Abstract
Here, percutaneous spinal cord injury (SCI) methods using a balloon catheter in adult rats are described. A balloon catheter was inserted into the epidural space through the lumbosacral junction and then inflated between T9-T10 for 10min under fluoroscopic guidance. Animals were divided into three groups with respect to inflation volume: 20 µL (n = 18), 50 µL (n = 18) and control (Fogarty catheter inserted but not inflated; n = 10). Neurological assessments were then made based on BBB score, magnetic resonance imaging and histopathology. Both inflation volumes produced complete paralysis. Gradual recovery of motor function occurred when 20 µL was used, but not after 50 µL was applied. In the 50 µL group, all gray and white matter was lost from the center of the lesion. In addition, supramaximal damage was noted, which likely prevented spontaneous recovery. This percutaneous spinal cord compression injury model is simple, rapid with high reproducibility and the potential to serve as a useful tool for investigation of pathophysiology and possible protective treatments of SCI in vivo.
Keywords: balloon compression, laminectomy-free, magnetic resonance imaging, percutaneous spinal cord injury, rat
Introduction
Although many studies have been conducted with the specific goal of developing new therapeutic methods for spinal cord injury (SCI), no substantial outcome has been translated into an effective clinical treatment. Animal models that accurately depict human clinical conditions are necessary for a greater understanding of SCI pathophysiology and the development of new therapeutic methods [3,11,14,17,18,24].
An animal model must produce consistent SCI in a simple and reproducible way with minimal surrounding tissue damage, including laminectomy and soft tissue damage. Moreover, the model should be representative of clinical cases [3,8,11,14,27]. Rat SCI models are classified as transection, contusion and compression models [14,24]. Transection models involve opening of the dura and cutting the spinal cord. Such models are different from human SCI because they are followed by a period of spinal cord compression. Therefore, many studies use the dura-intact compression and contusion models [1,3,19,24].
The most commonly used contusion model is the New York University impactor model [2,3,5,14,21,26,27]. Other contusion-compression models include the Ohio State University impactor model [2,4,7,9] Infinite Horizons impactor model [26], pneumatic device model [7], clip compression model [10,21,23] and balloon compression model [11,13,15,16,18, 22,25]. A previous study revealed that, among weight dropping, clip compression and balloon compression, balloon compression produced a closed injury that most resembled clinical cases [12]. Weight drop models do not create a persistent compression injury effect (the Infinite horizons model has a maximum of 60 s). However, clinical cases that involve events such as dislocations, fracture-dislocations and burst fractures demonstrate persistent injuries [21]. Weight drop and clip compression require a pre-injury laminectomy that results in open exposure of the spinal cord. Pre-injury laminectomy involves the removal of vertebrae, which minimizes secondary damage. Moreover, this technique produces undesirable surrounding soft tissue damage. Damaged soft tissue changes the regenerative environment that is vital to spinal cord regeneration and thus prone to undesirable effects in investigations of spinal cord regeneration [6,11,15,18].
Many studies have described SCI models without laminetomy by use of an embolectomy balloon catheter [11,15,16,17,20,22]. In addition, canine SCI models free from surgical intervention have been described [15,22], and a rat SCI model without laminectomy that involved laminotomy was reported. Laminotomy has also been shown to produce damage to the vertebrae and require surgical intervention to place a small hole in the vertebrae. Therefore, this method has some disadvantages, which include damage to the surrounding soft tissue and risk of infection [27].
Most rat SCI models including transection models feature spontaneously improved locomotor function [1,9,18, 27,28]. A balloon compression model produced variable degrees of injury via inflation volume and time [11,15,17,18]. One study used a 2Fr Forgaty catheter that was inserted into the epidural space and inflated for 5 min to a volume of 20 µL, which produced paralysis with spontaneous recovery noted 4 weeks after the SCI [27]. Another study used a saline filled endovascular occlusion catheter that was placed subdurally and inflated to 10~100 µL for 1~10 min. The group treated by application of 50 µL for 5 min displayed behavioral scores similar to transection; however, complete paralysis was not evident at 4 weeks after the SCI [18]. Spontaneous locomotor improvement in SCI models is a limitation in studies of new SCI therapeutic methods, as chronic SCI is associated with complete paralysis without return of locomotor function.
The rat is the most common laboratory animal used for SCI studies owing to their low cost, ease of handling, and sufficient size for monitoring of physiologic status [9,11,14,27]. The purpose of this study was to establish a minimally invasive SCI model by the percutaneous insertion of a Forgaty catheter into the epidural space, followed by catheter inflation to a volume for a time that produced complete paralysis with no spontaneous recovery.
Materials and Methods
Spinal cord injury
The experimental protocol adhered to the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University, Seoul, Korea. Forty six, male, 9-week-old, 300~330 g Sprague-Dawley rats were used. The rats were divided into three groups according to inflation volume: 20 µL (n = 18), 50 µL (n = 18), and control (n = 10; Fogarty catheter inserted but not inflated). Anesthesia was induced with 3% isoflurane (Forane; Choongwae, Korea) and maintained by 2.5% isoflurane. The rats were placed in the sternal recumbency position and the lumbosacral region was shaved prior to the application of povidone and alcohol.
The epidural space was entered using an 18G spinal needle (Weiss, Fixed Wing, Modified Tohy Point; BD, USA) via the lumbosacral joint under fluoroscopic guidance (Mobile C-RAM system; MCA-6100; Medison Xray, Korea), after which a 2F Forgaty balloon catheter (Edwards Lifesciences, USA) was inserted into the epidural space through the spinal needle under fluoroscopic guidance (Fig. 1A). The 2F Forgaty catheter was then filled with diluted iohexol with saline (1:1) (Omnioaque; Amersham Health, Cork, Ireland) and connected to a 50 µL Hamilton syringe (type 1705; Hamilton Company, USA). The tip of the balloon catheter was placed at T9 and inflated to the desired volume of 20 µL or 50 µL for 10 min using diluted iohexol with saline (1:1) (Figs. 1B and C). Balloon position and shape were confirmed by fluoroscopy, after which the catheter was rapidly deflated and removed. No antibiotics were administered post-procedure. Manual bladder expression was performed twice daily.
Fig. 1.
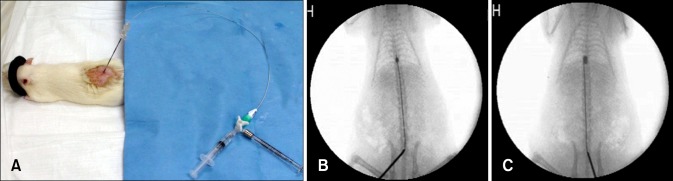
Details regarding the SCI procedure. (A) A spinal needle was placed between the lumbosacral junction and a balloon catheter was inserted into the epidural space through a spinal needle. The balloon was inflated to a volume of 20 µL (B) or 50 µL (C) using half-strength iohexol.
Behavioral assessment
Assessment of locomotor function was made based on the BBB score, which ranged from 0~21 [2]. During assessment, the gait of each rat was recorded with a video camera. Locomotor function analysis was first performed 2 h after SCI and then every week thereafter throughout the survival period.
Magnetic resonance imaging (MRI)
MRI was performed for two animals of each group 1 week after SCI using a 3-T MRI system (Oxford Medinus, Korea). MRI examinations were performed under 2% isoflurane-induced general anesthesia, during which time T1-weighted images (550.0/12.6 [TR/TE]; slice thickness: 1.5 mm no section gap) and T2-weighted images (4400/96 [TR/TE]; slice thickness: 1.5 mm no section gap) were obtained in transverse views.
Histopathological analysis
Histological examinations were performed at 7 days and 28 days after SCI. Briefly, the injured lesions were sectioned horizontally at the indicated time (days) [control n = 5 (7), 5 (28 days); 20 µL n = 5, (5); 50 µL n = 5, (5)] and transversely [20 µL n = 3, (5); 50 µL n = 3, (5)]. The sections had a thickness of 5 µm and were collected in series of five with a distance of 3 mm between individual sections. Additionally, the lumbosacral point of five animals randomly selected at 7 days after SCI were observed. Tissue staining was accomplished using hematoxylin and eosin stain, luxol fast blue/cresyl violet and Masson's Trichrome stain. Additionally, the total size of the lesion was determined for each horizontal section. Image analysis was performed using Image Tool for windows 3.00.
Statistical analyses
BBB scores of all animals in the same experimental group in the same week were averaged, and statistical analysis was performed using one-way ANOVA. Statistical analysis of the lesion area (rostrocaudally 5 mm from the epicenter of lesion) was performed using one-way ANOVA. Tukey's post hoc test was used to identify differences among groups.
Results
Behavioral outcomes
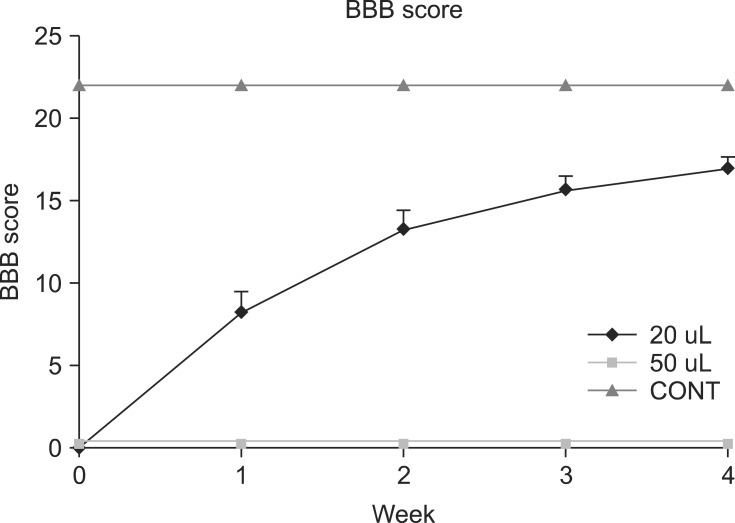
BBB score was measured in each rat until the end of survival (Fig. 2). In the control group, the locomotor function was normal after catheterization. Complete paralysis occurred in the group having an inflated volume of 20 µL immediately after the induced injury; however, various levels of spontaneously improved locomotor function were observed from the first week in most cases, and approximately half of the animals in this group showed a normal gait in the fourth week. For the group treated with an inflation volume of 50 µL, complete paralysis was also observed immediately after injury, and was maintained with no discernable improvement until the fourth week. In the 20 µL group of rats, no voluntary urination was evident immediately after injury, but manual bladder expression was not necessary at 1~2 weeks. In the 50 µL group, voluntary urination was impossible after injury and remained so until autopsy. Statistical analysis revealed significant differences between the control and injured groups (20 µL and 50 µL groups) immediately after SCI and among the control, 20 µL and 50 µL groups one week after SCI (Fig. 2).
Fig. 2.
Locomotor function after SCI graded based on modified BBB scores. Data points represent the group mean ± SEM. After 1 week, all intergroups revealed a significant difference (p < 0.05).
MRI findings
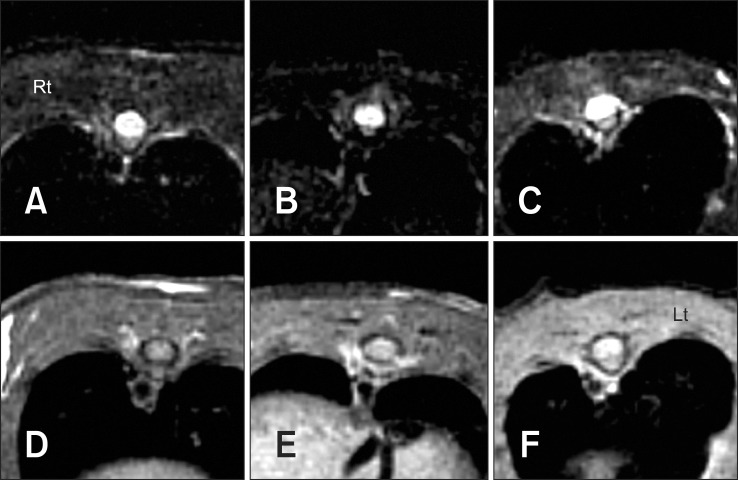
No lesions were evident in the control group. In the 20 µL and 50 µL groups, a hyperintensity signal in a lesion portion was identified in the T2-weighted transverse image and a hyperintense-to-isointense signal was identified in the T1-weighted transverse image. In addition, the center of the lesions had a completely hyperintense signal (Fig. 3), while a wedge shape was observed around the margin in the dorsal portion (Fig. 4)
Fig. 3.
MRI results at 1 week after SCI. Transverse T2-weighted image (A) and T1-weighted images (D) of a control group rat showing normal spinal cord parenchyma, central canal and cerebrospinal fluid. Transverse T2-weighted images of the center of a lesion from a rat in the 20 µL group (B) and the 50 µL group (C) showing a completely hyperintensity signal in spinal cord parenchyma. Transverse T1-weighted images of the center of a lesion in a rat of the 20 µL group (E) and the 50 µL group (F) showing a hyperintense to isointense signal in the spinal cord parenchyma.
Fig. 4.
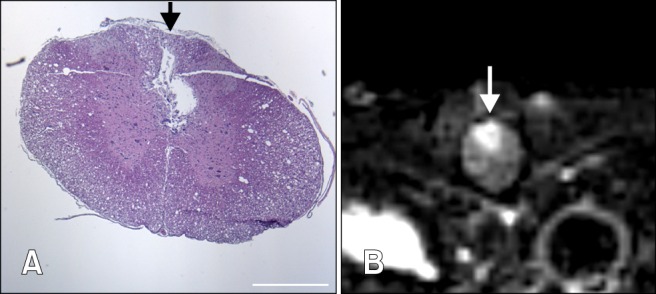
Observations from the 50 µL group of rats. (A) Transversal section of the margin of the lesion of the 50 µL group stained with H&E revealing cavitation and glial scar formation of the dorsal funiculus. Scale bar = 500 µm. (B) Transverse T2-weighted images of the margin of the lesion of the 50 µL group showing a wedge-shaped hyperintensity signal in the dorsal part.
Histopathological findings
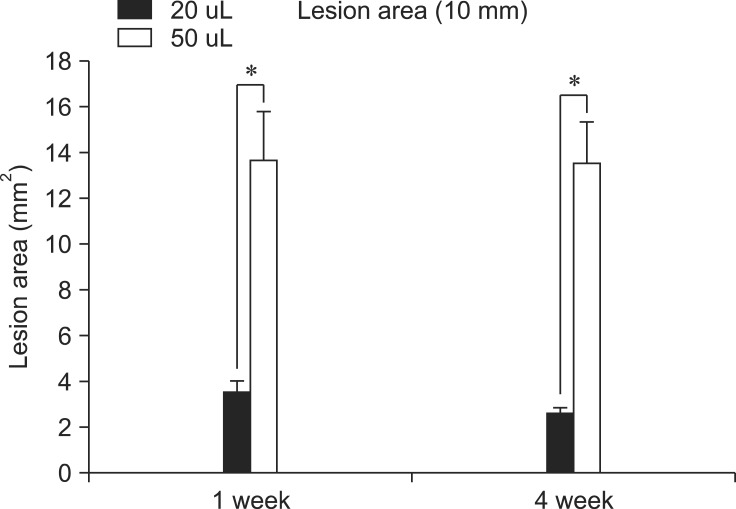
No lesions were detected in the lumbosacral portion of the rats. As expected, the control group did not show any lesions, while the 50 µL group showed more severe lesions than the 20 µL group in the horizontal section (Fig. 5). The gray matter at the center of the lesions was completely lost in the transverse section of the 20 µL rats, whereas some of the normal white matter remained and normal gray and white materials were observed in the rostrocaudal region 3 mm from the lesion center. Vacuolization was observed in the remaining white matter. The core lesion in the 50 µL group had no remaining normal tissue in the white and gray matter. From the 3 mm region, a small amount of remaining gray and white matter was observed, and a lesion was found around the dorsal funiculus (Fig. 4). In the damaged parts, cavitation was observed or fibrosis had already replaced the damage. Fibrosis was identified through Masson's Trichrome staining (Fig. 6). In horizontal sections, lesion areas of the 20 µL and 50 µL groups were measured, and a significant difference was observed between groups, although the difference between the first and fourth weeks was not significant (Fig. 7).
Fig. 5.

Histopahological findings for the horizontal section in Control (A), 20 µL (B) and 50 µL (C) groups stained with H&E at 4 weeks after SCI. In the control group there was no lesion, while the 50 µL group showed a more severe lesion than the 20 µL group.
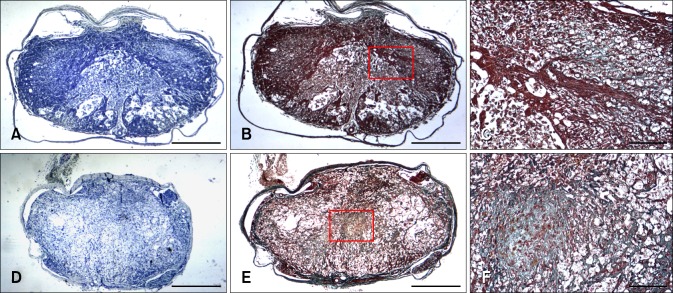
Fig. 6.
Histopahological findings at the injured epicenter in 20 µL (A~C) and 50 µL (D~F) groups 4 weeks after SCI. In the 20 µL group, the Luxol Fast blue/Cresyl violet stained section (A) revealed complete loss of gray matter, whereas the white matter remained partially present. The Masson Trichrome stained section (B) showed blue-stained fibrosis containing collagen. (C) Higher-magnification view of the red-outlined area. In the 50 µL group, the Luxol Fast blue/Cresyl violet stained section (D) revealed the complete loss of gray and white matter, while the Masson Trichrome stained section (E) revealed blue-stained fibrosis containing collagen. (F) Higher-magnification view of the red-outlined area. Scale bars = 500 µm.
Fig. 7.
Assessed area of a lesion in a 10 mm long section of contused spinal cord. Lesion areas of the 20 µL and 50 µL groups were measured and found to differ significantly. The difference in the first and fourth weeks was not significant. Columns represent the group mean ± SEM. *Significant differences (p < 0.05).
Discussion
The results of this study indicate that a complete paralysis model in rats can be produced using a balloon catheter without any surgical intervention such as laminectomy. Presently, the most commonly used SCI model is the weight drop model. However, this model requires extensive laminectomy [2,14,21]. Laminectomy is a potentially beneficial treatment for SCI [6]; therefore, the weight drop model is not considered to closely simulate injury of the human spinal cord owing to the unreduced dislocation or fracture dislocation of the vertebrae [27]. The latter study described an SCI model that did not involve laminectomy; however, this method requires surgical intervention to the spine, typically by laminotomy, which introduces the risk of infection and laminotomymediated spinal cord damage [27].
The present study used only percutaneous procedures, which pose the least risk of infection among rat SCI models developed to date. In addition, our model is less likely to unnecessarily damage the spinal cord and vessels, and is a very simple procedure compared to the SCI model of Vanicky et al. [27]. Our model can also be used to induce injury to any spinal cord segment and make multiple lesions without additional procedures.
MRI is the most important diagnostic method for examining diseases of the spinal cord, and is essential to understanding their pathophysiology. Indeed, MRI is a non-invasive technique that provides very important information regarding the pathophysiology in vivo. Although MRI was used to investigate SCI in rats in one study, only the sagital view was taken and the technique is not actively utilized in SCI research using rats due to the small size of the animals [18].
A wedge-shaped hyperintensity signal was observed on the marginal portion of the SCI, suggesting an infarction of the spinal cord due to obstruction of the dorsal vein. Histopathological examination identified a lesion of the dorsal funiculus (Fig. 4), which is considered to be equivalent to the pencil-shaped softenings described in previous studies [29]. This finding revealed that the use of a balloon catheter can induce spinal cord damage near an injury via infarction through a certain period of compression, which differs from the weight drop model. Thus, the balloon compression model is similar to human SCI that results from unreduced dislocation or fracture dislocation of the vertebrae [27]. The current invasive SCI methods require a surgical procedure, which causes surgical artifact in early stage MRI after SCI, hampering the initial study. In contrast, the present SCI model does not induce any unintentional damage to the spinal cord, and thus enables more information regarding the SCI pathophysiology using MRI.
The submaximal compression dose and treatment time conditions that blunted any spontaneous improvement in motor function were clarified in this study. It is well known that balloon catheter inflation volume and time significantly influence the degree of SCI [15,18,27]. However, the spontaneous improvement of motor function has substantially restricted the understanding of SCI treatment in previous models. In the present study, the balloon was inflated to a volume of 20 µL or 50 µL, and inflation was maintained for 10 min. For conditions of 20 µL and 5 min, spontaneous improvement of motor function was reported [20,27]. A previous study of the effect of volumes ranging from 10~50 µL for 5 min revealed that a 20 µL inflation volume did not impede the near-complete recovery of motor function, while a 50 µL volume produced only a slight recovery, which is consistent with our findings [18]. Taken together, the results of previous studies and those of the present study affirm the capability of an inflation volume of 50 µL for 10 min to be capable of causing virtually non-repairable SCI.
SCI treatment and pathophysiology studies have required different models, which has impeded comparison of the outcomes. Many studies have used the weight drop SCI model, which, while useful, is limited by inconsistency of results depending on the size and shape of the impounder as well as the modified posture and chest cavity movement [18,27]. In contrast, the presently developed SCI model has excellent reproducibility since the Fogarty catheter used for balloon compression is commercially available worldwide [27]. Overall, our model is a simple and rapid percutaneous SCI model that does not cause unintentional damage or infection. This model holds promising potential for future investigations of SCI treatment and pathophysiology.
Acknowledgments
This study was supported by Konkuk University in 2011.
References
- 1.Aoki M, Kishima H, Yoshimura K, Ishihara M, Ueno M, Hata K, Yamashita T, Iwatsuki K, Yoshimine T. Limited functional recovery in rats with complete spinal cord injury after transplantation of whole-layer olfactory mucosa. J Neurosurg Spine. 2010;12:122–130. doi: 10.3171/2009.9.SPINE09233. [DOI] [PubMed] [Google Scholar]
- 2.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 5.Behrmann DL, Bresnahan JC, Beattie MS. Modeling of acute spinal cord injury in the rat: neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Exp Neurol. 1994;126:61–75. doi: 10.1006/exnr.1994.1042. [DOI] [PubMed] [Google Scholar]
- 6.Benzel EC, Lancon JA, Bairnsfather S, Kesterson L. Effect of dosage and timing of administration of naloxone on outcome in the rat ventral compression model of spinal cord injury. Neurosurgery. 1990;27:597–601. doi: 10.1097/00006123-199010000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Bilgen M, Abbe R, Narayana PA. Dynamic contrastenhanced MRI of experimental spinal cord injury: in vivo serial studies. Magn Reson Med. 2001;45:614–622. doi: 10.1002/mrm.1083. [DOI] [PubMed] [Google Scholar]
- 8.Blight AR. Spinal cord injury models: Neurophysiology. J Neurotrauma. 1992;9:147–150. doi: 10.1089/neu.1992.9.147. [DOI] [PubMed] [Google Scholar]
- 9.Bresnahan JC, Beattie MS, Todd FD, 3rd, Noyes DH. A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Exp Neurol. 1987;95:548–570. doi: 10.1016/0014-4886(87)90299-8. [DOI] [PubMed] [Google Scholar]
- 10.Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda S, Nakamura T, Kishigami Y, Endo K, Azuma T, Fujikawa T, Tsutsumi S, Shimizu Y. New canine spinal cord injury model free from laminectomy. Brain Res Brain Res Protoc. 2005;14:171–180. doi: 10.1016/j.brainresprot.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Khan M, Griebel R. Acute spinal cord injury in the rat: comparison of three experimental techniques. Can J Neurol Sci. 1983;10:161–165. doi: 10.1017/s031716710004484x. [DOI] [PubMed] [Google Scholar]
- 13.Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S. A model for acute, chronic, and delayed graded compression of the dog cauda equina: presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine (Phila Pa 1976) 1995;20:2758–2764. doi: 10.1097/00007632-199512150-00019. [DOI] [PubMed] [Google Scholar]
- 14.Kwon BK, Oxland TR, Tetzlaff W. Animal models used in spinal cord regeneration research. Spine (Phila Pa 1976) 2002;27:1504–1510. doi: 10.1097/00007632-200207150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Choi CB, Chung DJ, Kang EH, Chang HS, Hwang SH, Han H, Choe BY, Sur JH, Lee SY, Kim HY. Development of an improved canine model of percutaneous spinal cord compression injury by balloon catheter. J Neurosci Methods. 2008;167:310–316. doi: 10.1016/j.jneumeth.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Lim JH, Jung CS, Byeon YE, Kim WH, Yoon JH, Kang KS, Kweon OK. Establishment of a canine spinal cord injury model induced by epidural balloon compression. J Vet Sci. 2007;8:89–94. doi: 10.4142/jvs.2007.8.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonjon N, Kouyoumdjian P, Prieto M, Bauchet L, Haton H, Gaviria M, Privat A, Perrin FE. Early functional outcomes and histological analysis after spinal cord compression injury in rats. J Neurosurg Spine. 2010;12:106–113. doi: 10.3171/2009.7.SPINE0989. [DOI] [PubMed] [Google Scholar]
- 18.Martin D, Schoenen J, Delrée P, Gilson V, Rogister B, Leprince P, Stevenaert A, Moonen G. Experimental acute traumatic injury of the adult rat spinal cord by a subdural inflatable balloon: methodology, behavioral analysis, and histopathology. J Neurosci Res. 1992;32:539–550. doi: 10.1002/jnr.490320409. [DOI] [PubMed] [Google Scholar]
- 19.Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17:1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- 20.Pedram MS, Dehghan MM, Soleimani M, Sharifi D, Marjanmehr SH, Nasiri Z. Transplantation of a combination of autologous neural differentiated and undifferentiated mesenchymal stem cells into injured spinal cord of rats. Spinal Cord. 2010;48:457–463. doi: 10.1038/sc.2009.153. [DOI] [PubMed] [Google Scholar]
- 21.Poon PC, Gupta D, Shoichet MS, Tator CH. Clip compression model is useful for thoracic spinal cord injuries: histologic and functional correlates. Spine (Phila Pa 1976) 2007;32:2853–2859. doi: 10.1097/BRS.0b013e31815b7e6b. [DOI] [PubMed] [Google Scholar]
- 22.Purdy PD, Duong RT, White CL, 3rd, Baer DL, Reichard RR, Pride GL, Jr, Adams C, Miller S, Hladik CL, Yetkin Z. Percutaneous translumbar spinal cord compression injury in a dog model that uses angioplasty balloons: MR imaging and histopathologic findings. AJNR Am J Neuroradiol. 2003;24:177–184. [PMC free article] [PubMed] [Google Scholar]
- 23.Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10:38–43. [PubMed] [Google Scholar]
- 24.Rosenzweig ES, McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004;17:121–131. doi: 10.1097/00019052-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, Konno S, Yabuki S, Mao GP, Olmarker K, Kikuchi S. A model for acute, chronic, and delayed graded compression of the dog cauda equina: neurophysiologic and histologic changes induced by acute, graded compression. Spine (Phila Pa 1976) 1995;20:2386–2391. doi: 10.1097/00007632-199511001-00003. [DOI] [PubMed] [Google Scholar]
- 26.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 27.Vanický I, Urdzíková L, Saganová K, Cízková D, Gálik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001;18:1399–1407. doi: 10.1089/08977150152725687. [DOI] [PubMed] [Google Scholar]
- 28.Wrathall JR, Pettegrew RK, Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985;88:108–122. doi: 10.1016/0014-4886(85)90117-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Nonaka H, Nagayama T, Hatori T, Ihara F, Zhang L, Akima M. Circulatory disturbance of rat spinal cord induced by occluding ligation of the dorsal spinal vein. Acta Neuropathol. 2001;102:335–338. doi: 10.1007/s004010100377. [DOI] [PubMed] [Google Scholar]