Abstract
Background
Intrathecal morphine forms granulomas that arise from the adjacent arachnoid membrane. We propose that these inflammatory cells exit the meningeal vasculature secondary to meningeal mast cell degranulation.
Methods
Three sets of experiments were accomplished in dogs. 1) Ex vivo Meningeal mast cell degranulation. Histamine release was measured ex vivo from canine dura incubated with opiates. 2) In vivo cutaneous mast cell degranulation. Flare areas on the dog abdomen were measured after subcutaneous opiates. 3) In vivo granuloma pharmacology. Dogs with lumbar intrathecal catheters received infusion of intrathecal saline or intrathecal morphine. Intrathecal morphine dogs received: i) No other treatment (Control); ii) Twice daily subcutaneous naltrexone; iii) Intrathecal co-infusion of cromolyn; or, iv) Twice daily subcutaneous cromolyn for the 24–28 day study course.
Results
1) Morphine but not fentanyl evoked dural histamine release, which was blocked by cromolyn but not naloxone. 2) Wheal/flare was produced by subcutaneous morphine, methadone, hydromorphone, but not fentanyl, and was unaffected by naltrexone but prevented by cromolyn. 3) Granulomas occurred in all dogs receiving intrathecal morphine (15/15); subcutaneous naltrexone had no effect on granulomas (6/6), but was reduced by concurrent intrathecal cromolyn (0/5) or twice daily subcutaneous cromolyn (1 of 5).
Conclusions
The pharmacology of cutaneous/dural MC degranulation and intrathecal granulomas are comparable, not mediated by opioid receptors, and reduced by agents preventing MC degranulation. If an agent produces cutaneous MC degranulation at concentrations produced by intrathecal delivery, the agent may initiate granulomas.
INTRODUCTION
By histopathology and MRI, intrathecal infusion of high concentrations of morphine in canine and ovine models had no effect upon spinal parenchyma, but led to an aseptic collection of inflammatory cells (granuloma). 1–4 Preclinical work shows granulomas have several attributes. i) Incidence is concentration dependent 2–5 and not observed after repeated bolus delivery. 6 ii) Using serial MRI’s in the dog, a mass may begin to form proximal to the catheter tip in an interval as short as 10 days; and, termination of morphine resulted in a progressive reduction in mass size over the ensuing 14 days.5 iii) The mass arises from the dura-arachnoid layer of the meninges adjacent to the infusion catheter tip 3. These observations are consistent with many human case series that have been reviewed. 7–9,10
An important question relates to the mechanism of this cellular accumulation. Bacterial presence has been reported; but, appears to be a rare, secondary, event (see 3,11). Solutions are typically prepared as to have cerebrospinal fluid compatible osmolarity and pH.3,12,13 Finally, infusion of vehicle (saline)2–4 or a variety of drugs (see, for example 14–16) fails to produce a granuloma in the dog. Substitution of saline for morphine infusate, after a mass has been established, reduces granuloma size. 5 Thus, the best characterized component appears to be morphine itself. We showed that several opioid molecules, including hydromorphone and methadone, resulted in intrathecal masses; whereas reduced, if any, granulomas were noted with high concentrations of fentanyl or the mu-opioid peptide [D-Ala2, N-MePhe4, Gly-ol5]-enkephalin (DAMGO), casting doubt as to whether this effect is opioid receptor mediated 12 (but see17). Whether the morphine granuloma is prevented or reversed by opiate antagonism is unknown.
Local accumulation of inflammatory cells arising from the meninges and its accessibility to the intrathecal infusate led us to consider that the mechanism might be a local drug effect on a meningeal target. The comparability of this phenomenon to plasma extravasation in other dural systems led us to consider the role of meningeal mast cells. Our hypothesis relating mast cell function to the morphine-induced granuloma is based on four considerations. 1) Mast cells are distributed in dura arachnoid. 18–20 2) Dural mast cell activation leads to local vasodilatation and release of chemoattractants that enhance cell migration. 21,22 3) Opiates degranulate cutaneous mast cells in skin, but not reliably mast cells in lung, intestine, heart or blood basophils. 23,24 4) As certain opiates (morphine) but not all (fentanyl) degranulate skin mast cells leading to dilation and plasma extravasation25–29 and because the ability of an opiate antagonist to prevent such degranulation is controversial,30,31 the role of opiate receptors in this phenomena appears unlikely. This profile suggests similarities to that of intrathecal morphine-evoked granulomas and is consistent with the hypothesis that granulomas involve a mast cell contribution. This hypothesis led to a series of studies to characterize opiate effects in ex vivo and in vivo models on mast cell degranulation and the contribution of this action to the granuloma initiated by continuous intrathecal morphine in the canine model.
MATERIALS AND METHODS
All studies described herein were accomplished under method protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. There are two principal components to this series of studies: ex vivo and in vivo.
Ex vivo studies
These studies sought to determine the pharmacology of meningeal mast cell degranulation. For these studies, mixed-breed hounds (2 male/3 female; approximately 25–35 kg) undergoing terminal experiments examining cardiac function were employed.
Meningeal harvest
The animals were deeply anesthetized to the minimum of a surgical plane anesthesia sufficiently deep to perform a laminectomy (sodium pentobarbital, 35 mg/kg IV, or propofol, 5–10 μg/kg/min, IV). The lumbar and thoracic vertebral bodies were exposed, cardiac arrest was initiated and the dura immediately harvested from the lumbar and thoracic levels. The dural fragments were immediately placed in an oxygenated iced Krebs ringer’s solution. The dural segments were cut into small fragments weighing approximately 10 mg. These were placed in individual Eppindorf tubes filled with Krebs ringer (1mL) that were oxygenated by bubbling with 95% O2/5% CO2 and warmed in a 37° C heating block. After an initial 20 min incubation, the solution was removed by aspiration and replaced. At this time, an aliquot of the test agent(s) was pipetted into each chamber. After a 30 min incubation, the solution was aspirated into individual containers and frozen until assay. The dural fragments were then gently blotted dry and weighed. The fragment was then placed in formalin and refrigerated. The dural fragment was mounted on a cryostat and tangential 10 μ sections were taken and mounted on glass slides.
Assay of Mast cell mediators
Each incubation sample (100μL) was assayed for histamine content. Histamine was analyzed using ELISA (Research Diagnostics, Inc, Flanders, NJ). The limit of detection of this assay was approximately 0.3 ng/mL. The intra- and interassay precision was <11.0 and <12.0 %CV, respectively (3–30 ng/mL).
Dural mast cell visualization
Mast cells in the dural sections were stained with Alcian Blue or with Naphthol-AS-D-chloroacetate esterase staining, a Fast Red staining technique, following methods provided by manufacturer (Sigma-Aldrich Corp, St. Louis, MO). Samples were surveyed under a microscope at 20X. In each fragment, 5 stained sections were then examined at 60X in a 100μ field taken in the center of each fragment.
In Vivo Studies – intrathecal infusion
Study design
To characterize the effects of intrathecal drug treatments on granuloma formation, groups of animals were prepared with chronic lumbar intrathecal catheters. They were then assigned to be entered into protocols. The groups and number of animals are listed in Table 1.
TABLE 1.
Summary of Intrathecal infusion treatment groups
| TREATMENT GROUPS | |||||
|---|---|---|---|---|---|
| Vehicle (1ml/d) | Morphine (12.5 mg/mL/d) | Morphine (12.5 mg/mL/d) + SQ naltrexone (0.9mg/kg/d) | Morphine (12.5 mg/mL/d) + IT Cromolyn (12 mg/kg/d) | Morphine (12.5 mg/mL/d) + SQ Cromolyn (7.5 mg/kg/d) | |
| Group Size | 5 | 15 | 6 | 5 | 5 |
Abbreviations: mg/mL/d: milligrams/milliliter/day; SQ: Subcutaneous; IT: intrathecal
Animals
Beagle dogs (Ridglan Farms Inc., Mt. Horeb, WI, or equivalent), 12 to 16 months of age (approximately 9–16 kilograms), were individually housed in runs with wood shavings and given ad libitum access to food and water. Animals were adapted for a minimum of 10 days prior to surgery. A nylon vest for pump placement was placed on each dog 48 hours prior to intrathecal catheter placement for acclimation.
Bolus subcutaneous drug delivery for systemic delivery
Drugs were injected subcutaneously in a shaved region of the nape of the neck using a 25 ga needle.
Continuous intrathecal drug delivery
Surgical preparation. Dogs were prepared with chronic intrathecal catheters by the surgical placement of the intrathecal catheter approximately 72 hours prior to dosing. Antibiotic sulfamethoxazole-trimethoprim (240mg tablet, 15–25mg/kg, oral, twice daily) was given 48 hours before and after surgery. Dogs received atropine (0.04mg/kg, IM) prior to sedation with xylazine (1.5mg/kg, IM). After intubation, anesthesia was maintained under spontaneous ventilation with 1.0–2.0% isoflurane and 60% N2O/40% O2. Intraoperatively, animals were continuously monitored for oxygen saturation, inspired and end tidal values of isoflurane, CO2, N2O and oxygen, and heart and respiratory rates. Surgical areas were shaved and prepared with chlorhexadine scrub and solution. Using sterile technique, the dura of the cisterna magna was exposed. Through a small incision (1–2mm), the intrathecal catheter was inserted and passed caudally a distance of approximately 40–42 cm to a level corresponding to the L2–3 segment. The catheter was fabricated of polyethylene or polyurethane tubing (0.61 mm OD), packaged, and sterilized by E-beam irradiation. Dexamethasone sodium phosphate (0.25mg/kg, IM) was administered just after catheter placement. The external catheter was tunneled to exit at the left scapular region. Upon closure of the incision, isoflurane was removed and the animal recovered. Butorphanol tartrate (Tobugesic®; 0.04mg/kg, IM; Fort Dodge Animal Health, New York, NY) was administered upon recovery and as necessary to relieve post-operative discomfort. Following recovery, a nylon vest was placed on the animal and an infusion pump (Minimed 507; Medtronic, Northridge, CA) was secured in the vest pocket where it was connected to the externalized end of the intrathecal catheter.
Initiation of continuous intrathecal morphine infusion. All intrathecal infusions were carried out at 40μL/hr = 960μl/d = 1 mL/day. As previously reported, the initiation of infusion of 12 mg/d morphine in the canine model resulted in behavioral depression, agitation and motor signs.3 It was found that a stepwise ramping of the infusion concentration from 3mg/d to 12/mg/d could be accomplished over a period of around 4–7 days and then continued to 24–28 days served to minimize adverse events. This protocol was followed in these studies.
Behavioral Observations. Specific behavioral indices of arousal (depressed to agitated: −3 to +3), muscle tone (flaccid to rigid: −3 to +3) and coordination (normal to impaired: 0 to 3) were assessed daily. These indices have been previously validated.32,33 For purposes of data presentation, overall sensorimotor impairment was presented as the cumulation of the absolute value of the observed muscle tone and coordination score at the time of euthanasia (e.g., 0 = no detectable sensorimotor abnormality; 6 being complete disability and requiring immediate euthanasia).
In a limited number of animals during the initial intrathecal dose incrementation phase noted above, hind limb thermal escape latencies were assessed with the initiation of the 3 mg/mL/d and 12 mg/mL/d infusion (N = 4). In animals that were to receive concurrent dosing with naltrexone, escape latencies were assessed with the start of the 12.5 mg/mL morphine infusion and the initial dosing with naltrexone (0.9 mg/kg, subcutaneous) (N = 4). Hindpaw thermal escape latencies were assessed using a Hargreaves-type stimulator wherein the hindpaws are placed over a glass surface under which is a focused projection bulb. The latency to paw removal was the response. In the absence of a response within 20 sec, the stimulus was terminated and that latency assigned as the response.34
Necropsy. Dogs were deeply anesthetized with an intravenous dose of propofol (5–10 mg/kg/min, IV) or sodium pentobarbital (35mg/kg or to effect). Animals were exsanguinated by perfusion with saline (~4L) followed by 10% formalin (approximately 4L) delivered by roller pump at approximately 100 mmHg. The spinal column was exposed by laminectomy of the spinal canal. The condition of the spinal cord and overlying dura was noted and the location of the catheter tip in situ. The spinal cord was cut (taking care to keep the dura intact) and removed in four blocks approximating the cervical, thoracic, lumbar (catheter tip region) and lower lumbar (below catheter tip).
Histochemistry. Spinal blocks from perfusion fixed animals were paraffin embedded, sectioned at approximately 4–8 μm thickness and stained with hematoxylin-eosin. To assess mast cell condition, a fragment of dura was additionally stained with either Fast Red or Alcian Blue. To estimate cross-sectional area of the granuloma, hematoxylin-eosin sections are taken at the level of the largest girth of the mass (typically at or near the lumbar catheter tip) and the cross-sectional area of the granuloma and the spinal cord was manually outlined using Image- Pro Plus 5.1 software (Acton Manufacturing Center c/o Media Cybernetics, Inc., Acton, MA) to measure area in the outlined pixels. Pixel count was converted into mm2.
Post-Mortem magnetic resonance imaging. In several dogs volume estimates and images were obtained using postmortem magnetic resonance imaging of cords from dogs that received 12 mg/day morphine. After perfusion fixation, cords were immersed in DMSO. Images were acquired with a 7T magnetic resonance imaging scanner using 3D gradient echo sequence (FSPGR) TR/TE=10/2.9,FA 20, FOV 15mm, matrix 256×256, slice thickness 100 μm. Cross-sectional images were created and granulomatous tissue areas manually outlined. Using Amira software (Visualization Sciences Group, Burlington, MA), these serial sections were used to volumetrically reconstruct the granuloma and adjacent spinal cord and to calculate granuloma volume.
In Vivo Studies – Intradermal drugs to study flare formation
Male beagles (approximately 7–12 kg, N = 4) were anesthetized with IV propofol (5 μg/kg/min). Animals were intubated, body temperature maintained with an underbody heating pad and continuously monitored as described above. The chest and abdomen were shaven and surgically prepped. Intradermal injections of drug solutions were delivered in 50μL at T = 0 at 12–14 sites (6–7/side). The injection sites were marked with ink. The diameter of the redness around each injection site was measured across its longest and narrowest axis and recorded without reference to drug treatment at T = 0, 10, 30 and 60 min. Flare area immediately after injection and at intervals were calculated in mm2 as an oval (3.14 x a x b where a = half length of long axis and b = half length of short axis). Animals were used at 5-day intervals. This protocol was similar to that previously described to assess flare in dogs.35,36 Each animal was used 5–7 times.
Drugs
Preservative-free, Food and Drug Administration–approved formulations of morphine sulfate (Infumorph; Abbott, Abbott Park, IL), hydromorphone hydrochloride (Dilaudid-HP for Injection; Abbott), and saline vehicle (0.9% NaCl; Abbott) were used. Other opioids employed were prepared from the powder. D/L methadone HCl and fentanyl HCl were obtained from the National Institute of Drug Abuse. The opiate antagonists, naloxone HCl, naltrexone HCl, the mast cell degranulating compound 48/80 and the mast cell stabilizers Cromolyn Sodium salt and nedocromil sodium were obtained from Sigma Chemical (St. Louis, MO). All drugs were prepared in saline (0.9%) unless otherwise stated. Doses and concentrations employed are indicated in the text.
Statistics
Comparisons across treatment groups for granuloma size and for sensorimotor scores employed the nonparametric Kruskal Wallis with post hoc comparisons to the saline treatment group using Dunn’s multiple comparison test. For the cutaneous flare, comparisons were made across treatment using one way ANOVA with post hoc comparison to the saline (vehicle control) group using Dunnett’s multiple comparison. For specific post hoc comparisons between the flare inducing agent alone and with a co-treatment, multiple two tailed t-tests were performed with a Bonferroni correction for alpha buildup performed for each set of tests. For analysis of thermal escape thresholds over time as a function of dose and treatment, a two-way repeated measures was performed with post hoc comparisons using the Bonferroni test. To relate degree of sensorimotor deficits with granuloma size, we undertook a linear regression of sensorimotor score at sacrifice versus cross-sectional area of the granuloma. Group comparisons having critical values corresponding to p<0.05 were considered to be statistically significant. All analyses employed the GraphPad Prism software package (v.4.0c for MAC OS X; GraphPad Prism Software Inc., La Jolla, CA).
RESULTS
In vivo spinal granuloma formation with intrathecal morphine
Granuloma formation
Previous work has shown that intrathecal infusion of saline has no untoward histopathologic reactions out though approximately 4 weeks (see FIGURE 1). In contrast, morphine at 12 mg/d (e.g. 12.5mg/mL delivered at 960μL/hr) in the dog led to the development of prominent granulomas inside 2 weeks in essentially all animals so infused.3,12 FIGURE 1 displays representative granulomas from three of the last 15 dogs receiving 12 mg/d morphine for approximately 28 days. Using ex vivo MRIs, volumetric reconstruction of the granuloma of 4 dogs that received 12 mg/d for greater than 24 days revealed calculated mass volumes of 0.42, 0.39, and 0.31, 0.36 cm3. As indicated by the typical volumetric reconstruction, the greatest girth of the mass was typically at the level of the catheter tip (FIGURE 2). In the animal imaged in FIGURE 2, dual catheters had been placed to permit infusion and sampling. As indicated, the granuloma developed around the infusion catheter and was largest at the tip of the infusion catheter (e.g. the drug delivery site). In the 15 dogs receiving intrathecal morphine (12 mg/d), the area of the granuloma at the level of largest girth was calculated. The median and inter quartile range of the maximum area of the pericatheter mass was 9.5 (8.1–14.0) mm2. The corresponding median percentage of the cross-sectional area with quartiles was 27.1% (23.3–33.1), emphasizing the compressive nature of these masses. The distribution of the cross-sectional area and percent of the cross-sectional area of these masses is presented in FIGURE 3 and will be discussed further below. As reviewed previously,3 examination of the granulomas revealed the presence of inflammatory cells with extensive immunoreactivity for CD68-ir macrophages and T cells (Leu4) (data not shown).
Figure 1.
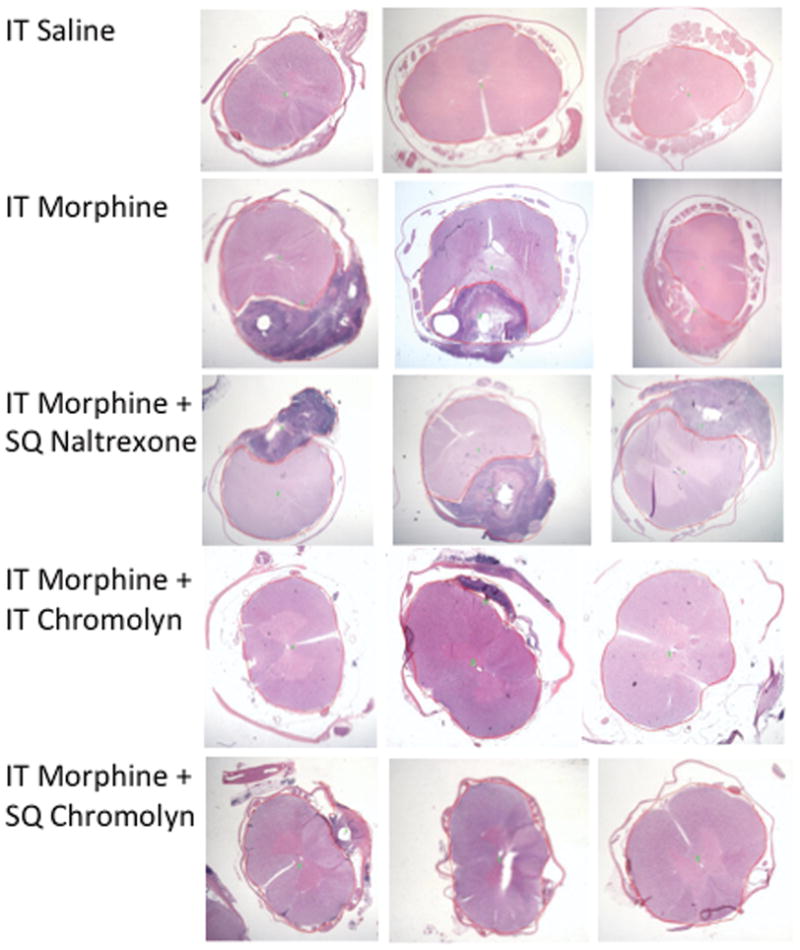
Representative lumbar H&E sections from 3 of the 5–15 dogs that received: 24–28 days of intrathecal (IT) saline, IT Mor (12 mg/mL), IT Mor (12 mg/mL) + BID (twice daily) naltrexone; IT Mor (12 mg/mL) + IT cromolyn (12 mg/mL), or IT Mor (12 mg/mL) + BID subcutaneous cromolyn (7.5mg/kg). Dark reaction product indicates granuloma.
Figure 2.
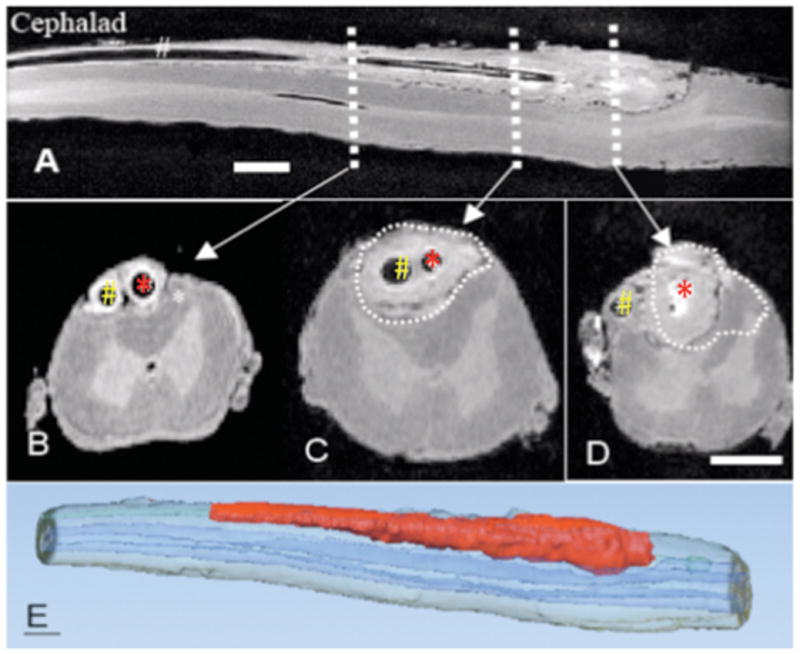
Ex Vivo MRI (magnetic resonance imaging) taken post mortem through the lumbar spinal cord of the dog with sampling catheter (red arrow) and infusion catheter (white arrow) that received a 28-day infusion of intrathecal morphine sulfate (12 mg/d). A: Mid-sagittal plane through the lumbar cord. B,C,D: shows three transverse sections taken at the levels indicated. In Figure B, sampling catheter and infusion catheter indicated by red and white arrows, respectively. Size bars are approximately 0.3 cm. The C and D sections correspond to the levels in the longitudinal section that are proximal to the respective catheter tip. E: Three dimensional reconstruction of dog spinal cord from ex vivo MRI. Red and blue volumes indicate granulomatous mass and spinal cord, respectively.
Figure 3.
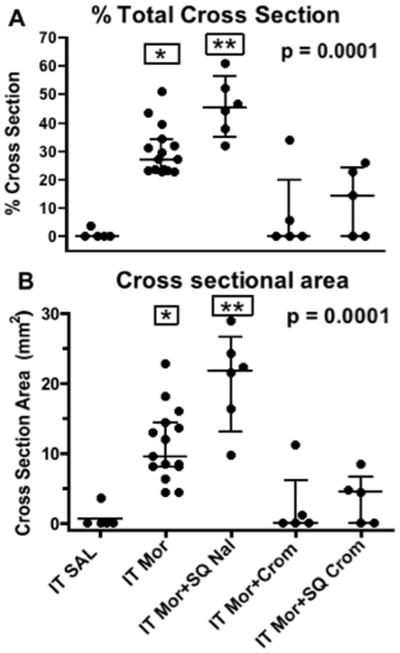
Scatter gram of A: % of cross-sectional area of mass to total spinal area and B: cross-sectional area (mm2) of the granulomatous mass at catheter tip for dogs receiving intrathecal (IT) saline, IT Morphine (Mor: 12mg/d), IT Mor (12 mg/d) + subcutaneous naltrexone (Nal: twice daily (BID): 0.9mg/kg); IT Mor (12 mg/d) +IT cromolyn (Crom: 12 mg/d) or IT Mor (12 mg/d) + subcutaneous cromolyn (Crom: BID 7.5mg/kg). Each point represent a single dog receiving approximately a 28-day IT infusion. Error bars indicate the median with 25th and 75th %. For numbers of animals in each group see Table 1. Kruskal Wallis analysis across treatment groups showed a significant main effect: p<0.0001. (Similar results were observed with a 1-way ANOVA.) Post hoc comparison to the saline treatment group using Dunn’s multiple comparison: * p<0.01; **:p<0.001.
Meningeal mast cell degranulation with morphine
Staining of cryostat sections of dural fragments with Alcian Blue or Fast Red revealed in the cervical, thoracic and lumbar dura of a normal dog the significant presence of dense granule-containing profiles of mast cells (as in FIGURE 4A). Typically in any given section, while some mast cells were found in the dura mater, the majority appeared to be present in the arachnoid layer. As shown in FIGURE 4B, they frequently were found to distribute along the axis of the numerous small blood vessels present in the arachnoid layer. Harvest of dura from dogs that had received either no infusion (control) or approximately 28 days of saline vehicle revealed a large number of intact mast cell profiles. However, after 28 days of morphine (12 mg/day) and displaying a granuloma proximal to the catheter tip, proximal dura typically revealed relatively few intact profiles in the lumbar region. In contrast, examination of spinal dura harvested from upper thoracic (e.g. T5-T10) and cervical (C2–3) levels, where no granuloma was present, revealed significant numbers of intact granule-containing profiles (FIGURE 4C). Similar staining profiles were observed with Naphthol-AS-D-chloroacetate esterase staining, a Fast Red staining technique (not shown).
Figure 4.
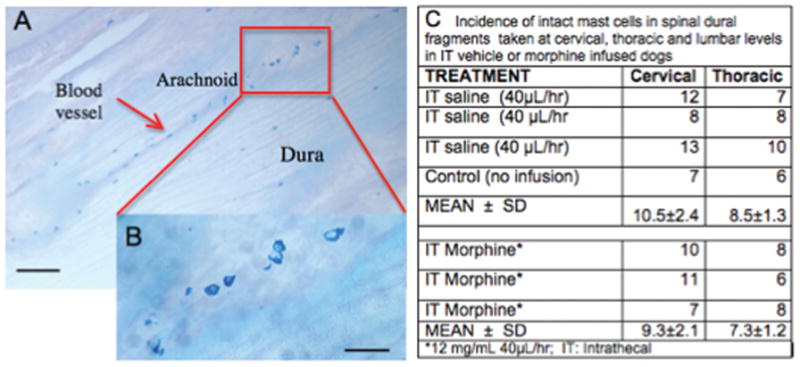
A: Section showing Alcian Blue stained lumbar dura mast cells in control animal at low (bar = 100μ). B. Inset showing enlargement of marked area at high (bar = 30μ) power. Note alignment of cells in arachnoid with blood vessel. C: Table presenting counts of intact Alcian Blue staining profiles in cervical, thoracic and lumbar dura in three animals receiving intrathecal (IT) saline or 3 animals receiving IT Morphine (12mg/d).
In vivo assessment of the cutaneous flare produced by intradermal opiates
Agonist degranulation
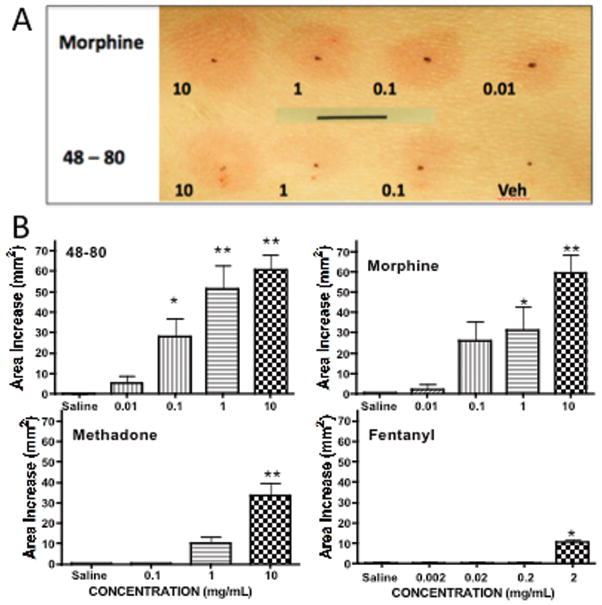
To determine the pharmacology of canine cutaneous mast cell degranulation, the flare producing effects of intradermal injections of Compound 48/80 and several opiates were assessed in the anesthetized dog. Subcutaneous injection of saline produced at most a small bleb at the injection site that typically resolved within 10 min with no incidence of reddening (flare). FIGURE 5A shows the visual image of the concentration-dependent fare produced by side by side subcutaneous injections of 50μL of increasing concentrations of Compound 48/80, morphine or saline (vehicle). FIGURE 5B summarizes the flare areas produced by subcutaneous Compound 48/80 (0.1–10 mg/mL), morphine (0.01–10 mg/mL) and methadone (0.1–10 mg/mL). These agents produced a maximum increase in flare area by 30 min that typically disappeared over the next several hours. In contrast, no change was observed with fentanyl. Ordering of the magnitude revealed that at the highest concentrations employed for morphine and methadone, effects were not different from those produced by the recognized mast cell degranulation agent Compound 48/80 (p>0.05). In contrast, fentanyl at the highest dose (2mg/mL) produced little flare, which, though different from saline, was difficult to discern. Higher doses of fentanyl were not examined, as at these concentrations, the multiple 50μL injections resulted in delivery of a dose equivalent to approximately 10–20 μg/kg and produced a decreased respiratory rate. (No clinically significant desaturation was, however, noted in those studies.)
Figure 5.
A: Representative photograph of dog abdomen showing cutaneous flares taken at 30 min after the subcutaneous injection of different concentrations (mg/mL) of morphine or 48–80 in 50μL. Bar indicates 10 mm. B: Histograms showing the flare area at approximately 30 min after subcutaneous injection of different concentrations of compound 48–80, morphine, methadone or fentanyl. Each histogram presents the mean ± SEM of 4–6 subcutaneous injections. 1-Way ANOVA analysis of each drug across its treatment doses showed a significant main effect for each drug: p<0.0001. Post hoc using Dunnett’s multiple comparison: * p<0.05 vs. saline, **p<0.01 vs. saline.
Mast cell stabilization
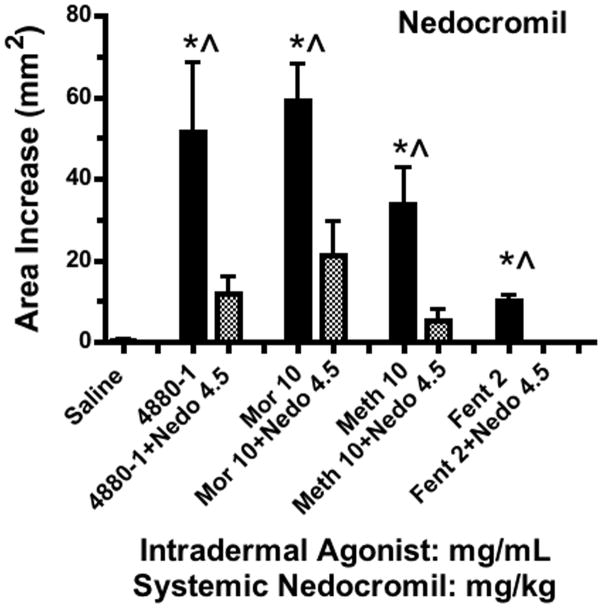
To determine if these effects were mediated by mast cell degranulation, dogs were pretreated 60 min in advance with the mast cell stabilizers cromolyn (7.5 mg/kg, IM) or nedocromil (4.5 mg/kg, IM). As indicated in FIGURES 6 and 7, both agents completely blocked the flare response otherwise produced by all agents. In a dose ranging study, a lower dose of cromolyn (2.5mg/kg, IM) incompletely reduced the flare after Compound 48/80 and morphine (data not shown). Diphenhydramine (10 mg/kg, IM), the H1 blocking agent, was examined on the flare produced by high concentrations of Compound 48/80 and morphine and again this dose completely prevented the observed flare response produced by these agents (data not shown).
Figure 6.
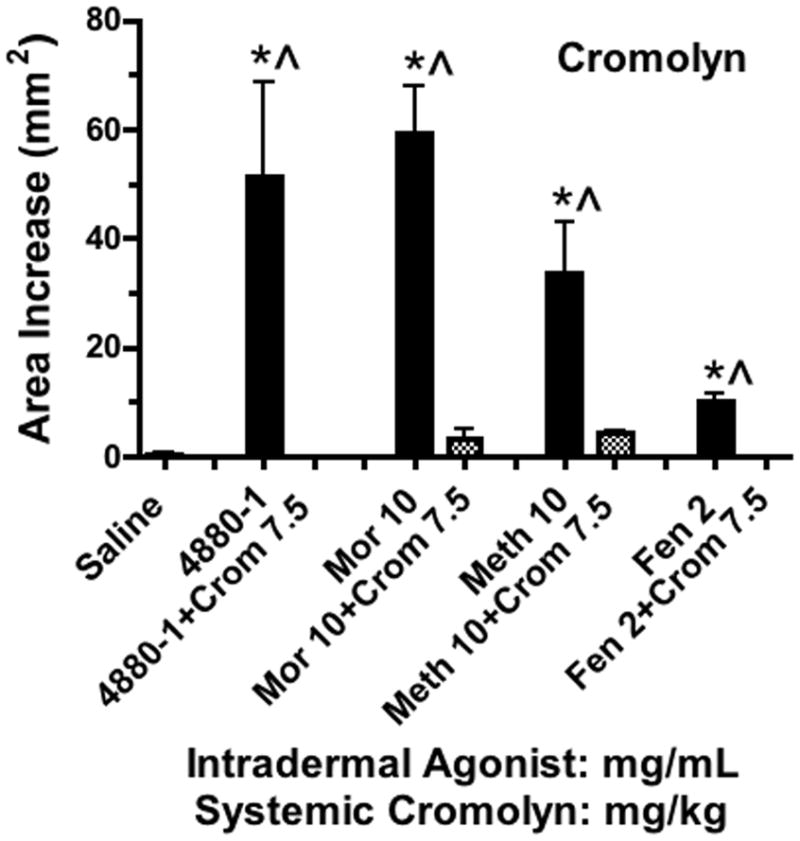
Histograms showing the cutaneous flare area at approximately 30 min after subcutaneous injection of 48–80, morphine, methadone or fentanyl (mg/mL)in 50μL in animals pretreated with cromolyn (7.5 mg/kg). Each histogram presents the mean ± SEM of 3–5 subcutaneous injections. 1-Way ANOVA analysis across treatments showed a significant main effect (p<0.0001). *Post hoc using Dunnett’s multiple comparison: * p<0.05 vs. saline. ^ Post hoc t-test with Bonferroni correction: drug alone vs. drug + cromolyn: p<0.05.
Figure 7.
Histograms showing the cutaneous flare area at approximately 30 min after subcutaneous injection of 48–80, morphine, methadone or fentanyl (mg/mL) in animals pretreated with nedocromil (4.5 mg/kg). Each histogram presents the mean ± SEM of 3–5 subcutaneous injections. 1-Way ANOVA analysis across treatments showed a significant main effect (p<0.0001). *Post hoc using Dunnett’s multiple comparison: * p<0.05 vs. saline. ^ Post hoc t-test with Bonferroni correction: drug alone vs. drug + nedocromil: p<0.05.
Opiate receptor antagonism
An important question was whether these effects from the opiates were affected by naloxone. Here, a very high dose of naloxone (10 mg/kg, IV) given 30 min prior to the agonists failed to block the flare change otherwise produced by each of the agents (FIGURE 8). The dose was evidently effective at blocking opiate receptor activation as the respiratory depression otherwise observed with the fentanyl was not observed.
Figure 8.
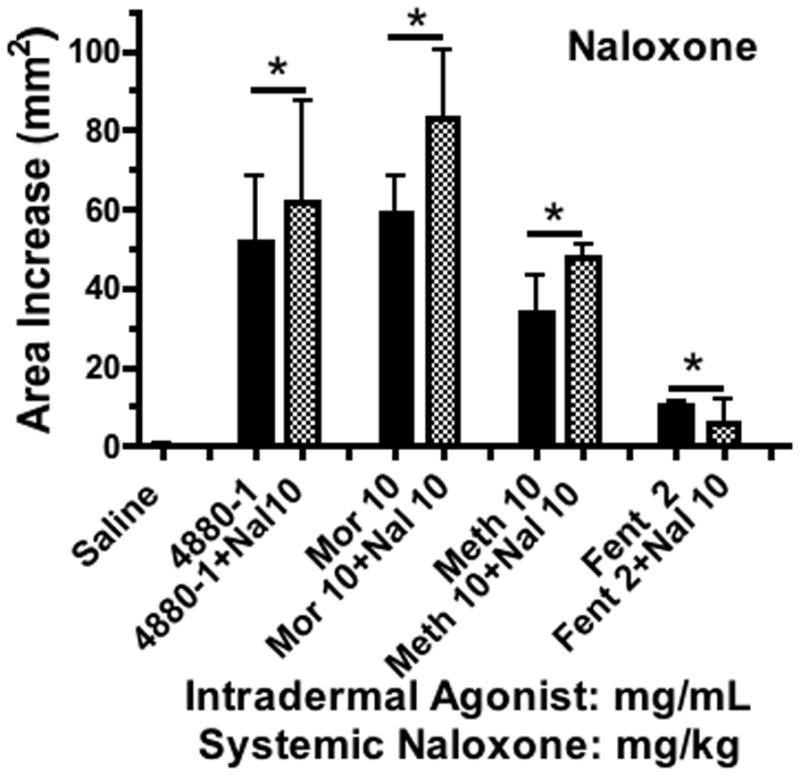
Histograms showing the cutaneous flare area at approximately 30 min after subcutaneous injection of different concentrations of 48–80, morphine, methadone or fentanyl (mg/mL) in animals pretreated with naloxone (10mg/kg). Each histogram presents the mean ± SEM of 3–5 subcutaneous injections. 1-Way ANOVA analysis across treatments showed a significant main effect (p<0.0001). *Post hoc using Dunnett’s multiple comparison: * p<0.05 vs. saline. Post hoc t-test with Bonferroni correction: drug alone vs. drug + naloxone were not different (p >0.05).
Ex vivo dural mast cell degranulation
To extend the observations in vivo on the pharmacology of opiate-induced degranulation of mast cells as measured by the flare and to compare dural degranulation with cutaneous degranulation, we examined ex vivo histamine release from canine dural fragments.
Dural histamine release
As shown in FIGURE 9, Dural fragments incubated for 30 min with morphine resulted in a significant concentration-dependent degranulation of mast cells (FIGURE 9A–D) and an increase in histamine in the dural supernatant (FIGURE 9A) over the range of morphine concentrations of 10 to 100μM. As indicated, morphine in this preparation is at least as potent as the release evoked by 100μM of Compound 48/80, a recognized mast cell degranulating agent. Importantly, the morphine-evoked histamine release was blocked by co-incubation with cromolyn but not by naloxone. Similar but more limited studies examining meningeal histamine release were carried out with other opioids at the 100 μM dose. As shown in TABLE 2, hydromorphone and methadone, but not fentanyl, exposed in a concentration of 100 μM yielded significant increases in histamine release different from saline and comparable to those observed with 100μM morphine. Histochemical examination of the mast cell population in the dural fragments after a 30 min incubation with vehicle revealed numerous complete mast cell profiles with dense intracellular granular staining (FIGURE 9A). Addition of morphine resulted in a prominent change in appearance of mast staining with an increasing fraction of the dural tissues showing varying degrees of disrupted cellular profiles with stain-free core indicative of degranulation (FIGURES 9C,D).
Figure 9.
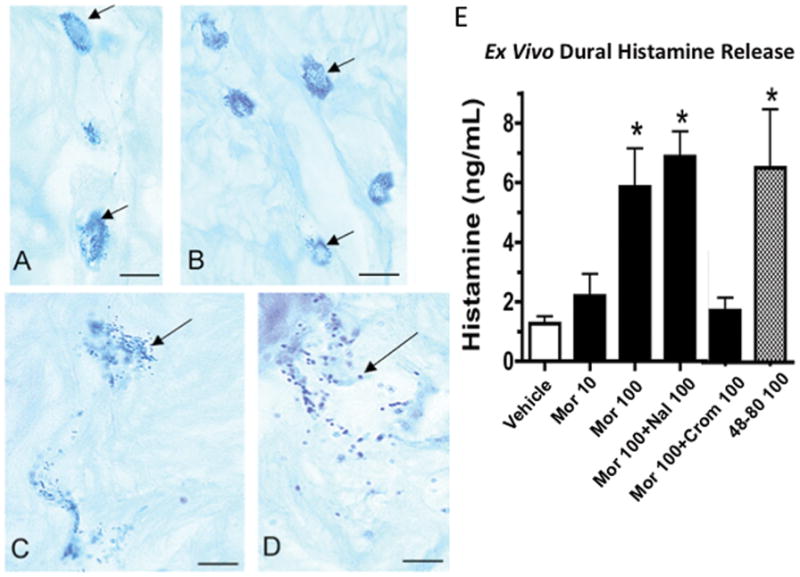
Histology shows single Alcian Blue stained mast cells (see arrows) found in dog lumbar meninges harvested after exposure to A: vehicle, B: 10μM morphine, C/D: 100μM morphine (Bar = 20μ) E. Ex vivo histamine release from dural fragments after treatment as indicated (Mor: morphine; Nal: naloxone; Crom: cromolyn). Doses indicated are in μM indicated in legend. Each bar presents the mean ± SEM of 4–8 samples. 1-Way ANOVA analysis across treatments showed a significant main effect: p<0.0001. Post hoc using Dunnett’s multiple comparison: * p<0.05 vs. vehicle (saline).
Table 2.
Histamine release Ex Vivo from dural fragments by agents
| DRUG* | Histamine (ng/mL) | Release (ng/mL) (mean ± SD) | Significance (vs. Saline)# |
|---|---|---|---|
| Saline^ | 1.5, 1.8,1.0, 0.8, 0.9 | 1.2 ± 0.4 | |
| Morphine^ | 3.9, 6.1, 5.1, 4.6, 10.6 | 6.1 ± 2.7 | p<0.01 |
| RS-Methadone | 5.8, 3.2, 4.7 | 4.6 ± 1.3 | P<0.05 |
| Hydromorphone | 6.1, 5.9, 3.8 | 5.3 ± 2.3 | P<0.05 |
| Fentanyl | 1.1, 2.1, 2.2 | 1.8 ± 0.6 | p>0.05 |
100 μM; 30 min incubation,
data same as in figure, p for comparison;
One-way ANOVA across treatment groups (p = 0.0018) with comparison vs. saline using Dunnetts.
In vivo effects of opiate antagonism on granuloma formation
Naltrexone antagonism of intrathecally infused morphine evoked analgesia
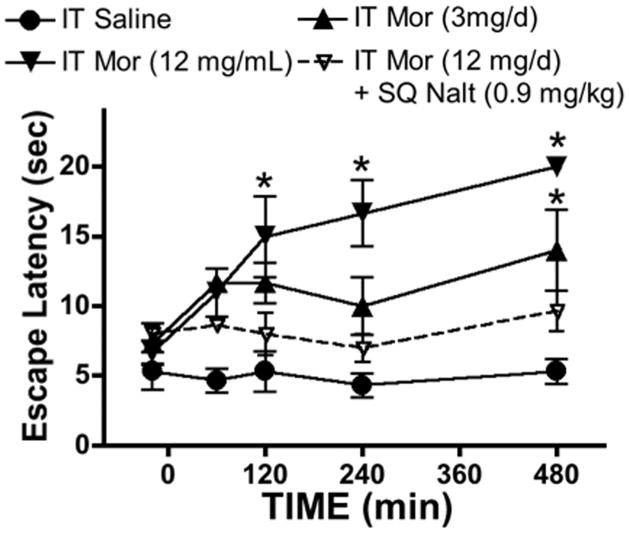
The following studies were performed to define the in vivo antagonism by naltrexone of the analgesic effects of a granuloma producing dose of intrathecal morphine in the dog. The intrathecal infusion of morphine 3 and 12 mg/d resulted in an increase in hind paw thermal escape latency as compared to vehicle (saline). In a separate group, a single injection of naltrexone (0.9 mg/kg) prevented the increase over this 8 hr interval (FIGURE 10).
Figure 10.
Thermal escape latency (sec) in dogs receiving continuous infusion of the doses (mg/mL/d) indicated of morphine (Mor) alone or morphine with bolus subcutaneous delivery of naltrexone (Nalt: 0.9 mg/kg). Each line represents the mean ±SEM for 4 dogs. Data were analyzed with a 2-way repeated ANOVA across time. Main effects for time and treatments were significant (p = 0.0002 and p = 0.0007, respectively). Individual comparisons across treatments at each time point vs. saline were accomplished with a post hoc Bonferroni test. * p<0.05 vs. saline.
To determine the effects of opiate antagonism on intrathecal morphine-initiated spinal granuloma formation, animals were injected twice daily with the opiate-antagonizing dose of naltrexone (0.9 mg/kg, sq.). As indicated in FIGURE 3, in animals receiving 12 mg/d morphine for approximately 4 weeks with this twice daily dose of naltrexone, the appearance (see FIGURE 1) and incidence of granuloma formation was not different from that observed with morphine alone. Thus, the median cross-sectional area with quartiles in dogs receiving intrathecal morphine alone or with naltrexone was 9.5 (8.1–14.0) mm2 and 21.9 (12.7–23.8) mm2, respectively, and both were statistically different from vehicle (p < 0.05).
In vivo effects of cromolyn on granuloma formation
Intrathecal cromolyn
To determine the effects of a spinally-delivered mast cell stabilizer on intrathecal morphine-initiated spinal granuloma formation, animals received an intrathecal delivery of morphine 12 mg/d with an admixture of cromolyn yielding cromolyn infusion concentrations of 12 mg/d (N = 5). These doses were based on the ex vivo work (FIGURE 9), wherein equal doses of cromolyn were observed to prevent morphine-evoked mast cell degranulation. In this series, the incidence and the median area of the pericatheter masses in animals receiving intrathecal 12 mg/mL morphine over an interval of 24–28 days were reduced by concurrent intrathecal cromolyn (1 of 5 with granuloma, median percentage of the cross-sectional area with quartiles = 0, 0.0–1.1 mm2), and were different from the intrathecal morphine alone group (p<0.05), but not different from the saline group (p > 0.05) (see FIGURE 3). Two dogs received an intrathecal infusion of cromolyn (12 mg/d) alone. In this limited series, no untoward effects on behavior were observed and hematoxylin-eosin staining showed no untoward signs (data not shown).
Subcutaneous cromolyn
To determine the effects of a systemically-delivered mast cell stabilizer on intrathecal morphine-initiated spinal granuloma formation, animals received an intrathecal delivery of morphine 12mg/d and twice daily dosing of cromolyn 7.5 mg/kg (N = 5). These doses were based on the in vivo degranulation work (FIGURE 6), wherein systemic cromolyn was observed to prevent intradermal morphine-evoked mast cell degranulation. In this series, the median percentage of the cross-sectional area and interquartile was 4.5 (0–4.8) mm2 (in animals receiving subcutaneous cromolyn) over an interval of 24–28 days and were different from the intrathecal morphine alone group (p<0.05), but not different from the saline group (p > 0.05).
Behavior
As previously described,3 the initiation of continuous infusion of 12 mg/d leads to an initial behavioral depression that is addressed by an incrementation of the infusion concentration over 3–7 days. Over the ensuing weeks, many animals receiving morphine, but not vehicle would display neurological signs that included hind limb spasticity, altered gait and an increased sensitivity to touch applied to the hind quarters. Bowel and bladder function were not impaired sufficiently to require early euthanasia. FIGURE 11 presents the sensorimotor score of all animals at the time of euthanasia. As indicated in FIGURE 11B, the animals receiving morphine only displayed a median score of 6 (maximum being 6) as compared to a median score of 0. Comparison across all treatment groups revealed a significant main effect, but only the morphine vs. saline comparison was significant. We plotted the motor score against the % cross-sectional area of the granuloma and observed a regression that, while statistically nonzero (slope with 95% CI: 0.047; 0.0189 – 0.075) with r2 = 0.253, was modest. This suggests that the degree of motor deficit observed with the evolution of the granuloma correlated poorly with the actual compressive extent to of the granuloma. Thus, several animals with significant compression showed virtually no deficits at the time of euthanasia. This was a conclusion that we previously reached.3
Figure 11.
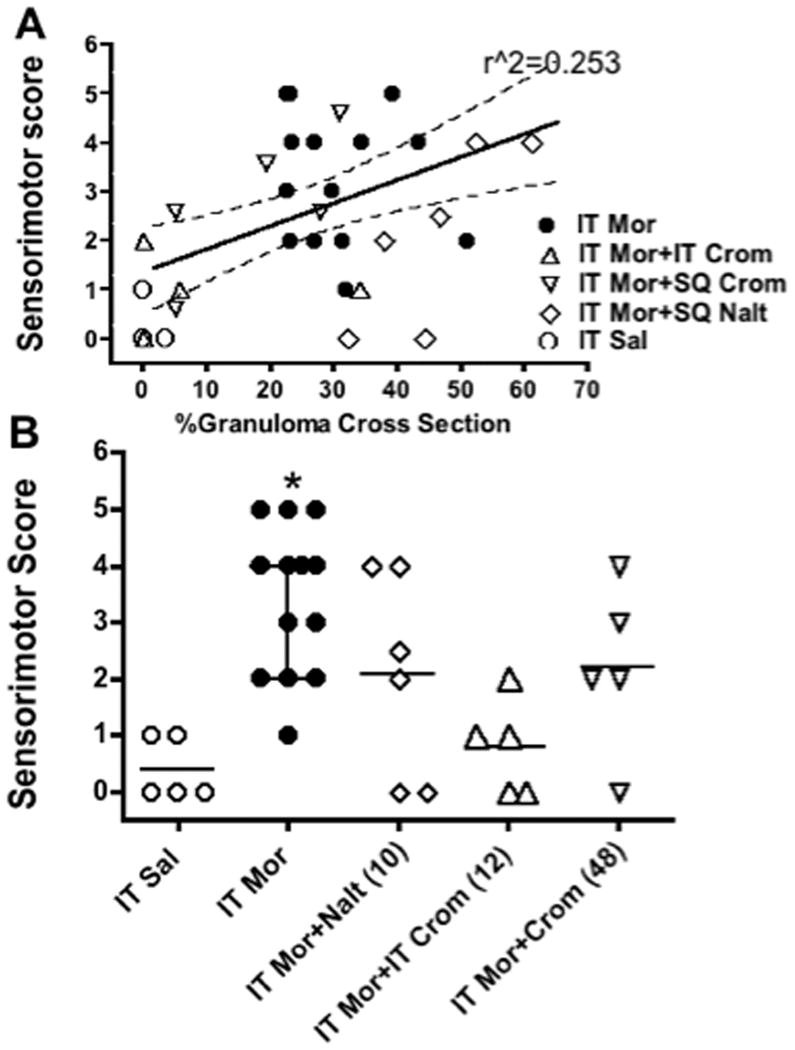
A: Regression line (with 95% confidence intervals) of sensorimotor scores vs. % cross-sectional area of granuloma in individual dogs. Higher scores indicate increased side effects. See text. Symbols indicate treatment received by the individual dog. (Regression line slope was 0.047 ± 0.014 with an r2 of 0.253). B: scattergram showing senori-motor score plotted vs. treatment. Kruskal-Wallace comparison across groups was significant (p = 0.0025). Post hoc comparison with a Dunn’s multiple comparison: * p<0.05 vs. saline.
DISCUSSION
Chronic intrathecal morphine initiates a volume-occupying accumulation of inflammatory cells deriving from the meningeal vasculature. We hypothesize that dural mast cell degranulation is a linkage between intrathecal opiates and the granuloma.
Opiate receptor activation and granuloma formation
In the dog, intrathecal infusion of approximately equi-analgesic concentrations of morphine, hydromorphone, or methadone, but not fentanyl, result in an intrathecal granuloma.5 This drug effect discrepancy, along with failure of naltrexone in the present work to alter the granuloma, argues against a simple opioid receptor-mediated effect. While higher doses of naltrexone might be required to reverse a possible interaction, we note the following: i) naltrexone and its metabolite, 6-beta-naltrexol, have half-lives of several hours with the metabolite being longer37 (but see38). 6 beta-naltrexol has opiate antagonist properties that may account for the long duration of blockade by the parent.39 ii) The dose employed resulted in a significant antagonism of the analgesic effects produced by the granuloma-producing dose for an excess of 8 hrs. Accordingly, twice daily dosing would result in excess of 16 hrs of receptor coverage. In previous work we showed that repeated daily bolus morphine dosing resulted in no granulomas,6 suggesting that periods between morphine injection were sufficient to presumably clear the agent and prevent granuloma formation, a cycle approximated by twice daily antagonist dosing. iii) We expect that size would be a sensitive index of granuloma formation and this was in fact numerically greater in the naltrexone group. Accordingly, we conclude that granuloma-inducing effects of intrathecal morphine is not dependent upon a “conventional” naltrexone-sensitive opiate receptor.
Spinal Meningeal Mast cells
Mast cells are distributed in spinal dura-arachnoid,19,20,40 and often aligned with local arachnoid vessels.41 The meningeal mast cells are subject to degranulation by the local milieu.42,43 Such activation releases low and high molecular weight compounds including vasodilators (histamine and serotonin), chemoattractants for T cells and mediators of increased permeability (tumor necrosis factor, tryptase and chymase)44–46 of the meningeal and cerebral vasculature.22,42,47,48 Tryptase is present in large amounts in all mast cells 49 and activates proteinase activated receptors (PARs)46 that disrupt vascular endothelial barrier integrity.50 This contribution to plasma extravasation and cell migration in dura led us to consider the role of MMCs in the intrathecal accumulation of inflammatory cells forming the granuloma.
Opiates and mast cell degranulation
Opiates degranulate mast cells and release vasodilating substances, notably histamine.25 In the skin where mast cells are located,36 degranulation results in a local cutaneous vasodilation, a flare. Work focusing on the pharmacology of this cutaneous effect shows that while a variety of opioids produce flares, this effect is not associated with an opiate receptor. Thus, subcutaneous injection of some opiate molecules (morphine, hydromorphone, methadone) but not other agents (fentanyl) 28,51,52 result in histamine release/flare in a manner that is largely refractory to opiate antagonism, but readily prevented by cromanes (e.g., cromolyn and nedocromil).25,29–31,51 Importantly, this structure activity series and failure of opiate antagonism to reverse the effects of dural or cutaneous mast cell degranulation suggests that classical opiate receptors play at most a minor role. Importantly, as shown here, meningeal mast cells are degranulated by morphine, but not by fentanyl and this effect is not antagonized by concentrations of naloxone which are larger than those required to block effects of opiate receptor activation in in vitro organ systems.53 This similarity between dural and cutaneous mast cell activation is interesting, in that in contrast, mast cells from the heart, lung and the gastrointestinal tract are reported to not show a strong response to opiates.24 The parallels between dura and cutaneous mast cells may reflect the fact that dura and skin arise embryologically from the somitic mesoderm and neural crest and their resident mast cells would potentially display similar pharmacological profiles.54,55
How do opiates act to degranulate mast cells? We note several possibilities. i) Mast cell degranulation may be initiated through activation of high-affinity receptors for the Fc region of IgE, through receptors on the cell surface.56,57 ii) Degranulation may occur through a receptor independent interaction mediated by cationic charges that act as receptor mimetic agents to trigger mast cells, by interacting with sialic acid residues of the cell surface and then with Gi-like proteins, activating phospholipase C and intracellular calcium mobilization.58–60 This pathway has been shown for morphine in mast cells.61 Mast cell stabilizers such as cromolyn may act by specifically preventing such G-protein activation61; iii) Opiates interact with the MD2 component of toll-like receptor 4, leading to activation.62–64 This effect is not stereospecific and is antagonized by the opiate receptor-inactive stereoisomer of naloxone.64 Toll-like receptor 4 activation can initiate mast cell degranulation 65,66. One caveat to this mechanism is that toll-like receptor activation is also apparently produced by fentanyl, which does not initiate a granuloma at therapeutic doses.64
Meningeal mast cell and spinal granuloma formation
Either co-intrathecal delivery of cromolyn or twice daily subcutaneous cromolyn resulted in a reduction in incidence and cross-sectional area of granulomas in dogs receiving continuous infusion of a granuloma-producing dose of morphine. Interestingly, intrathecal cromolyn in rats prevented dural mast cell degranulation otherwise noted after intraplantar carrageenan.40 Cromolyn can prevent degranulation by a variety of stimuli.67,68 Aside from preventing G-protein activation,61 cromolyn also has other effects, including i) block of chloride transport in mast and nerve cells,69 ii) reduced substance P binding and iii) reduced adenosine-induced plasma extravasation.70 What role these other actions play in the observed phenomena is not known. Additional studies defining the dose dependency of the cromolyn effect and the actions of other, non cromane, mast cell stabilizers to confirm the role of the presumed target would be desirable.
Corollaries of mast cell contributions to granuloma formation
The possibility that mast cells may be an intervening mechanism raises several issues.
Ligands that do not degranulate mast cells would be predicted to not produce granulomas. Fentanyl does not produce granulomas in dogs at intrathecal doses up to 2mg/mL/d. Consistent with this hypothesis, intradermal alfentanil had minimal effects upon mast cell degranulation when delivered by continuous intrathecal infusion at the maximum tolerable dose of 20mg/mL/d and resulted in minimal dural reactions.71 Anilinopiperidines are rarely employed for chronic intrathecal delivery because of their rapid clearance and associated significant plasma exposure in contrast to agents such as morphine with a low log P. As a caveat to the above commentary, we note in one case report that intrathecal fentanyl at a high daily dose of 2.7 mg/d (drug treatment history, infusate concentration and duration of exposure were not specified) was associated with an intrathecal mass.72 In a second report, intrathecal sufentanil (17μg/d) in a patient with a history of prior exposure to several agents including fentanyl, baclofen and ziconotide also developed a granuloma.73 These examples suggest the possible role of mechanisms in addition to potential contribution of mast cells, particularly at high concentrations.
Agents that block mast cell degranulation in the skin should be efficacious in blocking the granuloma. The present spinal work is limited to cromolyn. Stabilizers such as nedocromil 74–77 have different potencies and should have similar granuloma-sparing effects at lower doses and different pharmacokinetics. Alternately, Syk tyrosine kinases are important in mast cell degranulation.78 Inhibitors (e.g. BAY613606) reduce mast cell degranulation, histamine release and inflammation more potently than cromolyn.79 Interestingly, intrathecal BAY613606 blocked dural mast cell degranulation.40.
We propose that a potential screening approach is to determine whether the agent produces flares after local subcutaneous delivery. We suggest that the pharmacology of the cutaneous mast cell resembles that of the meningeal mast cell. A caveat to the use of the cutaneous flare is the issue of concentration. We did not examine the possibility that the failure of fentanyl to produce a granuloma (or flare) might represent its rapid subcutaneous/intrathecal clearance, although the highest tolerable doses were employed. A second caveat is that if the local drug target requires a low concentration relative to that required to degranulate mast cells then the therapeutic use of the agent that produced cutaneous flare might not be confounded by granuloma formation. Ziconotide produces histamine release 80, yet intrathecal ziconotide in preclinical safety evaluations 81,82 or in humans83 has not been reported to initiate granulomas. This observation may reflect the extremely low concentrations required of this agent for therapeutically effective degrees of calcium channel block. Conversely, as noted, while anilinopiperdines are not generally considered to degranulate mast cells, very high concentrations may have modest effects on vascular cells and this may reflect why they also may have some propensity to initiate a granuloma in humans.
In conclusion, while granuloma formation is often considered to be clinically infrequent, two observations are telling: i) there is an increasing incidence of observations/reports and in post-marketing surveillance reports describing these space-occupying masses and ii) amongst clinicians using intrathecal analgesics, 66% report a patient experiencing neurologic injury secondary to a granuloma (see for review9). Thus, this is not an effect to be dismissed. Our work seeks to address the mechanisms of this phenomenon. The present results must be considered as a contribution to a mechanistic understanding of the role of meningeal mast cells. The results do not exclude other mechanisms that may not engage mast cells. Further, the canine work demonstrates a mast cell effect with pretreatment. We do not know if blocking mast cell degranulation will reverse a granuloma. Finally, showing an effect of cromolyn absolutely cannot be construed as a safety evaluation of the drug by either route or the doses employed. Further preclinical work 84,85 defining such parameters and safety is required to evaluate the risk-benefit of this or any adjunctive therapy.
Summary statement.
Intrathecal morphine infusion leads to a meningeally derived granuloma. The present work in the dog points to a close correlation between the origin of these intrathecal granulomas and the degranulation of meningeal mast cells.
FINAL BOX SUMMARY.
What we already know about this topic
Intrathecal infusion of morphine is associated with granuloma formation in animals and humans, although the mechanisms for this effect and whether it can be prevented or predicted have not been examined
What this article tells us that is new
In dogs, intrathecal infusion of morphine induced granuloma formation which was prevented by an inhibitor of mast cell degranulation, but not by naltrexone
Subcutaneous injection of some, but not all opioids, induced mast cell degranulation, suggesting this may be a useful screen for potential granuloma formation with intrathecal infusion
Acknowledgments
Funding: This project was supported by National Institutes of Health grant NIDA-15353 (TLY), Bethesda, MD.
We thank the Alfred Mann Foundation (Santa Clarita, CA) for providing a number of the Minimed pumps used in these investigations. We would like to particularly acknowledge our debt to Dr. Chris Bernards who pointed us to the similarity of the meningeal phenomena to the events that occur in migraine, the aberrant effects of opiates in degranulating mast cells and the potential role of mast cells. Sadly, Dr. Bernards passed away on January 12, 2012. We would like to thank Nicole A. Tozier, Eddie Ruhland, Mary Ceccolini, B.S., Dasa Cizkova, D.V.M. and Ashley Wiese, D.V.M. for their technical contributions to these studies.
Footnotes
All studies were conducted in the Department of Anesthesiology, University of California San Diego.
These studies were reported in part in an abstract and presented at the Annual Meeting of the Society of Toxicology, March 21 2004, Baltimore, Maryland.
References
- 1.North RB, Cutchis PN, Epstein JA, Long DM. Spinal cord compression complicating subarachnoid infusion of morphine: Case report and laboratory experience. Neurosurgery. 1991;29:778–84. doi: 10.1097/00006123-199111000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Gradert TL, Baze WB, Satterfield WC, Hildebrand KR, Johansen MJ, Hassenbusch SJ. Safety of chronic intrathecal morphine infusion in a sheep model. Anesthesiology. 2003;99:188–98. doi: 10.1097/00000542-200307000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Yaksh TL, Horais KA, Tozier NA, Allen JW, Rathbun M, Rossi SS, Sommer C, Meschter C, Richter PJ, Hildebrand KR. Chronically infused intrathecal morphine in dogs. Anesthesiology. 2003;99:174–87. doi: 10.1097/00000542-200307000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Michael A, Buffen E, Rauck R, Anderson W, McGirt M, Mendenhall HV. An in vivo canine study to assess granulomatous responses in the MedStream Programmable Infusion System (TM) and the SynchroMed II Infusion System(R) Pain Med. 2012;13:175–84. doi: 10.1111/j.1526-4637.2011.01308.x. [DOI] [PubMed] [Google Scholar]
- 5.Allen JW, Horais KA, Tozier NA, Wegner K, Corbeil JA, Mattrey RF, Rossi SS, Yaksh TL. Time course and role of morphine dose and concentration in intrathecal granuloma formation in dogs: A combined magnetic resonance imaging and histopathology investigation. Anesthesiology. 2006;105:581–9. doi: 10.1097/00000542-200609000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Sabbe MB, Grafe MR, Mjanger E, Tiseo PJ, Hill HF, Yaksh TL. Spinal delivery of sufentanil, alfentanil, and morphine in dogs. Physiologic and toxicologic investigations. Anesthesiology. 1994;81:899–920. doi: 10.1097/00000542-199410000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Yaksh TL, Hassenbusch S, Burchiel K, Hildebrand KR, Page LM, Coffey RJ. Inflammatory masses associated with intrathecal drug infusion: A review of preclinical evidence and human data. Pain Med. 2002;3:300–12. doi: 10.1046/j.1526-4637.2002.02048.x. [DOI] [PubMed] [Google Scholar]
- 8.Coffey RJ, Burchiel K. Inflammatory mass lesions associated with intrathecal drug infusion catheters: Report and observations on 41 patients. Neurosurgery. 2002;50:78–86. doi: 10.1097/00006123-200201000-00014. discussion 86–7. [DOI] [PubMed] [Google Scholar]
- 9.Deer TR, Prager J, Levy R, Rathmell J, Buchser E, Burton A, Caraway D, Cousins M, De Andres J, Diwan S, Erdek M, Grigsby E, Huntoon M, Jacobs M, Kim P, Kumar K, Leong M, Liem L, McDowell G, Panchal S, Rauck R, Saulino M, Sitzman BT, Staats P, Stanton-Hicks M, Stearns L, Wallace M, Willis KD, Witt W, Yaksh T, Mekhail N. Polyanalgesic Consensus Conference-2012: Consensus on Diagnosis, Detection, and Treatment of Catheter-Tip Granulomas (Inflammatory Masses) Neuromodulation. 2012;15:483–96. doi: 10.1111/j.1525-1403.2012.00449.x. [DOI] [PubMed] [Google Scholar]
- 10.Aprili D, Bandschapp O, Rochlitz C, Urwyler A, Ruppen W. Serious complications associated with external intrathecal catheters used in cancer pain patients: A systematic review and meta-analysis. Anesthesiology. 2009;111:1346–55. doi: 10.1097/ALN.0b013e3181bfab9a. [DOI] [PubMed] [Google Scholar]
- 11.Lehmberg J, Scheiwe C, Spreer J, van Velthoven V. Late bacterial granuloma at an intrathecal drug delivery catheter. Acta Neurochir (Wien) 2006;148:899–901. doi: 10.1007/s00701-006-0810-9. [DOI] [PubMed] [Google Scholar]
- 12.Allen JW, Horais KA, Tozier NA, Yaksh TL. Opiate pharmacology of intrathecal granulomas. Anesthesiology. 2006;105:590–8. doi: 10.1097/00000542-200609000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Butt MT. Morphologic changes associated with intrathecal catheters for direct delivery to the central nervous system in preclinical studies. Toxicol Pathol. 2011;39:213–9. doi: 10.1177/0192623310391679. [DOI] [PubMed] [Google Scholar]
- 14.Sabbe MB, Grafe MR, Pfeifer BL, Mirzai TH, Yaksh TL. Toxicology of baclofen continuously infused into the spinal intrathecal space of the dog. Neurotoxicology. 1993;14:397–410. [PubMed] [Google Scholar]
- 15.Yaksh TL, Grafe MR, Malkmus S, Rathbun ML, Eisenach JC. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995;82:412–27. doi: 10.1097/00000542-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Chiari A, Yaksh TL, Myers RR, Provencher J, Moore L, Lee CS, Eisenach JC. Preclinical toxicity screening of intrathecal adenosine in rats and dogs. Anesthesiology. 1999;91:824–32. doi: 10.1097/00000542-199909000-00035. [DOI] [PubMed] [Google Scholar]
- 17.Johansen MJ, Satterfield WC, Baze WB, Hildebrand KR, Gradert TL, Hassenbusch SJ. Continuous intrathecal infusion of hydromorphone: Safety in the sheep model and clinical implications. Pain Med. 2004;5:14–25. doi: 10.1111/j.1526-4637.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- 18.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Artico M, Cavallotti C. Catecholaminergic and acetylcholine esterase containing nerves of cranial and spinal dura mater in humans and rodents. Microsc Res Tech. 2001;53:212–20. doi: 10.1002/jemt.1085. [DOI] [PubMed] [Google Scholar]
- 20.Michaloudi H, Batzios C, Chiotelli M, Grivas I, Papadopoulos GC. Mast cells populations fluctuate along the spinal dura mater of the developing rat. Brain Res. 2008;1226:8–17. doi: 10.1016/j.brainres.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 21.Miller HR, Pemberton AD. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology. 2002;105:375–90. doi: 10.1046/j.1365-2567.2002.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunder CA, St John AL, Abraham SN. Mast cell modulation of the vascular and lymphatic endothelium. Blood. 2011;118:5383–93. doi: 10.1182/blood-2011-07-358432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebertz JM, Hermens JM, McMillan JC, Uno H, Hirshman C, Hanifin JM. Functional differences between human cutaneous mast cells and basophils: A comparison of morphine-induced histamine release. Agents Actions. 1986;18:455–62. doi: 10.1007/BF01964946. [DOI] [PubMed] [Google Scholar]
- 24.Tharp MD, Kagey-Sobotka A, Fox CC, Marone G, Lichtenstein LM, Sullivan TJ. Functional heterogeneity of human mast cells from different anatomic sites: In vitro responses to morphine sulfate. J Allergy Clin Immunol. 1987;79:646–53. doi: 10.1016/s0091-6749(87)80162-8. [DOI] [PubMed] [Google Scholar]
- 25.Feldberg W, Paton WD. Release of histamine from skin and muscle in the cat by opium alkaloids and other histamine liberators. J Physiol. 1951;114:490–509. doi: 10.1113/jphysiol.1951.sp004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosman N, Jensen SM, Johansen FF. Histamine release from isolated rat mast cells induced by opiates: Effect of sterical configuration and calcium. Agents Actions. 1982;12:417–24. doi: 10.1007/BF01965920. [DOI] [PubMed] [Google Scholar]
- 27.Casale TB, Bowman S, Kaliner M. Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: Evidence for opiate and nonopiate receptor participation. J Allergy Clin Immunol. 1984;73:775–81. doi: 10.1016/0091-6749(84)90447-0. [DOI] [PubMed] [Google Scholar]
- 28.Levy JH, Brister NW, Shearin A, Ziegler J, Hug CC, Jr, Adelson DM, Walker BF. Wheal and flare responses to opioids in humans. Anesthesiology. 1989;70:756–60. doi: 10.1097/00000542-198905000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: An in vivo microdialysis study in human skin. Anesth Analg. 2004;98:364–70. doi: 10.1213/01.ANE.0000097168.32472.0D. [DOI] [PubMed] [Google Scholar]
- 30.Barke KE, Hough LB. Opiates, mast cells and histamine release. Life Sci. 1993;53:1391–9. doi: 10.1016/0024-3205(93)90581-m. [DOI] [PubMed] [Google Scholar]
- 31.Baldo BA, Pham NH. Histamine-releasing and allergenic properties of opioid analgesic drugs: Resolving the two. Anaesth and intensive care. 2012;40:216–35. doi: 10.1177/0310057X1204000204. [DOI] [PubMed] [Google Scholar]
- 32.Yaksh TL, Provencher JC, Rathbun ML, Myers RR, Powell H, Richter P, Kohn FR. Safety assessment of encapsulated morphine delivered epidurally in a sustained-release multivesicular liposome preparation in dogs. Drug Deliv. 2000;7:27–36. doi: 10.1080/107175400266768. [DOI] [PubMed] [Google Scholar]
- 33.Yaksh TL, Rathbun ML, Dragani JC, Malkmus S, Bourdeau AR, Richter P, Powell H, Myers RR, Lebel CP. Kinetic and safety studies on intrathecally infused recombinant-methionyl human brain-derived neurotrophic factor in dogs. Fundam Appl Toxicol. 1997;38:89–100. doi: 10.1006/faat.1997.2314. [DOI] [PubMed] [Google Scholar]
- 34.Wegner K, Horais KA, Tozier NA, Rathbun ML, Shtaerman Y, Yaksh TL. Development of a canine nociceptive thermal escape model. J Neurosci Methods. 2008;168:88–97. doi: 10.1016/j.jneumeth.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubinstein I, Nadel JA, Graf PD, Caughey GH. Mast cell chymase potentiates histamine-induced wheal formation in the skin of ragweed-allergic dogs. J Clin Invest. 1990;86:555–9. doi: 10.1172/JCI114744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker AB, Chung KF, McDonald DM, Lazarus SC, Frick OL, Gold WM. Mast cell heterogeneity in dog skin. Anat Rec. 1985;213:477–80. 530–1. doi: 10.1002/ar.1092130402. [DOI] [PubMed] [Google Scholar]
- 37.Langguth P, Khan PJ, Garrett ER. Pharmacokinetics of morphine and its surrogates. XI: Effect of simultaneously administered naltrexone and morphine on the pharmacokinetics and pharmacodynamics of each in the dog. Biopharm Drug Dispos. 1990;11:419–44. doi: 10.1002/bdd.2510110507. [DOI] [PubMed] [Google Scholar]
- 38.Garrett ER, el-Koussi Ae-D. Pharmacokinetics of morphine and its surrogates V: Naltrexone and naltrexone conjugate pharmacokinetics in the dog as a function of dose. J Pharm Sci. 1985;74:50–6. doi: 10.1002/jps.2600740114. [DOI] [PubMed] [Google Scholar]
- 39.Divin MF, Holden Ko MC, Traynor JR. Comparison of the opioid receptor antagonist properties of naltrexone and 6 beta-naltrexol in morphine-naive and morphine-dependent mice. Eur J Pharmacol. 2008;583:48–55. doi: 10.1016/j.ejphar.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xanthos DN, Gaderer S, Drdla R, Nuro E, Abramova A, Ellmeier W, Sandkuhler J. Central nervous system mast cells in peripheral inflammatory nociception. Mol Pain. 2011;7:42–58. doi: 10.1186/1744-8069-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: The role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–39. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 42.Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13:237–40. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 43.Messlinger K, Fischer MJ, Lennerz JK. Neuropeptide effects in the trigeminal system: Pathophysiology and clinical relevance in migraine. Keio J Med. 2011;60:82–9. doi: 10.2302/kjm.60.82. [DOI] [PubMed] [Google Scholar]
- 44.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 45.He S, Walls AF. Human mast cell tryptase: A stimulus of microvascular leakage and mast cell activation. Eur J Pharmacol. 1997;328:89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- 46.Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163:5064–9. [PubMed] [Google Scholar]
- 47.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: A role for neutrophil recruitment? J Immunol. 2010;184:6891–900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 48.Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhag. J Cereb Blood Flow Metab. 2010;30:689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611–5. [PubMed] [Google Scholar]
- 50.Itoh Y, Sendo T, Oishi R. Physiology and pathophysiology of proteinase-activated receptors (PARs): Role of tryptase/PAR-2 in vascular endothelial barrier function. J Pharm Sci. 2005;97:14–9. doi: 10.1254/jphs.fmj04005x3. [DOI] [PubMed] [Google Scholar]
- 51.Hermens JM, Ebertz JM, Hanifin JM, Hirshman CA. Comparison of histamine release in human skin mast cells induced by morphine, fentanyl, and oxymorphone. Anesthesiology. 1985;62:124–9. doi: 10.1097/00000542-198502000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Lagunoff D, Martin TW, Read G. Agents that release histamine from mast-cells. Ann Rev Pharmacol Toxicol. 1983;23:331–51. doi: 10.1146/annurev.pa.23.040183.001555. [DOI] [PubMed] [Google Scholar]
- 53.Kosterlitz HW, Waterfield AA. In vitro models in the study of structure-activity relationships of narcotic analgesics. Annu Rev Pharmacol. 1975;15:29–47. doi: 10.1146/annurev.pa.15.040175.000333. [DOI] [PubMed] [Google Scholar]
- 54.Catala M. Embryonic and fetal development of structures associated with the cerebro-spinal fluid in man and other species. Part I: The ventricular system, meninges and choroid plexuses. Arch Anat Cytol Pathol. 1998;46:153–69. [PubMed] [Google Scholar]
- 55.O’Rahilly R, Muller F. The meninges in human development. J Neuropathol Exp Neurol. 1986;45:588–608. [PubMed] [Google Scholar]
- 56.Pawankar R, Yamagishi S, Takizawa R, Yagi T. Mast cell-IgE-and mast cell-structural cell interactions in allergic airway disease. Curr Drug Targets Inflamm Allergy. 2003;2:303–12. doi: 10.2174/1568010033484016. [DOI] [PubMed] [Google Scholar]
- 57.Krishnaswamy G, Ajitawi O, Chi DS. The human mast cell: An overview. Methods Mol Biol. 2006;315:13–34. doi: 10.1385/1-59259-967-2:013. [DOI] [PubMed] [Google Scholar]
- 58.Mousli M, Hugli TE, Landry Y, Bronner C. Peptidergic pathway in human skin and rat peritoneal mast cell activation. Immunopharmacology. 1994;27:1–11. doi: 10.1016/0162-3109(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 59.Ferry X, Eichwald V, Daeffler L, Landry Y. Activation of betagamma subunits of G(i2) and G(i3) proteins by basic secretagogues induces exocytosis through phospholipase Cbeta and arachidonate release through phospholipase Cgamma in mast cells. J Immunol. 2001;167:4805–13. doi: 10.4049/jimmunol.167.9.4805. [DOI] [PubMed] [Google Scholar]
- 60.Shefler I, Zavaro O, Raz T, Baram D, Sagi-Eisenberg R. Inhibition of basic secretagogue-induced signaling in mast cells by cell permeable G alpha i-derived peptides. Int Arch Allergy Immunol. 2008;145:131–40. doi: 10.1159/000108138. [DOI] [PubMed] [Google Scholar]
- 61.Klinker JF, Seifert R. Morphine and muscle relaxants are receptor-independent G-protein activators and cromolyn is an inhibitor of stimulated G-protein activity. Inflamm Res. 1997;46:46–50. doi: 10.1007/s000110050058. [DOI] [PubMed] [Google Scholar]
- 62.Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010;167:880–93. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hutchinson MR, Loram LC, Zhang Y, Shridhar M, Rezvani N, Berkelhammer D, Phipps S, Foster PS, Landgraf K, Falke JJ, Rice KC, Maier SF, Yin H, Watkins L. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience. 2010;168:551–63. doi: 10.1016/j.neuroscience.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwamura C, Nakayama T. Toll-like receptors in the respiratory system: Their roles in inflammation. Curr Allergy Asthma Rep. 2008;8:7–13. doi: 10.1007/s11882-008-0003-0. [DOI] [PubMed] [Google Scholar]
- 66.Nie X, Cai G, Zhang W, Wang H, Wu B, Li Q, Shen Q. Lipopolysaccharide mediated mast cells induce IL-10 producing regulatory T cells through the ICOSL/ICOS axis. Clin Immunol. 2012;142:269–79. doi: 10.1016/j.clim.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Murphy S, Kelly HW. Cromolyn sodium - A review of mechanisms and clinical use in asthma. Drug Intel Clin Phar. 1987;21:22–35. doi: 10.1177/10600280870211p102. [DOI] [PubMed] [Google Scholar]
- 68.Altounyan RE. Review of clinical activity and mode of action of sodium cromoglycate. Clin Allergy. 1980;10 (Suppl):481–9. doi: 10.1111/j.1365-2222.1980.tb02162.x. [DOI] [PubMed] [Google Scholar]
- 69.Alton EWFW, Norris AA. Chloride transport and the actions of nedocromil sodium and cromolyn sodium in asthma. J Allergy Clin Immunol. 1996;98:S102–S105. [PubMed] [Google Scholar]
- 70.Crossman DC, Dashwood MR, Taylor GW, Wellings R, Fuller RW. Sodium cromoglycate - Evidence of tachykinin antagonist activity in the human skin. J Appl Physiol. 1993;75:167–72. doi: 10.1152/jappl.1993.75.1.167. [DOI] [PubMed] [Google Scholar]
- 71.Yaksh TL, Steinauer JJ, Williams SL, Malkmus SA. Alfentanil: Correlations between absence of effect upon subcutaneous mast cells and absence of granuloma formation after intrathecal infusion in the dog. Neuromodulation. 2012 doi: 10.1111/j.1525-1403.2012.00534.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zacest AC, Carlson JD, Nemecek A, Burchiel KJ. Surgical management of spinal catheter granulomas: Operative nuances and review of the surgical literature. Neurosurgery. 2009;65:1161–4. doi: 10.1227/01.NEU.0000359223.11215.D9. [DOI] [PubMed] [Google Scholar]
- 73.Gupta A, Martindale T, Christo PJ. Intrathecal catheter granuloma associated with continuous sufentanil infusion. Pain Med. 2010;11:847–52. doi: 10.1111/j.1526-4637.2010.00860.x. [DOI] [PubMed] [Google Scholar]
- 74.Janssen LJ, Wattie J, Betti PA. Effects of cromolyn and nedocromil on ion currents in canine tracheal smooth muscle. Eur Respir J. 1998;12:50–6. doi: 10.1183/09031936.98.12010050. [DOI] [PubMed] [Google Scholar]
- 75.Eady RP. The pharmacology of nedocromil sodium. Eur J Respir Dis Suppl. 1986;147:112–9. [PubMed] [Google Scholar]
- 76.Leung KB, Flint KC, Brostoff J, Hudspith BN, Johnson NM, Pearce FL. A comparison of nedocromil sodium and sodium cromoglycate on human lung mast cells obtained by bronchoalveolar lavage and by dispersion of lung fragments. Eur J Respir Dis Suppl. 1986;147:223–6. [PubMed] [Google Scholar]
- 77.Pearce FL, Al-Laith M, Bosman L, Brostoff J, Cunniffe TM, Flint KC, Hudspith BN, Jaffar ZH, Johnson N McI, Kassessinoff TA, Lau HYA, Lee PY, Leung KPB, Liu WL, Tainsh KR. Effects of sodium cromoglycate and nedocromil sodium on histamine secretion from mast cells from various locations. Drugs. 1989;37(Suppl 1):37–43. doi: 10.2165/00003495-198900371-00009. [DOI] [PubMed] [Google Scholar]
- 78.Siraganian RP, de Castro RO, Barbu EA, Zhang J. Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584:4933–40. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto N, Takeshita K, Shichijo M, Kokubo T, Sato M, Nakashima K, Ishimori M, Nagai H, Li YF, Yura T, Bacon KB. The orally available spleen tyrosine kinase inhibitor 2-[7-(3,4-dimethoxyphenyl)-imidazo[1,2-c]pyrimidin-5-ylamino]nicotinamide dihydrochloride (BAY 61–3606) blocks antigen-induced airway inflammation in rodents. J Pharmacol Exp Ther. 2003;306:1174–81. doi: 10.1124/jpet.103.052316. [DOI] [PubMed] [Google Scholar]
- 80.Bowersox SS, Singh T, Nadasdi L, Zukowska-Grojec Z, Valentino K, Hoffman BB. Cardiovascular effects of omega-conopeptides in conscious rats: Mechanisms of action. J Cardiovasc Pharmacol. 1992;20:756–64. [PubMed] [Google Scholar]
- 81.Skov MJ, Beck JC, de Kater AW, Shopp GM. Nonclinical safety of ziconotide: An intrathecal analgesic of a new pharmaceutical class. Int J Toxicol. 2007;26:411–21. doi: 10.1080/10915810701582970. [DOI] [PubMed] [Google Scholar]
- 82.Yaksh TL, de Kater A, Dean R, Best BM, Miljanich GP. Pharmacokinetic Analysis of Ziconotide (SNX-111), an Intrathecal N-Type Calcium Channel Blocking Analgesic, Delivered by Bolus and Infusion in the Dog. Neuromodulation. 2012;15:508–19. doi: 10.1111/j.1525-1403.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoederath P, Gautschi OP, Land M, Hildebrandt G, Fournier JY. Formation of two consecutive intrathecal catheter tip granulomas within nine months. Cent Eur Neurosurg. 2010;71:39–42. doi: 10.1055/s-0029-1202359. [DOI] [PubMed] [Google Scholar]
- 84.Yaksh TL. Spinal Delivery and Assessment of Drug Safety, Fundamental Neuropathology for Pathologists and Toxicologists. In: Bolon B, Butt MT, editors. Principles and Techniques. Hoboken: John Wiley & Sons, Inc; 2011. pp. 452–462. [Google Scholar]
- 85.Walker SM, Yaksh TL. Review article: Neuraxial analgesia in neonates and infants: A review of clinical and preclinical strategies for the development of safety and efficacy data. Anesth Analg. 2012;115:638–62. doi: 10.1213/ANE.0b013e31826253f2. [DOI] [PMC free article] [PubMed] [Google Scholar]