Abstract
The influenza M2 ectodomain (M2e) is poorly immunogenic and has some amino acid changes among isolates from different host species. We expressed a tandem repeat construct of heterologous M2e sequences (M2e5x) derived from human, swine, and avian origin influenza A viruses on virus-like particles (M2e5x VLPs) in a membrane-anchored form. Immunization of mice with M2e5x VLPs induced protective antibodies cross-reactive to antigenically different influenza A viruses and conferred cross protection. Anti-M2e antibodies induced by heterologous M2e5x VLPs showed a wider range of cross reactivity to influenza virus at higher levels than those by live virus infection, homologous M2e VLPs, or M2e monoclonal antibody 14C2. Fc receptors were found to be important for mediating protection by immune sera from M2e5x VLP vaccination. The present study provides evidence that heterologous recombinant M2e5x VLPs can be more effective in inducing protective M2e immunity than natural virus infection and further supports an approach for developing an effective universal influenza vaccine.
Keywords: M2e VLPs, M2e cross-reactivity, cross protection
1. Introduction
Current influenza virus vaccines mainly based on hemagglutinin (HA) are effective if there is a good match between the chosen vaccine strains and circulating variants. However, mutant influenza viruses continue to circulate and evolve in diverse natural hosts including wild birds, chickens, pigs, and humans. To accommodate circulating predicted variants, vaccines are updated annually. Recently, the 2009 outbreak of a swine origin H1N1 virus illustrates how fast a new pandemic virus can spread in the human population once it acquires the ability to transmit among humans (Nava et al., 2009; Solovyov et al., 2009). Also, there is a threat of a potential pandemic from highly pathogenic avian influenza viruses (Abdel-Ghafar et al., 2008).
The influenza M2 is a transmembrane protein forming a homotetramer (Sugrue and Hay, 1991). The extracellular domain of M2 (M2e) has 24 amino acid residues well conserved among human influenza A strains (Liu et al., 2005). However there are some residue differences in M2e depending on the host species such as pigs and birds (Fiers et al., 2004; Liu et al., 2005). M2e is poorly immunogenic, due to its small size, its low level of incorporation into virions, and possible shielding effects by larger viral surface proteins. Previous studies have used various M2e-conjugating carriers and adjuvant formulations to enhance the immunogenicity of M2e (Bessa et al., 2008; Eliasson et al., 2008; Fan et al., 2004; Fu et al., 2009b; Huleatt et al., 2008; Ionescu et al., 2006; Turley et al., 2011). Such M2e conjugate vaccines have been used to immunize mice multiple times, often together with experimental adjuvants such as complete or incomplete Freund’s adjuvant (Fan et al., 2004; Tompkins et al., 2007; Wu et al., 2009 ), cholera toxin subunits (Liu et al., 2004), monophosphoryl lipid A (Eliasson et al., 2008 ; Ernst et al., 2006), or heat-labile endotoxin (De Filette et al., 2006; Fiers et al., 2004; Heinen et al., 2002; Neirynck et al., 1999). Genetic fusion of M2e to the oligomerization domain of general control nondepressible 4 (M2e-GCN4) or the rotavirus fragment NSP4 was also applied to enhance its immunogenicity with adjuvants (monophosphoryl lipid A, or cholera toxin subunits, Freund’s adjuvant) (Andersson et al., 2012; De Filette et al., 2008). The wild type M2 presented in lipid enveloped virus-like particles (VLPs) was capable of inducing protective anti-M2e antibodies but its incorporation into VLPs and immunogenicity were low (Song et al., 2011c). A construct with multiple copies of homologous M2e, fused to the bacterial flagellin protein (4.M2e-tFliC) and expressed on VLPs, was shown to be immunogenic (Wang et al., 2012).
We engineered a construct with a tandem repeat of heterologous M2e sequences (M2e5x), which was expressed in a membrane-anchored form and presented on enveloped VLPs (M2e5x VLPs) (Walker and Faust, 2010). Here, the breadth and potency of cross reactivity of M2e5x VLP immune sera were investigated in comparison with live virus infection sera, homologous M2e VLPs, and M2e monoclonal antibody 14C2. Protective mechanisms by M2e immunity were also explored.
2. Materials and Methods
2.1 Cells, Viruses, and Reagents
Sf9 insect cells were maintained in serum-free SF900 II medium (Gibco-BRL). Influenza viruses, 2009 pandemic A/California/4/2009 virus (a gift from Dr. Richard Webby), and A/Philippines/2/1982 and A/PR/8/34 (gifts from Dr. Huan Nguyen), were propagated in 10 day-old embryonated chicken eggs (Quan et al., 2010). Formalin inactivated influenza virus was prepared as described using sucrose-gradient purification (Quan et al., 2008).
2.2 Characterization of M2e5x VLPs
The gene construct for encoding multiple M2e (M2e5x) was genetically designed to contain a melittin signal peptide, a polypeptide sequence derived from flagellin (amino acids 85–102, INNNLQRVRELAVQSANS), five copies of influenza virus M2e sequences from human type-SLLTEVETPIRNEWGSRSNDSSD (2x), swine type-SLLTEVETPTRSEWESRSSDSSD (1x, A/California/4/2009, H1N1), avian type I-SLLTEVETPTRNEWESRSSDSSD (1x, A/Vietnam/1203/04, H5N1) and avian type II-SLLTEVETLTRNGWGCRCSDSSD (1x, A/Hong Kong/156/97, H5N1), a tetramerizing leucine zipper derived from GCN4 (De Filette et al., 2008), and transmembrane and cytoplasmic domains of HA derived from influenza A/PR/8/34 virus (Walker and Faust, 2010) (Fig. 1A). SF9 insect cells were co-infected with rBVs expressing M2e5x protein and influenza M1 protein to produce VLPs containing M2e5x (M2e5x VLP) or infected with rBV expressing M1 alone to produce M1 VLPs (Song et al., 2011c; Walker and Faust, 2010). M2e5x VLPs or M1 VLPs were purified from culture supernatants using the hollow fiber ultrafiltration system and sucrose gradient ultracentrifugation, and characterized by Western blot using anti-M2 monoclonal antibody (14C2, Abcam Inc., Cambridge, MA) and anti-M1 monoclonal antibody (GA2B, Abcam Inc., Cambridge, MA) (Song et al., 2011a; Walker and Faust, 2010).
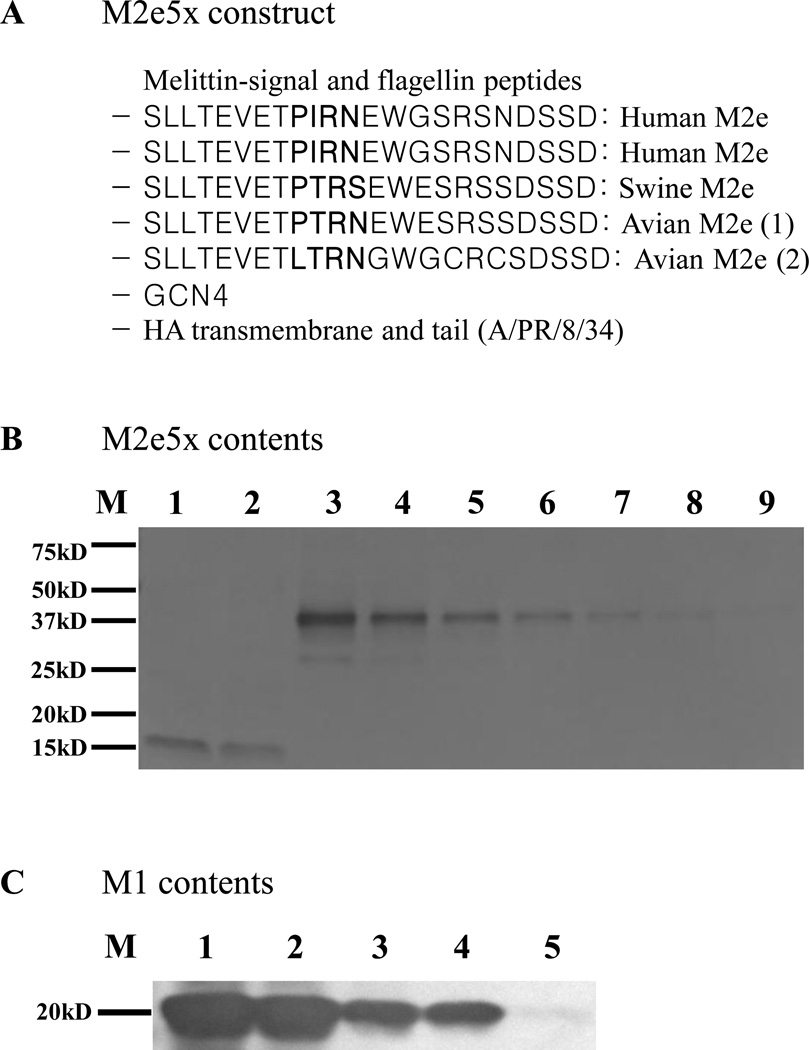
Fig. 1. M2e content and immunogenicity of M2e5x VLPs.
(A) The amino acid sequence of M2e5x construct. M2e5x VLPs indicate VLPs containing M2e5x in a membrane-anchored form. (B) M2e contents in M2e5x VLPs and influenza viruses. Different amounts of live influenza viruses and M2e5x VLPs were probed with anti-M2e monoclonal antibody (14C2) in Western blot. Lane 1: A/PR/8/34(H1N1) virus (10µg), Lane 2: A/Philippines/2/82(H3N2) virus (10µg), Lane 3–9; M2e5x VLP (100ng, 50ng, 25ng, 12.5ng, 6.25ng, 3.13ng, 1.56ng). (C) M1 contents in M2e5x VLPs and influenza viruses. Different amounts of live influenza viruses and M2e5x VLPs were probed with anti-M1 monoclonal antibody in Western blot. Lane 1–3: A/Philippines/2/82 (H3N2) virus (10µg, 5 µg, 1 µg, respectively), Lane 4–5; M2e5x VLP (1 µg, 100ng, respectively).
2.3 Immunization and challenge
Female BALB/c mice (6–8 weeks, Harlan Laboratories) were intramuscularly immunized with M2e5x VLPs (10µg) or M1 VLP (10 µg) at a 4-week interval. The mice (N=8) were bled to test immune responses at three weeks after prime or boost immunization. Four weeks after boost immunization, mice were intranasally challenged with 4xLD50 (50% mouse lethal dose LD50) of A/PR/8/34 influenza virus. The endpoint of euthanasia criteria was 25% body loss.
2.4 Determination of antibody responses
IgG and IgA antibody responses in immune sera were determined by ELISA using M2e peptides or inactivated H1N1 (A/PR8) or H3N2 (A/Philippines/82) influenza viruses as a coating antigen (4 µg/ml). Antibody concentrations were calculated by standard curves of purified mouse IgG and IgA antibodies (Southern Biotech, Birmingham, AL) as described (Quan et al., 2007; Song et al., 2011c).
Broncho-alveolar lavage fluids (BALF) were obtained at day 5 after challenge by infusing PBS buffer into the lungs via the trachea using a 25-gauge catheter. To compare M2e reactivity in infected or VLP immunized sera, ELISA plates were coated with human, swine, avian I or avian II type M2e peptide (4µg/ml).
2.5 Determination of cytokine or antibody secreting cellular responses
Interferon (IFN)-γ secreting cell spots of splenocytes and lung cells were determined on 96 well plates coated with cytokine specific capture antibodies (Song et al., 2010; Song et al., 2011b). Briefly, 0.5×106 spleen cells or 0.2×106 lung cells per well were cultured with human type M2e peptide (2µg/ml) or M2e5x VLP (2µg/ml) as an antigenic stimulator, and IFN-γ secreting cell spots were counted using an ELISpot reader. For antibody-secreting cell responses, spleen (0.5×106cells/well), bone marrow (0.3×106cells/well), or lung (0.2×106cells/well) cells were added to the culture plates and M2e peptide or M2e5x VLP-specific antibodies secreting cells were determined (Song et al., 2010).
2.6 Protective efficacy of immune sera
To determine in vivo protective efficacy of sera, naïve sera or immune sera from mice that were previously immunized with the M2e5x VLP or 4.M2e-tFliC VLP (Wang et al., 2012) were two fold diluted with PBS and heat-inactivated at 56°C for 30 min. The inactivated serum samples were mixed with influenza A virus and incubated at room temperature for 30 min as described (Quan et al., 2007; Quan et al., 2012; Song et al., 2011b). Naive mice (N=4) were intranasally infected with a mixture of influenza virus (3xLD50, A/California/2009) and immune sera, and body weight and survival rates were monitored for two weeks.
For testing the role of the Fc receptor in anti-M2 antibody mediated protection, BALB/c mice with genetic disruption of the Fc receptor common γ-chain (homozygous Fcer1g) were obtained from Taconic Farms (Germantown, NY). The M2e5x VLP immunized sera or naïve sera were mixed with influenza A/PR/8/34 virus (10xLD50) and incubated at room temperature for 30min. Naïve wild type and FcR knockout BALB/c mice (N=4) were intranasally infected with A/PR8/34 (5xDL50) virus and monitored daily for their weight changes and survival rates.
2.7 Statistical analysis
To determine the statistical significance, a two-tailed Student’s t-test was used when comparing two different groups. A p value less than 0.05 was considered to be significant.
3. Results
3.1 Characterization of heterologous tandem repeat M2e5x VLP
To improve the immunogenicity of M2e, VLPs containing a tandem repeat of M2e (M2e5x, Fig. 1A) from human, swine, and avian origin influenza A viruses were produced (Walker and Faust, 2010). Compared to the monomeric wild type M2 VLPs, this tandem repeat M2e5x was shown to be incorporated into VLPs at much higher levels (Kim et al., 2013). Here, the incorporation of M2e5x protein into VLPs (M2e5x VLPs) was compared with M2 in A/PR/8/34 and A/Philippines/2/82 influenza viruses (Fig. 1B). Even at hundred fold higher concentrations of virus (10 µg), the band intensity was less than that of 0.05 µg M2e5x VLPs. In contrast, M1 contents were found to be similar between M2e5x VLP and influenza virus per unit µg protein (column 3 and 4, Fig. 1C). Therefore, these results suggest that 14C2 recognizing M2e epitopes were incorporated into VLPs at several hundred fold higher levels compared to those in influenza viruses.
3.2 Immunogenicity of heterologous tandem repeat M2e5x VLP
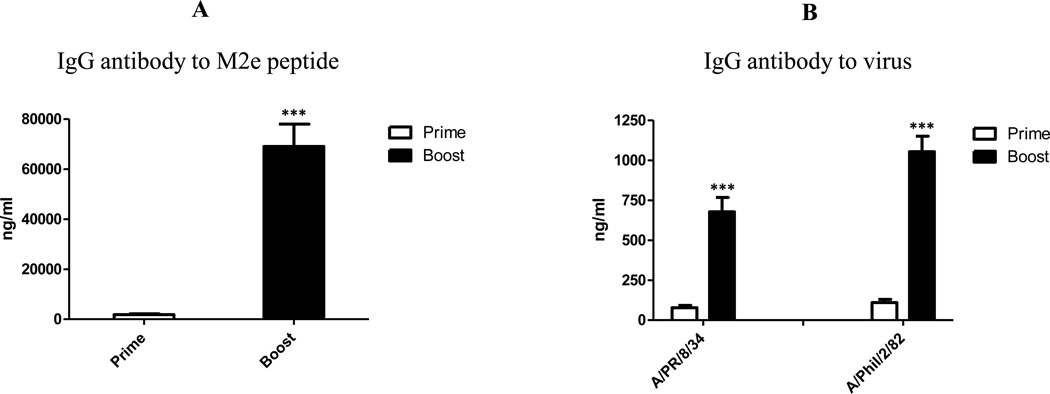
To evaluate the immunogenicity of M2e5x VLPs or M1 VLPs alone, mice (N=8) were immunized with M2e5x VLPs or M1 VLPs. After prime-boost immunization with M2e5x VLPs, the IgG antibodies specific for M2e (Fig. 2A) and H1N1 (A/PR/8/34) and H3N2 (A/Philippines/2/82) viral antigens (Fig. 2B) were significantly increased by approximately 37 and 7 to 9 folds respectively compared to those after priming. However, after prime-boost immunization with M1 VLPs, the IgG antibodies to M2e, H1N1 (A/PR/8/34), and H3N2 (A/Philippines/2/82) viral antigens were not detected (data not shown). These results indicate that M2e5x VLP is highly immunogenic, inducing antibodies recognizing different types of influenza viruses.
Fig. 2. Immunogenicity of M2e5x VLPs.
(A) IgG antibody responses reactive to M2e peptide (BALB/c mice, N=8). Levels of anti-M2e IgG antibodies were determined using human type M2e peptide-coated ELISA plates. (B) IgG antibody responses reactive to influenza viruses (BALB/c mice, N=8). Whole inactivated influenza A virus H1N1 subtype (A/PR/8/34) or H3N2 subtype (A/Philippines/2/82 abbreviated as A/Phil/2/82) were used as an ELISA coating antigen. Error bars indicates mean ± SEM (standard errors of mean). Asterisk indicates significant difference between prime and boost immunization groups (*** p<0.001).
3.3 M2e5x VLPs provide protection against influenza virus
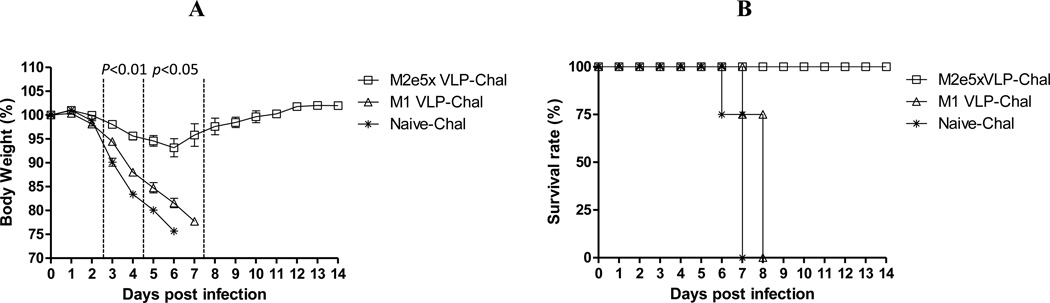
M2e5x VLP or M1 VLP-immunized or naive mice were infected with a lethal dose of A/PR/8/34 virus at 4 weeks after boosting. All mice in the naïve and M1-VLP groups lost over 25% in body weight and had to be euthanized although M1-VLP immunized mice showed a delay in weight loss and mortality (Fig. 3). However, the M2e5x VLP vaccinated mice showed less than 5% loss in weight resulting in 100% protection (Fig. 3). These results demonstrate that M2e5x VLPs are effective in conferring protection without adjuvant.
Fig. 3. M2e5x VLP vaccination induces protection against lethal infection.
Mice (N=8) that were immunized via intramuscular injection (IM) were intranasally challenged with a lethal dose (4×LD50) of influenza virus, A/PR/8/34 (H1N1), 4 weeks after boost vaccination. (A) Average body weight changes and (B) survival rates were monitored for 14 days. Error bars indicates SEM. LD, lethal dose.
3.4 Cross-reactive immune responses post challenge
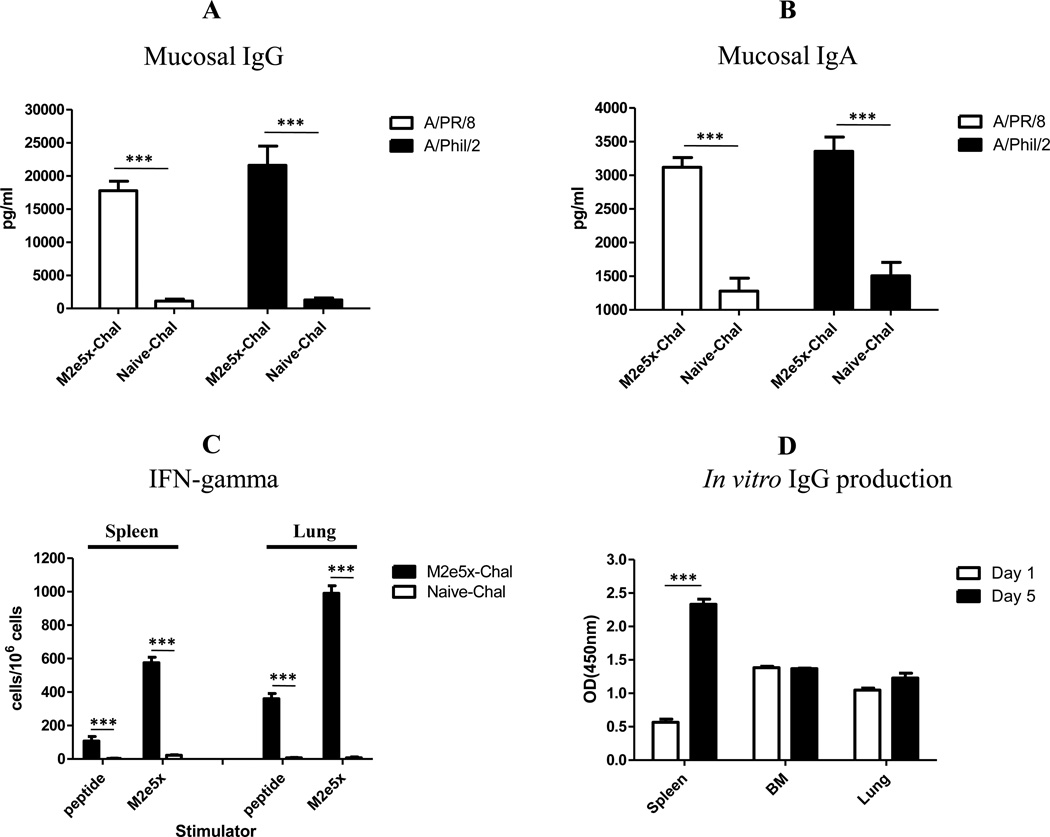
Higher levels of IgG and IgA antibodies cross-reactive to A/PR/8/34 and A/Philippines/2/82 viruses were detected in bronchoaveloar lavage fluids (BALF) compared to those from naïve infected mice at day 5 post-challenge (Fig. 4A,B). IFN-γ secreting T-cells in spleen and lungs were observed in vaccinated mice but not in naïve infected mice (Fig. 4C). The induction was more obvious in lung cells than that in spleen cells after challenge of vaccinated mice. These results suggest that mucosal antibodies and IFN-γ producing cellular response are induced after M2e5x VLP intramuscular vaccination.
Fig. 4. Cross reactive immune responses in M2e5x VLP immunized mice upon A/Phil/2/82 (H3N2) virus challenge.
(A–B) BALF was prepared on day 5 after challenge for cross reactive mucosal antibodies (N=4). Mucosal IgG antibodies (A) and IgA antibodies (B) reactive to influenza virus particles were determined by ELISA using H1N1 A/PR/8/34 or H3N2 A/Phil/2/82 as a coating antigen. (C) IFN-γ secreting T cells in spleen and lung cells (N=4). (D) In vitro antibody production by spleen, bone marrow (BM), and lung cells (N=4). M2e5x VLP specific IgG antibody levels produced during in vitro cultures for 1 day (Day 1) and 5 days (Day 5) were expressed as values of optical density (OD) determined by ELISA. Error bars indicates SEM (standard errors of mean). Asterisk or bar indicates significant difference (*** p<0.001).
There was a correlation between antibody secreting cell spots and in vitro antibody production (Kang et al., 2011; Song et al., 2010). As a measure of M2e5x VLP specific antibody secreting cell (ASC) responses, we harvested spleen, bone marrow and lung cells, cultured in vitro, and determined antibody levels. Higher levels of IgG antibodies were secreted into spleen cell culture supernatants at day 5 than those at day 1 (Fig. 4D). Cells from bone marrow and lungs produced substantial amounts of antibodies at day 1. These results indicate that vaccination with M2e5x VLPs can induce the generation of plasma cells in bone marrow and lung, as well as memory B cells in spleens which can differentiate into antibody secreting cells upon influenza virus infection.
3.5 M2e5x VLP immune sera show reactivity to heterologous M2e antigens
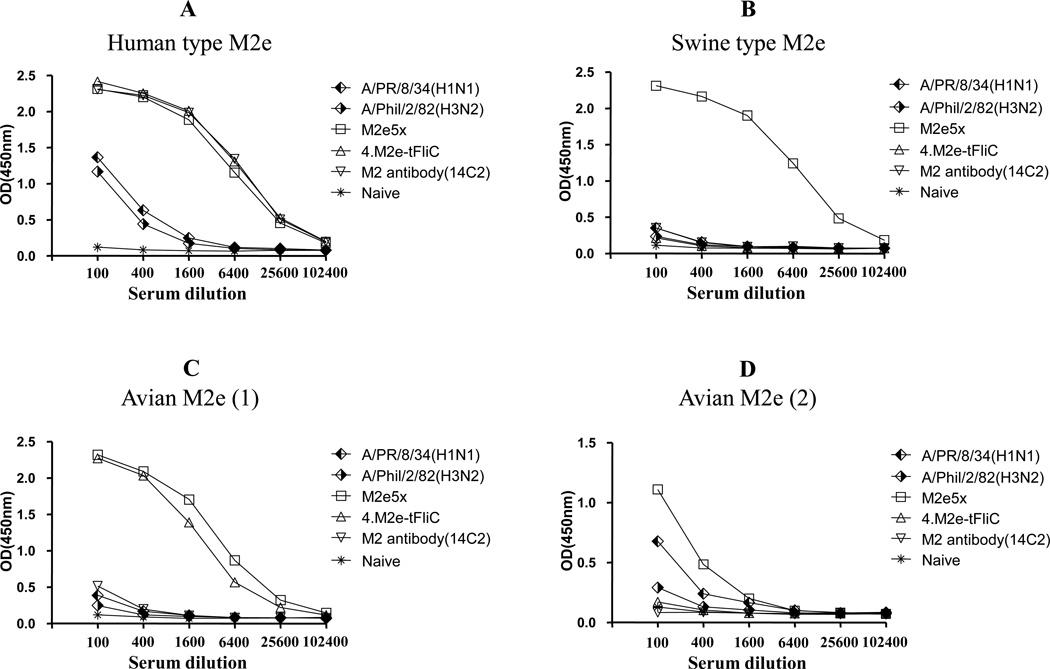
Wang et al. (2012) reported a fusion construct of bacterial flagellin and 4 homologous tandem repeats of the human M2e sequence (4.M2e-tFliC). Since the M2e5x construct contains heterologous tandem repeats, we compared cross-reactivity of M2e5x VLP, 4.M2e-tFliC VLP immune sera (Wang et al., 2012), and 14C2 antibody (Zebedee and Lamb, 1988). The 14C2 and immune sera of 4.M2e-tFliC VLP vaccination showed similarly high reactivity to M2e of human influenza A virus as observed with M2e5x VLP vaccination (Fig. 5A). However, both 4.M2e-tFliC VLP immune sera and 14C2 did not show reactivity to M2e from swine or avian H5N1 influenza A virus (A/Hong Kong/156/97) (Fig. 5B, D). Sera from infected mice with A/PR/8/34 (H1N1) or A/Philippines/2/82 (H3N2) influenza viruses were reactive to the human type M2e peptide antigen at lower levels and did not have reactivity to M2e derived from swine or avian isolates (Fig. 5B,C,D). M2e reactivity of M2e5x VLP immunized sera was approximately 64 times higher than sera from virus infection (Fig. 5A). Interestingly, immune sera from 4.M2e-tFliC VLP vaccination showed reactivity to M2e peptide of avian I influenza A virus (Fig. 5C). Importantly, only M2e5x VLP immune sera showed high cross-reactivity to different M2e peptides of human, swine, and avian origin isolates. These results indicate that M2e5x VLPs enable the induction of M2e antibodies with broader reactivity at higher levels than live influenza virus infection.
Fig. 5. Immune sera of M2e5x VLP vaccinated mice show a broader cross reactivity to different M2e antigens.
(A) Human type M2e polypeptide antigen. (B) Swine type M2e. (C) Avian type I M2e. (D) Avian type II M2e. A/PR/8/34 (H1N1): Pooled serum from mice infected with A/PR/8/34 virus, A/Phil/2/82(H3N2): Pooled serum from mice infected with A/Phil/2/82 virus, M2e5x: Pooled immune serum from mice vaccinated with M2e5x VLPs, 4.M2e-tFliC: Pooled immune serum from mice vaccinated with 4.M2e-tFliC VLPs, M2 antibody (14C2): M2 monoclonal antibody (50ug/ml), Naïve: naïve sera from unimmunized mice. Antibody reactivity was presented as optical densities at 450nm by ELISA coated with M2e peptide antigens. A representative data is shown out of 3 independent experiments.
3.6 Immune sera from M2e5x VLP immunization are reactive to influenza virus
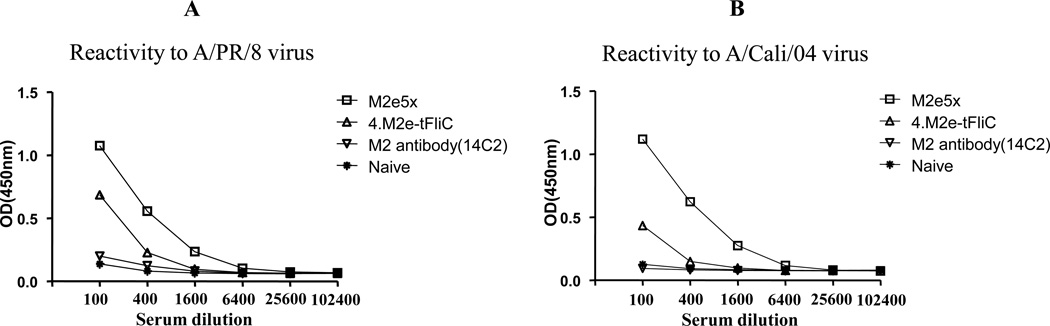
The 14C2 monoclonal antibody did not exhibit significant reactivity to A/PR/8/34 or A/California/2009 virions (Fig. 6A,B) despite its strong reactivity to M2e peptides of human type (Fig. 5A). The immune sera from 4.M2e-tFliC VLP vaccination showed low reactivity to A/PR/8/34 virus (Fig. 6A) and no reactivity to A/California/2009 virus (Fig. 6B). Importantly, M2e5x VLP immune sera showed significantly higher reactivity to both A/PR/8/34 and swine-origin A/California/2009 virus antigens (Fig. 6A,B). The results indicate that M2e5x VLP is much more effective in its capability to induce antibodies reactive to M2 on influenza virions compared to virus infection, homologous M2e VLP, or 14C2.
Fig. 6. M2e5x VLP immune sera are highly reactive with influenza viruses.
(A) Reactivity of M2e immune sera with A/PR/8/34 virus. (B) Reactivity of M2e immune sera with A/California/04/09 (H1N1) virus. M2e5x: Pooled immune serum from M2e5x VLP vaccinated mice, 4.M2e-tFliC: Pooled immune serum from 4.M2e-tFliC VLP vaccinated mice, M2 antibody (14C2): M2 monoclonal antibody (50ug/ml), Naïve: naïve sera from unimmunized mice. A representative data is shown out of 3 independent experiments.
3.7 M2e5x VLP immune sera confer better cross protection
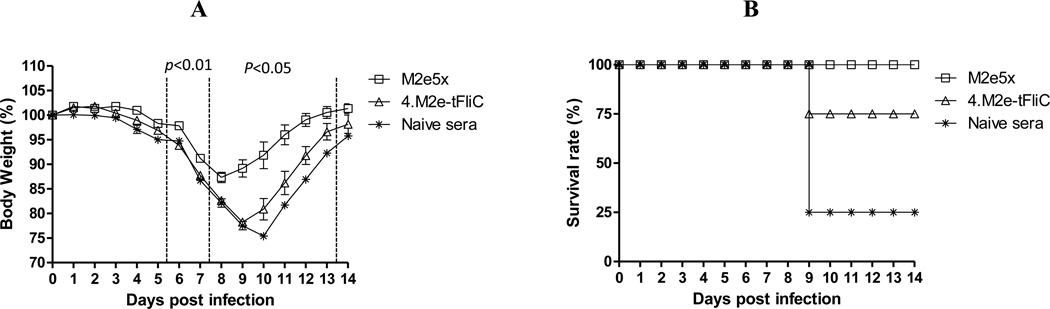
We tested whether sera with higher binding activity to M2e would confer better protection against influenza viruses with different M2. Groups of mice were infected with a lethal dose of swine-origin 2009 H1N1 influenza A virus mixed with sera from mice vaccinated with M2e5x VLPs or 4.M2e-tFliC VLPs (Fig. 7). Mice treated with M2e5x VLP immune sera showed moderate weight loss and all survived (Fig. 7A). However, mice with 4.M2e-tFliC VLP immune sera showed severe weight loss and 75% partial protection. Therefore, these results suggest that M2e5x VLPs containing heterologous M2e epitopes are capable of inducing antibodies conferring broader protection against influenza viruses with different residues in their M2e epitopes.
Fig. 7. M2e5x VLP immune sera are more effective in conferring broad protection against swine-origin 2009 H1N1 influenza A virus.
(A) Body weight. (B) Survival rates. Naïve BALB/c mice (N=4) were intranasally infected with A/California/4/09(H1N1) virus that was pre-mixed with M2e5x VLP or 4.M2e-tFlic VLP immune sera or naïve sera. Error bars indicates SEM (standard errors of mean).
3.8 Fc receptor plays an important role in M2e antibody mediated protection
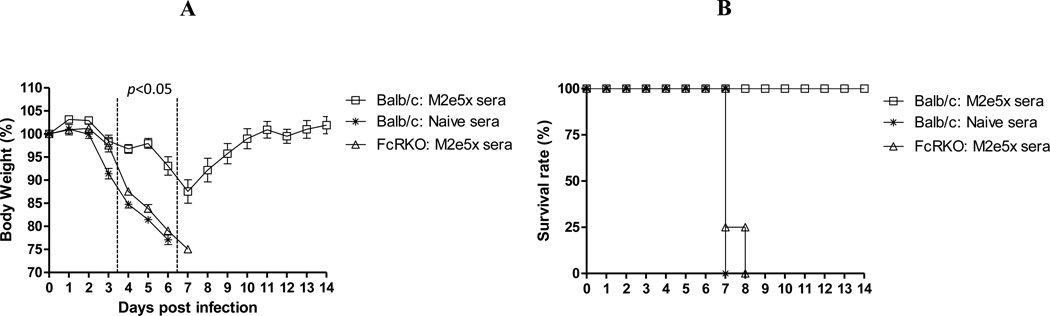
To determine a possible role of Fc receptor, we compared the protective efficacy of M2e5x VLP immune sera and unimmunized naïve sera using an FcR KO mouse model (Fig. 8). M2e5x VLP immune sera provided protection to wild type mice. However, M2e5x VLP immune sera did not confer any protection to FcR KO mice that were infected with the same virus. As a control, all mice infected with A/PR/8 virus mixed with unimmunized naive sera died (Fig. 8). Overall, these results support evidence that Fc receptor plays a crucial role in conferring protection via anti-M2e immune sera.
Fig. 8. Fc receptor is important for protection mediated by M2e5x VLP immune sera.
Survival rates (A) and body weight changes (B) of naïve FcR knockout or naïve wild type BALB/c mice (N=4) that were infected with A/PR/8/34 (H1N1) virus pre-mixed with sera. M2e5x sera: immune sera from M2e5x VLP vaccinated mice, Naïve: naïve sera from unimmunized mice.
Discussion
We observed that M2e5x VLPs conferred protection against lethal infection and prevented significant weight loss against diverse influenza A viruses with human type M2e sequences regardless of HA subtypes (H1, H3, H5) (Walker and Faust, 2010). However, it was not clear yet what immune components contribute to M2e-mediated cross protection. This study suggests that immune correlates contributing to improved protection by M2e5x VLPs could include increased immunogenicity of M2e5x VLPs, broader cross reactivity to different M2e peptides, the capacity of immune sera to bind to whole virus particles, and rapid recall immune responses at mucosal and systemic sites.
The first 9 amino acids of M2e are well conserved among human, swine, and avian influenza A viruses (Fiers et al., 2004; Liu et al., 2005). However, 6 amino acids out of the 15 remaining residues differ among diverse influenza A viruses (Fig. 1A). To overcome this heterogeneity of M2e of influenza A virus, we designed a tandem repeat construct consisting of representative M2e sequences from human, swine, and avian influenza virus. Vaccination with M2e5x VLPs was effective in inducing antibodies reactive to different M2e peptide antigens derived from human, swine, and avian species at higher levels than those detected in sera from live virus-infected mice, monoclonal antibody 14C2 (Zebedee and Lamb, 1988), or the immune sera from mice immunized with homologous human M2e tandem repeat 4.M2e-tFliC VLP immune sera (Wang et al., 2012) or individual wild type M2 VLP vaccine (Kim et al., 2013). Interestingly, immune sera from mice immunized with 4.M2e-tFlic VLP showed comparable reactivity to the avian M2e peptide but not to the swine M2e peptide. From this reactivity analysis, the changes in the residue 11 (from isoleucine to threonine) and 13 (from asparagine to serine) seem to significantly influence M2e reactivity of 4.M2e-tFlic VLP immune sera. Our current study provides evidence that broader reactivity of antibodies induced by vaccination with heterologous M2e5x VLPs might have contributed to improved cross protection compared to the homologous 4.M2e-tFlic VLP vaccine. In addition, heterologous M2e5x VLP was shown to confer much broader and stronger immunity than the individual human M2 VLP (Kim et al., 2013). Therefore, a strategy to include heterologous M2e sequences in a tandem repeat on VLPs would provide a promising approach for broader immunity.
M2 protein molecules are incorporated into the virions at a low level although they are expressed abundantly on the surface of infected cells (Lamb et al., 1985; Zebedee and Lamb, 1988). Induction of M2e antibodies reactive to virions would be advantageous in clearing virus particles. Most previous M2 vaccine studies did not report binding antibody titers to the virus particles probably due to no binding or less reactivity, which might be because of low amounts of M2 on virus surfaces and masking effects by the large viral glycoproteins. Immune sera from mice immunized with M2e-conjugate vaccines were shown to be reactive to the M2e peptide antigen but not to the influenza virus (Jegerlehner et al., 2004). We found that 14C2 and immune sera of 4.M2e.tFliC were as reactive to human M2e peptides as M2e5x VLP immune sera. In contrast, reactivity to the virus was observed at a high level in immune sera of M2e5x VLP but not with 14C2 or 4.M2e.tFliC VLP immune sera. Thus, not all antibodies binding to M2e polypeptides have the ability to recognize M2 on the influenza virus. The structural conformation of recombinant M2e vaccine constructs used to induce M2 antibodies could be an important factor contributing to differential reactivity between free M2e peptides and M2 on virus particles. This study supports a concept that M2-based vaccines that are capable of inducing antibodies binding to the virus will confer more effective protection (Fig. 6).
Mechanisms by which M2 antibodies provide protection have not been well characterized. M2 antibody mediated protection could act in concert with cellular effector functions. It was proposed that natural killer cells play an important role for M2e antibody mediated protection via antibody-dependent cytotoxicity (Jegerlehner et al., 2004). However, other studies demonstrated that natural killer cells were not required for M2 immune mediated protection (Fu et al., 2009a; Tompkins et al., 2007). In recent studies of passive immunization with M2e immune sera, alveolar macrophages and/or dendritic cells were shown to play a significant role in conferring protection (El Bakkouri et al., 2011; Song et al., 2011b; Song et al., 2011c). Also, it was reported that Fc receptors were essential for anti-M2e antibody-mediated immune protection against influenza A virus challenge (El Bakkouri et al., 2011). In line with a previous study (El Bakkouri et al., 2011) reporting the important role of Fc receptors, we found that protection by immune sera from M2e5x VLP vaccinated mice was not observed in mice that are deficient in the common γ-chain of the Fc receptor (Fig. 8). The common γ-chain is required for intracellular signaling of the several activatory Fc receptors (FcγRI, FcγRIII, and FcγRIV) (Nimmerjahn and Ravetch, 2005). Thus, it is possible that antibody mediated phagocytosis of viruses may play a role in protection by M2e5x VLP vaccination in addition to antibody dependent cell-mediated cytotoxicity.
In summary, (1) a broader cross reactivity and improved protection of M2 immunity can be obtained by a recombinant tandem repeat construct containing human, swine, and avian type M2e sequences compared to other M2e-based vaccines. (2) Higher levels of anti-M2e antibodies are induced by vaccination with M2e5x VLPs without using adjuvants compared to those induced by live virus infection. (3) Anti-M2e antibodies binding to influenza viruses can be induced by vaccination with M2e5x VLPs. (4) Signaling of several activatory Fc receptors play a critical role for anti-M2e antibody mediated protection by M2e5x VLP vaccination.
Acknowledgments
This work was supported by NIH/NIAID grants AI0680003 (R.W.C.), AI105170 (S.M.K.) AI093772 (S.M.K.), and AI087782 (S.M.K.), Georgia State University & Georgia Research Alliance research funds (S.M.K), TOBICO Co. & Dankook University (S.M.K), and Animal, Plant, and Fisheries Quarantine Inspection Agency grant (I-1541781-2012-15-01), Republic of Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N, Soeroso S, Uyeki TM. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358:261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Hakansson KO, Jensen BA, Christensen D, Andersen P, Thomsen AR, Christensen JP. Increased immunogenicity and protective efficacy of influenza m2e fused to a tetramerizing protein. PloS one. 2012;7:e46395. doi: 10.1371/journal.pone.0046395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa J, Schmitz N, Hinton HJ, Schwarz K, Jegerlehner A, Bachmann MF. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur J Immunol. 2008;38:114–126. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- De Filette M, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, Vandekerckhove J, Fiers W, Saelens X. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283:11382–11387. doi: 10.1074/jbc.M800650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filette M, Ramne A, Birkett A, Lycke N, Lowenadler B, Min Jou W, Saelens X, Fiers W. The universal influenza vaccine M2e-HBc administered intranasally in combination with the adjuvant CTA1-DD provides complete protection. Vaccine. 2006;24:544–551. doi: 10.1016/j.vaccine.2005.08.061. [DOI] [PubMed] [Google Scholar]
- El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, Van Rooijen N, Verbeek S, Fiers W, Saelens X. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. Journal of immunology. 2011;186:1022–1031. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- Eliasson DG, Bakkouri KE, Schon K, Ramne A, Festjens E, Lowenadler B, Fiers W, Saelens X, Lycke N. CTA1-M2e-DD: A novel mucosal adjuvant targeted influenza vaccine. Vaccine. 2008;26:1243–1252. doi: 10.1016/j.vaccine.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Ernst WA, Kim HJ, Tumpey TM, Jansen AD, Tai W, Cramer DV, Adler-Moore JP, Fujii G. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24:5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Fan J, Liang X, Horton MS, Perry HC, Citron MP, Heidecker GJ, Fu TM, Joyce J, Przysiecki CT, Keller PM, Garsky VM, Ionescu R, Rippeon Y, Shi L, Chastain MA, Condra JH, Davies ME, Liao J, Emini EA, Shiver JW. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22:2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Fiers W, De Filette M, Birkett A, Neirynck S, Min Jou W. A "universal" human influenza A vaccine. Virus Res. 2004;103:173–176. doi: 10.1016/j.virusres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Fu TM, Freed DC, Horton MS, Fan J, Citron MP, Joyce JG, Garsky VM, Casimiro DR, Zhao Q, Shiver JW, Liang X. Characterizations of four monoclonal antibodies against M2 protein ectodomain of influenza A virus. Virology. 2009a;385:218–226. doi: 10.1016/j.virol.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Fu TM, Grimm KM, Citron MP, Freed DC, Fan J, Keller PM, Shiver JW, Liang X, Joyce JG. Comparative immunogenicity evaluations of influenza A virus M2 peptide as recombinant virus like particle or conjugate vaccines in mice and monkeys. Vaccine. 2009b;27:1440–1447. doi: 10.1016/j.vaccine.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Heinen PP, Rijsewijk FA, de Boer-Luijtze EA, Bianchi AT. Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. J Gen Virol. 2002;83:1851–1859. doi: 10.1099/0022-1317-83-8-1851. [DOI] [PubMed] [Google Scholar]
- Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, Tang J, McDonald W, Song L, Evans RK, Umlauf S, Tussey L, Powell TJ. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26:201–214. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- Ionescu RM, Przysiecki CT, Liang X, Garsky VM, Fan J, Wang B, Troutman R, Rippeon Y, Flanagan E, Shiver J, Shi L. Pharmaceutical and immunological evaluation of human papillomavirus viruslike particle as an antigen carrier. J Pharm Sci. 2006;95:70–79. doi: 10.1002/jps.20493. [DOI] [PubMed] [Google Scholar]
- Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–5605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- Kang SM, Yoo DG, Kim MC, Song JM, Park MK, O E, Quan FS, Akira S, Compans RW. MyD88 Plays an Essential Role in Inducing B Cells Capable of Differentiating into Antibody-Secreting Cells after Vaccination. Journal of virology. 2011;85:11391–11400. doi: 10.1128/JVI.00080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, Kang SM. Virus-like Particles Containing Multiple M2 Extracellular Domains Confer Improved Cross-protection Against Various Subtypes of Influenza Virus. Mol Ther. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- Liu W, Peng Z, Liu Z, Lu Y, Ding J, Chen YH. High epitope density in a single recombinant protein molecule of the extracellular domain of influenza A virus M2 protein significantly enhances protective immunity. Vaccine. 2004;23:366–371. doi: 10.1016/j.vaccine.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Liu W, Zou P, Ding J, Lu Y, Chen YH. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 2005;7:171–177. doi: 10.1016/j.micinf.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Nava GM, Attene-Ramos MS, Ang JK, Escorcia M. Origins of the new influenza A(H1N1) virus: time to take action. Euro Surveill. 2009:14. doi: 10.2807/ese.14.22.19228-en. [DOI] [PubMed] [Google Scholar]
- Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82:1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim MC, Lee BJ, Song JM, Compans RW, Kang SM. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology. 2012;430:127–135. doi: 10.1016/j.virol.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Vunnava A, Compans RW, Kang SM. Virus-Like Particle Vaccine Protects against 2009 H1N1 Pandemic Influenza Virus in Mice. PLoS One. 2010;5:e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyov A, Palacios G, Briese T, Lipkin WI, Rabadan R. Cluster analysis of the origins of the new influenza A(H1N1) virus. Euro Surveill. 2009:14. doi: 10.2807/ese.14.21.19224-en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Choi CW, Kwon SO, Compans RW, Kang SM, Kim SI. Proteomic characterization of influenza H5N1 virus-like particles and their protective immunogenicity. J Proteome Res. 2011a;10:3450–3459. doi: 10.1021/pr200086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A. 2011b;108:757–761. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, Jin HT, Pekosz A, Compans RW, Kang SM. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011c;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue RJ, Hay AJ. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991;180:617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z, Hogan RJ, Wu Z, Benton KA, Tumpey TM, Epstein SL. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13:426–435. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, Kavita U, Stanberry L, Shaw A. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29:5145–5152. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Walker WT, Faust SN. Monovalent inactivated split-virion AS03-adjuvanted pandemic influenza A (H1N1) vaccine. Expert review of vaccines. 2010;9:1385–1398. doi: 10.1586/erv.10.141. [DOI] [PubMed] [Google Scholar]
- Wang BZ, Gill HS, Kang SM, Wang L, Wang YC, Vassilieva EV, Compans RW. Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a toll-like receptor ligand. Clinical and vaccine immunology : CVI. 2012;19:1119–1125. doi: 10.1128/CVI.00153-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Yuan XY, Li J, Chen YH. The co-administration of CpG-ODN influenced protective activity of influenza M2e vaccine. Vaccine. 2009;27:4320–4324. doi: 10.1016/j.vaccine.2009.04.075. [DOI] [PubMed] [Google Scholar]
- Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]