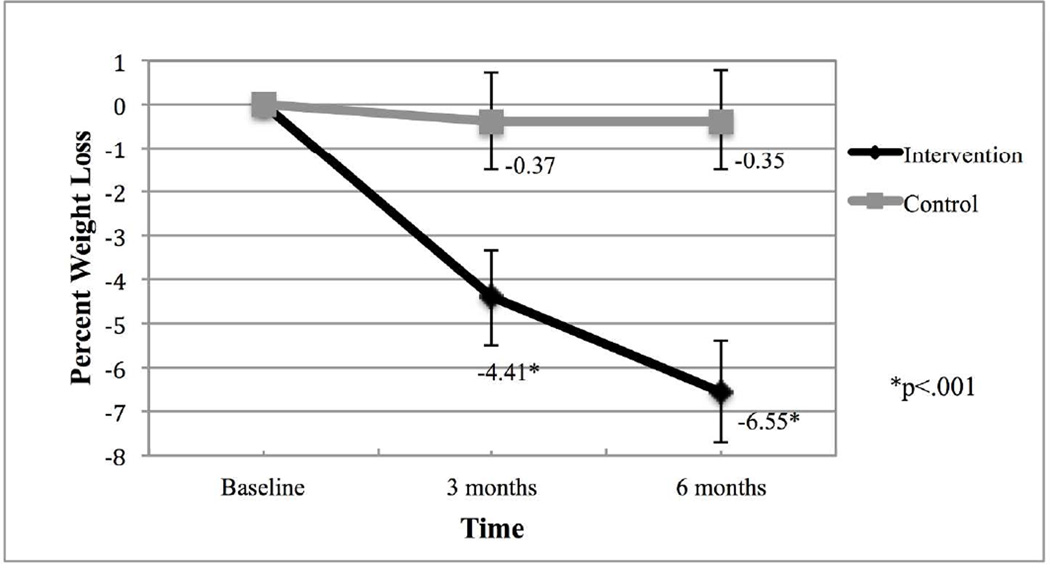
Using intent-to-treat analyses, the intervention group lost significantly more weight compared to the control group [Mean (95%CI); 3 months: −4.41%(−5.5, −3.3) vs. −0.37%(−1.5, .76); 6 months: −6.55%(−7.7, −5.4) vs. −0.35%(−1.5, .79); group×time interaction: p<.001] and a greater percentage achieved 5% (42.6% vs. 6.8%; p<.0001) and 10% (27.7% vs. 0%; p<.0001) weight loss. On average, the intervention group self-weighed more days/week (6.1±1.1 vs. 1.1±1.5; p<.0001) and consumed fewer calories/day compared to the control group [Mean (95% CI); 6 months: 1509 (1291,1728) vs. 1856 (1637,2074); group×time interaction: p=.006]. Among intervention participants, daily self-weighing was perceived positively.
Conclusions
These results indicate that an intervention focusing on daily self-weighing can produce clinically significant weight loss.
Introduction
Recent estimates indicate that 69% of Americans are overweight or obese.(1) This has major implications for the incidence and prevalence of chronic diseases such as heart disease and diabetes,(2) as well as some cancers.(3) Evidence indicates that even 5–10% weight losses can reduce risk factors for these diseases.(4&6) Standard behavioral weight loss interventions that include frequent face-to-face interactions with a trained weight loss counselor and detailed self-monitoring of diet and physical activity behaviors produce on average about 7–10% weight loss after 6–12 months,(7&9) however, the intensive nature of these interventions limits their potential for dissemination. This indicates a need for effective, lower intensity programs that reduce the burden of frequent face-to-face contacts and increases the potential for broader public health impact and reach.
One of the most effective strategies within most weight loss interventions is self-monitoring of diet, physical activity, and weight.(10, 11) Self-monitoring provides personal accountability and allows for greater awareness of how behaviors are impacting weight.(12) The mechanism of self-monitoring is suggested by Kanfer’s Model of self-regulation: self-monitoring provides feedback that allows for greater awareness, which can lead to greater self-efficacy, self-control, and self-initiated reinforcement.(13) Kanfer posits that individuals self-evaluate by comparing the feedback to the performance criterion, which allows for a judgment to be made that either results in positive or negative reinforcement of behaviors.(14, 15)
Despite being effective and theoretically grounded, detailed self-monitoring of diet and physical activity behaviors is difficult to sustain. Burke and colleagues found that adherence to self-monitoring protocols declines dramatically over time during participation in a behavioral treatment program for weight loss.(16, 17) Furthermore, qualitative evidence indicates that the labor intensive nature of self-monitoring leads to feelings of being overwhelmed, frustrated, and defeated.(18) Given poor adherence, a need exists for interventions that test simple and sustainable types of self-monitoring strategies.
Self-weighing is a simple self-monitoring behavior that has been shown to be useful for self-regulation of body weight.(19) Self-weighing provides feedback suggestive of how eating and exercise behaviors are impacting weight, and acts as a tool to allow individuals to make small adjustments to these behaviors to affect energy balance. Based on self-regulation theory, daily weighing is optimal over less frequent weighing because the feedback is more proximal, making it easier to attribute changes in weight to specific diet and physical activity behaviors. This allows for better self-regulation as small changes in body weight can be identified and resolved, likely leading to greater self-efficacy and empowerment over one’s ability to regulate their body weight.(20
A recent review of the self-weighing literature indicates that daily self-weighing is associated with greater weight loss compared to less frequent weighing.(21) The seminal study by Linde and colleagues found that individuals who reported daily self-weighing saw a significantly greater reduction in body mass index compared to those who reported weekly or never weighing.(22) Welsh and colleagues also examined the impact of self-weighing on weight loss post hoc within a phone-based weight loss intervention and found a similar dose response pattern.(23) These results suggest that daily self-weighing may be an effective self-monitoring strategy.
These studies examined the impact of daily weighing post hoc, which does not allow for strong control of the potential differences between those that chose to weigh daily and those that chose to weigh less often. Furthermore, a retrospective self-report measure of self-weighing frequency was used, which may have introduced systematic recall bias. Experimental evidence does indicate that daily self-weighing is effective for self-regulation of body weight during weight loss maintenance.(24) However, there is limited experimental evidence examining whether daily self-weighing is effective for weight loss, particularly when coupled with an intervention that does not include other forms of self-monitoring (25, 26)
The purpose of the WEIGH Study (Weighing Everyday to Improve and Gain Health) was to improve on previous evidence and test whether an intervention focusing on daily self-weighing as the main self-monitoring strategy can produce significantly greater weight loss compared to a delayed intervention control group. In order to test a lower intensity approach that emphasized daily self-weighing, we included no regular face-to-face contact, relied solely on tailored feedback, and did not emphasize self-monitoring of diet and physical activity behaviors. An objective measure of self-weighing frequency was utilized to provide an accurate assessment of this behavior. We hypothesized that the group receiving the intervention would have greater percent weight loss at 3 and 6 months compared to a delayed control group, as well as more positive changes in diet and physical activity behaviors.
Methods
Participants
Inclusion criteria included men and women ages 18–60 with a body mass index (BMI) between 25–40 kg/m2 and a maximum weight of 330 lbs. (the maximum weight allowable on the scales provided). Participants were also required to have access to the Internet to allow for weight tracking. Exclusion criteria included having a pre-existing medical condition (myocardial infarction within the past 2 years, cancer diagnosis (non-skin) within the past 5 years, uncontrolled high blood pressure, unstable thyroid disease, current treatment for psychiatric disorder other than depression, hospitalization for depression within the past year, or history of eating disorder), pregnant or nursing within the past 6 months or planning to become pregnant, currently undergoing treatment for substance abuse, or planning to move out of the area. Individuals were also excluded if, within the past 6 months, they participated in a structured weight-loss program or lost and kept off at least 10 lbs. of their body weight.
Recruitment
Participants were recruited via 1) an advertisement on a university listserv, 2) flyers posted around UNC Chapel Hill, and 3) flyers posted in medical offices in the Chapel Hill, NC area. Those who were eligible and interested were invited to an orientation session where they were provided with more details about the study and informed consent was obtained. Baseline measures were obtained prior to randomization being revealed to participants by blinded evaluation staff. Data were collected between February 2011 and November 2011 in Chapel Hill, NC. The University of North Carolina at Chapel Hill Institutional Review Board approved and monitored the study. Participants received $25 as an incentive for completion of follow-up assessments at 3 and 6 months.
Study Design and Intervention Description
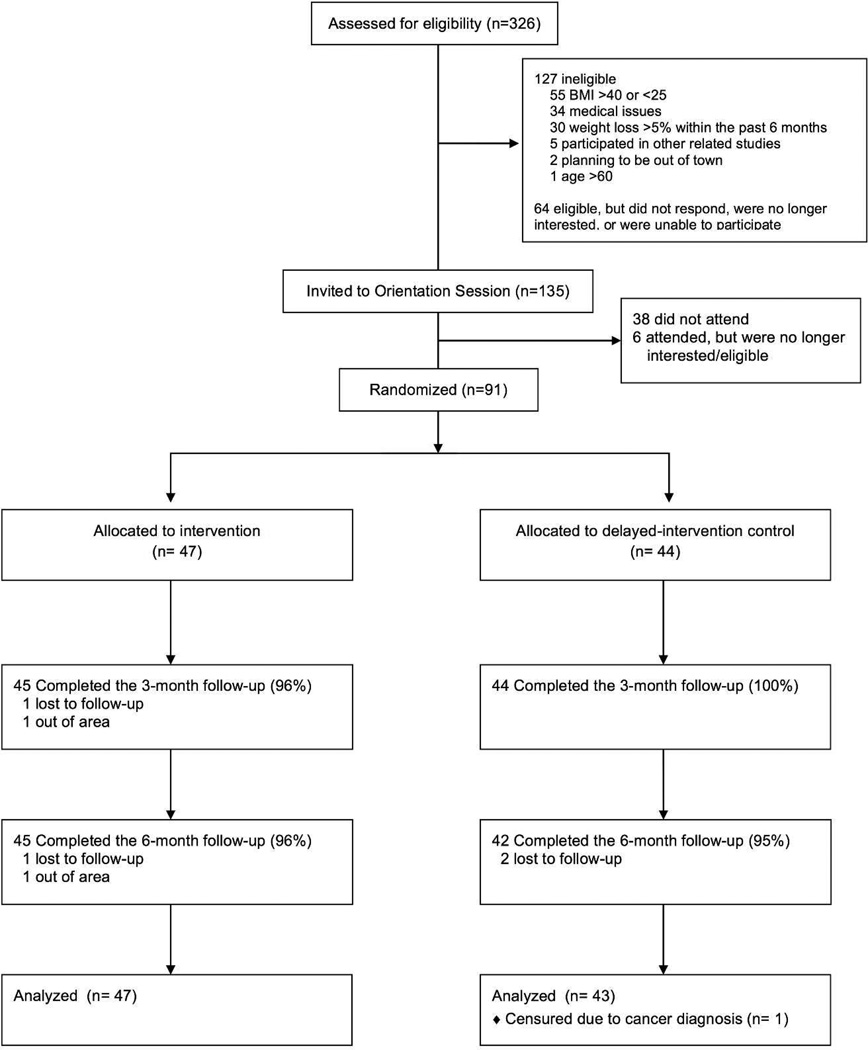
Participants were randomized to one of two treatment groups (Figure 1): An intervention group or a delayed intervention control group. The 6-month intervention consisted of 4 main components: (1) cellular-connected “smart” scales for daily weighing; (2) web-based graph of weight trends overtime; (3) weekly tailored feedback via email on self-weighing frequency and weight loss progress; and (4) 22 weekly lessons on behavioral weight control via email. Intervention participants were instructed to weigh daily at the same time each day using the smart scales. The smart scale displayed current weight and sent it directly to a website (www.bodytrace.com) via the wireless cellular network. The scales did not rely on an individual’s cell service, but rather were connected to the Bodytrace website via a separate cell service embedded in the scales. This allowed participants to use the scales in any location that had cell service. Participants were able to view on the website a graph of weight trends overtime.
Figure 1.
Study Enrollment and Retention Diagram
Weight and weighing data were accessible for each participant using a separate researcher interface. Each week a research assistant collected data on how often the participant weighed and their average and weekly weight loss. An algorithm was used to provide tailored feedback to each participant with the expected rate of weight loss at 0.5 lbs. per week and the expected self-weighing frequency at 6–7 days per week. Participants were placed in one of four categories each week based on their self-weighing frequency over the past week and average weight loss per week. They received messages and recommended strategies appropriate for that category. Participants who weighed daily and were losing at least 0.5 lbs. per week on average received feedback messages reinforcing their current behaviors.. Conversely, for those who were not losing weight or weighing daily, the emails included more specific strategies for adopting daily weighing and making changes to diet and physical activity behaviors.
Initially, participants attended a group session that included measurement of baseline weight, procurement of smart scales, and a 30-minute discussion about calorie balance. The weekly emailed lessons on behavioral weight control provided further skills training strategies for making changes to diet and physical activity behaviors. The lessons were initially derived from the Diabetes Prevention Program(27) and adapted from other online and face-to-face weight loss interventions(28&30) to be tailored to the goals of this study. Lessons were both informational and behavioral, and included topics such as portion control, restaurant eating, structured exercise, problem solving, stimulus control, and relapse prevention. Participants were not encouraged to self-monitor diet and exercise, but were provided with information about energy balance for weight loss. Specifically, participants were instructed to utilize the scale to determine if they should make adjustments to caloric intake and energy expenditure to achieve weight loss. In order to achieve weight loss, they were provided with recommendations to decrease their calories to an appropriate level (1200–1500 calories per day) and gradually increase physical activity overtime towards the goal 150–200 minutes of moderate-intensity exercise per week. Examples of calorie-controlled meal plans were also provided to further help guide food choices. The skills training and other feedback were included to insure that participants were well equipped to be able to make the behavior changes necessary for weight loss using the scale to guide their choices. After the study was complete, intervention group participants were followed up 3 months later (at 9 months) to assess short-term weight loss maintenance. During this maintenance period, intervention participants retained the smart scales but were provided with no further feedback or lessons.
The delayed intervention control group was also provided with the scales at baseline for evaluation purposes only and instructed to maintain their current self-weighing habits. Control group participants received no intervention during the study period and were provided with a modified version of the program after 6 months. They were blinded to the focus of daily weighing during the 6-month period.
Measures
Demographics
At baseline, a variety of demographic variables were collected to help characterize the sample including age, gender, race/ethnicity, education, marital status, occupation status, and comorbidities.
Anthropometrics
Height was collected at baseline using a wall-mounted stadiometer. Weight was measured to the nearest 0.2 lbs. using a digital scale wearing light clothes and no shoes at baseline, 3, and 6 months in the study center clinic. At 9 months, in order to assess maintenance effects within the intervention group, smart scale data were used to obtain an objective measure of weight after a 3-month period of no intervention.
Frequency of weighing
Self-weighing frequency was measured objectively in both groups via the smart scales throughout the 6-month study period. There were some technical problems with the scales that arose because of the reliance on cellular service to provide an objective measure of self-weighing frequency. However, such problems occurred in a small number of participants (n=4), and they were able find alternative places to weigh (e.g., work) to provide an objective measure of self-weighing frequency. Between 6–9 months, objective data on self-weighing frequency were obtained from intervention participants only. For those without data, we conservatively assumed that participants were not self-weighing.
Diet
Caloric intake was measured via two, 24-hour recalls on one weekday and one weekend day using the Automated Self-Administered 24-Hour Dietary Recall (ASA-24) from the National Cancer Institute (NCI) at baseline, 3 and 6 months.(31) Interviewer-administered 24-hour recalls have been shown to be a good estimate of changes in caloric intake using the automated multiple-pass method.(32
Physical Activity
At baseline, 3, and 6 months, exercise habits were assessed using the Paffenbarger Exercise Habits Questionnaire, which captures leisure time physical activity. Data were analyzed as energy expenditure from leisure time physical activity per week.(33) This questionnaire has moderate to high reliability,(34) and has been used in previous weight loss interventions to assess changes in physical activity.(35
Daily Self-weighing Perceptions
Within the intervention group, perceptions about daily self-weighing were assessed at 6 months via a questionnaire that was used in a previous daily self-weighing intervention for weight gain prevention.(36) Using an 8-point scale, where 8 was most favorable and 1 most unfavorable, participants were asked whether they found daily self-weighing to be easy to do, easy to remember, helpful, positive, and whether they were likely to continue doing it after completion of the study. Additionally, using a reverse-scored 8-point scale, participants were asked whether they found this behavior to be frustrating, anxiety provoking, or made them feel self-conscious. Average scores at 6 months were calculated.
Self-monitoring of diet and physical activity behaviors
Two self-report measures were used to assess self-monitoring of diet and physical activity behaviors at baseline, 3 and 6 months. The question asked was, “Over the past 3 months, how often have you used the following strategies to try to manage your weight? Recorded or graphed your physical activity? Recorded or wrote down the type and quantity of food eaten?” There were 5 response options that include never/hardly ever, some of the time, about half of the time, much of the time, and always or almost always with values of 1–5 attached to those responses. Average scores for each time period were calculated for both diet and physical activity to examine group differences in self-monitoring.
Statistical Analysis
Chi-square tests and one-way ANOVAs were conducted to compare differences in baseline characteristics. Intent-to-treat analyses using linear mixed models with random intercept and maximum likelihood estimates were conducted to examine the effect of treatment on weight loss and behavioral outcomes between groups over time. Separate models for the different outcomes were conducted looking at the effects of time within each group, group effects, and group by time interactions. All participants were included in the analyses with the assumption that any missing values were missing at random. Bonferroni corrections were included to account for multiple time point comparisons. Percent weight loss was used as the primary outcome variable to account for baseline weight. ANOVA was used to look at other continuous outcomes and chi-square tests were used to look at differences in dichotomous measures. Transformations were conducted for variables that did not meet the assumptions of normality. Caloric expenditure from physical activity was the only variable that required a natural log transformation. Raw means are presented with statistical tests performed using the transformed data. Analyses were conducted using SPSS for Mac (Version 19, Chicago, IL). A cut-off value of alpha <0.05 was used to assess statistical significance.
Results
Enrollment and Retention
Figure 1 outlines the study enrollment and retention. A total of 326 potential participants were screened online and by phone and 135 were invited to an orientation session. Of those, 38 did not attend and 6 decided not to participate. As a result, 91 participants were randomized to the intervention group (n=47) or a delayed intervention control group (n=44). Participant retention for the primary dependent variable of percent weight loss was 98% at 3 months and 96% at 6 months with no differences between groups (3 months: p=.50; 6 months: p=1.00). Retention at 9 months was 96% for weight loss using smart scale data (intervention group only).
Baseline Characteristics
Table 1 highlights the baseline characteristics across study groups. On average, participants were 44±11 years old, obese (BMI: 32.15± 3.8kg/m2) with an average weight of 90.5±15.2kg, female (75%), White (74%), college-educated (78%), and married (60%). About half the sample (46%) reported weighing less than weekly, 35% reported weighing weekly and 18% reported weighing daily over the past 3 months. About half of the sample reported any self-monitoring of diet less than half the time, and 83% reported any self-monitoring of physical activity less than half the time over the past 3 months. Baseline characteristics did not differ between groups on any variable with the exception of baseline weight (p=.008) and baseline BMI (p=.006), with the intervention group having higher average baseline weight and BMI. This difference was accounted for in the analysis by using percent weight loss as the main outcome variable.
Table 1.
Baseline demographic data by study group.
| Control | Intervention | P- value |
|
|---|---|---|---|
| n | 44 | 47 | |
| Age | 44.7 (± 10.6) | 43.0 (± 11.4) | 0.45 |
| Gender | 0.31 | ||
| Male | 9 (21) | 14 (30) | |
| Female | 35 (80) | 33 (70) | |
| Race/ethnicity | 0.76 | ||
| Black | 8 (18) | 6 (13) | |
| White | 31 (71) | 36 (77) | |
| Other | 5 (11) | 5 (10) | |
| Marital status | |||
| Not married | 17 (39) | 19 (40) | 0.86 |
| Married | 27 (61) | 28 (60) | |
| Education | |||
| High School, Vocational | 9 (21) | 11 (23) | 0.73 |
| Training, or Partial College | |||
| College Graduate or Greater | 35 (80) | 36 (77) | |
| Weight (kg) | 86.1 (± 13.4) | 94.5 (± 15.8) | .008 |
| BMI (kg/m2) | 31.05 (± 3.13) | 33.18 (± 4.03) | .006 |
| Self-weighing Frequency | 0.12 | ||
| Daily | 11 (25) | 5 (10.6) | |
| Several Times/Week | 12 (27) | 9 (20) | |
| One time/week | 3 (7) | 8 (17) | |
| Less than one time/week | 18 (41) | 24 (52) | |
| Self-monitoring Frequency of | |||
| Diet and Physical Activity | |||
| Behaviors | |||
| Diet | |||
| Less than Half the Time | 21 (48) | 28 (60) | .257 |
| Physical Activity | |||
| Less than Half the Time | 37 (84) | 39 (83) | .886 |
Data are M (±SD) or n (%) unless otherwise indicated
P-values in bold indicate statistical significance
Self-weighing Frequency
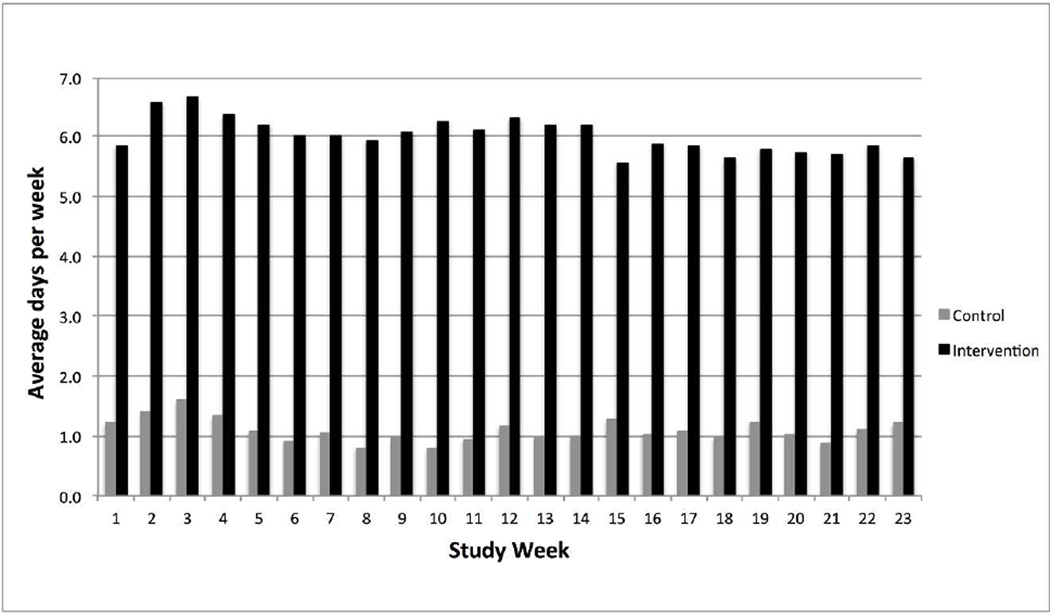
Figure 2 shows the average self-weighing frequency over time by study group using data derived from the smart scales. Over the 6-month study period, the intervention group self-weighed on average more days per week compared to the control group (6.1±1.1 vs. 1.1±1.5; p<.0001). Using all available data between 6–9 months, the intervention group self-weighed on average 4.0 ± 2.3 days per week, which is a significant decrease over time from the 6-month intervention period (p<.001). Regarding the percentage of intervention participants achieving self-weighing thresholds, 57% weighed on average 5 days or more per week and 6 participants (12.8%) had zero weights recorded during 6–9 months.
Figure 2.
Average Days Weighed Each Week by Study Group (n=91)
Weight Change
Weight loss over the study period was significantly different between groups (Figure 3). The intervention group lost on average more weight compared to the delayed control group at both 3 and 6 months [3 months: Mean (95%CI): −4.41%(−5.5, −3.3) vs. −0.37%(−1.5, .76); 6 months: Mean (95%CI): −6.55%(−7.7, −5.4) vs. −0.35%(−1.5, .79); group×time interaction: p<.001 at both time points]. Furthermore, a greater percentage of the intervention group achieved 5% (42.6% vs. 6.8%; p<.0001) and 10% (27.7% vs. 0%; p<.0001) weight loss at 6 months.
Figure 3.
Average Percent Weight Loss Over Time by Group [Mean (95%CI)] (n=91)
Using spearman correlation coefficients, smart scale weight data were strongly correlated with in-clinic weight data at both 3 and 6 months (3 months: r=.94, p<.001; 6 months r=.93, p<.001). Change in weight from baseline to the 9-month follow-up (using smart scale data) in the intervention group was −7.10% ± 8.55%, and was −0.92% ± 3.32% between 6 and 9 months (during which time, the intervention group received no feedback or lessons).
Diet and Physical Activity Behaviors
Table 2 shows the differences in diet and physical activity behaviors over time between groups. At both 3 and 6 months, intervention participants consumed on average fewer calories per day compared to control participants [3 months: Mean (95%CI): 1719 (1510, 1929) vs. 2101 (1885, 2318); group × time interaction: p=.003; 6 months: Mean (95%CI): 1509 (1291, 1728) vs. 1856 (1637, 2074); group × time interaction: p=.006]. There were no differences between groups with regard to calories expended per week from physical activity, although there was a trend towards greater physical activity over time among the intervention group, with the difference almost reaching statistical significance at 3 months (p=.052). Similarly, there were no significant differences over time between the intervention and control groups with regard to self-monitoring of physical activity [3 months: Mean (95%CI): 2.3 (1.9, 2.6) vs. 1.6 (1.2, 1.9); group×time interaction: p=.13; 6 months: Mean (95%CI): 1.9 (1.5, 2.3) vs. 1.7 (1.3, 2.0); group × time interaction: p=.78, however, there was a trend towards the intervention group reporting greater self-monitoring of diet at both 3 and 6 months compared to the control group [3 months: Mean (95%CI): 3.3 (2.9, 3.7) vs. 2.8 (2.4, 3.2); group × time interaction: p=.063; 6 months: Mean (95%CI): 2.2 (1.8, 2.6) vs. 1.6 (1.2, 2.0); group × time interaction: p=.062].
Table 2.
Average Caloric Intake and Expenditure by Study Group (n=91)
| P-value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Assessment Period | Time | Group × Time Interaction | ||||||
| Outcome Variable and Group |
BL (n=91) | 3mo (n=82) | 6mo (n=88) | 3mo vs. BL |
6mo vs. BL |
Group | BL to 3mo | BL to 6mo |
| Caloric Intake | ||||||||
| Intervention | 2014 (1808, 2220) | 1719 (1510, 1929) | 1509 (1293, 1725) | .018 | <.001 | .086 | .003 | .006 |
| Control | 1931 (1718, 2145) | 2101 (1885, 2318) | 1856 (1639, 2072) | .369 | 1.00 | |||
| Caloric Expenditure | ||||||||
| Intervention | 832 (536, 1128) | 1070 (769, 1371) | 1068 (768, 1369) | .052 | .523 | .702 | .155 | .178 |
| Control | 759 (453, 1065) | 933 (627, 1239) | 737 (427, 1048) | 1.00 | 1.00 | |||
Intention-to-treat, linear mixed models analysis with maximum likelihood estimation. Statistically significant p-values are shown in bold.
All values are means, 95% CIs in parentheses
P-values for Caloric Expenditure are based on the natural log transformed variable. Raw means are presented.
Abbreviations: BL = baseline; 3mo = 3 months; 6mo = 6 months
Perceptions of Daily Self-weighing
At 6 months, daily self-weighing was perceived positively within the intervention group. On average, participants felt that daily self-weighing was easy to do (6.9±1.5), easy to remember (7.2±1.2), helpful (6.9±1.6), positive (6.3±1.9) and they were likely to continue doing it after completion of the study (6.6±2.1). On average, they reported low scores for whether they found this behavior to be frustrating (2.4±1.6), anxiety provoking (3.1±1.8), or made them feel self-conscious (3.2±2.0).
Discussion
We found that a lower intensity weight loss intervention that focused on daily self-weighing as the main self-monitoring strategy and also included emailed tailored feedback and skills training with no regular face-to face-contact or focus on self-monitoring of diet and physical activity behaviors produced clinically significant weight losses of 6.13 kg on average, as well as reductions in caloric intake after 6 months. Including an objective measure of self-weighing allowed for a robust assessment of self-weighing frequency and a greater understanding of the feasibility of daily self-weighing. Participants adhered to the daily self-weighing prescription and reported positive responses, indicating that daily weighing is a behavior that will likely continue to be used for self-monitoring. Other studies that included daily weighing for weight control have found similar positive responses.(36, 37)
Previous weight loss trials that included daily self-weighing as a part of a comprehensive behavioral weight loss intervention have found similar effects as our study. Gokee-LaRose and colleagues examined the impact of an intensive behavioral self-regulation program that included daily self-weighing, weekly group sessions with a trained weight loss counselor, and instruction to self-monitor diet and physical activity behaviors. This group lost on average 6.5±5.5 kgs. after 20 weeks.(25) Our study found similar weight losses but tested a lower intensity approach that focused on daily weighing as the main self-monitoring strategy with no inclusion of face-to-face group sessions or detailed monitoring of diet and physical activity behaviors.
The ‘Weigh by Day’ trial examined the impact of a weight loss intervention that included telephone-based counseling with a home-based weight tele-monitoring system that was used to provide individualized feedback regarding progress during counseling calls compared to a delayed start control group. Participants were encouraged to daily weigh, as well as self-monitor diet and physical activity behaviors. Intervention participants lost on average 3.4±0.6 kgs. after 6 months.(40) Although similar to our study with its focus on daily weighing, the Weigh by Day intervention produced half the weight loss. While the study utilized innovated network connected scales, as in the present study, participants self-weighed approximately 3 days per week on average as compared to 6 days per week on average in our study. The main differences between these two studies include the modality and frequency of support (bi-weekly phone calls vs. weekly emails). It may be that the weekly feedback provided in our study, which emphasized and reinforced adherence to daily weighing, was important for increasing the rate of adherence. However, given the current study design, this cannot be experimentally determined. In our study, 79% of participants in our study weighed at least 6 days per week and 94% weighed at least 5 days per week during the 6-month intervention. Given this strong adherence, our findings may provide a better evaluation of the efficacy of a daily self-weighing intervention on weight loss. Similarly, more than half of participants maintained frequent weighing after the intervention was complete, although there was a significant decrease over time. Because no in-person assessment was conducted at 9 months, reasons for the change in self-weighing frequency between 6–9 months are not clear. We might hypothesize that this decrease may be due to participants opting not to self-weigh or a potential measurement issue (e.g., using a different location for self-weighing that did not allow for weights to be sent via the network, using a different scale) as technical support ceased at 6 months. More research is necessary, however, evaluating how to improve adherence rates post treatment.
Almost half of the participants in the WEIGH intervention group achieved the 5% weight loss threshold that is associated with positive changes in risk factors for chronic disease. The percent that achieved 5% is comparable to larger gold-standard clinical trials,(8, 9) and the average weight losses achieved are similar to more intensive interventions that included online counseling and detailed self-monitoring.(29, 38) However, the main difference is that our study was able to achieve these levels with no regular face-to-face interaction, only group tailored feedback via email, and no requirement for detailed self-monitoring of diet and physical activity behaviors. This approach, with its focus on self-regulation via daily weighing, may be more easily disseminated than higher intensity interventions.
Because the study did not isolate the effect of daily weighing, it is not possible to determine the direct impact of this behavior on weight loss. Our goal, however, was not to isolate daily weighing but to test it within the context of a low intensity intervention that included elements believed to be necessary for effectiveness. It was important that participants received the appropriate skills training and feedback to be able to learn the process of self-regulation using the scale to guide their choices. Previous studies found little or no effects for daily weighing in the absence of feedback or skills trailing on how to utilize the scale as a tool for self-regulation.(24, 37) These findings suggest that feedback around daily weighing is necessary for this strategy to be most effective and the results achieved in this study are likely a result of daily weighing in combination with the other components. Future studies using dismantling designs could be conducted to determine whether individuals can benefit from daily self-weighing alone, independent of feedback or skills training.
The use of a randomized-controlled design allowed us to examine the impact of a multi-component intervention that included daily weighing on weight loss among individuals who were instructed to daily weigh. Previous analyses examined the impact of this behavior within a multi-component weight loss intervention post hoc, which potentially introduces selection bias. The strong retention rate, inclusion of blinded evaluation staff, and use of intent-to-treat analyses strengthens our understanding of the effectiveness and feasibility of the intervention. Our study sample was highly educated and included only 25% minority and males, which limits generalizability of the results to other populations. Additional studies are necessary looking at more diverse samples, including those in various socioeconomic positions. Although we assessed weight loss maintenance at 3-months post intervention and found promising results for continued weight loss, future studies should examine whether these effects would be maintained over the long term.
Conclusion
Given the large percentage of Americans that are overweight or obese, there is a need to test the efficacy of weight loss interventions with high potential for sustainability and dissemination. This is important as a recent report from the U.S. Preventive Services Task Force indicates that lower-intensity interventions with limited face-to-face contact are not as effective as more intensive interventions.(39) In contrast to those findings, we found that an approach that included daily self- weighing along with a weekly email that included tailored feedback and skills training can be effective for producing clinically meaningful weight loss. Our results indicate that daily self-weighing can be an effective self-monitoring strategy that warrants inclusion in weight loss interventions.
Acknowledgements
The authors would like to thank the Lineberger Cancer Control Education Program Fellowship as part of the Cancer Control Education Program (#R25 CA057726) and the University of North Carolina at Chapel Hill, Gillings School of Public Health Dissertation Award for providing funding for this study. The authors would also like to gratefully acknowledge the study participants, the staff at the UNC Weight Research Program for their valuable support and Hannah Willmott for providing research assistance.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults: 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Archives of Internal Medicine. 2001;161(13):1581. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 3.Wolin KY, Carson K, Colditz GA. Obesity and Cancer. The oncologist. 2010;15(6):556. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Archives of Internal Medicine. 1987;147(10):1749. [PubMed] [Google Scholar]
- 5.Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. Archives of Internal Medicine. 1993;153(7):849. [PubMed] [Google Scholar]
- 6.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. American journal of clinical nutrition. 1992;56(2):320. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 7.Sarwer DB, von Sydow Green A, Vetter ML, Wadden TA. Behavior therapy for obesity: where are we now? Current Opinion in Endocrinology, Diabetes and Obesity. 2009;16(5):347. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- 8.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stunkard AJ, Wadden TA. Obesity: theory and therapy. New York: Raven press; 1993. [Google Scholar]
- 11.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. Journal of the American Dietetic Association. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behavior Therapy. 1993;24(3):377–394. [Google Scholar]
- 13.Carver CS, Scheier MF. Attention and self-regulation: A control-theory approach to human behavior. New York: Springer-Verlag; 1981. [Google Scholar]
- 14.Kanfer FH. Self-monitoring: Methodological limitations and clinical applications. Journal of consulting and clinical psychology. 1970;35(2):148–152. [Google Scholar]
- 15.Kanfer FH, Karoly P. Self-control: A behavioristic excursion into the lion's den. Behavior Therapy. 1972;3(3):389–416. [Google Scholar]
- 16.Burke LE, Choo J, Music E, et al. PREFER study: A randomized clinical trial testing treatment preference and two dietary options in behavioral weight management--rationale, design and baseline characteristics. Contemporary clinical trials. 2006;27(1):34–48. doi: 10.1016/j.cct.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Burke L, Elci O, Wang J, et al. Self-monitoring in behavioral weight loss treatment: SMART trial short-term results. Obesity. 2009;17(suppl 2):S273. [Google Scholar]
- 18.Burke LE, Swigart V, Warziski Turk M, Derro N, Ewing LJ. Experiences of Self- Monitoring: Successes and Struggles During Treatment for Weight Loss. Qualitative health research. 2009;19(6):815. doi: 10.1177/1049732309335395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuire M, Wing R, Klem M, Hill J. Behavioral strategies of individuals who have maintained long-term weight losses. Obesity research. 1999;7(4):334. doi: 10.1002/j.1550-8528.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 20.Boutelle K. Weighing the evidence: benefits of regular weight monitoring for weight control. Journal of nutrition education and behavior. 2006;38(3):131. doi: 10.1016/j.jneb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Vanwormer JJ, French SA, Pereira MA, Welsh EM. The impact of regular self-weighing on weight management: a systematic literature review. Int J Behav Nutr Phys Act. 2008;5:54. doi: 10.1186/1479-5868-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Annals of Behavioral Medicine. 2005;30(3):210–216. doi: 10.1207/s15324796abm3003_5. [DOI] [PubMed] [Google Scholar]
- 23.Welsh EM, Sherwood NE, VanWormer JJ, Hotop AM, Jeffery RW. Is frequent self-weighing associated with poorer body satisfaction? Findings from a phone-based weight loss trial. J Nutr Educ Behav. 2009;41(6):425–428. doi: 10.1016/j.jneb.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 25.Gokee-Larose J, Gorin AA, Wing RR. Behavioral self-regulation for weight loss in young adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2009;6:10. doi: 10.1186/1479-5868-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanWormer JJ, Martinez AM, Martinson BC, et al. Self-Weighing Promotes Weight Loss for Obese Adults. American Journal of Preventive Medicine. 2009;36(1):70–73. doi: 10.1016/j.amepre.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 27.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA. 2003;289(14):1833–1836. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 29.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Archives of Internal Medicine. 2006;166(15):1620. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 30.Jakicic JM, Tate DF, Lang W, et al. Effect of a stepped-care intervention approach on weight loss in adults: a randomized clinical trial. JAMA. 2012;307(24):2617–2626. doi: 10.1001/jama.2012.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subar AF, Thompson FE, Potischman N, et al. Formative research of a quick list for an automated self-administered 24-hour dietary recall. Journal of the American Dietetic Association. 2007;107(6):1002–1007. doi: 10.1016/j.jada.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Tran KM, Johnson RK, Soultanakis RP, Matthews DE. In-person vs Telephoneadministered Multiple-pass 24-hour Recalls in Women Validation with Doubly Labeled Water. Journal of the American Dietetic Association. 2000;100(7):777–783. doi: 10.1016/S0002-8223(00)00227-3. [DOI] [PubMed] [Google Scholar]
- 33.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. American Journal of Epidemiology. 1995;142(9):889. doi: 10.1093/oxfordjournals.aje.a117736. [DOI] [PubMed] [Google Scholar]
- 34.Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS., Jr Accuracy of the College Alumnus Physical Activity Questionnaire. J Clin Epidemiol. 1993;46(12):1403–1411. doi: 10.1016/0895-4356(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 35.Choo J, Elci OU, Yang K, et al. Longitudinal relationship between physical activity and cardiometabolic factors in overweight and obese adults. European journal of applied physiology. 2010;108(2):329–336. doi: 10.1007/s00421-009-1203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gokee LaRose J, Tate DF, Gorin AA, Wing RR. Preventing weight gain in young adults: a randomized controlled pilot study. Am J Prev Med. 2010;39(1):63–68. doi: 10.1016/j.amepre.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linde JA, Jeffery RW. Testing a brief self-directed behavioral weight control program. Behavioral medicine (Washington, DC) 2011;37(2):47–53. doi: 10.1080/08964289.2011.568992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gold BC, Burke S, Pintauro S, Buzzell P, Harvey-Berino J. Weight loss on the web: A pilot study comparing a structured behavioral intervention to a commercial program. Obesity (Silver Spring) 2007;15(1):155–164. doi: 10.1038/oby.2007.520. [DOI] [PubMed] [Google Scholar]
- 39.McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;139(11):933–949. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 40.VanWormer JJ, Martinez AM, Benson GA, et al. Telephone counseling and home telemonitoring: the Weigh by Day Trial. American journal of health behavior. 2009 Jul-Aug;33(4):445–454. doi: 10.5993/ajhb.33.4.10. [DOI] [PubMed] [Google Scholar]