Abstract
A miniature, planar, differential ion mobility spectrometer (DMS) was interfaced to an LCQ classic ion trap to conduct selective ion filtration prior to mass analysis in order to extend the dynamic range of the trap. Space charge effects are known to limit the functional ion storage capacity of ion trap mass analyzers and this, in turn, can affect the quality of the mass spectral data generated. This problem is further exacerbated in the analysis of mixtures where the indiscriminate introduction of matrix ions results in premature trap saturation with non-targeted species, thereby reducing the number of parent ions that may be used to conduct MS/MS experiments for quantitation or other diagnostic studies. We show that conducting differential mobility-based separations prior to mass analysis allows the isolation of targeted analytes from electrosprayed mixtures preventing the indiscriminate introduction of matrix ions and premature trap saturation with analytically unrelated species. Coupling these two analytical techniques is shown to enhance the detection of a targeted drug metabolite from a biological matrix. In its capacity as a selective ion filter, the DMS can improve the analytical performance of analyzers such as quadrupole (3-D or linear) and ion cyclotron resonance (FT-ICR) ion traps that depend on ion accumulation.
INTRODUCTION
The interfacing of the several ion mobility-based separation types with atmospheric pressure ionization mass spectrometry (API-MS) has created a powerful new class of hybrid instruments based on ion mobility methods complementary to mass spectrometry. While not viewed as a substitute for high-resolution chromatographic methods, ion mobility methods have unique features, including the ability to resolve ions not resolvable by MS, such as isobaric compounds, structural isomers, and, under special conditions, stereoisomers or chiral isomers. In addition, some techniques such as DMS / FAIMS operate in milliseconds (ms) so that complex scheduled MS operations are not affected. Pre-filtering of ion species on the front of mass spectrometers can substantially decrease the chemical noise in recorded mass spectra [1, 2, 3], and enhance the sensitivity of MS detection.
Currently there exist at least four types of ion mobility based separation methods, which function at ambient conditions. These are drift time of flight ion mobility spectrometry (DT-IMS) [4, 5] traveling wave ion mobility spectrometry (TWIMS) [6], differential ion mobility spectrometry (DMS/FAIMS) [7] and aspiration ion mobility spectrometry (AIMS) [8]. IMS and TWIMS operate in pulsed regimes with ion separation based on flight time in a drift tube or traveling wave structure. DMS and AIMS spectrometers can be classified as spatial type spectrometers, because these systems discriminate based on ion trajectories and operate continuously. Both DMS and AIMS methods offer advantages when used as an ion pre-filter for mass spectrometry because both operate continuously.
Conventional IMS in which ions travel through a drift tube under the influence of a weak DC electric field was first interfaced to a magnetic mass spectrometer [4, 5], and then to additional MS systems [9, 10] such as time of flight MS [11, 12], single [13] and triple quadrupole [14] MS, ion trap [15], and FT-ICR mass spectrometers [16]. Conventional IMS has some similarity to TOF-MS in that both are pulsed methods, although IMS operates in the millisecond time regime while the latter typically repeats scans at a higher kHz rate. In a reversed mode of coupling, a quadrupole ion trap has also been used for first stage ion accumulation before conventional IMS analysis [17, 18]. Ion pre-concentration in the ion trap provides increased dynamic range and higher IMS selectivity in applications involving the characterization of non-covalent complexes and separation of conformational isomers of aromatic ions.
A fundamental weakness of conventional IMS-MS is its low duty cycle (injection time/drift time) and ion losses between cycles, although this limitation can be addressed by multiplex techniques [19]. Without multiplexed operation, most ions from the ion source are lost between injections, neutralized on the walls of the ionization chamber or the shutter grids [20]. In addition, high IMS resolution requires longer drift times, further lowering the duty cycle. We believe that ion mobility techniques operating in a continuous mode are advantageous, and that the high selectivity for smaller organics and quick separations of DMS / FAIMS can provide significant advantages for mass spectrometry.
Differential ion mobility spectrometry (DMS / FAIMS) emerged in the early 1990’s as a method for ion separation and detection [21]. In differential mobility spectrometry, not unlike conventional ion mobility, ions are separated at pressures where ion motion is controlled by ionneutral collisions. Due to the complex chemical nature of ion-neutral interactions, the ion-neutral collisional cross-sectional area varies with field amplitude under intense oscillating electric fields [22, 23]. Ion mobility is modified by clustering/de-clustering reactions during high and low electric field portions of DMS fields, with ions being more clustered at low field than high, thus increasing the native differential mobility effect [24, 25].
Over the past fifteen years, differential ion mobility technology has been reported in multiple instrumentation designs and applications [26, 27, 28, 29, 30, 31, 32], and interfaced to a variety of mass spectrometers [33, 34]. One design, referred to as Field Asymmetric Ion Mobility Spectrometry (FAIMS), employs cylindrical co-axial tubes and is commercially available through Thermo Scientific [35] while SelexION™ Technology uses a planar configuration and is sold by AB SCIEX. The planar design used here has some unique features, including fast switching between filtering mode with full selectivity, and transparent mode where all ions are transmitted into the MS. Mode switching provides a convenient way to compare spectra with and without ion separation, a feature utilized in these studies. Planar and cylindrical designs have been compared by Shvartsburg et al. [36], where it was found that planar designs provide higher selectivity. In addition, planar designs enable the use of transport-gas modifiers – small organic molecules, also referred to as dopants – which enhance differential mobility separations by using reversible clustering to increase the difference between high and low field mobility [24, 25, 37, 38, 39].
When used as part of MS inlet systems, the 100% duty cycle of DMS allows efficient sample utilization. The presence of the DMS filter section itself has only about a 20% loss compared to the same MS without the DMS device mounted [3]. As a result, the filtering action of DMS can be used in combination with longer ion trap MS load times to extend the dynamic range and improve analytical sensitivity by loading the ion trap with a pre-filtered ion stream. Ion trap mass analyzers, most notably the three dimensional quadrupole ion traps, especially in miniaturized versions, suffer from limited ion storage capacity, which significantly limits their application range. In an ion trap system, ions accumulate within the trap to a load limited by the geometric and electrical properties of the trap. Ions are resonantly ejected to obtain the mass spectrum as RF fields are scanned. However, space charge effects limit the number of ions (due to coulombic repulsion) that an ion trap mass analyzer can store which in turn affects the quality of the mass spectral data generated. This problem is most acute in the analysis of mixtures where the indiscriminate introduction of matrix ions results in premature trap saturation due to the presence of irrelevant ions. The number of parent ions that may be used to conduct MS/MS experiments for quantitation or other diagnostic experiments is greatly reduced.
In an effort to extend the dynamic range of the ion trap, we have interfaced a planar, differential ion mobility spectrometer to an LCQ classic ion trap to conduct ion filtration prior to mass analysis. Conducting differential mobility-based separations prior to mass analysis allows an analyst to filter a population of ions created from a mixture by electrospray or other methods and selectively introduce targeted species into the trap for mass analysis. To evaluate whether differential mobility filtration can increase the number of targeted ions loaded in the trap, suppress matrix ions, and improve the dynamic range, experiments were conducted by varying the fill time of the trap with samples of known concentration. Direct comparisons between the uses of DMS filtration (selected-on vs. transparent mode) were conducted with particular emphasis on its applicability to the analysis of benzoylecgonine (BE), a metabolite of cocaine from a biological matrix.
EXPERIMENTAL SECTION
Experimental Conditions
The planar DMS system utilized during this research was developed by Sionex Corporation (now defunct) and has a filter gap 0.5mm high × 3.0mm wide × 10.0mm long. These dimensions balance the requirements for resolution as well as losses through diffusion for the measured inlet flow of approximately 0.6 liter/min. The electronics were manufactured by Sionex and provided the separation and compensation fields of the flyback type as described in Krylov et al. [40].
Samples were infused into the DMS-MS platform by ESI at the rate of 300 nl/min using a Harvard Apparatus syringe pump. A coated, 10µm PicoTip emitter (New Objective, Woburn, MA) was utilized for electrospray ionization. The electrospray voltage was held constant throughout the analysis at 2 kV. A bias voltage (50V) was applied across the desolvation region of the mobility filter to attract positive ions into the DMS inlet (Figure 1). Filtered ions of interest leaving the DMS filter are carried by gas dynamics / vacuum drag into the inlet orifice of the mass spectrometer. Ethyl acetate was introduced into the DMS analytical region as a drift gas modifier at a concentration of approximately 1.5% v/v, computed from a weight loss calibration. The (UHP N2) nitrogen heated desolvation gas was introduced at a flow rate of approximately 100 cc/min into the desolvation region, merging with the external airflow from the ESI system of about 500 cc/min. The DMS separation voltage employed was typically set at the maximum value of 1500V (mean to peak) while the compensation voltage (CV) was scanned from −43 to +15V to determine the voltage apex for the DMS-separated species. At the peak CV for ion transmission, the minimum number of ions is lost to wall collisions in the DMS device. Once the CV for the targeted ion was determined, the CV could be fixed at this value to optimize the isolation of the targeted ion from diverse ion populations of the electrosprayed mixture.
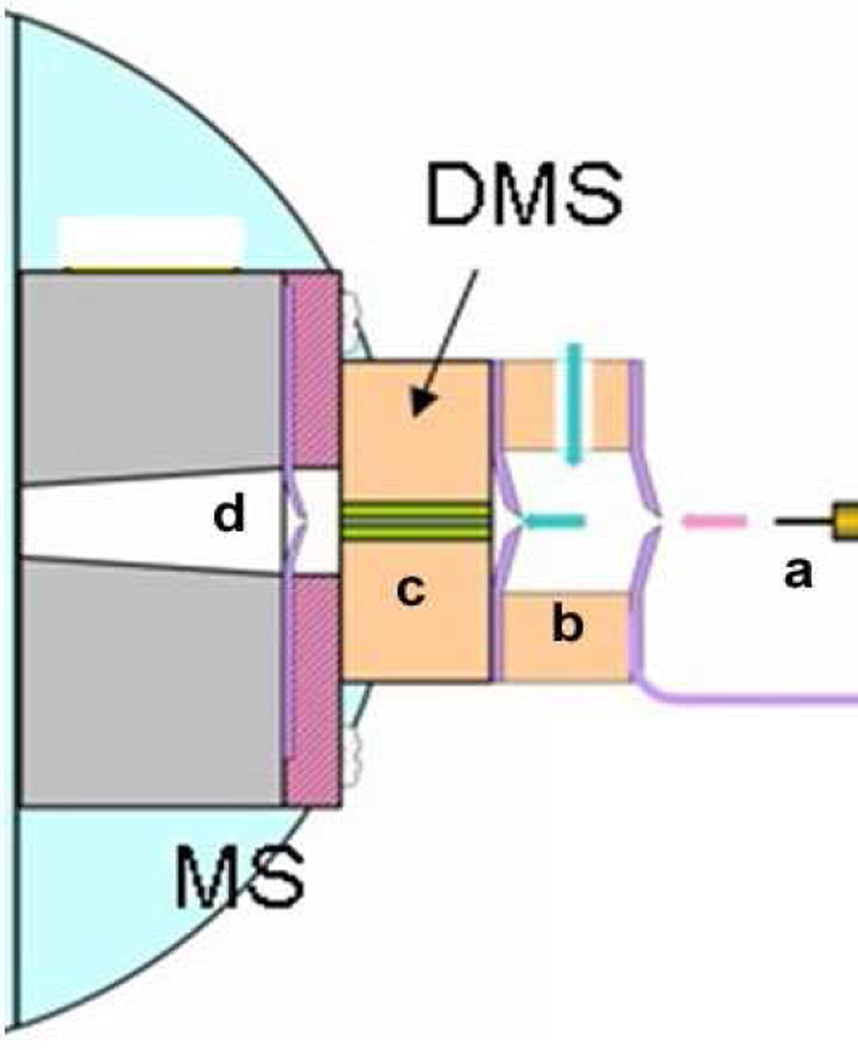
Figure 1.
Schematic of the DMS - MS inlet system. (a) ion source, (b) desolvation gas and modifier introduction, (c) DMS ion selection, (d) first stage of vacuum of mass spectrometer.
A Thermo-Finnigan, LCQ Classic served as the ion trap mass spectrometer for detection of the DMS-separated species. The DMS-resolved species entered the trap by pulsed electrostatic control of the ion gate. The period during which the ions were allowed to enter into the trap, also known as the ionization period, was evaluated by turning off the automatic gain control (AGC) feature and manually adjusting the fill time of the ion trap. The AGC on settings used for the experiments on this instrument were: full MS Target: 5×107 and SIM Target: 2×107 counts respectively with the maximum injection time set at 200 ms. Upon completion of the ionization period, fragmentation of the precursor species was conducted by collisional activation.
Materials and Methods
The stock concentration of the benzoylecgonine (BE) standard was 1mg/ml in MeOH (Cerilliant, Round Rock, TX). The internal standard utilized was a deuterated analog of benzoylecgonine (BE-d3) at a concentration of 100µg/ml in MeOH (Cerilliant, Round Rock, TX). The synthetic urine standard was also obtained from Cerilliant and served as a negative control for the preparation of the spiked samples.
Eight benzoylecgonine samples with final concentrations of: 25 ng/µl, 10 ng/µl, 7.5 ng/µl, 5ng/µl, 2.5 ng/µl, 1ng/µl, 0.5 ng/µl and 0.1 ng/µl were prepared in 1.0ml of synthetic urine (Cerilliant, Round Rock, TX). The concentration range chosen represents biological values at or below those determined by Cone et al. (2003) of the National Institute for Drug Abuse [41] for the quantitation of BE from biological samples using traditional GC or LC-based methodologies. The internal standard (BE-d3) was spiked into each of the urine samples at a concentration of 0.5 ng/µl prior to solid phase extraction. SPE was performed using 130mg Clean Screen Xcel I Columns (UCT, Bristol, PA). The columns were preconditioned with 2ml of MeOH. Each sample was mixed with 1mL of phosphate buffer (pH 6.0) and loaded onto the columns. The columns were washed with 1mL of 98% CH3OH / 2% CH3COOH (v/v) and eluted with 1mL CH2Cl2/IPA/NH4OH (78/20/2 v/v/v). The eluents were dried down under vacuum for 1 hour then capped and stored overnight at 4° C. Prior to DMS-MS analysis; the samples were reconstituted in 200 µl of mobile phase (70% MeOH/30% H2O/0.1% CH2O2 v/v/v).
RESULTS AND DISCUSSION
In addition to limiting the maximum number of ions that can be stored in the system, space charge effects in ion traps can adversely influence their ability to isolate and activate ions in order to obtain a spectrum with a specified resolution and acceptable mass accuracy [42]. Thus, in order to conduct quantitative analyses by MS/MS, an automatic gain control (AGC) is imposed which provides for space charge minimization and optimal conditions for spectral acquisition [15]. During typical LC-MS (or GC-MS) analyses, ions from matrix components (solvent, buffers, etc.) are introduced along with those of the analyte into the trap. The effectiveness of the DMS analyzer to conduct ion filtration was thus evaluated in terms of its ability to deal with complex mixtures in order to improve the performance of a 3D ion trap for quantitative analysis. Benzoylecgonine (BE), also known as 3-benzoyloxy-8-methyl-8-azabicyclo octane-4-carboxylic acid, is the primary metabolite of cocaine abuse. As such, it represents an analyte of interest to the forensic science community and was selected for this purpose. Samples of the metabolite in the range from 0.1 – 25 ng/µL in urine were prepared as described in the Experimental Section and analyzed by direct infusion into the DMS - MS/MS system. The performance of the DMS – MS system and the efficacy of ion filtration by DMS prior to mass analysis to improve the trap performance were thus investigated in the DMS-on and the DMS-transparent modes and compared with results obtained under normal operating conditions of the trap (AGC-on).
Ion Trap capacity as a function of fill time
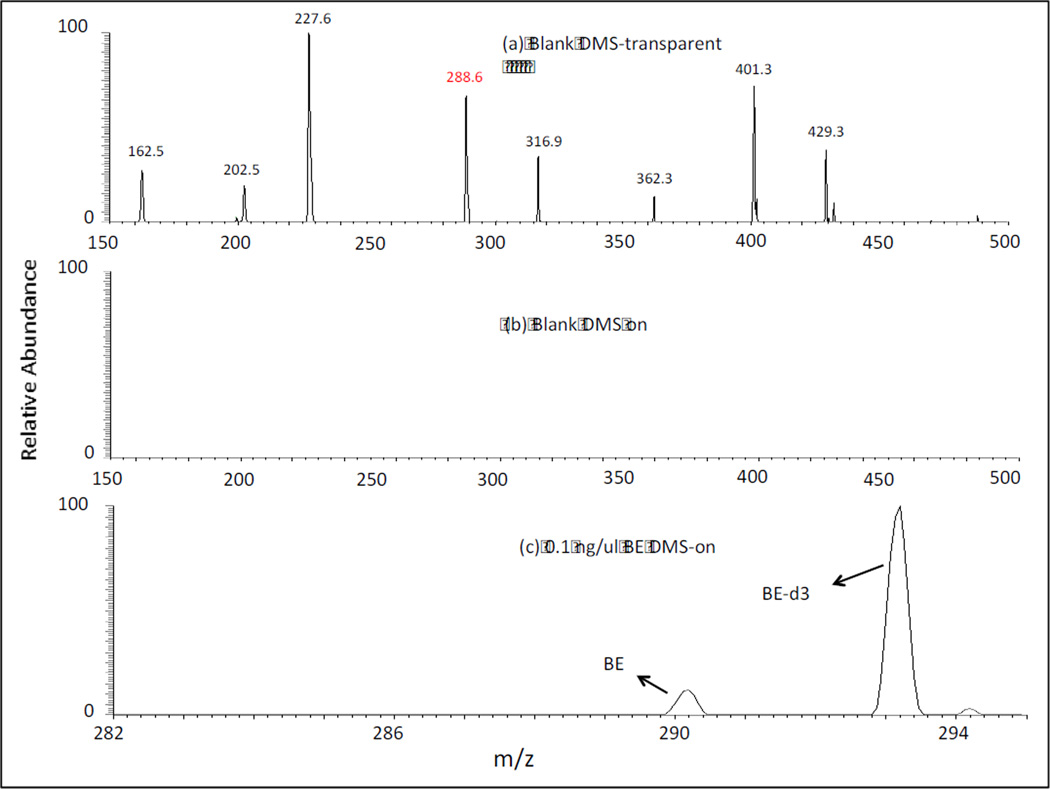
As an initial proof of concept, the selectivity of the DMS filter was tested by comparing the full scan mass spectra (MS1) of a matrix blank in the DMS-transparent and the DMS-on modes (Figure 2). For the latter conditions, the DMS compensation voltage was set at a value of CV = −16V corresponding to the transmission of m/z 290, the [M+H]+ ion of BE in the presence of ethyl acetate modifier. As shown in the Figure, DMS filtration resulted in complete elimination of the matrix background, and, most significantly, removal of m/z 289 a potential interference to the protonated ion of BE at m/z 290. It should be noted that this selectivity was demonstrated whether the AGC was on or off (data not shown). In a further test of this selectivity, the lowest BE concentration sample of 0.1 ng/µL was examined. As shown in Figure 2(c), operation of the system in the DMS-on mode, yielded a well-defined analyte signal along with one for the deuterated internal standard with the expected 10:1 IS/analyte ratio.
Figure 2.
Analysis of BE in urine by DMS-MS. (a) Full scan mass spectrum (m/z 150 – m/z 500) of urine blank solution in the DMS-transparent mode; (b) Full scan mass spectrum (m/z 150 – m/z 500) of previous solution with DMS-on set at CV = −16V showing removal of all matrix ions including potential interference at m/z 289; (c) Analysis of BE in urine (0.1ng/µL) with DMS set at CV = −16V showing the ratio of 1:10 analyte : internal standard. MS scan range (m/z 182 – m/z 296).
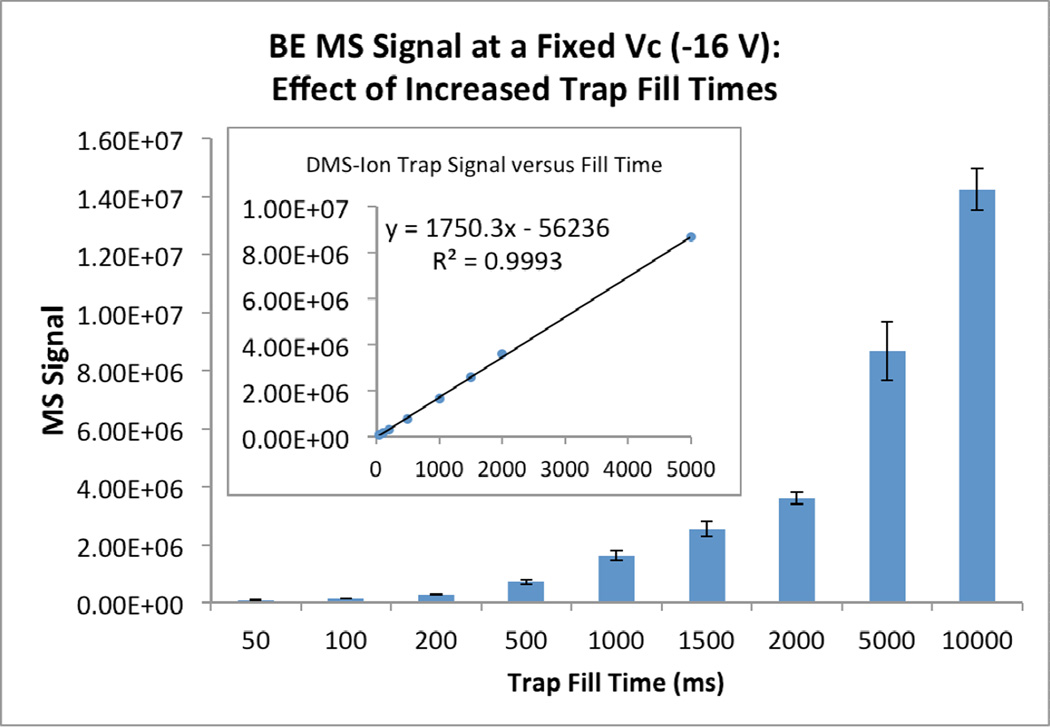
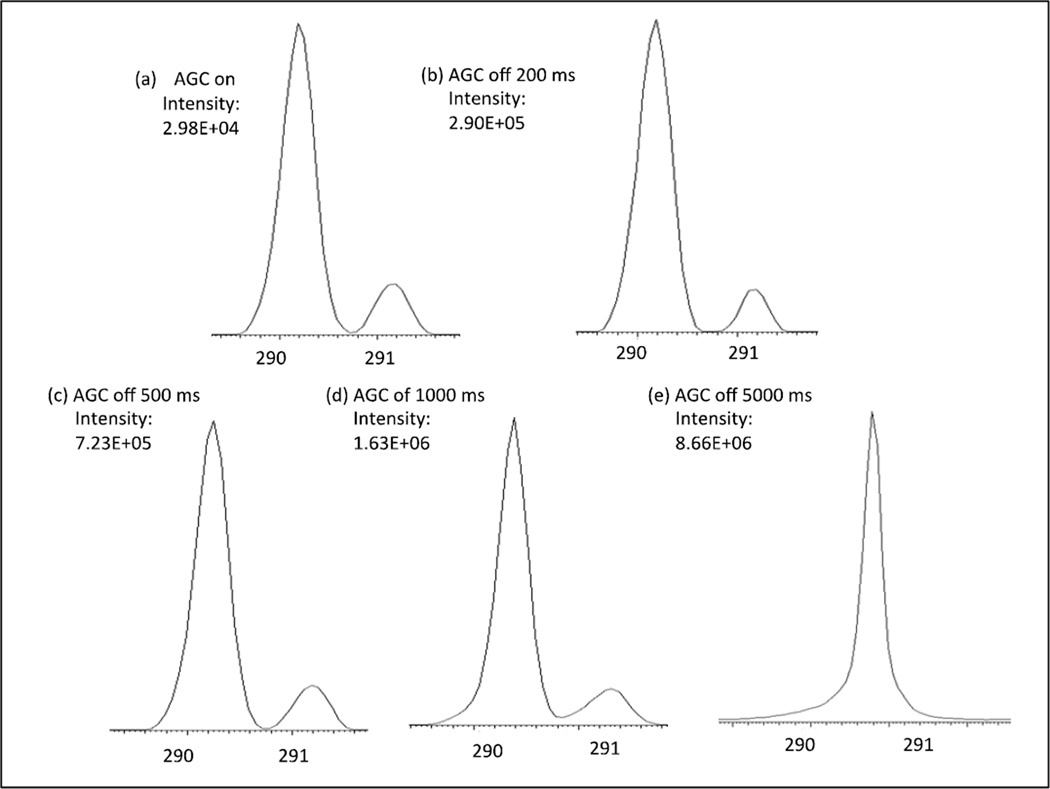
In view of the demonstrated selectivity of the DMS filter, we considered next the degree to which the trap fill time could be extended in order to increase the population of the parent ions of the BE metabolite when contained in a complex biological matrix. Trap fill times from 50 ms to 10,000 ms were tested and a linear dependence of ion accumulation as a function of fill time was observed upon infusion of a urine sample with a BE concentration of 7.5 ng/µl (Figure 3). While the improvement in ion accumulation was dramatic, a decrease in the resolving power of the trap also became apparent with overloading of the analyzer ion trap. As shown in Figure 4, deterioration of resolution began at fill times approaching 1,000 ms. Significantly, for this specific solution at a fill time of 500 ms, baseline resolution between these two peaks was observed and this was similar to that observed under the normal operating conditions of the ion trap, i.e., the AGC-on mode. Moreover, when the same mixture was introduced with the DMS transparent, the absence of ion filtration resulted in premature saturation of the ion trap due to the co-injection of matrix ions (data not shown). The difference in signal normalization between the AGC-on and manual trap fill time operation may be understood in terms of an extra normalization applied by the Xcalibur software when AGC is active that allows quantitative comparison of ion fluxes between different runs. The AGC-on count gives a value proportional to ion counts per time that can be used for quantitation without knowing the automatically adjusted trap fill time. When the trap fill time is set manually, no extra normalization is applied, as demonstrated by the linear dependence on trap fill time seen in Figure 3. Absolute signal values cannot be directly compared between AGC-on and AGC-off, but the LOD or LOQ values in the two conditions can be meaningfully compared.
Figure 3.
Parent ion signal intensities for BE [M+H]+ = 290 plotted as a function of varying the fill time of the ion trap. Inset: linear dependence of ion accumulation on fill time. The error bars represent the standard deviation of triplicate measurements.
Figure 4.
Separation of m/z 290 and m/z 291 ions of BE demonstrating loss of resolution with increasing trap fill time due to space charge effects
Quantitative analysis of benzoylecgonine from a biological matrix by MS/MS
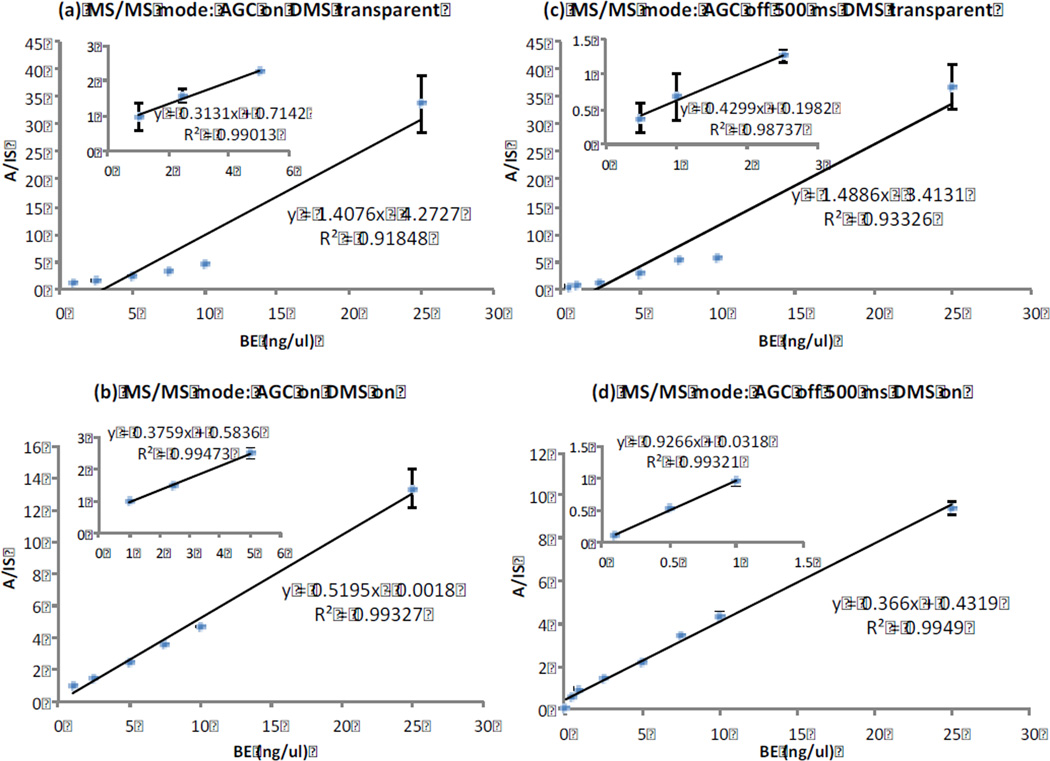
The previous experiment of monitoring the accumulation of the [M+H]+ ion of an analyte within a matrix, established the utility of combining selective ion filtration by DMS for increasing the ion-specific loading of an ion trap. However, typical uses of the ion trap revolve around its function as a vehicle for conducting tandem mass spectrometry experiments within a single mass analyzer. As a result, we sought next to assess the ability of DMS to separate a targeted species from a matrix followed by activation of the precursor ion to generate product ion spectra for quantitation. Moreover, from a general applicability perspective, this experiment addresses a typical problem encountered in many pharmacological (DMPK) studies involving the quantitation of drug metabolites from biological matrices. The transition m/z 290 → m/z 168 was monitored for this purpose. For this study, a trap fill time of 500 ms was used. This represents an extended time which maintains the mass resolving power of the trap obtained with AGC-on (see Figure 3). To further determine the potential advantages that may be realized by selective DMS filtration, the quantitation of BE by MS/MS on the m/z 290 → m/z 168 transition was determined under DMS-on and DMS-transparent conditions. (Figure 5 (a,b,c,d)). Panels (a,c) were obtained with no DMS filtration (DMS-transparent): (a) AGC-on, (c) AGC-off. Panels (b,d) were obtained using DMS filtration: (b) AGC-on, (d) AGC-off. Overall, calibration curve accuracies are much better with DMS filtration.
Figure 5.
Calibration curves for quantitation of benzoylecgonine in urine by MS/MS (m/z 290 → m/z 168). The error bars represent the standard deviation of triplicate analyses. (a,c) were obtained with no DMS filtration (DMS-transparent), and (a) AGC on. (c) AGC off. (b,d) were obtained using DMS filtration: (b) AGC on, (d) AGC off. Results are much better with DMS filtration. In addition, the use of an extended fill time allows the calibration curve to extend an extra factor of 10 lower in BE concentration.
In reviewing the data of Figure 5, most striking is the benefit derived from DMS filtration. In addition, the use of an extended fill time along with DMS filtration allows the calibration curve to extend lower in BE concentration than any other configuration. Dramatic improvement is evident when the DMS-on results are compared to the DMS-transparent mode where there is indiscriminate introduction of all sample ions into the trap. In addition to the much better linearity of the calibration curve, the result of higher loading of precursor ions due to DMS filtration is also shown by the improved sensitivity at the lower BE concentrations where the lowest point in the calibration curve is 0.1 ng/µL, a level that was unobservable with DMS transparent. This demonstrates that the matrix suppression provided by DMS filtration is especially useful in extending the quantitation range of ion trap analyses by MS/MS.
Further support for the use of DMS filtration is provided in the comparison with data obtained using normal operating conditions of an ion trap, i.e., AGC-on. Examination of Figure 5(a, b) shows that use of DMS filtration results in lower calibration data scatter and improved precision. This is believed to be due to more reproducible loading of the trap with the targeted ion, free of a fluctuating background of matrix ions and clusters, whose presence changes the target ion concentration, interferes with MS/MS selection and activation of the parent ion. The reported product ion intensity obtained at trap fill time of 500 ms is a factor of 25 higher when the AGC control function was overridden than with AGC on at a nominal time of 200 ms. [See Supplemental Figure 1.], but this is largely due to auto-scaling that is applied when AGC is on but not when AGC is off.
It should also be noted here that a wide scatter in the calibration curves was also observed when the entire DMS assembly was demounted from the mass spectrometer and the same solution was analyzed by direct infusion using the normal operating conditions of the trap, i.e., AGC-on. Despite some improvement in the overall total ion current due to the elimination of the DMS assembly and the shorter and more direct ion introduction path, the absence of selective ion filtration resulted in dramatic loss of resolution as shown in Supplemental Figure 2(a). This may be compared with the resolving power obtained with the DMS in place and the AGC-on. [Supplemental Figure 2(b)]. The effect of premature trap saturation with extraneous ions is further reflected in the lower R2 value of the calibration curve obtained when the DMS assembly was removed from the system. [Supplemental Figure 3]. Moreover, it should be pointed out that the loss of resolution failed to remove the background ion peak at m/z 288.6 (See Figure 2) further compromising the selection of the parent ion peak of m/z 290 and thereby introducing an additional level of uncertainty in the MS/MS data.
Losses in ion current due to the insertion of the DMS interface under different electrospray infusion rates has been examined experimentally in Schneider et al. [3]. There it was found that the physical presence of DMS interface introduced an average of 25–30% loss for flow rates of 20 µl/min and below in comparison to the standard AB SCIEX electrospray interface. For higher, standard LC flows of 200 µl/min and higher losses were approximately 75%. We recommend the use of DMS at lower infusion rates for higher efficiency either with micro / nano LC or with the use of a nanosplitter interface that provides high sensitivity and the benefit of sample collection for off-line analysis [43, 44, 45]. Lower flows are advantageous for DMS because ion desolvation tends to be more complete. DMS filtration requires full ion desolvation at atmospheric pressure while MS analysis at high liquid flows relies on desolvation in the transition to vacuum. The flows, voltages, and dimensions of the interface used in this work have not undergone any optimization, with the result that ion flux with this DMS mounted is about 20% of that with the DMS removed. Nonetheless, the lower loss level reported in Schneider et al. [3] should be achievable in an optimized design. The ion trap loading selectivity and improved LOD, are not affected because a longer loading time, with or without AGC, will populate the trap in a way that compensates for the lower ion flux. The results reported here describe the improved performance obtained by using the DMS in filter mode compared to the transparent mode. In DMS transparent mode, the interface is simply a rectangular tube with no applied fields, and thus has no selectivity.
Space Charge Effects and Potential Limits of DMS-MS/MS Operation
It is evident from the above results that differential mobility spectrometry in combination with an ion trap can improve the selectivity in ion selection from a biological matrix and thereby increase the parent ion content in the trap to improve its dynamic range for quantitative analysis by MS/MS. The trend observed in Figure 2 may also lead one to believe that a further increase in the trap fill time would result in continuous improvement in sensitivity. It must be realized, however, that space charge effects in the trap volume impose a limit to the improvement. Evidence to that effect is seen in Figure 3 which shows the decline of mass resolution of the parent ion peak as fill times approached 1,000 ms. It has been proposed that there are four primary space charge limits associated with an ion trap, namely, storage space, activation space, isolation space and spectral space charge limits [42]. The loss of resolution observed in Figure 3 is evidence that at fill times beyond 1,000 ms the system has reached (or exceeded) its “storage space charge” limit.
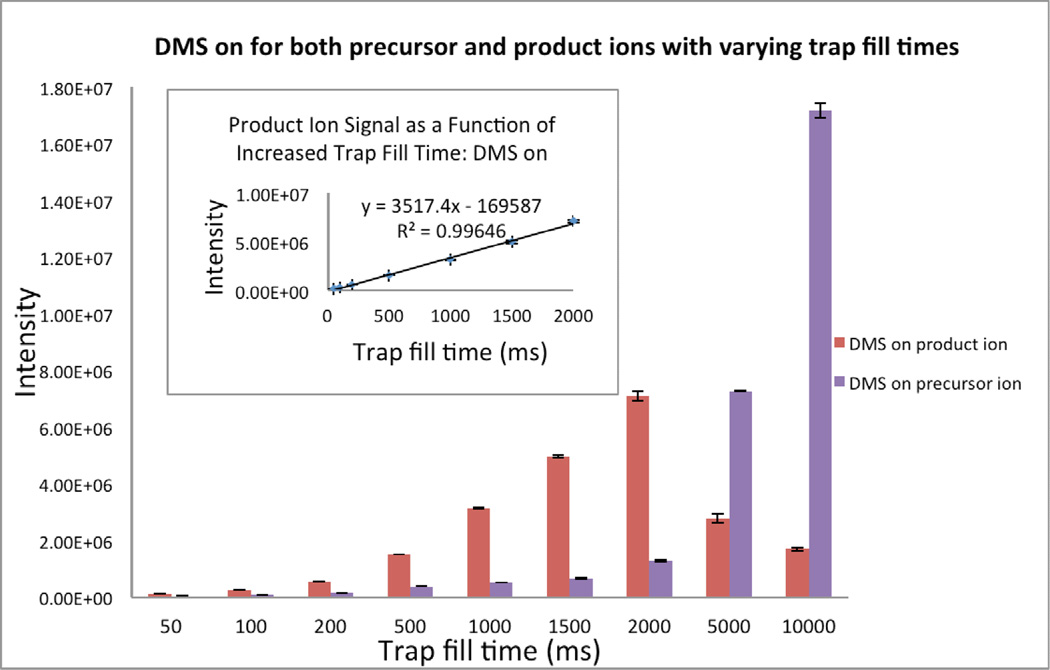
In order to ascertain the extent to which the same limitations might also apply to the MS/MS operation of the trap, the variation of precursor ion intensity as a function of fill time was compared to that of the product ion of the transition m/z 290 → m/z 168 of BE with the DMS fully engaged (DMS-on) for a sample of fixed concentration (2µg/ml urine) of the metabolite (Figure 6). As expected, the increase in trap fill time from 50 – 10,000ms increased the abundance of the [M+H]+ ion as shown by the purple bars. However, the increase in precursor ion signal was accompanied by a drop in product ion intensity at fill times in excess of 2,000 ms as shown by the red bars. While at first glance surprising, as mentioned earlier, this trend is consistent with the fundamental concept that there are different requirements (or limitations) associated with the maximum number of ions that can be stored while also maintaining the ability of the trap to activate ions for efficient MS/MS operation. The loss of signal intensity for the product ion of BE (red bars) suggests that above 2,000 ms the ion trap has reached its “spectral space charge limit” which governs MS/MS efficiency. On the other hand, it is also interesting to note the excellent linearity of the product ion signal at the lower fill times which support our observation that selective DMS filtration can be used to improve the overall analytical performance of ion traps.
Figure 6.
Effect of ion trap fill time on the abundance of the precursor [M+H]+ and product ions of benzoylecgonine (m/z 290 → m/z 168) from a urine matrix (2µg/ml urine) at varying trap fill times obtained using DMS filtration.
Conclusions
Interfacing differential ion mobility to a mass spectrometer provides an analyst with the ability to separate targeted species from a diverse population of electrospray-generated ions under ambient conditions without the necessity for chromatographic separations. In the absence of DMS, indiscriminate introduction of matrix ions from biological samples often results in premature trap saturation with irrelevant ion populations that dominate the trap contents, and prevent MS/MS for minor ions at moderate or low concentrations.
We have integrated a planar differential mobility filter onto the front end of an ion trap mass spectrometer and have demonstrated that the combination of ion filtration in tandem with extended trap fill times affords appreciable increases in signal intensities for both precursor (DMS-MS) and product (DMS-MS/MS) ion spectra for benzoylecgonine in a biological matrix. While the improvement in sensitivity observed by incorporation of DMS filtration with extended trap fill time for the analysis of BE in urine tested here was approximately 10-fold, this number is likely to be higher for more complex matrices and lower for less complex ones. It is expected, however, that the advantages will be best realized in trace level analyses or for improving the limits of detection in an assay as observed here for the quantitation of BE. Moreover, the use of DMS filtration and by-passing the AGC control functions adds a level of flexibility for conducting quantitative analyses or other related MS/MS operations in ion trap systems.
It should be noted that, in addition to improving the trap performance by selective DMS filtration and extension of trap fill times, the examples presented above demonstrate the rapid nature of the DMS – MS/MS approach in comparison with the traditional GC-MS or LC-MS separations for such analyses which may be prohibitively time consuming for high-volume screening. The difference in time requirement can be illustrated by our experience in creating calibration curves. An 8-point calibration curve showing excellent quantitative relationship (R2 = 0.9991) was prepared in triplicate in approximately 1.5 hours using DMS-MS while it would have required approximately 12 hours by GC-MS (data not shown). Our results show that, in its function as a selective ion filter, the DMS can improve the analytical performance of analyzers such as quadrupole (3-D or linear) and ion cyclotron resonance (ICR) ion traps that rely on ion accumulation.
Supplementary Material
REFERENCES
- 1.Levin DS, Vouros P, Miller RA, Nazarov EG. Using a Nanoelectrospray-Differential Mobility Spectrometer System for the Analysis of Oligosaccharides with Solvent Selected Control Over ESI Aggregate Ion Formation. J. Am. Soc. Mass Spectrom. 2007;18:502–511. doi: 10.1016/j.jasms.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coy SL, Krylov EV, Schneider BB, Covey TR, Brenner DJ, Tyburski JB, Patterson AD, Krausz KW, Fornace A, Nazarov EG. Detection of Radiation-Exposure Biomarkers by Differential Mobility Prefiltered Mass Spectrometry (DMS-MS) Int. J. Mass Spectrom. 2010;291:108–117. doi: 10.1016/j.ijms.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider BB, Covey TR, Coy SL, Krylov EV, Nazarov EG. Planar differential mobility spectrometer as a pre-filter for atmospheric pressure ionization mass spectrometry. Int. J. Mass Spectrom. 2010;298:45–54. doi: 10.1016/j.ijms.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDaniel EW, Martin DW, Barnes WS. Drift –Tube Mass Spectrometer for Studies of Low Energy Ion Molecule reaction. Review of Science Instruments. 1962;33:2–7. [Google Scholar]
- 5.Cohen MJ, Karasek FM. Plasma Chromatography TM-new dimension for gas chromatography and mass spectrometry. Journal of Chromatographic Science. 1970;8:330–337. [Google Scholar]
- 6.Giles K, Pringle SD, Worthington KR, Little D, Wildgoose JL, Bateman RH. Applications of a travelling wave-based radio-frequency-only stacked ring ion guide. Rapid Commun. Mass Spectrom. 2004;18:2401–2414. doi: 10.1002/rcm.1641. [DOI] [PubMed] [Google Scholar]
- 7.Buryakov IA, Krylov EV, Makas AL, Nazarov EG, Pervukhin VV, Rasulev UKh. Separation of ions according to their mobility in a strong alternating current electric field. Pis’ma Zh. Tech.Fiz. 199;17:61–65. (Russ) [Google Scholar]
- 8.Kotiaho T, Lauritsen FR, Degn H, Paakkanen H. Membrane inlet ion mobility spectrometry for on-line measurement of ethanol in beer and in yeast fermentation. Anal. Chim. Acta. 1995;309:317–325. [Google Scholar]
- 9.Kuk Y, Jarrold MF, Silverman P, Jj, Bower LE, Brown WL. Preparation and observation of Si10 clusters on a Au(001) (5×20) surface. Physical Review B: Condensed Matter. 1989;39:11168–11170. doi: 10.1103/physrevb.39.11168. [DOI] [PubMed] [Google Scholar]
- 10.Kemper P, Bowers MT. A hybrid double-focusing mass spectrometer-high-pressure drift reaction cell to study thermal energy reactions of mass-selected ions. J. Am. Soc. Mass Spectrom. 1989;1:197–207. [Google Scholar]
- 11.McAfee KB, Sipler DP, Edelson D. Mobilities and reactions of ions in argon. Physical Review. 1967;160:130–135. [Google Scholar]
- 12.Dwivedi WP, Klopsch SJ, Puzon GF, Xn L, Hill HH., Jr Metabolic profiling by ion mobility mass spectrometry (IMMS) Metabolomics. 2008;4:63–80. [Google Scholar]
- 13.Karasek FW, Kim SH, Hill HH., Jr Mass Identified Mobility Spectra of p-Nitrophenol and Reactant Ions in Plasma Chromatography. Anal. Chem. 1976;48:1133. doi: 10.1016/0021-9673(76)80009-x. [DOI] [PubMed] [Google Scholar]
- 14.Fahdil HA, Mathur D, Hasted JB. Mobilities of O+, O+* and O22+ in He and Ar from ion energy distribution measurements in an injected-ion drift tube. J. Phys. B: At. Mol. Opt. Phys. 1982;15:1443–1453. [Google Scholar]
- 15.March RE, Todd JF Jr, editors. Practical Aspects of Ion Trap Mass Spectrometry. Boca Raton, FL: CRC Press; 1995. [Google Scholar]
- 16.Bluhm BK, Gilling KJ, Russel DH. Development of a Fourier-transform ion cyclotron resonance mass spectrometer. Rev. Sci. Instrum. 2000;71:4078–4086. [Google Scholar]
- 17.Hoaglund CS, Valentine SJ, Clemmer DE. An Ion Trap Interface for ESI-Ion Mobility Experiments. Anal. Chem. 1997;69:4156–4161. [Google Scholar]
- 18.Creaser CS, Benyezzar M, Griffiths JR, Stygall JW. A Tandem Ion Trap/Ion Mobility Spectrometer. Anal. Chem. 2000;72:2724–2729. doi: 10.1021/ac991409d. [DOI] [PubMed] [Google Scholar]
- 19.Belov ME, Clowers BH, Prior DC, Danielson WF, Liyu AV, Petritis BO, Smith RD. Dynamically multiplexed ion mobility time-of-flight mass spectrometry. Anal. Chem. 2008;80:5873–5883. doi: 10.1021/ac8003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eiceman GA, Nazarov EG, Rodriguez E, Stone JA. Analysis of a drift tube at ambient pressure: Models and precise measurements in ion mobility spectrometry. Review of Scientific Instruments. 2001;72:3610–3621. [Google Scholar]
- 21.Buryakov IA, Krylov EV, Nazarov EG, Rasulev UKh. A New Method of Separation of Multi-Atomic Ions by Mobility at Atmospheric Pressure Using a High-Frequency Amplitude-Asymmetric Strong Electric Field. Int. J. Mass Spectrometry Ion Processes. 1993;128:143–148. [Google Scholar]
- 22.Mason EA, McDaniel EW. Transport Properties of Ions in Gases. New York: Wiley; 1988. [Google Scholar]
- 23.Mason EA. In: Plasma Chromatography. Carr TW, editor. Chapter 2. New York: Plenum Press; 1984. p. 49. [Google Scholar]
- 24.Schneider BB, Covey TR, Coy SL, Krylov EV, Nazarov EG. Control of chemical effects in the separation process of a differential mobility mass spectrometer system. Eur. J. Mass Spectrom. 2010;16:57–71. doi: 10.1255/ejms.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider BB, Covey TR, Coy SL, Krylov EV, Nazarov EG. Chemical Effects in the Separation Process of a Differential Mobility/Mass Spectrometer System. Anal. Chem. 2010;82:1867–1880. doi: 10.1021/ac902571u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purves RW, Guevremont R, Day S, Pipich CW, Matyjaszczyk MS. Mass spectrometric characterization of a high-field asymmetric waveform ion mobility spectrometer. Rev. Sci. Instrum. 1998;69:4094–4105. [Google Scholar]
- 27.Guevremont R, Purves RW. Atmospheric pressure ion focusing in a high-field asymmetric waveform ion mobility spectrometer. Rev. Sci. Instrum. 1999;70:1370–1383. [Google Scholar]
- 28.Guevremont R. High-field asymmetric waveform ion mobility spectrometry: a new tool for mass spectrometry. J. Chromatogr.,A. 2004;1058:3–19. [PubMed] [Google Scholar]
- 29.Guevremont R, Purves RW, Barnett DA, Ding L. Ion-Trapping at Atmospheric Pressure (760 torr) and Room Temperature with a High-Field Asymmetric Waveform Ion Mobility Spectrometer (FAIMS) Int. J. Mass Spectrom. 1999;193:45–56. [Google Scholar]
- 30.Shvartsburg AA, Tang K, Smith RD. Modeling the resolution and sensitivity of FAIMS analyses. J. Am. Soc. Mass Spectrom. 2004;15:1487–1498. doi: 10.1016/j.jasms.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Shvartsburg AA, Tang K, Smith RD. Optimization of the design and operation of FAIMS analyzers. J. Am. Soc. Mass Spectrom. 2005;16:2–12. doi: 10.1016/j.jasms.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Shvartsburg AA. Differential ion mobility spectrometry. Boca Raton: CRC Press, Inc.; 2008. p. 299. [Google Scholar]
- 33.Kolakowski B, Mester Z. Review of applications of high-field asymmetric waveform ion mobility spectrometry (FAIMS) and differential mobility spectrometry (DMS) Analyst. 2007;132:842–864. doi: 10.1039/b706039d. [DOI] [PubMed] [Google Scholar]
- 34.Makinen M, Nousiainen M, Sillanpaa M. Ion Spectrometric Detection Technologies for ultra-traces of explosives: A Review. Mass Spectrometry Reviews. 2011;30:940–973. doi: 10.1002/mas.20308. [DOI] [PubMed] [Google Scholar]
- 35.Thermo Fisher FAIMS TM Operator’s Manual 70111–97134, Revision A. Thermo Fisher Corporation; 2006. [Google Scholar]
- 36.Shvartsburg AA, Li F, Tang K, Smith RD. High-resolution field asymmetric waveform ion mobility spectrometry using new planar geometry analyzers. Anal. Chem. 2006;78:3706–3714. doi: 10.1021/ac052020v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin DS, Miller RA, Nazarov EG, Vouros P. Rapid Separation and Quantitative Analysis of Peptides Using a New Nanoelectrospray- Differential Mobility Spectrometer-Mass Spectrometer System. Anal. Chem. 2006;78:6422–6432. doi: 10.1021/ac060003f. [DOI] [PubMed] [Google Scholar]
- 38.Rorrer LC, Yost RA. Solvent vapor effects on Planar high –field asymmetric waveform ion mobility spectrometry. Int. Journal Mass Spectrom. 2011;3009:173–181. [Google Scholar]
- 39.Hall AB, Coy SL, Nazarov EG, Vouros P. Ultra-Rapid Separation of Cocaine and Cocaine Adulterants by Differential Mobility Spectrometry – Mass Spectrometry. J. Forensic Sci. 2012 doi: 10.1111/j.1556-4029.2011.02033.x. [DOI] [PubMed] [Google Scholar]
- 40.Krylov EV, Coy SL, Vandermey J, Schneider BB, Covey TR, Nazarov EG. Selection and generation of waveforms for differential mobility spectrometry. Review of Scientific Instruments. 2010;81:024101–024111. doi: 10.1063/1.3284507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cone EJ, Sampson-Cone AH, Darwin WD, Huestis MA, Oyler JM. Urine testing for cocaine abuse: Metabolic and excretion patterns following different routes of administration and methods for detection of false-negative results. Journal of Analytical Toxicology. 2003;27:386–401. doi: 10.1093/jat/27.7.386. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz JC, Senko MW, Syka JEP. A two- dimensional quadrupole ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2002;13:659–669. doi: 10.1016/S1044-0305(02)00384-7. [DOI] [PubMed] [Google Scholar]
- 43.Gangl ET, Annan M, Spooner N, Vouros P. Reduction of signal suppression effects in ESI-MS using a nanosplitting device. Anal Chem. 2001 Dec;73(23):5635–5644. doi: 10.1021/ac010501i. PubMed PMID: ISI:000172541200015. [DOI] [PubMed] [Google Scholar]
- 44.Gangl E, Vouros P, inventors. Northeastern U., assignee, assignee. Fluid NanoSplitter Device. 6,817,554. United States; United States Patent. 2002 Issued November 16, 2004.
- 45.Andrews CL, Li F, Yang E, Yu CP, Vouros P. Incorporation of a nanosplitter interface into an LC-MS-RD system to facilitate drug metabolism studies. J. Mass Spectrom. 2006 Jan;41(1):43–49. doi: 10.1002/jms.944. PubMed PMID: ISI:000235047300004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.