Editor’s note:
The World Health Organization predicts that depression, which already affects about 10 percent of the population in the United States, will be the world’s leading medical condition by 2030. Evidence suggests several causes for depression, including traumatic life events, disease, poison, and nutritional deficiencies. Many of these causes are associated with elevated levels of inflammatory biomarkers in the blood, which may in turn lead to inflammatory changes in the brain. Our authors examine what the latest research reveals about link between inflammation in the brain and depression, and how a better understanding of that link can play a critical first step in the personalization of care.
It is quite the rage these days to say that depression is an inflammatory disorder. This conclusion is based on hundreds of studies showing that groups of people with depression have elevated levels of a variety of peripheral inflammatory biomarkers, especially two cytokines—tumor necrosis factor (TNF) and interleukin (IL)-6—and an acute phase reactant called C-reactive protein (CRP).1 To a large degree, those of us who study the role of inflammation in depression are responsible for spreading the idea. But with the passage of time and the ongoing accumulation of findings from a variety of scientific disciplines, it is becoming increasingly apparent that this characterization, while attractive in the popular press, confuses as much as it clarifies, and is in deep need of refinement.
Suppose a patient comes to see the doctor with a painful, swollen right knee. The doctor suspects rheumatoid arthritis (RA), a classic inflammatory condition, and sends off an aspirate of synovial fluid from the offending knee for analysis. Given that concentrations of inflammatory cytokines such as IL-6 are typically many times higher in the synovial fluid of an affected RA joint than in the blood of a healthy adult, what would be the doctor’s conclusion if the joint fluid came back with normal cytokine levels? Almost certainly RA would be ruled out as the cause. No inflammation, no inflammatory disorder.
Now consider an analogous situation, but this time the patient complains of depression. The doctor orders a blood test to measure levels of inflammatory biomarkers. The results come back, and the doctor has a dilemma. The patient is weeping and utterly downcast; shows signs of apathy; can’t sleep, eat, or concentrate; and has suicidal thoughts. But all the patient’s inflammatory measures are completely normal. Does the doctor conclude that because depression is an inflammatory condition, the patient isn’t depressed, no matter how miserable the patient feels? Conversely, if the doctor orders a blood test on someone in good spirits, and the test comes back with evidence of increased inflammation, should the doctor insist that the patient is depressed, no matter how good the patient feels?
What these clinical vignettes illustrate is that increased inflammation—however measured—is neither necessary nor sufficient for the diagnosis of depression, and that, therefore, depression cannot be considered an inflammatory disorder without doing a fair amount of damage to the notion of what it means to be an inflammatory disorder. So how should we best understand the association between inflammation and depression? We believe the best available data suggest the following: depression is not an inflammatory disorder per se; rather, in some patients inflammatory processes appear to contribute significantly to the development and maintenance of symptoms of depression. This perspective unifies much of the scientific literature in the field, while simultaneously suggesting novel diagnostic and treatment approaches for the subgroup of depressed patients with increased inflammation.
The Unspoken Truth
A headline such as “Depression Is Associated with Increased Inflammation” reflects a failure to consider that studies generally indicate that the mean value for a given inflammatory biomarker tends to be higher in depressed groups than in groups of non-depressed individuals. This does not mean that all subjects with depression have higher inflammatory levels than all non-depressed comparison subjects, or that the inflammatory levels in depressed patients are abnormal. Indeed, the mean differences in inflammation between depressed and nondepressed individuals are modest, and mean values for inflammatory markers in groups of depressed individuals are typically in the “normal” range when such norms are established, as is the case with C-reactive protein.
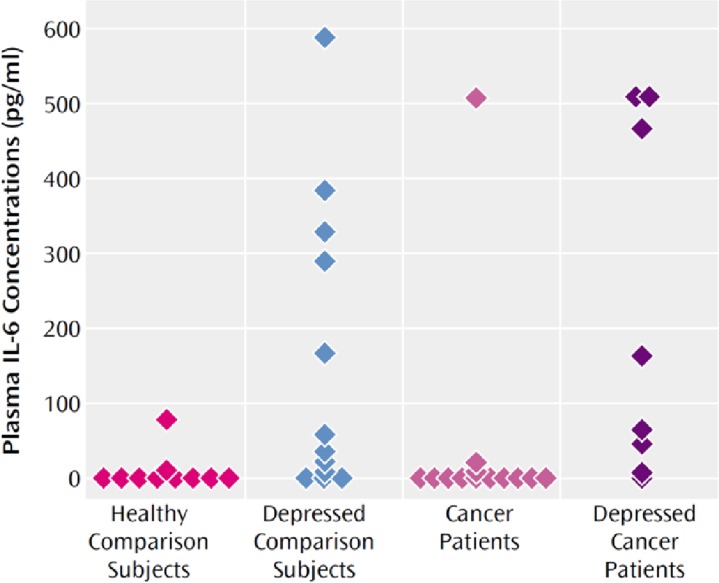
By way of illustrating this point, levels of C-reactive protein in people with autoimmune and inflammatory conditions or acute infection range from 10 to over 100 mg/L,2–4 versus depression or no diagnosis, where C-reactive protein ranges from 0 to 10 (with a CRP <3 mg/L generally considered the upper limit of normal).5 Figure 1 (see below) illustrates the other truth about inflammation and depression that is not always adequately emphasized. Despite differences in mean levels of C-reactive protein or IL-6 in this case, there is a huge overlap between people with depression and those without, and in any given study, the highest inflammatory value might be found in a control subject, while the lowest might be found in the depressed group.6
Figure 1.
Plasma Interleukin-6 (IL-6) Concentrations of Healthy Comparison Subjects, Comparison Subjects With Major Depression, and Cancer Patients With and Without Major Depression
Reproduced with permission from American Psychiatric Publishing (Musselman et al, Am J Psychiatry, Vol. 158, Issue 8, pages 1252–1257, 2001).
The first point—that the inflammatory activation observed in people with depression is modest—might tempt us to dismiss the potential relevance of inflammation to the pathophysiology of depression. But this would be both a serious mistake and a profound misunderstanding of the huge effect that small physiological differences can have over time if they are consistently skewed in one direction. As it turns out, depression is far from being alone as a condition characterized by reliable—but often only mildly increased—inflammatory activity. Other modern illnesses with evidence of moderately increased inflammatory signaling include cardiovascular disease, stroke, cancer, diabetes, and dementia. Conversely, even minor increases in inflammation—such as the ones observed in depression—are enough to strongly predict the development over time of many of these modern disease states, including depression. 5,7
The second point—that there is a high level of overlap of inflammatory biomarker levels between depressed and nondepressed groups—raises a more complex issue. When we say that groups of depressed people tend to have elevated levels of inflammatory biomarkers, what we really mean is that within any depressed group, there are individuals with levels that are significantly higher than those seen in the vast majority of healthy, nondepressed people, whereas there are many other depressed people with perfectly normal values. It is a dirty little secret of sorts that the one-third or so of depressed individuals with elevated inflammation have been pulling all their noninflamed, depressed colleagues along with them in publication after publication, giving the world a slightly misguided sense that depression—as a whole—is driven by increased inflammation.
The critical question is whether inflammation is relevant to depression as a whole or only to individuals with chronically elevated inflammatory biomarkers. And if depression is relevant only to those with increased inflammation, how much of an increase needs to exist before it reliably contributes to depressive pathogenesis? And might it be the case that people with depression and low levels of inflammation are just more sensitive to the depressogenic effects of inflammatory activity, so that even low levels disrupt brain functioning in ways that promote the disorder?
What Cytokine Antagonism Has Taught Us
A recent study from our group provides some surprising, tentative answers to these questions. Determined to see if peripheral inflammatory processes really contribute to depressive pathogenesis, we decided to put the theory to the test by examining whether blocking the inflammatory cascade would eradicate depression in patients who were otherwise medically stable. To test this as rigorously as possible, we elected to use a medication called infliximab, which is not believed to cross the blood-brain barrier—a tight layer of cells and tissue that separates the brain from the rest of the body—and has no biological effects other than to potently block the activity of TNF, the cytokine that along with IL-1beta is most responsible for initiating the inflammatory response.8 We measured pretreatment levels of peripheral inflammation in 60 patients with treatment-resistant depression, which has been shown to have a special relationship with increased inflammation, in part related to the ability of cytokines to sabotage and circumvent the mechanism of action of antidepressants. Patients were then randomized to receive three infusions of either infliximab or saline in a blinded manner over a six-week period. We followed depressive symptoms during this period and for six weeks following the final infusion.
The results were unequivocal. For the group as a whole, infliximab was no better than placebo—in fact, it was nearly identical. This strongly suggests that inflammatory processes are not directly causal for the entire spectrum of pathology that we currently characterize as depression. Interestingly, however, we found a significant relationship between pretreatment levels of inflammation and clinical response. Indeed, for people with baseline levels of C-reactive protein above 5 mg/L, infliximab outperformed placebo to the same degree that standard antidepressants do.
What we were also not expecting was that for depressed individuals with low levels of peripheral inflammation, placebo far outperformed infliximab, suggesting that blocking inflammation was not just neutral, but was actually doing something profoundly negative in these individuals. At the least it was blocking people’s ability to respond to placebo. More worrisome is the possibility that it was blocking potentially positive effects of inflammation. Perhaps we should not have been as surprised as we were. Animal work by psychobiologist Raz Yirmiya and others had already suggested that at lower concentrations, inflammatory molecules such as TNF play important roles in processes associated with positive brain functioning, including neurogenesis, synaptic scaling, and long-term potentiation.9 More recently, the antidepressant-like effect of deep brain stimulation (DBS) in rodents has been shown to be associated not with electrode placement or even with the passage of electric current, but rather with the local brain inflammation induced by needle placement.10 Consistent with this, the use of anti-inflammatory agents in humans seems to block the efficacy of DBS in patients with treatment-resistant depression. This negative association between anti-inflammatory agents and response was also noted with standard antidepressants in the STAR*D trial.11
Taken together, these results suggest that the relationship between inflammation and depression may be U shaped, with both very low levels and high levels of activity posing risks, albeit for different reasons. Nevertheless, the modern world is so replete with depression risk factors that also promote inflammation—including obesity, sedentary lifestyle, sleep loss, psychosocial stress, processed foods, air pollution, and perhaps hygienic practices (separating us from exposure to a range of coevolved microorganisms and parasites with powerful immunomodulatory and anti-inflammatory effects12)—that the association between inflammation and depression is hardly surprising. This is especially true given that relatively mild elevations in peripheral inflammatory activity—when chronic—seem sufficient to set in motion physiological mechanisms that are depressogenic, particularly in individuals made vulnerable by early environment and/or genetic inheritance.13 It is to these mechanisms that we now turn.
How Inflammation Produces Depression
When we started exploring how inflammation produces depression, we were convinced that depressogenic pathways unique to the immune system would be identified; leading to the conclusion that depression that occurs in response to stress or comes “out of the blue” is biologically different from depression induced by immune activation. We couldn’t have been more wrong.
One of the most surprising, and consistent, findings over the last decade has been that inflammatory processes induce depression because they are capable of tapping in to every known risk pathway. In this regard, inflammation functions like psychological stress, which has similar wide-ranging, and generally depressogenic, biological effects. In fact, inflammation causes most of the same changes in the brain and body that have been repeatedly observed in animals and humans exposed to stress, especially when the stress is chronic and of a psychosocial nature.1
One of the best models for understanding the impact of chronic inflammation on humans has been treatment with the cytokine interferon (IFN)-alpha for either cancer or hepatitis C virus infection. IFN-alpha causes full-blown depression in a respectable minority of patients, produces depressive symptoms in a far higher percentage, and produces an improvement in mood in no one but the unfortunate few who develop mania in response to the treatment.14 Our understanding of the central-nervous-system (CNS) effects of peripheral inflammation also have been augmented by studies of humans who became acutely inflamed as a result of receiving a dose of lipopolysaccharide or a typhoid vaccine.
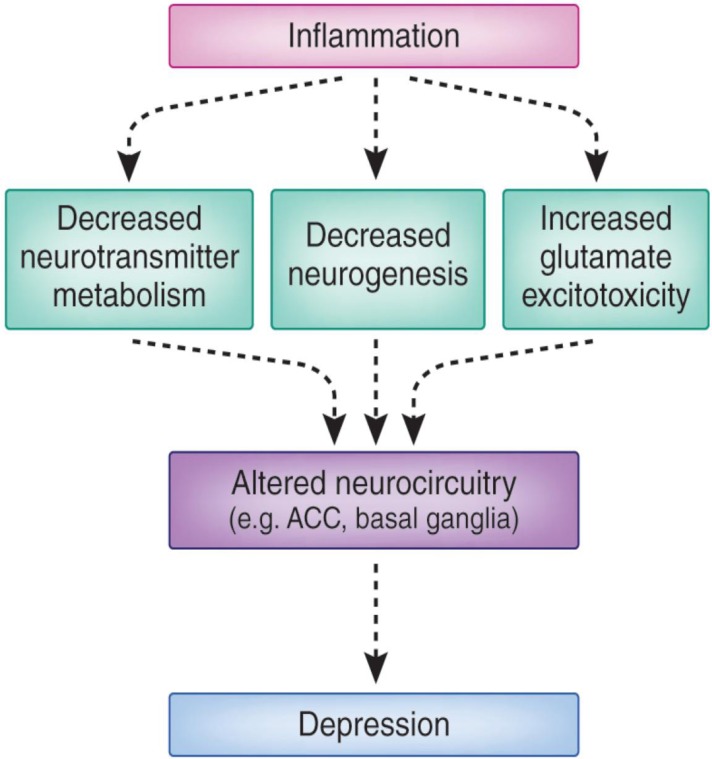
Figure 2 (see below) provides a schematic of the neurobiological pathways known to be influenced by both acute and chronic inflammatory stimuli in humans, ultimately leading to alterations in neurocircuitry and behavior. Neuroimaging studies reliably indicate that peripheral inflammation targets brain regions repeatedly implicated in the pathophysiology of depression, especially the anterior cingulate cortex (ACC) and basal ganglia.15 Depending on the nature of the stimulus, the impact of cytokines on the ACC is seen most strongly in either the subgenual or the dorsal area.16,17 Cytokine-induced increases in neural activity in these regions have been associated with the development of mood and anxiety symptoms. On the other hand, peripheral immune activation has been shown repeatedly to impair basal ganglia functioning in ways that are consistent with the known inhibitory effects of cytokines on dopamine signaling in the CNS.18,19 Reductions in basal ganglia activity have been noted in more posterior regions, where they associate with fatigue, and in more ventral regions (such as the nucleus accumbens), where they have been associated with the development of anhedonia, a psychological condition characterized by an inability to experience enjoyment in normally pleasurable acts.18
Figure 2.
Inflammation and Depression: Pathways to Pathology
Regarding the mechanisms of the effects of cytokines on these and other brain regions, cytokines have been shown repeatedly to alter neurotransmitter signaling in the CNS in ways that are relevant to the pathophysiology of depression and its treatment. For example, through activation of the intracellular signaling pathway mitogen-activated protein kinase, cytokines can increase the number and function of the reuptake pumps for serotonin, norepinephrine, and dopamine, which in turn can reduce the availability of these neurotransmitters within the synaptic cleft. This is relevant to depression and its treatment given that most currently available antidepressants act by blocking these reuptake pumps to increase neurotransmitter availability in the synapse.
Cytokines have other effects known to impact neurotransmitter availability. Indeed, by activating enzyme indoleamine 2, 3-dioxygenase, cytokines can shunt tryptophan away from the production of serotonin and into the production of kynurenine. Kynurenine is transported to the brain and can be converted by activated microglia (innate cells in the brain) to the neurotoxic metabolite quinolinic acid. The clinical relevance of this process has been shown by the association between cerebrospinal fluid levels of kynurenine and quinolinic acid, and the development of depression during treatment with IFN-alpha.1 Moreover, increased quinolinic acid has been found in activated microglia in the ACC of suicide victims who were depressed. Quinolinic acid can impact glutamate signaling in ways relevant to depression, including the stimulation of extrasynaptic N-methyl-D-aspartate (NMDA) receptors, which lead to the downregulation of the production of brain-derived neurotrophic factor (BDNF), a potent inducer of neurogenesis. Consistent with this and other activities of inflammatory cytokines, animal and human studies have demonstrated that increased inflammatory cytokines can reduce central levels of BDNF and neurogenesis, leading to depressive-like behavior.20
Depression is not just a brain disease. Indeed, many of the depression-related physiological abnormalities identified at the dawn of biological psychiatry involved the body’s stress system and especially the hypothalamic-pituitary-adrenal (HPA) axis. As a group, depressed individuals have been repeatedly reported to demonstrate increased circulating cortisol and concomitant glucocorticoid resistance (e.g. decreased sensitivity to the inhibitory effects of glucocorticoids on HPA axis regulation and inflammation).21 Depressed patients also show a flatter diurnal pattern of cortisol secretion than do healthy control subjects. Strikingly, inflammatory cytokines have been shown to be capable of producing all these abnormalities, including glucocorticoid resistance, and in the context of treatment with IFN-alpha, flattening of the cortisol slope strongly predicts the development of depression.22
Do Stress and Inflammation Always Cause Depression?
Further suggesting a link between inflammation and depression is the fact that psychosocial stress—which is a primary risk factor for depression development—reliably activates peripheral inflammatory pathways in humans. This activation is measured either as increases in plasma concentrations of inflammatory cytokines such as IL-1beta and IL-6 or as increased activation of the intracellular inflammatory transcription element nuclear factor-kappa beta (NFkB).1
Although not established in humans, psychological stressors in laboratory animals have been shown repeatedly to increase levels of proinflammatory cytokines in the CNS, especially in areas, such as the hippocampus, that are integrally involved in the mammalian stress response and repeatedly implicated in depression. Importantly, in these animal models, the behavioral and biological effects of stress (such as reductions in BDNF) can be made more tolerable by blocking the stress-induced increases in CNS inflammatory signaling.20 In animal models, stress has been shown to activate CNS microglial cells, which take on an inflammatory phenotype when activated.23 Of relevance to depression, several postmortem studies point to evidence of microglial activation in individuals who died by suicide.24 Also linking inflammation to depression is the fact that individuals at high risk for depression—such as those exposed to early-life adversity—respond to laboratory psychosocial stressors with more robust inflammatory responses than do others.25 Inflammatory responses to these types of stressors have, in turn, been shown to predict the future development of depression.
The Implications for Care
The science is clear: While depression is not an inflammatory condition, inflammation can cause depression, and inflammatory cytokines are clearly a factor. Although it is simple, this idea has profound implications. It suggests that inflammatory processes will be highly relevant to some individuals with depression, irrelevant to others, and perhaps even of benefit to a minority of depressed individuals. Recent data suggest that something as simple as the widely available blood test for C-reactive protein may identify individuals with greater or lesser likelihood of responding to anti-inflammatory therapeutic strategies and, by extension, individuals for whom inflammation is more or less of a causative factor. Such identification of patients with depression and increased inflammation represents a critical first step to the personalization of care, allowing specific treatments to be directed to specific pathologies that can then be monitored as a function of response. Such a development represents a game changer in psychiatry and truly emphasizes that when it comes to treatment for depression, one size does not fit all.
Footnotes
Article available online at http://www.dana.org/news/cerebrum/detail.aspx?id=44322
References
- 1.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaudo G, Marchesi S, Gerli R, Allegrucci R, Giordano A, Siepi D, Pirro M, Shoenfeld Y, Schillaci G, Mannarino E. Endothelial dysfunction in young patients with rheumatoid arthritis and low disease activity. Ann Rheum Dis. 2004;63:31–35. doi: 10.1136/ard.2003.007740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamouard P, Richert Z, Meyer N, Rahmi G, Baumann R. Diagnostic value of C-reactive protein for predicting activity level of Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:882–887. doi: 10.1016/j.cgh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Hoeboer SH, Groeneveld AB. Changes in circulating procalcitonin versus C-reactive protein in predicting evolution of infectious disease in febrile, critically ill patients. PLoS One. 2013;8:e65564. doi: 10.1371/journal.pone.0065564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG. Elevated C-Reactive Protein Levels, Psychological Distress, and Depression in 73 131 Individuals. Arch Gen Psychiatry. 2012:1–9. doi: 10.1001/2013.jamapsychiatry.102. [DOI] [PubMed] [Google Scholar]
- 6.Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 7.Couzin-Frankel J. Inflammation bares a dark side. Science. 2010;330:1621. doi: 10.1126/science.330.6011.1621. [DOI] [PubMed] [Google Scholar]
- 8.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Caballero L, Perez-Egea R, Romero-Grimaldi C, Puigdemont D, Molet J, Caso JR, Mico JA, Perez V, Leza JC, Berrocoso E. Early responses to deep brain stimulation in depression are modulated by anti-inflammatory drugs. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.63. [DOI] [PubMed] [Google Scholar]
- 11.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol Psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci U S A. 2010;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL, Miller AH. Chronic Interferon-alpha Decreases Dopamine 2 Receptor Binding and Striatal Dopamine Release in Association with Anhedonia-like Behavior in Non-Human Primates. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 22.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]