Abstract
This study was conducted to investigate whether administration of IH901, a ginseng intestinal metabolite, ameliorates exercise-induced oxidative stress while preserving antioxidant defense capability in rat skeletal muscles and lung. Eight adult male Sprague-Dawley rats per group were randomly assigned to the resting control, exercise control, resting with IH901 (25, 50, and 100 mg/kg) consumption (R/IH901), or exercise with IH901 (25, 50, and 100 mg/kg) consumption (E/IH901) group. The trained groups ran 35 min 2 days/week for 8 weeks. To analyze the IH901-training interaction, serum biochemical analysis, lipid peroxidation, citrate synthase, protein oxidation, antioxidant and superoxide dismutase in skeletal muscles and lung tissue were measured. Compared to the exercise control group, animals that consumed IH901 had significantly increased exercise endurance times (p < 0.05) and decreased plasma creatine kinase and lactate dehydrogenase levels (p < 0.05), while those in the E/IH901 groups had increased citrate synthase and anti-oxidant enzymes and decreased lipid peroxidation and protein oxidation (p < 0.05). In conclusion, IH901 consumption in aging rats after eccentric exercise has beneficial effects on anti-inflammatory and anti-oxidant activities through down-regulation of pro-inflammatory mediators, lipid peroxidation, and protein oxidation and up-regulation of anti-oxidant enzymes.
Keywords: anti-oxidant activity, eccentric exercise, IH901, lipid peroxidation, rat
Introduction
Reactive oxygen species (ROS) are a diverse class of radical species produced in all cells as a normal byproduct of metabolic processes [6]. The beneficial effects of ROS in biological systems include that they serve in energy production, phagocytosis, regulation of cell growth and intercellular signaling, as well as synthesis of biologically important compounds at physiological levels [14,33]. However, when overproduced, these compounds cause damage to organisms by attacking lipids, proteins, enzymes, carbohydrates and DNA, thus inducing oxidative stress, which is an imbalance between ROS and the defense and repair antioxidant system that leads to cell injury and death [11,15]. Indeed, more than 100 diseases have been reported to be related to ROS [27].
Cells counteract the detrimental effects of ROS by producing antioxidant molecules such as reduced glutathione (GSH) and thioredoxin (TRX), which reduce excessive levels of ROS to prevent irreversible cellular damage [12]. These free radicals are neutralized by an elaborate antioxidant defense system consisting of enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and numerous non-enzymatic antioxidants [35].
The benefits of regular physical exercise include a reduced risk of cardiovascular disease, cancer, osteoporosis, and diabetes [21,38,40] because of decreased alterations to antioxidant defenses. Conversely, exercise can produce an imbalance between ROS and antioxidants known as oxidative stress. Strenuous exercise-generated ROS seem to increase antioxidant enzyme activity and decrease GSH levels in the muscles, heart, and lungs, possibly as a compensatory mechanism to cope with oxidative stress.
Ginseng and extracts have been shown to have antioxidant and apoptotic effects [22,23]. IH901 [20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol or compound K] is known as the final intestinal bacterial metabolite of ginseng in humans. However, the roles of IH901 in exhaustive exercise-induced oxidative damage and inflammatory response in skeletal muscles and lung tissue are unclear. Therefore, this study was conducted to investigate whether IH901 administration has beneficial effects such as ameliorating exercise-induced oxidative stress and reserving antioxidant defense capabilities in rat skeletal muscles and lung tissue.
Materials and Methods
Animal care
Animal pathogen- and virus-free (APF/AVF) Sprague-Dawley (SD) rats were purchased from Samtako Bio Korea (Osan, Korea), which is certified by Institute for Laboratory Animal Research (USA). Eight 4-month-old adult male SD rats per group were randomly assigned to the resting control (RC) group, exercise control (EC) group, resting with IH901 (25, 50 and 100 mg/kg) consumption (R/IH901) group, or exercise with IH901 (25, 50 and 100 mg/kg) consumption (E/IH901) group. The low dose (25 mg/kg) was selected based on comparison of the mean body weight and metabolic rate of rats and humans (500~1,000 mg/kg). The rats were housed individually in a temperature-controlled (20 ± 2℃) room under a 12 : 12-hour light-dark cycle while provided with free access to water and their respective diet. The animal protocol was approved by the Laboratory Animal Care and Use Committee of the Laboratory Animal Research Center, Chungbuk National University (Chungju, Korea) (Approval No. CBNU-LAR-CA07-37).
Animal training program
The rats performed a graded treadmill run to fatigue on a customized rodent treadmill. The protocol consisted of having rats run up a 0% grade, beginning at a speed of 25 cm/sec (0 to 2 weeks). If the animal completed the required 15 min of running at this workload, the treadmill speed and grade were increased to a 10% and 30 cm/sec, respectively (2 to 4 weeks). If the animal completed the required 15 min of running at this workload, the treadmill speed and grade were increased to 15% and 35 cm/sec, respectively (4 to 8 weeks). This treadmill speed was then maintained until the rat reached the point of fatigue. An electric shock (<1 mA) grid at the back of the treadmill was used to encourage animals to run during the habituation period, which allowed for very minimal use of electric shock during the subsequent experimental exercise conditions. Skeletal muscle metabolic and antioxidant enzyme activities are unaffected by this type of treadmill habituation protocol [5].
Serum biochemistry
Blood samples were centrifuged at 1,400 × g and 4℃ for 10 min. The supernatants (serum) were then used to determine the aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), glucose, total cholesterol (TC), triacylglycerol (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and creatine kinase (CK) levels using an automatic analyzer (7170; Hitachi, Japan).
Tissue preparation
Two days after completion of the treadmill exercise, all animals were sacrificed by blood-letting via the abdominal artery, after which the lungs and selected skeletal muscles were harvested. Several skeletal muscles were taken from each rat so that the oxidative capacity could be estimated based on the activities of several enzymes. The soleus and gastrocnemius muscles and lung tissues were also exposed, excised and frozen at -80℃ for subsequent analysis.
Determination of CS activity in skeletal muscle
The citrate synthase (CS) activities of the tissue samples were measured using a commercial kit (Sigma-Aldrich, USA). Briefly, 8 µL of diluted sample were added to 182 µL of mixed substrate [30 mM acetyl-CoA, 10 mM 5,5'-dithiobis-(2-nitrobenzoic acid)]. Next, 10 µL of oxaloacetic acid was added to the mixture and CS was measured at 37℃ on a PowerWave XS at 412 nm for 90 seconds. The activity was expressed as units (µM/min/mL) of specific activity.
Determination of thiobarbituric acid reactive substances (TBARS) and protein carbonyl concentrations
TBARS were measured using a commercial kit (Cayman Chemical Company, USA). Briefly, a mixture of 100 µL of lung or skeletal muscle homogenate, 500 µL of sodium dodecyl sulfate (30 mg/mL), 2 mL of HCl (0.1 M), 300 µL of phosphotungstic acid (10 mg/mL) and 1 mL of 2-thiobarbituric acid (7 mg/mL) was incubated in boiling water for 30 min. After cooling, 5 mL of butanol was added. The organic layer was collected after centrifuging at 1,000 × g for 10 min at 4℃ and its absorbance at 532 nm was measured and compared with a standard curve constructed using known concentrations of 1,1,3,3,-tetramethoxypropane and expressed as µM malondialdehyde/mg protein. Protein oxidation was measured by estimation of the carbonyl groups according to the method described by Levine et al. [25], with slight modifications, using 2,4-dinitrophenylhydrazine (DNPH) reagent. The lung and skeletal muscle homogenate samples (150 µL) were treated with 500 µL of DNPH (10 mM) dissolved in HCl (2 M) as a sample or with 500 µL of HCl (2 M) as a control blank. The reaction mixtures were allowed to stand for 1 h at room temperature in the dark with stirring at 15 min intervals, after which 500 µL of ice cold 20% trichloroacetic acid was added and the sample was left on ice for 15 min. The tubes were then centrifuged at 10,010 × g for 5 min to obtain a protein pellet, which was subsequently washed 3× with 1 mL of ethanol-ethyl acetate (1:1) to remove the unreacted DNPH and lipid remnants. Each washing step was followed by centrifugation at 744 × g for 7 min, after which the final protein pellet was dissolved in 250 µL of guanidine hydrochloride (6 M) and incubated at 37℃ for 10 min. The carbonyl content was then calculated based on the absorption at 360 nm using an absorption coefficient (e) of 22,000 M-1cm-1. Each sample was read against the control sample (treated with 2.5 M HCl), and the protein carbonyl content was expressed as nmol/mg protein in the lung tissue and skeletal muscle.
Assay of antioxidants and SOD activities in lung and skeletal muscle tissues
Glutathione peroxidase (GPx) activities of the tissue samples were determined using a commercial kit (Cayman Chemical Company). Briefly, 20 µL of diluted sample was added to 150 µL of mixed substrate [nicotinamide adenine dinucleotide phosphate (NADPH), GSH, and glutathione reductase]. Next, 20 µL of cumene hydroperoxide (diluted in deionized water) was added to the mixture, GPx was measured at 37℃ on a PowerWave XS at 340 nm for 8 min and the specific activity was expressed as nmol/min/mL. Glutathione S-transferase (GST) activity in the tissue samples was determined using a commercial kit (Cayman Chemical Company). Briefly, 20 µL of diluted sample was added to 150 µL of glutathione, after which 20 µL of 1-chloro-2,4-dinitrobenzene was added, the GST was measured at 37℃ on a PowerWave XS at 340 nm for 5 min and the specific activity was expressed as nmol/min/mL for. Superoxide dismutase (SOD) activities in the tissue samples were measured using a commercial kit (Assay Designs; Ann Arbor, USA). Briefly, 25 mL of diluted sample was added to 175 µL of mixed substrate [2-(4-iodophenyl)-3-(4-nitrophenyl)-2H-tetrazolium] and 25 mL of xanthine was then added to the mixture. Next, the SOD was measured at 37℃ on a PowerWave XS at 450 nm for 5 min and expressed as percent inhibition of the specific activity.
Xanthine oxidase (XO) and myeloperoxidase (MPO) activities
A diluted tissue sample was added to 100 µM xanthine (dissolved in 50 mM sodium phosphate buffer, pH 7.5), after which XO activity was measured at 25℃ on a Powerwave XS spectrophotometer at 290 nm for 3 min. One unit of XO activity formed 1 mmol of urate per minute at 25℃. XO specific activity was expressed as unit/g protein. MPO activity was assayed by measuring the H2O2-dependent oxidation of 3,3',5,5'-tetramethylbenzidine (TMB). In its oxidized form, TMB has a blue color, which was measured spectrophotometrically at 650 nm. The reaction mixture for the analysis consisted of a 25 µL tissue homogenate sample, 25 µL TMB (final concentration 0.16 mM; Sigma-Aldrich) dissolved in dimethyl sulfoxide (DMSO) and 200 µL H2O2 (final concentration 0.24 mM; Merck, Germany) diluted in 0.08 M phosphate buffer (pH 5.4). The reaction was performed in a 96-well plate that was incubated for 5 min at 37℃, after which the reaction was stopped by the addition of 25 µL bovine catalase (final concentration, 13.6 µg/mL; Boehringer Mannheim, Germany). To ensure the linearity of the reaction during this time period, MPO standards (human leukocyte, MPO, 0.004~0.5 U/mL; the Green Corporation, Japan) were included in each assay. One unit of MPO activity was defined as the amount of enzyme reducing 1 µM peroxide/min.
Total protein concentration of lung and skeletal muscles
To express the antioxidant enzyme activities per milligram of protein, total protein concentrations of the tissue samples were spectrophotometrically estimated using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, USA).
Statistical analysis
Values were expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA) was used to evaluate differences between groups. SPSS software (version 12.0K; SPSS Institute, USA) was used to analyze the data. Differences were considered statistically significant when p < 0.05.
Results
Effect of IH901 on exercise endurance time
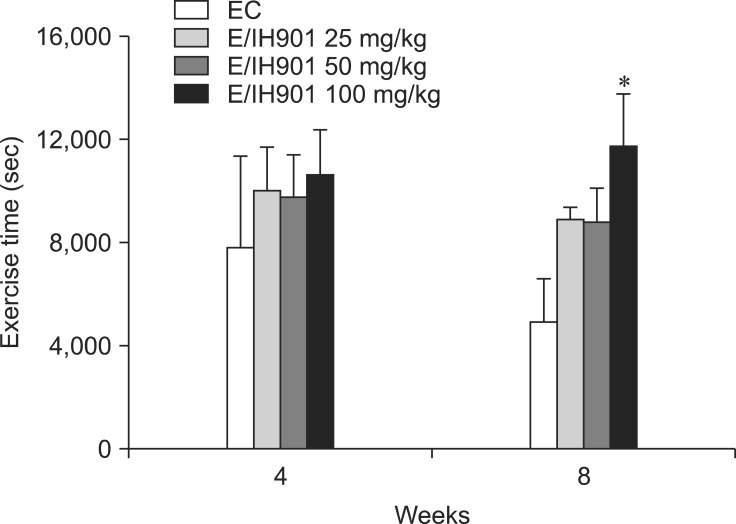
The mean endurance time of treadmill running to exhaustion was significantly (p < 0.05) increased in the E/IH901 100 mg/kg group at 8 weeks when compared to that of the EC group (Fig. 1).
Fig. 1.
The endurance time of treadmill running to exhaustion in rats treated with vehicle or IH901. Values are expressed as the mean ± SD (n = 8/group). The rats performed a graded treadmill run to fatigue on a customized rodent treadmill. The protocol consisted of having rats run upward at a speed of 25 cm/sec (0~2 weeks); 30 cm/sec (2~4 weeks) and 35 cm/sec (4~8 weeks). EC: exercise control. *p < 0.05 (vs. EC).
Effects of IH901 on plasma CK and LDH level
As shown in Table 1, all E/IH901 consumption groups showed a beneficial effect on the decrease of plasma CK and LDH levels at both 0 and 30 min relative to the EC group (p < 0.05). Interestingly, plasma CK and LDH levels decreased in a dose-dependent manner at both 4 and 8 weeks, especially in the 100 mg/kg IH901 consumption groups.
Table 1.
Plasma creatine kinase (CK) and lactate dehydrogenase (LDH) concentrations in rats after eccentric exercise
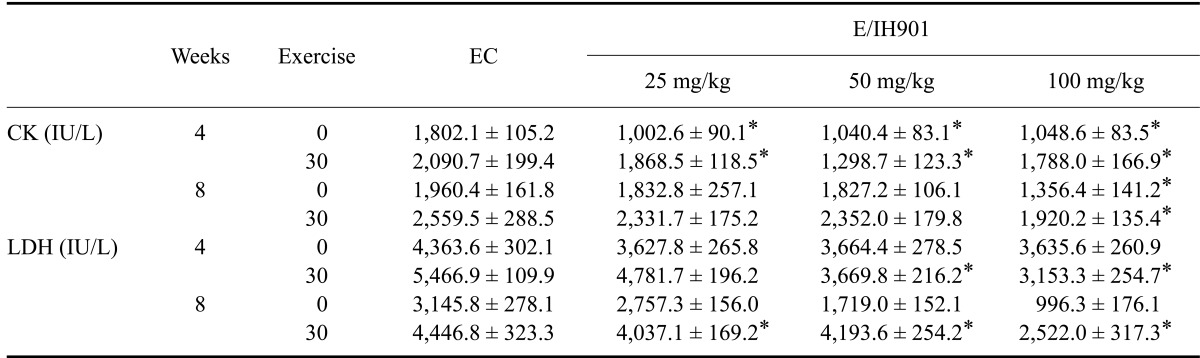
Values are expressed as the mean ± SD (n = 8/group). The rats performed a graded treadmill run to fatigue on a customized rodent treadmill. The protocol consisted of having rats run upward at a speed of 25 cm/sec (0~2 weeks); 30 cm/sec (2~4 weeks) and 35 cm/sec (4~8 weeks). Blood samples were collected at 0 (before exercise) and at 30 min after exercise. *p < 0.05 (vs. EC).
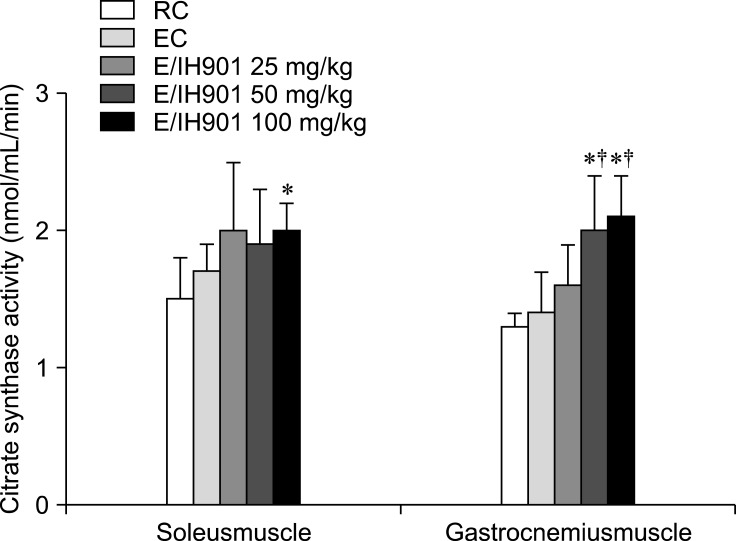
Effects of IH901 on CS level in skeletal muscles
CS activity in the skeletal muscles is shown in Fig. 2. The CS activity was not different between the resting and exercise control. In the soleus muscle, the CS activity was significantly higher in the 100 mg/kg IH901 consumption group with exercise than in the RC (p < 0.05). CS activity in the gastrocnemius muscle was significantly higher in both the 50 and 100 mg/kg IH901 consumption with exercise groups than in the control groups (p < 0.05).
Fig. 2.
Plasma creatine kinase (CK) and lactate dehydrogenase (LDH) concentrations after eccentric exercise. Values are expressed as the mean ± SD (n = 8/group). The rats performed a graded treadmill run to fatigue on a customized rodent treadmill. The protocol consisted of having rats run upward at a speed of 25 cm/sec (0~2 weeks); 30 cm/sec (2~4 weeks) and 35 cm/sec (4~8 weeks). Blood samples were collected before exercise (pre) and at 30 min after exercise (after). RC: resting control. *p < 0.05 (vs. EC), ‡p < 0.05 (vs. RC).
Effects of IH901 on TBARS and protein carbonyl levels in muscles or lung tissue
The TBARS and protein carbonyl levels in the skeletal muscles and lung tissue are shown in Table 2. The TBARS level was significantly higher in the EC group than the RC group in the soleus and gastrocnemius muscles and lung tissue (p < 0.05). The TBARS levels were decreased in the skeletal muscles and lung tissue of the IH901 consumption with or without exercise groups when compared to that of the EC group (p < 0.05). The protein carbonyl level in the EC group was significantly higher in the lung tissue than that of the RC group (p < 0.05). The protein carbonyl levels in the R/IH901 groups were significantly decreased in the soleus muscle and lung tissue relative to that of the EC group (p < 0.05) as well as in the gastrocnemius muscle with high doses (>50 mg/kg) of IH901 consumption when compared to the EC group (p < 0.05).
Table 2.
Thiobarbituric acid reactive substance (TBARS) and protein carbonyl levels in skeletal muscles and lung tissue of rats treated with vehicle or IH901
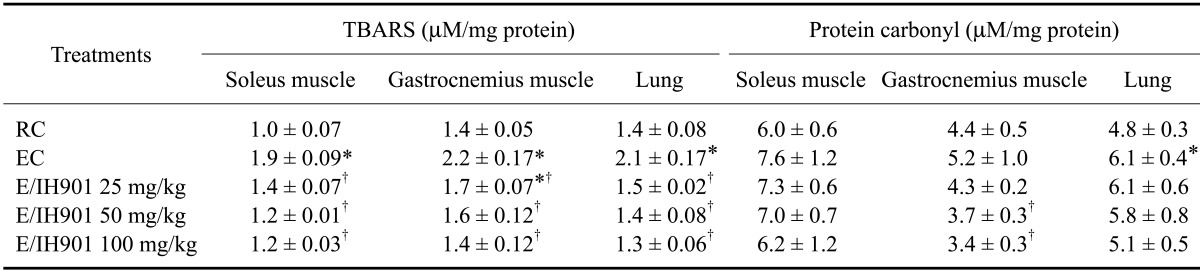
Values are expressed as the mean ± SD (n = 8/group). The selected skeletal muscles and lung tissues were collected from all rats two days after completion of the treadmill exercise. *p < 0.05 (vs. RC), †p < 0.05 (vs. EC).
Effects of IH901 on XO, MPO and nitric oxide (NO) levels in lung tissue
The effects of IH901 consumption on XO, MPO, and NO levels in the lung tissue of rats with or without exercise are shown in Table 3. The XO and MPO levels were significantly higher in the EC group than in the RC group (p < 0.05). The concentration of XO in the lung tissue was significantly lower in the 25 and 100 mg/kg IH901 consumption animals with exercise when compared to exercise animals without IH901 consumption (p < 0.05). When compared with resting animals, the MPO activity in lung tissue was significantly increased in exercise animals (p < 0.05). In exercise animals with 100 mg/kg IH901 consumption, the MPO activities in the lung tissue were significantly decreased when compared to that of the EC animals (p < 0.05); however, the pulmonary NO levels were not significantly different.
Table 3.
Xanthine oxidase (XO), myeloperoxidase (MPO) and nitric oxide (NO) levels in lung tissue of rats treated with vehicle or IH901
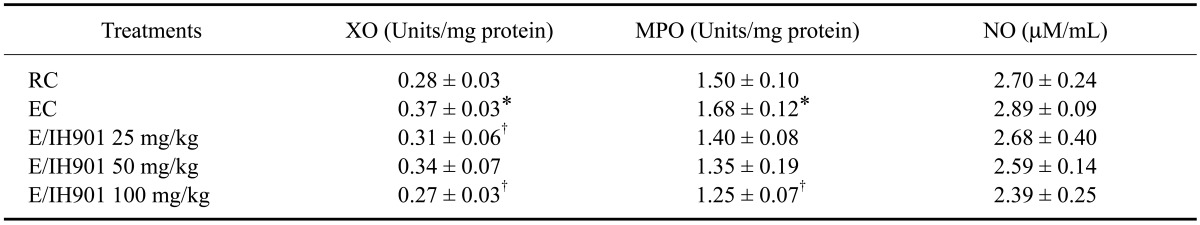
Values are expressed as the mean ± SD (n = 8/group). Lung tissues were collected from all rats two days after completion of the treadmill exercise. *p < 0.05 (vs. RC), †p < 0.05 (vs. EC).
Effects of IH901 on GPx, GST and SOD activities
The enzymatic activities of GPx, GST, and SOD in the skeletal muscles and lung tissue are shown in Table 4. The GPx level in both skeletal muscles and lung tissue did not differ between the RC and EC groups. Most of the IH901 consumption groups, especially the exercise groups, had significantly higher GPx activities than both RC and EC animals (p < 0.05). The SOD activity in the skeletal muscles was not different among treatment groups. However, the SOD activity in the lung tissue was significantly higher in the 50 and 100 mg/kg IH901 consumption groups with or without exercise than in the RC and EC groups (p < 0.05). There were no differences in GST activity among treatment groups except for the 100 mg/kg IH901 consumption with exercise animals (p < 0.05).
Table 4.
Antioxidant enzyme activities in skeletal muscles (soleus and gastrocnemius) and lung tissue of rats treated with vehicle or IH901
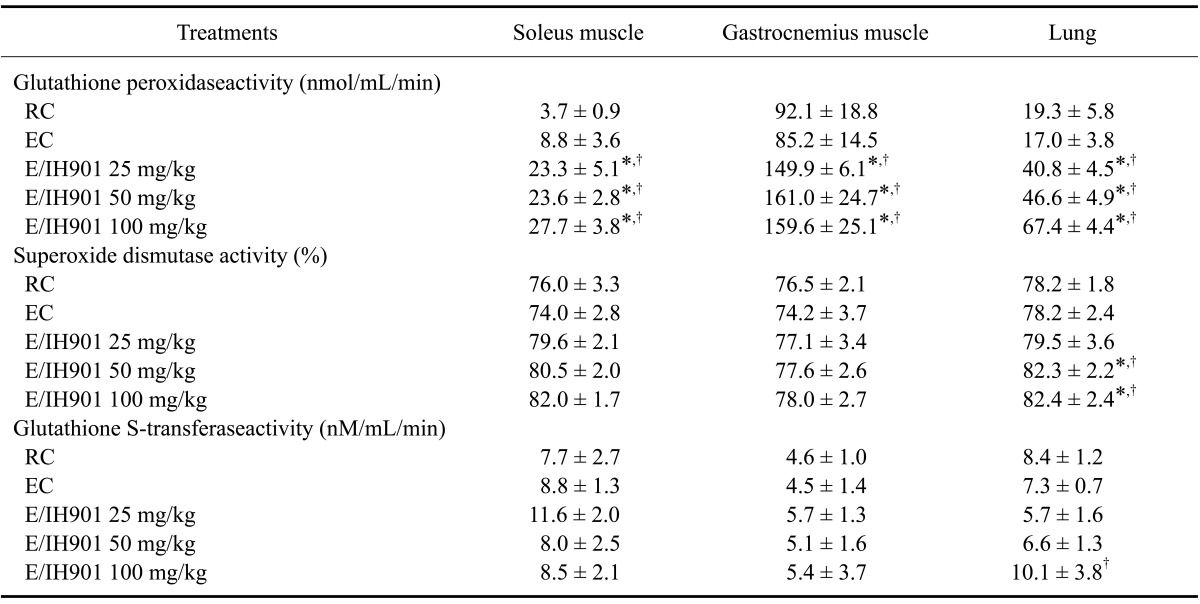
Values are expressed as the mean ± SD (n = 8/group). The selected skeletal muscles and lung tissues were collected from all rats at two days after completion of the treadmill exercise. *p < 0.05 (vs. RC), †p < 0.05 (vs. EC).
Discussion
This study was conducted to investigate whether IH901 administration has beneficial effects such as ameliorating exercise-induced oxidative stress and reserving antioxidant defense capabilities in rat skeletal muscles and lung tissue.
Exercise can produce an imbalance between ROS and antioxidants known as oxidative stress. Physical activity increases the generation of free radicals in several ways. A small amount of oxygen (2~5%) used in the mitochondria forms free radicals. In muscles, mitochondria are a source of reactive species including O2, H2O2, and HO [2,13]. Other sources of exercise-induced increases in free radicals include prostanoid metabolism, XO, NADPH oxidase, and several secondary sources such as the release of radicals by macrophages recruited to repair damaged tissue [18,20]. However, eccentric exercise can cause muscle damage through a cellular stress response that includes inflammatory cell activation and nitric oxide involvement [3,10].
The extent of muscle injury after exercise was measured by analyzing plasma CK and LDH levels as indicators of tissue injury and biochemical muscle fatigue markers [9]. In the present study, the increased plasma CK and LDH levels after eccentric exercise were significantly reduced by IH901 administration. However, other serum biochemical factors including albumin, AST, ALT, glucose, HDL, LDL, TC, TG, and UN did not differ among groups (data not shown).
Mitochondrial citrate synthase activities primarily occur in mitochondria, the major site of free radical generation. Free radicals play an important role as mediators of skeletal muscle damage and inflammation after strenuous exercise and in the presence of muscle disease [34]. In the present study, IH901 consumption did not affect CS activity, regardless of exercise or dose; however, IH901 consumption increased activity in the gastrocnemius muscles of the animals that exercised in a dose-dependent manner. It has been postulated that the generation of oxygen free radicals increases during exercise as a result of increased mitochondrial oxygen consumption and electron transport flux, inducing lipid peroxidation [1,19].
Eccentric exercise is known to induce free radical generation, which then causes muscle inflammation, and has been suggested to contribute to increased levels of lipid peroxidation due to macrophage reactions in the tissue [35]. Numerous studies have also shown that TBARS levels increase in the skeletal muscle, liver, plasma, and lungs after eccentric exercise [5,37]. Similarly, the TBARS concentration was significantly higher in both skeletal muscles and lung tissue after exercise in the present study. However, IH901 consumption led to a dose-dependent reduction in TBARS in both skeletal muscles and lung tissue, particularly in exercising animals.
Eccentric running of rats induces a significant 40% increase of protein carbonyls in the lung [30]. The most frequently used biomarker of protein damage is the carbonyl assay, which measures protein carbonyl groups [7,8]. In the present study, the protein carbonyl level was significantly reduced in lung tissue, but the level did not differ among skeletal muscles, regardless of exercise, except in the gastrocnemius muscle in the 100 mg/kg IH901 consumption group. These results are consistent with the results of previous studies that showed that IH901 inhibits the induction of protein carbonyls by inducing protein oxidation and aldehyde production [4,28].
There is some evidence that strenuous exercise may activate the XO pathway [36]. Additionally, XO formed as a result of inadequate supply of oxygen to exercising muscles might generate oxidative damage in other organs, including the lung, by causing increased circulating levels of XO, which binds in the lung to provide a pulmonary source of O2 radicals [39]. Shishehbor et al. [32] reported that human and animal studies have also demonstrated that MPO activity in tissues associated with eccentric exercise-induced tissue damage. Exercise training is also known to contribute to increased capillary blood flow, tissue oxygen utilization and up regulation of the antioxidants and NO systems in cardiovascular tissues [16,17]. Rubbo et al. [31] reported that NO actually inhibits peroxynitrite-induced lipid peroxidation. This report demonstrated that NO significantly enhances lipid peroxidation only when NO production rates approach or are equivalent to XO-induced O2- radical production rates.
IH901 promotes systemic antioxidant effects by suppressing distinct oxidation pathways, including both MPO and NO-derived oxidants [26,32]. The present study showed that XO activity in the lung tissue tended to decrease in both resting and exercise groups supplemented with various doses of IH901. However, the MPO activities in the lung tissue were significantly lower in animals that consumed IH901 with exercise than in the EC. All animals that consumed IH901 showed decreased pulmonary NO synthesis compared to the eccentric-exercised animals that did not consume IH901, except for those in the 50 mg/kg R/IH901 and 100 mg/kg E/IH901 groups. These results indicate that IH901 may be an inhibitor of lipid peroxidation as assessed by the XO and MPO activity and mildly increased pulmonary NO levels in the target tissues.
Antioxidant enzymes are considered the first line of defense against ROS generated during eccentric exercise. The increased levels of antioxidant enzyme activity may have been sufficient in muscles that experienced oxidative damage and lung tissue to cope with ROS generation and avoid lipid peroxidation. Several studies have demonstrated that GPx and SOD activity in muscles and lung tissue are affected by ginseng or ginseng substrates in a dose-dependent manner because of increased cytosolic and mitochondrial GPx and SOD activities [24,29]. Therefore, we examined the activities of GPx, SOD and GST in skeletal muscles and pulmonary tissues after eccentric exercise. In the present study, the GPx activities in animals that received all doses of the E/IH901 and the 100 mg/kg R/IH901 consumption groups were significantly increased in the skeletal muscles and lung tissue; however, the SOD activities were only significantly higher in the lung tissue. Finally, the GST activity in the muscles and lung tissue did not differ among groups.
In conclusion, IH901 consumption in aging rats after eccentric exercise has beneficial effects on potent anti-inflammatory and anti-oxidant activities through down-regulation of pro-inflammatory mediators, lipid peroxidation, and protein oxidation and up-regulation of anti-oxidant enzymes.
Acknowledgments
This work was supported by a Chungbuk National University Grant in 2010.
References
- 1.Alessio HM. Exercise-induced oxidative stress. Med Sci Sports Exerc. 1993;25:218–224. [PubMed] [Google Scholar]
- 2.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 3.Cabral de Oliveira AC, Perez AC, Merino G, Prieto JG, Alvarez AI. Protective effects of Panax ginseng on muscle injury and inflammation after eccentric exercise. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:369–377. doi: 10.1016/s1532-0456(01)00262-9. [DOI] [PubMed] [Google Scholar]
- 4.Cabral de Oliveira AC, Perez AC, Prieto JG, Duarte ID, Alvarez AI. Protection of Panax ginseng in injured muscles after eccentric exercise. J Ethnopharmacol. 2005;97:211–214. doi: 10.1016/j.jep.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Caillaud C, Py G, Eydoux N, Legros P, Prefaut C, Mercier J. Antioxidants and mitochondrial respiration in lung, diaphragm, and locomotor muscles: effect of exercise. Free Radic Biol Med. 1999;26:1292–1299. doi: 10.1016/s0891-5849(98)00342-6. [DOI] [PubMed] [Google Scholar]
- 6.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 7.Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res. 2000;33(Suppl):S99–S108. [PubMed] [Google Scholar]
- 8.Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 9.Dieli-Conwright CM, Spektor TM, Rice JC, Sattler FR, Schroeder ET. Hormone therapy attenuates exerciseinduced skeletal muscle damage in postmenopausal women. J Appl Physiol. 2009;107:853–858. doi: 10.1152/japplphysiol.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans WJ. Vitamin E, vitamin C, and exercise. Am J Clin Nutr. 2000;72:647S–652S. doi: 10.1093/ajcn/72.2.647S. [DOI] [PubMed] [Google Scholar]
- 11.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 12.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 13.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. J Biol Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 14.Harman D. Free radical theory of aging: origin of life, evolution, and aging. Age. 1980;3:100–102. [Google Scholar]
- 15.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 16.Husain K, Hazelrigg SR. Oxidative injury due to chronic nitric oxide synthase inhibition in rat: effect of regular exercise on the heart. Biochim Biophys Acta. 2002;1587:75–82. doi: 10.1016/s0925-4439(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 17.Husain K. Exercise conditioning attenuates the hypertensive effects of nitric oxide synthase inhibitor in rat. Mol Cell Biochem. 2002;231:129–137. doi: 10.1023/a:1014416915643. [DOI] [PubMed] [Google Scholar]
- 18.Jackson MS. Exercise and oxygen radical production by muscle. In: Hänninen O, Packer L, Sen CK, editors. Handbook of Oxidants and Antioxidants in Exercise. Amsterdam: Elsevier; 2000. pp. 57–68. [Google Scholar]
- 19.Kayatekin BM, Gönenç S, Açikgöz O, Uysal N, Dayi A. Effects of sprint exercise on oxidative stress in skeletal muscle and liver. Eur J Appl Physiol. 2002;87:141–144. doi: 10.1007/s00421-002-0607-3. [DOI] [PubMed] [Google Scholar]
- 20.Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2:38–44. doi: 10.1186/1550-2783-2-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000;151:293–299. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 22.Lee M, Sorn S, Baek S, Jang S, Kim S. Antioxidant and apoptotic effects of Korean white ginseng extracted with the same ratio of protopanaxadiol and protopanaxatriol saponins in human hepatoma HepG2 cells. Ann N Y Acad Sci. 2009;1171:217–227. doi: 10.1111/j.1749-6632.2009.04918.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee NJ, Lee JW, Sung JH, Lee YJ, Kang JK. In vitro antioxidant properties of a ginseng intestinal metabolite IH-901. Lab Anim Res. 2011;27:227–234. doi: 10.5625/lar.2011.27.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272:R363–R369. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- 25.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 26.Lin W, Yang S, Chen K, Huang C, Lee N. Protective effects of L-arginine on pulmonary oxidative stress and antioxidant defenses during exhaustive exercise in rats. Acta Pharmacol Sin. 2005;26:992–999. doi: 10.1111/j.1745-7254.2005.00155.x. [DOI] [PubMed] [Google Scholar]
- 27.Moskovitz J, Yim MB, Chock PB. Free radicals and disease. Arch Biochem Biophys. 2002;397:354–359. doi: 10.1006/abbi.2001.2692. [DOI] [PubMed] [Google Scholar]
- 28.Perez AC, de Oliveira ACC, Estevez E, Molina AJ, Prieto JG, Alvarez AI. Mitochondrial, sarcoplasmic membrane integrity and protein degradation in heart and skeletal muscle in exercised rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:199–206. doi: 10.1016/s1532-0456(02)00247-8. [DOI] [PubMed] [Google Scholar]
- 29.Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol. 1994;266:R375–R380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- 30.Radák Z, Nakamura A, Nakamoto H, Asano K, Ohno H, Goto S. A period of anaerobic exercise increases the accumulation of reactive carbonyl derivatives in the lungs of rats. Pflugers Arch. 1998;435:439–441. doi: 10.1007/s004240050537. [DOI] [PubMed] [Google Scholar]
- 31.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 32.Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–431. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 33.Sies H. Biochemistry of oxidative stress. Angew Chem Int Ed Engl. 1986;25:1058–1071. [Google Scholar]
- 34.Stangel M, Mix E, Zettl UK, Gold R. Oxides and apoptosis in inflammatory myopathies. Microsc Res Tech. 2001;55:249–258. doi: 10.1002/jemt.1174. [DOI] [PubMed] [Google Scholar]
- 35.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 36.Viña J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardó FV, Cuesta A, Ferrero JA, Terada LS, Repine JE. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000;49:539–544. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- 37.Voces J, Cabral de Oliveira AC, Prieto JG, Vila L, Perez AC, Duarte ID, Alvarez AI. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz J Med Biol Res. 2004;37:1863–1871. doi: 10.1590/s0100-879x2004001200012. [DOI] [PubMed] [Google Scholar]
- 38.Vogel T, Brechat PH, Leprêtre PM, Kaltenbach G, Berthel M, Lonsdorfer J. Health benefits of physical activity in older patients: a review. Int J Clin Pract. 2009;63:303–320. doi: 10.1111/j.1742-1241.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama Y, Beckman JS, Beckman TK, Wheat JK, Cash TG, Freeman BA, Parks DA. Circulating xanthine oxidase: potential mediator of ischemic injury. Am J Physiol. 1990;258:G564–G570. doi: 10.1152/ajpgi.1990.258.4.G564. [DOI] [PubMed] [Google Scholar]
- 40.Yung LM, Laher I, Yao X, Chen ZY, Huang Y, Leung FP. Exercise, vascular wall and cardiovascular diseases: an update (part 2) Sports Med. 2009;39:45–63. doi: 10.2165/00007256-200939010-00004. [DOI] [PubMed] [Google Scholar]